Abstract
Objective:
COVID-19 has emerged as a multi-system disease with the potential for endocrine dysfunction. We aimed to study the hormonal profile of hospitalized patients with COVID-19 at a tertiary care referral hospital at Jodhpur, India.
Design:
A hospital-based clinical study of endocrine profile of COVID-19 patients conducted from 15th May to 30th June 2020 after ethical approval.
Measurements:
Fasting blood samples for free thyroxine (T4), free tri-iodothyronine (T3), thyroid stimulating Hormone (TSH), serum prolactin; basal and 1 h post-intramuscular adrenocorticotropic hormone (ACTH) stimulated cortisol, interleukin-6 (IL-6), and high sensitivity C-reactive protein (hsCRP) were collected within 24 h of admission after written informed consent. All hormones and IL-6 were analyzed by chemiluminescent immunoassay. hsCRP was measured by immune-turbidimetric assay.
Results:
Of 235 patients studied, 14% had severe disease and 5.5% died. Adrenal insufficiency was present in 14%, most of whom had mild disease. A robust adrenal response was observed in those with severe disease. Basal and post-ACTH serum cortisol were significantly increased in severe disease or those who died compared to those who were mild or asymptomatic. Basal and post-ACTH serum cortisol showed a significant positive correlation with hsCRP but not with IL-6. Low T3 and low T4 syndrome were documented in 25% and 5%, respectively. Serum TSH and FT3 levels declined significantly from asymptomatic to severe category. Hyperprolactinemia was found in 21 patients. hsCRP showed a rising trend with disease severity while IL-6 did not.
Conclusions:
Endocrine dysfunction in the form of adrenal insufficiency, low T3, and low TSH syndrome and hyperprolactinemia were common COVID-19 hospitalized patients.
Keywords: Adrenal, cortisol, COVID-19, endocrine, hsCRP, prolactin, thyroid
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) has emerged as a global pandemic resulting in clinicians navigating new territories in diagnosis and management.[1] Early reports and previous experience from SARS suggested that the virus had a predilection for multisystem involvement.[2,3,4] This was further supported by the widespread presence of Angiotensin Converting Enzyme-2 (ACE-2), the receptor for SARS-CoV-2 spike proteins which serves as an entry point for the virus through spike protein mediated endocytosis.[2,5] Another important area of cross-talk between viral infections and endocrine system is the activation of inflammatory and cytokine pathways which appear to play a role in COVID-19 pathogenesis.[3,6] The hypothalamic–pituitary–adrenal (HPA) axis is the key player in stress responses while cytokines stimulate the HPA axis as well.[7]
While the presence of endocrine disorders like diabetes appears to be a risk factor for severe COVID-19 disease, the actual relationship between COVID-19 disease and the endocrine system remains unexplored. Several reports of endocrine organ involvement exist in SARS.[4] Few case reports and small studies of thyroid and pancreas involvement have emerged in SARS-Cov-2.[8,9,10,11,12,13,14,15] However, to the best of our knowledge, there are no comprehensive studies to evaluate endocrine dysfunction in COVID-19 patients. Hence, this study was designed to study the pituitary, thyroid, and adrenal function in COVID-19 patients admitted at tertiary care hospital at Rajasthan, India.
METHODS
This clinical study of endocrine profile of COVID-19 patients was conducted at All India Institute of Medical Sciences, Jodhpur, Rajasthan, India from 15 May to 30 Jun 2020. Our center serves as the tertiary care referral hospital providing inpatient care to COVID-19 patients in Western Rajasthan. All patients were evaluated as per the Indian Council of Medical Research (ICMR) guidelines and institutional protocol.[16] The presence of SARS-CoV-2 was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) using standard testing protocols.[17] All patients >18 years of age, admitted with COVID-19 were screened for inclusion. Of 286 screened, 235 who gave written informed consent were included in the study. The study protocol was approved by the institutional ethical committee (AIIMS/RES/2020/4550).
