Abstract
Introduction:
Coronavirus disease 2019 (COVID-19) pandemic is a worldwide public health crisis. During huge surge in COVID-19 cases, most of the patient arrived at Rajiv Gandhi Government General Hospital, Chennai were severe due to late presentation and also available evidence demonstrating that the delay in treatment is directly associated with increased mortality or poor patient outcome. As an innovative concept of Zero Delay COVID-19 Ward were set up to provide the required critical care for all severe COVID-19 cases. The experience of setting up of such Zero Delay COVID-19 Ward and profile of admitted COVID-19 patients were described in this paper.
Methods:
A total of 4515 laboratory-confirmed COVID-19 patients admitted at Zero Delay COVID-19 Ward was analyzed retrospectively from 7th July to 31st December 2020.
Results:
At the time of admission the frequency of dyspnea were 85.6% among them 99.1% recovered from dyspnea after the oxygen therapy and other management at Zero Delay COVID-19 Ward. Of the 4515 COVID-19 individuals, about 1829 (40.5%) had comorbidity, 227 (5%) had died. Multivariable logistic regression analysis, COVID-19 death was more likely to be associated with comorbidity (OR: 18.687; 95% CI: 11.229-31.1) than other variables.
Conclusions:
Comorbidity is an independent high risk factor for mortality of COVID-19 patients. From our observation, it is strongly recommended that effective zero delay covid-19 ward model will help for the prevention of mortality in current/expected waves of COVID-19.
Keywords: COVID-19, comorbidity, dyspnea, coronavirus, SARS-CoV-2, zero delay COVID-19 ward
Introduction
A novel coronavirus disease (COVID-19) caused by the SARS-CoV-2 virus was spreading in China in December 2019 to February 2020, and then was declared a global pandemic on March 11, 2020, by the World Health Organization (WHO). The number of people affected by COVID-19 is continuously increasing globally since then, resulting 168 040 871 confirmed cases, worldwide by May 27, 2021. 1 Early identification, early diagnosis, early isolation, and reducing mortality are crucial to combat with Covid-19 outbreaks. Countries continue to implement various action plans based on a whole of society approach and a realistic appraisal of what is feasible to achieve first in terms of diseases burden and reducing mortality.
Rajiv Gandhi Government General Hospital, a public sector tertiary care hospital designated for COVID-19 is located in Chennai, the capital city of State of Tamil Nadu, Southern India. The primary goal of which is to provide quality health services at free of cost. One of the defining features of COVID-19 is the huge stress placed on health systems and health care workers by the large proportion of COVID-19 patients who require quality clinical care. 2 Since the severity of disease is closely related to the prognosis, the early detection of high-risk and critically ill patients is an essential strategy to improve clinical outcome.3,4 Patients who died on account of COVID-19 were recorded having longer lingering time from the onset of symptoms to ICU admission than those who survived. 5 Patients with severe COVID-19 require a mean of approximately 13 days of respiratory support and care. 6 This was very much true during the initial days of pandemic in our center. Although we were stunned by the initial obstacles faced, we regained our composure and felt the need for reexpansion, redistribution, and restructuring of our health care resources.
During the peak when our institution was stormed by almost 1800 patients per day, most of them with late presentation and with “walking hypoxia,” the provision of mobile and the point of reception critical care services in reducing the morbidity and mortality of patients by initiating appropriate treatment as per treatment category as soon as they get admitted in the zero delay ward is an area of curious concern.
The choice of interventions ultimately depends on the relative feasibility of their implementation and their likely effectiveness in different social contexts. Henceforth we came up with the novel idea of Zero Delay COVID-19 ward which would address these issues at the door step, the very point of patient entry that might greatly mitigate the morbidity and mortality of COVID-19 on individuals who depended on essential and immediate care. In this setting, our effective strategy—Zero Delay COVID-19 ward is being set out in detail in this paper as well as the profile of demographics, clinical presentation, and laboratory investigations among COVID-19 hospitalized patients at Zero Delay COVID-19 ward.
