Abstract
The physical manifestations of neurofibromatosis type 1 (NF1) can cause chronic pain. This study investigated the impact of pain in youth with NF1 and plexiform neurofibromas (PNs) and its relationship to disease factors, social-emotional functioning, and quality of life (QOL) within a biopsychosocial framework. Caregivers of 59 children and adolescents with NF1 and PNs (6–18 years), and 41 of these youth (10–18 years), completed questionnaires assessing social-emotional functioning and QOL, including an item on pain interference. Measures of disease severity included total PN volume by percent body weight and number of disease complications. Both caregiver (73%) and self-report (59%) ratings indicated that pain interferes with the child’s daily functioning despite 33% taking pain medication. Based on caregivers’ behavior ratings, more symptoms of anxiety and larger tumor volumes predicted greater pain interference, while greater pain interference, worse depressive symptoms, and more disease complications predicted poorer QOL. As rated by adolescents, more symptoms of anxiety predicted greater pain interference, while greater pain interference and social stress predicted poorer QOL. Further, social-emotional problems mediate the relationship between pain interference and QOL. Thus, pain interferes with daily functioning in the majority of youth with NF1 and PNs even when using pain medication. The impact of pain interference, disease severity, and particularly social-emotional problems on QOL highlights the interaction between physical and psychological states in NF1. Future research and treatment of pain in this population should utilize a biopsychosocial approach and involve multidisciplinary therapies including psychological interventions that target social-emotional functioning.
Keywords: neurofibromatosis type 1, plexiform neurofibromas, pain, quality of life, children
INTRODUCTION
Neurofibromatosis type 1 (NF1) is one of the most common autosomal dominant genetic disorders, affecting approximately 1 in 3,000 [Friedman, 1999]. Individuals with NF1 have an increased risk of developing tumors of the central and peripheral nervous system, including plexiform neurofibromas (PNs), which are benign peripheral nerve sheath tumors that grow along the nerves [Korf, 1999; Ferner et al., 2007]. PNs and other physical manifestations of NF1 can result in severe and chronic pain. PNs may cause nerve [Citak et al., 2008], airway, and spinal cord compression, leg length discrepancies, and scoliosis [Kim et al., 2009]. Furthermore, pain is associated with PNs [Creange et al., 1999; Nguyen et al., 2011], which tend to grow most rapidly during childhood [Needle et al., 1997; Dombi et al., 2007]. Pain also may emerge after tumor removal [Creange et al., 1999]. Common non-tumor physical manifestations causing pain include skeletal complications [Elefteriou et al., 2009] and headaches [Creange et al., 1999; DiMario and Langshur, 2000].
Despite the potential for pain in the pediatric NF1 population, limited studies have focused on assessing the prevalence, severity, treatment, or impact of pain, with even fewer investigations of youth with PNs. In children with NF1 who have primarily mild to moderate disease severity, self-ratings of pain were significantly worse than reference values [Krab et al., 2009], and adolescents endorsed having pain for an average of four days during a 2-week period [Garwood et al., 2012]. Parents of children with NF1 complications reported significantly higher pain ratings on a general quality of life (QOL) scale compared to those without complications [Oostenbrink et al., 2007]. In a study including a subset of children with NF1 and PNs, approximately 30% of them reported pain [Nguyen et al., 2011]. Chart reviews of a pediatric sample enrolled in treatment trials for PNs, typically with large tumors and substantial morbidity, indicated that 53% reported the presence of pain [Kim et al., 2009]. Although studies evaluating the efficacy of analgesics or other pain management techniques in NF1 are limited, data suggest that 12% of children with PNs take narcotics [Kim et al., 2009] and over 70% of children and adults with NF1 use prescription pain medications [Creange et al., 1999].
In addition to the physical manifestations, youth with NF1 exhibit learning problems and cognitive deficits [Hofman et al., 1994; Koth et al., 2000; Hyman et al., 2005], and they display social-emotional difficulties, including higher rates of internalizing and externalizing disorders [Johnson et al., 1999; Barton and North, 2004; Graf et al., 2006; Martin et al., 2012], fewer friends [Barton and North, 2004; Noll et al., 2007], and more social problems [Dilts et al., 1996; Johnson et al., 1999; Barton and North, 2004] compared to normative samples [Barton and North, 2004; Graf et al., 2006; Martin et al., 2012] or their unaffected siblings [Dilts et al., 1996; Johnson et al., 1999]. While mechanisms for these functional problems have not been fully explored, the physical manifestations of NF1, along with cognitive and environmental factors, have been linked to social-emotional [Barton and North, 2004; Martin et al., 2012] and QOL outcomes [Wolkenstein et al., 2001; Graf et al., 2006; Krab et al., 2009]. Due to its relationship to disease severity [Page et al., 2006], pain may play a contributory role as well [Oostenbrink et al., 2007]. A recent study in adolescents with NF1 found that emotional functioning predicted overall QOL while physical complications, such as pain, predicted functional disability [Garwood et al., 2012]. These studies suggest that pain and its impact in NF1 may be best understood and treated within a biopsychosocial model, which conceptualizes pain as “the result of the dynamic interaction between physiological, psychological, and social factors” [Gatchel et al., 2007]. Thus, multiple factors can interact with physical pain to modulate its effect on daily functioning and QOL. While associations between various factors in this model have been demonstrated in other pediatric pain populations [Miro et al., 2009; Nieto et al., 2012], such research is needed in youth with NF1, including those with PNs who may have more severe disease complications. Measuring the impact of pain on daily functioning in children and understanding its relationship to the disease and other factors is critical for effective medical and psychological treatment of this complex disorder. Thus, the aims of this study were to (1) assess the degree to which pain interferes with daily functioning (pain interference); (2) describe the prevalence and type of pain medications used for treatment; and (3) examine the relationships between pain interference, disease factors, social-emotional functioning, and QOL in youth with NF1 and PNs. Our primary hypothesis was that social-emotional factors contribute to pain interference and overall QOL in this population above and beyond disease severity.
MATERIALS AND METHODS
Eligibility Criteria
Children and adolescents with NF1 and PNs from 6 to 18 years of age, who were enrolled on a natural history protocol at the National Cancer Institute (NCI) and completed the designated comprehensive psychological assessment, were eligible for this pain sub-study. Eligibility requirements for this protocol included a diagnosis of NF1 according to the NIH Consensus Conference criteria [Stumpf et al., 1988] or a confirmed NF1 germline mutation with analysis performed in a CLIA-certified laboratory. Sixty-four enrolled patients were within the target age range and had a PN. Of these, three children did not participate in the psychological assessment due to lack of interest by caregivers, and a child with autism was excluded because she could not complete the measures reliably. Thus, the final sample consisted of 60 patients with NF1 and PNs.
Measures
Demographic variables.
The primary caregiver (parent or legal guardian) completed a questionnaire assessing basic demographic data, such as race and years of parental education, as well as background information about the child, including sex, NF1 type (sporadic or familial), medical and psychiatric diagnoses, educational and therapeutic services, and pain medications.
NF1 symptom severity.
