Abstract
Antibiotic and herbicide resistance genes are the most common marker genes for plant transformation to improve crop yield and food quality. However, there is public concern about the use of resistance marker genes in food crops due to the risk of potential gene flow from transgenic plants to compatible weedy relatives, leading to the possible development of “superweeds” and antibiotic resistance. Several selectable marker genes such as aph, nptII, aaC3, aadA, pat, bar, epsp and gat, which have been synthesized to generate transgenic plants by genetic transformation, have shown some limitations. These marker genes, which confer antibiotic or herbicide resistance and are introduced into crops along with economically valuable genes, have three main problems: selective agents have negative effects on plant cell proliferation and differentiation, uncertainty about the environmental effects of many selectable marker genes, and difficulty in performing recurrent transformations with the same selectable marker to pyramid desired genes. Recently, a simple, novel, and affordable method was presented for plant cells to convert non-metabolizable phosphite (Phi) to an important phosphate (Pi) for developing cells by gene expression encoding a phosphite oxidoreductase (PTXD) enzyme. The ptxD gene, in combination with a selection medium containing Phi as the sole phosphorus (P) source, can serve as an effective and efficient system for selecting transformed cells. The selection system adds nutrients to transgenic plants without potential risks to the environment. The ptxD/Phi system has been shown to be a promising transgenic selection system with several advantages in cost and safety compared to other antibiotic-based selection systems. In this review, we have summarized the development of selection markers for genetic transformation and the potential use of the ptxD/Phi scheme as an alternative selection marker system to minimize the future use of antibiotic and herbicide marker genes.
Keywords: Genetic transformation, ptxD/Phi selection system, Selectable marker gene, Biosafety
Introduction
In genetic transformation, cells undergo a transformation treatment to introduce a foreign (exogenous) gene into the host genome. Transformation events are extremely low, so it is important to use efficient selectable marker genes along with the foreign gene of interest, which encode proteins that confer selection gain to transformed cells (Rosellini, 2012; Yang, Peng & Pan, 2019). A selectable marker gene has the properties for maximum selection of independent transformation events, minimum non-transformers or outliers, and easy detection of the marker gene and wide application in the plant species (Penna & Ganapathi, 2010). Usually, the selectable marker gene is positioned in the DNA vector construct along with the desired gene. In plant transformation, dominant selectable marker genes are developed based on genes that confer resistance to antibiotics or herbicides or have the ability to digest non-metabolizable substances (Miki & McHugh, 2004; Ravanfar et al., 2017). Resistance genes for antibiotics (hygromycin and kanamycin) and herbicides (bialaphos, glyphosate and the bar) have been used extensively as selection tools (Pandeya et al., 2018). However, many plant species are naturally resistant to antibiotics; for example, orchids are naturally resistant to antibiotics (Setiari et al., 2018). The sensitivity of various plant species to herbicides such as bialaphos and glyphosate cannot be overstated. Many commercially available genetically modified (GM) crops are bialaphos resistant (LibertyLink®), glyphosate resistant (Roundup Ready®), or have bar genes that confer resistance to the herbicide Basta (Breyer, Kopertekh & Reheul, 2014; Brown & Wernegreen, 2019). Herbicide-resistant genes show higher efficacy in performing plant transformation, but their use is limited by intellectual property restrictions and public perception (Nahampun et al., 2016). According to Ramessar et al. (2007) and (Molina-Márquez et al., 2019), there is a possibility of horizontal gene transfer associated with herbicide resistance traits in plant transformation processes, which draws the attention of researchers to search for an alternative selectable marker gene system. Public concern about herbicide-resistant markers, i.e., bar genes, is based on pollen flows from transgenic crops to compatible wild relatives, leading to the emergence of superweeds (Lal et al., 2020; Miki & McHugh, 2004; Pontiroli et al., 2007).
The use of antibiotic-resistant marker genes has been rejected by European Union (EU) and some other countries in both Asia and Africa because of the potential risk of horizontal gene transfer from plants to soil and gut microbes, which would lead to a large unintended spread of antibiotic-resistant genes (Pandeya et al., 2017). As a result, only a few thousand hectares (∼0.03% of world production) of genetically modified crops are grown in the EU (Brandt, 2003), likely reflecting European resistance to the technology. In contrast, food derived from GM crops is ubiquitous in the United States of America. In contrast, most animal feed used in Europe comes from imported plant material containing GM products. Similarly, GM cotton is used extensively as a raw material for the manufacture of clothing and related products (Key, Ma & Drake, 2008). Penna, Sági & Swennen (2002) pointed out that a major disadvantage of antibiotic resistant genes is that the selection scheme is based on the principle of negative selection, i.e., all non-transformed cells, which are much more numerous than the transformed cells, are successfully killed by the selection agent. Consequently, the majority of transformed cells cannot regenerate because dying or dead untransformed cells release growth inhibitors and lethal substances that interfere with the uptake of important elements from the growth medium by transformed cells. It has also been observed that some selection systems are more efficient than others for certain plant species and regeneration systems, simply because plants are sensitive to selection agents that are largely variable between plant species and tissues due to selection pressure (López-Arredondo & Herrera-Estrella, 2013; Wang et al., 2020). Therefore, an ideal marker system is needed that allows resistance to a lethal substance and, at the same time, can be converted into a substance essential for optimal growth and differentiation of cells in many plant species. The selection system allows to minimize or eliminate the risk of negative selection, as well as to recover false-positive clones that have escaped the selection system. In this scenario, it is important to design innovative and universally acceptable marker gene systems with higher transformation frequency. This can be achieved by stacking desirable genes to minimize both negative selection and the number of false-positive clones (Eckerstorfer et al., 2019; Pandeya et al., 2017).
The ptxD gene from Pseudomonas stutzeri WM88 encodes a NAD-dependent phosphite oxidoreductase (PTXD) (Costas, White & Metcalf, 2001). This enzyme is capable of catalyzing the oxidation of phosphite (Phi) to phosphate (Pi) and, in combination with Phi selection, offers the best option of a selection scheme for producing transgenic plants without the use of antibiotic- or herbicide-resistant genes, thus providing an innovative non-herbicidal mechanism for weed control (Changko et al., 2020; López-Arredondo & Herrera-Estrella, 2012; López-Arredondo & Herrera-Estrella, 2013). All plant cells require phosphorus (P) for reproduction, and cells harboring the ptxD are able to convert a nonmetabolizable P source (Phi) into a form of the compound (Pi) that they can readily incorporate into their metabolism. Selection of these cells should be easy over non-transformed cells that are unable to assimilate Phi as a P source. This unique property suggests the ptxD/Phi system as a possible general selection scheme to obtain efficient transgenic plants (González-Morales et al., 2020; López-Arredondo & Herrera-Estrella, 2013). In recent years, the ptxD/Phi system has been described as an effective selection marker in some important model organisms and crops such as Arabidopsis and tobacco (López-Arredondo & Herrera-Estrella, 2013), yeast (Kanda et al., 2014), sorghum (Che et al., 2018), cotton (Pandeya et al., 2017), rice (Manna et al., 2016), and maize Nahampun et al., 2016). It has been argued that this selection system is more beneficial than existing antibiotic and herbicide selection markers due to cost-effectiveness, efficiency and safety. This review summarizes the need for selectable marker genes for genetic transformation and the potential use of ptxD/Phi as an alternative selectable system to reduce the use of antibiotic and herbicide selectable marker genes in the future.
Survey Methodology
We focused on developments in the link between biosafety concerns and genetically modified crops. We conducted a literature search using PubMed (https://www.ncbi.nlm.nih.gov/PubMed), Google scholar (scholar.google.cn), and Science web (http://www.webofknowledge.com). Keywords such as genetic engineering, gene transformation, ptxD/Phi selection system, selectable marker genes and non-selectable marker genes, food safety and biosafety concerns, effects of phosphite and phosphate on weeds and plant growth were used while related articles were extracted to categorize and summarize the potential effects of genetically modified crops on living organisms and their environment. Based on the checklist and database of PRISMA guidelines for systematic reviews (Liberati et al., 2009), among others, three hundred literature sources were screened as appropriate data sources after an initial data search, identification and removal of duplicates. Included in this review study were publications comprising 171 reviews, 85 research articles and 44 book titles and conference proceedings. The search was further restricted to references that included molecular breeding, molecular genetics, biotechnology, plant science, and agronomy. The main focus was on concerns about the biosafety of genetically modified (GM) crops, the use of novel molecular markers and alternative selectable markers for genetic transformation to produce GM crops. The GM crops may be better able to withstand biotic and abiotic stresses and also ensure food security and a safe environment.
Need for marker gene selectable systems
Despite advances in gene transfer technology, transformation efficiency is very low, so that usually only a small proportion of transformed cells carry foreign DNA. Therefore, it is recommended to perform selection among the transformed cells or tissues from many non-transformed cells to regenerate genetically transformed plants (Penna & Ganapathi, 2010; Uddain & Subramaniam, 2020). Selectable marker genes are mainly genes used to recognize the transformed tissues or cells. These marker genes display a feature suitable for artificial selection of transformed tissues over non-transformed ones in a medium (Jekayinoluwa et al., 2020). Selectable marker genes are classified into many categories as they confer either positive or negative selection and selection is conditional or nonconditional depending on the presence of external substrates (Esland et al., 2018). According to Sundar & Sakthivel (2008), positive selection markers are used for genetic transformation to allow only transgenic cells to grow and develop and are necessary for efficient transformation, while negative selection marker genes lead to the death of transformed tissue. Depending on the functions of positive selection schemes, they can be categorized as conditional and non-conditional positive selection schemes (Esland et al., 2018). In conditional positive selection, a gene encodes a specific protein that confers resistance to a particular substrate that can be lethal to non-transformed plant cells or that only allows the development and differentiation of transformed tissues (García-Almodóvar et al., 2014). The conditional positive selection scheme includes antibiotics, herbicides, toxic and non-toxic chemicals, or a carbon source.
On the other hand, nonconditional positive selection schemes do not require external substrates but promote developmental selection and differentiation of transformed cells (Miki & McHugh, 2004; Rosellini, 2012). A typical example is the ipt gene, which promotes shoot growth by endogenously altering hormone levels in the plant. Babwah & Waddell (2000) and González-Morales et al. (2020) stated that once the selection technique does not depend on the substrate, it is called a nonconditional negative selection system. For example, the manifestation of a lethal protein such as a ribonuclease to remove certain cell types (Ma, 2005). When toxic gene activity requires a substrate to show toxicity, the method is called a conditional negative system (Babwah & Waddell, 2000; Wu et al., 2019a). Some examples of widely used positive selectable marker genes are phosphomannose isomerase (manA) and xylose isomerase (xylA) (Penna, Sági & Swennen, 2002; Zhang et al., 2019). Since these selectable marker genes regularly alter cell division and differentiation, there is a significant change in the development, morphology, and physiology of the transgenic plant (Table 1). Therefore, various tactics are necessary to reduce marker expression by using derivable promoters or generating plants without markers (Pandey et al., 2020; Penna, Sági & Swennen, 2002). The production of transgenic plants takes a long time, is labor intensive, expensive and obviously not an efficient process (Miki & McHugh, 2004; Tanner et al., 2020). This is the situation when dealing with important agricultural crops. The use of selectable marker genes speeds up the techniques of transformation and allows relatively early recovery of transgenic events (Lepais et al., 2020; Stoger et al., 2002). Selectable and visible marker reporter genes have little effect on the desired trait and also provide a valuable tool to determine the performance of transformed cells for the desired gene (Srivastava, 2019; Tuteja et al., 2012).
Table 1. Selectable markers for crop transformation.
Some markers that have been used for GM crops over the years are summarized in this review. Details comprise the conferred phenotype, and examples of organisms for which the use of the marker has been described.
| Gene | Phenotype | Organism | Reference |
|---|---|---|---|
| merA | Mercuric chloride | Peanut | Yang et al. (2003) |
| pflp | Erwinia chloride | Sweet pepper | You et al. (2003) |
| atlD | Arabital | Rice | LaFayette et al. (2005) |
| OASAID | 5-methyltryptophan (5-MT) | Rice | LaFayette et al. (2005) |
| xylA | Xylose | Maize | Guo et al. (2007) |
| ALS | Bispyribac sodium | Wheat | Ogawa et al. (2008) |
| dhlA | 1,2-dichloroethene | Rice | Moore & Srivastava (2008) |
| ALS | Bispyribac sodium | Soybean | Tougou et al. (2009) |
| pmi | Mannose | Sorghum | Gurel et al. (2009) |
| pmi | Mannose | Lettuce | Briza et al. (2010) |
| aadA | Streptomycin & Stectinomycin | Egg plant | Singh et al. (2011) |
| dadA | D-serine | Maize | Lai et al. (2011) |
| tflA | Toxoflavin | Rice | Koh et al. (2011) |
| Bar | Phosphinothricin | Soybean | Li et al. (2017) |
| Epsps | Hygromycin B phosphotransferase | Potato | Bakhsh et al. (2020) |
| nptII | Kanamycin | Potato | Bakhsh et al. (2020) |
Non-selectable marker genes in plants transformation
Non-selectable marker genes, also called reporter genes, encode molecules that are available visually or through biochemical controls and promote information about the cells or tissues that translate the inserted protein (Breyer, Kopertekh & Reheul, 2014; Olorunniji, Rosser & Stark, 2016). These genes are often used as “reporters” for gene expression by linking to other genes or promoters in GM products to express in the same manner as the linked gene or promoter (Lanigan, Kopera & Saunders, 2020; Miki & McHugh, 2004). They contain nontoxic proteins in plant materials and promote their physical assembly or controlled plant expression. The reporter genes uidA (gus) and gfp are commonly used to detect the activity of even a weak promoter (Cerezo et al., 2019). The uidA (gusA, or gus) gene is derived from E. coli, which converts the enzyme β-glucuronidase as a carbon and energy source (Elder et al., 2018; Porto et al., 2014). Expression of GUS from the inserted uidA gene can be detected in GM plant material using a GUS enzyme that produces a colored product when cleaved by GUS (Neumann, Kumar & Imani, 2020; Shrawat & Armstrong, 2018). The use of different growth media does not allow the sizing of the amount of protein available nor the visualization of the form of expression or distribution in the plant material (Banchi et al., 2019; Porto et al., 2014). The uidA gene with its associated protein is found in a wide variety of organisms, including E. coli and several other microbes, including other microorganisms of the digestive tract and soil bacteria (Porto et al., 2014; Riva et al., 2020). The work of GUS is common in all vertebrate tissues, with high activity in the kidney, liver and spleen (Xu et al., 2020). In turn, the activity of GUS is found in invertebrates such as mollusks, insects, and nematodes (Christiaens et al., 2018; Kumar et al., 2012). Moreover, low activity of GUS has been reported in more than forty different plant species and human food sources such as carrots, tomatoes and parsley etc (Mortensen et al., 2019). The gfp gene originates from the jellyfish (Aequorea victoria), which encodes green fluorescent protein (GFP) (Keutgen, Tomaszewska-Sowa & Keutgen, 2020) and emits a green light when exposed to blue or ultraviolet light. Zimmer (2002) revealed the main contribution of GFP helpers in bioluminescence of jellyfish. The green fluorescent protein is required as a gene expression marker for both GM animal and plant cells (Hoffman, 2015). Its expression in living tissue can be visualized by irradiation with ultraviolet or blue light without damaging the tissue. This allows observation of the intracellular location and mobility of related proteins in living cells (Franco-Bocanegra et al., 2019; Hanson & Köhler, 2001). Spontaneous modifications of the GFP gene sequence led to the emergence of a large number of variants with valuable properties (Su et al., 2019; Zimmer, 2002).