Methods
Study Design and Population
This is a retrospective study of patients with COVID-19 admitted in Zero Delay COVID-19 ward from July 7, 2020, to December 31, 2020 at Rajiv Gandhi Government General Hospital, Chennai, Tamil Nadu, India. The study included the patients diagnosed with COVID-19 by positive detection of SARS-CoV-2 RNA from nasopharyngeal/throat swabs using TaqPath™ COVID-19 Combo Kit (Thermo Fisher Scientific, USA), real-time reverse transcription-polymerase chain reaction (RT-PCR) kit as per the manufacturer instructions. The study was approved by the institutional ethics committee of the Government Madras Medical College, Chennai, Tamil Nadu.
Establishment of Zero Delay COVID-19 Wards at Rajiv Gandhi Government General Hospital
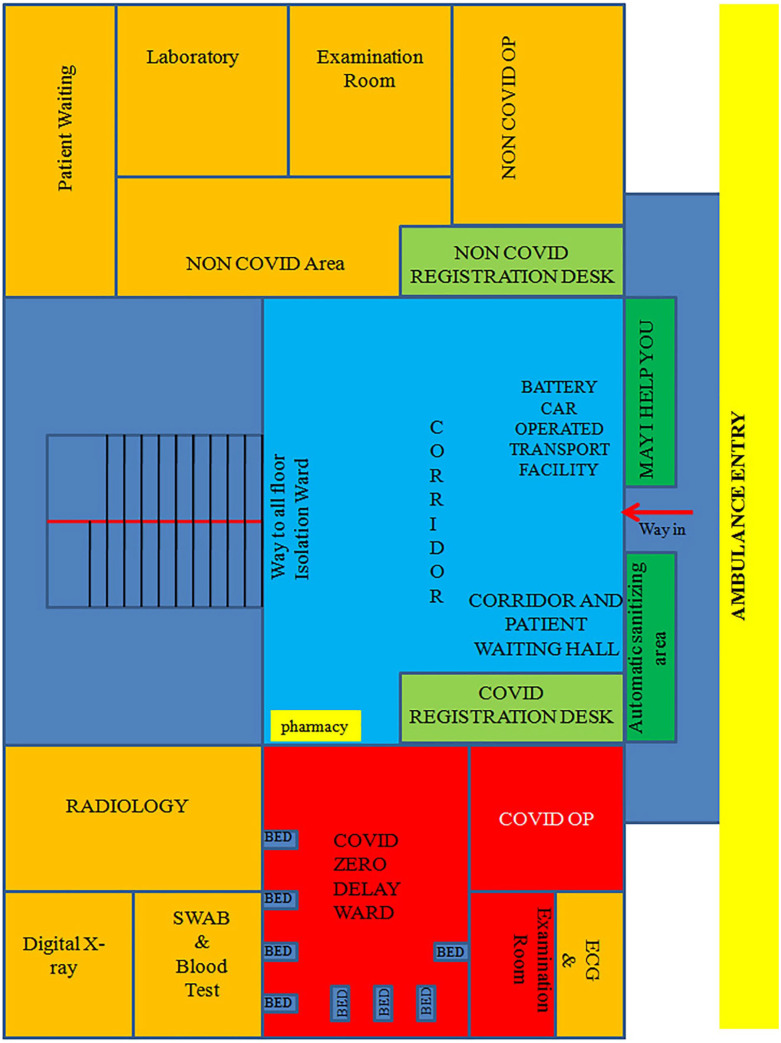
On July 7, 2020, a medical and administrative team from RGGGH, Chennai, created the novel concept of Zero Delay COVID-19 ward in Tower block III, which was originally constructed as an 8 story building exclusively dedicated for outpatient services of RGGGH, Chennai (Figure 1), to cope up with the rapidly increasing numbers of severe cases with COVID-19.
Figure 1.
COVID-19 hospital highlighting “COVID-19 zero delay ward.”
