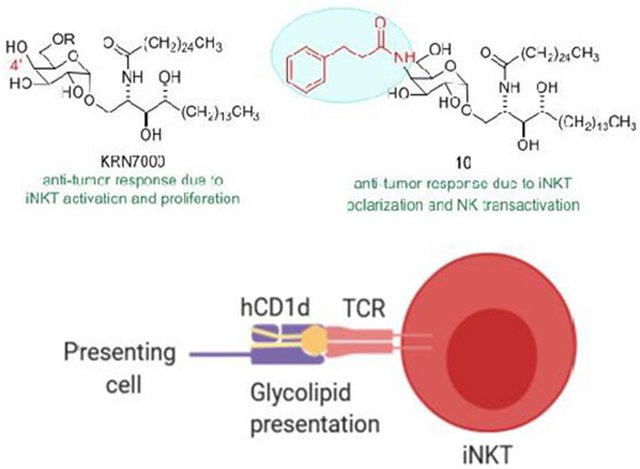
Abstract
Activation of invariant Natural Killer T cells by α-galactosylceramides stimulates strong immune responses and potent anti-tumor immunity. Numerous modifications of the glycolipid structure have been assessed to derive activating ligands for these T cells with altered and potentially advantageous properties in the induction of immune responses. Here, we synthesized variants of α-galactosylceramide with amide-linked phenyl alkane substitutions on the C4” position of the galactose ring. We show that these variants have weak iNKT cell stimulating activity in mouse models, but substantially greater activity for human iNKT cells. The most active of the C4”-amides in our study showed strong anti-tumor effects in a partially humanized mouse model for iNKT cell responses. In silico analysis suggested that the tether length and degree of flexibility of the amide substituent affected the recognition by iNKT cell antigen receptors of the C4”-amide substituted glycolipids in complex with their antigen presenting molecule CD1d. Our findings establish the use of stable amide linked additions to the sugar moiety for further exploration of the immunological effects of structural modifications of iNKT cell activating glycolipids and highlight the critical need for more accurate animal models for assessing these compounds for immunotherapeutic potential in humans.
Graphical Abstract
INTRODUCTION
Natural Killer T (NKT) cells have characteristics of both T lymphocytes and classical natural killer (NK) cells. In contrast to conventional T cells that recognize peptides presented by major histocompatibility complex (MHC) class I or class II proteins, NKT cells recognize glycolipid antigens presented by the MHC class I-like CD1d protein. NKT cells consist of several distinct subsets. The most extensively studied subpopulation, the invariant NKT (iNKT) cells, is characterized by the expression of an invariant TCRα chain (Vα14Jα18 in mice and Vα24Jα18 in humans) paired with a limited range of TCRβ chains. The prototypical iNKT cell antigen is KRN7000 (Figure 1A), an α-galactosylceramide (α-GalCer) derived from structure-activity relationship studies around glycolipids isolated from a marine sponge. Activation of iNKT cells by KRN7000-loaded CD1d elicits immune responses with implications for treating viral and bacterial infections, cancer and a variety of autoimmune conditions.1 KRN7000 has been investigated in clinical applications, including the treatment of solid and hematological malignancies and viral hepatitis.2 Key drawbacks of KRN7000 include its stimulation of both T helper 1 (Th1) and Th2 cytokines, which can have opposing effects. It has long been assumed that identifying ligands that selectively elicit Th1 or Th2 cytokines could greatly improve the prospects of developing effective therapeutic applications of iNKT cell activation.3 In addition, induction of anergy of iNKT cells and hepatic toxicity by KRN7000 have been identified as significant concerns.4
Figure 1. Glycolipid structures and synthesis of C4” amide linked derivatives of α-GalCer.
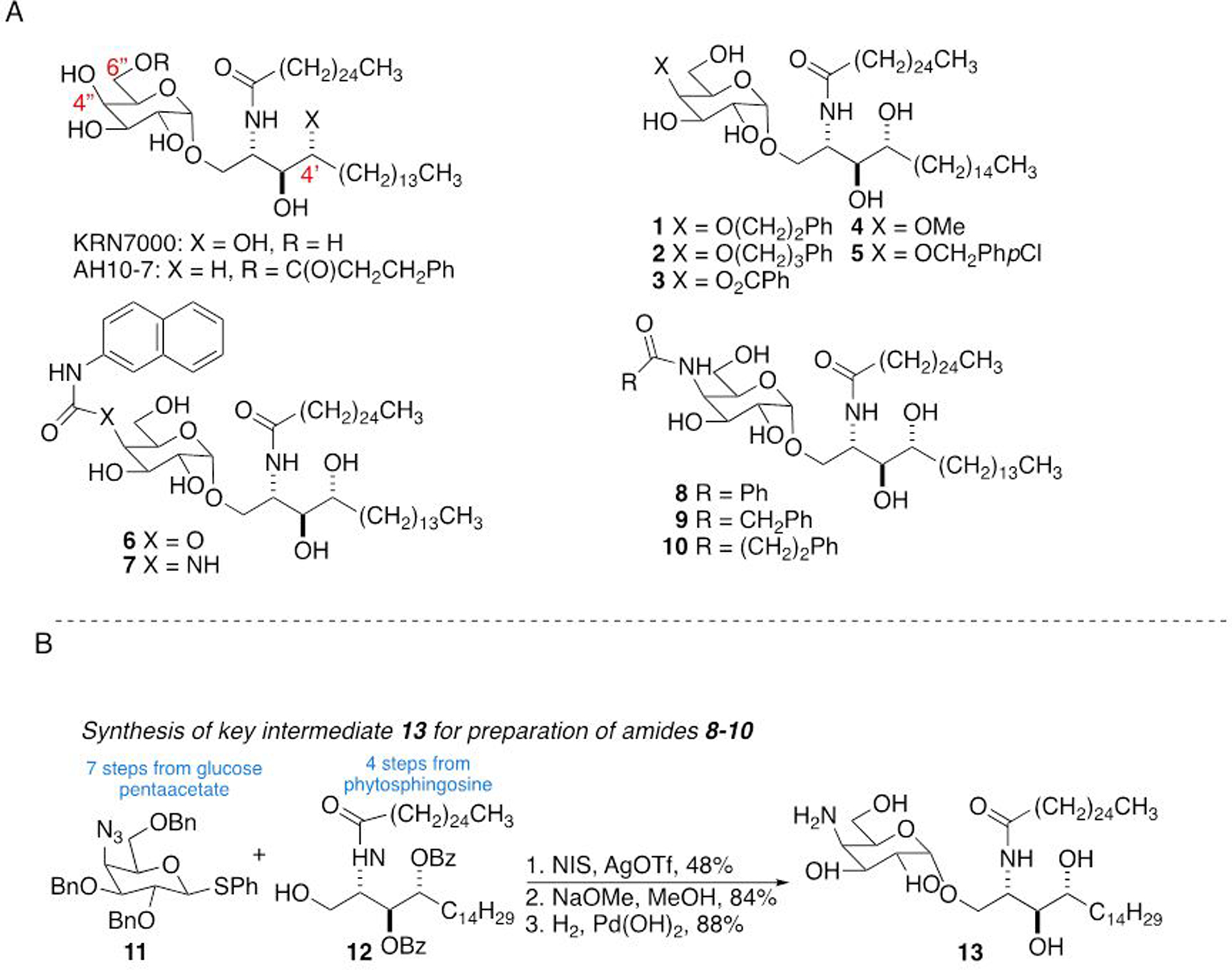
A) KRN7000 is the prototypical α-GalCer antigen for iNKT cells. AH10-7 is a C6”-modified α-GalCer prepared and evaluated by us previously. Compounds 1–7 are representative C4”-modified α-GalCer derivatives previously described in the literature (see text for references). Amides 8–10 are the focus of this work. B) Synthetic strategy for the preparation of 8–10.
Numerous analogs of KRN7000 have been synthesized in an effort to understand structure-activity relationships for iNKT cell glycolipid recognition, and also to produce specific iNKT cell ligands with more favorable activity profiles for potential therapeutic applications.5 These include variations in the acyl chain and the sphingoid base structures, as well as a variety of changes to the carbohydrate head group. For the latter, many active analogs with substitutions at the 6”-position of the sugar (see Figure 1A for numbering) have been described.3c, 6 Both before and after the structures of binary (glycolipid/CD1d)7 and ternary (glycolipid/CD1d/iNKT cell TCR)8 complexes were available, studies had shown that the C6”-hydroxyl is not directly involved in interactions critical for iNKT cell stimulation and that this position can be modified with a variety of adducts to enhance potency, alter the balance of cytokine production, and to incorporate fluorescent or biotin labels. The other sugar position that has seen significant exploration of substituents is the C2”-position, where essentially every alteration studied has resulted in inactive compounds.9 This is anticipated from the lipid bound CD1d/NKT TCR ternary structure,8 which shows that the C2”-OH is involved in hydrogen bond interactions with both CD1d and the T cell receptor, and that it is relatively boxed in by the proteins with little apparent room for accommodating bulkier substitutions. The 3”-OH is also involved in H-bond interactions as a donor to the CD1d protein and potentially also as an H-bond acceptor to the T cell receptor. From the crystal structure there seems to be little room around this position that would allow the introduction of additional atoms, suggesting that this is not likely to accommodate structural modifications. In contrast, the C4”-OH appears not to be essential for stabilizing glycolipid-protein interactions and is positioned in a location that may be more permissive to a range of substitutions.