Marker-free strategies or systems
Transformation without selectable marker genes (SMGs) is a suitable approach to obtain marker-free transgenic plants that increase consumer acceptance (Breyer, Kopertekh & Reheul, 2014). The development of GM plants without the use of SMGs has been reported in; Arabidopsis, barley, cassava, lime, potato, groundnut, tobacco, triticale (Chong-Pérez & Angenon, 2013; Manimaran et al., 2011; Schaart et al., 2011), alfalfa (Ferradini et al., 2011), apple (Malnoy et al., 2010), Prunus (Petri et al., 2011), orange (Ballester, Cervera & Pena, 2010), tomato (Xin & Guo, 2012) and wheat (Liu et al., 2011), (Table 2). Polymerase Chain Reaction (PCR) has been used in most cases to study the putative transformed events to detect the transgene (Breyer, Kopertekh & Reheul, 2014). Transformants selection can also be enhanced by the expression of a screenable marker: the uidA (GUS) or GFP gene, which makes the putative transformants a phenotypic asset associated with the gene expression of interest (GOI) (Bai et al., 2009), or it can be a direct screening of a product for the desired gene expression (Rukavtsova et al., 2013). Most studies on marker-free systems of experimental refinements have been used to improve some crucial factors, including the efficacy of DNA delivery technique and plant regeneration mechanism, for successful implementation of this process (Breyer, Kopertekh & Reheul, 2014). The refinements consist of improving treatment conditions to promote Agrobacterium-mediated transformation, e.g., vortex-mediated transformation of cold-treated seedlings (Rosellini & Veronesi, 2007; Weeks, Ye & Rommens, 2008), using A. tumefaciens strains that exhibit extremely high transformation efficiency (De Vetten et al. 2003). Most of these refinements take advantage of recent advances in identifying plant proteins and other factors within the transformation process and characterizing the molecular mechanism for successful T-DNA incorporation Anand, Vaghchhipawala & Mysore (2010); Barampuram & Zhang (2011).
Table 2. Examples of marker free crop plants detailing molecular techniques employed, marker genes used and crop species transformed are summarized in this review.
| Molecular techniques | Marker genes | Crop species | References |
|---|---|---|---|
| Co-transformation | Hpt and uidA | Wheat | Permingeat et al. (2003) |
| Inducible site-specific recombination sys. | Hpt, npt, codA, GUS | Strawberry | Schaart et al. (2004) |
| Two-border binary vector | Epsps-cp4, Epsp | Maize | Huang et al. (2004) |
| Co-transformation | Psy & phytoene | Rice | Parkhi et al. (2005) |
| R/Rs recombination system | CodA-npt II | Potato | Kondrak et al. (2006) |
| Cre/lox site specific recombination | GUS, npt II & gft | Tobacco | Jia et al. (2006) |
| Cre/lox P | Hpt, GUS & Gat | Soybean | Li et al. (2007) |
| Marker-free binary vector | Ipt | Potato | Bukovinszki et al. (2007) |
| MAT system | Ipt & npt II | Cassava | Saelim et al. (2009) |
| Marker-free binary vectors | Zmpsy or chitinase | Peanut | Bhatnagar et al. (2010) |
| Co-bombardment | Cry1B-Aa & hpt | Rice | Kumar, Arul & Talwar (2010) |
| MAT system | Ipt & wd | Rice | Khan et al. (2011) |
| Co-transformation | chi II, ap24, GUS & hpt | Rice | Rao et al. (2011) |
| Marker-free binary vector | At-CBF1 & npt II | Tomato | Singh et al. (2011) |
| Cre/lox site-specific recombination | Hph, gus & npt | Rice | Akbudak & Srivastava (2011) |
| Cre/lox site-specific recombination | Npt & GUS | Rice | Khattri et al. (2011) |
| Ipt-type MAT vector | GUS, ipt & chic | Potato | Khan et al. (2011) |
| Marker-free & vector free cassette | Acol | Melon | Hao et al. (2011) |
| Ipt-type MAT vector | Ipt and wd | Tomato | Khan et al. (2011) |
| CRISPR-Cas9 | ZmTMS5 | Maize | Chen et al. (2018) |
| CRISPR-Cas9 | ARGOS8 | Maize | Shi et al. (2017) |
| MFTID | Lox & bar | Wheat | Cao et al. (2020) |
| Co-transformation | Bar & GUS | Wheat | Liu et al. (2020) |
| Marker steroid-inducible recombinase | Npt II, gft & trfA | Banana | Kleidon (2019) |
In the development of genetically modified organisms (GMOs), many techniques have been elucidated to improve marker-free transgenic plants via the pollen tube pathway. This technique is widely practiced in China and effectively used in many crops such as maize, rice, wheat and cotton (Yang et al., 2009), melon (Hao et al., 2011) and soybean (Yang et al., 2011). The ovary-drip technique has also been used to achieve higher transformation in maize (Yang, Su & An, 2009). Marker-free production of GM products could be achieved via biolistic introduction of genes that bring unicellular microspores to pollen maturity and then use them for pollination (Aziz & Machray, 2003). Tuteja et al. (2012) reviewed several techniques or strategies that exclude marker-free product selection genes in transgenic development such as co-transformation, site-specific recombination, multi-auto transformation vector, system of transposition, and homologous recombination by (Puchta, 2000) and (Zubko, Scutt & Meyer, 2000). Among these techniques, co-transformation is the most widely used. Despite the continuous improvement of marker-free transgenes, the range of rescue of transformed events without the use of selectable marker genes remains highly susceptible to change and at least twofold or less than the use of antibiotic resistance marker genes (Breyer, Kopertekh & Reheul, 2014). In marker-free genetically transformed plants, selection events require a large number of GM transformants harboring the gene of interest (Bhatnagar et al., 2010). Another problem could be chimeric plants with only partially genetically transformed tissues due to lack of selection agents (Eckerstorfer et al., 2019; Li, Xie & Qiu, 2009; Joshi et al., 2009).
Technique for the co-transformation and segregation of marker genes
Co-transformation is a simple technique for obtaining transgenic products without marker genes (Breyer, Kopertekh & Reheul, 2014; Manimaran et al., 2011; Tuteja et al., 2012; Woo, Suh & Cho, 2011; Yau & Stewart, 2013). It involves the simultaneous transformation of a selectable marker gene and a desired gene from different T-DNAs into the genome prior to gene segregation in successive sexual generations. Four different approaches can be used in the co-transformation technique: (i) use of two different T-DNAs carrying two different plasmids in an Agrobacterium culture (Bahramnejad et al., 2019; McCormac et al., 2001; Sripriya, Raghupathy & Veluthambi, 2008); (ii) within an Agrobacterium culture, two different T-DNAs are placed on the same plasmid (Matthews et al., 2001; McCormac et al., 2001; Miller et al., 2002; Philips et al., 2019); (iii) the use of two T-DNAs in two different Agrobacterium groups (Quispe-Huamanquispe et al., 2019; Sripriya et al., 2011), and (iv) the two plasmids in one and identical biolistic delivery (Joyce & Sun, 2020; Kumar, Arul & Talwar, 2010; Prakash et al., 2009) (Fig. 1). The co-transformation technique involves the insertion of two T-DNAs into different genomic loci for segregation (Confalonieri & Sparvoli, 2019; Kausch et al., 2019; RamanaRao & Veluthambi, 2010). This technique offers unique advantages for the production of transgenic plants, i.e., it allows the simultaneous insertion of multiple genes of interest into a plant genome without many selectable marker genes, regardless of gene sequence (Miki & McHugh, 2004). In this technique, two to thirteen transgenes have been successfully incorporated simultaneously using biolistic gene gun (Low et al., 2018; Wu, 2007). The widespread use of biolistic methods may be critical to control multiple genetic traits using cloned genes, but extraction of marker genes from GM plants will be difficult (Ansari et al., 2020; Miki & McHugh, 2004).
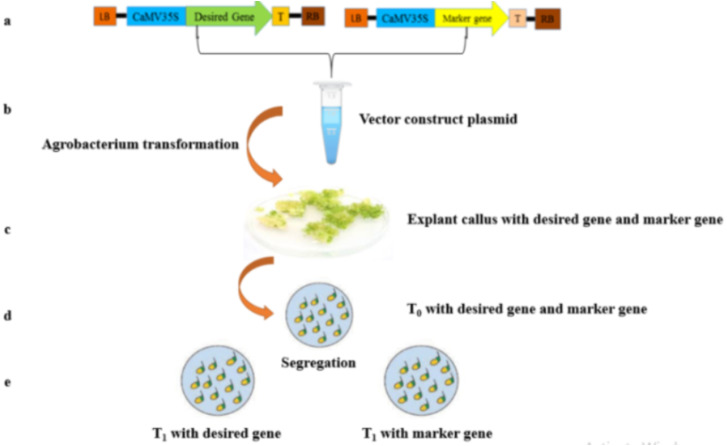
Figure 1. A scheme of genetic transformation procedure to produce marker free transgenic plants.
(A) Two T-DNA sections physical diagram, showing desired gene and marker gene. (B) Putative transformed calli with the desired gene and marker gene. (C) Desired gene and marker gene harbored by T0 putative transgenic plant. (D) Segregating two different plants; T1: Potentially harboring the desired target gene and other T2 with only marker gene.
Progress of GM plants without markers by co-transformation with Agrobacterium has been widely reported (Darbani et al., 2007; Manimaran et al., 2011; Narancio et al., 2020; Tuteja et al., 2012). This has been successfully used in crops like maize, rice, soybean and oilseeds. The method has also been used to develop first generation golden rice without hygromycin resistance marker gene (Al-Babili & Beyer, 2005). More recently, Holme et al. (2012) found that co-transformation has been used to generate cis-genic barley without markers. Theoretically, the technique appears to be simple and safe to generate non-marker GM plants compared to other techniques because it does not involve residual DNA sequences in GM products (Breyer, Kopertekh & Reheul, 2014). As an advantage of co-transformation by Agrobacterium compared to biolistic transformation, it is reported that the genes co-transformed by Agrobacterium are usually incorporated into the plant genome at different loci. Thus, different marker genes can be easily separated from the desired genes, which facilitates the generation of transgenic plants without markers (Ebinuma et al., 2001). Essentially, the co-transformation associated with the removal of selectable marker genes only applies to sexually reproducing species, but not to asexually propagated plants such as potato, date palm, sugarcane, and some woody plant species (Darbani et al., 2007; Manimaran et al., 2011; Parray, Mir & Shameem, 2019; Tuteja et al., 2012). Breyer and colleagues emphasized that the use of non-selectable marker genes seems to be the easier way to produce GM crop with reduced presence of introduced DNA sequence and associated biosafety issues (Breyer, Kopertekh & Reheul, 2014). This protocol has been successfully applied to the production of Bt cotton using the pollen tube pathway technique or the genetically transformed potato AV43-6-G7 through agrobacteria-mediated transformation. However, the main disadvantages of non-marker transformation techniques are the poor recovery rate of transformants, high labor, time and cost requirements, and the use of a large number of regenerated plants for PCR analysis (Breyer, Kopertekh & Reheul, 2014; Confalonieri & Sparvoli, 2019). In addition, this technique requires high transformation and regeneration efficiencies that are typically genotype-based, limiting its application to very limited organisms. The technique is not feasible on a commercial scale for generating crops with desired genes and agronomic traits, except that the efficiency of transformation protocols is significantly increased (Jansing et al., 2019; Tuteja et al., 2012).
Bio-safety concerns related to GM crops
The influence of GM plants on production and cropping patterns of agricultural species worldwide is becoming increasingly popular in modern times (Kadoić Balaško et al., 2020). However, the widespread adoption of GM plant varieties and their cultivation have raised notable concerns about biosafety issues in some parts of the world (Luna & Dowd-Uribe, 2020; Stewart, 2001). The most common biosafety concerns relate to the direct or indirect flow of toxic origin transgenes to non-target species; the possibility of unforeseen effects in transgene-environment interactions and leakage of transgenes from GM plant varieties into their weedy relatives is the most intense debate worldwide (Beacham, Sweet & Allen, 2017; Lu & Snow, 2005; O’Callaghan et al., 2005; Oliveira et al., 2007; Souza et al., 2019). In line with these concerns about GM products, the Food and Agriculture Organization (FAO), the World Health Organization (WHO), and the Organization for Economic Cooperation and Development (OECD) and other nations, through expert consultations, have accepted the following relevant health requirements that should be considered when reviewing a novel food (Krebs, 2000). These include: (i) the modified host organism should be described, including information on the composition of nutrients (antibiotics, toxins and allergenic potential, and other important changes during usual processing.); (ii) the organism used as a donor should be well described, with information on possible toxicity and related allergens, and the genes used and their products should be free of any public health risk; (iii) molecular description of the genetic transformation, with description of the modification process; (iv) documentation of the primary and any secondary gene products described, with an explanation of the foreign gene characteristics; (v) evaluation of the safety of the anticipated novel substances in food, with an assessment of any toxins formed directly by the modification; (vi) evaluation of novel food allergy possibilities; and (vii) evaluation of unintended effects on dietary composition and assessment of changes in nutrient enrichment. Considering the regulatory concerns of various countries around the globe towards GMOs, it is imperative to develop new and widely accepted marker gene systems to introduce more desirable genes for the production of the future GM crops. Therefore, the selectable markers i.e., ptxD/Phi are proposed as an alternative scheme for genetic transformation to develop safer crops.
The ptxD/Phi; alternative selectable marker for crops genetic transformation
To minimize the use of antibiotic or herbicide resistance genes, several alternative transformation systems have been proposed by bioscientists (Hu et al., 2016; Miki & McHugh, 2004; Patterson et al., 2019). Some of these markers are easily identified by the human eye and have been used as alternatives to antibiotic or herbicide markers (Boscaiu et al., 2019; Naing et al., 2015). For example, the anthocyanin gene is used as a substitute for strawberry, apple, and potato transformation selection of kanamycin (Anwar et al., 2018; Kortstee et al., 2011), mainly because anthocyanin is known to be an anti-cancer agent that may also provide health benefits (Breyer, Kopertekh & Reheul, 2014; Diaconeasa et al., 2020). The other most commonly used positive marker genes are the xylA gene from Streptomyces rubiginosus (Barampuram & Zhang, 2011; Tran et al., 2020) and Thermo-anaerobacterium thermosulfurogenes (Shrawat & Lörz, 2006), Xylose isomerase catalyzes the isomerization of xylose to d-xylulose, which can be used as a carbohydrate base in plant cells. These genes have been successfully used to develop transgenic potato, tobacco and tomato. However, d-xylose produces carbon, which is toxic to plant cells, so selection requires culture on a precise mixture of sucrose as well as d-xylose (Ghazanfar & Irfan, 2020; Haldrup, Noerremark & Okkels, 2001). Another positive marker gene, Phosphomannose isomerase (PMI), is considered credible and has been extensively studied (Penna, Sági & Swennen, 2002; Singh et al., 2019). Plant cells contain endogenous hexokinase that can convert mannose to mannose-6-phosphate. Therefore, cells expressing PMI can convert mannose-6-phosphate to fructose-6-phosphate, which can be used as a carbohydrate source in the plant (Penna, Sági & Swennen, 2002; Singh et al., 2019). Selection on the PMI/mannose scheme has been reported in the transformation of sugarcane, sorghum, sugar beet, rice, and maize (Penna, Sági & Swennen, 2002; Shi et al., 2020; Wu et al., 2019a). According to Lynch et al. (2019), this selection system is based on alternative carbon origin using mannose as a selection agent, where a specific mannose mixture is cultivated with one of the other sugars, which must be adapted to both the selection stage and the plant species to achieve optimal transformation efficiency.
