Abstract
α-mangostin has been confirmed to promote the apoptosis of MG-63 cells, but its specific pro-apoptosis mechanism in osteosarcoma (OS) remains further investigation. Here, we demonstrated that α-mangostin restrained the viability of OS cells (143B and Saos-2), but had little effect on the growth of normal human osteoblast. α-mangostin increased OS cell apoptosis by activating the caspase-3/8 cascade. Besides, α-mangostin induced endoplasmic reticulum (ER) stress and restrained the Wnt/β-catenin pathway activity. 4PBA (an ER stress inhibitor) or LiCl (an effective Wnt activator) treatment effectively hindered α-mangostin-induced apoptosis and the caspase-3/8 cascade. Furthermore, we also found that α-mangostin induced ER stress by promoting ROS production. And ER stress-mediated apoptosis caused by ROS accumulation depended on the inactivation of Wnt/β-catenin pathway. In addition, α-mangostin significantly hindered the growth of xenograft tumors, induced the expression of ER stress marker proteins and activation of the caspase-3/8 cascade, and restrained the Wnt/β-catenin signaling in vivo. In short, ROS-mediated ER stress was involved in α-mangostin triggered apoptosis, which might depended on Wnt/β-catenin signaling inactivation.
Keywords: α-mangostin, ER stress, ROS, apoptosis, osteosarcoma
Introduction
Osteosarcoma (OS) is the most common malignant bone tumor that primarily affects children and adolescents 1 . Although it has a lower prevalence than other solid tumors, it can be fatal if not detected and treated early 2 . It is characterized by rapid cell growth, high metastatic potential, and local infiltration of other organs 3,4 . Currently, the treatment of osteosarcoma includes surgery, radiation therapy and chemotherapy or a combination of these treatments, but its 5-year survival rate is still poor 5 . The inability to control the local metastasis of osteosarcoma has become a difficult point in its treatment, so it is necessary to find a new and effective anti-OS treatment drug.
In recent years, natural products have attracted much attention in cancer prevention and treatment due to their limited toxicity and various biological activities 6 . Mangosteen (Garcinia mangostana) is a tropical fruit growing in Southeast Asia, and its peel is a traditional medicine for many diseases 7 . α-mangostin is a natural compound isolated from mangosteen. It has various biological effects, such as anti-inflammatory, anti-oxidant, anti-fungal and anti-tumor 8 . α-mangostin has been confirmed to have anti-proliferative and pro-apoptotic effects in many cancers 9 –11 . It is reported that α-mangostin induces endoplasmic reticulum (ER) stress in human breast cancer cells 12 . In addition, α-mangostin induces apoptosis in MG63 cells 13 , while the specific mechanism by which α-mangostin induces apoptosis in OS cells is obscure.
Endoplasmic reticulum (ER) stress-activated unfolded protein response (UPR) is the main pre-survival response and plays an active role in canceration and tumor progression 14,15 . However, prolonged or severe UPR will induce cancer cell apoptosis 16 . ER stress involves three transmembrane receptors: protein kinase R-like endoplasmic reticulum kinase (PERK), inositolrequiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) 17 . PERK and IRE1 are activated by separation from CHOP (C/EBP homologous protein) and then induce phosphorylation of the eukaryotic translation initiation factor 2 subunit α (eIF-2α)/ATF-4/CHOP signaling pathway, which stimulates apoptosis and cell death through multiple downstream targets 17 . ER stress has been demonstrated to be involved in OS cell apoptosis 18 , revealing that induction of ER-dependent cell death may be an effective method to eliminate OS cells. Whether the mechanism of α-mangostin inducing apoptosis in human OS cells involves ER stress remains further investigation.
In this study, we found that α-mangostin inhibited cell viability and promoted apoptosis in 143B and Saos-2 cells. Subsequent mechanism studies indicated that ROS-mediated ER stress was involved in α-mangostin triggered apoptosis, which might depended on the inactivation of Wnt/β-catenin pathway.
Materials and Methods
Cell Culture and Treatment
143B, Saos-2 and hFOB cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in the DMEM (Thermo Fisher Scientific, Waltham, MA, USA) medium supplemented with 10% fetal calf serum (Gibco, Rockville, MD). All cell lines were cultured at 37°C in a cell incubator with a humidified atmosphere of 5% CO2.
α-Mangostin was purchased from Sigma-Aldrich (M3824) with a purity of > 98%.
Cell Viability Assay
Cell viability was measured with the MTT assay. Briefly, 2×104 cells were treated with 0, 10, 20, 30, 40, and 50 μM α-mangostin for either 24 or 48 h in 24-well plates. At the end of treatment, 10 µL MTT reagent (5 mg/ml in PBS) were added into each well, and the cells were incubated at 37°C for 4 h. Then, DMSO was added to each well and the absorbance was measured with a microplate reader (Tecan, Mannedorf, Switzerland) at a wavelength of 590 nm. The experiments were performed in triplicate.
Apoptosis Assay
Early apoptosis detection was performed using a FITC-labeled Annexin V/PI Apoptosis Detection kit (BD Bio-sciences, CA, USA). 2×105 cells were seeded in 6-cm dishes. After treatment, the cells were collected, and then fixed and stained in 1× binding buffer (140 mM NaCl, 10 mM HEPES/NaOH, and 2.5 mM CaCl) with 5 µL of FITC-conjugated Annexin V and 5 µL of PI solution for 30 min at room temperature in the dark. The apoptotic cells were detected with a flow cytometer (FACSCalibur, BD Biosciences) and the data were analyzed by using Cell Quest software.
