Abstract
Systemic sclerosis (SSc) has the potential to affect any component of the gastrointestinal (GI) tract. GI involvement in SSc is a leading cause of morbidity and overall decreased quality of life in this patient population, identifying a need for a concise approach to work-up. This literature review aims to present a systematic, anatomical approach and differential diagnosis of GI involvement in SSc for the general internist and rheumatologist. Each component of the luminal GI tract has its own specified section, beginning with a review of a clinical approach to diagnosis that includes a differential for clinicians to consider, followed by a discussion of the literature surrounding objective evaluation of these conditions (i.e. serologic studies, imaging, endoscopy). Additionally there is a focused discussion on an approach to GI bleeding in the patient with SSc.
Keywords: systemic sclerosis, scleroderma, clinical, gastrointestinal
1.0. INTRODUCTION
The evaluation of gastrointestinal (GI) complaints in patients with systemic sclerosis (SSc) can be a daunting task for both the general internist and the rheumatologist. A targeted and thorough assessment is essential, as the majority of complaints are often non-specific and can have a wide variety of potential therapies, depending on their etiology. Upwards of 90% of patients with SSc report some form of GI symptoms, with a significant subgroup of this population endorsing a noticeable reduced quality of life (65% of patients in this category meeting clinical criteria for depression).1 Additionally, GI manifestations of SSc are associated with significant morbidity and mortality, cited as the third leading cause of death following pulmonary arterial hypertension and interstitial lung disease.2 These factors reveal the importance of a thorough clinical evaluation and strategic approach to diagnostic testing to minimize the risk of poor outcomes in this patient population. The goal of this review is to provide a succinct, anatomically-focused, systematic approach to the clinical assessment and evaluation of common GI symptoms in patients with SSc for clinicians. The scope will remain mostly on the luminal issues of the GI tract, with an additional focused discussion for GI bleeds. Manifestations within the hepatobiliary system are not in the scope of this review.
1.1. Brief overview of pathogenesis
The proposed general mechanism of SSc involves structural and functional endothelial cell abnormalities, ultimately leading to the release of reactive species (cytokines, chemokines, and growth factors) and the recruitment of fibrocytes. These factors result in fibroproliferative vasculopathy and progressive tissue fibrosis.3 However, the response of the GI tissue to the SSc disease milieu appears to be somewhat distinct from that of other organ systems, as smooth muscle atrophy, rather than fibrosis, is often the most prominent feature.4 Numerous mechanistic processes for the development of GI dysfunction in SSc have been proposed, including a progressive vasculopathy, diffuse fibrosis, dysbiosis, and an autoantibody-driven neuropathic process.5,6 More recently many investigators have invoked a neuropathy as the initial pathologic event in SSc. For example, comparative studies of the lower esophageal sphincter in patients with SSc against control groups exhibited an abnormal muscular response to cholinergic neural stimulation, whereas direct muscle stimulation through gastrin and methacholine demonstrated a normal response.7–9 Preservation of muscle function was also further suggested by the reversal of esophageal motility in select patients with SSc when challenged with intraarterial reserpine.10 This hypothesis is further supported by the discovery of functional autoantibodies in these patients that interfere with cholinergic-mediated contraction via the M3R receptor, causing inhibition myopathy at the level of the smooth muscle within the GI tract. The extent and intensity of this binding appears to be associated with the duration of disease.3 In a cohort of patients with SSc and GI symptoms, ultrastructural studies of rectal biopsies revealed axonal and smooth muscle cell degeneration. Biopsies throughout the GI tract demonstrated patchy atrophy with progression to diffuse muscularis propria atrophy.8,11,12 Histologic examinations of autopsy specimens from esophageal tissue of patients with SSc have demonstrated a non-vascular distribution of muscular atrophy with an absence of inflammatory infiltrates, significant fibrosis, or ischemic necrosis.4 Therefore this “neurogenic hypothesis” suggests that a reduced neural stimulation of smooth muscle via autoantibodies ultimately leads to neural and muscle atrophy with varying degrees of fibrosis.8 These processes are thought to frame the pathophysiology behind many GI complaints.
2.0. THE ORAL CAVITY
2.1. Clinical approach to screening for oral complications in SSc
Symptoms involving the oral cavity are a result of both primary GI-SSc disease as well as secondary complications of cutaneous manifestations. Common presenting complaints include microstomia, xerostomia, periodontal disease, and fibrosis of the base of the tongue manifesting as tongue stiffness.2,13 Microstomia is a common, often disfiguring and functionally impactful complication of SSc. Patients may report peri-oral vertical creasing, as well as difficulty with fully opening the mouth when attempting daily dental hygiene or when biting a large sandwich. Such limitations may ultimately lead to poor nutrition and perioral care.2,11 Acquired microcheilia is reported is 50–80% of patients, and is reported to occur in the context of perioral cutaneous thickening.2,14
Xerostomia, or dry mouth, has been reported in 30–70% of patients with SSc.11,14,15 The majority of patients with SSc, however, do not meet criteria for Sjogren’s Syndrome (SS): one study of 133 patients with SSc found that 68% could be diagnosed with Sicca syndrome based on clinical symptoms and/or a Schirmer I test, yet only 20% fit diagnostic criteria for SS as defined by the American-European Consensus Group criteria. Interestingly this study did find an association between SS and the limited cutaneous subtype of SSc, with 18 of the 19 patients diagnosed with SS falling in this SSc subtype.16 Xerostomia may lead to lingual and buccal mucosal crenations, and an increased incidence of periodontal disease.15,17 This has been attributed to a reduction in salivary volume and enzymes which are essential in controlling oral bacterial populations.18,19 A case-control study of 109 patients (54 with SSc, 55 control) in Italy found that patients with SSc had a significant 2.95 increased risk (95% CI 1.26–6.84) for periodontal disease as defined by clinical attachment loss.20 Another case-control study of 394 patients (163 with SSc, 231 control) in Canada found a significant increase in the number of decayed teeth as well as periodontal disease defined by clinical attachment loss.21 However it should be noted there is conflicting data on the correlation between SSc and decayed teeth, as other studies have not found significant differences when comparing SSc patients to controls.15,22
2.2. Objective evaluation
The examiner should take care to assess for facial changes such as a decreased oral aperture, thinning and retraction of the lips (leading to a puckered or grimaced appearance), vertical wrinkling around the mouth, and a thickened sublingular frenulum.2,11 Diagnosis of microstomia is primarily a clinical diagnosis, with a review of systems positive for decreased mouth opening or limited range of motion of the mandible. Panoramic dental imaging can be utilized to confirm this diagnosis, however, this is unlikely to impact management from the standpoint of a generalist or rheumatologist.14 Physical limitations related to microstomia and microcheilia may preclude an effective routine examination for the general internist or rheumatologist, and therefore a referral for a focused dental exam may be warranted.23 The value of mouth stretching exercises for microstomia is still a subject of debate, as studies to date are limited by a number of factors including poor adherence to oral exercise regimens, parallel systemic therapy changes, and small study group sizes.24,25 The general consensus is that any benefit that may exist is quickly lost with non-adherence.25 Patients should be counseled with the knowledge that the true benefit is still ill-defined, yet it remains an intervention with minimal potential harm.