Recently, the phosphite oxidoreductase (ptxD) gene, a unique dominant marker for selection in transformation of plants and other organisms, has been reported (Heuer et al., 2017b; Hirota, Motomura & Kuroda, 2019; López-Arredondo & Herrera-Estrella, 2013). For example, the rice Actin2 promoter (OsAct2P) and terminator (OsAct2T) were used to clone the ptxD expression cassette, which was then incorporated into the pMDC99 vector for rice transformation (Manna et al., 2016). The constitutive promoter (rice actin2) was selected for a higher level of transgene expression, allowing rapid oxidation of Phi after it is absorbed by Pi transporters in the plant. Overall development, chlorophyll assembly, root development, PS-II activity, and Phi and Pi assembly were comparable in Phi-metabolizing transgenic plants developed in Phi media as the major P fertilizer compared with plants fed Pi. Phi-mediated growth inhibition has been observed in a variety of plant species in previous studies (Carswell, Grant & Plaxton, 1997; Hirosse et al., 2012; Thao et al., 2008a; Ticconi, Delatorre & Abel, 2001). For instance, application of Phi severely restricted root development of onion (Allium cepa) (Sukarno, Smith & Scott, 1993) and Brassica nigra (Carswell, Grant & Plaxton, 1997). In spinach, a decrease in the phosphate:phosphite ratio resulted in a decrease in shoot dry weight (Thao et al., 2008a). Similar results were observed in Brassica napus var Peruviridis (Thao et al., 2008b). Development, length and dry weight of sweetpotato plants were reduced with increasing Phi treatment (Hirosse et al., 2012). In addition, rice root and shoot biomass, root hair formation, and chlorophyll formation decreased when Phi concentrations in the media were increased (Manna et al., 2015). Ticconi, Delatorre & Abel (2001) observed similar results in Arabidopsis. However, López-Arredondo & Herrera-Estrella (2012) observed that Phi metabolizing transgenic Arabidopsis and tobacco plants expressing the bacterial ptxD gene resulted in improved phenotype and physiology in terms of biomass accumulation, yield as well as Phi build-up. This finding supported the hypothesis of Manna et al. (2016) that when rice plants evolved the ability to utilize Phi, there was a significant increase in growth, phenotype and physiology of rice seedlings, with better Pi build-up and lower Phi accumulation compared to wild rice plants grown in related Phi concentrations.
Furthermore, Che et al. (2018) evaluated PTXD/Phi in a ternary vector with pPHP70444 containing the ptxD gene driven by a maize ubiquitin promoter and intron. PTXD/Phi selection was imposed, resulting in more stringent selection and healthy callus growth. This resulted in transformation performance of up to 6% with no detectable outliers and a level of quality events of 47%. A novel non-antibiotic and non-herbicidal sorghum transformation scheme was developed using PTXD as a selectable gene. Despite the lower transformation frequency in sorghum, the PTXD/Phi selection scheme is a promising transformation system that can further increase the efficiency of selection in crops through codon optimization of the ptxD gene in monocotyledons (Che et al., 2018).
A similar study on cotton transformation using the same selection method showed that the ptxD/phosphite selection system provides a high-quality, effective, and simple method to produce GM cotton plants and addresses several concerns regarding the use of antibiotic- and herbicide-resistant genes in the development of transgenic organisms (Pandeya et al., 2017). The ptxD gene can be used to select transformed cells and produce transgenic cotton plants using a selection medium with Phi as the main source of P (Pandeya et al., 2017). A total of 3.43% transgenic events were obtained with the ptxD/Phi selection scheme, compared to only 0.41% with bar/phosphinothricin (PPT) selection. The nptII/kanamycin and hpt/hygromycin systems had event recovery rates of 2.88 and 2.47%, respectively (Pandeya et al., 2017). These results indicate that “clean” ptxD-expressing callus genotypes can be obtained by replicating subcultures and selecting the most evolving cultures without the use of visible, screenable marker genes. The selection mechanism under ptxD/Phi compared to selection based on the use of antibiotic and herbicide resistance genes in cotton transformation showed that ptxD/Phi selection produced a higher percentage (97%) of transformants compared to nptII/kanamycin and hpt/hygromycin using PCR analysis of putative transformants of regenerated events harboring the ptxD gene (Pandeya et al., 2017). It was observed that while Phi selection does not completely kill cultures lacking either the ptxD transgene or its expression, it does increase the growth of cells expressing the gene for selection (Nahampun et al., 2016; Pandeya et al., 2017). These results are in agreement with those of (López-Arredondo & Herrera-Estrella, 2012), who conducted a study to investigate the ability of medium containing Phi (1 mM) in the selection of ptxD-expressing organogenic shoots regenerating from leaf disks of tobacco after Agrobacterium- mediated transformation. Nahampun et al. (2016) transformed two maize genotypes: type I and Type II callus cultures separately with the same bacterial ptxD gene, and the transformants were subsequently selected on medium containing KH2PO3.
The novelty of ptxD/Phi selection is that the system allows the conversion of a toxic compound (Phi) into a valuable compound (Pi) for optimal plant growth. In this selection system, escape events are minimal and the nutritional value for the plant is also provided (López-Arredondo & Herrera-Estrella, 2012; López-Arredondo & Herrera-Estrella, 2013; Pandeya et al., 2018). The ptxD/Phi scaffold offers several advantages over currently used systems: (i) the Phi salts contain sodium and potassium, which are affordable and easily available from different companies, (ii) the phosphite salt is harmless to humans and animals, therefore no special precautions are required for its use, (iii) the phosphite salts are well soluble in thermal water and photostable, which makes them constant selection agents in tissue culture and greenhouses, and (iv) the ptxD/Phi method is suitable as a selection system in the greenhouse using inert substrates or different low-Pi soils by adding Phi in the soil or fertilizing to promote high-density experiments (Achary et al., 2017; López-Arredondo & Herrera-Estrella, 2012; López-Arredondo & Herrera-Estrella, 2013). Comparing the ptxD/Phi selection scheme to other selection systems, only the Pi ion in the culture medium needs to be replaced with a Phi ion to efficiently select cells expressing the ptxD gene, thereby eliminating all steps to modify the gene. In this scenario, the ptxD/Phi framework is considered to be a precise and beneficial addition to the plant transformation toolbox, eliminating the dependence on antibiotic and herbicide resistance genes, making it easier to overcome some problems related to the introduction of GM plants. Moreover, ptxD/Phi technology symbolizes one of the most exciting transforming selection systems of today, which not only provides an efficient way to generate transgenic plants, but also helps to address a number of concerns related to the use of antibiotic and herbicide resistance genes, as well as biosafety concerns in transgenic development.
However, plants treated with phosphite (Phi) rapidly accumulate Phi within their cells (McDonald, Grant & Plaxton, 2001). Phosphite is phloem mobile and accumulates in sink tissues (Leong, Lu & Chiou, 2018). Since Phi is not metabolized by plants, it remains in tissues for a long time and consequently disrupts the signal transduction chain that allows plants to detect and respond to Pi deficiency at the molecular level, thereby amplifying the negative effects of Phi (Mehta et al., 2021; Trejo-Téllez et al., 2019). In Pi-deficient tomato plants cultured in the presence of Phi, the expression of Phi-inducible genes such as PT1 and PT2 (high-affinity Pi transporters), PS2, and TPSI1 (novel genes) was strongly repressed. Moreover, Phi accumulates massively in the cytosol and blocks Pi efflux from the vacuole, while subsequent uptake of Pi into cells triggers an enormous transfer (Gómez-Merino & Trejo-Téllez, 2016). Phi is transported from the cytosol to the vacuole. Pi deficiency symptoms can be exacerbated by this suppression of Pi efflux from the vacuole, leading to increased programmed cell death in Pi-deficient or ptxD non-transgenic plants (Gómez-Merino & Trejo-Téllez, 2015).
The ptxD/Phi technology; a novel strategy for weeds control
To meet the food needs of the ever-growing population, the focus is on increasing crop productivity under challenging conditions (Catalá & Salinas, 2018). The main problem for farmers in this situation is to ensure optimal nutrient levels in the soil and control weeds (Catalá & Salinas, 2018). Typical weed control measures are tedious practices such as hand weeding, hoeing or ecologically aggressive methods such as tillage. The earlier discovery and application of herbicides such as 2,4-dichlorophenoxyacetic acid and Roundup eradicated most weeds growing in the soil (Armengot et al., 2015; Troyer, 2001). The overuse of these herbicides has led to the emergence of numerous herbicide-resistant weeds in fields. There are previous reports of 254 herbicide-resistant weed species, of which 41 are resistant to glyphosate Heap (2018). This worrisome condition has called for persistent research to find new strategies to control weeds in cereal fields. According to Pandeya et al. (2018), selective fertilization of transgenic plants carrying ptxD gene with Phi is an effective means to control the growth of weeds. The ptxD transgenic plants are able to metabolize Phi by converting it to Pi, a mechanism that enables the transgenic plants to outcompete many dicotyledonous and monocotyledonous weed species in both natural soils and substrates. More importantly, ptxD/Phi technology suppressed the growth of Amaranthus palmeri, a glyphosate-resistant weed that has devastating effects on many crops (Smith, Baker & Steele, 2000). In a study conducted on artificial inert soils and natural soils, Phi fertilization processes severely hindered A. palmeri emergence (Pandeya et al., 2018).
The ptxD/Phi system for weed control has been previously validated for other plant species, such as tobacco, which harbors the gene and effectively suppresses many weeds such as B. distachyon, I. purpurea, Brachiaria plantaginea, and Amaranthus hybridus when Phi was used instead of Pi (López-Arredondo & Herrera-Estrella, 2012). The ptxD/Phi system proved to be an efficient weed control tool in trials conducted under field conditions in Argentina (Heuer et al., 2017a). In another recent study on rice, Phi showed a positive effect against Amaranthus spinosus as a selective pre-emergence weed control (Manna et al., 2016). In addition, foliar application of Phi was more efficient in controlling widespread weeds such as Phyllanthus niruri, Euphorbia hirta, Portulaca oleracea and Chloris barbata, a monocot plant. The ptxD/Phi mechanism for weed control was not only effective but also provided an innovative molecular approach to reduce the overdependence on phosphate as a P source for current agriculture by expressing the ptxD gene in tobacco and Arabidopsis (López-Arredondo & Herrera-Estrella, 2012). The data confirmed that ptxD transgenic plants required 30–50% less phosphorus in greenhouse situations when supplemented in the form of Phi instead of Pi to achieve a biomass equivalent to the wild form fertilized by Pi (López-Arredondo & Herrera-Estrella, 2012). Other advantages include the lower fixation of Phi in the soil compared to Pi and the fact that few microbes can use Phi as a source of P (Heuer et al., 2017a; Lopez-Arredondo et al., 2014; McDonald, Grant & Plaxton, 2001). In addition, soil microbes, including algae, cannot utilize Phi as a major source of P. When phosphite is released from fields into water bodies, algal species cannot utilize it as a source of P, minimizing the effect of P fertilization to promote toxic algal blooms (Loera-Quezada et al., 2016) (Fig. 2). The effects of water eutrophication and toxic algal blooms have resulted in more than 400 lethal zones in many parts of the world’s oceans, covering more than 250,000 square kilometers of ocean surface (Hardy et al., 2016; Schmale et al., 2019). For this reason, the ptxD/Phi scheme can offer important ecological gains by reducing eutrophication of water bodies as well as hypoxia due to Pi and N fertilizer runoff into river bodies. Finally, it should be noted that Phi poses no risk to human or animal health and is massively used as an efficient fungicide in crop production (Achary et al., 2017). Thus, phosphite has a direct effect on phytopathogenic fungi by inhibiting mycelial proliferation and reducing conidiogenesis of Fusarium sp. isolated from the rhizosphere of plants (Solis-Palacios et al., 2021). In addition, Phi can act indirectly by activating the innate defense mechanisms of plants to limit pathogen growth (Cerqueira et al., 2017). Phi can also activate host defense genes, which helps plants ward off disease (Rampersad, 2020) and directly suppress the growth of pathogens such as Phytophthora (Huang et al., 2018). Phi is expected to interfere with the metabolic mechanisms of Phytophthora associated with phosphorus uptake. Phosphite accumulates around root tips in the area colonized by mycorrhizal fungi, which is transmitted through the phloem (Hunter, 2018). When phosphite accumulates in the system, it can induce necrosis causing damage to fine roots, resulting in the loss of ectomycorrhizal and arbuscular mycorrhizal production sites in plant species that form symbiotic interactions with mycorrhizal fungi (Plaxton & Carswell, 2018). Application of this Phi may damage the mycorrhizal interface because mycorrhizal fungi extend the lifespan of fine roots and active fine roots serve as a metabolic sink for photosynthates (and thus phosphite). Furthermore, any damage to immature roots would alter the pattern of root exudates and may lead to changes in the soil microbiota (Jorgensen, 2014), which may indirectly affect mycorrhizae (Jorgensen, 2014). Wu et al. (2019b) hypothesized that Phi treatment significantly increases isoflavonoid production, which explains why Phi-treated plants are more tolerant to various biotic stressors. Nevertheless, the expected expansive implementation of the ptxD/Phi program in the near future would require further studies to assess the likely and potential environmental impacts of Phi operation.
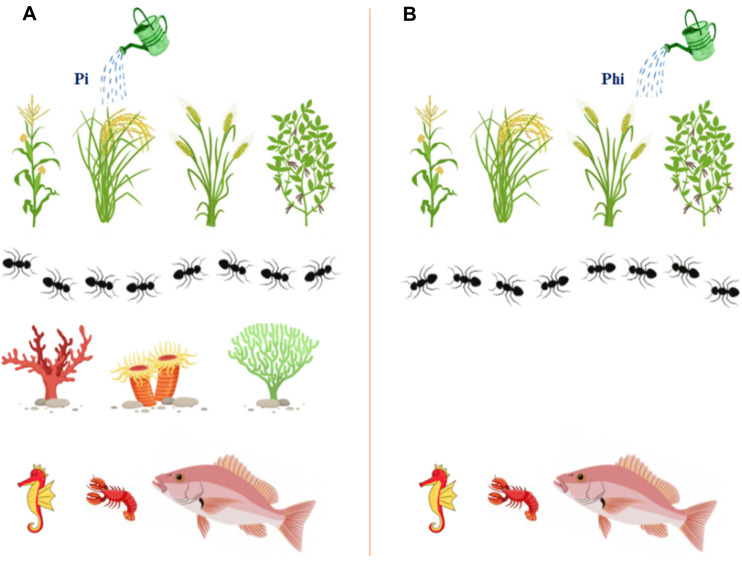
Figure 2. The graphical illustration to display the safety of ptxD/Phi selection system on different organisms and the environment, and showing the added advantages to enhance transgenic plants growth.
(A) Phosphate (Pi) fertilizer (B) Phosphite (Phi).
The transgenic plants carrying the ptxD gene can use phosphite (Phi) as the sole source of phosphorus (P) by converting Phi to phosphate (Pi) to achieve optimal growth and development, which can lead to higher yields (Fig. 2). Plants, soil and aquatic microbes cannot metabolize phosphite, so weeds cannot compete with crops for available nutrients, nor can aquatic life. Phosphite moves in water bodies and has no significant growth effect on aquatic microorganisms. Studies have shown that aquatic microorganisms such as algae, Ulva lactuca, Chlamydomonas reinhardtii, Botryococcus braunii, and Ettlia oleoabundans are unable to use Phi as a source of P and therefore have no negative effects on aquatic life (Lee et al., 2005; Loera-Quezada et al., 2015). However, accumulation of significant amounts of Pi in water bodies triggers algal blooms. This compound is leached from croplands that are heavily loaded with phosphate fertilizers. Rain is a means of washing these leachable compounds from the soil surface into water bodies, which eventually end up in large reservoirs such as lakes and oceans. Drainage systems discharge these nutrient components into rivers, and untreated raw sewage flows into water bodies and induces algal blooms.