Demographic profile and comorbidities were collected using a standardized data collection proforma. All patients were categorized as asymptomatic, mild, moderate, or severe according to guidelines published by Ministry of Health and Family Welfare (MoHFW), Government of India, and an additional category of critical as per World Health Organization (WHO) classification.[18,19]
The blood sample was collected in fasting state in sterile vacutainer within 24 h of admission and serum was separated and stored at -80°C. Thyroid function was evaluated by free triiodothyronine (FT3), free tetraiodothyronine (FT4), and thyroid stimulating hormone (TSH). Adrenal function was assessed by basal cortisol and 1 h post-intramuscular ACTH (adrenocorticotrophic hormone-250 μg) cortisol.[20,21] None of the patients had received dexamethasone for respiratory distress as per then existing protocols. Prolactin levels were included to assess pituitary function. Prolactin is a part of stress response and increased in various stressful conditions.[22] SARS-CoV-2 infection and its manifestations are related to inflammatory response, a form of stress. Hence, we hypothesized that prolactin levels will differ according to severity of COVID-19 and may be used to assess the severity. Growth hormone, gonadal functions, and posterior pituitary hormones were not evaluated in this study.
All hormones were analyzed by chemiluminescent immunoassay (Siemens Advia Centaur© immunoassay system, USA) and chemiluminescent immunoassay kits supplied by the same company. All Samples were thawed to 30°C before running the assays. Samples were processed according to the availability of kits because of international lockdown and transport restrictions.
The normal range of FT4, FT3, and TSH were 11.5–22.7 pmol/L, 3.5–6.2 pmol/L, and 0.35–5.5 mIU/L, respectively. Subjects with normal FT3, FT4, and TSH were considered euthyroid. Primary hypothyroidism was defined by low FT4 (<11.5 pmol/L) and high TSH (5.5 mIU/L) and subclinical hypothyroidism by FT4 within normal range and TSH >5.5 mIU/L. Low T4 and low T3 syndrome were defined by serum FT4 levels <11.5 pmol/L and FT3 levels <3.5 pmol/L with normal serum TSH levels. Central hypothyroidism was defined as serum TSH < 0.5 mIU/L and low FT4 for this study.
The normal range of morning basal cortisol was 4.30–22.40 μg/dL (7–9 AM). The range of measurement was 0.20–75.0 μg/dL, CV was 4.22–6.58%. There is no consensus on diagnosis of adrenal insufficiency in acute ill patients and varied levels of basal and stimulated cortisol have been used to define critically illness related corticosteroid insufficiency (CIRCI) in patients with severe sepsis and septic shock.[23,24] A previous study on SARS have used standard criteria to define adrenal insufficiency,[4] hence we have also defined adrenal insufficiency with basal cortisol <3 μg/dl or 1 h post-ACTH cortisol <18.0 μg/dl.[20,21] Additionally, delta cortisol (post stimulated cortisol minus basal cortisol) of <9 μg/dl was also considered to define CIRCI as recommended by current guideline.[23]
The normal range pf prolactin was 2.8–29.2 ng/ml (non-pregnant Females) and 2.1–17.7 ng/ml (Males). The CV was <5%. Hyperprolactinemia was defined as >20 ng/ml in males and >30 ng/ml in non-pregnant females.
The Interleukin-6 (IL-6) assay had analytical sensitivity of 2.7 pg/mL, with normal range of up to 4.4 pg/mL. The highly sensitive C-reactive protein (hsCRP) was measured by immune-turbidimetric test using Beckman Coulter-AU system, USA. The normal range of hsCRP was <1 mg/L and CV was <5%.
Statistical analysis
Data were analyzed using IBM SPSS Version 20.0, USA. All categorical data were expressed as number (%) and continuous data as mean ± SD (95% confidence interval). All hormonal data had skewed deviation; hence, statistical significance was calculated by the non-parametric Kruskal–Wallis test. Student 't' test was used for comparing data of inflammatory markers between two categories and ANOVA for more than two categories. All categorical data were compared by the Chi-square test. Pearson correlation coefficient was used to find the correlation between serum cortisol and serum IL-6 and hsCRP. A two-tailed P value of < 0.05 was considered statistically significant.