Facilities Created
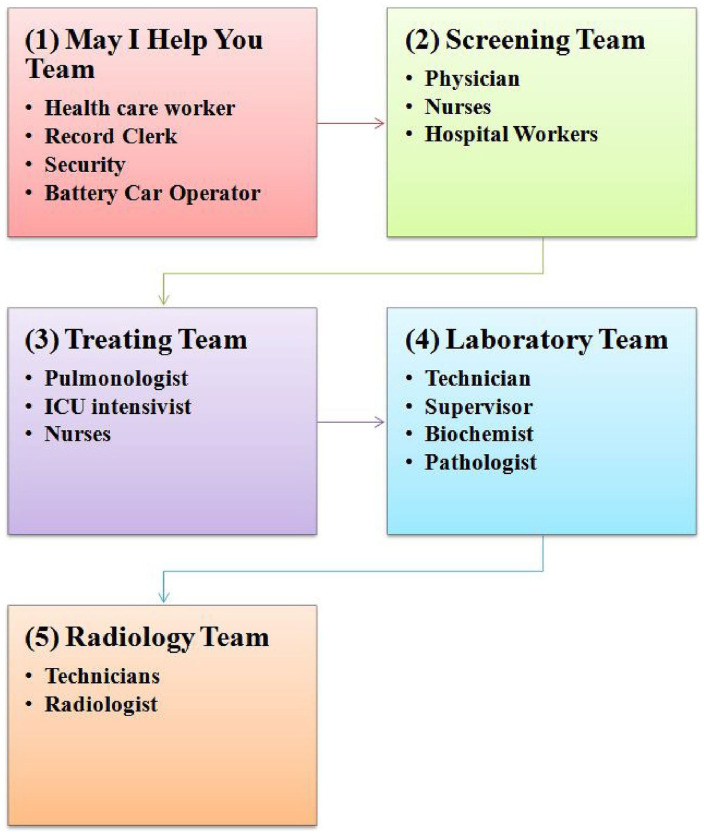
“May I help you” team, Screening team, Treating team
Battery car with oxygen support (Transport of critically ill patients is very critical; hence, the patients were transported in the battery car. Each battery car had an integrated oxygen support system)
10 beds with oxygen support
Emergency Medicines
Central laboratory services-dedicated COVID-19, Computed Tomography, ECG, Portable digital X-ray operated 24 × 7
Portable ventilator
The above facilities were created within a short period of 2 days with immense support from the local and state level administration.
Human Resources
We set up a multidisciplinary team with adequately qualified and experienced physicians, nurses, and an allied health professionals team to provide high-quality critical care services in the Zero Delay COVID-19 Ward. Residents from clinical departments with critical-care experience were posted in Zero Delay COVID-19 Ward while non-clinical residents were posted at screening desk. The detailed structure of the team is depicted in the Figure 2.
Figure 2.
Human resource at COVID-19 zero delay ward.
Functioning of Zero Delay COVID-19 Ward
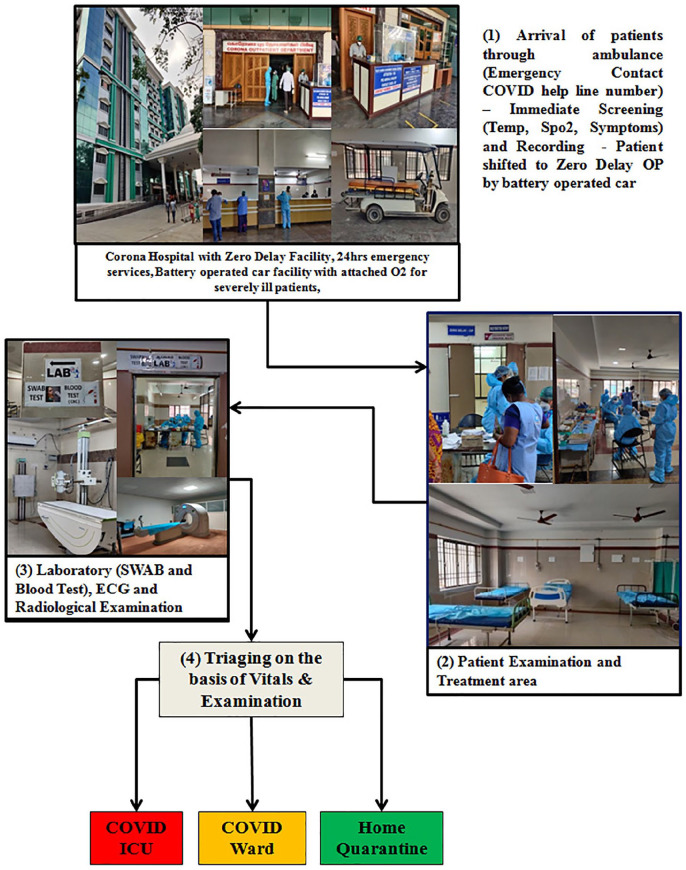
In the absence of effective antiviral treatments for COVID-19,7,8 one of the ways to reduce mortality was early intervention to prevent the progression of disease. The triad of interventions which helped us in reducing the morbidity and mortality are summarized as follows. Figure 3 shows the Zero Delay COVID-19 ward patient flow at the RGGGH, Chennai.