Initial studies by us and others looked at simple modifications of the C3”- and C4”-positions and concluded that changes at the C3”-position were markedly deleterious to murine iNKT cell stimulation while C4” substitutions may be better tolerated.9b, 10 Following availability of ternary structural data, Wang and coworkers reported C4”-modified analogs containing a limited range of small aliphatic ethers or acetamide which showed residual iNKT cell stimulating activity,11 and subsequently incorporated bulkier aromatic moieties on C4” via ether or ester linkages (compounds 1–3, Figure 1A), with a goal of picking up π-π stacking interactions with the iNKT cell TCR.12 Mouse iNKT cell hybridoma assays showed these compounds to be at least as stimulatory as KRN7000. Mouse in vivo cytokine profiles were less clear-cut, but did show a marked Th2 bias for 2. In 2016, Yu, Wong and coworkers reported that glucosyl variants of two potent α-GalCer analogs were more potent activators of human iNKT cells than the parent compounds, although they were relatively inactive in mice, thus suggesting significant species variation for variations of the C4” position of the glycolipid.13 Van Calenbergh and coworkers reported a series of C4”-analogs of KRN7000.14 These included ethers 4 and 5, carbamate 6 and urea 7 (Figure 1A). All of the analogs were found to be stimulatory in mice, although not as potent as KRN7000 (with the exception of methyl ether 4), and all except 4 were Th1 biasing. All of the compounds were able to stimulate human iNKT cells in vitro, again with only methyl ether 4 being slightly more antigenic than KRN7000. More recently, based on the polarization of iNKT cell cytokine responses seen with ethers 4 and 5, van Calenbergh and coworkers prepared an extensive library of 4”-O-alkylated α-GalCers with aromatic and cycloaliphatic moieties with differing tether lengths.15 None was as potent a stimulus as KRN7000, although several induced a proinflammatory response, suggesting that there could be applications for such compounds as cancer immunotherapeutics or as vaccine adjuvants. Taken together, these results support the hypothesis that modifying the 4”-OH could lead to potent iNKT cell activators that induce biased cytokine responses or other alterations of immunologic functions of iNKT cells.
In the current study, we have built on this background work by exploring the effects of amide linked phenyl or phenylalkyl substitutions of the C4”-OH of KRN7000. Although only weakly active in standard mouse models of iNKT cell responses, these compounds showed much greater activities for stimulation of human iNKT cells and in a partially humanized mouse model. The results support further investigation of C4”-modified amide derivatives as potential agents for manipulating iNKT cell responses in humans and emphasize the importance of accurate animal models for assessment of this family of potential therapeutic immunomodulators.
RESULTS AND DISCUSSION
Synthesis and assessment of iNKT cell stimulatory activity of C4” modified glycolipid amides.
Based on examination of the X-ray crystallographic structure of human CD1d and iNKT cell TCR in complex with KRN7000,8 our initial goal was to pick up π-π stacking between the glycolipid and the TCR. Our first set of compounds prepared to examine the effect of a phenyl group attached to linkers of different types and lengths was amides 8–10 (Figure 1A). In addition to incorporating an aromatic moiety, the amides could serve as an H-bond donor, acceptor or both. Although the area directly around the 4”-OH contains polar residues, there is a phenylalanine in what appears to be an accessible hydrophobic region. The approach to the syntheses of the target C4”-modified glycolipid amides 8–10 is illustrated in Figure 1B. The amides were prepared by acylation of amine-containing α-GalCer 13 (Figure 1B). Intermediate 13 was accessed by glycosylation of known disarmed ceramide 1216 with armed azidothioglycoside 11,11 followed by a two stage deprotection involving saponification of the benzoate esters and hydrogenolysis to cleave the benzyl ethers.
An initial screening for iNKT cell stimulating activity of C4”-modified variants of KRN7000 was carried out with well-established cell culture assays.17 Using interleukin-2 (IL-2) secretion by mouse T cell hybridomas cultured with mouse bone marrow-derived dendritic cells (DCs) as a measure of activation, we found little or no detectable response to the C4”-modified glycolipid amides over a wide range of concentrations. For reference, KRN7000 and the C6”-ester modified glycolipid AH10-7 (see Figure 1A) studied by us previously6l both gave robust iNKT cell activation over the same range of concentrations (Figure 2A, left). However, when tested with human iNKT cell clones cultured with human CD1d-transfected APCs (Figure 2A, right), the C4”-modified amides showed clear, dose-dependent stimulatory activity, as measured by cytokine secretion (interferon-γ (IFNγ) in this case, since that is the dominant cytokine produced by these human cell lines).17a Potency for stimulation of human iNKT cell lines was comparable to that for AH10-7, and less than for KRN7000. To assess stimulation of primary polyclonal iNKT cells, we cultured mouse spleen cells in the presence of KRN7000 or C4”-amide glycolipids and measured their release of IFNγ into the culture media. With wild type C57BL/6 splenocytes, this recapitulated the findings from mouse iNKT cell hybridomas, showing strong stimulation in response to KRN7000 but undetectable or comparatively weak responses to C4”-amide variants (Figure 2B, top). However, using splenocytes from human CD1d knock in (hCD1d-KI) mice, in which mouse CD1d is replaced by the homologous human protein,18 we could detect dose dependent responses to all three C4”-amide variants (Figure 2B, bottom).
Figure 2. Activation of iNKT cells by C4” amide variants of α-GalCer.
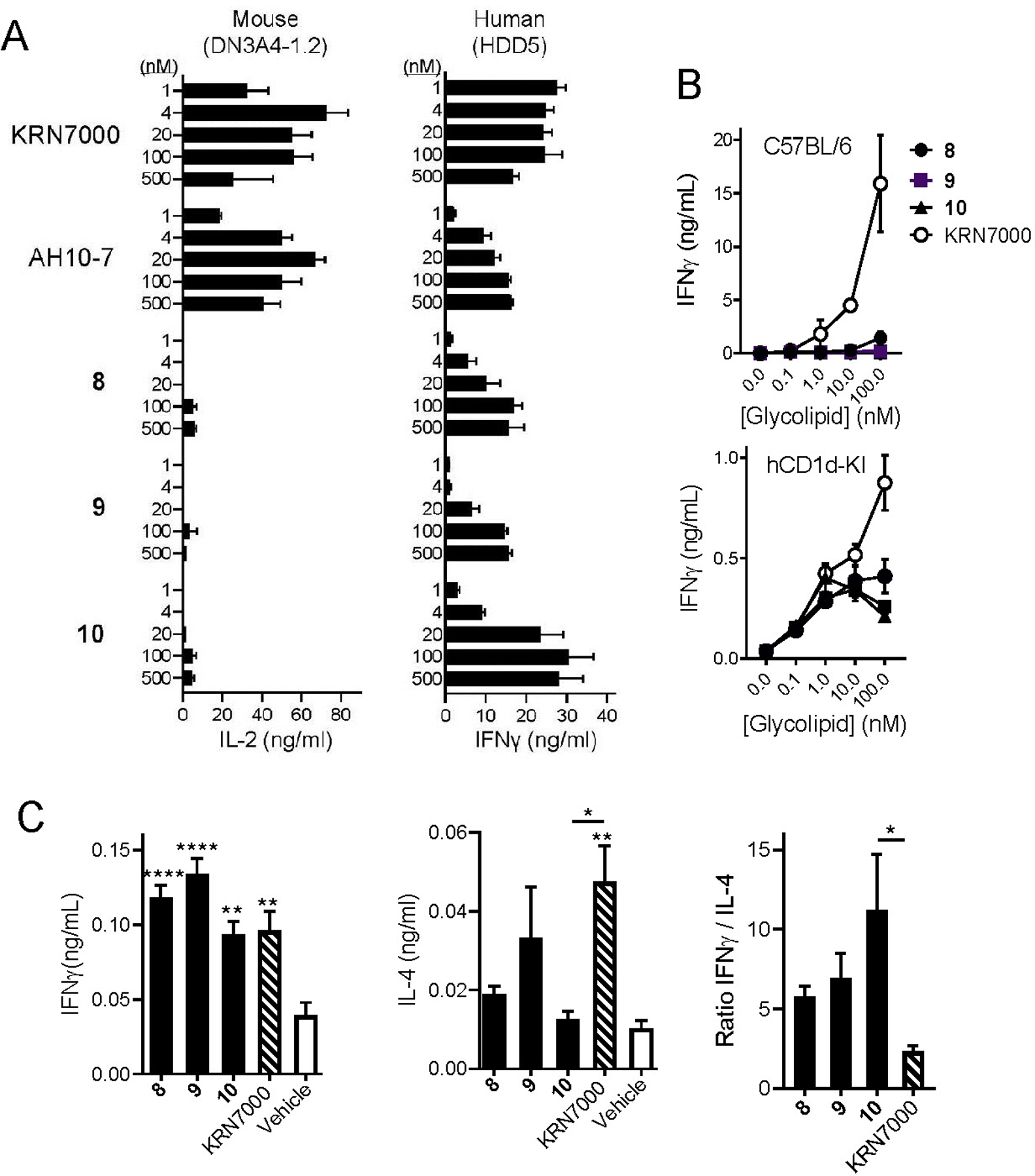
A) Mouse iNKT cell hybridoma DNA3.4-1.2 (left) or human iNKT cell clone HDD5 (right) were stimulated with a range of concentrations of the indicated glycolipids. Mouse iNKT hybridoma cells were cultured with glycolipids plus C57BL/6 strain bone marrow-derived dendritic cells, and supernatant levels of IL-2 were measured after 24 hours. Human iNKT cells were cultured with glycolipids plus HeLa cells transfected to express human CD1d, and supernatant levels of IFNγ were measured after 24 hours. Bars show mean values and one standard deviation for triplicate cultures. Results shown are representative of three experiments. B) Spleen cells from wild type C57BL/6 mice (top) or human CD1d knock-in mice (hCD1d-KI, bottom) were cultured with glycolipids at the concentrations indicated, and supernatant levels of IFNγ at 72 hours were determined by ELISA. Symbols show means and error bars represent ±1 SE for triplicate cultures. Results shown are representative of two separate experiments. C) Serum cytokine levels were determined in hCD1d-KI mice (N = 10 mice per group) following intravenous injection of 4 nanomoles of glycolipids or inert vehicle. Levels of IFNγ at 24 hrs (left) and of IL-4 at 2 hrs (center) post injection are shown. The ratio of these values for each glycolipid was calculated (right). Bars represent mean values ±1 SE. *, p < 0.05; **, p < 0.01; ***, p < 0.001 for comparisons with vehicle control group (ANOVA with Dunnet post-test for multiple comparisons). Results shown are representative of two experiments.