While a formal evaluation for SS (i.e. serologies, salivary gland functional testing, and biopsies) could be completed if there is concern for SSc-SS overlap, the history is likely to be sufficient for a diagnosis of xerostomia and more invasive studies are unlikely to change symptomatic management. One study compared the salivary gland biopsies of 202 patients with primary SS disease and 27 patients with SSc-SS overlap and noted that these processes appeared histologically identical.26 In addition, the presence of anti-Ro/SSA and anti-La/SSB dual antibody positivity (prevalence: 35% in primary SS vs 18.5% in SSc-SS overlap) was not associated with Sicca severity as measured by complications, adverse prognosis factors, and activity markers (levels of erythrocyte sedimentation rate, C-reactive protein, beta-2 glycoprotein, C4 serum complement, gammaglobulin, and cryoglobulin). Interestingly, the study did find that patients with SSc-SS overlap were less likely to have severe pulmonary fibrosis and peripheral neuropathy than patients with SSc alone.26 A dentist who has experience in the management of patients with SSc is an essential partner to optimize patient care.23 Additionally, identifying and limiting the use of medications which may further aggravate these problems is another important consideration for clinicians.
3.0. THE PHARYNX
3.1. Clinical approach to screening for pharyngeal complications in SSc
Pharyngeal involvement in SSc often presents as hoarseness, cough, and/or micro-aspirations. Pharyngeal complications in SSc are most frequently caused by uncontrolled gastroesophageal reflux disease (GERD) which is discussed below, or an overlapping inflammatory myositis leading to weakness of the pharyngeal muscles. Notably 42.6% of overlap myositis syndromes are associated with SSc (the most commonly associated connective tissue disease), and thus myositis is an important and relevant diagnosis to remain on any clinician’s differential.27
3.2. Objective evaluation
A history evaluating for symptoms of uncontrolled GERD, proximal muscle weakness, and other systemic manifestations of myositis should be performed to screen for pharyngeal involvement. Unfortunately little data exists on the workup of pharyngeal myositis specifically in the setting of SSc, and thus this topic warrants further investigation. However, for the general population any patient that describes new symptoms concerning for pharyngeal myopathy should also be screened for symptoms of respiratory muscle involvement. Some causes of proximal myopathy (particularly involving the pharyngeal or respiratory musculature) such as myasthenia gravis may overlap with SSc and should be quickly eliminated from the differential.28 One case report literature review identified 14 patients with observed myasthenia gravis-SSc overlap.29 Laboratory values that may be useful as screening for an inflammatory myositis include serum creatine kinase (CK), aldolase, and antibodies associated with myasthenia gravis such as anti-acetylcholine receptor.29,30 Swallow videofluoroscopy (modified barium swallow study) may also be useful in determining whether pharyngeal muscle involvement is contributing to dysphagia given the significant overlap of symptoms.31 Clinical suspicion for this diagnosis must remain high as it may guide the addition of certain therapeutics and provide opportunities to minimize aspiration risks.
4.0. THE ESOPHAGUS AND STOMACH
4.1. Clinical approach to screening for gastroesophageal complications in SSc
It is estimated that approximately 90% of patients with SSc have some form of esophageal involvement, whether it be symptomatic or subclinical, making the esophagus the most commonly involved region of the GI tract.2,8 Symptoms typically arise from GERD or esophageal dysmotility (which can be seen at any level: pharynx, upper esophageal sphincter, the lower two thirds of the esophagus, or the lower esophageal sphincter).11,14 The most common presenting complaint is that of heartburn, though additional complaints may include dysphagia, odynophagia, regurgitation, chronic cough, and hoarseness.14 Early diagnosis and treatment of esophageal involvement is important as chronic regurgitation and micro-aspiration are associated with the presence of interstitial lung disease, which is a leading cause of mortality in SSc.32,33 Chronic untreated or undertreated GERD or dysmotility may contribute to other complications, including esophagitis, ulcers, strictures, intestinal metaplasia, or esophageal adenocarcinoma.2,14 Many of these complications can present with reflux or dysphagia as well, therefore it is imperative for the clinician to maintain a wide differential when evaluating these patients. An additional diagnosis that presents similarly is eosinophilic esophagitis (EoE), with recent data supporting a connection between EoE and connective tissue diseases.34 A Utah population analysis in 2016 reported 11 patients with SSc-EoE overlap, and when matched with controls for age and gender, a 6-fold increased risk for SSc in patients diagnosed with EoE was identified.35
Approximately half of patients with SSc will present with symptoms suggestive of co-existing gastric involvement and report symptoms such as postprandial fullness, early satiety, bloating, nausea/vomiting, or epigastric pain.36 This has been attributed to delayed gastric emptying and/or abnormalities in gastric accommodation.37–39 In addition, as with the esophagus, it is suspected that there is a substantial subgroup of patients who have subclinical gastric disease. One study found that 89–90% of patients with SSc studied showed signs of gastric slow wave disturbances, although the clinical significance of this data is uncertain.37 Impaired gastric motility can also present more insidiously with weight loss secondary to decreased nutritional intake from early satiety, and thus should remain on a clinician’s differential when evaluated for malnutrition (discussed in more detail below).38
Gastric Helicobacter pylori infection is also an important consideration in patients with SSc. Presenting complaints include abdominal pain and dyspepsia, however some infections may present more insidiously with iron or vitamin B12 deficiency.40–42 A meta-analysis of articles studying the relationship between this bacterium and SSc found an increased incidence of H. pylori exposure in patients with SSc by ELISA testing (although notably an insignificant increase in cases detected by urea breath testing, which would indicate an active infection).43 Preliminary data comparing SSc patients with active H. pylori infection (diagnosed by urea breath testing and histology) to those SSc patients with negative testing demonstrated increased modified Rodnan skin scoring in the infected group.44 This data has been extrapolated to suggest eradication therapy may be beneficial in mitigating disease activity, however more research in this area is needed to support this hypothesis.45