RESULTS
The demographic characteristics of patients are depicted in Table 1. Pre-existing hypothyroidism was reported in 4.6% of patients who were on thyroxine replacement, but half of them had high TSH levels indicating suboptimal treatment. Two patients had a history of steroid (prednisolone) intake for other autoimmune diseases.
Table 1.
Basic characteristics of COVID-19 patients
| Parameter | n=235 | |
|---|---|---|
| Age (years) | 48.9±16.4 (18-87) | |
| Gender | ||
| Male | 147 (62.6%) | |
| Female | 88 (34.7%) | |
| Co-morbidities | ||
| Diabetes Mellitus | 38 (16.2%) | |
| Hypertension | 58 (24.7%) | |
| Coronary artery disease | 18 (7.7%) | |
| COPD/Asthma | 5 (2.1%) | |
| Malignancy | 3 (1.3%) | |
| Smoker | 30 (12.8%) | |
| Alcohol Intake | 30 (12.8%) | |
| Severity | WHO Criteria | MoHFW Criteria |
| Asymptomatic | 109 (46.4%) | 109 (46.4%) |
| Mild | 70 (29.8%) | 70 (29.8%) |
| Moderate | 23 (9.8%) | 23 (9.8%) |
| Severe | 22 (9.4%) | 33 (14.0%) |
| Critical | 11 (4.7%) | - |
| Outcome | ||
| Discharged | 222 (94.5%) | |
| Death | 13 (5.5%) | |
| Duration of Stay (days; n=159) | 10.3±4.9 (3-42) | |
| hsCRP (mg/L) | 34.3±60.3 (0.04-371) | |
| IL-6 (pg/ml, n=133) | 251±613 (1.4-5500) | |
WHO-World Health Organization, MoHFW- Ministry of Health & Family Welfare. hsCRP-Highly sensitive C-reactive protein, IL-6- Interleukin-6
About 76% of patients were either asymptomatic or had the mild disease. According to WHO classification, 9.8% had moderate disease and 4.7% were critical, whereas according to MoHFW criteria 14.0% had severe disease. Most of the patients have recovered and discharged (94.5%). There were 13 deaths (5.5%). Mean duration of hospital stay was significantly longer among non-survivors when compared to survivors (13.7 ± 7.2 vs. 10.1 ± 4.7 days; P = 0.011).
About one third of the patients (91 patients, 38.5%) had hs-CRP levels <3 mg/L and 84 patients (35.9%) had hs-CRP levels >10 mg/L. Serum hsCRP levels showed rising trends with increasing severity (P < 0.0001) (data not shown). Non-survivors had significantly high hs-CRP levels when compared with those who were discharged (124 ± 4 mg/dl vs. 22.9 ± 55.6 mg/dl; P < 0.0001).
A total of 17 patients (12.8%) had normal IL-6 levels (<4.4 pg/ml), and 47 patients (35.3%) had high IL-6 (>100 pg/ml) and rest were between 4.4 and 100 pg/ml. There was no significant difference in serum IL-6 levels among various categories of severity (data not shown). There was no difference in serum IL-6 levels between non-survivors when compared with those who survived (173.3 ± 189.0 pg/dl vs. 257.6 ± 635 pg/dl; P = 0.681).