Overall, these results from in vitro assays suggested a moderate level of activity for C4”-amide variants in human or partially humanized mouse iNKT cell stimulation. To analyze this in an in vivo setting, we assayed serum cytokine responses in hCD1d-KI mice following a single intravenous injection of glycolipids or inert control vehicle (Figure 2C). Levels of IFNγ were measured in sera obtained at 24 hours after stimulation, and IL-4 was measured in sera obtained at 2 hours post stimulation, which have been established in previous studies as the time points for peak production of these cytokines.19 All three C4”-amide variants showed IFNγ production similar in magnitude to KRN7000 in this assay, while showing lower levels of IL-4. This pattern has been previously described as representing iNKT cell activation with a Th1 bias,6l, 19 as reflected in the increased ratio of peak levels of IFNγ to IL-4. This ratio trended higher for compounds 8, 9 and 10 compared to KRN7000, achieving a significantly higher value relative to KRN7000 for 10 (Figure 2C, right).
Anti-tumor activity of C4”-amides.
The effects of α-GalCers on stimulating anti-tumor immunity through iNKT cell activation are well documented,20 and optimizing the structure of glycolipids for this activity remains a major translational goal. As an initial assessment of the anti-tumor activities of C4”-amides 8, 9 and 10, we tested these compounds in comparison to KRN7000 in the B16.F10 model of metastatic melanoma.18 We used hCD1d-KI mice for these experiments as we have done previously,6l given their ability to more accurately model the biology of iNKT cell responses in humans.18 In experiments using a single injection of glycolipids administered i.v. three days after tumor inoculation, we observed a potent anti-tumor effect of C4”-amide compound 10, which showed suppression of tumor burden in the lungs that was at least as great as KRN7000 and highly significant compared to mice receiving an inert vehicle injection (Figure 3A). The other C4”-amides (compounds 8 and 9) showed no significant anti-tumor activity in this model. A second experiment comparing KRN7000 and 10 directly in this model but using two injections of the glycolipids yielded a similar result with a trend toward superior activity for 10 versus KRN7000, although this did not achieve statistical significance (Figure 3B).
Figure 3. In vivo anti-tumor activity of C4”-amide glycolipids.
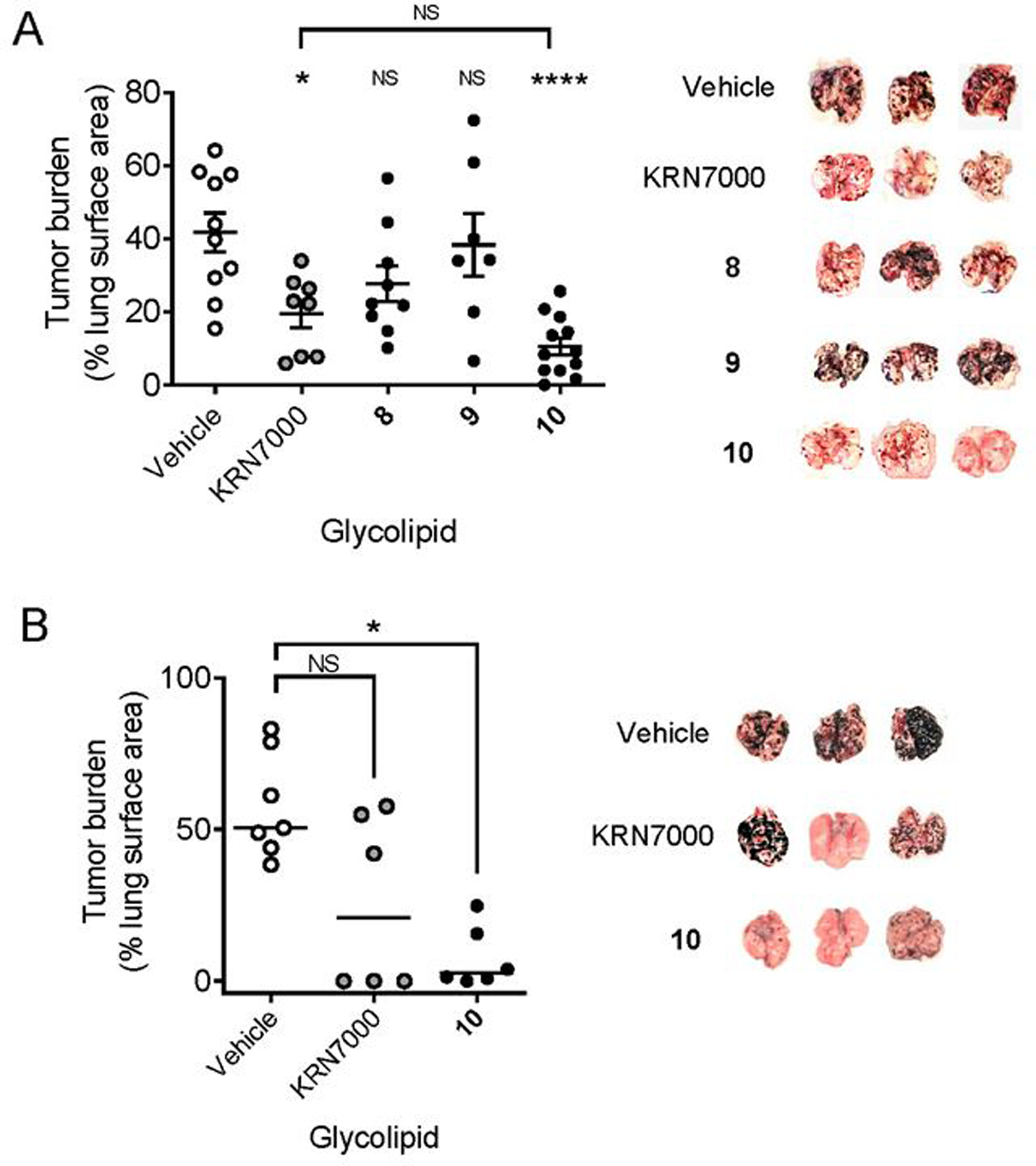
A) Partially humanized hCD1d-KI mice were injected i.v. with 5 × 105 B16.F10 melanoma cells. Three days later, groups of mice (minimum of 7 per group, range 7 – 12) received i.v. injections of the indicated glycolipids (nmol) or vehicle. Animals were sacrificed 15 days later, lungs were removed, and the percentage of lung surface covered with deeply pigmented metastatic nodules in each animal was determined using Image J analysis software. The graph on the left shows individual and median values for mice treated with glycolipids or control (vehicle). Images on the right are representative lungs from three animals in each of the treatment groups. B) Mice (hCD1d-KI mice, 6 – 7 per group) were injected i.v. with 5×105 B16.F10 melanoma cells, followed by two injections of glycolipids or vehicle administered at day 3 and day 7. Animals were sacrificed 15 days after the second glycolipid injection, lungs were removed and analyzed for tumor burden as in A. Values for individual mice and median values are shown. *, p < 0.05; ****, p < 0.0001; NS, not significant (ANOVA with Dunnet post-test for multiple comparisons between glycolipids and vehicle control). The experiment in A was carried out twice with similar results, and the experiment in B has been performed once.
Pronounced proinflammatory responses induced by C4”-amide compound 10.
To gain insight into the potential mechanisms for the strong anti-tumor effects of compound 10, we used flow cytometry to directly assess the induction of several important pro-inflammatory molecules by iNKT cells. Concurrently, we examined these markers on conventional T cells and NK cells, both of which have been shown to be indirectly stimulated following iNKT cell activation, a process referred to as transactivation which has been linked to proinflammatory and antitumor effects of iNKT cells.17a, 19 Groups of hCD1d-KI mice were injected once i.v. with either KRN7000, 10 or inert vehicle, and sacrificed at 5, 24, 72 and 168 hours post injection. Mononuclear cells were extracted from the livers of these animals, which represent a site of iNKT cell enrichment that facilitates analyses at the single cell level. Cell suspensions were stained with glycolipid-loaded human CD1d tetramers and with antibodies against cell surface and intracellular markers, followed by flow cytometry analysis with selective gating for iNKT cells, NK cells and conventional T cells (as illustrated in Supplemental Figure S1).