Much attention needs to be paid to measuring phosphate levels and monitoring water quality, as well as regulating algal phytoplankton dynamics against long-term environmental impacts. Phosphate enrichment of algae has been widely studied to determine their beneficial uses (De-Bashan & Bashan, 2004). The consequences of algal blooms (Mallin & Cahoon, 2020) include threats to human health/life: algal blooms contain toxins that reduce the suitability of water for human consumption. This claim is based on the fact that algal blooms in water contribute to rapid contamination of water, which poses a threat to human health and leads to severe irritation, itching and skin diseases when such contaminated water comes into contact with human skin. Fish and other aquatic life depend on dissolved oxygen in water. However, in the case of plants, heavy reproduction and dense growth in a very short period of time leads to increased competition for oxygen, resulting in an imbalance in the aquatic environment and suffocation of aquatic animals such as fish. Fertilizer contamination can lead to dead zones with little or no oxygen in the water, a scenario that makes it difficult for aquatic life to survive. This condition is called hypoxia. Oxygen-depleting algae blooms cause these dead zones as they die and decompose, which can lead to a large death of aquatic life, making the algae bloom region a dead zone with dead animals and plants. The resulting foul odor can affect the remaining aquatic life and drive them away further. Phosphate fertilization allows weeds to compete with crops such as cotton, cowpea, and other crops for the available phosphorus source, limiting their growth and development. Much of Pi is metabolized by microbes in the soil, making it unavailable to plants. Importantly, the soil microbiota and aquatic microbes cannot metabolize Phi, which greatly increases its availability to plants, which in turn reduces the risk of eutrophication of water bodies.
ptxD /Phi as selectable marker system in microorganisms for biological contamination control in cultivation systems
Mineral nutrient availability is a major limiting factor for microalgal culture production and conservation (Juneja, Ceballos & Murthy, 2013). Researchers and private companies have considered transgenic microalgae and cyanobacteria as highly potent green cell factories for the production of valuable bioproducts (Ng, Keskin & Tan, 2020). Therefore, the promoter (Pccg6) has been used to stimulate the expression of the bacterial phosphite oxidoreductase PTXD, which allows Trichoderma atroviride phosphite (Phi) as a major phosphorus source (Carreras-Villase nor et al., 2020). Recent research has shown that PTXD expression in both plants and microorganisms enables a very challenging climate that promotes the production of genetically modified organisms while limiting the production of diverse, weedy combinations of organisms (plants, fungi, microalgae, and bacteria) that are unable to metabolize Phi (Heuer et al., 2017a; Loera-Quezada et al., 2016; López-Arredondo & Herrera-Estrella, 2012; Pandeya et al., 2018). Trichoderma ptxD transgenes containing constructs of the ccg6OPT or pki1OPT genes showed positive phenotype developmental traits. Thus, the transgenic strain exhibits comparable growth kinetics on Pi and Phi containing media, suggesting that PTXD expressions in Trichoderma do not have destructive effects on its physiology (Carreras-Villase nor et al., 2020).
The use of PTXD/Phi has been shown to be an effective technique to control contaminating species (e.g., Kluyveromyces marxianus CBS 6556, S. cerevisiae Ethanol Red) in the fermentation of a number of S. cerevisiae and Y. lipolytica strains that metabolize phosphite using favorable raw materials (Shaw, Twilton & MacMillan, 2016). According to Silverman (2001), Trichoderma development or its biological properties are not affected by PTXD expression, resulting in a useful and observable phenotype for assessing the transcriptional function of the regulatory sequence. The metabolism of Phi gave T. atroviride a strategic advantage in overgrowth of harmful bacteria (Carreras-Villase nor et al., 2020). The phenotype remains vigorous and constant as transformants grow well even at high concentrations of Phi (Silverman, 2001). PTXD implements a simple enzymatic reaction involving the direct conversion of Phi to Pi using NAD + as a cofactor (Esland et al., 2018). The overall picture presented here can help in the development of fertilization strategies that maximize nutrient utilization, especially of P, while avoiding oversupply, which could reduce eutrophication of water bodies in case of wastewater discharge. This could be achieved by exploiting the physicochemical properties of Phi as Phi-metabolizing organisms (López-Arredondo & Herrera-Estrella, 2012). As a result, ptxD/Phi systems have the potential to minimize the cost of enzyme production using Trichoderma, as antibiotics and reactor sterilization are more expensive than Phi salts. The ptxD/Phi approach offers a more stable and cost-effective substitute for contamination control in the industrial development of Trichoderma and possibly other filamentous fungi, since Phi is FDA-approved for use as an agricultural fungicide and food additive (Carreras-Villase nor et al., 2020).
In addition, ptxD/Phi has been used for chloroplast transformation in Chlamydomonas reinhardtii and for the regulation of biological contaminants, expressing heterologous proteins in algal chloroplasts without affecting culture efficiency (Zhang, Wang & Wang, 2020). Consequently, ptxD/Phi selection can be used as a universal marker to transform C. reinhardtii wild-type microorganisms on medium containing Phi as a major source of P (Changko et al., 2020; Kanda et al., 2014). Combining phosphite fertilization with expression of the nuclear transgene ptxD provided an ideal substitute for herbicides in controlling weeds and pollution of algal cultures (Cutolo et al., 2020). Chloroplast expression of ptxD in Chlamydomonas reinhardtii has been proposed as a more environmentally friendly substitute for antibiotic resistance genes in plastid transformation (Day & Goldschmidt-Clermont, 2011). Transplastomic genotypes expressing the mutant PTXD type utilized NADP+ and NAD + to convert Phi to Pi more efficiently and evolved more rapidly than those expressing the wild-type protein (Cutolo et al., 2020). Loera-Quezada et al. (2016) reported that the production of genetically engineered microalgae capable of metabolizing Phi is a viable approach for developing a successful scheme for managing biological contaminants in microalgal development that can be combined with established biological and molecular strategies. The phosphite-based technique is important for closed photobioreactors in that one of the main advantages for Phi-metabolizing strains is that it eliminates the need for a sterile phase, which is the most expensive aspect of operating large photobioreactors (Loera-Quezada et al., 2016).
In addition, contamination by biological contaminants is a major obstacle to commercial microalgal processing using open culture systems (Lam et al., 2018). Due to low maintenance costs and relative ease of operation, Phi-based culture systems are ideal to meet the requirements of large-scale algal biomass production (Grobbelaar, 2012). Photosynthesis-dependent, phosphate-requiring CO2 metabolism could be more effectively maintained (Johnson & Alric, 2013), resulting in greater sugar and starch accumulation than the restricted wild-type enzyme, ultimately supporting more rapid selective development of algae in phi nutrient media. C. reinhardtii cells with mutant PTXD in their chloroplasts develop faster in phosphite media than cells expressing WT PTXD (Esland et al., 2018). As a result, Phi-based engineering will address contamination concerns in open pond systems and expand its application beyond microalgal species that can develop in harsh environments. Engineering Phi metabolism has biological consequences for current microalgal culture techniques, including: (i) the technique should allow closed photobioreactors to be operated at lower cost by removing the burden of sterilizing the media and reactor; (ii) the system should allow racecourse ponds to be used extensively for the development of microalgal biomass and derivatives; and (iii) the Phi scheme should allow the development of metabolic engineering in a variety of microalgal species.
Conclusions
Plant transformation methods rely on the use of selection agents due to the low efficiencies of transformation. Traditionally, these selection agents usually contain antibiotic-resistant and herbicide-resistant genes, which have raised increasing public concern about the use of antibiotic-resistant and herbicide-selectable marker genes from a food safety and environmental perspective. This raises concern when genetically modified crops and their products become known to the public. In this scenario, concerted efforts have been made to search for alternative selection systems that are safe and reliable to produce transgenic crops to address public perception about the acceptability of GM crops and their products. In recent years, alternative selection markers, such as positively selectable markers containing either non-antibiotic resistance or native plant genes, have been evaluated for plant transformation. Such markers have been successfully used in a variety of plant species.
In addition, several other techniques such as co-transformation, site-specific recombination, intra-chromosomal homologous recombination, and transposon-based techniques have been used to develop transgenic plants to satisfy public concerns and to address other biosafety issues. However, the processes involved in these techniques are tedious, time consuming and not well understood. In the present scenario, ptxD/Phi could serve as a very successful scheme to suppress weeds under natural, low-phosphorus soils, even those resistant to the herbicide glyphosate, while allowing plants with ptxD expression to develop optimally due to reduced competition from incapacitated weeds. Production of genetically engineered microalgae and other microorganisms capable of metabolizing Phi is also a viable approach for managing biological contaminants. Therefore, ptxD transformants could be evaluated in soils with low phosphorus levels using Phi as herbicide. As a selective agent, ptxD/Phi is less toxic to untransformed cells compared to antibiotics, herbicides, or drugs and therefore appears to produce greater frequencies for transformation. The ptxD/Phi technology offers one of the most favorable techniques for transforming crops and other organisms with desired genes and ensures that various issues related to biosafety of GM products are minimized. Ultimately, it remains to be determined whether it can provide greater safety than the selectable marker genes already in use.
Acknowledgments
The authors acknowledge the assistance of S.B. Mohammed and Peter Quandahor for their valuable suggestions and input in literature search, and manuscript reading and editing.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 32060502, 31960442), the Special Fund for Discipline Construction of Gansu Agricultural University, Lanzhou China (GAU-XKJS-2018-085, GAU-XKJS-2018-084), the special Fund for Talents of Gansu agricultural University, China (Grand No. 2017RCZX-44) and the Gansu Provincial Department of Education Fund (2019B-073). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Richard Dormatey conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Chao Sun conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Kazim Ali performed the experiments, prepared figures and/or tables, and approved the final draft.
Sajid Fiaz performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Derong Xu performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Alejandro Calderón-Urrea performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Zhenzhen Bi performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Junlian Zhang performed the experiments, prepared figures and/or tables, and approved the final draft.
Jiangping Bai conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
There is no raw data or code in this review article.
References
- Achary et al. (2017).Achary VMM, Ram B, Manna M, Datta D, Bhatt A, Reddy MK, Agrawal PK. Phosphite: a novel P fertilizer for weed management and pathogen control. Plant Biotechnology Journal. 2017;15:1493–1508. doi: 10.1111/pbi.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbudak & Srivastava (2011).Akbudak MA, Srivastava V. Improved FLP recombinase, FLPe, efficiently removes marker gene from transgene locus developed by Cre-lox mediated site-specific gene integration in rice. Molecular biotechnology. 2011;49:82–89. doi: 10.1007/s12033-011-9381-y. [DOI] [PubMed] [Google Scholar]
- Al-Babili & Beyer (2005).Al-Babili S, Beyer P. Golden Rice–five years on the road–five years to go? Trends in Plant Science. 2005;10:565–573. doi: 10.1016/j.tplants.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Anand, Vaghchhipawala & Mysore (2010).Anand A, Vaghchhipawala ZE, Mysore KS. Genomics of Agrobacterium–plant interaction: an approach to refine the plant transformation technology. In: Stewart CN, Touraev, A., Citovsky, V. and Tzfira, T A, Touraev A, Citovsky V, Tzfira T, editors. Plant Transformation Technologies. Wiley-Blackwell; Oxford, UK: 2010. pp. 31–49. [Google Scholar]
- Ansari et al. (2020).Ansari WA, Chandanshive SU, Bhatt V, Nadaf AB, Vats S, Katara JL, Sonah H, Deshmukh R. Genome editing in cereals: approaches, applications and challenges. International Journal of Molecular Sciences. 2020;21:4040. doi: 10.3390/ijms21114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar et al. (2018).Anwar M, Wang G, Wu J, Waheed S, Allan AC, Zeng L. Ectopic overexpression of a novel R2R3-MYB, NtMYB2 from Chinese narcissus represses anthocyanin biosynthesis in tobacco. Molecules. 2018;23:781. doi: 10.3390/molecules23040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengot et al. (2015).Armengot L, Berner A, Blanco-Moreno JM, Mäder P, Sans FX. Long-term feasibility of reduced tillage in organic farming. Agronomy for Sustainable Development. 2015;35:339–346. doi: 10.1007/s13593-014-0249-y. [DOI] [Google Scholar]
- Aziz & Machray (2003).Aziz N, Machray GC. Efficient male germ line transformation for transgenic tobacco production without selection. Plant Molecular Biology. 2003;51:203–211. doi: 10.1023/A:1021199718356. [DOI] [PubMed] [Google Scholar]