Adrenal function
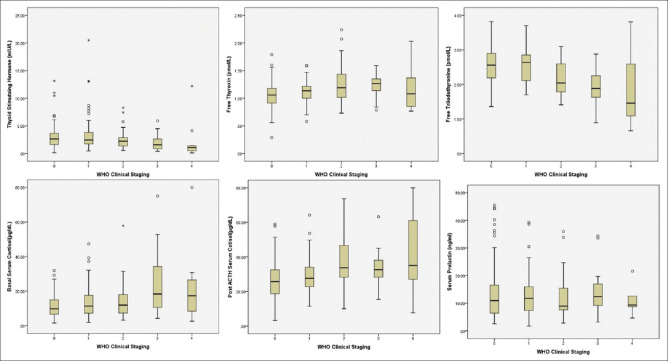
Adrenal insufficiency was present in 34 (14.5%) patients. There was no significant difference between genders [Male: 24 (18.2%) vs. Female: 10 (12.7%); P = 0.195]. Among the patients with AI, most belonged to asymptomatic or mild categories [30 (88%)] followed by one patient (3%) in moderate and three patients (9%) in severe MoHFW categories. A total of 17 patients (7.2%) had basal cortisol levels <3 μg/dL, of which 11 patients (64.7%) had post-ACTH cortisol value of < 18.0 μg/dL. CIRCI was present in 18.3% cases as defined by delta cortisol of <9 μg/dL. Mean basal cortisol levels among all patients were 13.9 ± 10.9 μg/dl (range 1.6–80.0) and post-ACTH cortisol levels were 29.5 ± 13.0 μg/dl (range 3.2–80.0). Basal and post-ACTH cortisol levels increased significantly with various categories of severity [Table 2, Figure 1]. Basal and post-ACTH cortisol levels were significantly higher among non-survivors when compared to those who survived (basal cortisol: 13.4 ± 9.9 μg/dl vs. 25.0 ± 21.7 μg/dl, P = 0.001; post-ACTH cortisol: 28.4 ± 11.2 μg/dl vs. 46.4 ± 11.2 μg/dl, P < 0.0001). Basal and post-ACTH cortisol has shown significant positive correlation with hsCRP (r = 0.319, P < 0.0001; and r = 0.215, P < 0.0001 respectively), but had no correlation with IL-6 levels (r = 0.028, P = 0.749; and r = 0.046, P = 0.619 respectively).
Table 2.
Serum cortisol levels according to severity of COVID-19
| WHO Criteria | MoHFW Criteria | |
|---|---|---|
| Basal Serum Cortisol (µg/dl) | ||
| Asymptomatic | 11.4±6.8 (10.1-12.7) | 11.4±6.8 (10.1-12.7) |
| Mild | 13.5±8.9 (11.4-15.6) | 13.5±8.9 (11.4-15.6) |
| Moderate | 15.2±12.0 (10.0-20.4) | 15.2±12.0 (10.0-20.4) |
| Severe | 23.1±17.9 (14.9-31.2) | 23.5±19.6 (16.0-30.9) |
| Critical | 24.5±24.7 (3.9-45.1) | - |
| P* | 0.017 | 0.008 |
| Post-ACTH Serum Cortisol (µg/dl) | ||
| Asymptomatic | 25.9±10.9 (23.8-28.0) | 25.9±10.9 (23.8-28.0) |
| Mild | 29.4±10.0 (26.8-31.9) | 29.4±10.0 (26.8-31.9) |
| Moderate | 37.5±15.3 (30.4-44.6) | 37.5±15.3 (30.4-44.6) |
| Severe | 32.3±10.8 (26.5-38.1) | 36.4±19.5 (28.4-44.5) |
| Critical | 43.8±28.7 (21.8-65.9) | - |
| P* | <0.0001 | <0.0001 |
*Kruskal-Wallis test. WHO-World Health Organization, MoHFW- Ministry of Health & Family Welfare
Figure 1.
Box plot of the hormones according to WHO staging: 0 = asymptomatic, 1 = mild, 2 = moderate, 3 = severe, 4 = critical. Two cases with TSH value of 94.3 and 43.4 mIU/L and serum prolactin value of 179.8 and 94.9 μg/L were excluded from TSH and prolactin graph respectively
Thyroid function
Primary hypothyroidism and subclinical hypothyroidism were present in 2.1% (5 patients) and 7.6% (18 patients), respectively. Low T4 and Low T3 syndrome were documented in 5.5% (13 patients) and 25.1% (59) patients. Central hypothyroidism was present in two patients, but possibility of sick euthyroid syndrome cannot be excluded. Eleven (4.6%) were hypothyroid at baseline of whom 2.5% were uncontrolled. Mean values for FT3, FT4, and TSH were 3.69 ± 0.9 pmol/L (1.1-8.53), 14.2 ± 3.9 pmol/L (3.9-36.69) and 3.6 ± 7.0 mIU/L (0.1-94.3), respectively. Six of patients who did not survive, had low T4 and Low T3 syndrome. Serum TSH and serum FT3 levels declined significantly from asymptomatic to severe categories, whereas serum FT4 increased significantly among various categories of severity [Table 3, Figure 1].