This analysis revealed that IFNγ production by iNKT cells was relatively weak compared to KRN7000 stimulation and was maximal at the earliest time point sampled (5 hours). Expansion of iNKT cells based on absolute counts was also minor or undetectable with 10 in contrast to KRN7000 stimulation, and a similar pattern was observed for granzyme B levels (Figure 4A). However, iNKT cells from animals receiving 10 showed a striking increase in intracellular staining for T-bet, the master transcription factor associated with proinflammatory Th1 polarization of responses (Figure 4B).21 Conversely, the expression of GATA-3, the master transcription factor opposing Th1 differentiation, was significantly reduced in iNKT cells stimulated with 10 compared to KRN7000 (Figure 4C). A parallel analysis of NK cells and conventional T cells in the same mice revealed enhanced transactivation of these lymphocyte subsets by 10 relative to KRN7000 (Figure 5). This included significantly increased magnitude and prolongation of IFNγ production, as well as significantly increased expansion of granzyme B expression by NK cells in the animals injected with 10 (Figure 5A). Similar effects, although less pronounced, were also observed for transactivation of conventional T cells (Figure 5B).
Figure 4. Proinflammatory Th1-biased responses to C4”-amide derivative 10.
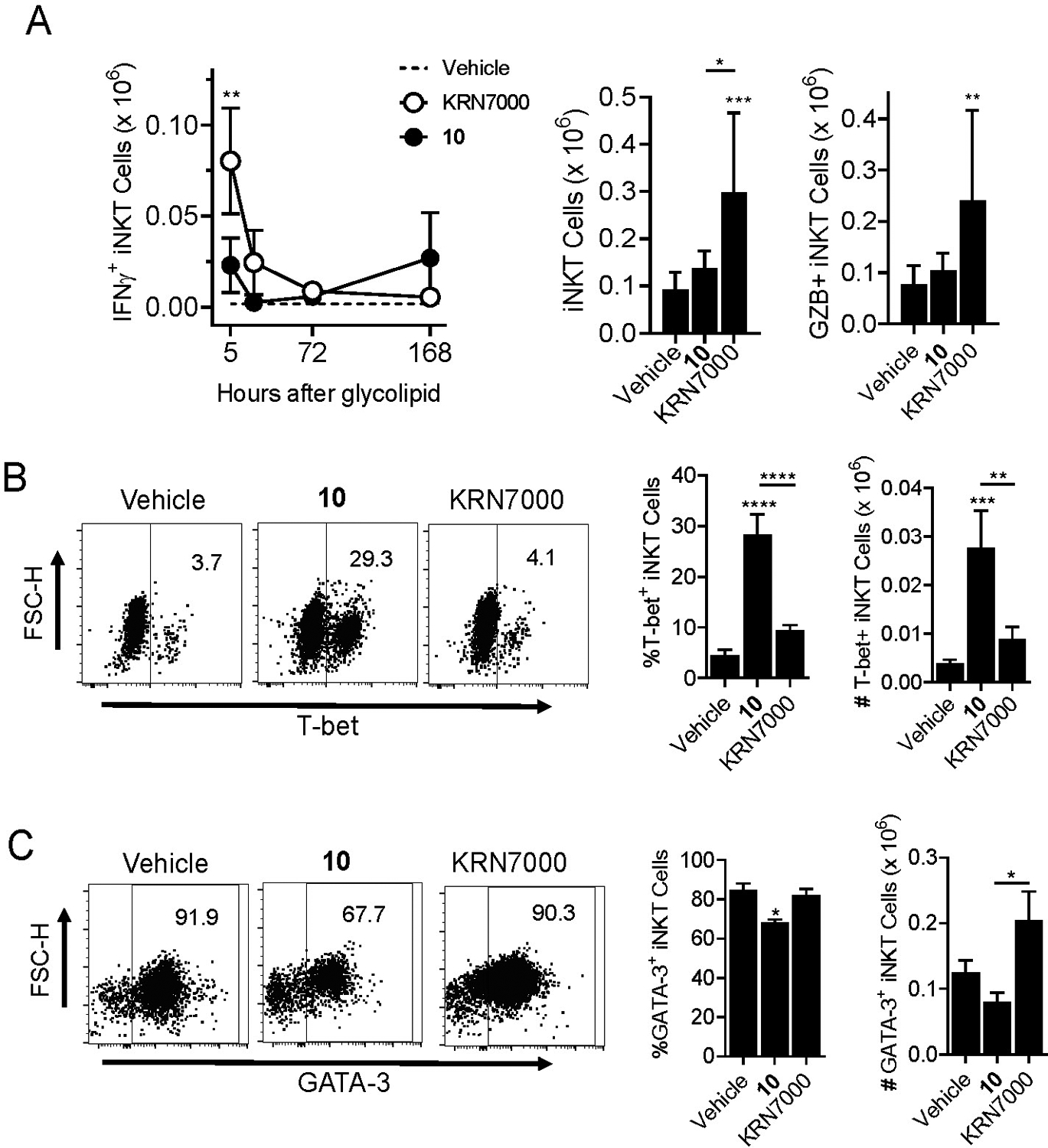
Groups of 20 hCD1d-KI mice were injected intravenously with either 4 nmol of KRN7000 or C4”-amide derivative 10, or inert control vehicle. Five mice from each group were sacrificed at 5, 24, 72 or 168 hours post glycolipid injection. Livers were removed and processed to generate cell suspensions for flow cytometry analysis of cell surface and intracellular markers of iNKT cells, NK cells and T cells. A) Analysis gated on α-GalCer-loaded CD1d tetramer stained iNKT cells. Graph on left shows time course of intracellular IFNγ staining in animals injected with KRN7000 versus compound 10. Dashed line indicates background staining level in control mice receiving vehicle. Bar graphs show absolute numbers of total iNKT cells (left) and of granzyme B stained iNKT cells (right) at 5 hours post glycolipid or vehicle injection. B) Analysis of intracellular staining of T-bet in gated iNKT cells at 72 hours post stimulation with the indicated glycolipid or inert control vehicle. C) Same as B except showing intracellular staining for GATA-3 in iNKT cells at 24 hours post glycolipid or vehicle administration. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****p < 0.0001 for comparisons to paired values from vehicle treated control mice (ANOVA with Dunnet post-test for multiple comparisons). See Supplemental Figure S1 for details on gating strategy for cell types and analysis of cell surface or intracellular markers.
Figure 5. Pronounced transactivation of NK cells and conventional T cells by C4”-amide derivative of α-GalCer.
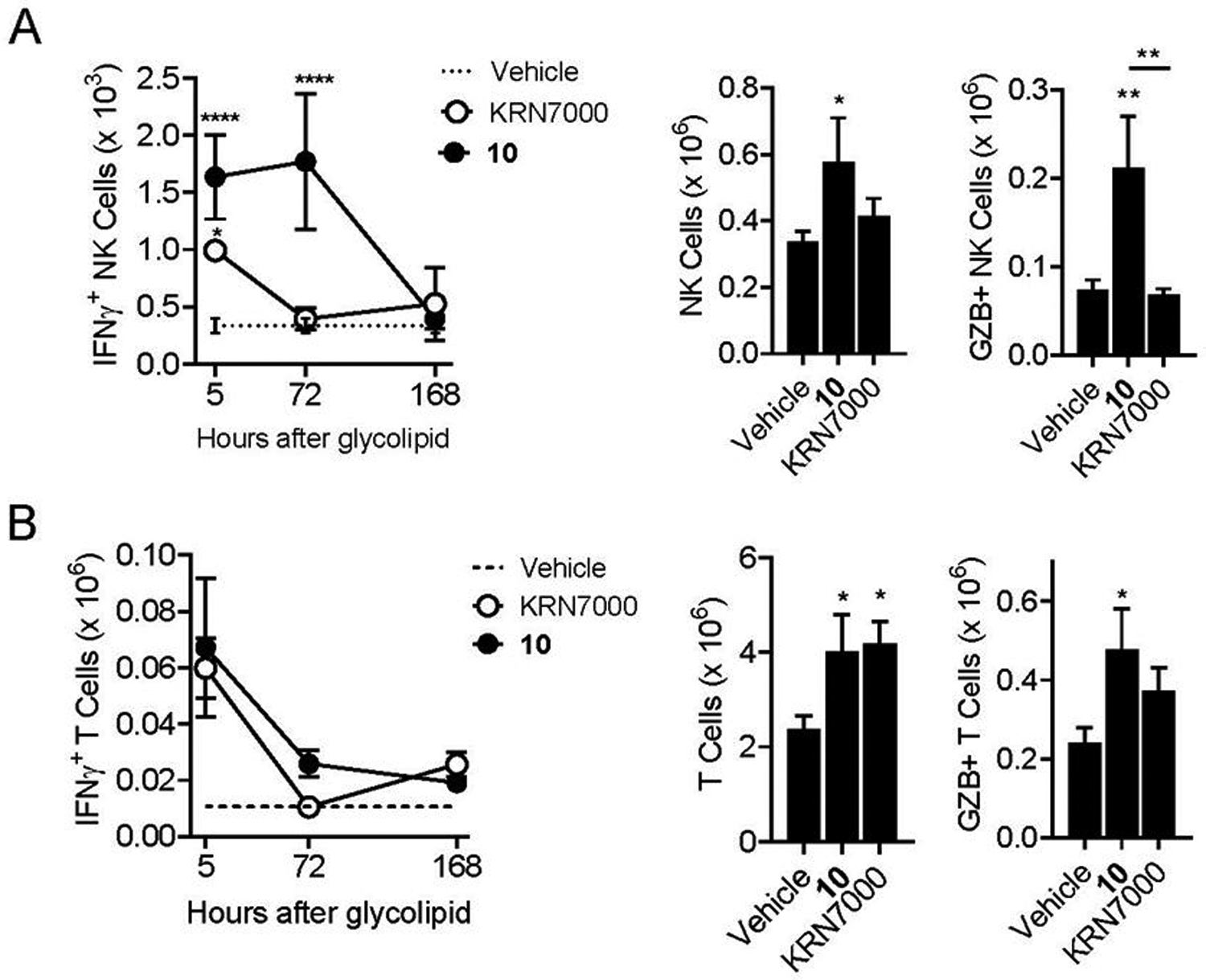
Results shown are from analysis of the same mice shown in Fig. 4, and graphs are as described in the previous figure. A) Analysis with gating on NK cells (B220 negative, TCRβ negative and NK1.1 positive lymphocytes. B) Analysis with gating on conventional T cells (B220 negative, TCRβ positive, CD1d tetramer negative). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****p < 0.0001 for comparisons to paired values from vehicle treated control mice, or for 10 versus KRN7000 where indicated (ANOVA with Dunnet post-test for multiple comparisons). See Supplemental Figure S1 for details on gating strategy for cell types and analysis of cell surface or intracellular markers.