- Babwah & Waddell (2000).Babwah A, Waddell C. Cytosine deaminase as a substrate-dependent negative selectable marker in Brassica napus. Theoretical and Applied Genetics. 2000;100:802–809. doi: 10.1007/s001220051355. [DOI] [Google Scholar]
- Bahramnejad et al. (2019).Bahramnejad B, Naji M, Bose R, Jha S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnology Advances. 2019;37:107405. doi: 10.1016/j.biotechadv.2019.06.004. [DOI] [PubMed] [Google Scholar]
- Bai et al. (2009).Bai Y, Guo Z, Wang X, Bai D, Zhang W. Generation of double-virus-resistant marker-free transgenic potato plants. Progress in Natural Science. 2009;19:543–548. doi: 10.1016/j.pnsc.2008.08.005. [DOI] [Google Scholar]
- Bakhsh (2020).Bakhsh A. Development of efficient, reproducible and stable Agrobacterium-mediated genetic transformation of five potato cultivars. Food Technology and Biotechnology. 2020;58:57–63. doi: 10.17113/ftb.58.01.20.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhsh et al. (2020).Bakhsh A, Hussain T, Rahamkulov I, Demirel U, Çalişkan ME. Transgenic potato lines expressing CP4-EPSP synthase exhibit resistance against glyphosate. Plant Cell, Tissue and Organ Culture (PCTOC) 2020;140:23–34. doi: 10.1007/s11240-019-01708-1. [DOI] [Google Scholar]
- Ballester, Cervera & Pena (2010).Ballester A, Cervera M, Pena L. Selectable marker-free transgenic orange plants recovered under non-selective conditions and through PCR analysis of all regenerants. Plant Cell, Tissue and Organ Culture (PCTOC) 2010;102:329–336. doi: 10.1007/s11240-010-9737-1. [DOI] [Google Scholar]
- Banchi et al. (2019).Banchi E, Candotto Carniel F, Montagner A, Bosi S, Bramini M, Crosera M, León V, Martín C, Pallavicini A, Vázquez E. Graphene-based materials do not impair physiology, gene expression and growth dynamics of the aeroterrestrial microalga Trebouxia gelatinosa. Nanotoxicology. 2019;13:492–509. doi: 10.1080/17435390.2019.1570371. [DOI] [PubMed] [Google Scholar]
- Barampuram & Zhang (2011).Barampuram S, Zhang ZJ. Recent advances in plant transformation. Methods in Molecular Biology. 2011;701:1–35. doi: 10.1007/978-1-61737-957-4_1. [DOI] [PubMed] [Google Scholar]
- Beacham, Sweet & Allen (2017).Beacham TA, Sweet JB, Allen MJ. Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment. Algal Research. 2017;25:90–100. doi: 10.1016/j.algal.2017.04.028. [DOI] [Google Scholar]
- Bhatnagar et al. (2010).Bhatnagar M, Prasad K, Bhatnagar-Mathur P, Narasu ML, Waliyar F, Sharma KK. An efficient method for the production of marker-free transgenic plants of peanut (Arachis hypogaea L.) Plant Cell Reports. 2010;29:495–502. doi: 10.1007/s00299-010-0838-4. [DOI] [PubMed] [Google Scholar]
- Boscaiu et al. (2019).Boscaiu M, Estruch M, Fita A, Plazas M, Prohens J, Rodríguez-Burruezo A, Verdeguer M, Vicente O. Creating products and services in plant biotechnology. In: Matei F, Zirra D, editors. Introduction to biotech entrepreneurship: from idea to business. Cham, Switzerland.PP: Springer; 2019. pp. 19–52. [Google Scholar]
- Brandt (2003).Brandt P. Overview of the current status of genetically modified plants in Europe as compared to the USA. Journal of Plant Physiology. 2003;160:735–742. doi: 10.1078/0176-1617-01031. [DOI] [PubMed] [Google Scholar]
- Breyer, Kopertekh & Reheul (2014).Breyer D, Kopertekh L, Reheul D. Alternatives to antibiotic resistance marker genes for in vitro selection of genetically modified plants–scientific developments, current use, operational access and biosafety considerations. Critical Reviews in Plant Sciences. 2014;33:286–330. doi: 10.1080/07352689.2013.870422. [DOI] [Google Scholar]
- Briza et al. (2010).Briza J, Rüzicková N, Niedermeierová H, Dusbábková J, Vlasák J. Phosphomannose isomerase gene for selection in lettuce (Lactuca sativa L.) transformation. Acta Biochimica Polonica. 2010;57:63–68. doi: 10.18388/abp.2010_2373. [DOI] [PubMed] [Google Scholar]
- Brown & Wernegreen (2019).Brown BP, Wernegreen JJ. Genomic erosion and extensive horizontal gene transfer in gut-associated Acetobacteraceae. BMC Genomics. 2019;20:1–15. doi: 10.1186/s12864-018-5379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovinszki et al. (2007).Bukovinszki, Divéki Z, Csányi M, Palkovics L, Balázs E. Engineering resistance to PVY in different potato cultivars in a marker-free transformation system using a ‘shooter mutant’A. tumefaciens. Plant cell reports. 2007;26:459–465. doi: 10.1007/s00299-006-0257-8. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2020).Cao X, Dong Z, Tian D, Dong L, Qian W, Liu X, Qin H, Zhai W, Gao C. Development and characterization of markerfree and transgene insertion sitedefined transgenic wheat with improved grain storability and fatty acid content. Plant Biotechnology Journal. 2020;18:129–140. doi: 10.1111/pbi.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Villaseñor et al. (2020).Carreras-Villaseñor N, Rico-Ruiz JG, Montes RAC, Yong-Villalobos L, López-Hernández JF, Martínez-Hernández P, Herrera-Estrella L, Herrera-Estrella A, López-Arredondo D. Assessment of the ptxD gene as a growth and selective marker in Trichoderma atroviride using P ccg6, a novel constitutive promoter. Microbial Cell Factories. 2020;19:1–19. doi: 10.1186/s12934-019-1269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell, Grant & Plaxton (1997).Carswell MC, Grant BR, Plaxton WC. Disruption of the phosphate-starvation response of oilseed rape suspension cells by the fungicide phosphonate. Planta. 1997;203:67–74. doi: 10.1007/s00050166. [DOI] [PubMed] [Google Scholar]
- Catalá & Salinas (2018).Catalá R, Salinas J. Tailoring crop nutrition to fight weeds. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:7456–7458. doi: 10.1073/pnas.1809311115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo et al. (2019).Cerezo S, Palomo-Ríos E, Mariem SB, Mercado JA, Pliego-Alfaro F. Use of fluorescent reporter genes in olive (Olea europaea L.) transformation. Acta Physiologiae Plantarum. 2019;41:49. doi: 10.1007/s11738-019-2839-4. [DOI] [Google Scholar]
- Cerqueira et al. (2017).Cerqueira A, Alves A, Berenguer H, Correia B, Gómez-Cadenas A, Diez JJ, Monteiro P, Pinto G. Phosphite shifts physiological and hormonal profile of Monterey pine and delays Fusarium circinatum progression. Plant Physiology and Biochemistry. 2017;114:88–99. doi: 10.1016/j.plaphy.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Changko et al. (2020).Changko S, Rajakumar PD, Young RE, Purton S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Applied Microbiology and Biotechnology. 2020;104:675–686. doi: 10.1007/s00253-019-10258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che et al. (2018).Che P, Anand A, Wu E, Sander JD, Simon MK, Zhu W, Sigmund AL, Zastrow-Hayes G, Miller M, Liu D. Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnology Journal. 2018;16:1388–1395. doi: 10.1111/pbi.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen R, Xu Q, Liu Y, Zhang J, Ren D, Wang G, Liu Y. Generation of transgene-free maize male sterile lines using the CRISPR/Cas9 system. Frontiers in plant science. 2018;9:1180. doi: 10.3389/fpls.2018.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong-Pérez & Angenon (2013).Chong-Pérez B, Angenon G. Strategies for generating marker-free transgenic plants. In: Sithole-Niang I, editor. Genetic Engineering (Intech Open). 2. 2013. pp. 17–48. [Google Scholar]
- Christiaens et al. (2018).Christiaens O, Dzhambazova T, Kostov K, Arpaia S, Joga MR, Urru I, Sweet J, Smagghe G. Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi-based GM plants. EFSA Supporting Publications. 2018;15:173. doi: 10.2903/sp.efsa.2018.EN-1424. [DOI] [Google Scholar]
- Confalonieri & Sparvoli (2019).Confalonieri M, Sparvoli F. Recent advances in Medicago spp. genetic engineering strategies. In: Frans J, de Bruijn, editors. The Model Legume Medicago Truncatula. Wiley; Hoboken: 2019. pp. 1149–1161. [DOI] [Google Scholar]
- Costas, White & Metcalf (2001).Costas AMG, White AK, Metcalf WW. Purification and characterization of a novel phosphorus-oxidizing enzyme from Pseudomonas stutzeri WM88. Journal of Biological Chemistry. 2001;276:17429–17436. doi: 10.1074/jbc.M011764200. [DOI] [PubMed] [Google Scholar]
- Cutolo et al. (2020).Cutolo E, Tosoni M, Barera S, Herrera-Estrella L, Dallósto L, Bassi R. A phosphite dehydrogenase variant with promiscuous access to nicotinamide cofactor pools sustains fast phosphite-dependent growth of transplastomic Chlamydomonas reinhardtii. Plants. 2020;9:473. doi: 10.3390/plants9040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbani et al. (2007).Darbani B, Eimanifar A, Stewart Jr CN, Camargo WN. Methods to produce marker-free transgenic plants. Biotechnology Journal: Healthcare Nutrition Technology. 2007;2:83–90. doi: 10.1002/biot.200600182. [DOI] [PubMed] [Google Scholar]
- Day & Goldschmidt-Clermont (2011).Day A, Goldschmidt-Clermont M. The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnology Journal. 2011;9:540–553. doi: 10.1111/j.1467-7652.2011.00604.x. [DOI] [PubMed] [Google Scholar]
- De-Bashan & Bashan (2004).De-Bashan LE, Bashan Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003) Water Research. 2004;38:4222–4246. doi: 10.1016/j.watres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- De Vetten et al. (2003).De Vetten N, Wolters A-M, Raemakers K, Meer Ivander, Stege Rter, Heeres E, Heeres P, Visser R. A transformation method for obtaining marker-free plants of a cross-pollinating and vegetatively propagated crop. Nature Biotechnology. 2003;21:439–442. doi: 10.1038/nbt801. [DOI] [PubMed] [Google Scholar]
- Diaconeasa et al. (2020).Diaconeasa Z, Ştirbu I, Xiao J, Leopold N, Ayvaz Z, Danciu C, Ayvaz H, Staˇnilaˇ A, Nistor M, Socaciu C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines. 2020;8:336. doi: 10.3390/biomedicines8090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinuma et al. (2001).Ebinuma H, Sugita K, Matsunaga E, Endo S, Yamada K, Komamine A. Systems for the removal of a selection marker and their combination with a positive marker. Plant Cell Reports. 2001;20:383–392. doi: 10.1007/s002990100344. [DOI] [PubMed] [Google Scholar]
- Eckerstorfer et al. (2019).Eckerstorfer MF, Dolezel M, Heissenberger A, Miklau M, Reichenbecher W, Steinbrecher RA, Waßmann F. An EU perspective on biosafety considerations for plants developed by genome editing and other new genetic modification techniques (nGMs) Frontiers in Bioengineering and Biotechnology. 2019;7:31. doi: 10.3389/fbioe.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder et al. (2018).Elder JR, Paul NC, Burin R, Guard J, Shah DH. Genomic organization and role of SPI-13 in nutritional fitness of Salmonella. International Journal of Medical Microbiology. 2018;308:1043–1052. doi: 10.1016/j.ijmm.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Esland, Larrea-Alvarez & Purton (2018).Esland L, Larrea-Alvarez M, Purton S. Selectable markers and reporter genes for engineering the chloroplast of Chlamydomonas reinhardtii. Biology. 2018;7:46. doi: 10.3390/biology7040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradini et al. (2011).Ferradini N, Nicolia A, Capomaccio S, Veronesi F, Rosellini D. A point mutation in the Medicago sativa GSA gene provides a novel, efficient, selectable marker for plant genetic engineering. Journal of Biotechnology. 2011;156:147–152. doi: 10.1016/j.jbiotec.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Franco-Bocanegra et al. (2019).Franco-Bocanegra DK, McAuley C, Nicoll JA, Boche D. Molecular mechanisms of microglial motility: changes in ageing and Alzheimer’s disease. Cell. 2019;8:639. doi: 10.3390/cells8060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Almodóvar et al. (2014).García-Almodóvar R, Petri C, Padilla I, Burgos L. Combination of site-specific recombination and a conditional selective marker gene allows for the production of marker-free tobacco plants. Plant Cell, Tissue and Organ Culture (PCTOC) 2014;116:205–215. doi: 10.1007/s11240-013-0396-x. [DOI] [Google Scholar]
- Ghazanfar & Irfan (2020).Ghazanfar M, Irfan M. Synthesis of iron oxide nanomaterials for biofuel applications. In: Manish S, Neha S, Mishra PK, Vijai Kumar G, editors. Nanomaterials in biofuels research. Singapore: Springer; 2020. pp. 275–307. [Google Scholar]
- Gómez-Merino & Trejo-Téllez (2015).Gómez-Merino FC, Trejo-Téllez LI. Biostimulant activity of phosphite in horticulture. Scientia Horticulturae. 2015;196:82–90. doi: 10.1016/j.scienta.2015.09.035. [DOI] [Google Scholar]
- Gómez-Merino & Trejo-Téllez (2016).Gómez-Merino FC, Trejo-Téllez LI. Conventional and novel uses of phosphite in horticulture: potentialities and challenges. Italus Hortus. 2016;23:1–13. [Google Scholar]
- González-Morales et al. (2020).González-Morales SI, Pacheco-Gutiérrez NB, Ramírez-Rodríguez CA, Brito-Bello AA, Estrella-Hernández P, Herrera-Estrella L, López-Arredondo DL. Metabolic engineering of phosphite metabolism in Synechococcus elongatus PCC 7942 as an effective measure to control biological contaminants in outdoor raceway ponds. Biotechnology for Biofuels. 2020;13:1–19. doi: 10.1186/s13068-019-1642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobbelaar (2012).Grobbelaar JU. Microalgae mass culture: the constraints of scaling-up. Journal of Applied Phycology. 2012;24:315–318. doi: 10.1007/s10811-011-9728-6. [DOI] [Google Scholar]
- Guo et al. (2007).Guo X-M, Zhang X-D, Liang R-Q, Zhang L-Q, Chen Y-F. Maize transformation using xylose isomerase gene as a selection marker. Journal of Plant Physiology and Molecular Biology. 2007;33:547–552. [PubMed] [Google Scholar]
- Gurel ey al. (2009).Gurel S, Gurel E, Kaur R, Wong J, Meng L, Tan H-Q, Lemaux PG. Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant cell reports. 2009;28:429–444. doi: 10.1007/s00299-008-0655-1. [DOI] [PubMed] [Google Scholar]
- Haldrup, Noerremark & Okkels (2001).Haldrup A, Noerremark M, Okkels FT. Plant selection principle based on xylose isomerase. In Vitro Cellular & Developmental Biology-Plant. 2001;37:114. doi: 10.1007/s11627-001-0022-1. [DOI] [Google Scholar]
- Hanson & Köhler (2001).Hanson MR, Köhler RH. GFP imaging: methodology and application to investigate cellular compartmentation in plants. Journal of Experimental Botany. 2001;52:529–539. doi: 10.1093/jexbot/52.356.529. [DOI] [PubMed] [Google Scholar]
- Hao et al. (2011).Hao J, Niu Y, Yang B, Gao F, Zhang L, Wang J, Hasi A. Transformation of a marker-free and vector-free antisense ACC oxidase gene cassette into melon via the pollen-tube pathway. Biotechnology Letters. 2011;33:55–61. doi: 10.1007/s10529-010-0398-2. [DOI] [PubMed] [Google Scholar]
- Hardy et al. (2016).Hardy FJ, Bouchard D, Burghdoff M, Hanowell R, LeDoux B, Preece E, Tuttle L, Williams G. Education and notification approaches for harmful algal blooms (HABs), Washington State, USA. Harmful Algae. 2016;60:70–80. doi: 10.1016/j.hal.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Heap (2018).Heap I. The international survey of herbicide resistant weeds. www.weedscience.com. [20 December 2019];2018
