Abstract
Humans and their microbiota have coevolved a mutually beneficial relationship in which the human host provides a hospitable environment for the microorganisms and the microbiota provides many advantages for the host, including nutritional benefits and protection from pathogen infection1. Maintaining this relationship requires a careful immune balance to contain commensal microorganisms within the lumen while limiting inflammatory anti-commensal responses1,2. Antigen-specific recognition of intestinal microorganisms by T cells has previously been described3,4. Although the local environment shapes the differentiation of effector cells3-5 it is unclear how microbiota-specific T cells are educated in the thymus. Here we show that intestinal colonization in early life leads to the trafficking of microbial antigens from the intestine to the thymus by intestinal dendritic cells, which then induce the expansion of microbiota-specific T cells. Once in the periphery, microbiota-specific T cells have pathogenic potential or can protect against related pathogens. In this way, the developing microbiota shapes and expands the thymic and peripheral T cell repertoire, allowing for enhanced recognition of intestinal microorganisms and pathogens.
In humans during the first three years of life, the composition of the microbiota progressively stabilizes to resemble that found in adults6. This corresponds to a period of expansion of the T cell repertoire7. The importance of the microbiota in the education of T cells can be shown in mouse models: antibiotic-treated and germ-free mice express distinct intestinal T cell receptor (TCR) repertoires compared to mice with a normal microbiota composition, suggesting that microbial antigens alter T cell development8.
Microbiota-specific T cells are found in the thymus
To address how gut bacteria drive the thymic development of T cells that are specific to commensal bacteria, we used segmented filamentous bacteria (SFB), a group of commensal microorganisms related to Clostridia that drive TH17 cell responses in mice4. SFB are one of the few commensal microorganisms for which a microorganism-specific T cell receptor has been identified and a defined SFB tetramer allows the tracking of SFB-specific T cells4. We colonized SFB-negative specific-pathogen-free (SPF) B6 mice with SFB at weaning (young) or at 12 weeks old (adult). Two weeks later, we examined T cell populations by flow cytometry. Using magnetic enrichment9 for tetramer-specific SFB-recognizing T cells (I-Ab/SFB3340 tetramer)4, we found that there was an expansion in SFB-specific CD4-single-positive (CD4+) T cells in the thymus of young, but not adult, SFB-colonized mice as compared to SFB-negative mice (Fig. 1a, b, Extended Data Fig. 1a, b). SFB colonization also resulted in an increase in the total number of thymic CD4+ T cells in SFB-specific TCR transgenic mice4 (Extended Data Fig. 1c).
Fig. 1 ∣. Commensal colonization leads to an expansion of bacteria-specific T cells in the thymus.
a–d, Mice were colonized with SFB at weaning (young) or at 12 weeks of age (adult) and two weeks later were compared to age-matched control mice. a, b, Flow plots (a) and counts (b) of thymic SFB-tetramer+ cells. c, d, Representative flow plots of RORγt and FOXP3 (c) and CD25 (d) in thymic SFB-tetramer+ cells. In a–d, n = 12 (control young, control adult, SFB adult); n = 15 (SFB young). e, RAG2-GFP mice (n = 7) were colonized with SFB at weaning. Representative flow plot of CD73 and RAG2 in thymic SFB-tetramer+ cells two weeks after colonization. f, Frequencies and counts of SFB-tetramer+ cells in the MLNs and ileum (control: n = 6 (MLNs young, ileum young), n = 5 (MLNs adult, ileum adult); SFB: n = 6 (MLNs young, ileum young), n = 10 (MLNs adult), n = 5 (ileum adult)). g, Young and adult mice were colonized with SFB and one week later were compared to age-matched control mice. SFB-specific qPCR in the thymus (control: n = 10 (young), n = 11 (adult); SFB: n = 10 (young), n = 11 (adult)). Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-way ANOVA with Tukey’s post hoc test (b, f, g).
Colonization with SFB did not induce the expansion of SFB-specific cells in other thymic subsets, or that of T cells specific to unrelated tetramers (Extended Data Fig. 1d, e). We detected no change in the total numbers or the maturation of thymocytes, indicating that this is an antigen-specific effect (Extended Data Fig. 1f-k).
The majority of SFB-specific CD4+ T cells were not thymic regulatory T cells (Treg cells) as they were FOXP3-negative and CD25-negative10 (Fig. 1c, d). Most SFB-specific CD4+ T cells were CD24−TCRβ+ and expressed variable levels of CD44 and CD69 (Extended Data Fig. 2a-c), suggesting that they are antigen-experienced positively selected T cells11,12.
To confirm that SFB-specific CD4+ T cells were not circulating blood contaminants, we labelled blood haematopoietic cells by injecting mice intravenously with an anti-CD45 antibody13. In contrast to splenic T cells–3% of which are contaminating blood cells–nearly all tetramer-positive cells were CD45−, verifying that they were not circulating (Extended Data Fig. 2d). To confirm that these cells were thymocytes, we colonized mice expressing green fluorescent protein (GFP) from the RAG2 promoter (hereafter, RAG2-GFP mice)14 and stained thymic SFB-specific CD4+ T cells with CD73, which marks recirculating mature T cells14. We found that, similar to most CD4+ thymic cells, SFB-specific CD4+ T cells were GFP+CD73− (Fig. 1e, Extended Data Fig. 2e), providing evidence that they are recently developed T cells and not recirculating cells14.
To further characterize thymic SFB-specific CD4+ T cells, we performed RNA sequencing (RNA-seq). The gene expression of SFB-specific and non-specific T cells corresponds to CD4+ T cells after selection15 (Extended Data Fig. 2f). SFB-specific cells exhibited higher expression of transcription, proliferation and TCR-signalling genes, again indicating that these are post-selection cells that are ready to emigrate from the thymus12 (Extended Data Fig. 2g, Supplementary Tables 1-3).
SFB colonization induces the peripheral expansion of SFB-specific TH17 cells4, and two weeks after colonization we found that these cells had expanded in the mesenteric lymph nodes (MLNs) and ileum of adult mice (Fig. 1f). However, in young mice, we did not detect a peripheral expansion until four weeks after colonization (Fig. 1f, Extended Data Fig. 2h), indicating differential peripheral T cell kinetics. At this time point, we continued to observe expanded thymic SFB-specific T cells; however, the majority were now CD73+ (Extended Data Fig. 2i-k), indicative of the migration of peripherally expanded cells to the thymus14. Further confirming the regulation of thymic T cells by commensal microorganisms, antibiotic-mediated depletion of the gut microbiota at weaning led to a reduction in thymic cellularity, in the number of CD4+ cells and in the number of thymic Treg cells in SPF mice, but not in germ-free mice (Extended Data Fig. 2l, m).
Microbiota DNA is found in the thymus
Intestinal microorganisms can penetrate to the MLNs16, but whether these microorganisms can reach other locations remains unclear. To investigate this question, young and adult mice were left uncolonized or were colonized with SFB. Intestinal colonization was comparable between groups as assessed by quantitative PCR (qPCR) with SFB-specific 16S rDNA primers (Extended Data Fig. 3a). SFB DNA was found in the thymus and MLNs of young, but not adult, SFB-colonized mice (Fig. 1g, Extended Data Fig. 3b). When we looked at additional organs, a minority of mice showed low levels of SFB DNA in the liver and heart, and no SFB DNA was detected in the spleen or lungs (Extended Data Fig. 3c), showing that the bacterial DNA in the thymus and MLNs was not due to microbial leak into the bloodstream. After colonization, SFB DNA was detected in the faeces for at least three weeks, whereas in the thymus and MLNs, SFB DNA reached a peak level by one week and was undetectable at two weeks after colonization (Extended Data Fig. 3d). In both uncolonized and SFB-colonized young mice–but not adult mice–we found total bacterial 16S DNA (that is, 16S DNA from all bacterial species) was present in the thymus and MLNs, with lower levels in other analysed tissues (Extended Data Fig. 3e, f). Unlike SFB 16S, total bacterial 16S DNA was found in the thymus and MLNs for at least three weeks after colonization (Extended Data Fig. 3g) and could reflect the colonization of extra-intestinal sites by the microbiota. To test whether this was common for other commensal microorganisms, we colonized young and adult mice with a mouse commensal strain of Escherichia coli. Using qPCR with E. coli-specific 16S rDNA primers, we found that intestinal colonization was similar between groups; however, as with SFB, E. coli DNA was found in the thymus and MLNs of young but not adult colonized mice (Extended Data Fig. 3h-j). We were unable to culture live E. coli or other bacteria from the thymus or MLNs.
