Abstract
Background and Aims:
Patients with chronic pulmonary aspergillosis (CPA) who discontinue antifungal therapy commonly exhibit disease recurrence. We aimed to evaluate the utility of the St. George’s respiratory questionnaire (SGRQ) in predicting the likelihood of clinical recurrence of CPA in patients who come off antifungal therapy.
Methods:
This audit included CPA patients for whom antifungal therapy was discontinued for at least 1 month. Comparisons were made between the quality of life scores at the time of discontinuation of treatment and at the time of diagnosis of clinical recurrence. The change in patients’ self-assessment scores was also compared.
Results:
There were 33 cases and 44 controls. Of the 33 cases, 22 (67%) were males with a mean age of 62 ± 13 years. The median for the symptom component of quality of life (QoL) changed from 78.4 at the time of discontinuation of therapy to 83.1 units at the time of diagnosis of clinical failure (p = 0.043), whereas that of the impact and activity components changed from 62.7 to 59.1 units (p = 0.387) and 85.0 to 85.9 units (p = 0.153), respectively. At 12 months, the symptoms domain of SGRQ was able to discriminate between cases of clinical recurrence and controls [area under the curve (AUC) 0.7, 95% confidence interval (CI): 0.6–0.8, p = 0.009]. The proportion of patients in very poor health status increased from 3/11 (9.1%) to 11/33 (33.3%) (p = 0.046).
Conclusion:
A deteriorating symptoms component of the SGRQ and a worsening of patients’ self-assessment are associated with clinical recurrence. Failure to improve by >8 units in the symptoms domain appear to be a marker of disease recurrence. We propose that the clinical approach to diagnose recurrent CPA would be a combination of clinical history, SGRQ scoring, chest imaging and a workup to exclude other causes of the patients’ symptoms.
Keywords: antifungal, chronic pulmonary aspergillosis, domains, recurrence, St. George’s respiratory questionnaire
Introduction
Chronic pulmonary aspergillosis (CPA) is a slowly progressive and destructive disease of the lungs, with radiological features characterised by cavity formation or enlargement of pre-existent cavities with or without intra-cavitary aspergilloma, parenchymal fibrosis or pleural thickening.1,2 Patients most commonly have a history of chronic obstructive pulmonary disease (COPD), prior pulmonary tuberculosis (TB) or non-tuberculous mycobacterial infection, or allergic bronchopulmonary aspergillosis (ABPA). 3
Long-term (⩾6 months) oral antifungal therapy is the mainstay of management, with response rates of about 50–60%.4–6 About half of CPA patients come off or have to change antifungal therapy for several reasons, but mainly triazole resistance, intolerance, lack of response (clinical failure) or a combination of these circumstances.6,7 However, patients who discontinue antifungal therapy commonly exhibit disease recurrence with worsening morbidity and mortality.4,8 The St. George Respiratory Questionnaire (SGRQ) in combination with the Medical Research Council (MRC) dyspnoea scale can be used to subjectively assess well-being and health status of CPA patients. 9 The SGRQ has 50 items that assess three domains, namely symptom, activity and impact domains, in addition to the patient’s evaluation of overall health status (self-assessment). The symptom domain assesses the frequency and severity of a respiratory condition while the activity domain assesses activities that cause, or are limited by, a respiratory condition. The impact domain evaluates social functioning and psychological disturbances resulting from a chronic respiratory disease. The scores range from 0 to 100, with higher scores indicating more severe disease. 10
We aimed to evaluate the utility of the SGRQ in predicting the likelihood of clinical recurrence of CPA in patients who come off antifungal therapy for various reasons.
Materials and methods
We carried out a retrospective clinical audit of CPA patients for whom antifungal therapy was discontinued for at least 1 month between June 2014 and May 2017 at the National Aspergillosis Centre (NAC), Manchester, United Kingdom (UK).
Patients diagnosed with clinical recurrence (defined as clinical deterioration while off antifungal therapy necessitating a clinical decision to recommence antifungal therapy) were identified. Clinical deterioration was any deterioration in systemic (e.g. fatigue, weight loss) or respiratory symptoms (e.g. breathlessness, productive cough and haemoptysis) of CPA, as reported by the patient and documented in the patient’s notes. This clinical deterioration should not have been attributable to other disease processes, notably concurrent pulmonary infection, overt deterioration in underlying disease or toxicity of non-antifungal drugs. The control group consisted of CPA patients who were off therapy for 12 months following discontinuation of treatment.