4.2. Objective evaluation
Given the significant symptom overlap with many of these manifestations, it is important to think broadly when planning the work-up. In patients with solitary reflux symptoms without dysphagia, empiric acid suppression therapy is a reasonable first step of management in clinics not equipped with direct endoscopy, as outlined by the Evidence-based Clinical Practice Guidelines for GERD 2015.46 In patients with dysphagia, or heartburn that is recurrent or unresponsive to high doses of proton pump inhibitors (PPIs) and/or H2 receptor blockers, the guidelines recommend esophagogastroduodenoscopy (EGD) as an important diagnostic and potentially therapeutic intervention (e.g. screening for erosive esophagitis and Barrett’s esophagus, ruling out EoE, management of esophageal strictures).34,46,47 One retrospective analysis of 13 SSc patients naïve to acid suppressant medications found that low grade esophagitis had a prevalence of 77%.48 Another study evaluated 133 SSc patients on long-term PPI therapy (median treatment of 6 years, total range 1–38 years) and demonstrated a lower prevalence of esophagitis at 32.3% compared to the previously mentioned acid suppressant-naïve study.49 In both studies a variety of complications aside from esophagitis were identified, including dysmotility, gastritis, H. pylori infection, esophageal candidiasis, and hyperplastic gastric polyps. The noted lower prevalence of visible esophagitis following PPI/H2 blocker therapy and the presence of these other abnormalities further reinforces the importance of acid suppression therapy.47–49 In the setting of a confirmed case of Barrett’s esophagus, routine follow up screening with EGD is typically recommended: every 3–5 years in Barrett’s without dysplasia, every 6–12 months for low-grade dysplasia, and every 3 months for high-grade dysplasia (based on 2008 American Journal of Gastroenterology guidelines).50 pH monitoring has been shown to be a useful study to diagnose non-erosive reflux disease in cases with normal mucosal findings on EGD.33,51 A retrospective study of 10 SSc patients with reflux referred for lung transplant found that abnormal pH was predictive for lower 1-year survival rates.52
Numerous expert consensus guidelines have identified high-resolution esophageal manometry (HREM) as the appropriate test to screen for aperistalsis, decreased amplitude of smooth muscle contractions within the esophageal body, or dysfunction within the lower esophageal sphincter.2,47,53–57 This is typically conducted following the exclusion of mechanical obstruction or mucosal disease via EGD, and can be done in conjunction with pH monitoring.58,59 HREM can also be utilized in combination with esophageal pressure topography (a space-time pressure plot) and a functional luminal imaging probe (measuring distensibility of the esophageal body) to isolate a dysfunctional component of the esophagus that is contributing to symptoms for diagnostic purposes.32,47,60 The accurate diagnosis of esophageal dysmotility may also have systemic implications, as one study of 79 SSc patients noted that abnormal contractility diagnosed by HREM was associated with increased severity of skin and lung disease.61 While multiple small studies have confirmed the utility of HREM for esophageal dysmotility diagnosis in the SSc patient population, additional research is needed to understand its implications.62–66
As discussed above, in patients who report symptoms suggestive of gastric involvement, such as early satiety, postprandial fullness, bloating, or nausea/vomiting that is unresponsive to medical management, further evaluation of gastric function should be pursued in conjunction with an esophageal workup. The American Journal of Gastroenterology in 2013 published guidelines on the diagnosis and management of gastroparesis provides examples of three separate tests useful in the detection of gastric dysmotility: four-hour gastric emptying scintigraphy, the wireless motility capsule, and C-octanoate or -spirulina breath testing. However, it should be noted that these same guidelines acknowledge that the latter two tests still require additional validation studies, and therefore scintigraphy still ultimately remains the reliable test.67 For this reason, a four-hour technetium-99 sulfur colloid gastric emptying study should be pursued when considering the diagnosis of impaired gastric transit in patients with SSc.2,68,69 Ideally, this should be a combined solid-liquid study, as the inclusion of liquids increases the overall sensitivity of the test by an estimated 25–36%.67
While there is no gold standard test, an assessment for H. pylori infection could include urea breath testing, as it has been studied in the SSc patient population and is proven to have high sensitivity and specificity in general (96 and 93%, respectively) while remaining noninvasive.70,71 While not specifically studied for SSc, stool antigen testing has also been proven to have high sensitivity and specificity for active infection (94 and 97%, respectively) and may also be a useful diagnostic tool given its ease of collection.71
5.0. THE SMALL BOWEL
5.1. Clinical approach to screening for small bowel complications in SSc
Many patients with SSc may also present with symptoms of small bowel involvement, including diarrhea, unintentional weight loss, distention, bloating, and malnutrition.2,14 The prevalence of small bowel dysmotility is estimated to be between 40–88% based on manometry studies. There is also increasing evidence that small bowel involvement precedes symptoms. For example, one landmark study of 17 SSc patients noted that 65% of patients with small bowel involvement by manometry were asymptomatic at the time of the study.37,72 A common complication of the small bowel in patients with SSc is small intestinal bacterial overgrowth (SIBO). While this is often attributed to small bowel dysmotility, it may also be a consequence of large bowel dysmotility with a weakened ileocecal valve and/or chronic gastric acid suppression.73 It is reported that the prevalence of SIBO in symptomatic patients is 30–62.5%, however these figures may be a high estimate due to inconsistencies in outcome measures both in terms of diagnostic modalities and symptom presentation.2,74 All of these complications may contribute to chronic malnutrition leading to long-term morbidity, such as dependence on total parenteral nutrition for adequate caloric intake.75 Therefore, the early identification of small bowel dysfunction and correction of malnutrition, SIBO, and/or dysmotility is essential.76
5.2. Objective evaluation
Prior to attributing these symptoms to SSc, it is important for clinicians to rule-out other causes of GI symptoms which are prevalent in the general population. Important considerations include infection, inflammatory or infiltrative bowel diseases, overlapping autoimmune bowel complications, and GI malignancies.