Measurement of Intracellular Reactive Oxygen Species (ROS)
2,7’-dichlorodihydrofluorescein diacetate (DCFDA; Invitrogen) was used to measure the intracellular ROS. Briefly, cells (2×105/mL) were seeded in 6-cm dishes, after treatment, the cells were collected and incubated with DCF-DA (10 μM) at 37 ºC for 30 min in the dark. After washing twice with PBS, the fluorescence intensity was measured by the microplate reader (Molecular Devices, CA, USA) at an excitation wave-length of 485 nm and an emission wavelength of 538 nm.
Western Blotting
Proteins from tissues and cells were extracted by using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA). Nuclear and Cytoplasmic Protein Extraction Kit (KeyGEN Biotech, Jiangsu, China) was used to extract nuclear and cytoplasmic protein. The protein concentration of each sample was detected using a BCA protein quantification kit. 20 µL of protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) at 120 V for 2 h, and then transferred onto PVDF membranes (Millipore, Boston, MA, USA) at 250 mA for 1.5-2.5 h. Next, the membranes were blocked for 2 h with 5% non-fat dry milk buffer at room temperature, and then incubated with the primary antibodies at 4°C overnight. After washing, the membranes were incubated with HRP-conjugated secondary antibody at room temperature for 1 h. Antibodies against β-actin (ab115777; 1:200), Histone H3 (ab1791; 1:4000), phosphorylated-glycogen synthase kinase 3β (p-Gsk3β (Ser9); ab131097; 1:1000), PERK (ab229912; 1:1000), ATF6 (ab37149; 1:800), and Gsk3β (ab93926; 1:1000) were purchased from Abcam (Cambridge, UK). Antibodies against β-catenin (#37447; 1:1000), Caspase-3 (#9662; 1:1000), Cleaved-Caspase-3 (#9654; 1:1000), Caspase-8 (#4790; 1:1000), Cleaved-Caspase-8 (#9748; 1:1000) and CHOP (#2895; 1:1000) were purchased from Cell Signaling Technology (Beverly, MA, USA). The reaction was visualized using an enhanced chemiluminescence (ECL) reagent (Millipore, Billerica, MA, USA). The protein abundances were detected using ChemiDoc XRS Imaging System (Bio-Rad, Hercules, CA, USA).
Xenografts
Adult female athymic BALB/c nude mice (18-20 g, 5 weeks old) were purchased from Vital River Laboratory Animal Technology (Beijing, China). Mice were fed under pathogen-free conditions (22±2°C; relative humidity, 50% ± 10%) with free access to sterile water and food under a 12 h dark/light cycle. They were housed in individually ventilated cages: six per cage, with 4-6 mm of disinfectant corncob bedding. 143B cells (5 × 106/0.1 ml/mouse) were suspended in sterile PBS and subcutaneously injected into mice. When the tumors had reached an average volume of 200 mm3, α-mangostin was intraperitoneally injected at 5 and 20 mg/kg (dissolved in 0.2 ml olive oil) once a day until the mice were killed. Mice in control group were intraperitoneally injected with olive oil once a day. Tumor growth was determined with vernier calipers every 3 days, and the tumor volume was estimated using the formula: tumor volume = 0.5 × length × width 2 . At the end of the experiment, mice were euthanized 24 h after the last administration, and the tumors were weighed. All procedures conducted were in accordance with the guidelines for the use and care of laboratory animals. The study was approved by the Ethics Committee of General Hospital of Ningxia Medical University (Yinchuan).
Statistical Analysis
Statistical analysis was performed using SPSS 22.0 software. Data were presented as the mean ± SEM and analyzed by using Student’s t-test and one-way analysis of variance (ANOVA). Significance was accepted at the P < 0.05.
Results
α-Mangostin Inhibits Cell Viability in Human Osteosarcoma Cells
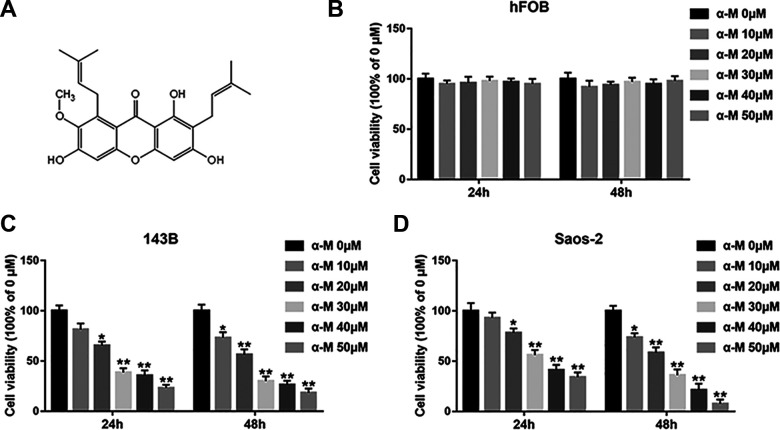
Researches have displayed that α-mangostin restrains the viability of many cancer cells. To explore the inhibitory effect of α-mangostin on cell viability in osteosarcoma cell lines and normal human osteogenic cells, the 143B, Saos-2 and hFOB cells were treated by α-mangostin (0, 10, 20, 30, 40, and 50 μM) for 24 and 48 h, and cell viability were assessed by MTT assay. The results showed that α-mangostin suppressed cell viability of 143B and Saos-2 cells in a concentration-dependent and time-dependent manner (Fig. 1C, D). No obvious changes in cell viability was measured in normal human osteoblasts (Fig. 1B), revealing that α-mangostin had little toxicity to normal cells.
Figure 1.
α-mangostin inhibits cell viability in human osteosarcoma cells. (A) Molecular structure of α-mangostin. (B–D) hFOB, 143B and Saos-2 cells were treated by different concentrations of α-mangostin (0, 10, 20, 30, 40, and 50 μM) for 24 and 48 h, and the cell viability was detected with MTT assay. N = 5, *P < 0.05, **P < 0.01.