Data collection
The web-based SGRQ scores were retrieved for both the eligible cases and controls. Clinical case-notes were reviewed to extract relevant data.
Statistical analysis
The Wilcoxon matched-pairs signed rank test was used to compare QoL scores at the time of discontinuation of treatment and at the time of diagnosis of clinical recurrence across the three domains of the SGRQ. The McNemar–Bowker test was used to compare the change in patients’ self-assessment scores. The QoL changes in controls were assessed from baseline to 12 months. A receiver operating characteristic (ROC) curve analysis was conducted to assess the discriminative ability of SGRQ to distinguish CPA patients with clinical recurrence. The SGRQ values were taken at the time of discontinuation of antifungal treatment, 3 and 12 months for the controls and at the time of discontinuation of antifungal treatment and diagnosis of clinical recurrence for the cases. Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). All tests were two-tailed and p ⩽ 0.05 was considered statistically significant.
Ethical statement
Ethical review was not required as data was collected as part of a clinical audit and only anonymized data was used.
Results
Demographic characteristics and underlying conditions
In total, 33 cases and 44 controls were included in this study. Of the 33 cases, 22 (67%) were males with a mean age of 62 ± 13 years. Mean age of the controls was 65 ± 11 years and 26 (59%) of the 44 controls were men.
COPD (27% versus 25%), tuberculous (9% versus 30%) and non-tuberculous (3% versus 3%) mycobacterial infections were the most common underlying conditions in both groups (45% versus 62%) (Table 1). The median time to re-initiation of treatment for cases was 5 (range: 1–11) months.
Table 1.
Underlying lung conditions among patients with CPA.
| Underlying disorder | Case | Control |
|---|---|---|
| Frequency (%) | Frequency (%) | |
| Chronic obstructive pulmonary disease | 9 (27) | 11 (25) |
| Pneumothorax | 4 (12) | 1 (2) |
| Non-tuberculous mycobacteria | 3 (9) | 3 (7) |
| Tuberculosis | 3 (9) | 13 (30) |
| Asthma | 3 (9) | 1 (2) |
| Lobectomy | 3 (9) | 3 (7) |
| Allergic bronchopulmonary aspergillosis | 2 (6) | 3 (7) |
| Asbestosis | 1 (3) | 1 (2) |
| Community acquired pneumonia | 1 (3) | – |
| Severe asthma with fungal sensitisation | 1 (3) | – |
| Sarcoidosis | 1 (3) | 2 (5) |
| Total | 33 (100) | (100) |
CPA, chronic pulmonary aspergillosis.
Change in SGRQ QoL domains
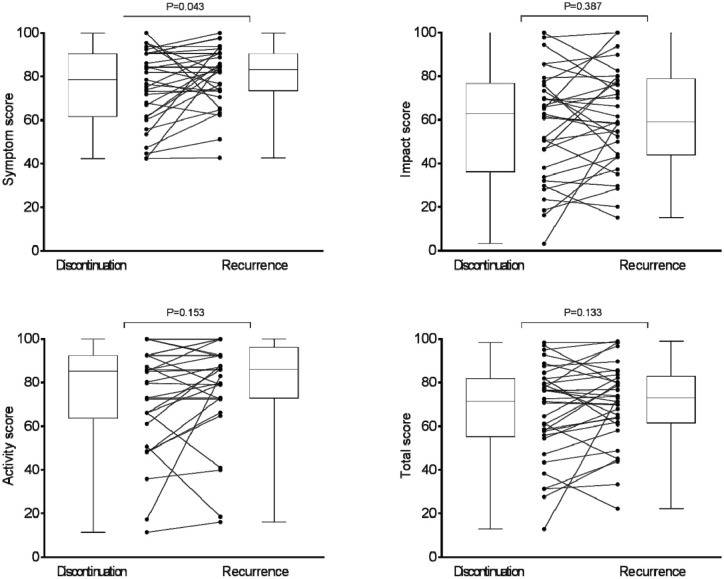
The median for the symptom component of QoL changed from 78.4 at the time of discontinuation of therapy to 83.1 units at the time of diagnosis of clinical failure (p = 0.043), whereas that of impact and activity components changed from 62.7 to 59.1 units (p = 0.387) and 85.0 to 85.9 units (p = 0.153), respectively (Figure 1). The median difference in the total score changed from 71.3 to 72.9 units (p = 0.133) (Figure 1).