Diagnostic modalities for the assessment of small bowel dysmotility are limited. An abdominal x-ray may identify extensive disease, demonstrating the classic “hide-bound” appearance indicative of tightly packed valvulae conniventes in the duodenum and jejunum, with dilated bowel loops.2,75 Small intestinal manometry has been used to demonstrate the presence of dysmotility in SSc, as evidenced by low-amplitude contractions with either absent or prolonged migrating motor complexes.77 Unfortunately this procedure has several clinical limitations, as it takes multiple hours to perform and is only able to assess the upper portions of the small bowel.2,75 Other types of studies that are increasingly utilized include scintigraphy, wireless motility capsules, and CT/MRI enterography.78 Clinicians should note that wireless motility capsules are to be avoided in patients with known, severe gastroparesis or bowel strictures.75
The gold standard for diagnosis of SIBO is jejunal culture, where >10^5 organisms/mL constitutes a positive test; however this test is limited in the SSc patient population as it is invasive and not infrequently cardiopulmonary complications of SSc prohibit routine anesthesia.74 The only diagnostic tests for SIBO that are specifically validated in this patient population are the hydrogen and methane breath tests after an oral glucose or lactulose bolus. While these tests have been shown to have reasonable specificity ranging from 78–100%, their sensitivities range from 62–93%, which make their use as screening tests sub-optimal.75 Clinicians can attempt to improve the sensitivity by preferentially ordering lactulose over glucose boluses (82 versus 62.5%).74 Ultimately diagnosis and treatment of SIBO may come down to strong clinical suspicion in the setting of a broad negative workup. Although not infrequently used, there is mixed data to support the practice of prescribing empiric antibiotic therapy; one meta-analysis of 10 studies found that while antibiotics were more effective than placebo to induce clinical improvement (as demonstrated by a normalized breath test), analysis of these studies was complicated by overall study heterogeneity and varying antibiotic choices.79,80 The most recent American Gastroenterological Association practice guidelines for SIBO, based on expert consensus, recommend the identification and correction of underlying causes and nutritional deficiencies with adjunct antibiotic use, taking note of the potential risk-benefit of empiric treatment. These guidelines similarly acknowledge the need for additional randomized control trials to further support specific antibiotic strategies.81
Concurrently with this workup, patients should be regularly screened for overall malnutrition, as this is indicative of worsened disease.75 The Malnutrition Universal Screening Tool (MUST) has been frequently utilized for preliminary screening in the SSc patient population for its ease of use, correlation with other screening tools, and validation in both the inpatient and outpatient setting.82–84 Positive screening with this tool has been shown to correlate with worsened outcomes.85 The prevalence of malnutrition in SSc has been variably described due to inconsistencies in the diagnostic criteria utilized, ranging from 5.3–55.6%.82,86,87 For this reason, the use of the ESPEN (European Society for Clinical Nutrition and Metabolism) criteria for malnutrition diagnosis following a positive screening should be highly considered, as these criteria were developed with a goal to define malnutrition terminology on a global level in line with the World Health Organization’s ICD system.82,88 A suggested initial evaluation for malabsorption or malnutrition in patients with SSc, based on expert consensus, should include hemoglobin, vitamin A, vitamin B12, folate, albumin, iron panel, carotene, and selenium.89–91
6.0. THE COLON
6.1. Clinical approach to screening for colonic complications in SSc
The prevalence of colonic manifestations in SSc is hypothesized to be up to 50% of all patients, but data is limited as these findings are often under-reported in the literature.37,92 The typical presentation of colonic involvement in SSc includes pain, distention, constipation, and tenesmus, with less common manifestations including fecal impaction and recurrent intestinal pseudoobstruction.2 Related long-term complications of constipation and colonic dysmotility may include pseudodiverticula, ulcerations, volvulus, and very rarely perforation or infarction.93
One additional GI tract complication to consider is pneumatosis cystoides intestinalis (PCI). This can present with abdominal pain, distention, nausea, or vomiting. PCI is a rare diagnosis and felt to be indicative of end-stage disease when associated with SSc and is therefore considered a poor prognostic sign.94 Most literature surrounding this diagnosis exists in the form of case reports with limited population data.93,95 A generalized review of a population with this diagnosis, not specific to SSc, found that the colon was the most commonly affected at 46% of cases, followed by small bowel (27%) and simultaneous small and large bowel disease (7%).96 Pneumoperitoneum is a frequent complication, with one case report series review of 37 patients finding an incidence rate of 87%. 97
6.2. Objective evaluation
As with the upper portions of the GI tract, complications of the large bowel can be evaluated by direct visualization through sigmoidoscopy/colonoscopy and manometry.93 Abdominal radiographs or computed tomography are beneficial in quickly identifying complications of dysmotility, pseudoobstruction, or fecal impaction such as colonic dilation and perforation. 94,98 Manometry of the large bowel comes with similar complications and limitations as small bowel manometry, including length of procedure time and cardiopulmonary limitations with anesthesia.74 Sitz markers (radiopaque markers that are used to assess colonic transit times) enable the assessment of colonic transit over several days, though concerns exist regarding pellet retention and risk of bowel perforation in patients with severe delays.99 One caveat to all available assessments of colonic dysmotility is that while multiple testing modalities exist, these are only able to identify the presence of dysmotility and their data does not consistently correlate with symptom severity.98,100 Both abdominal imaging and endoscopy also have the potential to visualize PCI and/or pneumoperitoneum, with direct visualization by endoscopy showing beaded, grape-like, or cobblestone abnormalities within the bowel wall.2,101
7.0. THE ANORECTUM
7.1. Clinical approach to screening anorectal complications in SSc
Following the esophagus, the anorectum is the second most commonly involved portion of the GI tract in patients with SSc. Up to 50–70% of patients report symptoms of dysfunction, with one survey reporting the most common symptom as fecal incontinence (38%), and a need for regular digital stimulation or evacuation of the rectum in 18% of patients.37,102 A case-control study found that 71.4% of patients within the SSc group had an impaired recto-anal inhibitory response (RAIR), which correlated with fecal incontinence symptoms. Notably there was no correlation found between various SSc subtypes, the duration of disease, or other GI symptoms.103 Another study of 44 SSc patients found that the patients reporting fecal incontinence symptoms exhibited a lower mean resting pressure of the internal anal sphincter compared to patients who were asymptomatic, suggesting hypotonia is a major contributor to symptoms; however, other studies did not confirm this findings.103,104 A differential for abnormal RAIR diagnosed in adulthood should also includeChagas’ disease, dermatomyositis, peripheral neuropathy (such as in diabetes), neurovascular insult, and post-surgical complication.104–107
7.2. Objective evaluation
History and physical exam is important in the diagnosis of anorectal SSc involvement. A digital rectal exam may be used to identify fecal impaction and evaluate anal sphincter tone, which is essential in differentiating anorectal from colonic involvement.11 Beyond the physical exam, anorectal manometry has also been utilized to assess the resting tone of the internal and external anal sphincters. An important distinction found on manometry in SSc is that the internal anal sphincter is preferentially affected, and the external sphincter is typically spared.2,108 This finding has been further supported by endoanal ultrasound, which typically shows a thin or atrophic internal anal sphincter – however this additional imaging beyond manometry is not typically needed for patient care.2,104
8.0. GASTROINTESTINAL BLEEDING
GI bleeding is a symptom that is relatively common in the general population, but warrants the consideration of a broader differential diagnosis when seen in the SSc population. As discussed above, many patients with SSc experience chronic GERD which can lead to esophagitis, and ulcers of the esophagus Barrett’s esophagus, and progression to esophageal adenocarcinoma, seen in 1.9% of patients.2,14 The presence of persistent reflux should also warrant consideration of peptic ulcer disease as a source of GI bleeding. In patients with SSc who utilize antibiotics frequently for SIBO or digital ulcers, H. pylori infection must be considered as well.109
An additional cause of bleeding that is more common in SSc patients relative to the general population is gastric antral vascular ectasia (GAVE). GAVE, also known as “watermelon stomach” given its appearance on endoscopy, is defined as vascular ectasia of the mucosal capillaries of the stomach, with focal thrombosis, spindle cell proliferation, and fibrohyalinosis seen histologically.2,110 While traditionally considered a rare diagnosis, its true prevalence is up for debate: a large retrospective study had previously defined the incidence of clinically significant gastric antral vascular ectasia as 5.7%, however a more recent study of asymptomatic patients (the SCOT trial) reported a much higher prevalence of 22.3%.111–113 An association between GAVE and anti-RNA polymerase-III antibodies is reported in several studies, though not all studies confirmed this association.112,114
Patients with SSc are also found to have a greater incidence of telangiectasias and angiodysplasia throughout the GI tract, from the oropharynx and throughout the bowel. As these vascular abnormalities, particularly those in the small bowel, can be difficult to diagnose and treat, they are frequently the culprit of recurrent bleeds.2,115,116 One capsule endoscopy study of 50 patients with SSc found that patients with GI vascular lesions were more likely to have the limited cutaneous subtype of SSc (73.3% limited cutaneous subtype vs. 26.7% diffuse cutaneous subtype, n=15).116 A standard GI bleeding workup should be pursued in all symptomatic patients or those with signs of iron deficiency anemia, starting with a bidirectional endoscopy, followed by consideration for capsule study, computed tomography or magnetic resonance enterography, and then push or balloon endoscopy.113,117,118 Given the complexities of using general anesthesia in SSc, patients should be referred to an experienced center for these latter studies.