- Heuer et al. (2017a).Heuer A, Thomson E, Schmidt C, Berninghausen O, Becker T, Hurt E, Beckmann R. Cryo-EM structure of a late pre-40S ribosomal subunit from Saccharomyces cerevisiae. Elife. 2017a;6:e30189. doi: 10.7554/eLife.30189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer et al. (2017b).Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M, Delhaize E, Rouached H. Improving phosphorus use efficiency: a complex trait with emerging opportunities. The Plant Journal. 2017b;90:868–885. doi: 10.1111/tpj.13423. [DOI] [PubMed] [Google Scholar]
- Hirosse et al. (2012).Hirosse EH, Creste JE, Custt4ht=øAÁdio CC, Machado-Neto NB. In vitro growth of sweet potato fed with potassium phosphite. Acta Scientiarum Agronomy. 2012;34:85–91. doi: 10.1007/978-981-10-8031-9_34. [DOI] [Google Scholar]
- Hirota, Motomura & Kuroda (2019).Hirota R, Motomura K, Kuroda A. Biological phosphite oxidation and its application to phosphorus recycling. In: Ohtake H, Tsuneda S, editors. Phosphorus recovery and recycling. Singapore: Springer; 2019. pp. 499–513. [DOI] [Google Scholar]
- Hoffman (2015).Hoffman RM. Application of GFP imaging in cancer. Laboratory Investigation. 2015;95:432–452. doi: 10.1038/labinvest.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme et al. (2012).Holme IB, Dionisio G, Brinch-Pedersen H, Wendt T, Madsen CK, Vincze E, Holm PB. Cisgenic barley with improved phytase activity. Plant Biotechnology Journal. 2012;10:237–247. doi: 10.1111/j.1467-7652.2011.00660.x. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2016).Hu L, Li H, Qin R, Xu R, Li J, Li L, Wei P, Yang J. Plant phosphomannose isomerase as a selectable marker for rice transformation. Scientific Reports. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2004).Huang S, Gilbertson L, Adams T, Malloy K, Reisenbigler E, Birr D, Snyder M, Zhang Q, Luethy M. Generation of marker-free transgenic maize by regular two-border Agrobacterium transformation vectors. Transgenic research. Transgenic research. 2004;13:451–461. doi: 10.1007/s11248-004-1453-3. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang Z, Carter N, Lu H, Zhang Z, Wang-Pruski G. Translocation of phosphite encourages the protection against Phytophthora infestans in potato: the efficiency and efficacy. Pesticide Biochemistry and Physiology. 2018;152:122–130. doi: 10.1016/j.pestbp.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Hunter (2018).Hunter SR. Determining the risk of phosphite tolerance in Phytophthora species in New Zealand and the United States: a case study on the implications of long-term use of phosphite to control Phytophthora cinnamomi in avocado (Persea americana) MS thesis, University of Waikato; 2018. [Google Scholar]
- Jansing et al. (2019).Jansing J, Schiermeyer A, Schillberg S, Fischer R, Bortesi L. Genome editing in agriculture: technical and practical considerations. International Journal of Molecular Sciences. 2019;20:2888. doi: 10.3390/ijms20122888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekayinoluwa et al. (2020).Jekayinoluwa T, Tripathi JN, Obiero G, Muge E, Tripathi L. Phytochemical Analysis and Establishment of Embryogenic Cell Suspension and Agrobacterium-mediated Transformation for Farmer Preferred Cultivars of West African Plantain (Musa spp.) Plants. 2020;9:789. doi: 10.3390/plants9060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia et al. (2006).Jia H, Pang Y, Chen X, Fang R. Removal of the selectable marker gene from transgenic tobacco plants by expression of Cre recombinase from a tobacco mosaic virus vector through agroinfection. Transgenic Research. 2006;15:375–384. doi: 10.1007/s11248-006-0011-6. [DOI] [PubMed] [Google Scholar]
- Johnson & Alric (2013).Johnson X, Alric J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: metabolic constraints for carbon partitioning between oil and starch. Eukaryotic Cell. 2013;12:776–793. doi: 10.1128/EC.00318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen (2014).Jorgensen JM. Masters Theses and Doctoral Dissertations. 2014. The effects of different concentrations of phosphite on ectomycorrhiza formation by Pisolithus tinctorius in Castanea dentata. [Google Scholar]
- Joshi et al. (2009).Joshi S, Miguel JS, Schaart J, Broggini GAL, Szankowski I, Jacobsen E, Krens F, Schouten HJ. Approaches for development of cisgenic apples. Transgenic Plant Journal. 2009;3:40–46. [Google Scholar]
- Joyce & Sun (2020).Joyce PA, Sun Y. Biolistics-mediated gene delivery in sugarcane. Biolistic DNA Methods in Molecular Biology. 2020;2124:217–228. doi: 10.1007/978-1-0716-0356-7_11. [DOI] [PubMed] [Google Scholar]
- Juneja, Ceballos & Murthy (2013).Juneja A, Ceballos RM, Murthy GS. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies. 2013;6:4607–4638. doi: 10.3390/en6094607. [DOI] [Google Scholar]
- Kadoić Balaško et al. (2020).Kadoić Balaško M, Mikac KM, Bažok R, Lemic D. Modern techniques in Colorado potato beetle (Leptinotarsa decemlineata Say) control and resistance management: history review and future perspectives. Insects. 2020;11:581. doi: 10.3390/insects11090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda et al. (2014).Kanda K, Ishida T, Hirota R, Ono S, Motomura K, Ikeda T, Kitamura K, Kuroda A. Application of a phosphite dehydrogenase gene as a novel dominant selection marker for yeasts. Journal of Biotechnology. 2014;182:68–73. doi: 10.1016/j.jbiotec.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Kausch et al. (2019).Kausch AP, Nelson-Vasilchik K, Hague J, Mookkan M, Quemada H, Dellaporta S, Fragoso C, Zhang ZJ. Edit at will: genotype independent plant transformation in the era of advanced genomics and genome editing. Plant Science. 2019;281:186–205. doi: 10.1016/j.plantsci.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Keutgen, Tomaszewska-Sowa & Keutgen (2020).Keutgen N, Tomaszewska-Sowa M, Keutgen A. Chlorophyll fluorescence of Nicotiana tabacum expressing the green fluorescent protein. Photosynthetica. 2020;58:460–467. doi: 10.32615/ps.2019.178. [DOI] [Google Scholar]
- Key, Ma & Drake (2008).Key S, Ma JK, Drake PM. Genetically modified plants and human health. Journal of the Royal Society of Medicine. 2008;101:290–298. doi: 10.1258/jrsm.2008.070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, Nakamura & Mii (2011).Khan RS, Nakamura I, Mii M. Development of disease-resistant marker-free tomato by R/RS site-specific recombination. Plant cell reports. 2011;30:1041–1053. doi: 10.1007/s00299-011-1011-4. [DOI] [PubMed] [Google Scholar]
- Khattri, Nandy & Srivastava (2011).Khattri A, Nandy S, Srivastava V. Heat-inducible Cre-lox system for marker excision in transgenic rice. Journal of biosciences. 2011;36:37–42. doi: 10.1007/s12038-011-9010-8. [DOI] [PubMed] [Google Scholar]
- Kleidon (2020).Kleidon J, Brinin A, Paul J-Y, Harding R, Dale J, Dugdale B. Production of selectable marker gene-free Cavendish banana (Musa spp.) using a steroid-inducible recombinase platform. Transgenic research. 2020;29:81–93. doi: 10.1007/s11248-019-00179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh et al. (2011).Koh S, Kim H, Kim J, Goo E, Kim YJ, Choi O, Jwa NS, Ma J, Nagamatsu T, Moon JS. A novel lightdependent selection marker system in plants. Plant Biotechnology Journal. 2011;9:348–358. doi: 10.1111/j.1467-7652.2010.00557.x. [DOI] [PubMed] [Google Scholar]
- Kondrák, van Der Meer & Bánfalvi (2006).Kondrák M, van Der Meer IM, Bánfalvi Z. Generation of marker-and backbone-free transgenic potatoes by site-specific recombination and a bi-functional marker gene in a non-regular one-border. Transgenic Research. 2006;15:729–737. doi: 10.1007/s11248-006-9021-7. [DOI] [PubMed] [Google Scholar]
- Kortstee et al. (2011).Kortstee A, Khan S, Helderman C, Trindade L, Wu Y, Visser R, Brendolise C, Allan A, Schouten H, Jacobsen E. Anthocyanin production as a potential visual selection marker during plant transformation. Transgenic Research. 2011;20:1253–1264. doi: 10.1007/s11248-011-9490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs (2000).Krebs J. GM food safety: facts, uncertainties, and assessment. Final report on the OECD Edinburgh conference on the scientific and health aspects of genetically modified foods; 2000. http://www.oecd.org/subject/biotech/edinburgh.htm . [Google Scholar]
- Kumar, Arul & Talwar (2010).Kumar S, Arul L, Talwar D. Generation of marker-free Bt transgenicindica rice and evaluation of its yellow stem borer resistance. Journal of Applied Genetics. 2010;51:243–257. doi: 10.1007/BF03208854. [DOI] [PubMed] [Google Scholar]
- Kumar et al. (2012).Kumar S, Verma AK, Das M, Dwivedi PD. Allergenic diversity among plant and animal food proteins. Food Reviews International. 2012;28:277–298. doi: 10.1080/87559129.2011.635391. [DOI] [Google Scholar]
- LaFayette et al. (2005).LaFayette P, Kane P, Phan B, Parrott W. Arabitol dehydrogenase as a selectable marker for rice. Plant cell reports. 2005;24:596–602. doi: 10.1007/s00299-005-0015-3. [DOI] [PubMed] [Google Scholar]
- Lai et al. (2011).Lai et al. (2011) F-M, Privalle L, Mei K, Ghoshal D, Shen Y, Klucinec J, Daeschner K, Mankin LS, Chen N, Cho S. Evaluation of the E. coli D-serine ammonia lyase gene (Ec. dsdA) for use as a selectable marker in maize transformation. In Vitro Cellular & Developmental Biology-Plant. 2011;47:467. doi: 10.1007/s11627-011-9351-x. [DOI] [Google Scholar]
- Lal et al. (2020).Lal M, Bhardwaj E, Chahar N, Dangwal M, Das S. (Trans) gene flow: mechanisms, biosafety concerns and mitigation for containment. In: Tandon R, Shivanna K, Koul M, editors. Reproductive ecology of flowering plants: patterns and processes. Singapore: Springer; 2020. pp. 335–394. [Google Scholar]
- Lam et al. (2018).Lam TP, Lee T-M, Chen C-Y, Chang J-S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresource Technology. 2018;252:180–187. doi: 10.1016/j.biortech.2017.12.088. [DOI] [PubMed] [Google Scholar]
- Lanigan, Kopera & Saunders (2020).Lanigan TM, Kopera HC, Saunders TL. Principles of genetic engineering. Gene. 2020;11:291. doi: 10.3390/genes11030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2005).Lee TM, Tsai PF, Shyu YT, Sheu F. The effects of phosphite on phosphate starvation responses of Ulva Lactuca (Ulvales, Chlorophyta) 1. Journal of Phycology. 2005;41:975–982. doi: 10.1111/j.1529-8817.2005.00119.x. [DOI] [Google Scholar]
- Leong, Lu & Chiou (2018).Leong SJ, Lu W-C, Chiou T-J. Phosphite-mediated suppression of anthocyanin accumulation regulated by mitochondrial ATP synthesis and sugars in Arabidopsis. Plant and Cell Physiology. 2018;59:1158–1169. doi: 10.1093/pcp/pcy051. [DOI] [PubMed] [Google Scholar]
- Lepais et al. (2020).Lepais O, Chancerel E, Boury C, Salin F, Manicki A, Taillebois L, Dutech C, Aissi A, Bacles CF, Daverat F. Fast sequence-based microsatellite genotyping development workflow. PeerJ. 2020;8:e9085. doi: 10.7717/peerj.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Xie & Qiu (2009).Li B, Xie C, Qiu H. Production of selectable marker-free transgenic tobacco plants using a non-selection approach: chimerism or escape, transgene inheritance, and efficiency. Plant Cell Reports. 2009;28:373–386. doi: 10.1007/s00299-008-0640-8. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li S, Cong Y, Liu Y, Wang T, Shuai Q, Chen N, Gai J, Li Y. Optimization of Agrobacterium-mediated transformation in soybean. Frontiers in plant science. 2017;8:246. doi: 10.3389/fpls.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2007).Li Z, Xing A, Moon BP, Burgoyne SA, Guida AD, Liang H, Lee C, Caster CS, Barton JE, Klein TM. Cre/loxP-mediated self-activating gene excision system to produce marker gene free transgenic soybean plants. Plant Molecular Biology. 2007;65:329–341. doi: 10.1007/s11103-007-9223-2. [DOI] [PubMed] [Google Scholar]
- Liberati et al. (2009).Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2020).Liu H-Y, Ke W, Jing W, DU L-p, PEI X-w, YE X-g. Genetic and agronomic traits stability of marker-free transgenic wheat plants generated from Agrobacterium-mediated co-transformation in T2 and T3 generations. Journal of Integrative Agriculture. 2020;19:23–32. doi: 10.1016/S2095-3119(19)62601-8. [DOI] [Google Scholar]
- Liu et al. (2011).Liu X, Jin W, Liu J, Zhao H, Guo A. Transformation of wheat with the HMW-GS 1Bx14 gene without markers. Russian Journal of Genetics. 2011;47:182–188. doi: 10.1134/S1022795411010066. [DOI] [PubMed] [Google Scholar]
- Loera-Quezada et al. (2015).Loera-Quezada MM, Leyva-González MA, López-Arredondo D, Herrera-Estrella L. Phosphite cannot be used as a phosphorus source but is non-toxic for microalgae. Plant Science. 2015;231:124–130. doi: 10.1016/j.plantsci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Loera-Quezada et al. (2016).Loera-Quezada MM, Leyva-González MA, Velázquez-Juárez G, Sanchez-Calderón L, Do Nascimento M, López-Arredondo D, Herrera-Estrella L. A novel genetic engineering platform for the effective management of biological contaminants for the production of microalgae. Plant Biotechnology Journal. 2016;14:2066–2076. doi: 10.1111/pbi.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo & Herrera-Estrella (2012).López-Arredondo DL, Herrera-Estrella L. Engineering phosphorus metabolism in plants to produce a dual fertilization and weed control system. Nature Biotechnology. 2012;30:889–893. doi: 10.1038/nbt.2346. [DOI] [PubMed] [Google Scholar]
- López-Arredondo & Herrera-Estrella (2013).López-Arredondo DL, Herrera-Estrella L. A novel dominant selectable system for the selection of transgenic plants under in vitro and greenhouse conditions based on phosphite metabolism. Plant Biotechnology Journal. 2013;11:516–525. doi: 10.1111/pbi.12063. [DOI] [PubMed] [Google Scholar]
- Lopez-Arredondo et al. (2014).Lopez-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L. Phosphate nutrition: improving low-phosphate tolerance in crops. Annual Review of Plant Biology. 2014;65:95–123. doi: 10.1146/annurev-arplant-050213-035949. [DOI] [PubMed] [Google Scholar]
- Low et al. (2018).Low L-Y, Yang S-K, Kok D-XA, Ong-Abdullah J, Tan N-P, Lai K-S. New Visions in Plant Science. IntechOpen; London, UK: 2018. Transgenic plants: gene constructs, vector and transformation method; pp. 41–61. [Google Scholar]
- Lu & Snow (2005).Lu B-R, Snow AA. Gene flow from genetically modified rice and its environmental consequences. BioScience. 2005;55:669–678. doi: 10.1641/0006-3568(2005)055[0669:GFFGMR]2.0.CO;2. [DOI] [Google Scholar]
- Luna & Dowd-Uribe (2020).Luna JK, Dowd-Uribe B. Knowledge politics and the Bt cotton success narrative in Burkina Faso. World Development. 2020;136:105127. doi: 10.1016/j.worlddev.2020.105127. [DOI] [Google Scholar]