The same primary caregiver also rated their child’s overall NF1 symptoms as mild, moderate, or severe based on the presence and severity of tumors, pain, motor deficits, and/or learning problems, and the extent to which these symptoms impact activities of daily living using the NF1 Symptom Severity Scale. The authors slightly modified the original scale by Ablon [1996] to include possible effects of PNs on daily functioning, such as pain and problems with mobility, posture, and vision.
Total PN volume.
PN volumes were obtained from whole-body Short T1-Inversion Recovery Magnetic Resonance Imaging (STIR MRI) using a sensitive and reliable semi-automated technique for detecting tumors that are not well-defined [Solomon et al., 2004]. Total tumor burden (TTB) was calculated by dividing the sum of each patient’s PN volumes by body weight and expressed as a percentage to account for the broad age range and variability in body mass [Dombi et al., 2007]. The TTB data were positively skewed; therefore, a natural log transformation was conducted, resulting in an approximately normal distribution (LogTTB).
Disease-related complications.
The nurse practitioner conducting the history and physical exams completed a rating form of 17 NF1-related diagnoses and disease complications, such as PNs, scoliosis, spinal fusion, vision problems, headaches, seizures, and limb length discrepancy, as previously described [Martin et al., 2012]. These ratings were summed to produce a total disease complications severity score, ranging from 0 to 17.
Pain interference and quality of life.
Caregivers and adolescents completed the Impact of Pediatric Illness (IPI) Scale, a general QOL scale that assesses the effects of pediatric chronic illness on the domains of adaptive, emotional, physical, and cognitive functioning. It includes a parent proxy-report form for primary caregivers of children ages 6–18 years and parallel self-report forms for adolescents ages 10–18 years and adults ages 18 years and older. Items are rated on a 5-point Likert scale (1–5; “not at all” to “a lot”). To compute the total score, negative items were reversed, individual item ratings (equally weighted) were transformed to a scale of 0–100, and the mean was calculated. Higher mean total scores indicate better QOL.
The IPI Scale is reliable and valid in NF1 [Wolters et al., 2010; Wolters et al., 2013]. Internal consistency of the total scale for both the parent and adolescent forms is good (coefficient α reliability estimates were 0.91 and 0.84, respectively). Children with mild NF1 symptoms as rated by parents had significantly higher parent proxy and self-report mean total IPI Scale scores compared to children with moderate/severe NF1 symptoms, demonstrating construct validity.
Pain interference was assessed by one item on the IPI Scale forms that asks the extent to which the individual experiences pain that interferes with his/her daily functioning (“My child has pain that interferes with his/her daily functioning” or “I have pain that keeps me from doing what I want”). High scores on this item indicate greater pain interference.
Social-emotional functioning.
The anxiety, depression, and withdrawal subscales of the Behavior Assessment System for Children-2nd Edition [Reynolds and Kamphaus, 2004] Parent Rating Scale (BASC-II-P) were chosen a priori to assess the social-emotional functioning of the children and adolescents, ages 6–18 years, by parent report for this sub-study. The anxiety, depression, and social stress subscales of the BASCII-Self-Report (BASC-II-SR), which assess similar domains to the parent form, were chosen a priori to assess the self-rated social-emotional functioning of the adolescents, ages 10–18 years, who also completed the self-report IPI Scale. For both measures, raw scores are converted to T-scores (mean = 50; SD = 10). Scores between 60 and 69 are considered in the “at risk” (AR) range, and scores of 70 or higher are in the “clinically significant” (CS) range.
Procedures
The NF1 Natural History protocol, a longitudinal study designed to characterize both tumor and non-tumor manifestations of the disease, was approved by the NCI Institutional Review Board. Referrals came from physicians primarily around the United States or from primary caregivers who obtained information about the study from the internet or other sources. Prior to enrollment, an investigator obtained informed consent from the child’s primary caregiver or adult patient and minor assent from children ages 7–17 years. During multi-day outpatient clinic visits, participants completed detailed multidisciplinary evaluations including a comprehensive psychological evaluation while primary caregivers completed the parent questionnaires. A nurse practitioner conducted standardized history and physical exams. The children also underwent a whole body STIR MRI scan to assess total tumor burden.
Statistical Analyses
Descriptive statistics were computed to summarize the demographic and medical data as well as pain interference ratings for the total sample and various subgroups including age and sex. Analyses of variance (ANOVAs) were conducted to compare the pain severity ratings between different subgroups such as young versus older age groups, male versus female, and patients taking pain medications on a regular basis versus not taking pain medications. Using ANOVAs with repeated measures, caregiver and self-report ratings were compared in the 40 caregiver–adolescent pairs on the pain interference and overall QOL measures, which consist of parallel items, but not on the BASC-II parent and self-report forms that contain different items.
Pearson product moment correlations were calculated in order to estimate the bivariate relationships between pain interference, social-emotional functioning, disease variables, and quality of life. These correlations were examined within the various caregiver-rated measures and also within the various self-reported measures but not between the caregiver and self-report measures. To determine the extent to which biopsychosocial factors predict pain interference and overall QOL, standard multiple regression analyses were conducted. Since the interaction of both physiological and psychological factors may impact the effects of pain on daily functioning, the predictor variable with the most significant bivariate correlation was selected from each of the biopsychosocial domains assessed (e.g., disease severity and social-emotional functioning) and entered into the multiple regressions. To specifically explore the role of social-emotional factors as mediators of the relationship between pain interference and QOL, the Sobel test [Lockhart et al., 2011] and bootstrapping procedure [Hayes, 2009] were conducted. For all these analyses, the pain interference item was not included in the total IPI Scale score; deleting this one item did not change the internal consistency of the total scale appreciably for either the adolescent or parent form. Since this is an exploratory study investigating possible relationships between these domains and a limited number of variables were selected for various analyses, α was set at 0.05.
RESULTS
Demographic Variables
The total sample consisted of 60 youths with NF1 and PNs, ages 6.3–18.8 years (mean age = 12.7 years; SD = 3.6). The demographic characteristics of the total sample, including the adolescent (n = 42) and child (n = 18) subgroups, are listed in Table I. Missing data included one Parent IPI Scale for an 18-year old whose caregiver did not attend his clinic visit, one Adolescent IPI Scale that inadvertently was not administered to a 13-year old, and one tumor volume for a 12-year old who did not have a baseline whole body MRI scan. Thus, 59 youths had Parent IPI Scale data, including the pain interference item, and 41 adolescents, ages 10.6–18.8 years (mean age = 14.5 years; SD 2.4) had self-report IPI Scale data. The 59 caregivers who completed the measures about their child’s pain and behavior consisted of 44 mothers (75%), 12 fathers (20%), and 3 other legal guardians (5%). No significant relationships were found between any of the demographic variables (child’s age, sex, parent’s years of education, NF1 type) and the caregivers’ ratings of their child’s pain interference, overall QOL, and three social-emotional subscales, or the disease variables (LogTTB, number of complications). Similar negative findings were found for the child’s self-report except on the Anxiety subscale, which was rated higher for children with familial versus sporadic NF1 (F = 5.81; P < 0.05); however, the mean T-scores of both groups were within normal limits (53.9 vs. 47.8, respectively).
Table I.