To define the diversity of microorganisms in the tissue we used 16S sequencing. We detected a broad range of microbial phyla within both the MLNs and the thymus, which overlapped with caecal microbial phyla (Extended Data Fig. 3k). As expected, antibiotic treatment at weaning reduced the levels of bacterial DNA in all analysed tissues (Extended Data Fig. 3l-n).
CX3CR1+dendritic cells expand thymic T cells
We next sought to identify the antigen-presenting cells (APCs) that induce the thymic expansion of microbiota-specific T cells. As mice reach adulthood, thymic T cell subsets expand until week 5 before declining7 (Extended Data Fig. 4a, b). The number of thymic dendritic cells (DCs) also increases until week 5 (Extended Data Fig. 4c). As with T cells, we found that antibiotic-mediated depletion of the gut microbiota at weaning reduced the number of thymic DCs at week 5 (Extended Data Fig. 4d). Colonization with SFB or E. coli in young but not adult mice increased the total number of thymic DCs (Extended Data Fig. 4e, f). Several intestinal populations of APCs exist, which can be subdivided on the basis of their expression of markers including the integrin CD103 and the chemokine receptor CX3CR117. It has previously been shown that the microbiota regulates the migration of CX3CR1+ DCs from the intestine to the MLNs16. However, migration to the thymus has not so far been characterized. We detected an increased number of thymic CX3CR1+ DCs–but not CD103+ DCs–after weaning, which decreased by 12 weeks of age (Extended Data Fig. 4g). We confirmed that thymic CX3CR1+ DCs were not blood contaminants by injecting mice intravenously with an anti-CD45 antibody as above (Extended Data Fig. 4h). Colonization with SFB or E. coli in young but not adult mice increased the number and percentage of CX3CR1+ DCs, but not CD103+ DCs, in the thymus and MLNs (Fig. 2a, Extended Data Fig. 4i-l). Antibiotic-mediated depletion of the gut microbiota led to a decrease in subsets of thymic DCs (Extended Data Fig. 4m).
Fig. 2 ∣. Enrichment of CX3CR1+ DCs in the thymus after colonization with commensal microorganisms.
a, Young and adult mice were colonized with SFB or E. coli (EC) and compared two weeks later to age-matched control mice. Frequencies and counts of thymic CX3CR1+ DCs (n = 15 (control young), n = 20 (control adult, SFB young, SFB adult), n = 12 (EC young), n = 16 (EC adult)). b, Wild-type (WT) mice and mice depleted of CX3CR1+ cells (CX3-DTR) were colonized with SFB at weaning and compared two weeks later. Frequencies and counts of thymic SFB-tetramer+ cells (n = 5 (control), n = 6 (SFB WT), n = 8 (SFB CX3-DTR)). c, d, Caeca from young (n = 5) and adult (n = 9) KikGR33 transgenic mice were exposed to 405-nm-wavelength light and two days later the thymus, MLNs and spleen were collected. c, Representative flow plot of RFP+MHCII+ cells in young mice. d, Composition of RFP+MHCII+ cells. Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-way ANOVA (a) or one-way ANOVA (b) with Tukey’s post hoc test.
Other populations of APCs (B cells and plasmacytoid dendritic cells) remained unchanged after SFB or E. coli colonization in young mice, and only B cells showed an increase in adult and antibiotic-treated mice (Extended Data Fig. 4n-q).
To understand the role of CX3CR1+ DCs in the thymic expansion of microbiota-specific T cells, we used mice that express the diphtheria toxin receptor under the control of the CX3CR1 promoter (hereafter, CX3CR1-DTR mice18). All CX3CR1+ cells are depleted after treatment with diphtheria toxin. We treated CX3CR1-DTR and littermate controls with diphtheria toxin, followed by SFB colonization at weaning. Two weeks later we found that thymic SFB-specific T cell expansion was reduced in the absence of CX3CR1+ cells (Fig. 2b, Extended Data Fig. 5a). Depletion of CX3CR1+ cells at weaning reduced the total level of bacterial 16S DNA in the thymus and MLNs (Extended Data Fig. 5b, c). In mice colonized with SFB or E. coli at weaning, depletion of CX3CR1+ cells reduced the levels of SFB or E. coli DNA in the thymus without affecting intestinal colonization (Extended Data Fig. 5d-g). We also used mice in which CX3CR1+ DCs and macrophages can be selectively depleted16 and generated mice in which CD103+ DCs were depleted (Extended Data Fig. 5h). Depletion of CX3CR1+ DCs, but not CD103+ DCs, reduced the levels of thymic E. coli-specific DNA and total bacterial 16S DNA in E. coli-colonized mice (Extended Data Fig. 5i, j). To determine whether antigen presentation by CX3CR1+ cells was required for the thymic expansion of microbiota-specific T cells, we used mice in which CX3CR1+ cells lose their expression of MHCII expression after treatment with tamoxifen19, and found a decreased expansion of thymic SFB-specific T cells without changes in SFB DNA levels or in the thymic numbers of CX3CR1+ DCs (Extended Data Fig. 5k-m).
Thymic DCs can be resident XCR1+ cells or migratory SIRPα+ cells that localize in the cortex and perivascular regions of the thymus20. All thymic CX3CR1+ DCs express SIRPα+ (Extended Data Fig. 6a).
To further characterize cell migration from the intestine to the thymus, we used KikGR33 transgenic mice in which all cells express GFP that photoconverts to red fluorescent protein (RFP) after exposure to light of 405-nm wavelength21. We photoconverted the caecum of young and adult KikGR33 mice and analysed distal tissues 48 h later. In both groups, we observed RFP+ cells in the thymus, MLNs and spleen; the majority of these RFP+ cells were MHCII+ APCs, which indicates the steady-state migration of caecal APCs to these tissues (Fig. 2c, Extended Data Fig. 6b-e). However, the composition of migratory APCs differed between groups. In young mice, the majority of the thymus migratory cells were CX3CR1+ DCs; by contrast, in adult mice these cells were mainly CX3CR1− DCs and B cells. In young mice, most of the MLN migratory cells were CX3CR1− DCs and most of the spleen migratory cells were B cells, whereas in adult mice, B cells constituted the majority of migrating APCs to both the MLNs and the spleen (Fig. 2d, Extended Data Fig. 6f).
To understand this differential migration as mice aged, we analysed the expression of chemokines in the thymus and CX3CR1+ DC chemokine receptors in the colon. We found that the thymic expression of CCR5 ligands (CCL3 and CCL5) and CX3CL1 decreased in adult mice compared to young mice (Extended Data Fig. 6g). In parallel, we found a selective loss of intestinal CCR5+CX3CR1+ DCs as mice aged (Extended Data Fig. 6h). To test for the requirement of CX3CR1 or CCR5 for cell migration from the gut to the thymus, we treated SFB-colonized young wild-type or CX3CR1-deficient mice with TAK-779 (an antagonist of CCR5 and CCR222) or an antagonistic anti-CCL2 antibody. Loss of CX3CR1 or inhibition of CCR5 decreased the levels of thymic SFB DNA, and no SFB DNA was found in TAK-779-treated CX3CR1-deficient mice (Extended Data Fig. 6i).