- Lynch et al. (2019).Lynch KM, Zannini E, Wilkinson S, Daenen L, Arendt EK. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Comprehensive Reviews in Food Science and Food Safety. 2019;18:587–625. doi: 10.1111/1541-4337.12440. [DOI] [PubMed] [Google Scholar]
- Ma (2005).Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annual Review of Plant Biology. 2005;56:393–434. doi: 10.1146/annurev.arplant.55.031903.141717. [DOI] [PubMed] [Google Scholar]
- Mallin & Cahoon (2020).Mallin MA, Cahoon LB. The Hidden Impacts of Phosphorus Pollution to Streams and Rivers. BioScience. 2020;70:315–329. doi: 10.1093/biosci/biaa001. [DOI] [Google Scholar]
- Malnoy et al. (2010).Malnoy M, Boresjza-Wysocka EE, Norelli JL, Flaishman MA, Gidoni D, Aldwinckle HS. Genetic transformation of apple (Malus x domestica) without use of a selectable marker gene. Tree Genetics & Genomes. 2010;6:423–433. doi: 10.1007/s11295-009-0260-7. [DOI] [Google Scholar]
- Manimaran et al. (2011).Manimaran P, Ramkumar G, Sakthivel K, Sundaram R, Madhav M, Balachandran S. Suitability of non-lethal marker and marker-free systems for development of transgenic crop plants: present status and future prospects. Biotechnology Advances. 2011;29:703–714. doi: 10.1016/j.biotechadv.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Manna et al. (2016).Manna M, Achary VMM, Islam T, Agrawal PK, Reddy MK. The development of a phosphite-mediated fertilization and weed control system for rice. Scientific Reports. 2016;6:24941. doi: 10.1038/srep24941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna et al. (2015).Manna M, Islam T, Kaul T, Reddy CS, Fartyal D, James D, Reddy MK. A comparative study of effects of increasing concentrations of phosphate and phosphite on rice seedlings. Acta Physiologiae Plantarum. 2015;37:258. doi: 10.1007/s11738-015-2016-3. [DOI] [Google Scholar]
- Matthews et al. (2001).Matthews PR, Wang M-B, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV. Marker gene elimination from transgenic barley, using co-transformation with adjacenttwin T-DNAs’ on a standard Agrobacterium transformation vector. Molecular Breeding. 2001;7:195–202. doi: 10.1023/A:1011333321893. [DOI] [Google Scholar]
- McCormac et al. (2001).McCormac AC, Fowler MR, Chen D-F, Elliott MC. Efficient co-transformation of Nicotiana tabacum by two independent T-DNAs, the effect of T-DNA size and implications for genetic separation. Transgenic Research. 2001;10:143–155. doi: 10.1023/A:1008909203852. [DOI] [PubMed] [Google Scholar]
- McDonald, Grant & Plaxton (2001).McDonald AE, Grant BR, Plaxton WC. Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. Journal of Plant Nutrition. 2001;24:1505–1519. doi: 10.1081/PLN-100106017. [DOI] [Google Scholar]
- Mehta et al. (2021).Mehta D, Ghahremani M, Pérez-Fernández M, Tan M, Schläpfer P, Plaxton WC, Uhrig RG. Phosphate and phosphite have a differential impact on the proteome and phosphoproteome of Arabidopsis suspension cell cultures. The Plant Journal. 2021;105:924–941. doi: 10.1111/tpj.15078. [DOI] [PubMed] [Google Scholar]
- Miki & McHugh (2004).Miki B, McHugh S. Selectable marker genes in transgenic plants: applications, alternatives and biosafety. Journal of Biotechnology. 2004;107:193–232. doi: 10.1016/j.jbiotec.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2002).Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao Z-Y. High efficiency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA binary system. Transgenic Research. 2002;11:381–396. doi: 10.1023/A:1016390621482. [DOI] [PubMed] [Google Scholar]
- Molina-Márquez et al. (2019).Molina-Márquez A, Vila M, Vigara J, Borrero A, León R. The Bacterial Phytoene Desaturase-Encoding Gene (CRTI) is an Efficient Selectable Marker for the Genetic Transformation of Eukaryotic Microalgae. Metabolites. 2019;9:49. doi: 10.3390/metabo9030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore & Srivastava (2008).Moore SK, Srivastava V. A bacterial haloalkane dehalogenase (dhlA) gene as conditional negative selection marker for rice callus cells. In Vitro Cellular & Developmental Biology-Plant. 2008;44:468–473. [Google Scholar]
- Mortensen et al. (2019).Mortensen S, Bernal-Franco D, Cole LF, Sathitloetsakun S, Cram EJ, Lee-Parsons CW. EASI transformation: an efficient transient expression method for analyzing gene function in Catharanthus roseus seedlings. Frontiers in Plant Science. 2019;10:755. doi: 10.3389/fpls.2019.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahampun et al. (2016).Nahampun HN, López-Arredondo D, Xu X, Herrera-Estrella L, Wang K. Assessment of ptxD gene as an alternative selectable marker for Agrobacterium-mediated maize transformation. Plant Cell Reports. 2016;35:1121–1132. doi: 10.1007/s00299-016-1942-x. [DOI] [PubMed] [Google Scholar]
- Naing et al. (2015).Naing KW, Nguyen XH, Anees M, Lee YS, Kim YC, Kim SJ, Kim MH, Kim YH, Kim KY. Biocontrol of Fusarium wilt disease in tomato by Paenibacillus ehimensis KWN38. World Journal of Microbiology and Biotechnology. 2015;31:165–174. doi: 10.1007/s11274-014-1771-4. [DOI] [PubMed] [Google Scholar]
- Narancio et al. (2020).Narancio R, Ding Y-L, Lin Y-H, Sahab S, Panter S, Hayes M, John U, Anderson H, Mason J, Spangenberg G. Application of linked and unlinked co-transformation to generate triple stack, marker-free, transgenic white clover (Trifolium repens L.). Plant Cell. Tissue and Organ Culture (PCTOC) 2020;142:635–646. doi: 10.1007/s11240-020-01891-6. [DOI] [Google Scholar]
- Neumann, Kumar & Imani (2020).Neumann K-H, Kumar A, Imani J. Genetic problems and gene technology. In: Neumann K, editor. Plant cell and tissue culture–a tool in biotechnology. Cham: Springer; 2020. pp. 337–435. [DOI] [Google Scholar]
- Ng, Keskin & Tan (2020).Ng IS, Keskin BB, Tan SI. A critical review of genome editing and synthetic biology applications in metabolic engineering of microalgae and cyanobacteria. Biotechnology Journal. 2020;15:1900228. doi: 10.1002/biot.201900228. [DOI] [PubMed] [Google Scholar]
- O’Callaghan et al. (2005).O’Callaghan M, Glare TR, Burgess EP, Malone LA. Effects of plants genetically modified for insect resistance on nontarget organisms. Annual Review of Entomology. 2005;50:271–292. doi: 10.1146/annurev.ento.50.071803.130352. [DOI] [PubMed] [Google Scholar]
- Ogawa et al. (2008).Ogawa T, Kawahigashi H, Toki S, Handa H. Efficient transformation of wheat by using a mutated rice acetolactate synthase gene as a selectable marker. Plant cell reports. 2008;27:1325–1331. doi: 10.1007/s00299-008-0553-6. [DOI] [PubMed] [Google Scholar]
- Oliveira et al. (2007).Oliveira AR, Castro TR, Capalbo DM, Delalibera I. Toxicological evaluation of genetically modified cotton (Bollgard®) and Dipel® WP on the non-target soil mite Scheloribates praeincisus (Acari: Oribatida) Experimental and Applied Acarology. 2007;41:191–201. doi: 10.1007/s10493-007-9059-0. [DOI] [PubMed] [Google Scholar]
- Olorunniji, Rosser & Stark (2016).Olorunniji FJ, Rosser SJ, Stark WM. Site-specific recombinases: molecular machines for the Genetic Revolution. Biochemical Journal. 2016;473:673–684. doi: 10.1042/BJ20151112. [DOI] [PubMed] [Google Scholar]
- Pandey et al. (2020).Pandey P, Srivastava S, Pandey AK, Dubey RS. Abiotic-stress tolerance in plants-system biology approach. In: Tripathi DK, Singh VP, Chauhan DK, Sharma S, Prasad SM, Dubey NK, Ramawat N, editors. Plant life under changing environment. Academic Press, Amsterdam: Elsevier; 2020. pp. 577–609. [Google Scholar]
- Pandeya et al. (2017).Pandeya D, Campbell LM, Nunes E, Lopez-Arredondo DL, Janga MR, Herrera-Estrella L, Rathore KS. ptxD gene in combination with phosphite serves as a highly effective selection system to generate transgenic cotton (Gossypium hirsutum L.) Plant Molecular Biology. 2017;95:567–577. doi: 10.1007/s11103-017-0670-0. [DOI] [PubMed] [Google Scholar]
- Pandeya et al. (2018).Pandeya D, López-Arredondo DL, Janga MR, Campbell LM, Estrella-Hernández P, Bagavathiannan MV, Herrera-Estrella L, Rathore KS. Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:e6946–E6955. doi: 10.1073/pnas.1804862115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhi et al. (2005).Parkhi V, Rai M, Tan J, Oliva N, Rehana S, Bandyopadhyay A, Torrizo L, Ghole V, Datta K, Datta S. Molecular characterization of marker-free transgenic lines of indica rice that accumulate carotenoids in seed endosperm. Molecular Genetics and Genomics. 2005;274:325–336. doi: 10.1007/s00438-005-0030-7. [DOI] [PubMed] [Google Scholar]
- Parray, Mir & Shameem (2019).Parray JA, Mir MY, Shameem N. Agriculture: biotechniques in plant biology. Singapore: Springer; 2019. Plant genetic engineering and GM crops: merits and demerits; pp. 155–229. [DOI] [Google Scholar]
- Patterson et al. (2019).Patterson EL, Saski C, Küpper A, Beffa R, Gaines TA. Omics potential in herbicide-resistant weed management. Plants. 2019;8:607. doi: 10.3390/plants8120607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna & Ganapathi (2010).Penna S, Ganapathi TR. Engineering the plant genome: prospects of selection systems using nonantibiotic marker genes. GM Crops. 2010;1:128–136. doi: 10.4161/gmcr.1.3.12383. [DOI] [PubMed] [Google Scholar]
- Penna, Sági & Swennen (2002).Penna S, Sági L, Swennen R. Positive selectable marker genes for routine plant transformation. In Vitro Cellular & Developmental Biology-Plant. 2002;38:125–128. doi: 10.1079/IVP2001272. [DOI] [Google Scholar]
- Permingeat et al. (2003).Permingeat HR, Alvarez ML, Cervigni GD, Ravizzini RA, Vallejos RH. Stable wheat transformation obtained without selectable markers. Plant Molecular Biology. 2003;52:415–419. doi: 10.1023/A:1023969501440. [DOI] [PubMed] [Google Scholar]
- Petri et al. (2011).Petri C, Hily JM, Vann C, Dardick C, Scorza R. A high-throughput transformation system allows the regeneration of marker-free plum plants (Prunus domestica) Annals of Applied Biology. 2011;159:302–315. doi: 10.1111/j.1744-7348.2011.00499.x. [DOI] [Google Scholar]
- Philips et al. (2019).Philips JG, Dudley KJ, Waterhouse PM, Hellens RP. The rapid methylation of T-DNAs upon Agrobacterium inoculation in plant leaves. Frontiers in Plant Science. 2019;10:312. doi: 10.3389/fpls.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton & Carswell (2018).Plaxton WC, Carswell MC. Metabolic aspects of the phosphate starvation response in plants. In: Lerner HR, editor. Plant responses to environmental stresses. Routledge; 2018. pp. 349–372. [Google Scholar]
- Pontiroli et al. (2007).Pontiroli A, Simonet P, Frostegard A, Vogel TM, Monier J-M. Fate of transgenic plant DNA in the environment. Environmental Biosafety Research. 2007;6:15–35. doi: 10.1051/ebr:2007037. [DOI] [PubMed] [Google Scholar]
- Porto et al. (2014).Porto MS, Pinheiro MPN, Batista VGL, dos Santos RC, De Albuquerque Melo Filho P, De Lima LM. Plant promoters: an approach of structure and function. Molecular Biotechnology. 2014;56:38–49. doi: 10.1007/s12033-013-9713-1. [DOI] [PubMed] [Google Scholar]
- Prakash et al. (2009).Prakash NS, Bhojaraja R, Shivbachan S, Priya GH, Nagraj T, Prasad V, Babu VS, Jayaprakash T, Dasgupta S, Spencer TM. Marker-free transgenic corn plant production through co-bombardment. Plant Cell Reports. 2009;28:1655. doi: 10.1007/s00299-009-0765-4. [DOI] [PubMed] [Google Scholar]
- Puchta (2000).Puchta H. Removing selectable marker genes: taking the shortcut. Trends in Plant Science. 2000;5:273–274. doi: 10.1016/S1360-1385(00)01684-8. [DOI] [PubMed] [Google Scholar]
- Quispe-Huamanquispe et al. (2019).Quispe-Huamanquispe DG, Gheysen G, Yang J, Jarret R, Rossel G, Kreuze JF. The horizontal gene transfer of Agrobacterium T-DNAs into the series Batatas (Genus Ipomoea) genome is not confined to hexaploid sweetpotato. Scientific Reports. 2019;9:1–13. doi: 10.1038/s41598-019-48691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamanaRao & Veluthambi (2010).RamanaRao MV, Veluthambi K. Selectable marker elimination in the T 0 generation by Agrobacterium-mediated co-transformation involving Mungbean yellow mosaic virusTrAP as a non-conditional negative selectable marker and bar for transient positive selection. Plant Cell Reports. 2010;29:473–483. doi: 10.1007/s00299-010-0836-6. [DOI] [PubMed] [Google Scholar]
- Ramessar et al. (2007).Ramessar K, Peremarti A, Gómez-Galera S, Naqvi S, Moralejo M, Munoz P, Capell T, Christou P. Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: a case of the science not supporting the politics. Transgenic Research. 2007;16:261–280. doi: 10.1007/s11248-007-9083-1. [DOI] [PubMed] [Google Scholar]
- Rampersad (2020).Rampersad SN. Pathogenomics and management of Fusarium diseases in plants. Pathogens. 2020;9:340. doi: 10.3390/pathogens9050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao et al.(2011).Rao MVR, Parameswari C, Sripriya R, Veluthambi K. Transgene stacking and marker elimination in transgenic rice by sequential Agrobacterium-mediated co-transformation with the same selectable marker gene. Plant Cell Reports. 2011;30:1241–1252. doi: 10.1016/S1360-1385(00)01684-8. [DOI] [PubMed] [Google Scholar]
- Ravanfar et al. (2017).Ravanfar SA, Orbovic V, Moradpour M, Aziz MAbdul, Karan R, Wallace S, Parajuli S. Improvement of tissue culture, genetic transformation, and applications of biotechnology to Brassica. Biotechnology and Genetic Engineering Reviews. 2017;33:1–25. doi: 10.1080/02648725.2017.1309821. [DOI] [PubMed] [Google Scholar]
- Riva et al. (2020).Riva F, Riva V, Eckert EM, Colinas N, Di Cesare A, Borin S, Mapelli F, Crotti E. An environmental escherichia coli strain is naturally competent to acquire exogenous DNA. Frontiers in Microbiology. 2020;11:574301. doi: 10.3389/fmicb.2020.574301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosellini (2012).Rosellini D. Selectable markers and reporter genes: a well furnished toolbox for plant science and genetic engineering. Critical Reviews in Plant Sciences. 2012;31:401–453. doi: 10.1080/07352689.2012.683373. [DOI] [Google Scholar]
- Rosellini & Veronesi (2007).Rosellini D, Veronesi F. Safe genetically engineered plants. Journal of Physics: Condensed Matter. 2007;19:395005–395012. [Google Scholar]
- Rukavtsova et al. (2013).Rukavtsova E, Lebedeva A, Zakharchenko N, Buryanov YI. The ways to produce biologically safe marker-free transgenic plants. Russian Journal of Plant Physiology. 2013;60:14–26. doi: 10.1134/S1021443712060131. [DOI] [Google Scholar]
- Saelim et al. (2009).Saelim L, Phansiri S, Suksangpanomrung M, Netrphan S, Narangajavana J. Evaluation of a morphological marker selection and excision system to generate marker-free transgenic cassava plants. Plant cell reports. 2009;28:445–455. doi: 10.1007/s00299-011-1033-y. [DOI] [PubMed] [Google Scholar]