Figure 3.
Functioning of COVID-19 zero delay ward.
Pre arrival intimation
Immediate arrival screening
Treatment initiation without delay
Pre arrival intimation
Pre arrival notification is a service wherein the hospital is alerted about the condition of the patients before their arrival. This was a well known practice followed for victims of accident and other medical emergencies under the Tamil Nadu Accident and Emergency care Initiation (TAEI). This service was extended and implemented to COVID-19 also. In order to diffuse the knowledge of Zero delay COVID-19 ward and other set up available at RGGGH, the Tamil Nadu government made all efforts to the reach public via social media, SMS, caller tune, television, posters at local bodies, door to door delivery of health services, and education through health visitors and doctors in the PHC. Pre-intimation contact mobile number was shared with the corporation and ambulance drivers, so they were able to tell exactly how many patients would be coming which helped us in effective deployment of health resources before the patient’s arrival to the hospital. Pre arrival intimation also helped the screening and rapid response team to get ready the required appropriated treatment of the arriving severe patient even stroke and myocardial infarction were also addressed with specialized rapid referral expert team (Figure 4).
Figure 4.

Patient pre arrival information.
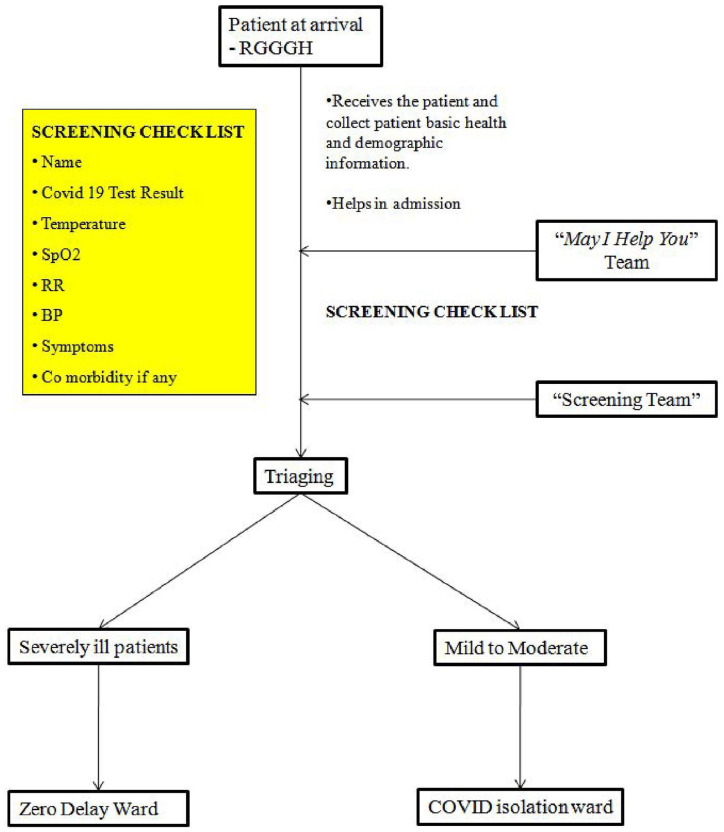
Immediate arrival screening
After arrival of the patients to RGGGH at the Zero Delay entrance, may I help you team received the patients, collected patient’s basic health and demographic information and were helping the patients in the admission process. Simultaneously, all patients were screened immediately with screening check list such as temperature, respiratory rate (RR), SpO2 or any clinical signs of impending organ failure by the screening team. Screening was skipped if severity of the patients were already intimated by the ambulance driver or transferring health care center. Patients with stable COVID-19 who did not require oxygen were isolated and treated in the separate wards, whereas patients who required oxygen were immediately initiated on oxygen support and were transferred to Zero delay COVID-19 ward by battery car (Figure 5).
