Abstract
Background
We aimed to study the association of suspected versus confirmed infection with the novel SARS-CoV2 virus with the prevalence of acute kidney injury (AKI) in critically ill children.
Methods
Sequential point-prevalence study of children and young adults aged 7 days to 25 years admitted to intensive care units under investigation for SARS-CoV2 infection. AKI was staged in the first 14 days of enrollment using KDIGO creatinine-based staging. SARS-CoV2 positive (CONFIRMED) were compared to SUSPECTED (negative or unknown). Outcome data was censored at 28-days.
Results
In 331 patients of both sexes, 179 (54.1%) were CONFIRMED, 4.2% (14) died. AKI occurred in 124 (37.5%) and severe AKI occurred in 63 (19.0%). Incidence of AKI in CONFIRMED was 74/179 (41.3%) versus 50/152 (32.9%) for SUSPECTED; severe AKI occurred in 35 (19.6%) of CONFIRMED and 28 (18.4%) of SUSPECTED. Mortality was 6.2% (n = 11) in CONFIRMED, but 9.5% (n = 7) in those CONFIRMED with AKI. On multivariable analysis, only Hispanic ethnicity (relative risk 0.5, 95% CI 0.3–0.9) was associated with less AKI development among those CONFIRMED.
Conclusions
AKI and severe AKI occur commonly in critically ill children with SARS-CoV2 infection, more than double the historical standard. Further investigation is needed during this continuing pandemic to describe and refine the understanding of pediatric AKI epidemiology and outcomes.
Trial registration
Impact
What is the key message of the article?
AKI occurs in children exposed to the novel SARS-CoV2 virus at high prevalence (~40% with some form of AKI and 20% with severe AKI).
What does it add to the existing literature?
Acute kidney injury (AKI) occurs commonly in adult patients with SARS-CoV2 (COVID), very little data describes the epidemiology of AKI in children exposed to the virus.
What is the impact?
A pediatric vaccine is not available; thus, the pandemic is not over for children. Pediatricians will need to manage significant end-organ ramifications of the novel SARS-CoV2 virus including AKI.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) is associated with multiple end-organ effects in critically ill patients. Available, though still sporadic, data indicate children suffer infection at high rates and with increased morbidity, just as in adults1–6. Recent data indicate organ dysfunction in the setting of SARS-CoV2 can occur in all populations, including children7–10, however, the nature of this relationship (SARS-CoV2 and organ injury) continues to be delineated. A large proportion (~50%) of SARS-CoV2 infected adults requiring admission to intensive care units (ICU) develop acute kidney injury (AKI), with nearly 20% receiving renal replacement therapy (RRT). Those with AKI have increased morbidity versus similar patients without AKI11–17 and mortality is 2- to 9-fold higher. Delineation and detail of organ dysfunction in critically ill pediatric patients with SARS-CoV2 remain limited by the population size studied. Thus far, only preliminary data have been published describing AKI in the context of SARS-CoV23,18–22. Data from China indicate a total of 13 of 2133 (0.6%) children in the Hubei province required ICU admission, though no data related to AKI is mentioned23. A convenience sample of nearly 50 children admitted to ICUs in the United States indicates 86% of children experienced at least one organ failure but AKI was not explicitly mentioned5.
We conducted a multinational, prospective, point-prevalence study in children and young adults admitted to pediatric ICU with suspected or confirmed SARS-CoV2 infection to study its implications with AKI. The aim of the study was to determine the difference in AKI prevalence in critically ill children between those with confirmed versus suspected SARS-CoV2 infection. The hypothesis, as in adults, was that in confirmed SARS-CoV2 infection would be associated with more and worse severity AKI and, consequently, more downstream morbidity and mortality compared to patients with suspected (i.e., unconfirmed) infection. In our initial, preliminary analysis we identified potential associations with chronic medical conditions and race with AKI in critically ill children suspected of infection with SARS-CoV222—thus a secondary aim of this full study was to expand and strengthen or refute these findings.
Materials and methods
Study design
Multinational, prospective, point-prevalence study conducted from April 15, 2020 through June 24, 2020 (NCT:04466306, SARS-CoV2 Pediatric AKI Registry, and Collaborative—SPARC). Patient data were collected weekly from 57 participating centers and entered directly into a cloud-based data entry platform. Study data were collected and managed using REDCap (Research Electronic Data Capture)24,25. The study was approved at each center by local Investigational Review Boards (with a requirement for patient consent waived) and data use agreements for data transfer were in place.
Study population
Overall, 57 participating centers throughout North America, South America, Europe, the Middle East, and Asia participated in SPARC-1 (Supplemental Table 1). All critically ill children >1 week and ≤25 years of age admitted to the pediatric ICU receiving care in non-neonatal pediatric intensive care units (ICUs) (medical-surgical pediatric and cardiac) on the days of data capture were screened. Inclusion criteria were the following for patients in the ICU on the day of intake: (1) tested for SARS-CoV2 with symptomatology specific to respiratory disease or infectious appearance, (2) “enhanced droplet” precautions in place due to symptomatology (per institutional policy), (3) infectious disease service involvement for SARS-CoV2 evaluation (per institutional policy), or (4) unexplained symptomatology concerning for SARS-CoV2 without having been tested yet (fever, shortness of breath, chest tightness, altered neurologic status). These determinations were made by the care provider in the local ICU and not subject to adjudication by the SPARC investigators (local or lead). There were no other exclusion criteria.
At 28-days after enrollment, diagnosis of SARS-CoV2 infection was confirmed on chart review for confirmation of polymerase chain reaction (PCR+) or antibody testing (Ab+) results based on local protocols. The tests used for detection changed through the course of the study (12 weeks) at each center as the pandemic evolved. A majority of the centers used multiple tests for the purpose of both PCR and Ab testing—these tests had evolving and improved sensitivity and specificity for detection of acute (PCR) or historical (Ab+) infection. Patients positive for either test were listed as CONFIRMED, while negative or unknown were listed as SUSPECTED (given the high proportion of false-negative rates related to the quality of testing available during the time of this study)26.
Outcomes
The primary outcome of interest was AKI prevalence defined by the weekly rate of AKI in SUSPECTED or CONFIRMED children for that given week and then overall for the entirety of the study. Kidney Disease Improving Global Outcomes (KDIGO) criteria based on serum creatinine rise relative to baseline creatinine was used to denote AKI. Weekly serum creatinine values were obtained while patients were in the study. Baseline creatinine was defined as the lowest serum creatinine within the three months prior to hospital admission. If baseline creatinine was not available, the validated height-independent equations for age and sex were used to back-calculate creatinine, assuming normal pre-admission estimated glomerular filtration rate (eGFR) (120 ml/min/1.73 m2 for children ≥2 years of age; median normative-based eGFR-for-age in children <2 years old)27. Secondary outcomes included AKI severity (Stage based by KDIGO scoring), critical care resources used: vasoactive, invasive ventilator support, renal replacement therapy (RRT), extracorporeal membrane oxygenation (ECMO), length of hospitalization (dichotomized at less than or greater than 14 days), and 28-days hospital mortality.
Covariates
Patient demographic data, center-level data, and additional clinical factors obtained on individual patients included comorbidities, baseline serum creatinine and eGFR, admission serum creatinine, and categories of reasons for ICU admission (shock/hemodynamic instability, sepsis/infection, respiratory distress, neurologic symptoms, gastrointestinal symptoms, other).