Demographic Characteristics of the Total Sample
| Characteristic | Total sample (N = 60) | Adolescents (n = 42) | Children (n = 18) | |||
|---|---|---|---|---|---|---|
| Mean age in years (SD) (range) | 12.7 (3.6) (6.3–18.8) | 14.5 (2.4) (10.6–18.8) | 8.3 (1.5) (6.3–10.6) | |||
| Mean parent education in years (SD) (range) | 14.2 (2.2) (9.0–20.0) | 14.0 (2.3) (9.0–20.0) | 14.5 (2.0) (12.0–18.0) | |||
| Sex | n | % | n | % | n | % |
| Male | 39 | 65% | 27 | 64% | 12 | 67% |
| Female | 21 | 35% | 15 | 36% | 6 | 33% |
| Race | ||||||
| Caucasian | 47 | 78% | 32 | 76% | 15 | 83% |
| African-American | 3 | 5% | 2 | 5% | 1 | 6% |
| Hispanic | 2 | 3% | 2 | 5% | 0 | 0% |
| Other | 8 | 14% | 6 | 14% | 2 | 11% |
| NF1 type | ||||||
| Familial | 28 | 47% | 21 | 50% | 7 | 39% |
| Sporadic | 32 | 53% | 21 | 50% | 11 | 61% |
| NF1 symptom severity ratings | ||||||
| Mild | 18 | 30% | 14 | 33% | 4 | 22% |
| Moderate/severe | 42 | 70% | 28 | 67% | 14 | 78% |
Ratings of Pain Interference
Children of all ages, from 6 to 18 years, had caregiver ratings indicating that pain was interfering with their daily activities to some degree. Seventy-three percent (43 out of 59) of caregivers rated that pain interfered “a little” to “a lot” with their child’s daily functioning in the past month, which was not significantly different between children (83%; 15 out of 18) and adolescents (68%; 28 out of 41) (X2 = ns). By self-report, 59% (24 out of 41) of the adolescents rated having pain that interfered “a little” to “a lot” with their functioning in the past month. Table II presents the breakdown of the caregivers’ and adolescents’ pain interference ratings.
Table II.
Ratings of Pain Interference
| Pain interference rating | Caregiver proxy-report | Self-report | ||||||
|---|---|---|---|---|---|---|---|---|
| Total sample (N = 59) | Adolescents (n = 41) | Children (n = 18) | Adolescents (n = 41) | |||||
| n | % | n | % | n | % | n | % | |
| “None” | 16 | 27% | 13 | 32% | 3 | 17% | 17 | 41% |
| “A little” | 13 | 22% | 7 | 17% | 6 | 33% | 9 | 22% |
| “Some” | 20 | 34% | 13 | 32% | 7 | 39% | 9 | 22% |
| “Pretty much” | 9 | 15% | 7 | 17% | 2 | 11% | 4 | 10% |
| “A lot” | 1 | 2% | 1 | 2% | 0 | 0% | 2 | 5% |
The IPI Scale pain interference item is rated on a 5-point Likert scale from 1 (“none”) to 5 (“a lot”).
When examining the 40 caregiver–adolescent pairs, the caregivers’ ratings of their child’s pain interference (mean = 2.45; SD = 1.2; range = 1–5) did not differ significantly from the adolescents’ self-report ratings (mean = 2.18; SD = 1.2; range = 1–5; F = 2.81; P = 0.10). There also was no significant difference between the caregivers’ ratings of pain interference between the child and adolescent IPI Scale age groups (2.44 vs. 2.42, respectively; F =.01; P = 0.92). When comparing disease severity groups, pain interference was significantly higher in youth with moderate/severe NF1 disease severity compared to those with mild disease severity, by both proxy (2.8 vs. 1.5; F = 21.22; P < 0.0001) and self-report (2.5 vs. 1.4; F = 8.87; P = 0.005).
Pain Medication
Parents reported that 33% (20/60) of all the participants, including 27% of the children (5/18) and 36% of the adolescents (15/42), were taking pain medication on a regular basis. Of those, 10% (n = 2) were taking only over-the-counter (OTC) pain relievers regularly, such as ibuprofen or acetaminophen, while 90% (n = 18) were taking prescription pain medications or a combination of prescription and OTC pain medications. As listed in Table III, a wide variety of prescription pain medications were reported. Despite taking pain medication on a regular basis, pain was rated as interfering with functioning to at least some degree by 93% (14/15) of these adolescents and 100% (20/20) of their caregivers.
Table III.
Pain Medications
| Type of pain medication | Number of patients (N = 60) |
|---|---|
| No regular pain medication | 40 |
| Over-the-counter only (acetaminophen, ibuprofen) | 2 |
| Prescription (with/without OTC medication) | 18 |
| Opioids | |
| Morphine | 1 |
| Tylenol with codeine | 5 |
| Vicodin/hydracodone | 1 |
| Anticonvulsants | |
| Neurontin | 6 |
| Gabapentin | 2 |
| Pregabalin | 1 |
| Tegretol | 1 |
| Topiramate | 1 |
| Antidepressants | |
| Amitriptyline | 4 |
| Rizatriptan | 1 |
| Zolmitriptan | 1 |
| Topical/local anesthetics | |
| Lidocaine patch | 3 |
Seven patients were taking more than one type of prescription pain medication at the same time.
Disease Characteristics
As obtained from the whole body MRI scans, the mean total PN volume was 1,393 ml (SD = 1,919; range 4–12,975) and the mean TTB (expressed as percent of body weight) for the total sample was 3.61% (SD = 4.26; range = 0.01–25.02). After the log transformation, the mean LogTTB was 0.43 (SD = 1.70; range = −4.61 to 3.22). Based on the nurse practitioner’s assessment, the mean number of disease complications was 4.6 (SD 1.6) with a range of 2–9. The most common disease complications besides PNs (100%) were spinal neurofibromas (90%), scoliosis (58%), visual impairments (62%), and headaches (33%).
Quality of Life and Social-Emotional Functioning
In the 40 caregiver-adolescent pairs, the caregivers’ ratings of their children’s overall QOL (mean total IPI score 68.7; SD = 12.7; range = 45.4–92.1) were not significantly different from the with moder ate/severe NF1 symptoms, those with mild NF1 symptoms had higher total IPI scores as rated by both caregivers (n = 59; 64.2 vs. 79.2; F = 29.5, P < 0.0001) and adolescents (n = 41; 65.3 vs. 74.8; F = 7.87, P < 0.01), indicating better overall QOL in children with less severe symptoms.
As rated by caregivers on the BASC-II-P (n 59), the mean T-scores on the depression (55.3; SD 10.7; range = 37–79), anxiety (53.0; SD = 11.1; range 33–86), and withdrawal (53.1; SD 10.5; range = 36–79) subscales were within normal limits. However, 32% of scores on the depression subscale, 20% of scores on the anxiety subscale, and 29% of the scores on the withdrawal subscale were in the at risk/clinically significant (AR/CS) range. As rated by the adolescents (ages 8–18 years; n 47) on the BASC-II-SR, mean T-scores on the depression (49.6; SD = 8.9; range = 40–82), anxiety (50.7; SD = 9.0; range = 34–70), and social stress (48.1; SD = 8.3; range = 34–75) subscales were within normal limits. However, 8.5% of the scores on the depression subscale, 19% of scores on the anxiety subscale, and 8.5% of the scores on the social stress subscale were in the AR/CS range.