Figure 5.
Patient arrival and screening.
Treatment initiation without further delay
We centralized the diagnosis and treatment within single area. The treatment protocols were formed, circulated to sensitize physicians and treatment were made accordingly. Rapid response team played a vital role in the commencement of appropriate treatment for all patients who were referred with clinical features suggestive of presumptive COVID-19 pneumonia or RT-PCR confirmed COVID-19 cases or radiological features suggestive of COVID-19 (CORADS-4 and above/Grade 1). Those patients who required oxygen at the time of screening were immediately started on appropriate mode of oxygen therapy and administered a combo of remdesivir, dexamethasone, and Low molecular weight heparin (LMWH) prophylactic dose within 20 min of screening (Figure 6).
Figure 6.
Treatment protocol at COVID-19 zero delay ward.
Simultaneously Central laboratory technicians started the diagnostic confirmation of COVID-19 and other blood profile and radiological tests of the admitted patient, the diagnostic procedures were facilitated and results can be obtained in 5 to 10 min. Physician and staff nurses monitored the progression of disease by measuring respiratory rate, temperature, oxygen saturation, and blood pressure multiple times per day. Clinically improved patients had been transferred to COVID-19 isolation wards. If patients condition worse or met following clinical criteria, they were quickly transferred to designated COVID-19 ICU: respiration rate of 24 beats/min or higher; blood oxygen saturation of 94% or lower; a partial pressure of arterial oxygen to fraction of inspired oxygen ratio of 300 mm Hg or less; lung imaging showing a greater than 50% progression of lesions; or the identification or development of severe chronic diseases, including hypertension, diabetes, coronary heart disease, cancer, structural lung disease, pulmonary heart disease, or immunosuppression.
All kinds of resources, including frontline medical staff and medical protective materials, were mobilized and deployed uniformly to guarantee patients’ medical care at Zero delay COVID-19 ward. More than 15 Clinical staff invested in patient’s treatment and care, and 30 clinical staffs were reserved for unexpected needs. Adequate material and human resources were provided. Education and proper training to all staffs were helped to treat the COVID-19 patients. Support of Government of Tamil Nadu played an immense role for controlling this pandemic of COVID-19 management in Zero Delay COVID-19 Ward.
Data Collection
The information of each patients were extracted from Zero Delay COVID-19 Ward nominal records, successive demographic, clinical characteristics, laboratory, radiography findings, and comorbidities were retrieved from the paper based medical records of internal medicine, community medicine, pathology laboratory, and biochemical laboratory records and patient’s medical records by the research team of Multidisciplinary Research Unit, Madras Medical College. The clinical outcomes of each patient (ie, nonsurvived, discharged) were also collected from medical record department registry.
Clinical Definitions
The clinical criteria of COVID-19 were based on guidelines given by the Indian Ministry of Health and Family Welfare. We graded severity of pneumonia based on computed tomography (CT) scan imaging of chest as percentage of total lung volume involved ≤25 as Grade 1; 26%-50% as Grade 2; 51%-75% as Grade 3; ≥76% as Grade 4.
Statistical Analysis
All statistical analyses were performed using SPSS version 15.0. We used descriptive statistics and expressed continuous variables as median and interquartile range (IQR) and categorical data as frequencies and percentages. We compared mean ranks using the Mann-Whitney test was used. Difference between survivors and non-survivors based on age group, CT chest abnormalities were compared using Pearson’s chi-squared test. We used multivariable logistic regression analysis to compute odds ratio (OR) with 95% confidence interval (95% CI) for analysis of potential risk factors for non-survivor COVID-19 patients. A 2-sided P < .05 was defined as statistically significant.
Results
In total 4515 COVID-19 patients admitted at Zero Delay COVID-19 Ward from July 7, 2020 to December 31, 2020. Significant difference was found between the gender and mortality groups, more men died (173; 76.2%) compared to women (54; 23.8%) (P < .0001). Of the 4515 patients, 1829 (40.5%) reported having at least 1 comorbidity. Compared with the survivors, non-survivors were more likely to have underlying comorbidities (208; 91.6%) (P < .0001) (Table 1). Patients exhibited various symptoms on admission, most common symptom was dyspnea (3864/4515, 85.6%). Dyspnea were more likely to occur in patients who were non-survivors (P < .004). The dyspnea recovery rate at Zero Delay COVID-19 Ward was not significant between survivors and non-survivors (P < .920). There was a significant association between the disease outcome and initial treatment with low molecular weight heparin and steroids (P < .039). When compared with those survivors to that of those non survivors, there was no significant differences for most of the hematological and biochemical parameters (Table 1).