α-Mangostin Induces Apoptosis in 143B and Saos-2 Cells
It has been reported that α-mangostin induces apoptosis and inhibits epithelial-mesenchymal transition (EMT) in MG-63 cells 13 . We aimed to investigate the effect of α-mangostin on apoptosis of 143B and Saos-2 cells. Cells were treated by different concentrations of α-mangostin (0, 10, 20, and 30 μM) for 24 h. Cell apoptosis was increased with the increasing concentration of α-mangostin (Fig. 2A). Subsequent research found that α-mangostin notably upregulated the expression of cleaved-caspase-3 and -8 (Fig. 2B). Moreover, the pretreatment of Z-VAD (a pan-caspase inhibitor) on 143B and Saos-2 cells for 2 h markedly reversed the effect of α-mangostin on cell viability (Fig. 2C), suggesting that α-mangostin induced apoptosis by activating the caspase pathway in 143B and Saos-2 cells.
Figure 2.
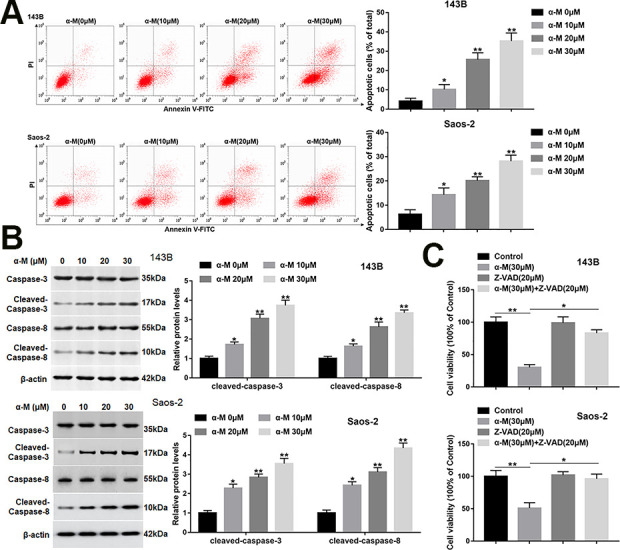
α-mangostin induces apoptosis in 143B and Saos-2 cells. 143B and Saos-2 cells were treated by different concentrations of α-mangostin (0, 10, 20, 30 μM) for 24 h. (A) Cell apoptosis was detected with Annexin V/PI staining by flow cytometry analysis. (B) The protein levels of cleaved-caspase-3/8 were detected by Western blotting. (C) Z-VAD (20 μM) was applied to the 143B and Saos-2 cells which were treated or untreated by α-mangostin (30 μM), and the cell viability was detected by MTT assay. N = 5, *P < 0.05, **P < 0.01.
α-Mangostin Induces Endoplasmic Reticulum (ER) Stress in 143B and SAOS-2 Cells
To explore if α-mangostin induced ER stress in OS cells, the protein levels of CHOP, and the UPR markers were detected. The data showed that the protein levels of CHOP, PERK and ATF6 were upregulated following α-mangostin treatment in 143B and Saos-2 cells (Fig. 3A). Next, to further investigate the role of ER stress in α-mangostin-induced apoptosis, an ER stress inhibitor (4PBA) was applied to the cells which were treated or untreated by α-mangostin. As shown in Fig. 3B, 4PBA treatment observably reversed the increased expression of CHOP, PERK and ATF6 induced by α-mangostin. As a comparison, 4PBA treatment alone did not alter the protein levels of the above proteins (Fig. 3B). Furthermore, 4PBA treatment notably restrained the effect of α-mangostin on apoptosis and cleaved-caspase-3/8 expression (Fig. 3C, D). While, 4PBA treatment alone had no significant effect on apoptosis and caspase-3/8 expression.
Figure 3.
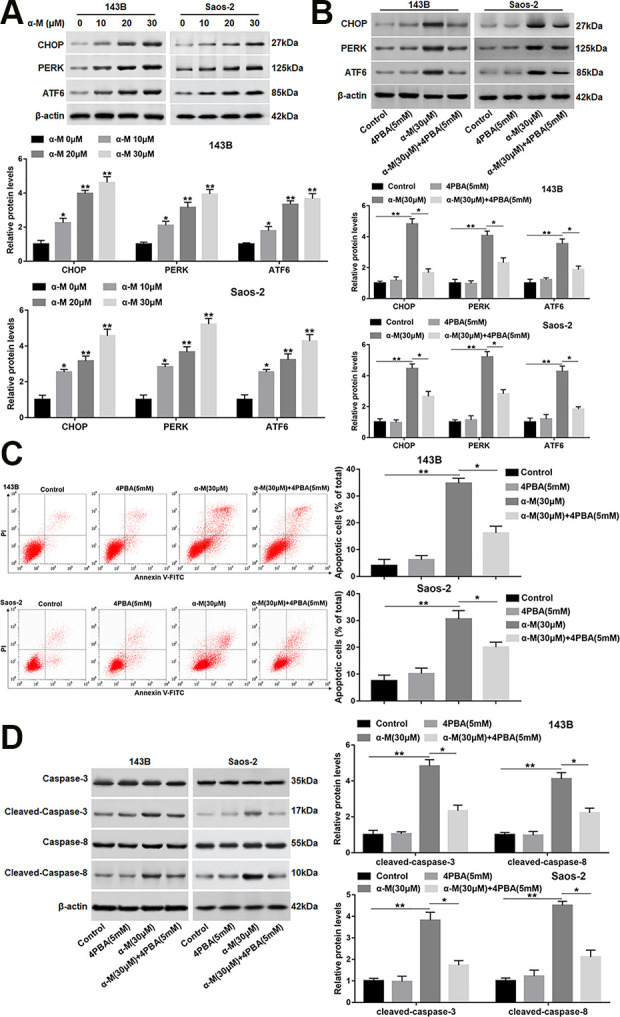
α-mangostin induces ER stress in 143B and Saos-2 cells. (A) 143B and Saos-2 cells were treated by different concentrations of α-mangostin (0, 10, 20, 30 μM) for 24 h, and the protein levels of CHOP, PERK, and ATF6 were detected. 4PBA (5 mM) was applied to the cells treated or untreated by α-mangostin (30 μM), and (B) the protein levels of CHOP, PERK and ATF6 were analyzed. (C) Cell apoptosis was determined with Annexin V/PI staining by flow cytometry analysis. (D) The protein expression of cleaved-caspase-3/8 was measured. N=5, *P < 0.05, **P < 0.01.