Thymic microbiota-specific T cells are functional
To test whether expanded thymic microbiota-specific T cells responded to their cognate antigens in peripheral tissues, we used the T cell transfer model of colitis, in which naive T cells transferred to an immunodeficient host cause intestinal microorganism-dependent pathology23. We colonized mice at weaning with SFB or E. coli. Two weeks later, we isolated naive thymic T cells and transferred them into SFB or E. coli-colonized Rag2−/− mice (Supplementary Table 4). SFB-colonized recipients exhibited increased weight loss and intestinal pathology, together with increased immune infiltration with a higher proportion of TH17 cells, after transfer of thymic T cells from SFB-colonized donors compared to uncolonized donors (Fig. 3a-c, Extended Data Fig. 7a-d). We also found an increase in the proportion and number of SFB-specific colonic T cells, most of which expressed the TH17 transcription factor RORγt (Fig. 3d, Extended Data Fig. 7e, f). A similar phenotype was observed when thymic T cells from E. coli-colonized donors were transferred into E. coli-colonized recipients, with increased weight loss, pathology and immune infiltration (Fig. 3e-g, Extended Data Fig. 7g, h). In contrast to colonization with SFB, a higher proportion of colonic T cells in recipients that were colonized with E. coli expressed the TH1 transcription factor T-bet (Fig. 3h, Extended Data Fig. 7i). We performed parallel experiments using adult mice as T cell donors. Colonized recipient mice that received naive thymic T cells from SFB- or E. coli-colonized adult donors did not develop exacerbated colitis (Extended Data Fig. 8a-n). To confirm a role for CX3CR1+ DCs, we colonized diphtheria-toxin-treated CX3CR1-DTR and littermate control mice at weaning with SFB and transferred naive thymic T cells as above. SFB-colonized recipient mice exhibited reduced weight loss, colitis, colonic immune infiltration and SFB-specific T cells when they received T cells from young CX3CR1+-cell-deficient SFB-colonized donors as compared to wild-type littermate control donors (Fig. 3i-k, Extended Data Fig. 9a-d). Likewise, thymic T cell transfer from SFB-colonized mice in which CX3CR1+ cells lack MHCII expression induced less weight loss and colitis, with fewer SFB-specific T cells (Extended Data Fig. 9e-k). Transferring thymic T cells from donors that were treated with antibiotics at weaning induced colitis to a more limited extent compared to T cells from donors with a SPF microbiota (Extended Data Fig. 9l-q).
Fig. 3 ∣. Thymic microbiota-specific T cells are functional in distal tissues.
a–h, Mice were colonized with SFB or E. coli at weaning and two weeks later thymic CD4+ T cells were transferred to SFB- or E. coli-colonized Rag2−/− mice. Recipient mice were followed for four weeks and disease severity was compared to Rag2−/− mice that received age-matched control thymocytes. a, e, Relative weight change in SFB (a) or E. coli (e) recipient mice. b, c, f, g, Representative haematoxylin and eosin (H&E) staining (b, f) and colitis score (c, g) in SFB (b, c) or E. coli (f, g) recipient mice. d, h, Frequencies and counts of colonic SFB-tetramer+ cells (d) and TH1 cells (h). In a–d, n = 8 (control), n = 13 (SFB); in e–h, n = 8 (control), n = 15 (EC). i–k, Similarly, thymocytes from SFB-colonized diphtheria-toxin-treated littermate wild-type and CX3-DTR mice were transferred to SFB-colonized Rag2−/− mice. i, Relative weight change (n = 13 (WT); n = 14 (CX3-DTR)). j, k, Representative H&E staining (j) and colitis score (k) (n = 6 (WT); n = 10 (CX3-DTR)). l, Mice were colonized with E. coli (n = 11) at weaning and two weeks later were infected with S. Typhimurium (ST) and survival was compared to infected control mice (n = 11). A subset of mice colonized with E. coli received anti-CD4 antibody (n = 10) or isotype control (IgG) (n = 8) injections before and during infection with S. Typhimurium. Kaplan–Meier plot of survival. Each replicate is a biologically independent sample. Data are shown as mean ± s.e.m with P values by two-way ANOVA with Fisher’s LSD post hoc test (a, e, i) or are shown as individual values and mean, with P values by two-tailed unpaired t-test (c, d, g, h, k) or log-rank (Mantel–Cox) test (l). Scale bars, 100 μm (b, f, j).
Although these experiments show that microbiota-specific T cells can be pathogenic, we also wanted to understand whether expanded microbiota-specific T cells could protect from infection. Salmonella enterica subsp. enterica serovar Typhimurium is closely related to E. coli, with cross-reactive humoral responses24. We found E. coli cross-reactive T cells in Salmonella-infected mice (Extended Data Fig. 9r). To test whether expanded E. coli-specific T cells protected mice against infection with Salmonella, we left mice uncolonized or colonized them with E. coli at weaning. Two weeks later, we infected mice with a lethal dose of Salmonella. Although both groups had comparable levels of Salmonella colonization, mice that were colonized with E. coli mice exhibited increased survival after Salmonella infection, which was abrogated after depletion of CD4+ T cells (Fig. 3l, data not shown).
Discussion
These results show that in young mice, gut microbial colonization drives the thymic expansion of T cells that are specific to commensal microorganisms (Extended Data Fig. 10). Intestinal microorganisms are trafficked in a CX3CR1- and CCR5-dependent manner from the intestine to the thymus by intestinal CX3CR1+ DCs, which present microbiota-derived antigens to induce the expansion of microbiota-specific T cells. This is in contrast to medullary thymic epithelial cells and other thymus-resident APCs that facilitate clonal deletion and/or the induction of thymic Treg cells25,26. Although the molecular mechanisms that underlie this expansion remain unclear, we believe that microbial non-self-antigens might act in concert with unique co-stimulatory signals induced by the gut microenvironment. These data indicate that the thymus has a role in the expansion of microbiota-responsive T cells with diverse effector potential after antigen re-encounter. It will be important to test whether similar thymic trafficking of microorganisms that induce Treg cells5 occurs and whether this drives the expansion of thymic T cells or Treg cells. As a developmental window for the expansion of skin-microbiota-specific T cells has previously been described27,28, future work should explore whether microbiota from non-intestinal sites also educate thymic T cells. Understanding the development of bacteria-specific T cells in early life–including how to reopen the development window and restart the thymic generation of bacteria-specific T cells in older hosts–could provide new approaches to treat immune disorders that exacerbate bacteria-specific responses, such as inflammatory bowel disease, and to boost immune responses against pathogens.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-021-03531-1.
Methods
Data reporting
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Mice
All mice were bred in-house under standard conditions at the animal facility of Baylor College of Medicine or Memorial Sloan Kettering Cancer Center and maintained as SFB- and E. coli-free. The colony is routinely tested by qPCR and immunophenotyping to confirm lack of SFB and E. coli colonization. C57BL/6J (Jax 000664), CD11c-Cre (JAX 008068), MHCII-conditional (JAX 013181)29, CX3CR1-GFP (JAX 005582)30, Rag2−/− (JAX 008449), C57BL/6-Tg(Tcra,Tcrb)2Litt/J (JAX 027230)4, CX3CR1-CreERT2 (JAX 021160)31 and Tg(CAG-KikGR)33Hadj/J (JAX 013753)21 mice were originally purchased from The Jackson Laboratory before being bred in-house. FVB-Tg(RAG2-EGFP)1Mnz/J (JAX 005688) mice were provided by M. van den Brink. CX3CR1-STOP-DTR (JAX 025629)16 and CX3CR1-DTR18 were previously described. For inducible CD103-DTR mice, we introduced a loxP-floxed stop cassette followed by DTR into the CD103 locus. Mice were subsequently crossed to CD11c-Cre mice, allowing for deletion of the stop cassette in CD11c+ cells and expression of DTR in CD103+ cells. All mice were crossed at least 12 generations to the C57BL/6J background. Germ-free C57BL/6J mice were bred at the germ-free animal facility of University of Utah School of Medicine. All mouse experiments were performed with mice between 3 and 16 weeks of age with males and females at similar ratios, unless otherwise specified. Littermate controls were used for each experiment and mice were randomly assigned to experimental groups. All experiments were performed in accordance with approved protocols by the Institutional Animal Care and Usage Committee at Baylor College of Medicine, Memorial Sloan Kettering Cancer Center and University of Utah.