Table 1.
Demographic, Baseline and Clinical Characteristics Comparison Between Survivors and Non-Survivors of COVID-19 Patients at Zero Delay COVID-19 Ward.
| Characteristics | Total cases (N = 4515) | Survivors (n = 4288) | Non-survivors (n = 227) | P value |
|---|---|---|---|---|
| Male € | 2857 (63.3%) | 2684 (62.6%) | 173 (76.2%) | .0001 $ |
| Age (in years) & | 57 (45-67) | 57 (45-66) | 63 (54-72) | .0001* |
| Age >40 years € | 3704 (82%) | 3489 (81.4%) | 215 (94.7%) | .0001 $ |
| Comorbidity (yes) € | 1829 (40.5%) | 1621 (37.8%) | 208 (91.6%) | .0001 $ |
| Dyspnea (yes) € | 3864 (85.6%) | 3655 (85.2%) | 209 (92.1%) | .004 $ |
| Abnormal CT chest € | 3388 (75%) | 3179 (74.1%) | 209 (92.1%) | .0001 $ |
| Injection (yes) € | 4282 (94.8%) | 4060 (94.7%) | 222 (97.8%) | .039 $ |
| Dyspnea Recovered € | 3819 (99.2%) | 3621 (99.1%) | 198 (99%) | .920 $ |
| WBC (cells/mm3) & | 7200 (5500-9800) | 7100 (5300.00-10 200) | 8000 (5900-11 150) | .385* |
| Hemoglobin (g/dL) & | 12.4 (11-13.9) | 12.1 (10.3-13.7) | 11.6 (10.25-12.75) | .09* |
| Platelets (×106/μL) & | 2.32 (1.79-2.94) | 2.39 (1.88-3.06) | 2.68 (2.44-3.05) | .071* |
| Neutrophils & (%) | 64 (53.1-77.3) | 64.3 (55.65-76.15) | 64.7 (48.5-74.15) | .995* |
| Lymphocytes & (%) | 25.4 (14.4-34.5) | 24.3 (13.35-32.75) | 22.9 (13.9-41.2) | .797* |
| NLR & | 2.43 (1.5-5.14) | 2.65 (1.61-5.325) | 2.82 (1.165-4.97) | .609* |
| Urea (mg/dL) & | 24 (19-36) | 25 (18.00-36.50 | 22 (18.50-45.00) | .15* |
| Creatinine (mg/dL) & | 0.8 (0.7-1.1) | 0.8 (0.7-1.1) | 0.8 (0.550-1.1) | .25* |
| Sodium (m.eq/L) & | 138 (135-141) | 138 (134-141) | 138 (133.5-145.5) | .09* |
| Potassium(m.eq/L) & | 4.2 (3.8-4.5) | 4.2 (3.9-4.6) | 4.4 (3.95-4.6) | .191* |
| TGL-C (mg/dL) & | 129 (91-182) | 139 (96.5-204) | 131 (75.5-156) | .2* |
| LDH (IU/L) & | 241 (192-322) | 253 (199-343) | 193 (185.5-270) | .527* |
| Ferritin (ng/mL) & | 186 (71.7-472) | 202 (64.5-553.4) | 116.5 (42.5-191.05) | .216* |
| CRP (mg/L) & | 7 (1.6-57.0) | 9.2 (1.50-64.65) | 1.8 (1.0-110.5) | .496* |
| SpO2 after & (%) | 97 (96-98) | 98 (96-98) | 97.5 (96-99) | .002* |
| SpO2 before & (%) | 95 (90-98) | 97 (95-98) | 92 (89-93.25) | .0001* |
Abbreviations: CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; SpO2, oxygen saturation; TGL–C, triglyceride cholesterol; WBC, white blood cell.
SpO2 After means, after treatment at zero delay COVID-19 ward, SpO2 Before means, at the time of admission.