α-Mangostin Impedes the Activation of WNT/β-Catenin Signaling in 143B and SAOS-2 Cells
Several reports indicate that Wnt/β-catenin pathway plays a pivotal role in the apoptosis inhibition. Zhao at al. have shown that β-Elemonic acid suppressed the growth of human Osteosarcoma by inducing ER stress and restraining Wnt/β-catenin signaling 18 . Hence, we examined whether this pathway is functionally involved in the pro-apoptotic effect of α-mangostin. The results revealed that α-mangostin treatment restrained the protein levels of Wnt3a and p-Gsk3β (Ser9), while increased Gsk3β protein expression (Fig. 4A). Moreover, α-mangostin suppressed the β-catenin protein level in nucleus in a dose-dependent fashion (Fig. 4B).
Figure 4.
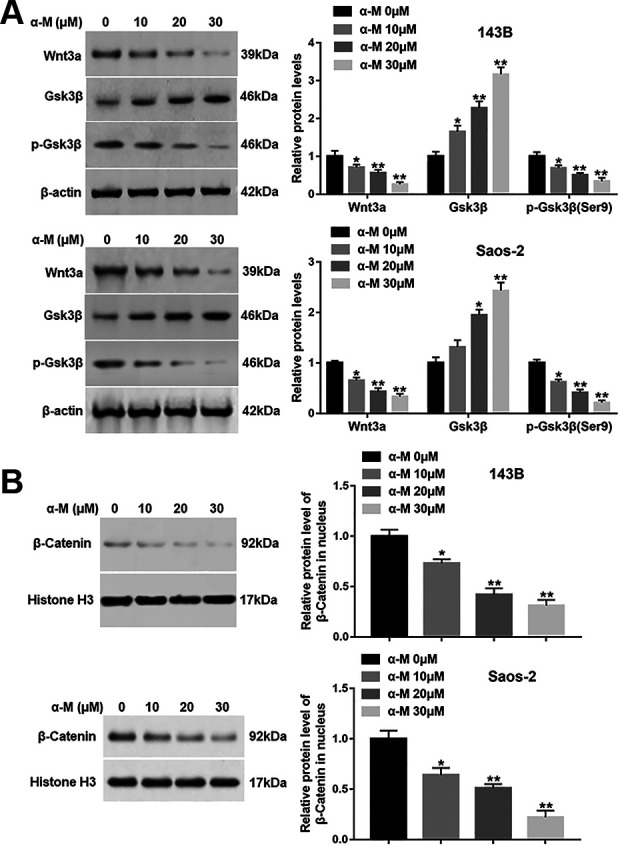
α-mangostin impedes the activation of Wnt/β-catenin signaling in 143B and Saos-2 cells. 143B and Saos-2 cells were treated by different concentrations of α-mangostin (0, 10, 20, 30 μM) for 24 h, and (A) the protein levels of Wnt3a, p-Gsk3β (Ser9) and Gsk3β in cytoplasm, and (B) the expression of β-catenin in nucleus were measured. N = 5, *P < 0.05.
ER Stress-Mediated Apoptosis Induced by α-Mangostin Depends on Wnt/β-Catenin Signaling Inactivation
To further study the role of Wnt/β-catenin pathway in α-mangostin-induced apoptosis, 143B and Saos-2 cells were treated by α-mangostin alone or together with LiCl (an inhibitor of Gsk3β). We found that LiCl treatment reversed the upregulation of Gsk3β protein expression and the reduction of nuclear β-catenin expression caused by α-mangostin treatment (Fig. 5A, B). In addition, cell apoptosis and the protein levels of cleaved-caspase-3/8 were declined in α-mangostin and LiCl co-treated group compared with α-mangostin treated group (Fig. 5C, D).
Figure 5.