Table 3.
Thyroid function tests according to severity of COVID-19
| WHO Criteria | MoHFW Criteria | |
|---|---|---|
| Thyroid stimulating hormone (mIU/L) | ||
| Asymptomatic | 3.4±4.4 (2.5-4.2) | 3.4±4.4 (2.5-4.2) |
| Mild | 4.7±11.4 (2.0-7.5) | 4.7±11.4 (2.0-7.5) |
| Moderate | 2.8±2.0 (1.9-3.6) | 2.8±2.0 (1.9-3.6) |
| Severe | 2.0±1.4 (1.3-2.6) | 2.1±2.3 (1.2-2.9) |
| Critical | 2.3±3.7 (-0.3-4.9) | - |
| P* | 0.004 | 0.002 |
| Free thyroxin (pmol/L) | ||
| Asymptomatic | 14.2±3.9 (12.9-14.2) | 14.2±3.9 (12.9-14.2) |
| Mild | 14.2±2.6 (14.2-15.4) | 14.2±2.6 (14.2-15.4) |
| Moderate | 16.7±5.1 (14.2-18.0) | 16.7±5.1 (14.2-18.0) |
| Severe | 15.4±2.6 (14.2-16.7) | 15.4±3.9 (12.9-16.7) |
| Critical | 15.4±5.1 (11.6-20.6) | - |
| P* | 0.003 | 0.002 |
| Free tri-iodothyronine (pmol/L) | ||
| Asymptomatic | 4.0±0.9 (3.8-4.1) | 4.0±0.9 (3.8-4.1) |
| Mild | 4.0±0.8 (3.7-4.1) | 4.0±0.8 (3.7-4.1) |
| Moderate | 3.4±0.8 (3.1-3.7) | 3.4±0.8 (3.1-3.7) |
| Severe | 3.1±0.8 (2.8-3.4) | 2.9±1.1 (2.6-3.4) |
| Critical | 2.8±1.4 (1.7-3.8) | - |
| P* | <0.0001 | <0.0001 |
Krushal Wallis test. WHO-World Health Organization, MoHFW- Ministry of Health & Family Welfare
Serum prolactin
Twenty-one (8.5%) patients had evidence of hyperprolactinemia. Two patients had very high serum prolactin levels (179.8 and 94.8 μg/L). They had no symptoms related to hyperprolactinemia and were not further evaluated presently. There was no difference between genders [Male: 13 patients (8.8%) vs. Female: 8 patients (9.1%); P = 0.568]. Mean prolactin levels were 15.2 ± 16.9 μg/L (1.8–179.8) among all patients. Serum prolactin levels did not differ among various categories of severity (P = 0.791) [Figure 1] and between survivors and non-survivors (15.1 ± 17.1 vs. 17.2 ± 6.2; P = 0.786).
DISCUSSION
This cross-sectional study was designed to systematically explore the pituitary, adrenal, and thyroid hormone profile of patients presenting COVID-19. Among pituitary functions, we chose serum prolactin as it is only secreted by the pituitary, has no intermediary for systemic effect like growth hormone which acts through the GH-IGF-1 axis (Insulin-like growth factor-1) and is related to stress response.[22]
Adrenal insufficiency was present in 14% of our patients most of whom belonged to asymptomatic or mild categories (88%) and CIRCI in 18.3% patients. In a study on SARS patients, 39% were reported to have adrenal insufficiency, of which 83% were considered central based on low ACTH levels.[4] One reason for such a high frequency of AI appears to be the criteria used to define AI, that is, basal cortisol < 138 nmol/L and post-ACTH < 551.7 nmol/L as per the then prevailing guidelines. On applying this older definition, we found that 23% of our patients had AI. Mechanisms for hypocortisolism in SARS-CoV-2 infections include critical illness related corticosteroid insufficiency or functional hypopituitarism secondary to inflammatory stress response.[23] Molecular mimicry between SARS viral proteins and ACTH, leading to anti-ACTH antibodies with destruction of ACTH positive cells resulting in central hypopituitarism has been hypothesized.[25,26] However, due to short duration of illness this mechanism is unlikely in our patients.