Number (percentage).
Median (25th-75th interquartile range).
Chi square.
Mann-Whitney.
There was a significant association between the mortality and age distribution (P < .001). Higher death rates were reported in older age groups: patients aged more than 40 years died frequently (215; 94.7%) compared to groups below 40 years (12; 5.3%) and highest percentage was noted in more than 60 years (127/227; 55.9%) among them (Table 2).
Table 2.
Patient’s Age Group and Mortality.
| Age group (4515) | Survivors (n = 4288) (%) | Non-survivors (n = 227) (%) | P value $ |
|---|---|---|---|
| <18 (50) | 49 (1.1) | 1 (0.4) | .0001 |
| 19-30 (292) | 288 (6.7) | 4 (1.8) | |
| 31-40 (469) | 462 (10.8) | 7 (3.1) | |
| 41-50 (807) | 778 (18.1) | 29 (12.8) | |
| 51-60 (1129) | 1070 (25.0) | 59 (26.0) | |
| >60 (1768) | 1641 (38.3) | 127 (55.9) |
Chi square.
Non survivors showed more abnormalities in chest CT images (209; 92.1%) than survivors (18; 7.9%). The chest CT imaging of patients among non-survivors showed more severe radiological changes as compared to survivors, grade 4, grade 3, and grade 2 CT changes were present among non-survivors 48/209; (21.1%), 43 (18.9%), and 56 (24.7%) respectively (Table 3).
Table 3.
Patient’s CT Chest Abnormality and Mortality.
| Mortality group | Grading of chest CT based on total lung volume | P value $ | ||||
|---|---|---|---|---|---|---|
| Normal (n = 1087) (%) | Grade 1 (n = 2137) (%) | Grade 2 (n = 799) (%) | Grade 3 (n = 236) (%) | Grade 4 (n = 256) (%) | ||
| Survivors (n = 4288) | 1069 (24.9) | 2075 (48.4) | 743 (17.3) | 193 (4.5) | 208 (4.9) | .0001 |
| Non-survivors (n = 227) | 18 (7.9) | 62 (27.3) | 56 (24.7) | 43 (18.9) | 48 (21.1) | |
Chi square.
Multivariate logistic regression analysis showed that patients with comorbidity were associated with higher odds of mortality with COVID-19 (OR = 18.687; 95% CI, 11.229-31.1) than other significant risk factors including age, male gender, dyspnea, chest CT abnormalities (Table 4).
Table 4.
Multivariate Logistic Regression Analysis: Risk Factors Association With COVID-19 Mortality.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age (>40) | 1.956 (0.998-3.833) | .051 |
| Sex (male) | 1.845 (1.338-2.544) | .0001 |
| Comorbidity (yes) | 18.687 (11.229-31.1) | .0001 |
| Dyspnea (yes) | 0.376 (0.111-1.270) | .115 |
| Abnormal CT chest (yes) | 5.587 (1.685-18.518) | .005 |
| Injection (yes) | 0.137 (0.041-0.455) | .001 |
Pseudo R2 = .214.
Discussion
The main focus of this paper is to describe about the Zero Delay COVID-19 Ward and to analyze the profile of admitted patients. We reported 4515 COVID-19 hospitalized patients and described demographic, clinical characteristics and laboratory markers by their outcome status. Around 40% of the patients had significant comorbidity. We divided the patients into 2 groups; survivors and non-survivors patients. Non survivors patients were having higher age and notably, 94.7% of non survivors patients were more than 40 years. Most of the non survivors had complications associated with COVID-19 respiratory syndrome. The common symptoms at admission to Zero Delay COVID-19 Ward in our study was dyspnea, which is the most reported symptom in severe COVID-19 patients and consistent with the common symptom among critically ill patients reported in similar study. 9
We did not find any significant differences in laboratory characteristics between survivors and non survivors patients. We suspected that this may be due to initial evaluation of laboratory characteristics at the time of admission. We found that the abnormal chest CT images were associated with mortality and tended to have high grade of abnormal chest CT manifestations among deceased patients. This was similar to those demonstrated in previous reports.3,10
The death rate in the 4515 COVID-19 patients was 5.02%, being lower than for 20% (18%-23%) in the US, 23% (19%-27%) in the Europe, and 11% (7%-16%) for China reported in systematic review and meta-analysis of 77 studies among severe COVID-19. 11 The COVID-19 mortality risk reported consistently to male patients, older age and patients with comorbidity, similar to the findings in our study. However, the main observation of our study that comorbidity was identified as more significant risk factor for mortality in patients with COVID-19 than age, gender. Many previous studies also demonstrated that the presence of comorbidity is associated with high risk of worst clinical outcomes. 12 Another study from Lombardy region of Italy showed 49% were having systemic hypertension, 13 in USA: the Coronavirus Disease 2019—Associated Hospitalization Surveillance Network (COVID-NET) also showed hypertension was present among 49.7% of patients affected with COVID-19. 14 An association between COVID-19 severity and comorbidity has also been reported in China, 15 and the USA. 16 Comorbidity were risk analysis also done in the previous Indian studies.17,18 In the current study, we found that the comorbidity persisted as high-risk factor for mortality. This has important implications for a patient’s special isolation, care, and decision making.