9.0. CONCLUSIONS
Clinicians should maintain a wide differential in the evaluation of GI symptoms in patients with SSc, taking care to rule out diagnoses most prevalent in the general population prior to pursuing a workup more specific to SSc-linked processes. The possibility of multi-level disease throughout the GI tract must be entertained. As mentioned previously, GI involvement in SSc not only has a significant impact on quality of life, but, when severe, is associated with increased mortality. While the treatments for these specific complications are outside the scope of this review, successful identification of the underlying process of symptoms is imperative to allow for appropriate targeted therapies.
Unfortunately research in this area has been limited due to an incomplete understanding of the underlying GI disease mechanism, a lack of biomarkers to differentiate GI disease activity from damage, and the high cost of studies needed to objectively measure GI motility. There is also limited data specific to this patient population for some of the symptoms described in this review, driving the need for extrapolation from more generalized studies and guidelines. As a mechanistic understanding of SSc and its impact on the GI tract is further established and risk stratification improves, this may potentially allow for more targeted assessment in the future.
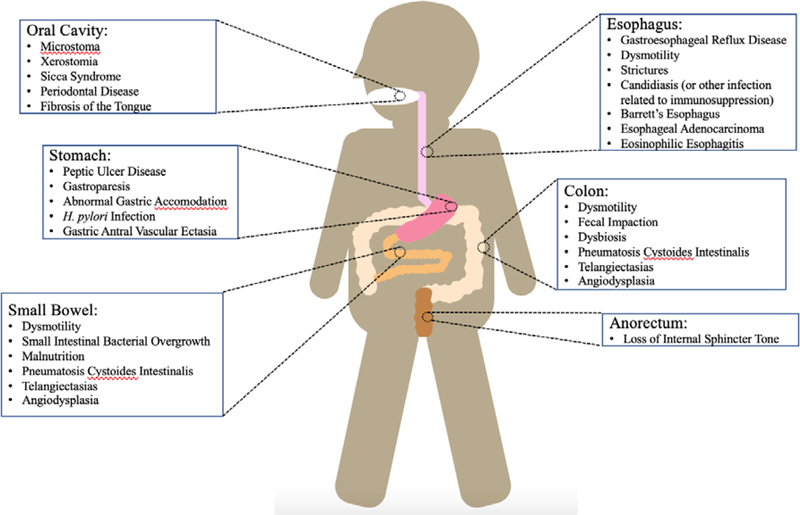
Figure 1.
Differential for GI manifestations of SSc
Acknowledgments
Funding Statement
NIH/NIAMS K23 AR071473 to ZM; Scleroderma Research Foundation to ZM; Jerome L Greene Foundation to ZM; Scleroderma Research Foundation
Footnotes
Conflict of interest statement: None of the authors received any financial support or other benefits from commercial sources for the work reported in this manuscript, nor do any of the authors have any financial interests, which could create a potential conflict of interest or appearance thereof.
REFERENCES
- 1.Yang H, Xu D, Li MT, et al. Gastrointestinal manifestations on impaired quality of life in systemic sclerosis. J Dig Dis. 2019;20(5):256–261. [DOI] [PubMed] [Google Scholar]
- 2.Kroner PT, Tolaymat OA, Bowman AW, Abril A, Lacy BE. Gastrointestinal manifestations of rheumatological diseases. Am J Gastroenterol. 2019;114(9):1441–1454. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Singh J, Rattan S, DiMarino AJ, Cohen S, Jimenez SA. Review article: Pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment Pharmacol Ther. 2017;45(7):883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts CG, Hummers LK, Ravich WJ, Wigley FM, Hutchins GM. A case-control study of the pathology of oesophageal disease in systemic sclerosis (scleroderma). Gut. 2006;55(12):1697–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distler O, Assassi S, Cottin V, et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J. 2020;55(5):1902026. doi: 10.1183/13993003.02026-2019. Print 2020 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology. 2002;123(4):1144–1150. [DOI] [PubMed] [Google Scholar]
- 8.Rose S, Young MA, Reynolds JC. Gastrointestinal manifestations of scleroderma. Gastroenterol Clin North Am. 1998;27(3):563–594. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Fisher R, Lipshutz W, Turner R, Myers A, Schumacher R. The pathogenesis of esophageal dysfunction in scleroderma and raynaud’s disease. J Clin Invest. 1972;51(10):2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willerson JT, Thompson RH, Hookman P, Herdt J, Decker JL. Reserpine in raynaud’s disease and phenomenon. short-term response to intra-arterial injection. Ann Intern Med. 1970;72(1):17–27. [DOI] [PubMed] [Google Scholar]
- 11.Shah AA, Wigley FM. Often forgotten manifestations of systemic sclerosis. Rheum Dis Clin North Am. 2008;34(1):221–38; ix. [DOI] [PubMed] [Google Scholar]
- 12.Malandrini A, Selvi E, Villanova M, et al. Autonomic nervous system and smooth muscle cell involvement in systemic sclerosis: Ultrastructural study of 3 cases. J Rheumatol. 2000;27(5):1203–1206. [PubMed] [Google Scholar]
- 13.Bali V, Dabra S, Behl AB, Bali R. A rare case of hidebound disease with dental implications. Dent Res J (Isfahan). 2013;10(4):556–561. [PMC free article] [PubMed] [Google Scholar]
- 14.McFarlane IM, Bhamra MS, Kreps A, et al. Gastrointestinal manifestations of systemic sclerosis. Rheumatology (Sunnyvale). 2018;8(1): 10.4172/2161-1149.1000235. Epub 2018 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood RE, Lee P. Analysis of the oral manifestations of systemic sclerosis (scleroderma). Oral Surg Oral Med Oral Pathol. 1988;65(2):172–178. [DOI] [PubMed] [Google Scholar]
- 16.Avouac J, Sordet C, Depinay C, et al. Systemic sclerosis-associated sjogren’s syndrome and relationship to the limited cutaneous subtype: Results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum. 2006;54(7):2243–2249. [DOI] [PubMed] [Google Scholar]
- 17.Eversole LR, Jacobsen PL, Stone CE. Oral and gingival changes in systemic sclerosis (scleroderma). J Periodontol. 1984;55(3):175–178. [DOI] [PubMed] [Google Scholar]
- 18.Baron M, Hudson M, Tatibouet S, et al. Relationship between disease characteristics and orofacial manifestations in systemic sclerosis: Canadian systemic sclerosis oral health study III. Arthritis Care Res (Hoboken). 2015;67(5):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalewska A, Knaś M, Gińdzieńska-Sieśkiewicz E, et al. Salivary antioxidants in patients with systemic sclerosis. J Oral Pathol Med. 2014;43(1):61–68. [DOI] [PubMed] [Google Scholar]
- 20.Isola G, Williams RC, Lo Gullo A, et al. Risk association between scleroderma disease characteristics, periodontitis, and tooth loss. Clin Rheumatol. 2017;36(12):2733–2741. [DOI] [PubMed] [Google Scholar]
- 21.Baron M, Hudson M, Tatibouet S, et al. The canadian systemic sclerosis oral health study: Orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology (Oxford). 2014;53(8):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andonopoulos AP, Drosos AA, Skopouli FN, Moutsopoulos HM. Sjögren’s syndrome in rheumatoid arthritis and progressive systemic sclerosis. A comparative study. Clin Exp Rheumatol. 1989;7(2):203–205. [PubMed] [Google Scholar]