A report by Tan et al.,[12] suggests that basal serum cortisol cut-off of 744 nmol/L correlated well with 15-day mortality in 535 patients studied. Our study has shown similar results wherein the median serum cortisol was 858 nmol/L in those who died and 676 nmol/L in those with severe disease suggesting that baseline serum cortisol could serve as a marker for disease severity as well. Another interesting speculation maybe that increased endogenous steroids may contribute to poor prognosis by delaying viral clearing. However, steroids may prove beneficial in the cytokine storm syndrome by limiting unregulated inflammation for which they are currently recommended in treatment of critically-ill COVID-19 patients.[4,27] Also, steroids may have some local membrane-stabilizing anti-inflammatory action in the alveoli.[28] Both the basal cortisol and post-ACTH response were associated with disease severity and hsCRP levels. This agrees with the usual course in most viral infections.[29] However, there was no correlation between IL-6 levels and basal or post-ACTH cortisol response.
Our findings in the thyroid hormone profile suggest that primary hypothyroidism and subclinical hypothyroidism were present in 2.1 and 7.6%, respectively. Low FT3 and low FT4 syndrome were present in 5.5% and 25.1%, respectively. It is pertinent to note that 6 of 13 non-survivors had low FT3 and low FT4 syndrome. Serum TSH and FT3 levels declined significantly from asymptomatic to severe categories while serum FT4 showed an increasing trend. Similar findings have been reported from two clinical studies from China which suggested low T3 low T4 and low TSH in COVID-19 patients.[9,13] Patients with thyroid dysfunction were also demonstrated to take longer for seroconversion and thyroid dysfunction positively co-related with various severity parameters.[13] Our findings also support the trend of lower TSH and T3 in patients with severe disease. Based on these finding we can consider the role of TSH and FT3 as a severity marker of COVID morbidity. These findings are similar to sick euthyroid syndrome reported with other viral infections.[30] Two reports highlight subacute thyroiditis with SARS-CoV-2 infection.[9,14] Muller et al.[15] have recently demonstrated that the frequency of atypical thyroiditis in a high dependency unit went up from 0.5% in 2019 to 10% in 2020 when COVID-19 patients were admitted, and follow-up of eight patients showed clinical hypothyroidism in two patients. Further follow-up of our patients is required to assess if immune medicated thyroid dysfunction develops as a long-term consequence of infection.
Twenty-one patients had elevated serum prolactin. There was no relationship between disease severity and serum prolactin. We speculate that hyperprolactinemia may be stress induced in this context.[22]
Our study has several limitations. Firstly, we have studied a limited number of patients in a single center and most of whom were categorized as asymptomatic or mild. This distribution of patients in agreement with previously published studies.[13,31] Secondly, we have not measured plasma ACTH, GH-IGF-1 and gonadal axis. Lastly, all the patients could not undergo complete evaluation, hence are labelled as presumptive diagnosis. A complete evaluation is planned in post-COVID clinic at future date after due ethical approval.
CONCLUSIONS
In conclusion, on evaluating 235 patients with COVID-19, endocrine dysfunctions were present in up to 15% of them. Adrenal insufficiency, low T3 and low TSH syndrome, and hyperprolactinemia occurred in COVD-19 patients. One salient finding was a high prevalence of AI among asymptomatic and mild COVID-19 patients and not in severe or critical patients. This suggests that serum cortisol or post-ACTH cortisol is unlikely to serve as a guide to administer dexamethasone in COVID-19 patients. Thyroid dysfunction represents spectrum of euthyroid sick syndrome.[30] The complete clinical implications of these findings are not apparent currently. Further clinical studies with follow-up are ongoing and expected to reveal the long-term endocrine effects of COVID-19.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the COVID-19 team of AIIMS Jodhpur, which made the necessary support system available for the care of patients. We also are thankful to all junior and senior resident doctors as well as nursing officers who were involved in patient care.