Colonization of mice with SFB and E. coli
SFB colonization was performed by two consecutive oral gavages with faecal pellets from SFB-monocolonized mice. SFB-monocolonized faecal pellets were a gift from D. Littman. E. coli were cultured overnight in LB medium with ampicillin at 50 μg/ml. Mice with a SPF microbiota were colonized with a single gavage of 108 colony-forming units (CFU) of mouse commensal E. coli. Colonization was confirmed by qPCR with SFB or E. coli-specific primers using Il23r (for tissues) or 16S (for faecal pellets) as housekeeping gene. Primers used were: Il23r F: GCAAATGGA AATGTCAGCAGAGCC, Il23r R: GCAGCTCACTTTCAGTAATCTGGG32, 16S F: CGGTGAATACGTYCGG, 16S R: GGWTACCTTGTTACGACTT33, SFB F: GACGCTGAGGCATGAGAGCAT, SFB R: GACGGCACGGATTGTTATTCA32, E. coli F: GGTAGAGCACTGTTTTGGCA, E. coli R: TGTCTCCCGTGATAACTTTCT34.
Depletion of the gut microbiota
SPF wild type C57BL/6J mice were gavaged a single dose of 20 mg streptomycin (Sigma) and provided ampicillin (Sigma) at 1 g/l in their drinking water for 1–2 weeks19. Bacterial 16S was assessed by qPCR as above. Germ-free mice received streptomycin and ampicillin in their drinking water for 2 weeks at the same concentrations as SPF mice.
RNA and DNA isolation and qPCR analysis
For bacterial DNA identification thymus, MLNs, liver, spleen, heart, lungs, and faecal pellets were collected one week after the specified treatment and stored at −80 °C. DNA was isolated using the DNeasy PowerSoil Kit according to the manufacturer’s protocol (Qiagen). qPCR was performed using SYBR Green Supermix (Bio-Rad Laboratories) in the Bio-Rad thermocycler 384-well plates. The thermocycling program was 40 cycles at 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s, with an initial cycle of 95 °C for 120 s. The primers used were described above. The relative expression of the target gene was determined using a standard curve to calculate the number of nucleotides per sample and the ΔΔCT method. For formatting purposes, a ΔΔCT value of 1 × 10−5 (limit of detection) was represented as 0 in qPCR plots for bacterial identification. For chemokine expression analysis, thymic RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s protocol. DNA was removed using a DNA-free DNA Removal Kit (Thermo Fisher Scientific). cDNA was generated using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). qPCR was performed as above using β-actin (Actb) as a housekeeping gene. Primers used were: Actb F: GTGACGTTGACATCCGTAAAGA, Actb R: GCCGGACTCATCGTACTCC, Ccl3 F: TGAAACCAGCAGCCTTTGCTC, Ccl3 R: AGGCATTCAGTTCCAGGTCAGTG, Ccl5 F: AGATCTCTGCAGCTGCCCTCA, Ccl5 R: GGAGCACTTGCTGCTGGTGTAG, Ccl2 F: GCATCCACGTGTTGGCTCA, Ccl2 R: CTCCAGCCTACTCATTGGGATCA, Ccl4 F: CCATGAAGCTCTGCGTGTCTG, Ccl4 R: GGCTTGGAGCAAAGACTGCTG, Xcl1 F: TCTTGATCGCTGCTTTCACC, Xcl1 R: GAAGTCCTAGAAGAGAGTAGC, Ccl19 F: GCTAATGATGCGGAAGACTG, Ccl19 R: ACTCACATCGACTCTCTAGG, Ccl21b F: GCTGCCTTAGTACAGCCAG, Ccl21b R: GTGTCTGTTCAGTTCTCTTGC, Ccl25 F: GCTTTTTGCCTGCCTGGTTG, Ccl25 R: TCAGTCTGAGAGTCTGAGGC, Cx3cl1 F: CGCGTTCTTCCATTTGTGTA, Cx3cl1 R: CTGTGTCGTCTCCAGGACAA.
Cell isolation
Intestinal lamina propria cells were isolated as previously described16,19,35. In brief, mouse small or large intestines were washed in PBS, once with freshly prepared 30mM EDTA and 1 mM DTT and once with 30 mM EDTA (both at 37 °C), and then digested at 37 °C in collagenase 8 (100 U/ml, Sigma-Aldrich) and DNase (150μg/ml, Sigma-Aldrich) containing medium with 10% fetal bovine serum. Digested material was separated on a 40%/80% Percoll gradient. Thymus, MLNs and spleen were digested as above, washed in PBS and passed through a 40-μm strainer.
Tetramer magnetic enrichment
Analysis of tetramer+ cells was performed as previously described with minor modifications9,36-38. In brief, cells were blocked with CD16/32 (93) and stained with PE-, APC-, or PE- and APC-conjugated tetramers specific against SFB (SFB3340 200-210, NIH tetramer core facility) at room temperature for 20 min and at 4 °C for 40 min in the dark. Next, cells were incubated with anti-PE or anti-APC nanobeads (BioLegend) for 15 min at 4 °C and the PE+ or APC+ magnetically labelled fraction was retained using a MojoSort Magnet (BioLegend). We then proceeded to surface staining with antibodies as in Extended Data Fig. 1a. Using the same methodology we tested PE-conjugated tetramers specific against 2W1S, Helicobacter hepaticus and human peptides (Ealpha 52-68 variant, HH1713 172-186, and class II-associated invariant chain peptide, NIH tetramer core facility).
Antibodies, cell staining and flow cytometry
Anti-CD45 antibody (30-F11, 2 μg/ml) was injected intravenously 3 min before euthanasia to exclude blood cell contaminants in the organs when indicated13,39. Cells isolated from thymus, MLNs, spleen, blood, ileum and colon lamina propria were blocked with CD16/32 (Fc receptor) to prevent non-specific antibody binding. Then, cells were surface-stained in FACS buffer (PBS, 2 mM EDTA) for 30 min in the dark at 4 °C with fluorescently conjugated antibodies from BD, Thermo Fisher Scientific or BioLegend specific to CD45 (30-F11), CCR5 (HM-CCR5), XCR1 (ZET), SIRPα (P84), CD69 (H1.2F3), TCRβ (H57-597), CD44 (IM7), CD24 (30-F1), CD11b (M1/70), CD11c (N418), MHCII (M5/114.15.2), Ly6C (HK1.4), CD103 (2E7), CX3CR1 (SA011F11), Ly6G (1A8), CD19 (1D3), B220 (RA3-6B2), IgM (RMM-1), PDCA-1 (927), CD73 (TY/11.8), CD25 (PC61.5), CD3 (145-2C11), CD4 (GK1.5) and CD8 (53-6.7). For identification of T cell subsets, after staining for surface antigens, cells were fixed and permeabilized using a Fixation/Permeabilization Solution Kit (Thermo Fisher Scientific) overnight at 4 °C and stained with antibodies specific to mouse FOXP3 (FJK-16s), T-bet (4B10) or RORγt (B2D) for 45 min at room temperature. For RNA-seq of SFB-specific T cells, thymic SFB+ CD4+ T cells were sorted. Cells were identified as MHCII−DAPI−CD62L− (MEL-14) Qa-2− (695H1-9-9) CD25−CD73−CD69+CD24+CD3+CD4+CD8−Tetramer+. For SFB non-specific T cells, the same markers (except for tetramer) were used to isolate CD4+ T cells from uncolonized mice. For T cell transfer experiments, CD8 cells were depleted from the thymus by MACS using biotin anti-mouse CD8α (53-6.7). Next, thymic SFB+ CD4+ T cells were sorted. Cells were identified as CD45RB+ (C363.16A)CD25−CD73−CD4+CD8−. Cell sorting was performed on an Aria Cell Sorter (BD Biosciences). Flow cytometry analysis was performed on an LSR II (BD Biosciences) or Aurora (Cytek) and analysed using FlowJo software v.10.7.1 (Tree Star). DAPI or UV live/dead fixable dead cell stain (Thermo Fisher Scientific) was used to exclude dead cells. Total cell counts were determined with Precision Count Beads (BioLegend) or in the Aurora’s SpectroFlo Software.