Variables Associated With Taking Pain Medication
Participants who took pain medication on a regular basis had significantly higher mean caregiver proxy-report (3.30 vs. 1.97; F = 28.07, P < 0.0001) and adolescent self-report (3.3 vs. 1.5; F = 39.34, P < 0.0001) ratings of pain interference compared to those who did not take such medication regularly. Children taking regular pain medication also had poorer overall QOL (62.0 vs. 72.8; F = 11.77, P < 0.01) and more symptoms of depression (60.3 vs. 52.9, F = 6.29, P < 0.05) and anxiety (57.9 vs. 50.5, F = 6.33, P < 0.05) as rated by caregivers, as well as poorer self-rated QOL (60.2 vs. 75.6; F = 18.79, P < 0.0001), than those not taking pain medication.
When examining disease variables, LogTTB was not significantly different between children taking pain medication regularly and those who were not (.87 vs. 0.21; F = 2.01, P = 0.16). The mean number of disease complications tended to be higher in children taking pain medications but not significantly so (5.1 vs. 4.3; F = 3.35; P = 0.07).
Relationship of Disease Severity and Social-Emotional Functioning to Pain Interference
As shown in Table IV, higher LogTTB, but not the number of disease complications, was significantly related to higher pain interference as rated by caregivers. Neither measure of disease severity was related to the adolescent self-report pain interference ratings. More social-emotional problems in the selected subscales on both the BASC-II-P and BASC-II-SR were significantly associated with greater pain interference.
Table IV.
Correlations Between Measures of Pain Interference, Disease Severity, Social-Emotional Functioning and Overall Quality of Life (QOL)
| Pain Interference | Overall QOLa | |||
|---|---|---|---|---|
| Measures | Caregiver proxy-report | Adolescent self-report | Caregiver proxy-report | Adolescent self-report |
| Pain interferenceb | −0.61**** | −0.68**** | ||
| Disease severityb | ||||
| LogTTB | 0.29* | 0.24 | −0.23 | −0.26 |
| Disease complicationsc | 0.16 | 0.25 | −0.28* | −0.29† |
| Social-emotionalb | ||||
| BASC-II depression | 0.41** | 0.35* | −0.65**** | −0.54*** |
| BASC-II anxiety | 0.44*** | 0.53*** | −0.42*** | −0.61**** |
| BASC-II withdrawal-P or social stress-SR | 0.29* | 0.49** | −0.57**** | −0.65**** |
P; parent; SR; self-report; caregiver BASC-II scores were correlated with IPI Scale parent proxy ratings, and self-report BASC-II scores were correlated with IPI Scale self-report ratings; LogTTB; log transformation of the total PN volume expressed as percentage of body weight.
The pain interference item was deleted from the overall QOL score for these correlations and higher QOL scores mean better functioning.
Higher pain interference, disease severity, and social-emotional scores indicate more problems.
Number of disease complications as rated by the nurse practitioner.
p < .05,
p < .01,
p < .001,
p < .0001,
p = .07.
Relationship of Pain Interference, Disease Variables, and Social-Emotional Functioning to Overall QOL
Also shown in Table IV, less pain interference and better social-emotional functioning in the selected domains were associated with better overall QOL (without the pain interference item) as rated by both the caregivers and adolescents. Fewer NF1 disease complications were significantly related to better proxy-reported QOL while the relation to self-reported QOL was a non-significant trend (r = 0.29, P = 0.07).
Multiple Regression Results
The results of both the caregiver and adolescent multiple regression models examining the extent to which variables of disease severity and social-emotional functioning predict degree of pain interference are presented in Table V. For the caregiver ratings, the model consisting of their child’s overall tumor burden and anxiety was significant (F = 9.15, P < 0.001; Adj R2 = 0.22) and predicted 22% of the variance in pain interference. Both predictor variables accounted for a significant amount of unique variance. For the adolescents, the model including total disease complications and self-reported anxiety predicted self-reported pain interference (F = 7.34, P < 0.01; Adj R2 = 0.26) and accounted for 26% of the variance; only anxiety was a significant predictor.
Table V.
Multiple Regression Analyses for Predicting Pain Interference Scores From Disease Variables and Social-Emotional Measures
| β Weights | Uniqueness indices | |||||
|---|---|---|---|---|---|---|
| Predictor variablesa | β | t | P | Index | F | P |
| Caregiver proxy-report | ||||||
| BASC-II-P Anxiety | 0.41 | 3.50 | <0.001 | 0.17 | 12.26 | <0.001 |
| LogTTB | 0.24 | 2.06 | <0.05 | 0.06 | 4.26 | <0.05 |
| Adolescent self-report | ||||||
| BASC-SR anxiety | 0.49 | 3.25 | <0.01 | 0.22 | 10.53 | <0.01 |
| Disease complications | 0.14 | 0.89 | ns | 0.02 | 0.80 | ns |
P, parent rating of child; SR, self-report rating; LogTTB, log transformation of the total PN volume expressed as percentage of body weight.
Caregiver-rated measures were used to predict caregiver ratings of their child’s QOL, and self-report measures were used to predict self-report ratings of QOL.
The results of both the caregiver and child models examining the extent to which pain interference, disease severity, and social-emotional functioning predict overall QOL are presented in Table VI. For the caregivers’ ratings, the model consisting of their children’s pain interference, disease complications, and depressive symptoms was significant (F = 26.8, P < 0.0001; Adj R2 = 0.57) and predicted 57% of the variance in overall QOL. Each of the predictor variables accounted for a significant amount of unique variance. For the adolescents’ ratings, the model including pain interference, disease complications, and social stress was significant (F = 20.20, P < 0.0001; Adj R2 = 0.62), and predicted 62% of the variance in overall QOL; only self-reported social stress and pain interference accounted for significant amounts of unique variance.
Table VI.
Multiple Regression Analyses for Predicting Overall QOL Scores From Pain Interference, Disease Variables, and Social-Emotional Measures
| β Weights | Uniqueness indices | |||||
|---|---|---|---|---|---|---|
| Predictor variablesa | β | t | P | Index | F | P |
| Caregiver proxy-report | ||||||
| BASC-II-P depression | −0.48 | −5.03 | <0.0001 | 0.19 | 25.35 | <0.001 |
| IPI-P pain interference | −0.39 | −4.04 | <0.001 | 0.12 | 16.34 | <0.001 |
| Disease complicationsb | −0.18 | −2.06 | <0.05 | 0.03 | 4.25 | <0.05 |
| Adolescent self-report | ||||||
| BASC-SR social stress | −0.43 | −3.61 | 0.001 | 0.14 | 13.02 | <0.001 |
| IPI-SR pain interference | −0.42 | −3.43 | <0.01 | 0.13 | 1176 | <0.01 |
| Disease complicationsb | −0.21 | −1.90 | ns | 0.04 | 3.61 | ns |
P, parent rating of child; SR, self-report rating.
Caregiver-rated measures were used to predict caregiver ratings of their child’s QOL, and self-report measures were used to predict self-report ratings of QOL.
Number of disease complications as rated by the nurse practitioner.