Statistical analyses
The prevalence of AKI amongst SUSPECTED or CONFIRMED SARS-CoV2 infection in critically ill children was analyzed using descriptive statistics. Comparisons were made with Chi-square, Fisher’s exact test, and unpaired t-tests as appropriate.
Among those CONFIRMED with SARS-CoV2 infection, further exploration was conducted to evaluate for independent risk factors of AKI development. We used chi-square, and Fisher’s Exact test as appropriate, to evaluate for differences between those with and without AKI. Univariate risk differences and risk ratios with corresponding 95% confidence intervals were calculated. We present both risk differences and risk ratios for univariate analysis, but only risk ratios for multivariable analysis. A log-binomial regression model was used for multivariable analysis among those CONFIRMED. For multivariate analyses, we included covariates with a p-value of ≤0.2 in univariate analyses, and a priori forced the following potential confounders into the model (center, age categories, sex, and presence or absence of comorbidities). Continuous variables were also condensed to larger categorical groupings to allow model stability. Alpha-level of <0.05 was considered statistically significant. A sensitivity analysis was conducted for the multivariable model among the entire cohort (SUSPECTED and CONFIRMED). All statistical analyses were performed in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Characteristics of cohort
A total of 331 patients were included from 42 of the participating centers in 15 countries (Table 1). In these patients, 179/331 (54.1%) had CONFIRMED SARS-CoV2 infection. Over 70% (n = 233) had at least one chronic condition (108 (71.1%) SUSPECTED versus 125 (69.8%) CONFIRMED). The most common chronic conditions included central nervous system dysfunction and pulmonary disease. Sex, age, and body mass index were not different between those with CONFIRMED infection versus SUSPECTED infection. However, those of racial/ethnic minorities were more likely to have CONFIRMED infection (36.9% among Hispanics, 27.9% among Blacks) versus SUSPECTED infection (12.5% among Hispanics, 22.5% among Blacks), p-values < 0.0001 and 0.04, respectively.
Table 1.
Epidemiology of SARS-CoV2 infection in critically ill children and young adults.
| Characteristics | Total | Suspected | Confirmed |
|---|---|---|---|
| N | 331 | 152 | 179 |
| Date of enrollment | |||
| Week 1 (April 15) | 47 (14.2) | 13 (8.6) | 34 (19.0) |
| Week 2 (April 22) | 39 (11.8) | 23 (15.1) | 16 (8.9) |
| Week 3 (April 29) | 29 (8.8) | 17 (11.2) | 12 (6.7) |
| Week 4 (May 6) | 29 (8.8) | 13 (8.6) | 16 (8.9) |
| Week 5 (May 13) | 43 (13.0) | 20 (13.2) | 23 (12.9) |
| Week 6 (May 20) | 33 (10.0) | 13 (8.6) | 20 (11.2) |
| Week 7 (May 27) | 21 (6.3) | 11 (7.2) | 10 (5.6) |
| Week 8 (June 3) | 23 (7.0) | 15 (9.9) | 8 (4.5) |
| Week 9 (June 10) | 23 (7.0) | 10 (6.6) | 13 (7.3) |
| Week 10 (June 17) | 17 (5.1) | 7 (4.6) | 10 (5.6) |
| Week 11 (June 24) | 27 (8.2) | 10 (6.6) | 17 (9.5) |
| Age, years (median, IQR) | 11 (3, 16) | 10 (2, 16) | 12 (4, 16) |
| Age categories | |||
| Infants/young children (0–<5 years) | 100 (30.2) | 52 (34.2) | 48 (26.8) |
| School-aged children (5–<13 years) | 87 (26.3) | 38 (25.0) | 49 (27.4) |
| Adolescents/young adults (13–25 years) | 144 (43.5) | 62 (40.8) | 82 (45.8) |
| Sex | |||
| Female | 146 (44.1) | 64 (42.1) | 82 (45.8) |
| Male | 185 (55.9) | 88 (57.9) | 97 (54.2) |
| Race | |||
| Caucasian | 159 (48.0) | 86 (56.6) | 73 (40.8) |
| Black | 84 (25.4) | 34 (22.4) | 50 (27.9) |
| Other | 9 (2.7) | 3 (2.0) | 6 (3.4) |
| Unknown | 79 (23.9) | 29 (19.1) | 50 (27.9) |
| Ethnicity | |||
| Non-Hispanic, non-Latino, non-Spanish | 195 (58.9) | 108 (71.1) | 87 (48.6) |
| Hispanic, Latino, Spanish | 85 (25.7) | 19 (12.5) | 66 (36.9) |
| Unknown | 51 (15.4) | 25 (16.5) | 26 (14.5) |
| Body mass index categoriesa | |||
| Underweight | 36 (10.9) | 16 (10.5) | 20 (11.2) |
| Normal weight | 118 (35.7) | 57 (37.5) | 61 (34.1) |
| Overweight | 30 (9.1) | 9 (5.9) | 21 (11.7) |
| Obese | 79 (23.9) | 29 (19.1) | 50 (27.9) |
| Location | |||
| United States | 298 (90.0) | 125 (82.2) | 173 (96.7) |
| Canada | 13 (3.9) | 13 (8.6) | 0 (0) |
| Western Europe | 9 (2.7) | 7 (4.6) | 2 (1.1) |
| Eastern Europe/Russia | 10 (3.0) | 6 (4.0) | 4 (2.2) |
| Middle East | <5 | n/a | n/a |
| Any chronic conditionb | 233 (70.4) | 108 (71.1) | 125 (69.8) |
| No chronic condition | 98 (29.6) | 44 (29.0) | 54 (30.2) |
| Asthma | 42 (12.7) | 19 (12.5) | 23 (12.8) |
| Seizures/epilepsy | 44 (13.3) | 22 (14.5) | 22 (12.3) |
| Congenital heart disease (corrected and uncorrected) | 30 (9.1) | 12 (7.9) | 18 (10.1) |
| Cancer (in therapy, remission) | 29 (8.8) | 12 (7.9) | 17 (9.5) |
| Cerebral palsy/encephalopathy | 34 (10.3) | 18 (11.8) | 16 (8.9) |
| Chronic ventilation | 36 (10.9) | 21 (13.8) | 15 (8.4) |
| Diabetes | 18 (5.4) | 6 (3.9) | 12 (6.7) |
| Hypertension | 13 (3.9) | 8 (5.3) | 5 (2.8) |
| Baseline eGFR, median (IQR)c | 125 (93, 180) | 126 (94, 198) | 125 (91, 157) |
| Baseline serum creatinine, mg/dl, median (IQR)c | 0.38 (0.23, 0.66) | 0.32 (0.22, 0.66) | 0.47 (0.23, 0.66) |
| Admission serum creatinine, mg/dl, median (range)d | 0.52 (0.33, 0.81) | 0.50 (0.30, 0.81) | 0.59 (0.37, 0.80) |
| Admission reasons | |||
| Shock/hemodynamic instability | 91 (27.5) | 32 (21.1) | 59 (33.0) |
| Sepsis/infection | 77 (23.3) | 39 (25.7) | 38 (21.2) |
| Respiratory distress | 159 (48.0) | 69 (45.4) | 90 (50.3) |
| CNS symptoms | 39 (11.8) | 23 (15.1) | 16 (8.9) |
| Metabolic derangements | 18 (5.4) | 7 (4.6) | 11 (6.2) |
| Gastrointestinal symptoms | 10 (3.0) | 3 (2.0) | 7 (3.9) |
| Other | 48 (14.5) | 26 (17.1) | 22 (12.3) |
| Acute kidney Injury | |||
| None | 207 (62.5) | 102 (67.1) | 105 (58.7) |
| Mild (stage 1) | 61 (18.4) | 22 (14.5) | 39 (21.8) |
| Severe (stage 2/3) | 63 (19.0) | 28 (18.4) | 35 (19.6) |
Data presented as N (column percentages) except where indicated.