16S rDNA high-throughput sequencing
Thymi, MLNs and caeca were collected in a sterile manner at weaning and stored at −80 °C. Samples were processed and analysed by the Center for Metagenomics and Microbiome Research at Baylor College of Medicine. 16S rDNA gene sequencing and analysis was performed as previously described39. In brief, the V4 region of the 16S rDNA was amplified by PCR and sequenced in the MiSeq platform (Illumina) using the 2 × 250-bp paired-end reads protocol. Reads were merged using USEARCH v.7.0.1090, allowing zero mismatches and a minimum overlap of 50 bases. Merged reads with >0.05 expected errors were discarded. The 16S rDNA gene sequences were clustered into operational taxonomic units (OTUs) at a cut-off value of 97%. OTUs were mapped to an optimized version of the SILVA database. A rarefied OTU table from the output files generated in the previous two steps was used for further analysis. For thymus and MLNs, individual samples were pooled.
RNA-seq and analysis
Samples for RNA-seq were sorted (as described above) from 10 pooled SFB-colonized mice (SFB tetramer+) or individual uncolonized mice directly into Trizol LS (Thermo Fisher Scientific). The volume was adjusted to 1 ml with PBS and samples were frozen and stored at −80 °C. RNA was extracted using the RNeasy Mini Kit (Qiagen) as per the manufacturer’s instructions. After ribogreen quantification and quality control by Agilent BioAnalyzer, total RNA was amplified using the SMART-seq v.4 (Clonetech) ultralow-input RNA kit for sequencing (12 cycles of amplification for 2–10 ng of total RNA). Subsequently, 10 ng of amplified cDNA was used to prepare Illumina Hiseq libraries with the Kapa DNA library preparation chemistry (Kapa Biosystems) using 8 cycles of PCR. Samples were barcoded and run on a Hiseq 4000, in a 50-bp/50-bp paired-end run, using the TruSeq SBS Kit v.3 (Illumina). The output data (FASTQ files) were aligned and mapped to the mouse reference genome (GRCm38) using the rnaStar aligner40,41 (star/STAR-STAR_2.5.0a). The expression count matrix from the mapped reads was computed using HTSeq42 (https://htseq.readthedocs.io/en/master/) and processed using DESeq (http://www-huber.embl.de/users/anders/DESeq) to normalize the full dataset and analyse differential expression between sample groups. Principal component analysis was obtained by clustering the data using the normalized counts of all genes for our samples and the published database15 (GSE148973). The top 200 enriched genes obtained from differential gene expression analysis between SFB-non-specific and SFB-specific CD4+ cells were analysed in the database for annotation, visualization and integrated discovery (DAVID; v.6.8) using KEGG PATHWAY as a reference database43.
Administration of diphtheria toxin, 4-hydroxytamoxifen, TAK-779 and neutralizing anti-CCL2 antibody
Mice were injected every other day with 200 ng diphtheria toxin (Sigma) in PBS. (Z)-4-hydroxytamoxifen, 98% Z isomer (4-OHT, Sigma) was resuspended to 20 mg/ml in ethanol by heating to 37 °C. 4-OHT was then diluted in corn oil (Sigma) to 2 mg/ml and mice were injected every 3 days with 100 μl. Mice were injected twice a week with 2 mg/kg anti-CCL2 antibody (2H5, BioLegend) in PBS. Mice were injected every other day with 150 μg TAK-779 (Sigma) in 5% mannitol (Sigma). All injections were intraperitoneal.
Intestinal cell trafficking by photoconversion of KikGR33 mice
Photoconversion of intestinal tissue of SPF KikGR33 mice was performed as previously described21 using a violet laser source (405 nm, peak power 5 mW) for 5 min. In brief, KikGR33 transgenic mice were anaesthetized with 2.5% isoflurane (vol/vol) delivered in 2 l/min of O2 and maintained at 37 °C throughout the procedure. A 3-cm incision was made in the abdominal wall and the caecum was exposed. Intestinal tissue was kept hydrated by applying sterile saline throughout the procedure. The caecum was returned back to the abdomen, and the incision sutured. Forty-eight hours after the surgery, cells in the thymus, MLNs, spleen and caecum were analysed by flow cytometry for the presence of photoconverted RFP+ cells.
Adoptive transfer colitis
The induction of colitis by the transfer of non-Treg T cells into an immunodeficient recipient was performed as previously described19,23 with minor modifications as outlined in Supplementary Table 4. In brief, thymi of 5-week-old SPF mice were collected and depleted of CD8+ cells using Magnisort SAV negative-selection beads (Thermo Fisher Scientific) and biotin-conjugated anti-CD8α antibodies (53-6.7, BioLegend). Next, CD4+CD8−CD45RBhiCD25−CD73− T cells were isolated by FACS. A total of 4 × 105 T cells were transferred into 8–10-week-old Rag2−/− mice by intraperitoneal injection. Mice were weighed weekly and analysed at four or eight weeks after transfer as indicated for each condition.
Ex vivo measurement of T cell responses against Salmonella
Five-week-old SPF wild-type mice were orally infected with 3 × 108 CFU of attenuated S. Typhimurium ΔaroA. Twelve days later, cells from the MLNs were isolated. A total of 2.5 × 105 cells per well in a 96-well plate were stimulated with 8 ng/ml of heat-killed Salmonella or E. coli in complete RPMI. Twenty-four hours later, supernatants were collected and IFNγ levels were measured by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (BD OptEIA set mouse IFNγ (AN-18)).
Salmonella Typhimurium survival assay
SPF wild-type female mice were colonized with 1 × 108 CFU of E. coli at weaning by oral gavage. Two weeks later, mice were infected with 1 × 105 CFU of wild type Salmonella (SL1344). Mice were monitored and weighed daily. Mice with a loss of more than 20% of their initial body weight, hypothermia, listlessness, inability to get food or water, hunched posture, ruffled or matted fur, and ocular discharge were considered moribund and were euthanized.
Histology
Distal colons and ilea were collected from Rag2−/− mice four or eight weeks after thymic T cell transfer. Tissues were fixed in 10% neutral buffered formalin, routinely processed, sectioned at 6 mm and stained with H&E for light microscopic examination. Images were taken at 20× magnification using a Nikon Ti Eclipse microscope. Samples were assessed in a blinded fashion and scored 0 to 4 based on the criteria previously described44: grade 0, no change from normal tissue; grade 1, one or a few multifocal mononuclear cell infiltrates in lamina propria with minimal epithelial hyperplasia and slight to no depletion of mucus from goblet cells; grade 2, more tissue involved or more frequent lesions than grade 1 including increased neutrophils along with mild epithelial hyperplasia and mucin depletion and occasional epithelial erosions with rare submucosal involvement; grade 3, large area of mucosa involvement or more frequent lesions than grade 2 with moderate inflammation and submucosal involvement along with rare transmural and/or crypt abscesses and moderate epithelial hyperplasia, mucin depletion and ulcers; grade 4, involvement of most of the intestinal section and more severe than grade 3 including transmural lesions, marked epithelial hyperplasia and marked mucin depletion along with abscesses and ulcers.
Statistics and reproducibility
Statistical analysis was performed using GraphPad Prism v.9.0.2. All data are presented as individual values and mean or mean ± s.e.m in weight loss panels. A two-tailed unpaired Student’s t-test using a 95% confidence interval was used to evaluate the difference between two groups. For more than two groups, a one-way ANOVA was used. For more than two groups under different conditions, a two-way ANOVA was used. A probability value of P < 0.05 was considered significant. Statistical significance is indicated in each figure. Sequencing experiments were performed once. All remaining experiments were repeated independently at least twice with similar results. Representative flow plots and micrographs were selected from the biological replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Extended Data
Extended Data Fig. 1 ∣. Characterization of SFB-tetramer+ CD4+ T cells.
a, Gating strategy used to identify SFB-tetramer+ CD4+ T cells after magnetic enrichment. Live, CD45+ single cells negative for CX3CR1, Ly6G, B220, Ly6C, MHCII and CD8 were further analysed. From the CD3+CD4+ population, SFB-specific cells were identified as double positive for APC- and PE- (Fig. 1) or single positive for APC- or PE-conjugated tetramers specific for SFB peptide QFSGAVPNKTD (all other figures). b, Mice were colonized with SFB at weaning and two weeks later thymic T cells were compared to age-matched control (CTRL) mice. Frequencies and counts of SFB-tetramer+ cells in thymic flow-through after magnetic enrichment (n = 3 per group). c, 7B8 TCR transgenic mice were left uncolonized or SFB-colonized at weaning and two weeks later CD4+ counts in the thymus were compared (control n = 6; SFB n = 7). d–f, Mice were colonized with SFB at weaning and two weeks later thymic T cells were compared to age-matched control mice. d, Frequencies and counts of SFB-tetramer+ cells in CD8+ (single-positive; SP) and CD4+CD8+ (double-positive; DP) T cells (n = 10 per group). e, Frequencies and counts of no tetramer (NT), 2W1S, H. hepaticus (HH) and human (HU) tetramer+ cells in CD4+ T cells (n = 5 per group). f, Frequencies and counts of CD4+, CD8+ and CD4+CD8+ thymic subsets. g–k, Representative flow plots of control and SFB CD4+ T cells for RORγt and FOXP3 (g), CD25 (h), CD44 (i); CD24 and TCRβ (j) and CD69 and TCRβ (k). In f–k, n = 15 per group. Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-tailed unpaired t-test (b, c) or two-way ANOVA with Sidak’s post hoc test (d–f).