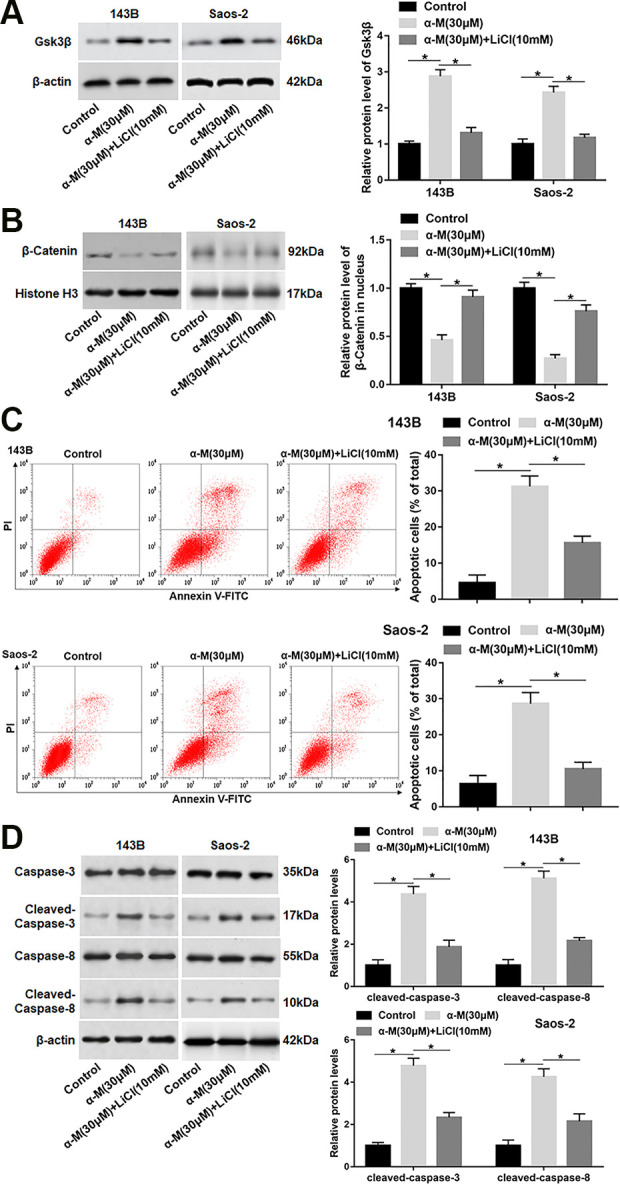
ER stress-mediated apoptosis induced by α-mangostin depends on Wnt/β-catenin signaling inactivation. 143B and Saos-2 cells were treated by α-mangostin (30 μM) alone or together with LiCl (10 mM) for 24 h. (A, B) The protein levels of Gsk3β and nuclear β-catenin were analyzed. (C) Cell apoptosis was detected with flow cytometry. (D) The protein levels of cleaved-caspase-3/8 were determined. N=5, *P < 0.05, **P < 0.01.
α-Mangostin Increases Intracellular ROS Levels in 143B and SAOS-2 Cells
A research shows that α-mangostin enhances ROS generation, leads to ASK1/p38 activation, and induces cervical cancer cell apoptosis 19 . We evaluated the effect of α-mangostin on ROS level. As expected, the ROS production was increased following α-mangostin treatment in a dose-dependent manner in 143B and Saos-2 cells (Fig. 6A). Moreover, NAC (a ROS scavenger) observably reduced α-mangostin-induced ROS generation (Fig. 6B). Besides, NAC also rescued α-mangostin induced increase in apoptosis, activation in the caspase-3/8 cascade, and upregulation in CHOP and ATF6 expression (Fig. 6C–E). Moreover, NAC could not reverse the down-regulated expression of Wnt3a caused by α-mangostin. In addition, the protein levels of p-Gsk3β (Ser9) and nuclear β-catenin were increased in NAC and α-mangostin co-treated group compared with α-mangostin treatment group (Fig. 6F, G). However, NAC treatment alone had no significant effect on apoptosis, ER stress and Wnt/β-catenin pathway activity (Fig. 6C–G).
Figure 6.
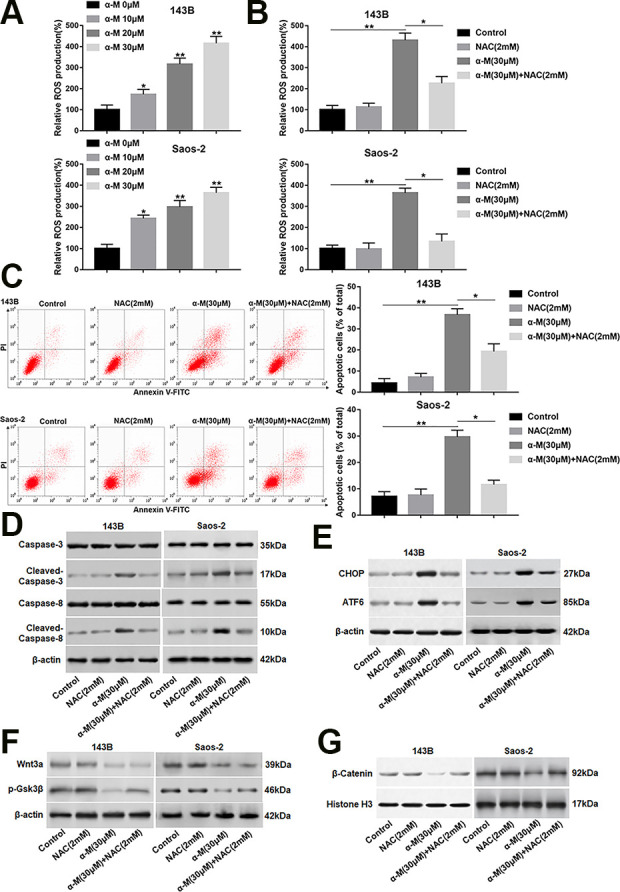
α-Mangostin increases intracellular ROS level in 143B and Saos-2 cells. (A) 143B and Saos-2 cells were treated with different concentrations of α-mangostin (0, 10, 20 and 30 μM) for 24 h, and the ROS production was detected by DCF-DA staining. 143B and Saos-2 cells were pre-treated by NAC (2 mM) for 2 h, then co-treated with α-mangostin (30 μM) for 24 h. (B) The ROS production was measured by DCF-DA staining. (C) Cell apoptosis was analyzed with flow cytometry. (D, E) The protein levels of cleaved-caspase-3/8, CHOP and ATF6 were determined. (F, G) The expression of Wnt3a, p-Gsk3β and nuclear β-catenin was analyzed. N = 5, *P < 0.05, **P < 0.01.
