Abstract
The current coronavirus disease (COVID-19) pandemic is showing no signs of abatement and result in significant morbidity and mortality in the infected patients. Many therapeutic agents ranging widely between antivirals and anti-inflammatory drugs have been used to mitigate the disease burden. In the deluge of the drugs being used for COVID-19 infection, glucocorticoids (GCs) stand out by reducing mortality amongst in-hospital severe-to-critically ill patients. Health-care practitioners have seen this as a glimmer of hope and started using these drugs more frequently than ever in clinical practice. The fear of mortality in the short term has overridden the concern of adverse long-term consequences with steroid use. The ease of availability, low cost, and apparent clinical improvement in the short term have led to the unscrupulous use of the steroids even in mild COVID-19 patients including self-medication with steroids. The use of GCs has led to the increasing incidence of hyperglycemia and consequent acute complications of diabetic ketoacidosis and mucormycosis in COVID-19 patients. There is an urgent need to dissipate information about optimum management of hyperglycemia during steroid use. In view of this, the Endocrine Society of India has formulated this position statement about the diagnosis and management of hyperglycemia due to the use of GCs in patients with COVID-19 infection.
Keywords: COVID-19, diabetes, glucocorticoids, hyperglycemia, insulin, oral anti-diabetic drugs, steroid
BACKGROUND
The current coronavirus disease (COVID-19) pandemic has stirred the scientific community because of its enormous proportions causing significant morbidity and mortality particularly amongst people with diabetes. Recent evidence suggests a mortality benefit amongst in-hospital severe-to-critically ill COVID-19 patients with glucocorticoid (GC) therapy.[1] The steroid regimens recommended by World Health Organization include dexamethasone 6 mg/day or prednisone 40 mg/day or hydrocortisone 200 mg/day for a period of 10 days. Contrary to recommendations, continuation of steroids for a prolonged time after discharge is not uncommon in the hope of prevention of post-COVID pulmonary fibrosis. These supraphysiological doses may exacerbate hyperglycemia in individuals with diabetes, unmask diabetes in population at-risk, and precipitate acute complications like hyperglycemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA).
Hyperglycemia increases the incidence and severity of COVID-19 infection, prolonged hospital and intensive care unit (ICU) stay; contributes to poor disease outcomes; and is associated with higher mortality.[2,3] An appropriate management of in-hospital and postdischarge (home-isolation) hyperglycemia is need of the hour for optimizing outcomes in COVID-19 patients. Prior position statements and recommendations have elaborated on the management of diabetes in the COVID-19 pandemic.[4,5,6,7] However, the evidence and consensus for the diagnosis and management of steroid-associated hyperglycemia in the COVID-19 pandemic are lacking. Thus, the purpose of this statement is to provide expert recommendations elucidating the causes and management of steroid-associated hyperglycemia amongst in-hospital and domiciliary care of COVID-19 patients.
HYPERGLYCEMIA IN COVID-19 PATIENTS
Hyperglycemia observed in the COVID-19 patients could be divided into the following four categories:
COVID-induced diabetes
Patients not known to have diabetes with HbA1c <6.5%, but random blood glucose ≥200 mg/dl (repeated) at presentation and/or fasting blood glucose (FBG) ≥126 mg/dl (without precipitating drugs like steroids).[8]
Preexisting diabetes
Hyperglycemia in a known patient with diabetes mellitus may exacerbate on contracting COVID-19 disease.
COVID treatment-related hyperglycemia
Patients not known to have diabetes at presentation (HbA1c <6.5%) but with random blood glucose >140 mg/dl (repeated values) after the inception of steroids for COVID-19 disease.[9]
Stress hyperglycemia
Transient hyperglycemia (FBG ≥126 mg/dl or random blood glucose ≥200 mg/dl) during severe illness with HbA1c <6.5% in people without previous diabetes that revert to normoglycemia at discharge from hospital.[10]
GC-ASSOCIATED HYPERGLYCEMIA
The use of GC worsens hyperglycemia in preexisting diabetes and may precipitate new-onset diabetes in predisposed individuals. More than two-thirds of patients develop hyperglycemia within the first 1–2 days after initiation of GC. Steroids are associated predominantly with post-prandial hyperglycemia and an overreliance on FBG may underestimate the incidence of hyperglycemia. The salient features of GC-associated hyperglycemia (GAH) are given below:
Definition
Random blood glucose >140 mg/dl (repeated values) (within first 24–48 h of initiation of GCs) after the inception of steroids for COVID-19 disease, with HbA1c <6.5% at presentation, is considered as “steroid associated hyperglycemia”.