AKI acute kidney injury, eGFR estimated glomerular filtration rate (ml/min/1.73 m2), IQR interquartile range.
aBMI/weight-for-height data missing for 68 patients. BMI categories based on CDC definitions; weight-for-height for those <2 years of age, BMI percentile for those 2–20 years, and adult BMI categories for those >20 years.
bCommon chronic conditions presented for those where at least 10 patients had the condition.
cBaseline eGFR (ml/min/1.73 m2)/creatinine (mg/dl) missing for 204 patients, so for the remainder, it was estimated based on standard estimating equations (see “Methods” section).
dAdmission creatinine value missing on four patients.
Prevalence and risk factors for acute kidney injury in the context of SARS-CoV2 infection
AKI was common in the patients enrolled (n = 124, 37.4%) (Table 2). No statistically significant differences were found in sex, presence of chronic conditions, or body mass index between those with or without AKI for the entire cohort. Racial and ethnic minorities had a higher percentage with AKI than White patients (p-value = 0.04 for Blacks, 0.03 for Hispanics).
Table 2.
AKI prevalence in critically ill children and young adults with suspected SARS-CoV2 infection.
| Characteristics | SUSPECTED no AKI | SUSPECTED AKI | CONFIRMED no AKI | CONFIRMED AKI |
|---|---|---|---|---|
| N | 102 (30.8%) | 50 (15.1%) | 105 (31.7%) | 74 (22.4%) |
| Date of enrollment | ||||
| Week 1 (April 15) | 9 (8.8) | 4 (8.0) | 17 (16.2) | 17 (23.0) |
| Week 2 (April 22) | 15 (14.7) | 8 (16.0) | 7 (6.7) | 9 (12.2) |
| Week 3 (April 29) | 12 (11.8) | 5 (10.0) | 8 (7.6) | 4 (5.4) |
| Week 4 (May 6) | 7 (6.9) | 6 (12.0) | 9 (8.6) | 7 (9.5) |
| Week 5 (May 13) | 14 (13.7) | 6 (12.0) | 11 (10.5) | 12 (16.2) |
| Week 6 (May 20) | 9 (8.8) | 4 (8.0) | 11 (10.5) | 9 (12.2) |
| Week 7 (May 27) | 7 (6.9) | 4 (8.0) | 6 (5.7) | 4 (5.4) |
| Week 8 (June 3) | 12 (11.8) | 3 (6.0) | 4 (3.8) | 4 (5.4) |
| Week 9 (June 10) | 7 (6.9) | 3 (6.0) | 11 (10.5) | 2 (2.7) |
| Week 10 (June 17) | 3 (2.9) | 4 (8.0) | 8 (7.6) | 2 (2.7) |
| Week 11 (June 24) | 7 (6.9) | 3 (6.0) | 13 (12.4) | 4 (5.4) |
| Age, years (median, IQR) | 7 (2, 16) | 13 (9, 17) | 11 (3, 15) | 14 (6, 17) |
| Age categories | ||||
| Infants/young children (0–<5 years) | 44 (43.1) | 8 (16.0) | 30 (28.6) | 18 (24.3) |
| School-aged children (5–<13 years) | 20 (19.6) | 18 (36.0) | 33 (31.4) | 16 (21.6) |
| Adolescents/young adults (13–25 years) | 38 (37.3) | 24 (48.0) | 42 (40.0) | 40 (54.1) |
| Sex | ||||
| Female | 43 (42.2) | 21 (42.0) | 50 (47.6) | 32 (43.2) |
| Male | 59 (57.8) | 29 (58.0) | 55 (52.4) | 42 (56.8) |
| Race | ||||
| Caucasian | 62 (60.8) | 24 (48.0) | 45 (42.9) | 28 (37.8) |
| Black | 24 (23.5) | 10 (20.0) | 23 (21.9) | 27 (36.5) |
| Other | 2 (2.0) | 1 (2.0) | 2 (1.9) | 4 (5.4) |
| Unknown | 14 (13.7) | 15 (30.0) | 35 (33.3) | 15 (20.3) |
| Ethnicity | ||||
| Non-Hispanic, non-Latino, non-Spanish | 75 (73.5) | 33 (66.0) | 40 (38.1) | 47 (63.5) |
| Hispanic, Latino, Spanish | 16 (15.7) | 3 (6.0) | 48 (45.7) | 18 (24.3) |
| Unknown | 11 (10.8) | 14 (28.0) | 17 (16.2) | 9 (12.2) |
| Body mass index categoriesa | ||||
| Underweight | 10 (9.8) | 6 (12.0) | 10 (9.5) | 10 (13.5) |
| Normal weight | 40 (39.2) | 17 (34.0) | 34 (32.4) | 27 (36.5) |
| Overweight | 7 (6.9) | 2 (4.0) | 11 (10.5) | 10 (13.5) |
| Obese | 20 (19.6) | 9 (18.0) | 33 (31.4) | 17 (23.0) |
| Location | ||||
| United States | 87 (85.3) | 38 (76.0) | 102 (97.1) | 71 (96.0) |
| Canada | 7 (6.9) | 6 (12.0) | 0 (0) | 0 (0) |
| Western Europe | 4 (3.9) | 3 (6.0) | 2 (1.9) | 0 (0) |
| Eastern Europe/Russia | 4 (3.9) | 2 (4.0) | 1 (1.0) | 3 (4.1) |
| Middle East | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) |
| Any chronic conditionb | 72 (70.6) | 36 (72.0) | 73 (69.5) | 52 (70.3) |
| No chronic condition | 30 (29.4) | 14 (28.0) | 32 (30.5) | 22 (29.7) |
| Asthma | 13 (12.8) | 6 (12.0) | 12 (11.4) | 11 (14.9) |
| Seizures/epilepsy | 17 (16.7) | 5 (10.0) | 12 (11.4) | 10 (13.5) |
| Congenital heart disease (corrected and uncorrected) | 9 (8.8) | 3 (6.0) | 9 (8.6) | 9 (12.2) |
| Cancer (in therapy, remission) | 5 (4.9) | 7 (14.0) | 7 (6.7) | 10 (13.5) |
| Cerebral palsy/encephalopathy | 12 (11.8) | 6 (12.0) | 9 (8.6) | 7 (9.5) |
| Chronic ventilation | 16 (15.7) | 5 (10.0) | 9 (8.6) | 6 (8.1) |
| Diabetes | 1 (1.0) | 5 (10.0) | 7 (6.7) | 5 (6.8) |
| Hypertension | 5 (4.9) | 3 (6.0) | 1 (1.0) | 4 (5.4) |
| Baseline eGFR, median (IQR)c | 140 (101, 198) | 103 (74, 182) | 126 (99, 149) | 113 (81, 166) |
| Baseline serum creatinine, mg/dl, median (IQR)c | 0.31 (0.20, 0.44) | 0.44 (0.28, 0.85) | 0.47 (0.29, 0.60) | 0.47 (0.20, 0.87) |
| Admission serum creatinine, mg/dl, median (range)d | 0.34 (0.23, 0.54) | 1.00 (0.60, 1.56) | 0.45 (0.31, 0.62) | 0.82 (0.60, 1.50) |
| Admission reasons | ||||
| Shock/hemodynamic instability | 14 (13.7) | 18 (36.0) | 24 (22.9) | 35 (47.3) |
| Sepsis/infection | 19 (18.6) | 20 (40.0) | 24 (22.9) | 14 (18.9) |
| Respiratory distress | 53 (52.0) | 16 (32.0) | 50 (47.6) | 40 (54.1) |
| CNS symptoms | 21 (20.6) | 2 (4.0) | 13 (12.4) | 3 (4.1) |
| Metabolic derangements | 2 (2.0) | 5 (10.0) | 9 (8.6) | 2 (2.7) |
| Gastrointestinal symptoms | 1 (1.0) | 2 (4.0) | 4 (3.8) | 3 (4.1) |
| Other | 16 (15.7) | 10 (20.0) | 15 (14.3) | 7 (9.5) |
Data presented as N (column percentages) except where indicated.