- 23.Yuen HK, Hant FN, Hatfield C, Summerlin LM, Smith EA, Silver RM. Factors associated with oral hygiene practices among adults with systemic sclerosis. Int J Dent Hyg. 2014;12(3):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzo G, Scardina GA, Messina P. Effects of a nonsurgical exercise program on the decreased mouth opening in patients with systemic scleroderma. Clin Oral Investig. 2003;7(3):175–178. [DOI] [PubMed] [Google Scholar]
- 25.Yuen HK, Nelson SL. Test--retest reliability of oral health impact profile (OHIP-49) in adults with systemic sclerosis. Spec Care Dentist. 2014;34(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salliot C, Mouthon L, Ardizzone M, et al. Sjogren’s syndrome is associated with and not secondary to systemic sclerosis. Rheumatology (Oxford). 2007;46(2):321–326. [DOI] [PubMed] [Google Scholar]
- 27.Paik JJ. Muscle disease in scleroderma. Curr Opin Rheumatol. 2018;30(6):576–580. [DOI] [PubMed] [Google Scholar]
- 28.Suresh E, Wimalaratna S. Proximal myopathy: Diagnostic approach and initial management. Postgrad Med J. 2013;89(1054):470–477. [DOI] [PubMed] [Google Scholar]
- 29.Zivković SA, Medsger TA Jr. Myasthenia gravis and scleroderma: Two cases and a review of the literature. Clin Neurol Neurosurg. 2007;109(4):388–391. [DOI] [PubMed] [Google Scholar]
- 30.Bohlmeyer TJ, Wu AH, Perryman MB. Evaluation of laboratory tests as a guide to diagnosis and therapy of myositis. Rheum Dis Clin North Am. 1994;20(4):845–856. [PubMed] [Google Scholar]
- 31.Shapiro J, Martin S, DeGirolami U, Goyal R. Inflammatory myopathy causing pharyngeal dysphagia: A new entity. Ann Otol Rhinol Laryngol. 1996;105(5):331–335. [DOI] [PubMed] [Google Scholar]
- 32.Tetreault MP, Kahrilas P. GI manifestations with a focus on the esophagus: Recent progress in understanding pathogenesis. Curr Rheumatol Rep. 2019;21(8):42–019-0841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denaxas K, Ladas SD, Karamanolis GP. Evaluation and management of esophageal manifestations in systemic sclerosis. Ann Gastroenterol. 2018;31(2):165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abonia JP, Wen T, Stucke EM, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132(2):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frech TM, Boynton K, Downs-Kelly E, Jones B, Kriesel JD, Peterson K. Eosinophilic esophagitis in two patients with systemic sclerosis. Case Rep Rheumatol. 2016;2016:6410421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domsic R, Fasanella K, Bielefeldt K. Gastrointestinal manifestations of systemic sclerosis. Dig Dis Sci. 2008;53(5):1163–1174. [DOI] [PubMed] [Google Scholar]
- 37.Sallam H, McNearney TA, Chen JD. Systematic review: Pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma). Aliment Pharmacol Ther. 2006;23(6):691–712. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Semin Arthritis Rheum. 1994;24(1):29–39. [DOI] [PubMed] [Google Scholar]
- 39.Maddern GJ, Horowitz M, Jamieson GG, Chatterton BE, Collins PJ, Roberts-Thomson P. Abnormalities of esophageal and gastric emptying in progressive systemic sclerosis. Gastroenterology. 1984;87(4):922–926. [PubMed] [Google Scholar]
- 40.Rocha GA, Queiroz DM, Mendes EN, Barbosa AJ, Lima Júnior GF, Oliveira CA. Helicobacter pylori acute gastritis: Histological, endoscopical, clinical, and therapeutic features. Am J Gastroenterol. 1991;86(11):1592–1595. [PubMed] [Google Scholar]
- 41.Yuan W, Li Y, Yang K, et al. Iron deficiency anemia in helicobacter pylori infection: Meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45(6):665–676. [DOI] [PubMed] [Google Scholar]
- 42.Mwafy SN, Afana WM. Hematological parameters, serum iron and vitamin B(12) levels in hospitalized palestinian adult patients infected with helicobacter pylori: A case-control study. Hematol Transfus Cell Ther. 2018;40(2):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yong WC, Upala S, Sanguankeo A. Helicobacter pylori infection in systemic sclerosis: A systematic review and meta-analysis of observational studies. Clin Exp Rheumatol. 2018;36 Suppl 113(4):168–174. [PubMed] [Google Scholar]
- 44.Radić M, Martinović Kaliterna D, Bonacin D, Morović Vergles J, Radić J. Correlation between helicobacter pylori infection and systemic sclerosis activity. Rheumatology (Oxford). 2010;49(9):1784–1785. [DOI] [PubMed] [Google Scholar]
- 45.Magen E, Delgado JS. Helicobacter pylori and skin autoimmune diseases. World J Gastroenterol. 2014;20(6):1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51(8):751–767. [DOI] [PubMed] [Google Scholar]
- 47.Carlson DA, Hinchcliff M, Pandolfino JE. Advances in the evaluation and management of esophageal disease of systemic sclerosis. Curr Rheumatol Rep. 2015;17(1):475–014-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thonhofer R, Siegel C, Trummer M, Graninger W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatol Int. 2012;32(1):165–168. [DOI] [PubMed] [Google Scholar]
- 49.Marie I, Ducrotte P, Denis P, Hellot MF, Levesque H. Oesophageal mucosal involvement in patients with systemic sclerosis receiving proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24(11–12):1593–1601. [DOI] [PubMed] [Google Scholar]
- 50.Wang KK, Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of barrett’s esophagus. Am J Gastroenterol. 2008;103(3):788–797. [DOI] [PubMed] [Google Scholar]
- 51.Gyawali CP, Azagury DE, Chan WW, et al. Nonerosive reflux disease: Clinical concepts. Ann N Y Acad Sci. 2018;1434(1):290–303. [DOI] [PubMed] [Google Scholar]
- 52.Fisichella PM, Reder NP, Gagermeier J, Kovacs EJ. Usefulness of pH monitoring in predicting the survival status of patients with scleroderma awaiting lung transplantation. J Surg Res. 2014;189(2):232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gyawali CP, de Bortoli N, Clarke J, et al. Indications and interpretation of esophageal function testing. Ann N Y Acad Sci. 2018;1434(1):239–253. [DOI] [PubMed] [Google Scholar]