Exploratory Testing for Indirect Effects
As shown in Figures 1 and 2, pain interference was significantly associated with overall QOL (path c’) and with proxy-report of depressive symptoms and self-report of social stress (path a). When controlling for pain interference, social-emotional problems were significantly associated with QOL (path b). Furthermore, tests of the indirect effects of pain interference on overall QOL through social-emotional functioning (proxy-rated depression and self-rated social stress) were significant according to the Sobel test (t = −2.1, P < 0.001; t = −2.3, P < 0.05; respectively) and boot strapping analyses (95%CIs: −3.37 to −1.2, estimated effect of depressive symptoms = −2.1; 95%CIs: −3.3 to −0.78, effect stress estimated interference remained of social 1.76), and pain significantly related to QOL (path c’). These results suggest that social-emotional problems partially mediate the effects of pain interference on overall QOL. Thus, children who have greater pain interference have poorer QOL, in part, because of the relationship between social-emotional difficulties and pain interference.
FIG. 1.
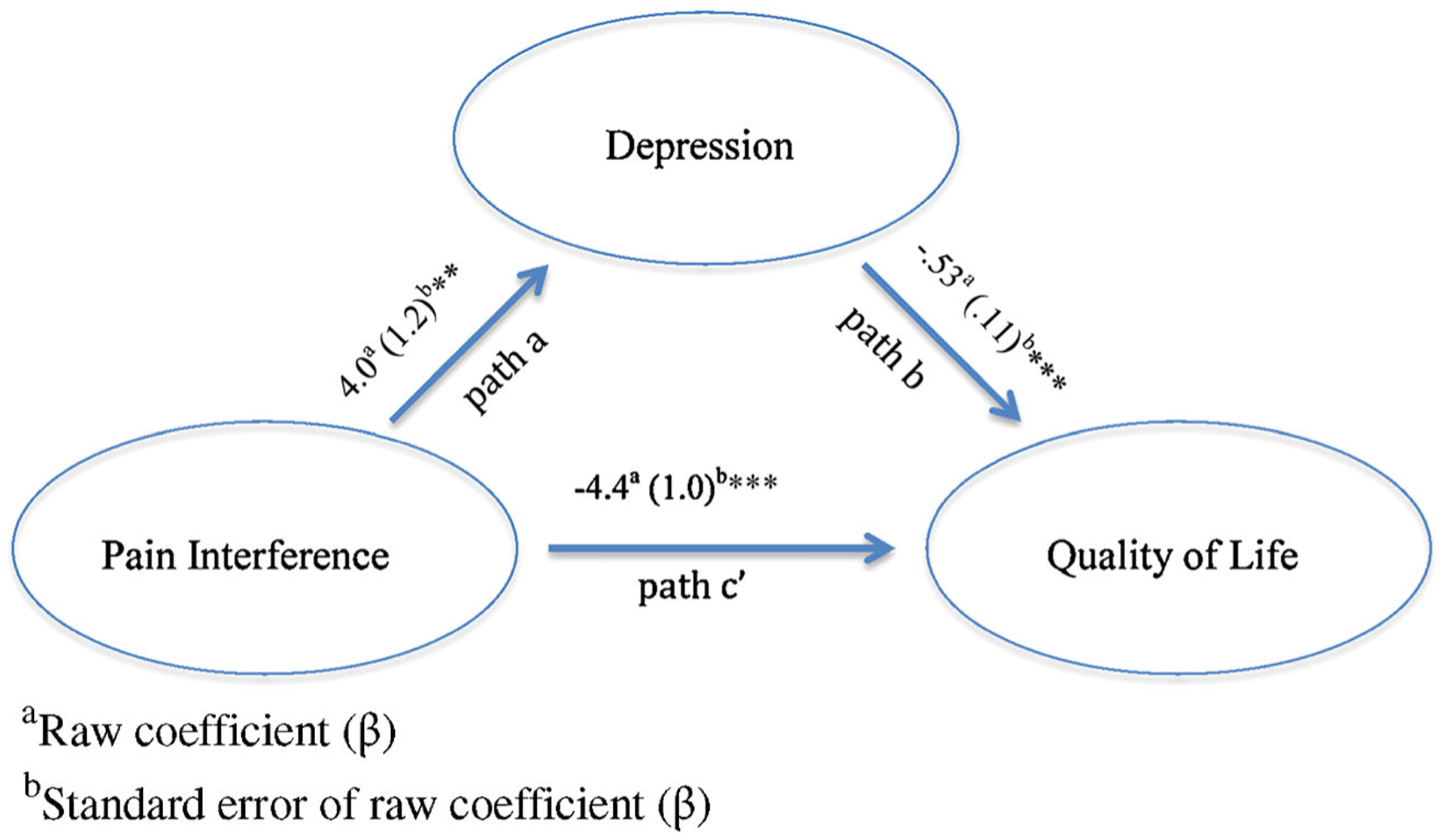
Mediation model of the indirect effects of pain interference on overall QOL via depressive symptoms as rated by caregivers.
FIG. 2.
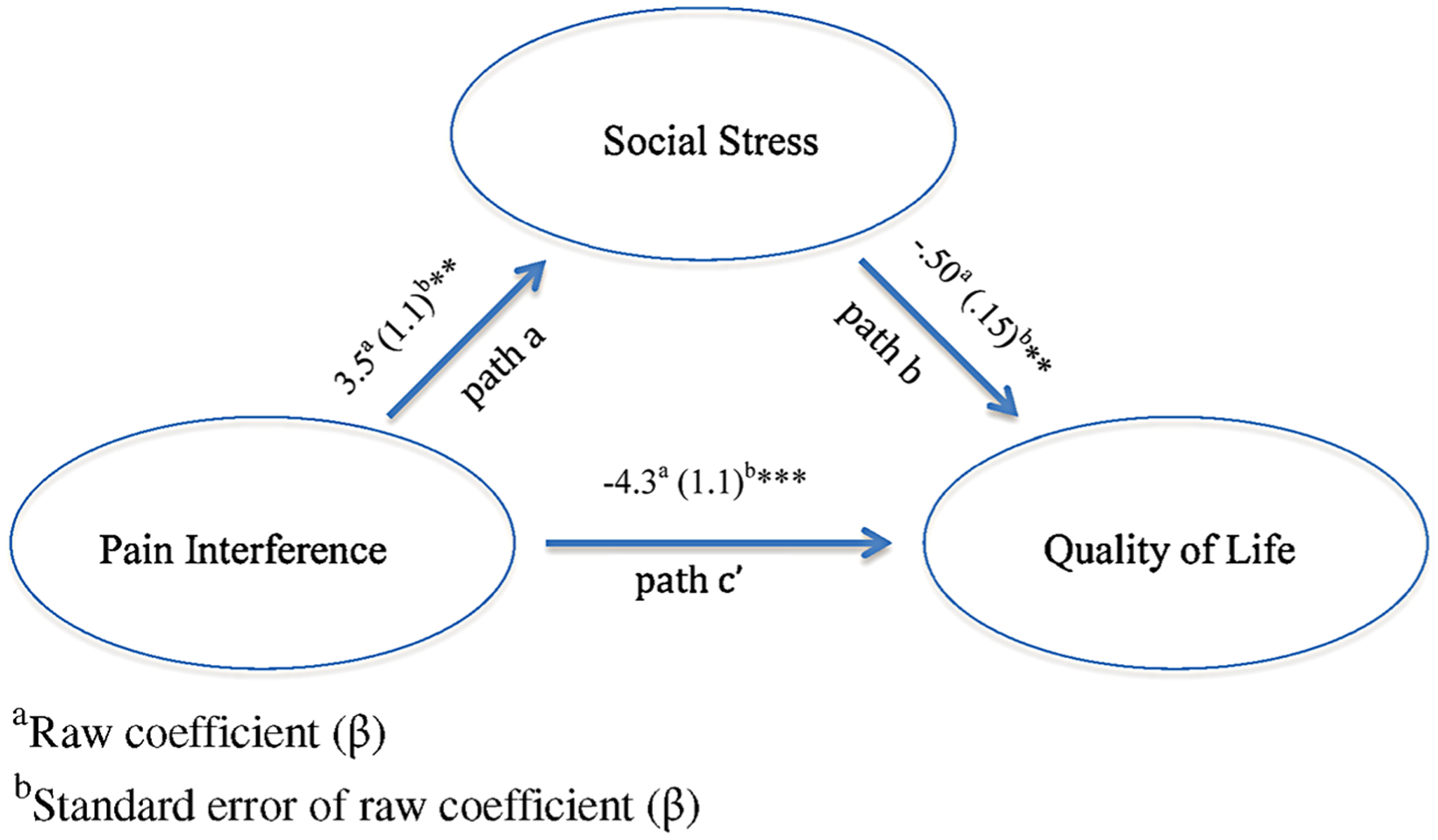
Mediation model of the indirect effects of pain interference on overall QOL via social stress as rated by adolescents.