AKI acute kidney injury, eGFR estimated glomerular filtration rate (ml/min/1.73 m2), IQR interquartile range.
aBMI/weight-for-height data missing for 68 patients. BMI categories based on CDC definitions; weight-for-height for those <2 years of age, BMI percentile for those 2–20 years, and adult BMI categories for those >20 years.
bCommon chronic conditions presented for those where at least 10 patients had the condition.
cBaseline eGFR (ml/min/1.73m2)/creatinine (mg/dl) missing for 204 patients, so for the remainder, it was estimated based on standard estimating equations (see “Methods” section).
dAdmission creatinine value missing on four patients.
AKI occurred in 74/179 (41.3%) CONFIRMED patients compared to 50/152 (32.9%) SUSPECTED patients (Table 2). Among those with SUSPECTED SARS-CoV2, AKI occurred more frequently in older children compared to younger children (p-value < 0.001), but this association was not found among those with CONFIRMED SARS-CoV2 (p-value = 0.08). Hispanic ethnicity was found to be protective against AKI among CONFIRMED patients (p-value < 0.001), but not among SUSPECTED patients (p-value = 0.27). AKI was associated with shock to a greater percentage than without shock among both cohorts (p-value < 0.01 among SUSPECTED, p-value < 0.001 among CONFIRMED). However, other admission diagnoses associated with increased risk of AKI were only significant among the SUSPECTED cohort and not the CONFIRMED cohort (sepsis/infection, respiratory distress, central nervous system symptoms, and metabolic derangements). In univariate analyses, Hispanic ethnicity was protective (p-value < 0.001), while invasive respiratory support and vasopressor use were associated with an increased risk of AKI development (p-value 0.02 and <0.01, respectively) (Table 2). The use of vasopressors was associated with a 23.8% absolute increase in the risk of developing AKI compared to no vasopressor use (risk difference 23.8%, 95% CI 6.9–40.6%).
After adjustment for covariates, only the presence of Hispanic ethnicity was associated with AKI in CONFIRMED patients (protective), adjusted RR 0.5 (95% CI 0.3–0.9, Table 3). Vasopressor use was not associated with an increased risk of AKI development (p-value = 0.92).
Table 3.
Independent risk factors for AKI in critically ill children and young adults with suspected SARS-CoV2 infection.
| Relative risks | p-value | |
|---|---|---|
| Age category | ||
| <5 years | Reference | |
| 5–13 years | 1.1 (0.8–1.6) | 0.5 |
| ≥13 years | 0.6 (0.4–1.1) | 0.09 |
| Gender | ||
| Male | 1.2 (0.9–1.6) | 0.3 |
| Female | Reference | |
| Race | ||
| Black | 1.5 (0.7–3.0) | 0.3 |
| White | Reference | |
| Ethnicity | ||
| Hispanic | 0.7 (0.4–1.2) | 0.19 |
| Not-Hispanic | Reference | |
| Presence of Comorbidities | 0.9 (0.6–1.3) | 0.6 |
| Healthy | Reference | |
| Admission diagnosis | ||
| CNS-related | 0.4 (0.1–0.9) | 0.03 |
| Not CNS-related | Reference | |
| Vasopressor use | 1.4 (0.9–2.0) | 0.10 |
| No vasopressor use | Reference | |
| Invasive respiratory Support | 1.3 (0.9–1.9) | 0.11 |
| No invasive respiratory support | Reference | |
The above data demonstrate the relative risks versus embedded reference value for the association of SARS-CoV2 infection with the outcome of AKI.
Variables in the model included center, age category, gender, race, ethnicity, presence or absence of comorbidities, CNS (central nervous system)-associated admission diagnosis, invasive respiratory use, and vasopressor use.
Additional analyses were done to evaluate adjusted risk ratios for AKI development among the entire cohort (CONFIRMED and SUSPECTED) (Table 4). Adjusting for the same variables as in the confirmed cohort, we found that Hispanic ethnicity was no longer a protective factor (p-value = 0.19), yet the presence of CNS-related admission diagnosis was protective (p-value = 0.03). This suggests that there is effect modification of SARS-CoV2 confirmation and the risk factors associated with the development of AKI.
Table 4.
Estimates of effect size for AKI development among critically ill children and young adults with confirmed SARS-CoV2 infection.