Conclusion
On a group of hospitalized COVID-19 patients, we documented that comorbidity correlated with a higher mortality. Altogether, these findings suggest that patients with comorbidity are a much more vulnerable population in the COVID-19 outbreak. From our experience, there is a significant association in the recovery of patients who had been initiated on steroids and low molecular weight heparin in the zerodelay ward. At this critical moment in the global outbreak of COVID-19 pandemic, we hope our valid management in Zero delay COVID-19 ward can help us achieve the victory in the battle against COVID-19 pandemic as well as future epidemics and disasters. These measures would surely prevent the mortality in the long run.
Limitations
Our study should also consider some limitations. First, this is a single center experience. However, we assume that these observations may be the most typical scenario encountered in other centers in the state of Tamil Nadu and India as well. Second, we have only analyzed the clinical parameters and CT chest manifestations at the time of admission. Further study including clinical follow ups are required to determine if the findings in the specified group are sustained throughout the course of illness. Third, the retrospective nature of the study—we were not able to determine the presence of other co-infections on the observed CT chest findings. Last, patients who were classified under mild, very mild, and asymptomatic were not included in our study. Hence the severe or moderate disease rate in a real-world scenario might be even lower.
Acknowledgments
We gratefully acknowledge the Government of India, Ministry of Health and Family Welfare, Department of Health Research, Multidisciplinary Research Unit (MRU), Virus Research and Diagnostic Laboratory (VRDL).
Footnotes
Author Contributions: Conception of the study, overall study supervision (TE), study supervision (SZH), Acquisition of the data, drafting of manuscript, Statistical analysis and interpretation of data (KR), assistance in drafting of the article, data collection (AR), assistance in editing and reviewing of the manuscript (MN), assistance in data collection (NV, KE, GA, GM), acquired the data (PP, JP, MR, GA) and study supervision, reviewing of manuscript and approved the final version of the manuscript (GN).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Karthick Rajendran  https://orcid.org/0000-0003-3938-3347
https://orcid.org/0000-0003-3938-3347
References
- 1. Coronavirus disease (COVID-19). Data as received by WHO from national authorities. https://covid19.who.int/
- 2. World Health Organization. COVID-19 strategy update. https://www.who.int/docs/default-source/coronaviruse/covid-strategy-update-14april2020.pdf?sfvrsn=29da3ba0_19
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30-e31. doi: 10.1016/S0140-6736(20)30304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. doi: 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395: 507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191. doi: 10.1371/journal.pone.0243191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan W-J, Liang W-H, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garg S, Kim L, Whitaker M, et al. Hospitalized rates and characteristics of patients with laboratory-confirmed Coronavirus Disease 2019—COVIDNET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464. doi: 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025-1031. doi: 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta N, Agrawal S, Ish P, et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90(1). doi: 10.4081/monaldi.2020.1294 [DOI] [PubMed] [Google Scholar]
- 18. Mishra V, Burma AD, Das SK, Parivallal MB, Amudhan S, Rao GN. COVID-19-hospitalized patients in Karnataka: survival and stay characteristics. Indian J Public Health. 2020;64:S221-S224. doi: 10.4103/ijph.IJPH_486_20. [DOI] [PubMed] [Google Scholar]