Molecular Modeling of TCR recognition of C4”-amides.
To understand general features at the level of TCR-ligand recognition that could correlate with the observed biological activities of the C4”-amide derivatives of α-GalCer, we performed molecular dynamics (MD) simulations of a fully solvated hCD1d-TCR complex bound to 8, 9, 10, and KRN7000. For each ternary complex, the dynamic nature of protein-ligand interactions was captured using a Simulation Interaction Diagram (SID) to evaluate the occurrence and type of ligand-protein interactions. These results, displayed in Figure 6, provide percentages that indicate the fraction of time a given residue interacted with the ligand. The most notable difference between 10 and the other amides was that the phenyl group in 10 made no systematic contacts with CD1d or TCR. In contrast, this group interacted with CD1d-His68 via π-π stacking in 8 and π-π stacking with CD1d-Trp153 and TCR-Phe51 in 9 (see also structures in Figure 7). In 8 and 9, the amide NH group was involved in hydrogen bonds, but this was not the case for 10. These observations suggested that the alkyl-phenyl group was more flexible and freely positioned in 10 than in 8 and 9. Analysis of the dihedral angle formed by rotation of the bond adjacent to the amide carbonyl group showed a larger range of angles throughout the MD simulation in 10 compared with the other two compounds (Figure 7, lower panel). Such flexibility was also evident in the visualization of the movement around this bond in the dynamic simulations (see movies included in Supplemental Information). As far as the galactose moiety and the glycosidic linkage, which are common to all ligands including KRN7000, 10 exhibited a more similar set of interactions compared to KRN7000. Among these interactions, the strongest were the two hydrogen bonds with both CD1d-Asp151 and the CD1d-Asp80. In contrast, these interactions were less frequent in 8 and 9. Therefore, it appeared that the greater potency of 10 may be related to having a flexible substituent (i.e. lack of substantial π-π or aromatic hydrogen bond interactions), while retaining a similar set of interactions as KRN7000 for the rest of the glycolipid. Thus, the enhanced tumor protection and Th1 biasing effects of 10 could have resulted from other favorable properties, such as solubility and its uptake or distribution in antigen presenting cells.
Figure 6. In silico analysis of ternary complexes.
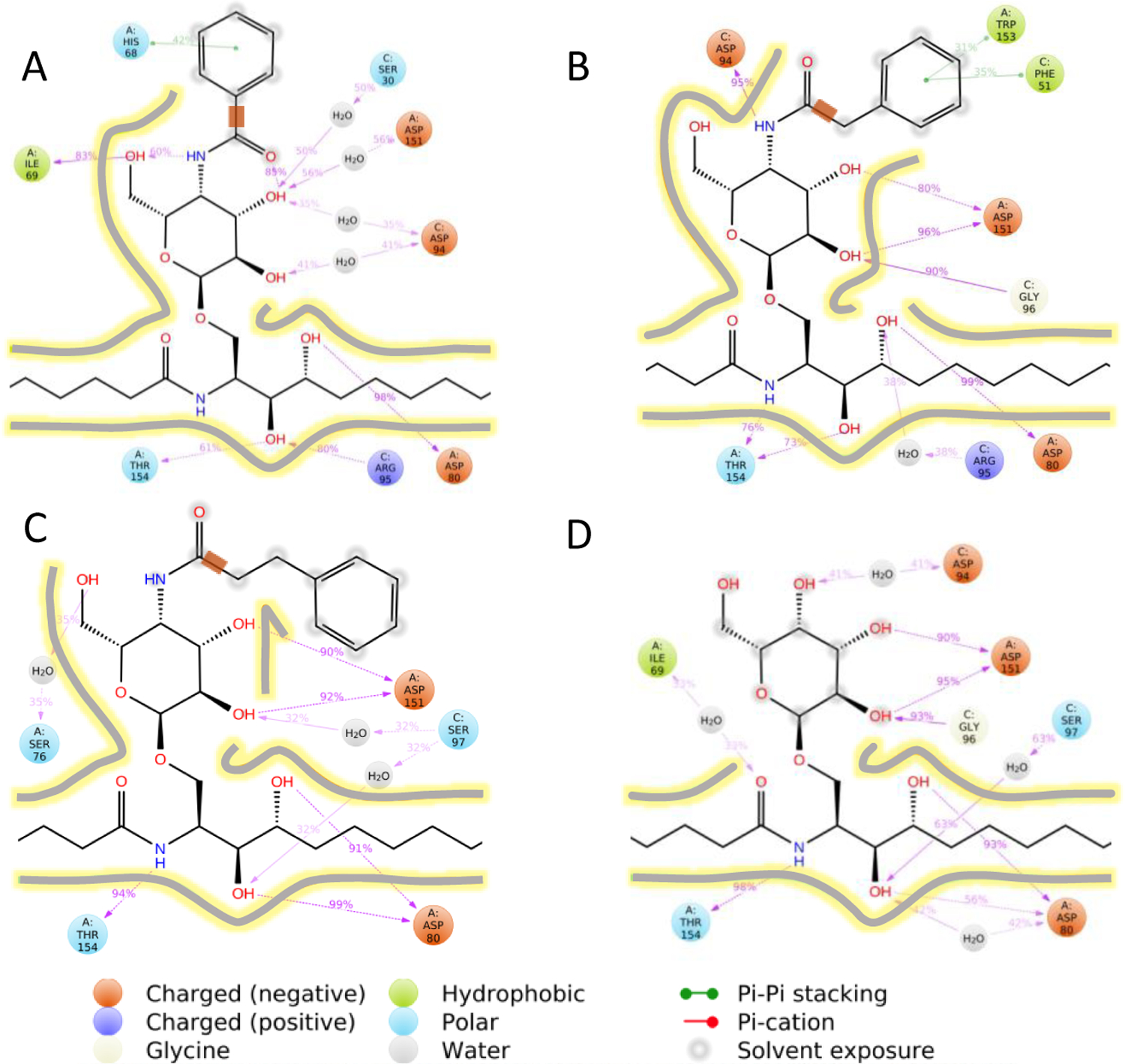
Molecular Dynamics simulation interaction diagrams (SIDs) are shown for the human CD1d-TCR complex containing 8 (A), 9 (B), 10 (C) or KRN7000 (D). Percentages indicate the fraction of time a given residue interacts with the ligand. Only interactions with an occurrence larger than 30% are displayed. The bonds adjacent to the amide’s carbonyl group are highlighted as a thick orange line in panels A, B, and C for further discussion in Figure 7. Contour lines give an approximation to the protein cavity based on the strength of the ligand-protein interactions.
Figure 7. Positioning of glycolipid ligands predicted by molecular dynamics.

Representative snapshots are shown from the MD simulation for the human CD1d-TCR complex containing C4”-amide α-GalCer derivatives 8 (A), 9 (B), and 10 (C). Yellow dashed lines indicate hydrogen bonds and light blue dashed lines indicate π-π interactions. The C-C bonds of bound glycolipids are colored green, nitrogen atoms are blue and oxygen atoms are red. Residues of the CD1d and TCR proteins in close apposition to the glycolipid are shown in grey. Water molecules within 4 Å from the ligand are also displayed. Lower panels show the distribution of the dihedral angles throughout the MD simulation along the bond adjacent to the amide’s carbonyl group (highlighted in orange in Figure 6). The broad distribution in 10 indicated larger displacements of the alkyl-phenyl group.