| Potential variables of interesta | Total N (%) | Risk of AKI | Univariate risk difference (95% CI) | Univariate risk ratio (95% CI) | p-value | Multivariate risk ratio (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Age categories | |||||||
| 0–5 years | 48 (26.8) | 37.5% | Reference | Reference | Reference | ||
| 5–13 years | 49 (27.4) | 32.7% |
−4.9% (−23.8, 14.1) |
0.9 (0.5–1.5) | 0.62 | 1.5 (0.9–2.3) | 0.13 |
| ≥13 years | 82 (45.8) | 48.8% |
11.3% (−6.2, 28.7) |
1.3 (0.8–2.0) | 0.21 | 0.9 (0.5–1.8) | 0.76 |
| Gender | |||||||
| Male | 97 (54.2) | 43.3% |
4.3% (−10.2, 18.7) |
1.1 (0.8–1.6) | 0.56 | 1.2 (0.8–1.8) | 0.39 |
| Race | |||||||
| Caucasian | 73 (40.8) | 38.4% | Reference | Reference | Reference | ||
| Black | 50 (27.9) | 54.0% |
15.6% (−2.1, 33.4) |
1.4 (1.0–2.1) | 0.09 | 1.8 (0.6–5.4) | 0.29 |
| Other | 6 (3.4) | 66.7% |
28.3% (−11.0, 67.6) |
1.7 (0.9–3.3) | 0.22 | ||
| Ethnicity | |||||||
| Hispanic | 66 (36.9) | 27.3% |
−26.8% (−41.8, −11.8) |
0.5 (0.3–0.8) | <0.001 | 0.5 (0.3-0.9) | 0.03 |
| Body mass index categoriesb | |||||||
| Underweight | 20 | 50.0% |
5.7% (−19.5, 31.0) |
1.1 (0.7–1.9) | 0.65 | ||
| Normal weight | 61 | 44.3% | Reference | Reference | |||
| Overweight | 21 | 47.6% |
3.4% (−21.4, 28.1) |
1.1 (0.6–1.8) | 0.79 | ||
| Obese | 50 | 34.0% |
−10.3% (−28.4, 7.8) |
0.8 (0.5–1.2) | 0.27 | ||
| Any comorbidities | 125 (69.8) | 41.6% |
0.9% (−14.8, 16.6) |
1.0 (0.7–1.5) | 0.91 | 0.7 (0.5–1.2) | 0.18 |
| Admission diagnosisc | |||||||
| Sepsis/infection | 38 (21.2) | 36.8% |
−5.7% (−23.1, 11.7) |
0.9 (0.5–1.4) | 0.53 | ||
| CNS-related | 16 (8.9) | 18.8% |
−24.8% (−45.4, −4.2) |
0.4 (0.2–1.2) | 0.06 | 0.4 (0.1–1.3) | 0.12 |
| Metabolic | 11 (6.2) | 18.2% |
−24.7% (−48.7, −0.7) |
0.4 (0.1–1.5) | 0.13 | ||
| Gastrointestinal symptoms | 7 (3.9) | 42.9% |
1.6% (−35.8, 39.0) |
1.0 (0.4–2.5) | 1.00 | ||
| Other | 22 (12.3) | 31.8% |
−10.9% (−31.8, 10.1) |
0.7 (0.4–1.4) | 0.33 | ||
| Nephrotoxic medication used | 73 (40.8) | 41.1% |
−0.4% (−15.1, 14.3) |
1.0 (0.7–1.4) | 0.96 | ||
| Invasive respiratory support | 47 (26.3) | 55.3% |
19.0% (2.5, 35.4) |
1.5 (1.1–2.1) | 0.02 | 1.2 (0.8–1.8) | 0.45 |
| Vasopressor use | 42 (23.5) | 59.5% |
23.8% (6.9, 40.6) |
1.7 (1.2–2.3) | <0.01 | 1.0 (0.6–1.6) | 0.92 |
| ECMO use | 4 (2.2) | 75.0% |
34.4% (−8.6, 77.5) |
1.8 (1.0–3.3) | 0.31 | ||
Table presents the univariate risk differences and risk ratios with corresponding 95% confidence intervals. The multivariate model includes age category, gender, center, race, ethnicity, presence or absence of comorbidities, CNS-associated admission diagnosis, invasive respiratory use, and vasopressor use.
AKI acute kidney injury, CI confidence interval, CNS central nervous system, ECMO extracorporeal membrane oxygenation.
aFor variables where a reference category is not specified in the table, the references are as follows: Gender reference is female. The ethnicity reference is non-Hispanic. Comorbidities reference is no comorbidities. Reference for admission diagnoses is the absence of that diagnosis. Reference for nephrotoxic medication use is the absence of use, and similarly for invasive respiratory support, vasopressor use, and ECMO use.
bBody mass index categories missing for 27 patients.
cAdmission diagnosis categories were not mutually exclusive and patients could have multiple diagnoses.
dNephrotoxic medications were defined based on the nephrotoxic injury negated by the just-in-time action (NINJA) initiative list.
Severity of acute kidney injury and outcomes in the context of SARS-CoV2 infection
Injury severity varied amongst those with AKI, with 18.4% having mild or stage 1 AKI and 19.0% having severe (stage 2 or 3) AKI (Table 5). Among the entire cohort, children with severe AKI or mild AKI were older compared to those without AKI (p-value 0.01 and 0.002, respectively). In the CONFIRMED population, 74 had any severity of AKI in the first 14 days (41.4%).
Table 5.
Severity of AKI in critically ill children and young adults with suspected SARS-CoV2 infection.
| Characteristics | Total | No AKI | AKI stage 1 | AKI stage 2–3 | |
|---|---|---|---|---|---|
| N | 331 | 207 (62.5) | 61 (18.4) | 63 (19.0) | |
| SARS-CoV2 status | |||||
| Confirmed positive | 179 (54.1) | 105 (50.7) | 39 (63.9) | 35 (55.6) | |
| Suspected, tested negative | 87 (26.3) | 59 (28.5) | 14 (23.0) | 14 (22.2) | |
| Suspected, unknown test results | 65 (19.6) | 43 (20.8) | 8 (13.1) | 14 (22.2) | |
| Date of enrollment | |||||
| Week 1 (April 15) | 47 (14.2) | 26 (12.6) | 11 (18.0) | 10 (15.9) | |
| Week 2 (April 22) | 39 (11.8) | 22 (10.6) | 9 (14.8) | 8 (12.7) | |
| Week 3 (April 29) | 29 (8.8) | 20 (9.7) | 7 (11.5) | 2 (3.2) | |
| Week 4 (May 6) | 29 (8.8) | 16 (7.7) | 5 (8.2) | 8 (12.7) | |
| Week 5 (May 13) | 43 (13.0) | 25 (12.1) | 6 (9.8) | 12 (19.1) | |
| Week 6 (May 20) | 33 (10.0) | 20 (9.7) | 3 (4.9) | 10 (15.9) | |
| Week 7 (May 27) | 21 (6.3) | 13 (6.3) | 4 (6.6) | 4 (6.4) | |
| Week 8 (June 3) | 23 (7.0) | 16 (7.7) | 2 (3.3) | 5 (7.9) | |
| Week 9 (June 10) | 23 (7.0) | 18 (8.7) | 3 (4.9) | 2 (3.2) | |
| Week 10 (June 17) | 17 (5.1) | 11 (5.3) | 4 (6.6) | 2 (3.2) | |
| Week 11 (June 24) | 27 (8.2) | 20 (9.7) | 7 (11.5) | 0 (0) | |