Figure 1.
Individual patient scores and box and whisker plots for each of the SGRQ’s QoL domains at the time of discontinuation of antifungal treatment and at the time of diagnosis of clinical recurrence requiring recommencement of antifungal treatment. Scores range from 0 to 100, high score = poor quality of life.
QoL, quality of life; SGRQ, St. George’s respiratory questionnaire.
ROC analysis of symptoms domain at 3 and 12 months
At 3 months, the symptoms domain of SGRQ was unable to discriminate between cases of clinical recurrence and controls [area under curve (AUC) = 0.6, 95% confidence interval (CI): 0.4–0.7, p = 0.294]. However, this was possible at 12 months (AUC 0.7, 95% CI: 0.6–0.8, p = 0.009) (Figure 2). With a change of >8 units used to define clinical recurrence, the sensitivity was 90.9% with specificity of 87.0%, Positive predictive value (PPV) of 55.6% and negative predictive value (NPV) of 45.5% (Table 2).
Figure 2.
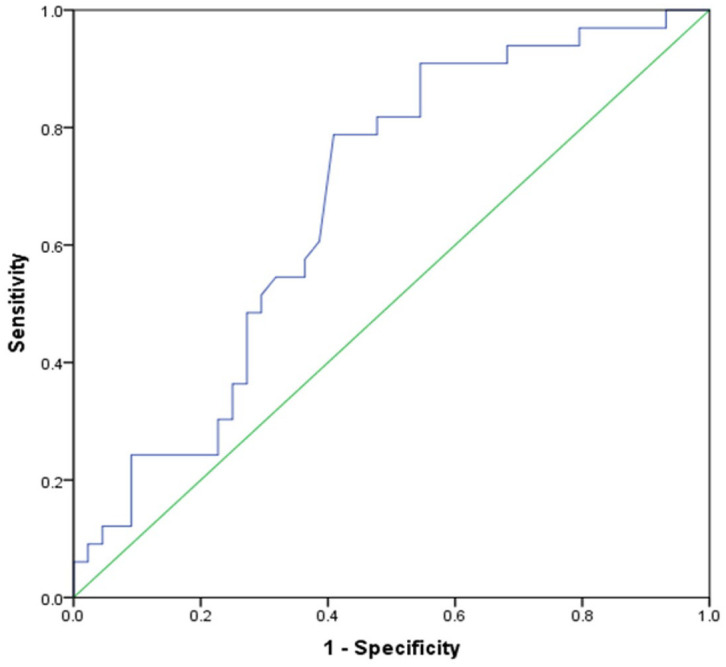
A ROC curve for the symptoms domain scores of SGRQ for predicting clinical recurrence at 12-months. AUC is 0.675 (95% CI: 0.555–0.795; p = 0.009).
AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic; SGRQ, St. George’s respiratory questionnaire.
Table 2.
Diagnostic performances of the different minimum clinically significant difference in the symptoms scores of the SGRQ.
| Category | 12-month change in SGRQ scores | Sensitivity | Specificity | PPV | NPV | J index |
|---|---|---|---|---|---|---|
| Improvement | >12 | 90.9 | 83.3 | 50.8 | 34.1 | 0.742 |
| >8 | 90.9 | 87.0 | 55.6 | 45.5 | 0.779 | |
| >4 | 81.8 | 78.6 | 55.1 | 50.0 | 0.604 | |
| Deterioration | <4 | 81.8 | 39.1 | 49.1 | 75.0 | 0.209 |
| <8 | 90.9 | 28.3 | 47.6 | 81.3 | 0.192 | |
| <12 | 90.9 | 23.9 | 46.2 | 78.6 | 0.148 |
NPV, negative predictive value; PPV, positive predictive value; SGRQ, St. George’s respiratory questionnaire.