REFERENCES
- 1.Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020;382:1268–9. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19, and multiorgan response? Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. doi:10.1016/j.cpcardiol. 2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 4.Leow MK-S, Kwek DS-K, Ng AW-K, Ong K-C, Kaw GJ-L, Lee LS-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–4. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 7.Silverman MN, Pearce BD, Biron CA, Miller AH. Immune Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Viral Infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: A mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico? J Clin Endocrinol Metab. 2020;105:dgaa346. doi: 10.1210/clinem/dgaa346. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Su X, Ding Y, Fan W, Su J, Chen Z, et al. Thyroid function abnormalities in COVID-19 patients. medRxiv. doi: 10.3389/fendo.2020.623792. Published online June 16, 2020. 2020.06.15.20130807. doi: 10.1101/2020.06.15.20130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection? J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaney AI, Griffin GD, Simon EL. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020;38:2291. doi: 10.1016/j.ajem.2020.05.114. e3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8:659–60. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: A potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020:1–2. doi: 10.1007/s40618-020-01312-7. doi: 10.1007/s40618-020-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8:739–41. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [[Last accessed on 2020 Apr 29]];Indian Council of Medical Research, New Delhi. Availablefrom: https://www.icmr.gov.in/ [Google Scholar]
- 17.Testing Strategy. [[Last accessed on 2020 Apr 29]]. Available from: https://www.icmr.gov.in/cteststrat.html .
- 18.Clinical management of COVID-19. [[Last accessed 2020 Jul 27]]. Available from: https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19 .
- 19.MoHFW | Home. [[Last accessed on 2020 Apr 17]]. Available from: https://www.mohfw.gov.in/dashboard/index.php .
- 20.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:364–89. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundgurthi A, Garg MK, Dutta MK, Pakhetra R. Intramuscular ACTH stimulation test for assessment of adrenal function. J Assoc Physicians India. 2013;61:320–4. [PubMed] [Google Scholar]
- 22.Levine S, Muneyyirci-Delale O. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically Ill patients (Part I): Society of critical care medicine (SCCM) and european society of intensive care medicine (ESICM) 2017. Crit Care Med. 2017;45:2078–88. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 23.Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically Ill patients (Part I): Society of critical care medicine (SCCM) and european society of intensive care medicine (ESICM) 2017. Crit Care Med. 2017;45:2078–88. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 24.Téblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. 2019;15:417–27. doi: 10.1038/s41574-019-0185-7. [DOI] [PubMed] [Google Scholar]
- 25.Wheatland R. Molecular mimicry of ACTH in SARS – implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63:855–62. doi: 10.1016/j.mehy.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei L, Sun S, Zhang J, Zhu H, Xu Y, Ma Q, et al. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS) Biochem Cell Biol. 2010;88:723–30. doi: 10.1139/O10-022. [DOI] [PubMed] [Google Scholar]
- 27.Kolilekas L, Loverdos K, Giannakaki S, Vlassi L, Levounets A, Zervas E, et al. Can steroids reverse the severe COVID-19 induced “cytokine storm”? J Med Virol. 2020;92:2866–9. doi: 10.1002/jmv.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S253–7. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- 29.Steenblock C, Todorov V, Kanczkowski W, Eisenhofer G, Schedl A, Wong ML, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol Psychiatry. 2020;25:1611–7. doi: 10.1038/s41380-020-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Farwell AP. Euthyroid sick syndrome. Compr Physiol. 2016;6:1071–80. doi: 10.1002/cphy.c150017. [DOI] [PubMed] [Google Scholar]
- 31.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]