| Age, years (median, IQR) | 11 (3, 16) | 10 (2, 16) | 14 (7, 17) | 13 (6, 16) | |
| Age categories | |||||
| Infants/young children (0–<5 years) | 100 (30.2) | 74 (35.8) | 11 (18.0) | 15 (23.8) | |
| School-aged children (5–<13 years) | 87 (26.3) | 53 (25.6) | 16 (26.2) | 18 (28.6) | |
| Adolescents/young adults (13–25 years) | 144 (43.5) | 80 (38.7) | 34 (55.7) | 30 (47.6) | |
| Sex | |||||
| Female | 146 (44.1) | 93 (44.9) | 27 (44.3) | 26 (41.3) | |
| Male | 185 (55.9) | 114 (55.1) | 34 (55.7) | 37 (58.7) | |
| Race | |||||
| Caucasian | 159 (48.0) | 107 (51.7) | 27 (44.3) | 25 (39.7) | |
| Black | 84 (25.4) | 47 (22.7) | 13 (21.3) | 24 (38.1) | |
| Other | 9 (2.7) | 4 (1.9) | 4 (6.6) | 1 (1.6) | |
| Unknown | 79 (23.9) | 49 (23.7) | 17 (27.9) | 13 (20.6) | |
| Ethnicity | |||||
| Non-Hispanic, non-Latino, non-Spanish | 195 (58.9) | 115 (55.6) | 38 (62.3) | 42 (66.7) | |
| Hispanic, Latino, Spanish | 85 (25.7) | 64 (30.9) | 12 (19.7) | 9 (14.3) | |
| Unknown | 51 (15.4) | 28 (13.5) | 11 (18.0) | 12 (19.1) | |
| Body mass index categoriesa | |||||
| Underweight | 36 (10.9) | 20 (9.7) | 6 (9.8) | 10 (15.9) | |
| Normal weight | 118 (35.7) | 74 (35.8) | 24 (39.3) | 20 (31.8) | |
| Overweight | 30 (9.1) | 18 (8.7) | 9 (14.8) | 3 (4.8) | |
| Obese | 79 (23.9) | 53 (25.6) | 13 (21.3) | 13 (20.6) | |
| Location | |||||
| United States | 298 (90.0) | 189 (91.3) | 54 (88.5) | 55 (87.3) | |
| Canada | 13 (3.9) | 7 (3.4) | 2 (3.3) | 4 (6.4) | |
| Western Europe | 9 (2.7) | 6 (2.9) | 2 (3.3) | 1 (1.6) | |
| Eastern Europe/Russia | 10 (3.0) | 5 (2.4) | 2 (3.3) | 3 (4.8) | |
| Middle East | <5 | n/a | n/a | n/a | |
| Any chronic conditionb | 233 (70.4) | 145 (70.1) | 48 (78.7) | 40 (63.5) | |
| No chronic condition | 98 (29.6) | 62 (30.0) | 13 (21.3) | 23 (36.5) | |
| Asthma | 42 (12.7) | 25 (12.1) | 12 (19.7) | 5 (7.9) | |
| Seizures/epilepsy | 44 (13.3) | 29 (14.0) | 7 (11.5) | 8 (12.7) | |
| Congenital heart disease (corrected and uncorrected) | 30 (9.1) | 18 (8.7) | 7 (11.5) | 5 (7.9) | |
| Cancer (in therapy, remission) | 29 (8.8) | 12 (5.8) | 9 (14.8) | 8 (12.7) | |
| Cerebral palsy/encephalopathy | 34 (10.3) | 21 (10.1) | 5 (8.2) | 8 (12.7) | |
| Chronic ventilation | 36 (10.9) | 25 (12.1) | 7 (11.5) | 4 (6.3) | |
| Diabetes | 18 (5.4) | 8 (3.9) | 5 (8.2) | 5 (7.9) | |
| Hypertension | 13 (3.9) | 6 (2.9) | 3 (4.9) | 4 (6.3) | |
| Baseline eGFR, median (IQR)c | 125 (93, 180) | 129 (100, 181) | 112 (77, 159) | 108 (79, 196) | |
| Baseline serum creatinine, mg/dl, median (IQR)c | 0.38 (0.23, 0.66) | 0.34 (0.22, 0.58) | 0.39 (0.27, 1.11) | 0.50 (0.20, 0.72) | |
| Admission serum creatinine, mg/dl, median (range)d | 0.52 (0.33, 0.81) | 0.40 (0.26, 0.60) | 0.76 (0.50, 1.00) | 1.27 (0.74, 2.37) | |
| Admission reasons | |||||
| Shock/hemodynamic instability | 91 (27.5) | 38 (18.4) | 21 (34.4) | 32 (50.8) | |
| Sepsis/infection | 77 (23.3) | 43 (20.8) | 11 (18.0) | 23 (36.5) | |
| Respiratory distress | 159 (48.0) | 103 (49.8) | 30 (49.2) | 26 (41.3) | |
| CNS symptoms | 39 (11.8) | 34 (16.4) | 2 (3.3) | 3 (4.8) | |
| Metabolic derangements | 18 (5.4) | 11 (5.3) | 5 (8.2) | 2 (3.2) | |
| Gastrointestinal symptoms | 10 (3.0) | 5 (2.4) | 2 (3.3) | 3 (4.8) | |
| Other | 48 (14.5) | 31 (15.0) | 8 (13.1) | 9 (14.3) | |
Data presented as N (column percentages) except where indicated.
AKI acute kidney injury, eGFR estimated glomerular filtration rate (ml/min/1.73 m2), IQR interquartile range.
aBMI/weight-for-height data missing for 68 patients. BMI categories based on CDC definitions; weight-for-height for those <2 years of age, BMI percentile for those 2–20 years, and adult BMI categories for those >20 years.
bCommon chronic conditions presented for those where at least 10 patients had the condition.
cBaseline eGFR (ml/min/1.73 m2) / creatinine (mg/dl) missing for 204 patients, so for the remainder it was estimated based on standard estimating equations (see “Methods” section).
dAdmission creatinine value missing on four patients.
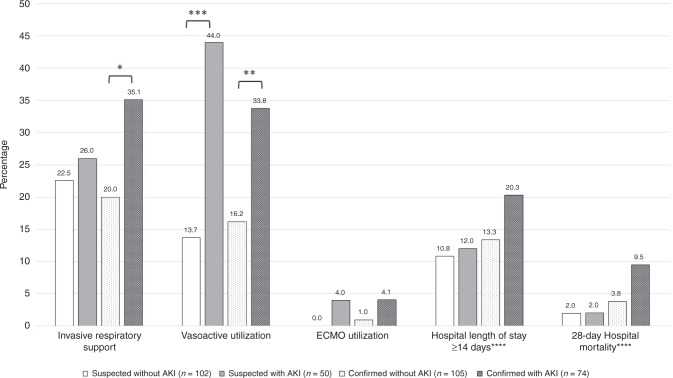
AKI in the setting of SUSPECTED and CONFIRMED SARS-CoV2 infection, was associated with increased morbidity (Fig. 1). Among CONFIRMED patients, a greater percentage of those with AKI required respiratory and vasoactive support compared to those without AKI, 35.1% versus 20.0% (p-value < 0.05) and 33.8% versus 16.2% (p-value < 0.01), respectively. While among those with SUSPECTED SARS-CoV2 infection, only vasoactive support occurred more frequently in those with AKI compared to those without AKI (44.0% versus 13.7%, p-value < 0.001). The majority of children in the study had hospital length of stay (LOS) under 14 days, regardless of AKI status. The confirmation of SARS-CoV2 infection or AKI did not confer a higher proportion of patients with a LOS >14 days. Mortality was 4.2% (n = 14) in the overall study population. Though this trend increased to 6.2% (n = 11) for patients with CONFIRMED infection, and further to 9.5% (n = 7) among those CONFIRMED and AKI, these did not reach statistical significance between groups (p = 0.09 in CONFIRMED versus SUSPECTED and p = 0.049 in CONFIRMED versus ALL).
Fig. 1. Critical care outcomes for critically ill children and young adults with confirmed and suspected SARS-CoV2 infection and by AKI status.