α-Mangostin Inhibits Tumor Growth in 143B Xenografted Mice
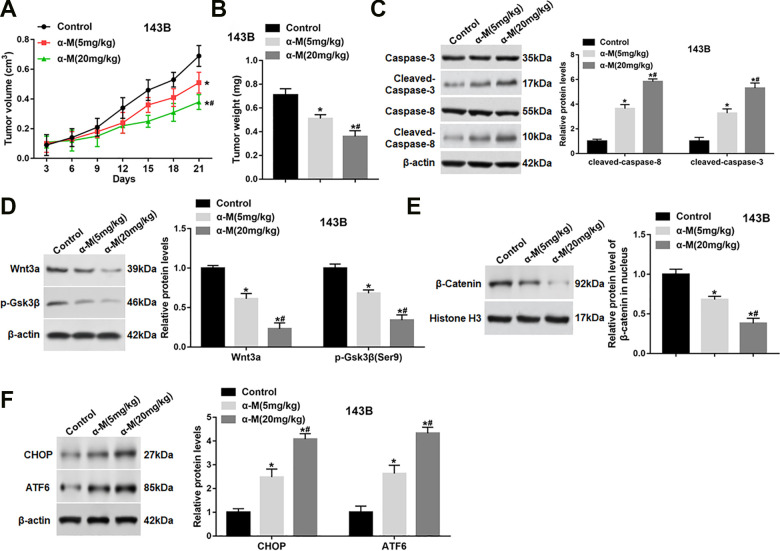
To evaluate whether α-mangostin had an inhibitory effect on tumor growth in vivo, 143B cells were subcutaneously injected into nude mice. When the tumors had reached an average volume of 200 mm3, α-mangostin were intraperitoneally injected daily at a concentration of 5 and 20 mg/kg. Our data displayed that administration with α-mangostin resulted in a remarkable suppression of tumor volume and weight in a dose-dependent manner (Fig. 7A, B). Besides, the expression of cleaved-caspase-3/8 in tumor tissues were observably up-regulated after the injection of α-mangostin (Fig. 7C). Moreover, the protein levels of Wnt3a and p-Gsk3β (Ser9) in tumor tissues were significantly down-regulated (Fig. 7D), and the nuclear accumulation of β-catenin was also reduced after administration with α-mangostin (Fig. 7E). In addition, the protein expression of CHOP and ATF6 was observably increased after α-mangostin treatment (Fig. 7F).
Figure 7.
α-mangostin inhibits tumor growth in 143B xenografted mice. 143B cells (5 × 106/0.1 ml/mouse) were subcutaneously injected into mice. When the tumors had reached an average volume of 200 mm3, α-mangostin of different concentrations were intraperitoneally injected at 5 and 20 mg/kg (dissolved in 0.2 ml olive oil) once a day until the mice were killed. (A) Tumor volume was measured every 3 days for 21 days. (B) At the end of the experiment, mice were sacrificed, and tumors were excised to measure the weight. (C–F) The protein levels of cleaved-caspase-3/8, Wnt3a, p-Gsk3β, nuclear β-catenin, CHOP and ATF6 were determined. N = 10, *P < 0.05, **P < 0.01.
Discussion
Our results indicated that α-mangostin induced ER stress by promoting the production of ROS, and then suppressed the nuclear transfer of β-catenin, which in turn activated the caspase3/8 cascade, thereby promoting OS cell apoptosis. Moreover, α-mangostin also inhibited the nuclear accumulation of β-catenin by restraining Wnt3a (Fig. 8).
Figure 8.
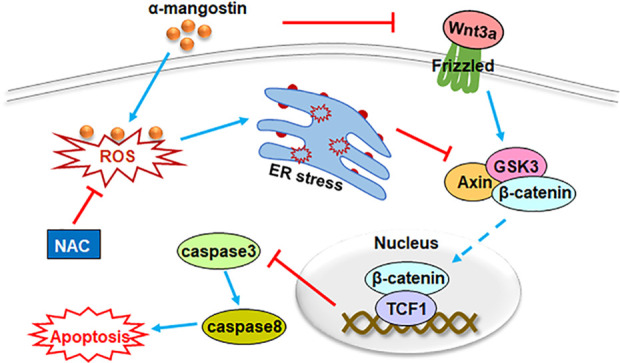
A schematic diagram for the role of α-mangostin on osteosarcoma cell apoptosis. By increasing ROS level, α-mangostin induced ER stress and impeded the nuclear accumulation of β-catenin. Then, the caspase3/8 cascade was activated, and OS cell apoptosis was increased. Moreover, α-mangostin also inhibited the nuclear transport of β-catenin by restraining Wnt3a. NAC treatment could effectively eliminate the generation of ROS induced by α-mangostin.
The peel of mangosteen contains a high concentration of flavonoids, and α-mangiferin has been identified as the most abundant flavonoid 20 . It is attractive because of its abundance and broad prospects for development. α-mangostin has anti-cancer and anti-proliferative effects on many types of cancers 21 . Existing evidence indicates that α-mangostin generally induces cell cycle arrest and apoptosis in cancer cells 22 . α-mangostin accelerates the apoptosis of mouse skin tumor cells induced by 9,10-dimethylbenz[a]anthracene (DMBA)/TPA Perish 7 . In athymic nude mice, α-mangostin induces apoptosis and cell cycle arrest in prostate cancer cells 23 . Besides, α-mangostin inhibits cell viability and induces apoptosis in MG63 cells 13 . In addition, α-mangostin inhibits tumor growth in vivo in some cancers, such as prostate cancer, hepatoma and breast cancer 21,23 . In this study, we found that α-mangostin suppressed cell viability of 143B and Saos-2 cells in a concentration-dependent and time-dependent manner. Moreover, α-mangostin notably upregulated the expression of cleaved-caspase-3/8, and a pan-caspase inhibitor (Z-VAD) markedly reversed the effect of α-mangostin on cell viability, suggesting that α-mangostin induced apoptosis by activating caspase pathway. The in vivo experiments demonstrated that α-mangostin resulted in a remarkable suppression on tumor volume and weight of 143B xenografts.