From a structural point of view, it is unclear why 8, 9, and 10 are much less active in standard mouse iNKT cell hybridoma screening assays. From the simulation interaction diagram generated using the mouse CD1d and TCR (see Supplemental Figure S2), we inferred that the type and frequency of the interactions were roughly similar to those found in human CD1d-TCR complexes, with the noteworthy exception that in 8 the galactose adopted a non-standard orientation that was highly constrained via a cation-π interaction with TCR-Lys68 (Supplemental Figure S3). This highly distorted positioning of the sugar would be anticipated to compromise TCR recognition and may have contributed to the extremely weak responses to compound 8 in fully murine assays. However, our analysis did not predict this conformation to be prominent for murine complexes containing 9 or 10, and the explanation for their extremely poor stimulatory activity in mice remains unclear.
CONCLUSIONS
In the current study, we synthesized a series of three analogues of KRN7000 containing aromatic groups linked with no spacer or by one or two CH2 groups to the C4” position via an amide bond. Significantly, we found that, although analogs 8–10 were only weak activators of mouse iNKT cell responses, these all showed at least moderate potency for human iNKT cell activation in cell cultures, as well as in vivo activity in the human CD1d knock-in mouse model. We were able to take advantage of the partially humanized iNKT cell system of human CD1d knock-in mice to perform detailed investigation of in vivo responses, showing pronounced anti-tumor effects that were dependent on the length of the alkane linker and associated with enhanced proinflammatory responses. Overall, our results confirm the notion that the C4” position of the sugar of α-GalCer represents a relevant position for structural modification of glycolipid ligands of iNKT cells based on the prototypical KRN7000 structure. In addition, our use of the relatively stable amide linker provides a straightforward approach for linking a variety of substituents to the C4” of the carbohydrate group.
Several strategies for incorporating substituents on the sugar have yielded varying levels of success in generating active iNKT cell ligands. For example, Zhang et al. concluded that the introduction of aromatic groups on the C4” position with ether or ester linkers did not block iNKT cell TCR ligand recognition, and in some cases might create analogues with potencies comparable to KRN7000.12 The group of van Calenbergh reached similar conclusions in their studies of 4”-O-alkylated α-GalCer analogs, initially identifying a C4”- linked p-chlorobenzyl ether as an analogue with promising immunostimulating properties although less potent than KRN7000 based on studies in mice.14 More recently, they reported synthesis and biologic evaluation in mice of a large panel of 4”-O alkylated derivatives, concluding that while ether-linked substitutions to the C4” position decreased the immunogenic potential in mice relative to KRN7000, benzyl‐modified glycolipids with this structure are able to produce a distinct pro‐inflammatory immune response that may be useful for particular applications.15
It is important to note that all studies published to date on the biologic activities of C4”-modified α-GalCer analogues have relied mainly or exclusively on analyses using cultured mouse iNKT cells, and in vivo evaluation has been limited to standard laboratory mouse strains. In light of the striking species related differences that we observed in the current study, this suggests that past screening efforts have been largely inadequate for characterizing the true biologic activities of C4”-modified forms of α-GalCer for human iNKT cell responses. Our results indicated that the reduced activity of C4”-amides in assays based on mouse iNKT cells was at least partly reversed by using human CD1d knock-in mice. These animals express human CD1d in place of the endogenous mouse protein and accurately replicate the tissue distribution and levels of iNKT cells that are typical for humans.6l, 18 Although the TCRs of iNKT cells in these mice are composed of endogenous mouse TCRα and β chains, they undergo selection for recognition of human CD1d during development, possibly leading also to more human-like receptor structure and specificity. In this model, we found that the C4”-amide glycolipids showed activity both in vitro and in vivo that was nearly equal to KRN7000 with respect to stimulation of cytokine production.
Significant anti-tumor activity could also be demonstrated for compound 10, but not for the other two C4” amides, apparently reflecting a fine tuning of the overall immune response that depends on subtle variations in the glycolipid structure. It has been suggested previously that appending phenyl substituents to the C4” position could alter the affinity of TCR interaction with the glycolipid-CD1d complex, either through π-π stacking as suggested by Zhang et al,12 or through an extra hydrophobic interaction between the benzyl moiety and the α2-helix of CD1d.15 However, our modeling and molecular dynamics analysis of ternary complexes containing 8, 9 and 10 did not suggest a uniform effect for the benzyl modification in enhancing interactions within the ternary complex. Thus, it appeared that 10, which in general was the most active of the C4”-amides in the assays applied, did not pick up substantial π-π or aromatic hydrogen bond interactions, whereas 8 and 9 did. Conversely, molecular dynamics simulations suggested that the most characteristic feature of 10 was the relatively unconstrained movement of the longer C4” linker and its attached phenyl group. This suggests that enhanced Th1 cytokine bias, sustained transactivation of NK cells and the associated anti-tumor immunity could be the result of other properties of 10, such as overall solubility of the glycolipid in physiologic conditions or its partitioning into different subcellular compartments, as previously shown for other α-GalCer analogues.6l, 17b, 19, 22 In other words, a non-interacting aromatic group may confer more favorable immunologic effects than an interacting one in the context of C4”-substituted amides.
MATERIALS AND METHODS
Glycolipids, synthetic procedures and compound characterization
KRN7000 was obtained from a commercial source (Avanti Polar Lipids). The synthesis of AH10-7 has been previously reported.6l All chemicals, solvents and deuterated solvents were purchased from Sigma-Aldrich, Alfa-Aesar, Oakwood Chemicals or Fisher Scientific and used as received unless noted. Methylene chloride (DCM) was dried over CaH2. Deuterated chloroform (CDCl3) was dried over activated 4Å molecular sieves. All reactions, unless specified, were conducted under an atmosphere of N2 in glassware that had been oven or flame dried. 1H NMR spectra were recorded at 400 MHz and/or at 500 MHz and calibrated to the residual CHCl3 peak at 7.27 ppm. 13C NMR spectra were recorded at 100 MHz and/or at 125 MHz and calibrated to the CDCl3 peak at 77.23 ppm. Chemical shifts are reported in units of parts per million (ppm). Infrared (IR) spectra were recorded on an FT-IR spectrophotometer and are reported in cm−1. High-resolution mass spectra were obtained on an AccuTOF instrument at the University of Connecticut. Specific rotations [a]D were obtained on a JASCO P-2000 polarimeter, using the sodium D-line as a source, and the concentration (c) is expressed in g per 100 mL. Flash chromatography was performed on silica gel, 40 microns, 32–63 flash silica. Thin layer chromatography was performed on silica gel (silica gel 60 F254) glass plates, and the compounds were visualized by UV and/or 5% phosphomolybdic acid in ethanol. Experimental details for the preparation of new compounds and proton and carbon NMR spectra of purified intermediates and final products are available in Supplemental Data.
Mice
C57BL/6 mice were purchased from Jackson Laboratory, and hCD1dKI mice on a C57BL/6 background18 were bred and maintained in the animal facilities of the Albert Einstein College of Medicine Animals were 6 – 10 weeks of age at the time they were entered into experiments, and only female mice were used. Animals were maintained according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, and studies involving mice were specifically approved by the Institutional Animal Care and Use Committees (IACUCs) of the Albert Einstein College of Medicine.
Reconstitution of glycolipids for in vitro and in vivo use
For in vitro assays, glycolipid stock solutions were prepared at 100 mM in DMSO (Sigma). Immediately before use, these stocks were heated to 70°C, sonicated for 5 min and then diluted to 1 mM in pre-warmed (37°C) culture medium (RPMI-1640 with 10% FCS). This stock was further diluted with culture medium immediately before adding to cell cultures to give the desired final glycolipid concentrations ranging from 0.01 – 1000 nM and a final DMSO concentration of 1%. For in vivo injection into mice, glycolipids were first dissolved to 20 mM in DMSO and then further diluted to 200 μM using PBS + 0.5% Tween-20. This solution was diluted 1:10 with pre-warmed (80°C) PBS immediately before injection of mice. Injection of 200 μl delivered 4 nmol of glycolipid in a vehicle with final composition of PBS + 0.1% DMSO + 0.05% Tween-20.
Monoclonal antibodies and flow cytometry
Fluorochrome-conjugated monoclonal antibodies for Flow Cytometry analyses were obtained from commercial vendors (BD Biosciences except where indicated otherwise) and included the following: anti-GATA-3 (BUV395 conjugate, clone L50-823), anti-NK1,1 (BV605 conjugate, clone PK136), anti-CD4 (BV786 conjugate, clone RM4–5), anti-TCRβ (FITC conjugate, clone H57–597), anti-B220 (APC-Cy7 (conjugate, clone RA3–6B2) anti-Granzyme B (PE-CFS597 conjugate, clone GB11), anti-IFN-γ (AF700 conjugate, clone XMG1.2), and anti-T-bet (PE-Cy7 conjugate, clone eBio4B10, from eBiosciences). Human or mouse CD1d tetramers loaded with glycolipid PBS-57 were either APC or PE conjugated and were obtained from the NIH Tetramer Core Facility. Samples were also stained with Zombie NIR™ Fixable Viability Kit (Biolegend) to distinguish live versus dead cells. For flow cytometry analyses, samples of cell suspensions were resuspended in PBS and stained with Zombie NIR™ and subsequently stained with fluorochrome-conjugated mAbs against surface markers diluted to predetermined optimal concentrations in PBS containing 2% fetal bovine serum (FBS) and sodium azide (0.05%; Sigma-Aldrich). For experiments involving staining of intracellular molecules (T-bet, GATA-3, Granzyme B, and intracellular cytokines), cells were fixed using paraformaldehyde (2% in PBS; Electron Microscopy Sciences), permeabilized using Fixation & Permeabilization Buffer (eBiosciences), and stained with the relevant mAb fluorochrome conjugates. Multiparameter FACS analyses were carried out using an Aurora multispectral flow cytometer (Cytek), and data analysis was done using FlowJo™ software (BD Biosciences).