Percentage of patients in the given category presented on Y axis. X axis presents various clinical outcomes by cohort; suspected of SARS-CoV2 without AKI, n = 331; suspected of SARS-CoV2 with AKI; confirmed with SARS-CoV2 without AKI, n = confirmed with SARS-CoV2 with AKI, n = 74. *p-value < 0.05. **p-value < 0.01. ***p-value < 0.001. ****Length of hospitalization among survivors, but missing for 46 survivors and hospital mortality outcome missing for 34 patients. AKI acute kidney injury, ECMO extracorporeal membrane oxygenation.
Clinical management details do not seem to suggest differences between the groups. Nephrotoxin medication use was slightly higher in those with AKI among SUSPECTED SARS-CoV2 infection but it was not statistically significant (60.0% versus 44.1%, p-value = 0.06) and there was no difference in nephrotoxic medication use between those with and without AKI among the CONFIRMED cohort (40.5% versus 41.0%, p-value = 0.96). Remdisivir was only used in 9 patients in the entire cohort, all with confirmed SARS-CoV2 infection (5.0%), and only 3 of those with AKI (4.1%). Maximum fluid overload percentage was similar between those with and without AKI in CONFIRMED cohort (median 2.8%, IQR 0.1–8.4%; median 2.9%, IQR 0.3–9.1%, respectively) and the SUSPECTED cohort (median 1.1%, IQR 0–5.4%; median 20%, IQR 0.3–4.1%).
Discussion
AKI is common in pediatric and young adult patients in the setting of potential infection with the SARS-CoV2 virus. The AKI we describe in children tested for SARS-CoV2 infection appears to be higher in incidence versus historical norms—both in terms of overall percentage and severity. Compared to the global epidemiology study of AKI in 4914 critically ill children (AWARE28), the overall AKI incidence in SPARC was 41.4% (versus 26.2% in AWARE) and was 19% for severe AKI (versus 11.6% in AWARE). We found that children with CONFIRMED infection had a higher, but not statistically significant, prevalence of AKI than those with SUSPECTED infection. In a sepsis sub-cohort analysis of the AWARE data set (n = 765), AKI occurred in 40.5% of children and 22.3% was severe AKI29. Mortality in this analysis was 4.2% overall (6.2% in CONFIRMED children, 9.5% in those with CONFIRMED and AKI) compared to historical numbers (AWARE: 2.5% in ICU patients and 11.0% patients with AKI). A similar association between sepsis-associated AKI and mortality was reported in AWARE (7.7%) versus the current study with CONFIRMED and AKI. In a point-prevalence study of pediatric sepsis, 25% of 569 patients captured had AKI30,31. Additionally, we identified a cohort of children requiring RRT, as in adult AKI associated with SARS-CoV2.
We were able to further refine risk factors for AKI in the context of SARS-CoV2 infection. We found a similar AKI occurrence rate in our patients with confirmed SARS-CoV2 infection (41.4% for any AKI and 19.6% for severe AKI). In the available literature related to AKI in the context of SARS-CoV2, we note that our data elaborates on the preliminary findings that we had reported earlier in this cohort22. Our study expands on our previous data and provides more granularity to findings from smaller pediatric studies, which showed variability in AKI rates (from 0 to 40% AKI rate in cohorts <75 total patients studied5,32,33). It remains unclear at this time how the AKI epidemiology in children affected by the novel virus has changed during subsequent waves as there remains very little published data. Finally, distinct from our interim analysis, we found that Hispanic ethnicity and primary diagnosis of central nervous system dysfunction was associated with less AKI, different than our preliminary analysis, which was limited by population size for the purpose of adjusting associations for multivariate analysis. The rationale for the protection is unclear as it is not completely congruous with data relating risks to pediatric AKI. Hispanic race in AWARE showed no association with increased or decreased protection from AKI. A protective association may have been delineated in this sample of patients based on an increased proportion of Hispanic patients testing positive for SARS-CoV2 on incidental testing (versus symptomatic). Central nervous system dysfunction as a primary diagnosis also may actually be associated with “lower” AKI prevalence. Many patients with altered mental status, seizures, or drug ingestions changing CNS status are at lower risk for AKI34. Emerging data indicate SARS-CoV2 is associated with an increased risk of new or additive CNS dysfunction, but this is potentially outside the scope of AKI35.
The role of chronic disease and SARS-CoV2 has been controversial. In our preliminary analysis of this data, we had indicated an early connection between chronic respiratory and neurologic disease and AKI in the setting of viral infection, but we did not show such an association in our expanded findings. Interestingly, CONFIRMED or SUSPECTED infection was not common in those cardiomyopathy and heart failure. We also note that infants and young children (<5 years of age) had a lower prevalence of AKI than older children and young adults. While our exclusion of those hospitalized in Neonatal Intensive Care Units may have confounded these findings, this rate is lower than reported rates of AKI in other populations of septic neonates36,37. An ongoing study is currently reporting AKI prevalence in infants with suspected or confirmed SARS-CoV238 and should serve to expand and refine our findings in this age demographic. Further, we had more ability to show the relationships between age, ethnicity, and other chronic conditions in relation to both severity of illness in SARS-CoV2 and also with AKI in this setting.
Multi-inflammatory syndrome in children (MIS-C) is now recognized as a distinct manifestation of the virus21,39. This post-infectious dysregulated inflammatory response syndrome is under-represented in this cohort as it was designed and conducted in the early portion of the pandemic. As such, we were not able to delineate patients with MIS-C as we only required site investigators to note “CONFIRMED” as inclusive of both PCR and Ab testing. The inclusion of both populations, however, is relevant and important as we compare cardiogenic shock in the context of SARS-CoV2 and AKI. Further studies that evaluate the relationship between MIS-C and AKI are needed—thus far the data mirror findings of a high prevalence of severe AKI40. It is possible, that even in the absence of negative PCR testing, retrospective adjudication would result in increased prevalence of MIS-C and associated organ dysfunction41,42.
Our study has notable strengths and limitations. This report is taken from participating centers and thus should be placed in the context of the evolution of this pandemic. Our study was in the first three months of the pandemic—with a majority of the patients identified from centers located in the Region of the Americas as defined by the World Health Organization. We limited our enrollment only to those suspected or confirmed to have SARS-CoV2 infection, thus it is quite possible selection bias is introduced and may actually affect our findings of both AKI prevalence and associations—and should be considered when comparing to the general pediatric ICU population. To this point, the evolution of the testing standards used likely impacted some of our results—as a majority of the participating centers adopted multiple tests for acute (PCR) and historical (Ab) based detection strategies. It was not possible to harmonize all the different tests utilized for comparison. It is possible providers were more likely to suspect SARS-CoV2 with a negative test result in more severely ill patients than those who were less severely ill. We also may not have captured all suspected patients due to the challenges of doing remote research during an active pandemic. For this reason, we limited the analysis of AKI severity as the detailed analysis of AKI severity would require the inclusion of duration, change over time, and other associated confounding factors related to AKI in individual patients—data we did not capture in a point-prevalence method. In statistical modeling, collinearity existed between diagnosis: shock-vasopressor use and between respiratory distress-invasive respiratory support; only vasopressor use and invasive respiratory support were included as these were viewed to be more objective. To prevent overfitting, country of location and admission diagnosis of metabolic derangements were dropped.
Conclusions
The SARS-CoV2 virus is associated with a higher than baseline prevalence of AKI in critically ill children. The prevalence and severity of AKI in the context of viral infection are similar to children with sepsis. As the first year of the COVID pandemic leads into the second year, the absence of a vaccine available for use across pediatric patients creates the potential for an ongoing pandemic. Future studies are needed to study the relationship of the virus and AKI, including understanding the pathobiology of infection and the severity of dysfunction.