- Schaart et al. (2004).Schaart JG, Krens FA, Pelgrom KT, Mendes O, Rouwendal GJ. Effective production of marker-free transgenic strawberry plants using inducible site-specific recombination and a bifunctional selectable marker gene. Plant Biotechnology Journal. 2004;2:233–240. doi: 10.1111/j.1467-7652.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Schaart et al. (2011).Schaart JG, Krens FA, Wolters AMA, Visser RG. Transformation methods for obtaining marker-free genetically modified plants. In: Steward CN, Touraev A, Citovsky V, Tzfira T, editors. Plant Transformation Technologies. Wiley-Blackwell; Oxford: 2011. pp. 229–242. [Google Scholar]
- Schmale et al. (2019).Schmale DG, Ault AP, Saad W, Scott D, Westwrick J. Perspectives on Harmful Algal Blooms (HABs) and the cyberbiosecurity of freshwater systems. Frontiers in Bioengineering and Biotechnology. 2019;7:128. doi: 10.3389/fbioe.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiari et al. (2018).Setiari N, Purwantoro A, Moeljopawiro S, Semiarti E. Micropropagation of Dendrobium phalaenopsis orchid through overexpression of embryo gene AtRKD4. AGRIVITA. Journal of Agricultural Science. 2018;40:284–294. [Google Scholar]
- Shaw, Twilton & MacMillan (2016).Shaw MH, Twilton J, MacMillan DW. Photoredox catalysis in organic chemistry. The Journal of Organic Chemistry. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2020).Shi F, Tan M, Mo Q, Chen X, Zhao Y, Guo X, Jiang N. Application of the phosphomannose-isomerase/mannose selection system in the Agrobacterium-mediated transformation of Lonicera hypoglauca Miq. Journal of Plant Biochemistry and Biotechnology. 2020;29:528–538. doi: 10.1007/s13562-020-00570-z. [DOI] [Google Scholar]
- Shi et al. (2017).Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE. ARGOS 8 variants generated by CRISPRCas9 improve maize grain yield under field drought stress conditions. Plant Biotechnology Journal. 2017;15:207–216. doi: 10.1111/pbi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2017).Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE. ARGOS 8 variants generated by CRISPRCas9 improve maize grain yield under field drought stress conditions. Plant Biotechnology Journal. 2017;15:207–216. doi: 10.1111/pbi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawat & Armstrong (2018).Shrawat AK, Armstrong CL. Development and application of genetic engineering for wheat improvement. Critical Reviews in Plant Sciences. 2018;37:335–421. doi: 10.1080/07352689.2018.1514718. [DOI] [Google Scholar]
- Shrawat & Lörz (2006).Shrawat AK, Lörz H. Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnology Journal. 2006;4:575–603. doi: 10.1111/j.1467-7652.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- Silverman (2001).Silverman RB. Abstracts, division of biological chemistry, 222nd national meeting of the American Chemical Society, August 26-29, 2001. Biochemistry. 2001;40:8607–8664. doi: 10.1021/bi0151420. [DOI] [Google Scholar]
- Singh et al. (2019).Singh BR, Gupta VK, Deeba F, Bajpai R, Pandey V, Naqvi AH, Upreti DK, Gathergood N, Jiang Y, Enshasy HAEl. Non-toxic and ultra-small biosilver nanoclusters trigger apoptotic cell death in fluconazole-resistant Candida albicans via Ras signaling. Biomolecules. 2019;9:47. doi: 10.3390/biom9020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al. (2011).Singh S, Rathore M, Goyary D, Singh RK, Anandhan S, Sharma DK, Ahmed Z. Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant cell reports. 2011;30:1019–1028. doi: 10.1007/s00299-011-1007-0. [DOI] [PubMed] [Google Scholar]
- Smith, Baker & Steele (2000).Smith DT, Baker RV, Steele GL. Palmer amaranth (Amaranthus palmeri) impacts on yield, harvesting, and ginning in dryland cotton (Gossypium hirsutum) Weed Technology. 2000;14:122–126. doi: 10.1614/0890-037X(2000)014[0122:PAAPIO]2.0.CO;2. [DOI] [Google Scholar]
- Solis-Palacios et al. (2021).Solis-Palacios R, Hernández-Ramírez G, Salinas-Ruiz J, Hidalgo-Contreras JV, Gómez-Merino FC. Effect and Compatibility of Phosphite with Trichoderma sp. Isolates in the Control of the Fusarium Species Complex Causing Pokkah Boeng in Sugarcane. Agronomy. 2021;11:1099. doi: 10.3390/agronomy11061099. [DOI] [Google Scholar]
- Souza et al. (2019).Souza CS, Marucci RC, Chaves DR, Mendes SM. Effects of genetically modified plants with Bt toxins on natural enemies. In: Souza B, Vázquez L, Marucci R, editors. Natural enemies of insect pests in neotropical agroecosystems. Cham: Springer; 2019. pp. 489–496. [DOI] [Google Scholar]
- Sripriya, Raghupathy & Veluthambi (2008).Sripriya R, Raghupathy V, Veluthambi K. Generation of selectable marker-free sheath blight resistant transgenic rice plants by efficient co-transformation of a cointegrate vector T-DNA and a binary vector T-DNA in one Agrobacterium tumefaciens strain. Plant Cell Reports. 2008;27:1635. doi: 10.1007/s00299-008-0586-x. [DOI] [PubMed] [Google Scholar]
- Sripriya et al. (2011).Sripriya R, Sangeetha M, Parameswari C, Veluthambi B, Veluthambi K. Improved Agrobacterium-mediated co-transformation and selectable marker elimination in transgenic rice by using a high copy number pBin19-derived binary vector. Plant Science. 2011;180:766–774. doi: 10.1016/j.plantsci.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Srivastava (2019).Srivastava N. Molecular and biotechnological tools in developing abiotic stress tolerance in wheat. In: Hasanuzzaman M, Nahar K, Hossain M, editors. Wheat production in changing environments. Singapore: Springer; 2019. pp. 283–341. [DOI] [Google Scholar]
- Stewart (2001).Stewart C. The utility of green fluorescent protein in transgenic plants. Plant Cell Reports. 2001;20:376–382. doi: 10.1007/s002990100346. [DOI] [PubMed] [Google Scholar]
- Stoger et al. (2002).Stoger E, Sack M, Perrin Y, Vaquero C, Torres E, Twyman RM, Christou P, Fischer R. Practical considerations for pharmaceutical antibody production in different crop systems. Molecular Breeding. 2002;9:149–158. doi: 10.1023/A:1019714614827. [DOI] [Google Scholar]
- Su et al. (2019).Su J, Jiang J, Zhang F, Liu Y, Ding L, Chen S, Chen F. Current achievements and future prospects in the genetic breeding of chrysanthemum: a review. Horticulture Research. 2019;6:1–19. doi: 10.1038/s41438-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukarno, Smith & Scott (1993).Sukarno N, Smith S, Scott E. The effect of fungicides on vesicular–arbuscular mycorrhizal symbiosis: I. The effects on vesicular–arbuscular mycorrhizal fungi and plant growth. New Phytologist. 1993;125:139–147. doi: 10.1111/j.1469-8137.1993.tb03872.x. [DOI] [PubMed] [Google Scholar]
- Sundar & Sakthivel (2008).Sundar IK, Sakthivel N. Advances in selectable marker genes for plant transformation. Journal of Plant Physiology. 2008;165:1698–1716. doi: 10.1016/j.jplph.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tanner et al. (2020).Tanner JD, Chen KY, Bonnart RM, Minas IS, Volk GM. Considerations for large-scale implementation of dormant budwood cryopreservation. Plant Cell, Tissue and Organ Culture (PCTOC) 2020;144:35–48. doi: 10.1007/s11240-020-01884-5. [DOI] [Google Scholar]
- Thao et al. (2008a).Thao HTB, Yamakawa T, Myint AK, Sarr PS. Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.) Soil Science & Plant Nutrition. 2008a;54:761–768. doi: 10.1111/j.1747-0765.2008.00290.x. [DOI] [Google Scholar]
- Thao et al. (2008b).Thao HTB, Yamakawa T, Shibata K, Sarr PS, Myint AK. Growth response of komatsuna (Brassica rapa var. peruviridis) to root and foliar applications of phosphite. Plant and Soil. 2008b;308:1–10. doi: 10.1007/s11104-008-9598-0. [DOI] [Google Scholar]
- Ticconi, Delatorre & Abel (2001).Ticconi CA, Delatorre CA, Abel S. Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiology. 2001;127:963–972. doi: 10.1104/pp.010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougou et al. (2009).Tougou M, Yamagishi N, Furutani N, Kaku K, Shimizu T, Takahata Y, Sakai J-i, Kanematsu S, Hidaka S. The application of the mutated acetolactate synthase gene from rice as the selectable marker gene in the production of transgenic soybeans. Plant cell reports. 2009;28:769–776. doi: 10.1007/s00299-009-0679-1. [DOI] [PubMed] [Google Scholar]
- Tougou et al. (2009).Tougou M, Yamagishi N, Furutani N, Kaku K, Shimizu T, Takahata Y, Sakai J-i, Kanematsu S, Hidaka S. The application of the mutated acetolactate synthase gene from rice as the selectable marker gene in the production of transgenic soybeans. Plant cell reports. 2009;28:769–776. doi: 10.1007/s00299-009-0679-1. [DOI] [PubMed] [Google Scholar]
- Tran et al. (2020).Tran PHN, Ko JK, Gong G, Um Y, Lee S-M. Improved simultaneous co-fermentation of glucose and xylose by Saccharomyces cerevisiae for efficient lignocellulosic biorefinery. Biotechnology for Biofuels. 2020;13:12. doi: 10.1186/s13068-019-1641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Téllez et al. (2019).Trejo-Téllez LI, Estrada-Ortiz E, Gómez-Merino FC, Becker C, Krumbein A, Schwarz D. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Frontiers in Plant Science. 2019;10:371. doi: 10.3389/fpls.2019.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer (2001).Troyer JR. In the beginning: the multiple discovery of the first hormone herbicides. Weed Science. 2001;49:290–297. doi: 10.1614/0043-1745(2001)049[0290:ITBTMD]2.0.CO;2. [DOI] [Google Scholar]
- Tuteja et al. (2012).Tuteja N, Verma S, Sahoo RK, Raveendar S, Reddy IBL. Recent advances in development of marker-free transgenic plants: regulation and biosafety concern. Journal of Biosciences. 2012;37:167–197. doi: 10.1007/s12038-012-9187-5. [DOI] [PubMed] [Google Scholar]
- Uddain & Subramaniam (2020).Uddain J, Subramaniam S. Encapsulation-based a novel antibiotic selection technique for Agrobacterium-mediated genetic transformation of Dendrobium Broga Giant orchid. Gene Reports. 2020;21:100806. doi: 10.1016/j.genrep.2020.100806. [DOI] [Google Scholar]
- Wang et al. (2020).Wang W, Xu J, Fang H, Li Z, Li M. Advances and challenges in medicinal plant breeding. Plant Science. 2020;298:110573. doi: 10.1016/j.plantsci.2020.110573. [DOI] [PubMed] [Google Scholar]
- Weeks, Ye & Rommens (2008).Weeks JT, Ye J, Rommens CM. Development of an in planta method for transformation of alfalfa (Medicago sativa) Transgenic Research. 2008;17:587–597. doi: 10.1007/s11248-007-9132-9. [DOI] [PubMed] [Google Scholar]
- Woo, Suh & Cho (2011).Woo H-J, Suh S-C, Cho Y-G. Strategies for developing marker-free transgenic plants. Biotechnology and Bioprocess Engineering. 2011;16:1053–1064. doi: 10.1007/s12257-011-0519-3. [DOI] [Google Scholar]
- Wu (2007).Wu K. Monitoring and management strategy for Helicoverpa armigera resistance to Bt cotton in China. Journal of Invertebrate Pathology. 2007;95:220–223. doi: 10.1016/j.jip.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2019a).Wu H, Acanda Y, Canton M, Zale J. Efficient biolistic transformation of immature citrus rootstocks using phosphomannose-isomerase selection. Plants. 2019a;8:390. doi: 10.3390/plants8100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2019b).Wu L, Gao X, Xia F, Joshi J, Borza T, Wang-Pruski G. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiological and Molecular Plant Pathology. 2019b;106:49–56. doi: 10.1016/j.pmpp.2018.12.001. [DOI] [Google Scholar]
- Xin & Guo (2012).Xin C, Guo J. An efficient marker-free vector for clean gene transfer into plants. African Journal of Biotechnology. 2012;11:3751–3757. doi: 10.5897/AJB11.3163. [DOI] [Google Scholar]
- Xu et al. (2020).Xu Y, Guan X, Zhou R, Gong R. Melanocortin 5 receptor signaling pathway in health and disease. Cellular and Molecular Life Sciences. 2020;77:3831–3840. doi: 10.1007/s00018-020-03511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Su & An (2009).Yang A, Su Q, An L. Ovary-drip transformation: a simple method for directly generating vector-and marker-free transgenic maize (Zea mays L.) with a linear GFP cassette transformation. Planta. 2009;229:793–801. doi: 10.1007/s00425-008-0871-5. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2009).Yang A, Su Q, An L, Liu J, Wu W, Qiu Z. Detection of vector-and selectable marker-free transgenic maize with a linear GFP cassette transformation via the pollen-tube pathway. Journal of Biotechnology. 2009;139:1–5. doi: 10.1016/j.jbiotec.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Yang, Nairn & Ozias-Akins (2003).Yang H, Nairn J, Ozias-Akins P. Transformation of peanut using a modified bacterial mercuric ion reductase gene driven by an actin promoter from Arabidopsis thaliana. Journal of Plant Physiology. 2003;160:945–952. doi: 10.1078/0176-1617-01087. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2011).Yang S, Li G, Li M, Wang J. Transgenic soybean with low phytate content constructed by Agrobacterium transformation and pollen-tube pathway. Euphytica. 2011;177:375–382. doi: 10.1007/s10681-010-0262-4. [DOI] [Google Scholar]
- Yang, Peng & Pan (2019).Yang X, Peng J, Pan J. Nourseothricin N-acetyl transferase (NAT), a new selectable marker for nuclear gene expression in Chlamydomonas. Plant Methods. 2019;15:1–8. doi: 10.1186/s13007-018-0385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau & Stewart (2013).Yau Y-Y, Stewart CN. Less is more: strategies to remove marker genes from transgenic plants. BMC Biotechnology. 2013;13:36. doi: 10.1186/1472-6750-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You et al. (2003).You S-J, Liau C-H, Huang H-E, Feng T-Y, Prasad V, Hsiao H-h, Lu J-C, Chan M-T. Sweet pepper ferredoxin-like protein (pflp) gene as a novel selection marker for orchid transformation. Planta. 2003;217:60–65. doi: 10.1007/s00425-002-0970-7. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang K, Lu X, Li Y, Jiang X, Liu L, Wang H. New technologies provide more metabolic engineering strategies for bioethanol production in Zymomonas mobilis. Applied Microbiology and Biotechnology. 2019;103:2087–2099. doi: 10.1007/s00253-019-09620-6. [DOI] [PubMed] [Google Scholar]
- Zhang, Wang & Wang (2020).Zhang M-P, Wang M, Wang C. Nuclear transformation of Chlamydomonas reinhardtii: a review. Biochimie. 2020;181:1–11. doi: 10.1016/j.biochi.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Zimmer (2002).Zimmer M. Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chemical Reviews. 2002;102:759–782. doi: 10.1021/cr010142r. [DOI] [PubMed] [Google Scholar]
- Zubko, Scutt & Meyer (2000).Zubko E, Scutt C, Meyer P. Intrachromosomal recombination between attP regions as a tool to remove selectable marker genes from tobacco transgenes. Nature Biotechnology. 2000;18:442–445. doi: 10.1038/74515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
There is no raw data or code in this review article.