In vitro and in vivo activation of iNKT cells.
Mouse iNKT hybridoma line from C57BL/6 mice (DN3A4-1.2) were stimulated using standard conditions with mouse BMDCs as APCs, and supernatants were harvested after 24 h for determination of levels of IL-2 by capture ELISA.17b Cloned human iNKT cell line HDD5 was co-cultured in 96 well plates at 2 × 104 cells/well with 2 × 104 human CD1d-transfected HeLa cells in 200 μL of RPMI-1640 supplemented with 10% FBS at 37°C.23 Glycolipid antigens were added at concentrations ranging from 1 – 500 nM. Supernatants were harvested after 24 h of culture, and concentrations of human IFNγ were measured by capture ELISA as described.17 For stimulation of primary iNKT cells in splenocyte cultures, 106 spleen cells from C57BL/6 or hCD1dKI mice were cultured with different glycolipid concentrations for 72 h in culture and mouse IFNγ was measured by ELISA. For in vivo stimulation of iNKT cells, C57BL/6 and hCD1dKI mice were injected i.v. via the retro-orbital plexus with 4 nmol of glycolipids in vehicle (PBS + 0.05% Tween-20 + 0.1% DMSO). Mice were bled 2 h and 24 h later, and serum samples were stored at −80 °C before cytokine measurement by ELISA. For in vivo tracking of iNKT, NK and T cell activation, glycolipids were injected i.v. via retro-orbital plexus, and organs (spleen and liver) were harvested at a range of time intervals from 5 h to 7 days for preparation of cell suspensions that were analyzed by flow cytometry.
Determination of B16-F10 melanoma lung metastases
B16-F10 metastasis assays were performed as previously described.6l, 18 Wild type C57BL/6 mice or C57BL/6-hCD1dKI mice were injected with 5 × 105 B16-F10 (from ATCC Passage 3) in 200 μL of PBS. After 3 days, 4 nmol of glycolipids were administrated. Two weeks after challenge, mice were sacrificed, lungs removed, and the area of melanized nodules in the lung surface was measured with the use of Image J software. Results were expressed as percentage of the total lung surface area that was occupied by melanized tumor.
Modeling and computational methods
Compounds 8, 9 and 10 were docked into the mCD1d-TCR and hCD1d-TCR complexes from PDB structures 3HE6 and 2PO6, respectively. These receptor complexes were prepared (e.g., H atoms added, protonation and tautomeric states assigned, and H-bond donor/acceptor groups reoriented) using the Protein Preparation application in Maestro version 10.6.014. Structures of glycolipids 8, 9 and 10 were prepared by replacing the C4”-hydroxyl of KRN7000 with phenylamides having 0, 1, or 2 carbon spacers between the ipso carbon of the phenyl and the carbonyl carbon of the amide.
Semi-flexible docking was performed with standard precision Glide. The galactose and glycosidic linkage were treated flexibly, while torsions along the sphingoid base and fatty acid chains were fixed to the crystallographic values. This docking protocol was previously successful in reproducing the pose for KRN7000 and AH10-7 found in crystallographic ternary complexes.6l The top ten docking poses for each substituted structure were conformationally clustered into distinct poses. The overall best-ranked pose for each ligand in the ternary mouse or human complex served as the starting point for molecular dynamics (MD) simulations.
MD simulations were performed with Desmond. The protein was solubilized with the spherical point-charge (SPC) water model within a 10 Å buffer from the protein. This resulted in a total of ~110000 atoms (~30000 water molecules) for the various models. All simulations were carried out with the NPT relaxation protocol consisting of: 100 ps of NVT Brownian dynamics at T = 10 °K, 12 ps of NVT Berendsen dynamics at T = 10 °K, and consecutive 500 ps NPT Martyna, Tobias, and Klein (MTK) dynamics at 10, 50, 100, and 200 °K. Finally, the production period was performed with 50 ns of NPT MTK dynamics at 310 °K with a 2 fs RESPA integrator time step. A total of 1000 snapshots at 310 °K were recorded and analyzed for each MD simulation using the Simulation Interaction Diagram (SID) software within the Schrodinger 2019–4 suite.
Statistical analysis
Data are shown as mean values with error bars representing one standard error (SE). Levels of significance were P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), P < 0.0001 (****). Statistical analyses were done using GraphPad Prism software. Data with multiple groups were analyzed for overall significance using one-way ANOVA, and level of significance for pairwise comparisons of selected groups was calculated using Dunnet post-test. Group sizes (N) for individual experiments are defined in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by NIH grants R01 GM111849 (to ARH, JAG, SAP) and R01 AI045889 (to SAP). Flow cytometry studies were carried out using resources of FACS Core Facility of the Einstein Cancer Center which is supported by NIH/NCI Cancer Center Service Grant P30 CA13330. The Cytek Aurora flow cytometer used in this study was purchased with funds from NIH shared instrumentation grant 1S10OD026833-01. Biorender was used to create the graphical abstract.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and methods, characterization data, proton and carbon NMR, three supplemental figures and three movies of molecular dynamics simulations.
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Brennan PJ; Brigl M; Brenner MB, Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013, 13 (2), 101–17; [DOI] [PubMed] [Google Scholar]; (b) Carreno LJ; Saavedra-Avila NA; Porcelli SA, Synthetic glycolipid activators of natural killer T cells as immunotherapeutic agents. Clin Transl Immunology 2016, 5 (4), e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair S; Dhodapkar MV, Natural Killer T Cells in Cancer Immunotherapy. Front Immunol 2017, 8, 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Yu KO; Im JS; Molano A; Dutronc Y; Illarionov PA; Forestier C; Fujiwara N; Arias I; Miyake S; Yamamura T; Chang YT; Besra GS; Porcelli SA, Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A 2005, 102 (9), 3383–8; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miyamoto K; Miyake S; Yamamura T, A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 2001, 413 (6855), 531–4; [DOI] [PubMed] [Google Scholar]; (c) Li X; Chen G; Garcia-Navarro R; Franck RW; Tsuji M, Identification of C-Glycoside Analogues that Display a Potent Biological Activity against Murine and Human Invariant Natural Killer T Cells. Immunol. 2009, 127, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh VV; Wilson MT; Olivares-Villagomez D; Singh AK; Wu L; Wang CR; Joyce S; Van Kaer L, Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest 2005, 115 (9), 2572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent X; Bertin B; Renault N; Farce A; Speca S; Milhomme O; Millet R; Desreumaux P; Henon E; Chavatte P, Switching invariant natural killer T (iNKT) cell response from anticancerous to anti-inflammatory effect: molecular bases. J Med Chem 2014, 57 (13), 5489–508. [DOI] [PubMed] [Google Scholar]