Patients’ self-assessment of health status
The proportion of patients who classified themselves as being in a very poor health status increased from 3/11 (9.1%) at the time of discontinuation of therapy to 11/33 (33.3%) at the time of diagnosis of clinical recurrence (p = 0.046) (Figure 3).
Figure 3.
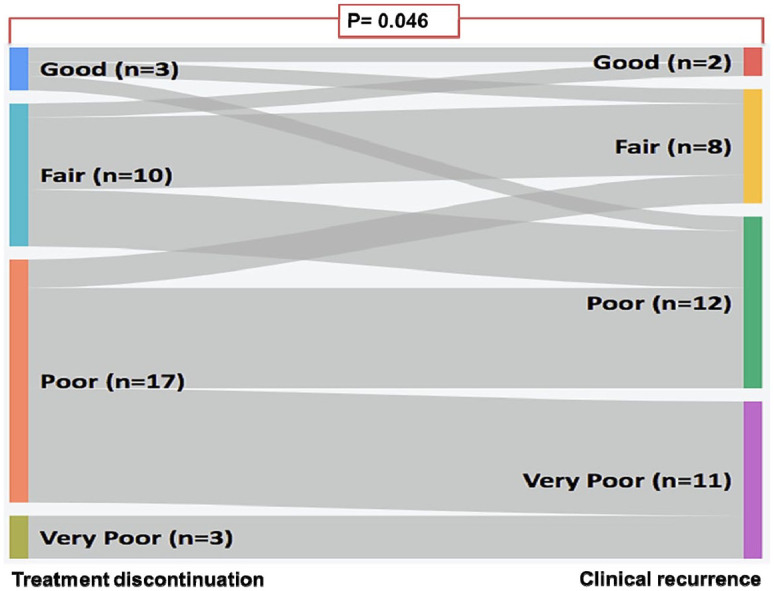
Sankey diagram for the difference in the patients’ global self-assessment of their conditions at the time of discontinuation of treatment and at the time of diagnosis of clinical failure evaluated by McNemar–Bowker test.
Discussion
Our data suggest that a deteriorating symptoms component of the SGRQ and a worsening of patients’ self-assessment are associated with clinical recurrence. Failure to improve by ⩾8 units in the symptoms domain appears to be a marker of disease recurrence.
The common symptoms of CPA include fatigue, breathlessness, productive cough, weight loss and haemoptysis. 11 Clinicians who treat patients with CPA often have to assess such symptoms in a clinic setting. Tools such as the SGRQ introduce some objectivity into the assessment of these softer and more subjective indices of clinical well-being and participation in daily living.
In a retrospective review of 39 patients who had come off their antifungals after achieving resolution of clinical and radiographic manifestations of CPA, 14 (35.9%) had a recurrence of CPA within 12 months of discontinuing triazole therapy. 4 The rate of recurrence of CPA is likely to be higher among patients who do not achieve resolution of their disease but have to discontinue antifungal therapy. Currently, there is no way to determine the risk of relapse among CPA patients who come off antifungals, thereby giving relevance to our findings.
These findings confirm the vital role of SGRQ in the management of CPA patients. Given the frequency of recurrence of CPA following discontinuation of antifungals, having a tool that identifies those at risk of recurrence is vital. The SGRQ takes 8–15 min to complete and has been validated for use in respiratory conditions such as asthma, COPD and bronchiectasis. For obstructive airway disease, the overall SGRQ can be used to assess the efficacy of treatment modalities with a mean change in the overall score of 4 units being interpreted as a slightly efficacious treatment. Mean changes of 8 units and 12 units are interpreted as moderately and very efficacious change, respectively.10,12
Whereas there was a statistically significant change in the median for the symptom component of the QoL at the time of discontinuation of therapy in our study, this was not the case with the impact and activity components. It is unclear why the change in the symptom component was not matched with a commensurate deterioration in the scores for the impact and activity components.