Extended Data Fig. 2 ∣. Thymic expansion of bacteria-specific T cells after colonization with commensal microorganisms at weaning.
a–c, Thymic SFB-tetramer+ cells from young mice were analysed by flow cytometry (n = 15). a, CD24 and TCRβ. b, CD44. c, CD69 and TCRβ. d, Mice were colonized with SFB at weaning for two weeks and were injected intravenously with anti-CD45 before euthanasia. Flow cytometry of CD45 in thymic SFB-tetramer+ cells and spleen (n = 5). e, RAG2-GFP mice were colonized with SFB at weaning and two weeks later thymi were collected. Flow cytometry of thymic CD4+ cells (n = 7). f, g, Thymic SFB-non-specific and SFB-specific CD4+ T cells from young mice two weeks after colonization were analysed by RNA-seq. f, Principal component analysis of SFB-non-specific and SFB-specific CD4+ T cells (circled) compared to thymocytes at different developmental stages (GSE148973). g, Heat map showing row-standardized z-scores of mRNA expression for genes in enriched KEGG pathways (P < 0.05). In f, g, n = 5 (SFB-non-specific T cells); n = 3 (SFB-specific T cells). Each SFB-specific T cell replicate is a pool of 10 thymi. h–j, Mice were colonized with SFB at weaning and were compared four weeks later to age-matched control mice. h, Flow cytometry and counts of SFB-tetramer+ cells in the MLNs (n = 5 (CTRL; n = 15 (SFB)), spleen (SPL; n = 5 per group), ileum (n = 5 (CTRL); n = 10 (SFB)) and colon (n = 5 per group). i, j, Flow cytometry (i) and counts (j) of thymic SFB-tetramer+ cells (n = 5 (CTRL); n = 15 (SFB)). k, RAG2-GFP mice were colonized with SFB at weaning and four weeks later thymi were collected. Flow cytometry of thymic CD4+ cells (n = 7). l, m, SPF and germ-free mice were treated with antibiotics (ABX) at weaning and compared two weeks later to age-matched control mice. Counts of total thymocytes, CD4+, CD8+ and thymic Treg cells in SPF mice (l; n = 15 (CTRL); n = 9 (ABX)) and germ-free mice (m; n = 3 (CTRL); n = 4 (ABX)). Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-tailed unpaired t-test (j, l, m) or two-way ANOVA with Sidak’s post hoc test (h).
Extended Data Fig. 3 ∣. Bacterial DNA is found in the thymus after early-life colonization.
a, b, h–j, Mice were colonized with SFB or E. coli at weaning (young) or at 12 weeks of age (adult) and one week later were compared to age-matched control mice. a, b, SFB-specific qPCR in faecal pellets (FP) (a; young: CTRL, SFB n = 8; adult: CTRL, SFB n = 6); and MLNs (b; young: CTRL, SFB n = 9; adult: CTRL, SFB n = 7). h–j, E. coli-specific qPCR in the thymus (h; young: CTRL n = 10, EC n = 15; adult: CTRL, EC n = 15), MLNs (i; young: CTRL, EC n = 8; adult: CTRL, EC n = 7) and faecal pellets (j; young: CTRL, EC n = 8; adult: CTRL, EC n = 10). Mice were colonized with SFB at weaning and compared one week later to age-matched control mice. c, e, SFB-specific qPCR (c; n = 6 per group) and 16S qPCR (e) in the thymus, MLNs, liver, spleen, heart and lungs (in e, thymus, MLNs: CTRL, SFB n = 12; liver, SPL, heart, lungs: CTRL, SFB n = 6). d, g, Mice were colonized with SFB at weaning and analysed up to three weeks later. SFB-specific PCR (d) and 16S qPCR (g) in the thymus, MLNs and faecal pellets (n = 5 per group). f, Mice were colonized with SFB at 12 weeks of age and compared one week later to age-matched control mice. 16S qPCR in thymus and MLNs (thymus: CTRL n = 16, SFB n = 11; MLNs CTRL, SFB n = 7). k, Mice were euthanized at 4 weeks of age, tissues were collected, DNA was isolated and 16S rDNA sequencing was performed. Bacterial phyla and class relative abundance in the thymus, MLNs and caecum (n = 5 per group; thymus and MLNs were pooled). l–n, Mice were treated with antibiotics at weaning and compared one week later to age-matched control mice. 16S qPCR in the thymus (l; n = 7 (CTRL), n = 8 (ABX)); MLNs (m; n = 16 (CTRL), n = 9 (ABX)) and faecal pellets (n; n = 10 per group). Each replicate is a biologically independent sample. Data are shown as mean ± s.e.m. (d, g) or as individual values and mean; P values by two-tailed unpaired t-test (l–n) or two-way ANOVA with Tukey’s (a, b, f, h–j), Sidak’s (c, e) or Fisher’s LSD (e) post hoc test.
Extended Data Fig. 4 ∣. CX3CR1+ DCs are enriched in the thymus after colonization with commensal microorganisms in young mice.
a–c, g, Thymic cell populations were analysed at weaning (W3), one (W4) and two (W5) weeks after weaning, and at 12 weeks of age (W12). Frequencies and counts of CD4+, CD8+ and CD4+CD8+ subsets (a); Treg cells (b) (in a, b, W3 n = 5; W4 n = 11; W5 n = 15; W12 n = 8); CD11c+MHCII+ DCs (c); and CD103+ and CX3CR1+ DC subsets (g) (in c, g, n = 10 (W3); n = 15 (W4, W5, W12)). d, m, p, q, Mice were treated with antibiotics at weaning and thymi were compared two weeks later to age-matched control mice. d, m, Frequencies and counts of CD11c+MHCII+ DCs (d) and CD103+ and CX3CR1+ DC subsets (m) (n = 23 per group). p, q, Frequencies of plasmacytoid DCs (pDCs) (p) and B cells (q) (n = 15 per group). e, f, i–l, n, o, Mice were colonized with SFB or E. coli at weaning (young) or at 12 weeks of age (adult) and thymi were compared two weeks later to age-matched control mice. e, f, Flow cytometry (e) and counts (f) of CD11c+MHCII+ DCs. i, Representative flow plot of CX3CR1+ DCs. j, Frequencies and counts of CD103+ DCs in the thymus (in e, f, i, j, young: CTRL n = 15; SFB n = 20; EC n = 12. Adult: CTRL n = 20; SFB n = 20; EC n = 16). k, l, Frequencies of CD11c+MHCII+ DCs (k) and CX3CR1+ DC subsets (l) in the MLNs (young: CTRL n = 12; SFB n = 15; EC n = 10. Adult: CTRL n = 8; SFB n = 12; EC n = 8). n, o, Frequencies of pDCs (n) and B cells (o) in the thymus (n = 12 per group). h, Mice were colonized with SFB at weaning for two weeks and were injected intravenously with anti-CD45 before euthanasia. Flow cytometry of CD45 in thymic CX3CR1+ DCs and spleen (n = 5). Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-tailed unpaired t-test (d, p, q), one-way ANOVA with Tukey’s post hoc test (b, c) or two-way ANOVA with Tukey’s (a, f, g, j–l, n, o) or Sidak’s (m) post hoc test.