Supplementary information
Supplemental Table 1 - Collaborators for SPARC
Acknowledgements
The principal investigators of the SPARC collaborative would like to acknowledge the research coordinators at each participating site for their contributions to this work (Supplemental File). The cloud-based data entry into Redcap is supported by a Center for Clinical and Translational Science and Training grant at the University of Cincinnati School of Medicine (2UL1TR001425-05A1).
Author contributions
R.K.B., E.C.B., K.A.K., M.Z., D.A., and S.L.G.: concept and design, data acquisition, analysis, interpretation, drafting, and final approval. K.M.G., M.S., P.K., and R.C.: interpretation, drafting, and final approval.
Competing interests
The authors declare no competing interests.
Footnotes
A full list of SPARC Investigators members appears in the Supplementary Information.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-021-01667-4.
References
- 1.Joshi K, et al. Cardiac dysfunction and shock in pediatric patients with covid-19. JACC Case Rep. 2020;2:1267–1270. doi: 10.1016/j.jaccas.2020.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soni, S. L. et al. Demographic & clinical profile of patients with covid-19 at a tertiary care hospital in North India. Indian J. Med. Res.10.4103/ijmr.IJMR_2311_20 (2020). [DOI] [PMC free article] [PubMed]
- 3.Davies P, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with Sars-Cov-2 (Pims-Ts) in the UK: a multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oualha M, et al. Severe and fatal forms of covid-19 in children. Arch. Pediatr. 2020;27:235–238. doi: 10.1016/j.arcped.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekerdemian, L. S. et al. Characteristics and outcomes of children with coronavirus disease 2019 (covid-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 174, 868–873 (2020). [DOI] [PMC free article] [PubMed]
- 6.Sachdeva R, et al. The impact of coronavirus disease 2019 pandemic on U.S. And Canadian Picus. Pediatr. Crit. Care Med. 2020;21:e643–e650. doi: 10.1097/PCC.0000000000002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagarro, A. et al. Screening and severity of coronavirus disease 2019 >(covid-19) in children in Madrid, Spain. JAMA Pediatr.10.1001/jamapediatrics.2020.1346 (2020). [DOI] [PMC free article] [PubMed]
- 8.Peng S, et al. Early versus late acute kidney injury among patients with covid-19-A multicenter study from Wuhan, China. Nephrol. Dial. Transpl. 2020;35:2095–2102. doi: 10.1093/ndt/gfaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, et al. Multi-organ dysfunction in patients with covid-19: a systematic review and meta-analysis. Aging Dis. 2020;11:874–894. doi: 10.14336/AD.2020.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim MA, et al. Multiorgan failure with emphasis on acute kidney injury and severity of covid-19: systematic review and meta-analysis. Can. J. Kidney Health Dis. 2020;7:2054358120938573. doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y, et al. Kidney disease is associated with in-hospital death of patients with covid-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch, J. S. et al. Acute kidney injury in patients hospitalized with covid-19. Kidney Int. 98, 209–218 (2020). [DOI] [PMC free article] [PubMed]
- 15.Gupta S, et al. Aki treated with renal replacement therapy in critically Ill patients with covid-19. J. Am. Soc. Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chueh, T. I., Zheng, C. M., Hou, Y. C. & Lu, K. C. Novel evidence of acute kidney injury in covid-19. J. Clin. Med. 9, 3547(2020). [DOI] [PMC free article] [PubMed]
- 17.Gagliardi, I. et al. Covid-19 and the kidney: from epidemiology to clinical practice. J. Clin. Med. 9, 2506 (2020). [DOI] [PMC free article] [PubMed]
- 18.Wang X, et al. Be aware of acute kidney injury in critically ill children with covid-19. Pediatr. Nephrol. 2021;36:163–169. doi: 10.1007/s00467-020-04715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raina R, Chakraborty R, Sethi SK, Bunchman T. Kidney replacement therapy in covid-19 induced kidney failure and septic shock: a pediatric continuous renal replacement therapy [Pcrrt] position on emergency preparedness with resource allocation. Front. Pediatr. 2020;8:413. doi: 10.3389/fped.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deep, A., Bansal, M. & Ricci, Z. Acute kidney injury and special considerations during renal replacement therapy in children with coronavirus disease-19: perspective from the critical care nephrology section of the european society of paediatric and neonatal intensive care. Blood Purif. 50, 150–160 (2020). [DOI] [PMC free article] [PubMed]
- 21.Lee, M., Hilado, M., Sotelo, S., Opas, L. M. & Im, D. D. Acute kidney injury in multisystem inflammatory syndrome in children (Mis-C): a case report. SN Compr. Clin. Med.10.1007/s42399-020-00647-9 (2020). [DOI] [PMC free article] [PubMed]
- 22.Bjornstad, E. C. et al. Preliminary assessment of acute kidney injury in critically ill children associated with Sars-Cov-2 infection: a multicenter cross-sectional analysis. Clin. J. Am. Soc. Nephrol. 16, 446–448 (2020). [DOI] [PMC free article] [PubMed]
- 23.Dong, Y. et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics10.1542/peds.2020-0702 (2020).
- 24.Harris PA, et al. The redcap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, et al. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green, D. A. et al. Clinical performance of Sars-Cov-2 molecular tests. J. Clin. Microbiol.58, e00995-20 (2020). [DOI] [PMC free article] [PubMed]
- 27.Hoste L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol. Dial. Transpl. 2014;29:1082–1091. doi: 10.1093/ndt/gft277. [DOI] [PubMed] [Google Scholar]
- 28.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu, R. K. et al. Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr. Res.10.1038/s41390-021-01363-3 (2021). [DOI] [PMC free article] [PubMed]
- 30.Weiss SL, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 31.Weiss SL, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart DJ, et al. Renal dysfunction in hospitalised children with covid-19. Lancet Child Adolesc. Health. 2020;4:e28–e29. doi: 10.1016/S2352-4642(20)30178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu H, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (covid-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu RK, Kaddourah A, Goldstein SL, Investigators AS. Assessment of a renal Angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc. Health. 2018;2:112–120. doi: 10.1016/S2352-4642(17)30181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaRovere KL, et al. Neurologic involvement in children and adolescents hospitalized in the United States for covid-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jetton JG, et al. Incidence and outcomes of neonatal acute kidney injury (awaken): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health. 2017;1:184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starr MC, et al. The impact of increased awareness of acute kidney injury in the neonatal intensive care unit on acute kidney injury incidence and reporting: results of a retrospective cohort study. J. Perinatol. 2020;40:1301–1307. doi: 10.1038/s41372-020-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gale C, et al. Characteristics and outcomes of neonatal Sars-Cov-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc. Health. 2021;5:113–121. doi: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldstein LR, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deep A, et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from Picus across United Kingdom. Crit. Care Med. 2020;48:1809–1818. doi: 10.1097/CCM.0000000000004662. [DOI] [PubMed] [Google Scholar]
- 41.Alsaied T, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 42.Sperotto F, et al. Cardiac manifestations in Sars-Cov-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021;180:307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 - Collaborators for SPARC