- 54.Savarino E, de Bortoli N, Bellini M, et al. Practice guidelines on the use of esophageal manometry - A GISMAD-SIGE-AIGO medical position statement. Dig Liver Dis. 2016;48(10):1124–1135. [DOI] [PubMed] [Google Scholar]
- 55.Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91(2):213–227.e6. [DOI] [PubMed] [Google Scholar]
- 56.Liu LWC, Andrews CN, Armstrong D, et al. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. J Can Assoc Gastroenterol. 2018;1(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung HK, Hong SJ, Lee OY, et al. 2019. seoul consensus on esophageal achalasia guidelines. J Neurogastroenterol Motil. 2020;26(2):180–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: Moving from research into clinical practice. Gut. 2008;57(3):405–423. [DOI] [PubMed] [Google Scholar]
- 59.Klingler PJ, Hinder RA, Wetscher GJ, et al. Accurate placement of the esophageal pH electrode for 24-hour pH monitoring using a combined pH/manometry probe. Am J Gastroenterol. 2000;95(4):906–909. [DOI] [PubMed] [Google Scholar]
- 60.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111(12):1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimmel JN, Carlson DA, Hinchcliff M, et al. The association between systemic sclerosis disease manifestations and esophageal high-resolution manometry parameters. Neurogastroenterol Motil. 2016;28(8):1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aggarwal N, Lopez R, Gabbard S, Wadhwa N, Devaki P, Thota PN. Spectrum of esophageal dysmotility in systemic sclerosis on high-resolution esophageal manometry as defined by chicago classification. Dis Esophagus. 2017;30(12):1–6. [DOI] [PubMed] [Google Scholar]
- 63.Luciano L, Granel B, Bernit E, et al. Esophageal and anorectal involvement in systemic sclerosis: A systematic assessment with high resolution manometry. Clin Exp Rheumatol. 2016;34 Suppl 100(5):63–69. [PubMed] [Google Scholar]
- 64.Roman S, Hot A, Fabien N, et al. Esophageal dysmotility associated with systemic sclerosis: A high-resolution manometry study. Dis Esophagus. 2011;24(5):299–304. [DOI] [PubMed] [Google Scholar]
- 65.Raja J, Ng CT, Sujau I, Chin KF, Sockalingam S. High-resolution oesophageal manometry and 24-hour impedance-pH study in systemic sclerosis patients: Association with clinical features, symptoms and severity. Clin Exp Rheumatol. 2016;34 Suppl 100(5):115–121. [PubMed] [Google Scholar]
- 66.Ogliari C, Piazza O Sed N, Vecchi M. High resolution manometry in scleroderma patients. Clin Gastroenterol Hepatol. 2017;15(10):1640–1641. [DOI] [PubMed] [Google Scholar]
- 67.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology. Clinical guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37; quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller JB, Gandhi N, Clarke J, McMahan Z. Gastrointestinal involvement in systemic sclerosis: An update. J Clin Rheumatol. 2018;24(6):328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104(6):1640–1647. [DOI] [PubMed] [Google Scholar]
- 70.Reinauer S, Goerz G, Ruzicka T, Susanto F, Humfeld S, Reinauer H. Helicobacter pylori in patients with systemic sclerosis: Detection with the 13C-urea breath test and eradication. Acta Derm Venereol. 1994;74(5):361–363. [DOI] [PubMed] [Google Scholar]
- 71.Wang YK, Kuo FC, Liu CJ, et al. Diagnosis of helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21(40):11221–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marie I, Levesque H, Ducrotté P, et al. Manometry of the upper intestinal tract in patients with systemic sclerosis: A prospective study. Arthritis Rheum. 1998;41(10):1874–1883. [DOI] [PubMed] [Google Scholar]
- 73.Chander Roland B, Mullin GE, Passi M, et al. A prospective evaluation of ileocecal valve dysfunction and intestinal motility derangements in small intestinal bacterial overgrowth. Dig Dis Sci. 2017;62(12):3525–3535. [DOI] [PubMed] [Google Scholar]
- 74.Sawadpanich K, Soison P, Chunlertrith K, et al. Prevalence and associated factors of small intestinal bacterial overgrowth among systemic sclerosis patients. Int J Rheum Dis. 2019;22(4):695–699. [DOI] [PubMed] [Google Scholar]
- 75.Sakkas LI, Simopoulou T, Daoussis D, Liossis SN, Potamianos S. Intestinal involvement in systemic sclerosis: A clinical review. Dig Dis Sci. 2018;63(4):834–844. [DOI] [PubMed] [Google Scholar]
- 76.Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994;37(9):1265–1282. [DOI] [PubMed] [Google Scholar]
- 77.Marie I, Ducrotté P, Denis P, Hellot MF, Levesque H. Outcome of small-bowel motor impairment in systemic sclerosis--a prospective manometric 5-yr follow-up. Rheumatology (Oxford). 2007;46(1):150–153. [DOI] [PubMed] [Google Scholar]
- 78.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: Position paper of the american and european neurogastroenterology and motility societies. Neurogastroenterol Motil. 2011;23(1):8–23. [DOI] [PubMed] [Google Scholar]
- 79.Quigley EM. Small intestinal bacterial overgrowth: What it is and what it is not. Curr Opin Gastroenterol. 2014;30(2):141–146. [DOI] [PubMed] [Google Scholar]
- 80.Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38(8):925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quigley EM, Murray JA, Pimentel M. AGA clinical practice update on small intestinal bacterial overgrowth: Expert review. Gastroenterology. 2020. [DOI] [PubMed] [Google Scholar]
- 82.Caimmi C, Caramaschi P, Venturini A, et al. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clin Rheumatol. 2018;37(4):987–997. [DOI] [PubMed] [Google Scholar]
- 83.Baron M, Hudson M, Steele R, Canadian Scleroderma Research Group. Malnutrition is common in systemic sclerosis: Results from the canadian scleroderma research group database. J Rheumatol. 2009;36(12):2737–2743. [DOI] [PubMed] [Google Scholar]
- 84.Stratton RJ, Hackston A, Longmore D, et al. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92(5):799–808. [DOI] [PubMed] [Google Scholar]
- 85.Codullo V, Cereda E, Crepaldi G, et al. Disease-related malnutrition in systemic sclerosis: Evidences and implications. Clin Exp Rheumatol. 2015;33(4 Suppl 91):S190–4. [PubMed] [Google Scholar]