DISCUSSION
In this sample of youth with NF1 and PNs, ages 6–18 years, a substantial portion (73%) of caregivers indicated that pain interferes with their child’s everyday functioning, ranging from “a little” to “a lot,” while the majority of adolescents (59%) self-rated such levels of pain interference. One third of these youth regularly take pain medications including prescription drugs, yet despite such medication use, almost all of this subset continue to report that pain interferes with everyday functioning. Thus, pharmacologic treatment does not appear to be controlling their pain sufficiently. Furthermore, the children and adolescents regularly taking pain medication have significantly poorer overall QOL and exhibit more symptoms of depression and anxiety, but not significantly greater disease severity, than those not taking pain medication. Thus, managing and coping with pain are critical but unmet needs for these youth. Although other studies have reported the presence of pain in up to 53% of children with NF1 and PNs [Kim et al., 2009; Nguyen et al., 2011], this is the first published study to our knowledge that assessed prospectively the degree of pain interference in this population.
Consistent with our hypothesis and the biopsychosocial model, greater pain interference was associated with a variety of factors including more internalizing problems, such as depression, anxiety, and socialization difficulties, greater PN tumor burden, and poorer overall QOL. Furthermore, social-emotional functioning, particularly anxiety, is a primary predictor of pain interference that accounts for a significant amount unique variance. In addition, pain interference and social-emotional factors are significant predictors of overall QOL; in the caregiver analyses, the number of disease complications also contributes to QOL but to a lesser extent than the other variables. Importantly, social-emotional problems (caregiver-rated depressive symptoms and self-rated social stress) partially mediated the effects of pain interference on overall QOL. Related research in children with other chronic health conditions, such as cystic fibrosis, sickle cell disease, and juvenile idiopathic arthritis, have described similar relationships between pain, social-emotional functioning, and impact on daily living activities [Palermo et al., 2006; Barakat et al., 2008; Connelly et al., 2012]. These studies, as well as the current results, highlight the complex interaction of physical pain and affective states as conceptualized by the biopsychosocial model and supported by neurobiological research [Gatchel et al., 2007; Lumley et al., 2011]. Neuroimaging studies have demonstrated brain pathways that link pain and emotional regulation while an imbalance of various neurotransmitters may contribute to the chronic pain state as well [Gatchel et al., 2007; Garland, 2012]. Importantly, the current results suggest that pain interference as well as social-emotional functioning should be targets for intervention in NF1, both in terms of pharmacologic treatment and psychological therapies to help address concurrent physical and emotional symptoms. The interdependence of these factors indicate that treatment for chronic pain must involve interdisciplinary and multi-modal therapies, including mindfulness-based and other mind-body techniques, in order to be the most effective [Gatchel et al., 2007; Zeidan et al., 2012].
Pain interference in this sample of youth with NF1 and PNs was not significantly affected by age, gender, SES, or familial versus sporadic NF1. While healthy samples have reported a higher prevalence of pain and pain interference in older versus younger adolescents [Roth-Isigkeit et al., 2005] and females [Fouladbakhsh et al., 2012], such age and sex differences in disease-related pain and functional disability typically have not been described in youth with NF1 or other chronic medical conditions [Koh et al., 2005; Kritzberger et al., 2011; Garwood et al., 2012; Lundberg et al., 2012]. Socioeconomic status (SES), measured by years of parental education in the current study, was not related to pain interference. However, higher SES was related to less bodily pain [Krab et al., 2009] and better QOL in various domains [Oostenbrink et al., 2007] in other studies of children with NF1. These discrepant results may be related to the various outcomes assessed and different samples; the current study included only youth with PNs, and the size of the PN tumors was associated with pain interference but not parental level of education (r = −0.12; ns). Studies in children with other medical conditions have found that higher SES is associated with lower pain [King et al., 2011] and better clinical outcomes [Quittner et al., 2010]; thus, SES should be considered in research exploring factors related to such outcomes in NF1 as well. Familial NF1 has been related to better QOL in some domains in children, but not specifically pain [Graf et al., 2006; Oostenbrink et al., 2007; Krab et al., 2009], consistent with the current study that did not find a difference in pain interference between familial and sporadic NF1.
When comparing caregiver and self-reports, there were no significant differences in the ratings of pain interference or overall QOL. However, previous research assessing QOL in pediatric medical conditions consistently have found differences between parent and child reports [Upton et al., 2008; Lundberg et al., 2012]. In NF1 studies, the agreement between parents and their children have ranged from low to high, with the lowest concordance being in the behavioral domains and the highest on the physical domains [Graf et al., 2006; Krab et al., 2009]. It is possible that differences may be found when examining the specific domains of QOL in children with NF1 and PNs, which was not the focus of the current study. In the regression analyses, there were some differences in the variables found to be the primary predictors of the caregivers’ and patients’ data, which supports the use of self-report measures to understand the adolescents’ perceptions and guide treatment planning.
In examining the relationships of pain interference and QOL to disease factors, greater proxy-reported pain interference was related to larger PN tumor volume but not number of disease complications. In contrast, proxy-reported QOL was related to the number of NF1 disease complications but not to tumor burden. Thus, larger PNs may be one of the main disease manifestations contributing to pain that interferes with daily functioning in these youth, while their multiple disease complications have more of an impact on overall QOL. Other reports have found that individuals with PNs report the presence of pain [Citak et al., 2008; Kim et al., 2009; Nguyen et al., 2011] and that higher bodily pain and poorer QOL were related to parent perceptions of the presence of NF1 complications [Graf et al., 2006; Oostenbrink et al., 2007].