There has been considerable interest in identifying biomarkers for assessing treatment response in CPA. A recent study by Sehgel and colleagues involving 126 consecutive CPA treatment-naïve patients showed that Aspergillus fumigatus-specific IgG and serum galactomannan inconsistently decreased following treatment and may not be useful indicators for assessing for treatment response in CPA. 13 Therapy with oral itraconazole for at least 6 months continues to be advocated for CPA with voriconazole being an alternative in case of poor response or intolerance to itraconazole and both posaconazole and isavuconazole being options for salvage therapy. 14 Amphotericin B and echinocandins are intravenous therapeutic options for CPA in those with either treatment failure or who are intolerant to oral antifungal agents. 14
Our study has important limitations. Firstly, the retrospective, single-centre nature of the study design limits its generalization. Secondly, the small sample size makes the findings less precise and underpowered to answer the research question. A prospective, multicentre study would be useful to confirm our findings and better define the utility of this simple tool in predicting CPA recurrence.
In conclusion, we have demonstrated that a deteriorating symptoms component of the SGRQ and a worsening of patients’ self-assessment are associated with clinical progression of CPA disease. However, with the small AUC (~7), the results from this study should be interpreted with caution. The SGRQ should not be used alone as a marker of CPA progression, as its performance is below optimal in the discrimination of cases and controls. The suggested clinical approach to diagnose recurrent CPA would be a combination of clinical history, SGRQ scoring, chest imaging and a workup to exclude other causes of the patients’ symptoms.
Acknowledgments
We greatly acknowledge the support from the clinical team, administrators and nurses at the National Aspergillosis Centre, Manchester, UK.
Footnotes
Author contributions: FB conceived the idea. FB and AO extracted the data. Both authors discussed the results and contributed to the final manuscript
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Akaninyene Otu  https://orcid.org/0000-0002-6009-2707
https://orcid.org/0000-0002-6009-2707
Contributor Information
Felix Bongomin, The National Aspergillosis Centre, 2nd Floor Education and Research Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK; Department of Medical Microbiology and Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda.
Akaninyene Otu, The National Aspergillosis Centre, 2nd Floor Education and Research Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK; Department of Internal Medicine, College of Medical Sciences, University of Calabar, Calabar, Cross River State, Nigeria.
References
- 1. Denning DW, Riniotis K, Dobrashian R, et al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003; 37: S265–S280. [DOI] [PubMed] [Google Scholar]
- 2. Tochigi N, Ishiwatari T, Okubo Y, et al. Histological study of chronic pulmonary aspergillosis. Diagn Pathol 2015; 10: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 2011; 37: 865–872. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal R, Vishwanath G, Aggarwal AN, et al. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses 2013; 56: 559–570. [DOI] [PubMed] [Google Scholar]
- 5. Jain LR, Denning DW. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J Infect 2006; 52: e133–e137. [DOI] [PubMed] [Google Scholar]
- 6. Bongomin F, Harris C, Hayes G, et al. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS One 2018; 13: e0193732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kosmidis C, Muldoon EG. Challenges in the management of chronic pulmonary aspergillosis. Med Mycol 2017; 55: 63–68. [DOI] [PubMed] [Google Scholar]
- 8. Koyama K, Ohshima N, Suzuki J, et al. Recurrence of chronic pulmonary aspergillosis after discontinuation of maintenance treatment by antifungal triazoles. J Infect Chemother 2014; 20: 375–379. [DOI] [PubMed] [Google Scholar]
- 9. Al-Shair K, Atherton GTW, Kennedy D, et al. Validity and reliability of the St. George’s Respiratory Questionnaire in assessing health status in patients with chronic pulmonary aspergillosis. Chest 2013; 144: 623–631. [DOI] [PubMed] [Google Scholar]
- 10. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19: 398–404. [DOI] [PubMed] [Google Scholar]
- 11. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Postgr Med J 2015; 91: 270–277. [DOI] [PubMed] [Google Scholar]
- 12. Jones PW. Quality of life, symptoms and pulmonary function in asthma: long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Nedocromil Sodium Quality of Life Study Group. Eur Respir J 1994; 7: 55–62. [DOI] [PubMed] [Google Scholar]
- 13. Sehgal IS, Dhooria S, Choudhary H, et al. Monitoring treatment response in chronic pulmonary aspergillosis: role of clinical, spirometric and immunological markers. Clin Microbiol Infect 2019; 25: 1157.e1–e7. [DOI] [PubMed] [Google Scholar]
- 14. Sehgal IS, Dhooria S, Muthu V, et al. An overview of the available treatments for chronic cavitary pulmonary aspergillosis. Expert Rev Respir Med 2020; 14: 715–727. [DOI] [PubMed] [Google Scholar]