- 86.Rosato E, Gigante A, Gasperini ML, et al. Nutritional status measured by BMI is impaired and correlates with left ventricular mass in patients with systemic sclerosis. Nutrition. 2014;30(2):204–209. [DOI] [PubMed] [Google Scholar]
- 87.Krause L, Becker MO, Brueckner CS, et al. Nutritional status as marker for disease activity and severity predicting mortality in patients with systemic sclerosis. Ann Rheum Dis. 2010;69(11):1951–1957. [DOI] [PubMed] [Google Scholar]
- 88.Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. [DOI] [PubMed] [Google Scholar]
- 89.Dupont R, Longué M, Galinier A, et al. Impact of micronutrient deficiency & malnutrition in systemic sclerosis: Cohort study and literature review. Autoimmun Rev. 2018;17(11):1081–1089. [DOI] [PubMed] [Google Scholar]
- 90.Emmanuel A Current management of the gastrointestinal complications of systemic sclerosis. Nat Rev Gastroenterol Hepatol. 2016;13(8):461–472. [DOI] [PubMed] [Google Scholar]
- 91.Baron M, Bernier P, Côté LF, et al. Screening and therapy for malnutrition and related gastro-intestinal disorders in systemic sclerosis: Recommendations of a north american expert panel. Clin Exp Rheumatol. 2010;28(2 Suppl 58):S42–6. [PubMed] [Google Scholar]
- 92.Brandler JB, Sweetser S, Khoshbin K, Babameto M, Prokop LJ, Camilleri M. Colonic manifestations and complications are relatively under-reported in systemic sclerosis: A systematic review. Am J Gastroenterol. 2019;114(12):1847–1856. [DOI] [PubMed] [Google Scholar]
- 93.Gyger G, Baron M. Systemic sclerosis: Gastrointestinal disease and its management. Rheum Dis Clin North Am. 2015;41(3):459–473. [DOI] [PubMed] [Google Scholar]
- 94.Vischio J, Matlyuk-Urman Z, Lakshminarayanan S. Benign spontaneous pneumoperitoneum in systemic sclerosis. J Clin Rheumatol. 2010;16(8):379–381. [DOI] [PubMed] [Google Scholar]
- 95.Wu LL, Yang YS, Dou Y, Liu QS. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19(30):4973–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679–82; discussion 682–3. [DOI] [PubMed] [Google Scholar]
- 97.Kaneko M, Sasaki S, Teruya S, et al. Pneumatosis cystoides intestinalis in patients with systemic sclerosis: A case report and review of 39 japanese cases. Case Rep Gastrointest Med. 2016;2016:2474515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang SJ, Lan JL, Chen DY, Chen YH, Hsieh TY, Lin WY. Colonic transit disorders in systemic sclerosis. Clin Rheumatol. 2001;20(4):251–254. [DOI] [PubMed] [Google Scholar]
- 99.Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. 2016;13(5):295–305. [DOI] [PubMed] [Google Scholar]
- 100.Staller K, Barshop K, Ananthakrishnan AN, Kuo B. Number of retained radiopaque markers on a colonic transit study does not correlate with symptom severity or quality of life in chronic constipation. Neurogastroenterol Motil. 2018;30(5):e13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sattar B, Chokshi RV. Colonic and anorectal manifestations of systemic sclerosis. Curr Gastroenterol Rep. 2019;21(7):33–019-0699–0. [DOI] [PubMed] [Google Scholar]
- 102.Trezza M, Krogh K, Egekvist H, Bjerring P, Laurberg S. Bowel problems in patients with systemic sclerosis. Scand J Gastroenterol. 1999;34(4):409–413. [DOI] [PubMed] [Google Scholar]
- 103.Heyt GJ, Oh MK, Alemzadeh N, et al. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): An association with fecal incontinence. Dig Dis Sci. 2004;49(6):1040–1045. [DOI] [PubMed] [Google Scholar]
- 104.Thoua NM, Abdel-Halim M, Forbes A, Denton CP, Emmanuel AV. Fecal incontinence in systemic sclerosis is secondary to neuropathy. Am J Gastroenterol. 2012;107(4):597–603. [DOI] [PubMed] [Google Scholar]
- 105.Jorge JM, Wexner SD. Anorectal manometry: Techniques and clinical applications. South Med J. 1993;86(8):924–931. [DOI] [PubMed] [Google Scholar]
- 106.Deen KI, Premaratna R, Fonseka MM, De Silva HJ. The recto-anal inhibitory reflex: Abnormal response in diabetics suggests an intrinsic neuroenteropathy. J Gastroenterol Hepatol. 1998;13(11):1107–1110. [DOI] [PubMed] [Google Scholar]
- 107.Zhang HW, Han XD, Wang Y, Zhang P, Jin ZM. Anorectal functional outcome after repeated transanal endoscopic microsurgery. World J Gastroenterol. 2012;18(40):5807–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wald A Clinical practice. fecal incontinence in adults. N Engl J Med. 2007;356(16):1648–1655. [DOI] [PubMed] [Google Scholar]
- 109.Yamaguchi K, Iwakiri R, Hara M, et al. Reflux esophagitis and helicobacter pylori infection in patients with scleroderma. Intern Med. 2008;47(18):1555–1559. [DOI] [PubMed] [Google Scholar]
- 110.Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marie I, Ducrotte P, Antonietti M, Herve S, Levesque H. Watermelon stomach in systemic sclerosis: Its incidence and management. Aliment Pharmacol Ther. 2008;28(4):412–421. [DOI] [PubMed] [Google Scholar]
- 112.Hung EW, Mayes MD, Sharif R, et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J Rheumatol. 2013;40(4):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parrado RH, Lemus HN, Coral-Alvarado PX, Quintana LÃ3pez G. Gastric antral vascular ectasia in systemic sclerosis: Current concepts. Int J Rheumatol. 2015;2015:762546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ingraham KM, O’Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: Demographics and disease predictors. J Rheumatol. 2010;37(3):603–607. [DOI] [PubMed] [Google Scholar]
- 115.Khanlou H, Malhotra A, Friedenberg F, Rothstein K. Jejunal telangiectasias as a cause of massive bleeding in a patient with scleroderma. Rev Rhum Engl Ed. 1999;66(2):119–121. [PubMed] [Google Scholar]
- 116.Marie I, Antonietti M, Houivet E, et al. Gastrointestinal mucosal abnormalities using videocapsule endoscopy in systemic sclerosis. Aliment Pharmacol Ther. 2014;40(2):189–199. [DOI] [PubMed] [Google Scholar]
- 117.Ko CW, Siddique SM, Patel A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020. [DOI] [PubMed] [Google Scholar]
- 118.Otani K, Watanabe T, Shimada S, et al. Clinical utility of capsule endoscopy and double-balloon enteroscopy in the management of obscure gastrointestinal bleeding. Digestion. 2018;97(1):52–58. [DOI] [PubMed] [Google Scholar]