Extended Data Fig. 5 ∣. MHCII expression on CX3CR1+ cells is required for the thymic expansion of bacteria-specific T cells.
a–g, Wild-type and CX3-DTR mice were colonized with SFB or E. coli at weaning or left untreated and tissues were compared one week (qPCR) or two weeks (flow cytometry analysis) later. a, Flow cytometry of thymic SFB-tetramer+ cells (CTRL: n = 5; SFB: WT n = 6; CX3-DTR n = 8). b, c, 16S qPCR in the thymus (b; n = 8 (WT); n = 9 (CX3-DTR)); and MLNs (c; n = 13 (WT); n = 12 (CX3-DTR)). d, e, SFB-specific qPCR in the thymus (d; n = 5 per group) and faecal pellets (e; n = 10 (WT); n = 5 (CX3-DTR)). f, g, E. coli-specific qPCR in the thymus (f; WT n = 7; CX3-DTR n = 12) and faecal pellets (g; n = 10). h, Diphtheria-toxin-treated littermate wild-type mice and mice depleted of CD103+ DCs (CD103-DTR CD11c-Cre) were analysed two weeks after weaning. Flow cytometry of CD103+ and CX3CR1+ DC subsets in colon lamina propria (n = 5). i, j, Diphtheria-toxin-treated littermate wild-type, CX3-DTR CD11c-Cre and CD103-DTR CD11c-Cre mice were colonized with E. coli at weaning and thymi were compared one week later. i, E. coli-specific qPCR (n = 8 (WT); n = 10 (CX3-DTR); n = 8 (CD103-DTR)). j, 16S qPCR (n = 24 (WT); n = 10 (CX3-DTR); n = 8 (CD103-DTR)). k–m, 4-OHT-treated MHCIIfl/+ (WT) and MHCIIfl/− (KO) CX3-Cre mice were colonized with SFB at weaning and thymi were compared two weeks later. k, Frequencies and counts of SFB-tetramer+ cells (n = 10 per group). l, SFB-specific qPCR (n = 5 per group). m, Frequencies of CD103+ and CX3CR1+ DC subsets (n = 10 per group). Each replicate is a biologically independent sample. Data are shown as individual values and mean P values by two-tailed unpaired t-test (b–g, k, l) or one-way ANOVA with Tukey’s (i, j) or Sidak’s (m) post hoc test.
Extended Data Fig. 6 ∣. Intestinal CX3CR1+ DCs migrate to the thymus.
a, Thymic DCs were analysed at two weeks after weaning for XCR1 and SIRPα. Flow cytometry of total DCs, CX3CR1+ DCs, CD103+ DCs and pDCs (n = 5). b–f, Caeca from KikGR33 transgenic mice were exposed to 405-nm-wavelength light or left untreated at 5 (young) or 14 (adult) weeks of age and two days later the thymus, MLNs and spleen were collected. b, Flow cytometry of RFP+MHCII+ cells in KikGR33 mice without light exposure (n = 5). c, Frequencies and counts of RFP+MHCII+ cells in young mice. d, e, Flow cytometry (d) and counts (e) of RFP+MHCII+ cells in adult mice. f, Flow cytometry of RFP+MHCII+ cells subsets in young and adult thymi. In c–f, n = 5 (young); n = 9 (adult). g–i, Mice were analysed at weaning (young) or at 12 weeks of age (adult) and thymus chemokine gene expression or colon lamina propria DC populations were analysed. g, Relative expression of chemokines in the thymus (n = 5 (young); n = 15 (adult)). h, CCR5+ cell frequencies of CX3CR1+ DCs in the colon (n = 10 per group). Wild-type and CX3CR1GFP/GFP (KO) mice were colonized with SFB at weaning and treated with TAK-779 or anti-CCL2 antibody and thymi were compared one week later. i, SFB-specific qPCR (n = 17 (WT); n = 13 (anti-CCR5 + anti-CCR2); n = 5 (WT anti-CCR2); n = 10 (KO); n = 5 (KO anti-CCR5 + anti-CCR2)). Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-tailed unpaired t-test (g, h) or one-way ANOVA with Fisher’s LSD post hoc test (i).
Extended Data Fig. 7 ∣. Bacteria-specific T cells from the thymus of young mice are functional in distal tissues.
Wild-type donor mice were colonized with SFB or E. coli at weaning and two weeks later CD4+ T cells from the thymus were sorted and transferred to SFB- or E. coli-colonized Rag2−/− recipient mice. Recipient mice were followed for four weeks and disease severity was compared to Rag2−/− mice receiving age-matched control donor thymocytes. a–f, SFB (n = 8 (CTRL); n = 13 (SFB)). a, Colon length. b, Immune infiltration in colon lamina propria. c, d, Flow cytometry (c) and counts (d) of TH17 cells. e, Representative flow plot of SFB-tetramer+ cells. f, Flow cytometry of RORγt and T-bet expression in SFB-tetramer+ cells. g–i, E. coli (n = 8 (CTRL); n = 15 (EC)). g, Colon length. h, Immune infiltration in colon lamina propria. i, Representative flow plot of TH1 cells. Each replicate is a biologically independent sample. Data are shown as individual values and mean; P values by two-tailed unpaired t-test (a, b, d, g, h).
Extended Data Fig. 8 ∣. Bacteria-specific T cells from the thymus of adult mice do not increase pathology in distal tissues.
Wild-type donor mice were colonized with SFB or E. coli at 12 weeks of age and two weeks later CD4+ T cells from the thymus were sorted and transferred to SFB- or E. coli-colonized Rag2−/− recipient mice. Recipient mice were followed for four weeks and disease severity was compared to Rag2−/− mice receiving age-matched control donor thymocytes. a–h, SFB. a, Relative weight change after transfer (n = 5 (CTRL); n = 7 (SFB)). b, Colon length. c, d, Representative H&E (c) and colitis score (d). e, Immune infiltration in colon lamina propria. f, g, Frequencies and counts of TH17 cells (f) and SFB-tetramer+ cells (g). h, Flow cytometry of RORγt and T-bet expression in SFB-tetramer+ cells. In b–h, n = 4 (CTRL); n = 7 (SFB). i–n, E. coli (n = 7 (CTRL); n = 9 (EC)). i, Relative weight change after transfer. j, Colon length. k, l, Representative H&E (k) and colitis score (l). m, Immune infiltration in colon lamina propria. n, Frequencies and counts of TH1 cells. Each replicate is a biologically independent sample. Data are shown as mean ± s.e.m., with P values by two-way ANOVA with Fisher’s LSD post hoc test (a, i), or are shown as individual values and mean, with P values by two-tailed unpaired t-test (b, d–g, j, l–n). Scale bars, 100 μm (c, k).