Several limitations of this study should be considered when interpreting the results. First, pain interference was assessed using a single item from a general QOL scale for children with chronic illnesses. This question inquires only about the extent to which pain interferes with overall daily functioning and does not assess the impact of pain on specific activities or the frequency and intensity of pain, which would provide a more comprehensive assessment. Furthermore, the response period of the scale was over the past month whereas measures assessing pain during a shorter time period (e.g., in the past week or in real-time) may provide more accurate data. In addition, the study was cross-sectional; thus, the results of the mediational analyses are considered exploratory since longitudinal data are required to make more definitive statements of mediation [Kraemer et al., 2008]. Finally, the generalizability of our findings is limited by our specific patient population: the sample consisted only of youth with PNs, many of whom were referred to the NCI for PN treatment trials and may have larger tumors and more severe complications than other children with NF1.
Despite these limitations, the current study extends the literature in several ways. First, it prospectively assessed the degree of pain interference in children and adolescents with NF1 and PNs to provide initial yet important information about the impact of pain on their everyday functioning. In addition, it used quantitative methods of disease severity and specific measures of social-emotional functioning to investigate the relationships of these factors with pain interference and QOL. This study indicated that social-emotional problems are unique predictors of pain interference and QOL, and partially mediate the relationship between these variables; thus, interventions for these youth should teach strategies to increase emotional awareness and improve their affective states in conjunction with medical therapies. Importantly, the current study applied theoretical and mediational models to begin to explain and organize the multiple factors that may influence the experience and impact of pain in NF1. The findings support the use of the biopsychosocial model to design future research and guide the treatment of pain in this population.
It is recommended that future studies continue to assess various aspects of pain in NF1, including location, source, frequency, triggers, and pain quality, which will help to guide treatment efforts. It will also be useful to investigate whether pain in this population is associated with disturbances in specific activities of daily living, such as school attendance [Dick and Pillai, 2010], participation in hobbies [Roth-Isigkeit et al., 2005], and sleep [Roth-Isigkeit et al., 2005; Citak et al., 2008]. In addition, studies are needed to understand the contributions of other variables in the biopsychosocial model, such as environmental stress, family functioning, coping styles, and cognitive processes, which may impact pain interference and QOL in medically ill children [Gatchel et al., 2007; Barakat et al., 2008; Lewandowski et al., 2010]. Finally, longitudinal studies are required to reveal how these biopsychosocial variables change over time in NF1 and determine how they interact with one another and influence outcomes throughout development using mediational analyses [Kraemer et al., 2008; Nieto et al., 2012]. Most importantly, psychological interventions should be developed and evaluated for children and adolescents affected by NF1 and PNs that will address their social-emotional difficulties and help them cope with their pain as a means to improve their QOL.
CONCLUSIONS
Pain interferes with the daily functioning of the majority of children and adolescents with NF1 and PNs, including those regularly taking pain medication. Total tumor volume and social-emotional problems predict pain interference while disease complications, pain interference, and social-emotional problems predict overall QOL. These results highlight the complex interactions between the physical manifestations of pain and psychological functioning in this population and target areas for future research and interventions that apply the biopsychosocial model. The development and investigation of therapeutic interventions that utilize a multidisciplinary approach and include psychological interventions need to be a priority for youth with NF1 and PNs.
ACKNOWLEDGMENTS
The authors thank the children and caregivers who participated in this study. We also thank all members of the Pediatric Oncology Branch Psychology Group and NF Team for their assistance with the data collection and care of patients on the NF1 Natural History study. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. In addition, this project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- Ablon J 1996. Gender response to neurofibromatosis 1. Soc Sci Med 42:99–109. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Patterson CA, Daniel LC, Dampier C. 2008. Quality of life among adolescents with sickle cell disease: mediation of pain by internalizing symptoms and parenting stress. Health Qual Life Outcomes 6:60. doi: 10.1186/1477-7525-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B, North K. 2004. Social skills of children with neurofibromatosis type 1. Dev Med Child Neurol 46:553–563. [DOI] [PubMed] [Google Scholar]
- Citak EC, Oguz A, Karadeniz C, Okur A, Memis L, Boyunaga O. 2008. Management of plexiform neurofibroma with interferon alpha. Pediatr Hematol Oncol 25:673–678. [DOI] [PubMed] [Google Scholar]
- Connelly M, Bromberg MH, Anthony KK, Gil KM, Franks L, Schanberg LE. 2012. Emotion regulation predicts pain and functioning in children with juvenile idiopathic arthritis: An electronic diary study. J Pediatr Psychol 37:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creange A, Zeller J, Rostaing-Rigattieri S, Brugieres P, Degos JD, Revuz J, Wolkenstein P. 1999. Neurological complications of neurofibromatosis type 1 in adulthood. Brain 122:473–481. [DOI] [PubMed] [Google Scholar]
- Dick BD, Pillai Riddell R. 2010. Cognitive and school functioning in children and adolescents with chronic pain: A critical review. Pain Res Manag 15:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts CV, Carey JC, Kircher JC, Hoffman RO, Creel D, Ward K, Clark E, Leonard CO. 1996. Children and adolescents with neurofibromatosis 1: A behavioral phenotype. J Dev Behav Pediatr 17:229–239. [PubMed] [Google Scholar]
- DiMario FJ, Langshur S. 2000. Headaches in patients with neurofibromatosis-1. J Child Neurol 15:235–238. [DOI] [PubMed] [Google Scholar]
- Dombi E, Solomon J, Gillespie AJ, Fox E, Balis FM, Patronas N, Korf BR, Babovic-Vuksanovic D, Packer RJ, Belasco J, Goldman S, Jakacki R, Kieran M, Steinberg SM, Widemann BC. 2007. NF1 plexiform neurofibroma growth rate by volumetric MRI: Relation to age and body weight. Neurology 68:643–647. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Kolanczyk M, Schindeler A, Viskochil DH, Hock JM, Schorry EK, Crawford AH, Friedman JM, Little D, Peltonen J, Carey JC, Feldman D, Yu X, Armstrong L, Birch P, Kendler DL, Mundlos S, Yang FC, Agiostratidou G, Hunter-Schaedle K, Stevenson DA. 2009. Skeletal abnormalities in neurofibromatosis type 1: Approaches to therapeutic options. Am J Med Genet A 149A:2327–2338. [DOI] [PubMed] [Google Scholar]
- Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, Towers R, Gleeson M, Steiger C, Kirby A. 2007. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet 44:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladbakhsh JM, Vallerand AH, Jenuwine ES. 2012. Self-treatment of pain among adolescents in an urban community. Pain Manag Nurs 13:80–93. [DOI] [PubMed] [Google Scholar]
- Friedman JM. 1999. Epidemiology of neurofibromatosis type 1. Am J Med Genet 89:1–6. [PubMed] [Google Scholar]