Recent studies have shown that ER stress is associated with neurodegenerative diseases, inflammatory diseases, metabolic disorders and cancers 24 . Besides, ER stress is considered to be an important regulator of many cellular pathological processes, including cancer cell death pathways under the action of anticancer drugs 25 . Increasing evidence has suggested that ER stress plays an important role in the regulation of apoptosis 26 . It has been reported that increased ER stress causes OS cell apoptosis. Wang et al. found that induction of OS cell apoptosis is enhanced by stimulation of enhanced ER stress via the PERK/eIF2α/ATF4/CHOP pathway 27 . ER stress is involved in nimbolide-induced OS cell apoptosis 28 . Furthermore, α-mangiferin induces ER stress and autophagy in human breast cancer cells 12 . Our data revealed that α-mangostin induced ER stress by increasing the protein levels of CHOP, PERK and ATF6. Besides, an ER stress inhibitor (4PBA) notably restrained the effect of α-mangostin on apoptosis and the caspase-3/8 cascade, indicating that α-mangostin might increase apoptosis through inducing ER stress.
ROS plays an important role in cancer progression by stimulating cell growth and genetic instability 5 . ROS profoundly affects many cellular responses, including protein kinase activation, cell cycle progression, and apoptotic cell death 29 . Basic levels of ROS are essential for cell physiological functions, but excessive ROS formation induce apoptosis and cell cycle arrest in cancers 30 . Increasing data display that ROS interferes with ER protein folding and induces ER stress, thereby activating UPR to resolve the protein folding defect 29 . A recent research shows that a chemotherapeutic agent induce necrotic or apoptotic cell death of cancer cells by stimulating ROS generation 5 . Therefore, ROS has been identified as a potential target for finding new anticancer drugs. We demonstrated that the ROS production was increased following α-mangostin treatment in a dose-dependent manner. Besides, a ROS scavenger (NAC) rescued α-mangostin induced ER stress, ROS generation and cell apoptosis, as well as the activation of the caspase-3/8 cascade. In addition, NAC also reversed the decrease of p-Gsk3β (Ser9) and nuclear β-catenin expression caused by α-mangostin. However, NAC had no significant effect on the down-regulated expression of Wnt3a caused by α-mangostin.
Excessive activation of Wnt/β-catenin pathway induces abnormal cell proliferation and inhibit apoptosis, which is helpful for tumorigenesis and development 31 . This pathway is involved in the regulation of OS cell behavior, including cell proliferation, apoptosis, metastasis and chemical resistance. For instance, zinc promotes apoptosis in osteosarcoma by activating the Wnt-3a/β-catenin pathway 32 . The protein methyltransferase SETD2 inhibits the growth of osteosarcoma cells by inhibiting the Wnt/β-catenin signaling 33 . Several researches have revealed the correlation between ER stress and Wnt pathway. Horndasch et al. demonstrate that CHOP is an inhibitor of the classical Wnt pathway in Xenopus embryos and mammalian cells 34 . Song et al. find that endoplasmic reticulum stress may lead to the possibility of p-Gsk3β (Ser9) dephosphorylation 35 . Our results displayed that α-mangostin impeded the activation of Wnt/β-catenin signaling by restraining Wnt3a, p-Gsk3β (Ser9) and nuclear β-catenin expression and increasing Gsk3β expression. Moreover, LiCl prominently reversed α-mangostin-induced increase in apoptosis and activation in the caspase-3/8 cascade, suggesting that α-mangostin-mediated apoptosis depended on Wnt/β-catenin signaling inactivation.
In the present study, we testified that ER stress was involved in α-mangostin-induced apoptosis, which mediated by ROS and depended on Wnt/β-catenin signaling inactivation. Moreover, α-Mangosteen had a significant inhibitory effect on the growth of 143B xenograft. Our findings might provide a novel idea for the treatment of osteosarcoma with plant compounds.
Footnotes
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the General Hospital of Ningxia Medical University of ETHICS COMMITTEE’S (APPROVAL NUMBER: 201900351) approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fei Ren  https://orcid.org/0000-0001-7190-3117
https://orcid.org/0000-0001-7190-3117
References
- 1. Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH, He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, Yang T, et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther. 2015;9:1555–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ying J, Xu H, Wu D, Wu X. Emodin induces apoptosis of human osteosarcoma cells via mitochondria-and endoplasmic reticulum stress-related pathways. Int J Clin Exp Pathol. 2015; 8(10):12837–12844. [PMC free article] [PubMed] [Google Scholar]
- 3. Pridgeon MG, Grohar PJ, Steensma MR, Williams BO. Wnt signaling in ewing sarcoma, osteosarcoma, and malignant peripheral nerve sheath tumors. Curr Osteoporos Rep. 2017;15(4):239–246. [DOI] [PubMed] [Google Scholar]
- 4. Wang L, Xue GB. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem Biophys Res Commun. 2018; 495(1):27–34. [DOI] [PubMed] [Google Scholar]
- 5. Lee CH, Shih YL, Lee MH, Au MK, Chen YL, Lu HF, Chung JG. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase-and mitochondria-dependent signaling pathways. Molecules. 2017;22(3):437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vue B, Zhang S, Chen QH. Synergistic effects of dietary natural products as anti-prostate cancer agents. Nat Prod Commun. 2015;10(12):2179–2188. [PubMed] [Google Scholar]