Extended Data Fig. 9 ∣. Requirement for antigen presentation by CX3CR1+ cells for thymic T cell pathogenicity in distal tissues.
a–d, Diphtheria-toxin-treated littermate SPF wild-type and CX3-DTR donor mice were colonized with SFB at weaning and two weeks later CD4+ T cells from the thymus were sorted and transferred to SFB-colonized Rag2−/− recipient mice. Recipient mice were followed for four weeks and disease severity was assessed. a, Colon length (n = 12 (WT); n = 13 (CX3-DTR). b, Immune infiltration in colon lamina propria. c, d, Flow cytometry (c) and counts (d) of SFB-tetramer+ cells. In b–d, n = 8 per group. e–k, 4-OHT-treated MHCIIfl/+ (WT) and MHCIIfl/− (KO) CX3-Cre mice were colonized with SFB at weaning and two weeks later CD4+ T cells from the thymus were sorted and transferred to SFB-colonized Rag2−/− recipient mice. Recipient mice were followed for four weeks and disease severity was assessed. e, Relative weight change after transfer. f, Colon length. g, h. Representative H&E (g) and colitis score (h). i, Immune infiltration in colon lamina propria. j, k, Flow cytometry (j) and counts (k) of SFB-tetramer+ cells. In e–k, n = 8 (WT); n = 10 (KO). l–q, SPF wild-type donor mice were treated with antibiotics at weaning and two weeks later CD4+ T cells from the thymus were sorted and transferred to untreated Rag2−/− recipient mice. Recipient mice were followed for eight weeks and disease severity was compared to Rag2−/− mice receiving age-matched control donor thymocytes. l, Relative weight change after transfer. m, Colon length. n, o, Representative H&E (n) and colitis score (o). p, Immune infiltration in colon lamina propria. q, Frequencies and counts of TH1 cells. In l–q, n = 7 (CTRL); n = 9 (ABX). r, Wild-type mice were infected at 5 weeks of age with S. Typhimurium. MLNs were collected and cells restimulated with S. Typhimurium or E. coli. IFN-γ levels in culture supernatants (n = 7 (ST); n = 5 (EC)). Each replicate is a biologically independent sample. Data are shown as mean ± s.e.m., with P values by two-way ANOVA with Fisher’s LSD post hoc test (e, l), or are shown as individual values and mean, with P values by two-tailed unpaired t-test (a, b, d, f, h, i, k, m, o–r). Scale bars, 100 μm (g, n).
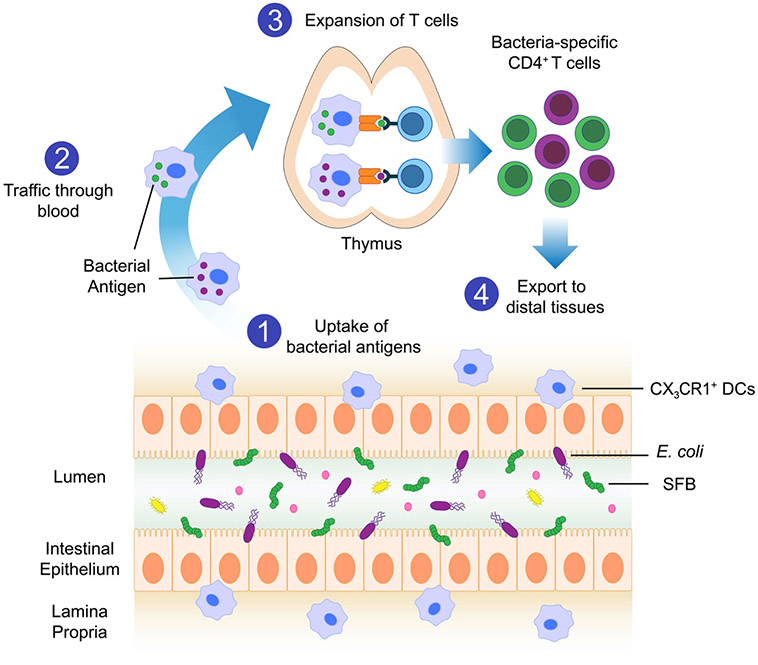
Extended Data Fig. 10 ∣. Proposed model for early-life thymic expansion of microbiota-specific T cells.
At weaning we propose that CX3CR1+ DCs take up intestinal commensal microorganisms (1) and migrate to the thymus (2), where they present bacterial antigens to CD4+ T cells. This induces the expansion of bacteria-specific CD4+T cells (3) that are later exported to peripheral organs (4).
Supplementary Material
Acknowledgements
This work was supported by NIH grants R01AI136963 (G.E.D. and M.L.B.), R01AI125264 (G.E.D.), R01DK114456 (M.L.B.), R01AI130152 (T.E.) and R01 DK114252 (R.S.L.); the Kleberg Foundation (G.E.D. and M.L.B); the Kenneth Rainin Foundation (G.E.D.); the Leukemia and Lymphoma Society Scholar Award (T.E.); and the Cytometry and Cell Sorting Core at Baylor College of Medicine, with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672) and the NIH (P30 CA125123 and S10 RR024574) and with the assistance of J. M. Sederstrom. We thank the Baylor College of Medicine Genetically Engineered Mouse Core supported by the Cancer Center Grant (P30 CA125123); the Mouse Embryonic Stem Cell Core at Baylor College of Medicine; D. Littman and C.-S. Hsieh for critical reading of the manuscript; N. Ajami for advice on 16S; and the NIH Tetramer Facility, which is supported by contract HHSN272201300006C from the NIAID.
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41586-021-03531-1.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing interests The authors declare no competing interests.
Data availability
All data generated and supporting the findings of this study are available within the paper. The RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE171279. The 16S sequencing data have been submitted to the Sequence Read Archive (SRA) under the BioProject ID 718898. Additional information and materials will be made available upon request. Source data are provided with this paper.
References
- 1.Hooper LV, Littman DR & Macpherson AJ Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zegarra-Ruiz DF et al. A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 25, 113–127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu M et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510, 152–156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lathrop SK et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bäckhed F et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Boehm T & Swann JB Thymus involution and regeneration: two sides of the same coin? Nat. Rev. Immunol 13, 831–838 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Cebula A et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497, 258–262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotov DI & Jenkins MK Peptide:MHCII tetramer-based cell enrichment for the study of epitope-specific CD4+ T cells. Curr. Protoc. Immunol 125, e75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol 6, 345–352 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Egawa T & Littman DR ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat. Immunol 9, 1131–1139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogquist KA, Xing Y, Hsu F-C & Shapiro VS T cell adolescence: maturation events beyond positive selection. J. Immunol 195, 1351–1357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KG et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protocols 9, 209–222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen DL et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol 20, 195–205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopp LB et al. An integrated epigenomic and transcriptomic map of mouse and human αβ T cell development. Immunity 53, 1182–1201 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl GE et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494, 116–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varol C, Zigmond E & Jung S Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol 10, 415–426 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Longman RS et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med 211, 1571–1583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M et al. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity 49, 151–163 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadeiba H & Butcher EC Thymus-homing dendritic cells in central tolerance. Eur. J. Immunol 43, 1425–1429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowotschin S & Hadjantonakis A-K Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev. Biol 9, 49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohy D et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J. Biol. Chem 284, 31270–31279 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH & Powrie F Regulatory T cells and intestinal homeostasis. Immunol. Rev 204, 184–194 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Di Padova FE et al. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect. Immun 61, 3863–3872 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ & Laufer TM Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med 194, 427–438 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein L, Kyewski B, Allen PM & Hogquist KA Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol 14, 377–391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leech JM et al. Toxin-triggered interleukin-1 receptor signaling enables early-life discrimination of pathogenic versus commensal skin bacteria. Cell Host Microbe 26, 795–809 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharschmidt TC et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43, 1011–1021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto K, Joshi SK & Koni PA A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis 32, 152–153 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Jung S et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol 20, 4106–4114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhurst CN et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano T et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163, 381–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchan A, Hadden M & Suzuki MT Development and application of quantitative-PCR tools for subgroups of the Roseobacter clade. Appl. Environ. Microbiol 75, 7542–7547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chern EC, Siefring S, Paar J, Doolittle M & Haugland RA Comparison of quantitative PCR assays for Escherichia coli targeting ribosomal RNA and single copy genes. Lett. Appl. Microbiol 52, 298–306 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Valdez Y et al. Nramp1 expression by dendritic cells modulates inflammatory responses during Salmonella Typhimurium infection. Cell. Microbiol 10, 1646–1661 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon JJ et al. Tracking epitope-specific T cells. Nat. Protocols 4, 565–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon JJ et al. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu HH, Moon JJ, Kruse AC, Pepper M & Jenkins MK Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J. Immunol 185, 4705–4713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viladomiu M et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci. Transl. Med 9, eaaf9655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engström PG et al. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat. Methods 10, 1185–1191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anders S, Pyl PT & Huber W HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 35, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W, Sherman BT & Lempicki RA Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Berg DJ et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Invest 98, 1010–1020 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and supporting the findings of this study are available within the paper. The RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE171279. The 16S sequencing data have been submitted to the Sequence Read Archive (SRA) under the BioProject ID 718898. Additional information and materials will be made available upon request. Source data are provided with this paper.