- 6.(a) Guillaume J; Seki T; Decruy T; Venken K; Elewaut D; Tsuji M; Calenbergh S, Synthesis of C6′′-modified α- C -GalCer analogues as mouse and human iNKT cell agonists. Organic & Biomolecular Chemistry 2017, 15 (10), 2217–2225; [DOI] [PubMed] [Google Scholar]; (b) Pauwels N; Aspeslagh S; Elewaut D; Van Calenbergh S, Synthesis of 6”-Triazole-substituted α-GalCer Analogues as Potent iNKT Cell Stimulating Ligands. Bioorg. Med. Chem 2012, 20, 7149–7154; [DOI] [PubMed] [Google Scholar]; (c) Aspeslagh S; Nemcovic M; Pauwels N; Venken K; Wang J; Van Calenbergh S; Zajonc DM; Elewaut D, Enhanced TCR Footprint by a Novel Glycolipid Increases NKT-Dependent Tumor Protection. J. Immunol 2013, 191 (6), 2916–2925; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Birkholz A N. M; Yu ED; Girardi E; Jing J K. A; Pauwels N; Franck RW; Tsuji M; Howell AR; Van Calenbergh S; Kronenberg M; Zajonc DM, Structural Modifications of αGalCer in Both Lipid and Carbohydrate Moiety Influence Activation of Murine and Human iNKT Cells. J. Biol. Chem 2015, 290, 17206–17217; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhou X-T; Forestier C; Goff RD; Li C; Teyton L; Bendelac A; Savage PB, Synthesis and NKT Cell Stimulating Properties of Fluorophore- and Biotin-Appended 6”-Amino-6”-deoxy-galactosylceramides. Org. Lett 2002, 4, 1267–1270; [DOI] [PubMed] [Google Scholar]; (f) Trappeniers M; Van Beneden K; Decruy T; Hillaert U; Linclau B; Elewaut D; Van Calenbergh S, 6’-Derivatised a-GalCer Analogues Capable of Inducing Strong CD1d-Mediated Th1-Biased NKT Cell Responses in Mice. J. Am. Chem. Soc 2008, 130, 16468–16469; [DOI] [PubMed] [Google Scholar]; (g) Tashiro T; Nakagawa R; Inoue S; Shiozaki M; Watari H; Taniguchi M; Mori K, RCAI-61, the 6’-O-Methylated Analog of KRN7000: Its Synthesis and Potent Bioactivity for Mouse Lymphocytes to Produce Interferon-gamma in vivo. Tetrahedron Lett. 2008, 49, 6827–6830; [Google Scholar]; (h) Pauwels N; Aspeslagh S; Vanhoenacker G; Sandra K; Yu ED; Zajonc DM; Elewaut D; Linclau B; Van Calenbergh S, Divergent Synthetic Approach to 6”-Modified α-GalCer Analogues. Org. Biomol. Chem 2011, 9, 8413–8421; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Hsieh M-H; Hung J-T; Liw Y-W; Lu Y-J; Wong C-H; Yu AL; Liang P-H, Synthesis and Evaluation of Acyl-chain- and Galactose-6”-modified Analogues of α-GalCer for NKT Cell Activation. ChemBioChem 2012, 13, 1689–1697; [DOI] [PubMed] [Google Scholar]; (j) Jervis PJ; Graham LM; Foster EL; Cox LR; Porcelli SA; Besra GS, New CD1d agonists: Synthesis and biological activity of 6”-triazole-substituted α-galactosyl ceramides. Bioorg. Med. Chem. Lett 2012, 22 (13), 4348–4352; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Guillaume J; Seki T; Decruy T; Venken K; Elewaut D; Tsuji M; Calenbergh S, Synthesis of C6′′-Modified α-C-GalCer Analogues as Mouse and Human iNKT Cell Agonists. Org. Biomol. Chem 2017, 15 (10), 2217–2225; [DOI] [PubMed] [Google Scholar]; (l) Chennamadhavuni D; Saavedra-Avila NA; Carreno LJ; Guberman-Pfeffer MJ; Arora P; Yongqing T; Pryce R; Koay HF; Godfrey DI; Keshipeddy S; Richardson SK; Sundararaj S; Lo JH; Wen X; Gascon JA; Yuan W; Rossjohn J; Le Nours J; Porcelli SA; Howell AR, Dual Modifications of alpha-Galactosylceramide Synergize to Promote Activation of Human Invariant Natural Killer T Cells and Stimulate Anti-tumor Immunity. Cell Chem Biol 2018, 25 (5), 571–584 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Zajonc DM; Cantu III C; Mattner J; Zhou D; Savage PB; Bendelac A; Wilson IA; Teyton L, Structure and Function of a Potent Agonist for the Semi-invariant Natural Killer T Cell Receptor. Nature Immunol. 2005, 6, 810–818; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Koch M; Stronge VS; Shepherd D; Gadola SD; Matthew B; Ritter G; Fersht AR; Besra GS; Schmidt RR; Jones EY; Cerundolo V, The Crystal Structure of Human CD1d with and without a-Galactosylceramide. Nature Immunol. 2005, 6, 819–826. [DOI] [PubMed] [Google Scholar]
- 8.Borg NA; Wun KS; Kjer-Nielsen L; Wilce MCJ; Pellicci DG; Koh R; Besra GS; Bharadwaj M; Godfrey DI; McCluskey J; Rossjohn J, CD1d-lipid-antigen Recognition by the Semi-invariant NKT T-cell Receptor. Nature 2007, 448, 44–49. [DOI] [PubMed] [Google Scholar]
- 9.(a) Barbieri L; Costantino V; Fattorusso E; Mangoni A; Aru E; Parapini S; Taramelli D, Immunomodulatory a-Galactoglycosphingolipids: Synthesis of a 2’-O-Methyl-a-Gal-GSL and Evaluation of Its Immunostimulating Capacity. Eur. J. Org. Chem 2004, 468–473; [Google Scholar]; (b) Wu D; Xing G-W; Poles MA; Horowitz A; Kinjo Y; Sullivan B; Bodmer-Narkevitch V; Plettenburg O; Kronenberg M; Tsuji M; Ho DD; Wong C-H, Bacterial Glycolipids and Analogs as Antigens for CD1d-restricted NKT Cells. Proc. Natl. Acad. Sci. U.S.A 2005, 102, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raju R; Castillo BF; Richardson SK; Thakur M; Severins R; Kronenberg M; Howell AR, Synthesis and Evaluation of 3”- and 4”-Deoxy and -Fluoro Analogs of the Immunostimulatory Glycolipid, KRN7000. Bioorg. Med. Chem. Lett 2009, 19, 4122–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia C Z. W; Zhang Y; Chen W; Nadas J; Severin R; Woodward R;; Wang B W. X; Kronenberg M; Wang PG, The Roles of 3’ and 4’ Hydroxy Groups in alpha-Galactosylceramide Stimulation of Invariant Natural Killer T Cells. ChemBioChem 2009, 4, 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W X. C; Nadas J; Chen W; Gu L; Wang PG, Introduction of Aromatic Group on 4’-OH of α-GalCer Manipulated NKT Cell Cytokine Production. Bioorg. Med. Chem 2011, 19, 2767–2776. [DOI] [PubMed] [Google Scholar]
- 13.Wu T-N; Lin K-H; Wu Y-T; Huang J-H; Hung J-T; Wu J-C; Chen C-Y; Chu K-C; Lin N-H; Yu AL; Wong C-H, Phenyl Glycolipids with Different Glycosyl Groups Exhibit Differences in Murine and Human iNKT Cell Activation. ACS Chem. Biol 2016, 11, 3431–3441. [DOI] [PubMed] [Google Scholar]
- 14.Janssens J; Decruy T; Venken K; Seki T; Krols S; Van der Eycken J; Tsuji M; Elewaut D; Van Calenbergh S, Efficient Divergent Synthesis of New Immunostimulant 4”-Modified α-Galactosylceramide Analogues. ACS Med. Chem. Lett 2017, 8 (6), 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssens J; Bitra A; Wang J; Decruy T; Venken K; van der Eycken J; Elewaut D; Zajonc DM; van Calenbergh S, 4”-O-Alkylated alpha-Galactosylceramide Analogues as iNKT-Cell Antigens: Synthetic, Biological, and Structural Studies. ChemMedChem 2019, 14 (1), 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Xia C; Yao Q; Schumann J; Rossy E; Chen W; Zhu L; Zhang W; Libero GD; Wang PG, Synthesis and Biological Evaluation of a-Galactosylceramide (KRN7000) and Isoglobotrihexosylceramide (iGb3). Bioorg. Med. Chem. Lett 2006, 16, 2195–2199; [DOI] [PubMed] [Google Scholar]; (b) Bi J; Wang J; Zhou K; Wang Y; Fang M; Du Y, Synthesis and Biological Activities of 5-Thio-α-GalCers. ACS Med. Chem. Lett 2015, 6 (4), 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Bricard G; Venkataswamy MM; Yu KO; Im JS; Ndonye RM; Howell AR; Veerapen N; Illarionov PA; Besra GS; Li Q; Chang YT; Porcelli SA, Alpha-galactosylceramide analogs with weak agonist activity for human iNKT cells define new candidate anti-inflammatory agents. PLoS One 2010, 5 (12), e14374; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Im JS; Arora P; Bricard G; Molano A; Venkataswamy MM; Baine I; Jerud ES; Goldberg MF; Baena A; Yu KO; Ndonye RM; Howell AR; Yuan W; Cresswell P; Chang YT; Illarionov PA; Besra GS; Porcelli SA, Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 2009, 30 (6), 888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen X; Rao P; Carreno LJ; Kim S; Lawrenczyk A; Porcelli SA; Cresswell P; Yuan W, Human CD1d knock-in mouse model demonstrates potent antitumor potential of human CD1d-restricted invariant natural killer T cells. Proc Natl Acad Sci U S A 2013, 110 (8), 2963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora P; Baena A; Yu KO; Saini NK; Kharkwal SS; Goldberg MF; Kunnath-Velayudhan S; Carreno LJ; Venkataswamy MM; Kim J; Lazar-Molnar E; Lauvau G; Chang YT; Liu Z; Bittman R; Al-Shamkhani A; Cox LR; Jervis PJ; Veerapen N; Besra GS; Porcelli SA, A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity 2014, 40 (1), 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey DI; Le Nours J; Andrews DM; Uldrich AP; Rossjohn J, Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48 (3), 453–473. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ; Wang H; Starrett GJ; Phuong V; Jameson SC; Hogquist KA, Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity 2015, 43 (3), 566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora P; Venkataswamy MM; Baena A; Bricard G; Li Q; Veerapen N; Ndonye R; Park JJ; Lee JH; Seo KC; Howell AR; Chang YT; Illarionov PA; Besra GS; Chung SK; Porcelli SA, A rapid fluorescence-based assay for classification of iNKT cell activating glycolipids. J Am Chem Soc 2011, 133 (14), 5198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spada FM; Koezuka Y; Porcelli SA, CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med 1998, 188 (8), 1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