- 7. Wang F, Ma H, Liu Z, Huang W, Xu X, Zhang X. alpha-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed Pharmacother. 2017;92:672–680. [DOI] [PubMed] [Google Scholar]
- 8. Zhang C, Yu G, Shen Y. The naturally occurring xanthone alpha-mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J Biol Sci. 2018; 25(6):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Li Y, Wang W, Deng L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: a review. Expert Opin Ther Pat. 2018;28(5):415–427. [DOI] [PubMed] [Google Scholar]
- 10. Zhang KJ, Gu QL, Yang K, Ming XJ, Wang, Jx. Anticarcinogenic effects of α-mangostin: a review. Planta Med. 2017; 83(3-04):188–202. [DOI] [PubMed] [Google Scholar]
- 11. Scolamiero G, Pazzini C, Bonafè F, Guarnieri C, Muscari C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int J Med Sci. 2018;15(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang W, Liang Y, Ma X. Alpha-mangostin induces endoplasmic reticulum stress and autophagy which count against fatty acid synthase inhibition mediated apoptosis in human breast cancer cells. Cancer Cell Int. 2019;19:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park SJ, Park BS, Yu SB, Kang HM, Kim HJ, Kim IR. Induction of Apoptosis and Inhibition of Epithelial Mesenchymal Transition by alpha-Mangostin in MG-63 Cell Lines. Evid Based Compl Alternat Med. 2018;2018:3985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front Oncol. 2017;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hazari YM, Bashir A, ul Haq E, Fazili KM. Emerging tale of UPR and cancer: an essentiality for malignancy. Tumour Biol. 2016;37(11):14381–14390. [DOI] [PubMed] [Google Scholar]
- 16. Alasiri G, Fan LYN, Zona S, Goldsbrough IG, Ke HL, Auner HW, Lam EWF. ER stress and cancer: the FOXO forkhead transcription factor link. Mol Cell Endocrinol. 2018;462(Pt B):67–81. [DOI] [PubMed] [Google Scholar]
- 17. Xu C, Bailly Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115(10):2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao A, Zhang Z, Zhou Y, Li X, Li X, Ma B, Zhang Q. β-Elemonic acid inhibits the growth of human Osteosarcoma through endoplasmic reticulum (ER) stress-mediated PERK/eIF2α/ATF4/CHOP activation and Wnt/β-catenin signal suppression. Phytomedicine. 2020;69:153183. [DOI] [PubMed] [Google Scholar]
- 19. Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, Hsieh YH. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. 2017;8(29):47425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuda M, Sakashita H, Hayashi H, Shiono J, Miyake G, Komine Y, Taira F, Sakashita H. Synergism between α-mangostin and TRAIL induces apoptosis in squamous cell carcinoma of the oral cavity through the mitochondrial pathway. Oncol Rep. 2017;38(6):3439–3446. [DOI] [PubMed] [Google Scholar]
- 21. Hsieh SC, Huang MH, Cheng CW, Hung JH, Yang SF. Hsieh YH. α-Mangostin induces mitochondrial dependent apoptosis in human hepatoma SK-Hep-1 cells through inhibition of p38 MAPK pathway. Apoptosis. 2013;18(12):1548–1560. [DOI] [PubMed] [Google Scholar]
- 22. Ding YY, Luan JJ, Fan Y, Olatunji OJ, Song J, Zuo J. α-Mangostin reduced the viability of A594 cells in vitro by provoking ROS production through downregulation of NAMPT/NAD. Cell Stress Chaperones. 2020; 25(1):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33(2):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie WY, Zhou XD, Yang J, Chen LX, Ran DH. Inhibition of autophagy enhances heat-induced apoptosis in human non-small cell lung cancer cells through ER stress pathways. Arch Biochem Biophys. 2016;607:55–66. [DOI] [PubMed] [Google Scholar]
- 25. Zhu GY, Li YW, Tse AKW, Hau DKP, Leung CH, Yu ZL, Fong WF. 20 (S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol. 2011;668(1-2):88–98. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Y, Shu F, Liang X, Chang H, Shi L, Peng X, Zhu J, Mi M. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PloS one. 2014;9(2):e89021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, Yin F, Xu J, Zhang T, Wang G, Mao M, Wang Z, Sun W, Han J, Yang M. CYT997 (Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J Exp Clin Cancer Res. 2019;38(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu JF, Hou CH, Lin FL, Tsao YT, Hou SM. Nimbolide induces ROS-regulated apoptosis and inhibits cell migration in osteosarcoma. Int J Mol Sci. 2015;16(10):23405–23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou L, Jiang L, Xu M, Liu Q, Gao N, Li P, Liu EH. Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. Sci Rep. 2016; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin C-L, Lee C-H, Chen C-M, Cheng C-W, Chen P-N, Ying T-H, Hsieh Y-H. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell Physiol Biochem. 2018;46(1):322–334. [DOI] [PubMed] [Google Scholar]
- 31. Xu X, Rajamanicham V, Xu S, Liu Z, Yan T, Liang G, Guo G, Zhou H, Wang Y. Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed Pharmacother. 2019;115:108922. [DOI] [PubMed] [Google Scholar]
- 32. Gao K, Zhang Y, Niu J, Nie Z, Liu Q, Lv C. Zinc promotes cell apoptosis via activating the Wnt-3a/β-catenin signaling pathway in osteosarcoma. J Orthop Surg Res. 2020;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang C, He C, Wu Z, Li F, Xiao J. Histone methyltransferase SETD2 regulates osteosarcoma cell growth and chemosensitivity by suppressing Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2018;502(3):382–388. [DOI] [PubMed] [Google Scholar]
- 34. Horndasch M, Lienkamp S, Springer E, Schmitt A, Pavenstädt H, Walz G, Gloy J. The C/EBP homologous protein CHOP (GADD153) is an inhibitor of Wnt/TCF signals. Oncogene. 2006;25(24):3397–3407. [DOI] [PubMed] [Google Scholar]
- 35. Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277(47):44701–44708. [DOI] [PubMed] [Google Scholar]