- Garland EL. 2012. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Prim Care 39:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood MM, Bernacki JM, Fine KM, Hainsworth KR, Davies WH, Klein-Tasman BP. 2012. Physical, cognitive, and psychosocial predictors of functional disability and health-related quality of life in adolescents with neurofibromatosis-1. Pain Res Treat 2012:975364.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel RJ, Bo Pen Y, Peters ML, Fuchs PN, Turk DC. 2007. The biopsychosocial approach to chronic pain: Scientive advances and future directions. Psychol Bull 133:581–624. [DOI] [PubMed] [Google Scholar]
- Graf A, Landolt MA, Mori AC, Boltshauser E. 2006. Quality of life and psychological adjustment in children and adolescents with neurofibromatosis type 1. J Pediatr 149:348–353. [DOI] [PubMed] [Google Scholar]
- Hayes AF. 2009. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr 76:408–420. [Google Scholar]
- Hofman KJ, Harris EL, Bryan RN, Denckla MB. 1994. Neurofibromatosis type 1: The cognitive phenotype. J Pediatr 124:S1–S8. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. 2005. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65:1037–1044. [DOI] [PubMed] [Google Scholar]
- Johnson NS, Saal HM, Lovell AM, Schorry EK. 1999. Social and emotional problems in children with neurofibromatosis type 1: Evidence and proposed interventions. J Pediatr 134:767–772. [DOI] [PubMed] [Google Scholar]
- Kim A, Gillespie A, Dombi E, Goodwin A, Goodspeed W, Fox E, Balis FM, Widemann BC. 2009. Characteristics of children enrolled in treatment trials for NF1-relatedplexiformneurofibromas.Neurology 73:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. 2011. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 152:2729–2738. [DOI] [PubMed] [Google Scholar]
- Koh JL, Harrison D, Palermo TM, Turner H, McGraw T. 2005. Assessment of acute and chronic pain symptoms in children with cystic fibrosis. Pediatr Pulmonol 40:330–335. [DOI] [PubMed] [Google Scholar]
- Korf BR. 1999. Plexiform neurofibromas. Am J Med Genet 89:31–37. [DOI] [PubMed] [Google Scholar]
- Koth CW, Cutting LE, Denckla MB. 2000. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol 6:185–194. [DOI] [PubMed] [Google Scholar]
- Krab LC, Oostenbrink R, De Goede-Bolder A, Aarsen FK, Elgersma Y, Moll HA. 2009. Health-related quality of life in children with neurofibromatosis type 1: Contribution of demographic factors, disease-related factors, and behavior. J Pediatr 154:420–425. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. 2008. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol 27:S101–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzberger CJ, Antiel RM, Wallace DP, Zacharia JD, Brands CK, Fischer PR, Harbeck-Weber C. 2011. Functional disability in adolescents with orthostatic intolerance and chronic pain. J Child Neurol 26:593–598. [DOI] [PubMed] [Google Scholar]
- Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. 2010. Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain 11:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart G, MacKinnon DP, Ohlrich V. 2011. Mediation analysis in psychosomatic medicine research. Psychosom Med 73:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. 2011. Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol 67:942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg V, Lindh V, Eriksson C, Petersen S, Eurenius E. 2012. Health-related quality of life in girls and boys with juvenile idiopathic arthritis: Self- and parental reports in a cross-sectional study. Pediatr Rheumatol Online J 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Wolters P, Baldwin A, Gillespie A, Dombi E, Walker K, Widemann B. 2012. Social-emotional functioning of children and adolescents with neurofibromatosis type 1 and plexiform neurofibromas: Relationships with cognitive, disease, and environmental variables. J Pediatr Psychol 37:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro J, Raichle KA, Carter GT, O’Brien SA, Abresch RT, McDonald CM, Jensen MP. 2009. Impact of biopsychosocial factors on chronic pain in persons with myotonic and facioscapulohumeral muscular dystrophy. Am J Hosp Palliat Care 26:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needle MN, Cnaan A, Dattilo J, Chatten J, Phillips PC, Shochat S, Sutton LN, Vaughan SN, Zackai EH, Zhao H, Molloy PT. 1997. Prognostic signs in the surgical management of plexiform neurofibroma: The Children’s Hospital of Philadelphia experience, 1974–1994. J Pediatr 131:678–682. [DOI] [PubMed] [Google Scholar]
- Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. 2011. Plexiform neurofibromas in children with neurofibromatosis type 1: Frequency and associated clinical deficits. J Pediatr 159:652–655, e652. [DOI] [PubMed] [Google Scholar]
- Nieto R, Raichle KA, Jensen MP, Miro J. 2012. Changes in pain-related beliefs, coping, and catastrophizing predict changes in pain intensity, pain interference, and psychological functioning in individuals with myotonic muscular dystrophy and facioscapulohumeral dystrophy. Clin J Pain 28:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll RB, Reiter-Purtill J, Moore BD, Schorry EK, Lovell AM, Vannatta K, Gerhardt CA. 2007. Social, emotional, and behavioral functioning of children with NF1. Am J Med Genet A 143A:2261–2273. [DOI] [PubMed] [Google Scholar]
- Oostenbrink R, Spong K, de Goede-Bolder A, Landgraf JM, Raat H, Moll HA. 2007. Parental reports of health-related quality of life in young children with neurofibromatosis type 1: Influence of condition specific determinants. J Pediatr 151:182–186, 186 e181–182. [DOI] [PubMed] [Google Scholar]
- Page PZ, Page GP, Ecosse E, Korf BR, Leplege A, Wolkenstein P. 2006. Impact of neurofibromatosis 1 on Quality of Life: A cross-sectional study of 176 American cases. Am J Med Genet A 140A:1893–1898. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Harrison D, Koh JL. 2006. Effect of disease-related pain on the health-related quality of life of children and adolescents with cystic fibrosis. Clin J Pain 22:532–537. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. 2010. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest 137: 642–650. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. 2004. Behavior assessment system for children, 2nd edition. Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. 2005. Pain among children and adolescents: Restrictions in daily living and triggering factors. Pediatrics 115:e152–e162. [DOI] [PubMed] [Google Scholar]
- Solomon J, Warren K, Dombi E, Patronas N, Widemann BC. 2004. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph 28:257–265. [DOI] [PubMed] [Google Scholar]
- Stumpf DA, Alksne JF, Annegers JF, Brown SS, Conneally M, Housman D, Leppert MF, Miller JP, Moss ML, Pileggi AJ, Rapin I, Strohman RC, Swanson LW, Zimmerman A. 1988. Neurofibromatosis conference statement. National Institutes of Health Consensus Development Conference Bethesda, MD. Arch Neurol 45:575–578. [PubMed] [Google Scholar]
- Upton P, Lawford J, Eiser C. 2008. Parent-child agreement across child health-related quality of life instruments: A review of the literature. Qual Life Res 17:895–913. [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplege A. 2001. Quality-of-life impairment in neurofibromatosis type 1. Arch Dermatol 137:1421–1425. [DOI] [PubMed] [Google Scholar]
- Wolters PL, Martin S, Toledo-Tamula MA, Gillespie A, Baldwin A, Widemann B. 2013. Quality of life of adolescents with neurofibromatosis type 1 (NF1) and plexiform neurofibromas (PNs): Reliability and validity of the Impact of Pediatric Illness (IPI) Scale self-report form. The National Conference in Pediatric Psychology, New Orleans, LA. [Google Scholar]
- Wolters PL, Martin S, Walker K, Widemann BC. 2010. Impact of Illness (IPI) Scale parent form: Validity and reliability data for children with neurofibromatosis type 1 (NF1) and plexiform neurofibromas (PNs). Children’s Tumor Foundation NF Conference, Baltimore, MD. [Google Scholar]
- Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. 2012. Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 520:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]