GC-induced diabetes (GID) is considered on prolonged (>12 weeks) use of supraphysiologic doses of GCs (>5 mg/day of prednisolone or its equivalent) and satisfying either of the below mentioned criteria[9]:
FBG ≥126 mg/dl
Random glucose ≥200 mg/dl (multiple occasions)
Blood glucose ≥200 mg/dl 2 h after an oral glucose load
HbA1c ≥6.5%
However, GID has not yet been defined in the context of COVID-19 disease.
Epidemiology
The odds ratio of new-onset diabetes following steroid therapy ranges from 1.36 to 2.31.[11]
Oral or parenteral GCs increase the risk of diabetes (incidence 2% in a primary care population). A minimal association of incident diabetes is observed when GCs are administered by other routes (inhalational/topical/intra-articular). The potency and half-life of commonly used GC drugs are given in Table 1.
Amongst in-hospital patients, more than half of the patients receiving high-dose steroids develop hyperglycemia (86% have at least one episode of hyperglycemia and 48% presenting a mean blood glucose ≥140 mg/dl).[12]
Table 1.
Potency of various glucocorticoid
| Type of glucocorticoid | Potency (dose equivalent) | Half-life (h) |
|---|---|---|
| Hydrocortisone | 1 | 8 |
| Prednisolone | 5 | 16-30 |
| Methylprednisolone | 6 | 18-36 |
| Betamethasone | 30 | 36-54 |
| Dexamethasone | 30 | 36-54 |
Predisposing factors
The dose and the duration of GC use is an important risk factor leading to the GAH. A dose of prednisolone 5 mg or equivalent of other steroids is likely to cause hyperglycemia.[9] However, some patients may develop hyperglycemia even at lower doses necessitating close vigilance. Glucose levels rise within 4–8 h of administration of GC and the predisposing factors for GAH are given below:
Dose and duration of GC treatment
Age (elderly)
Previous glucose intolerance or gestational diabetes
Abdominal obesity
Family history of diabetes
Race or ethnicity (South Asian)
Prior history of hyperglycemia with GCs
Pathogenesis
The various pathophysiological mechanisms for GAH are elucidated in Table 2.
Table 2.
Pathophysiological mechanisms of glucocorticoid-associated hyperglycemia
| Pathophysiological mechanisms |
| Increase endogenous glucose production (hepatic gluconeogenesis) |
| Impaired insulin sensitivity (antagonizing metabolic actions of insulin) |
| Reduce insulin-mediated actions on muscle and adipose tissue: |
| Reduce peripheral glucose uptake |
| Decrease GLUT-4 translocation to cell membrane |
| Decrease muscle glycogen synthesis[13] |
| Post-receptor insulin signaling defect |
| Enhance the effects of other counter-regulatory hormones (glucagon and epinephrine) that increase endogenous gluconeogenesis |
| Inhibit the production and secretion of insulin from pancreatic β-cells |
| Reduction of β-cell GLUT-2 and glucokinase receptor expression |
| Increasing activity of glucose-6-phosphate dehydrogenase |
| Alteration in β-oxidation |
| Reduce the effect of incretins on β-cell to decrease insulin secretion |
| Lipotoxicity-induced β-cell failure indirectly (induce lipolysis elevating triglycerides and free fatty acids) |
| Expression of the nuclear receptor peroxisome proliferator-activated receptor α is altered |
MANAGEMENT CONSIDERATIONS FOR GAH IN COVID-19
Treatment of hyperglycemia due to GC use in patients suffering from COVID can be divided into management during home isolation and management in hospital settings.
Management of hyperglycemia due to GC use during home isolation
Many patients are prescribed steroids during mild or moderate COVID infection in domiciliary settings. Majority of these patients receive a single dose of oral steroid as a short course. Rarely, patients are prescribed with multiple doses of oral or parenteral steroids at home.
Methylprednisolone, dexamethasone, prednisolone, and hydrocortisone are the commonly prescribed steroids for patients during home isolation.
The patients receiving steroids at home are managed as per Figure 1.
The suggested choice of insulin[9] based on the GC being taken by the patient is given in Table 3.
The glycemic monitoring is done using the capillary blood glucose (CBG) and the doses of the insulin or noninsulin therapies are adjusted to keep the CBG between 140 and 180 mg/dl.
Continuous glucose monitoring system may be used for monitoring of glycemia if appropriate.
Figure 1.
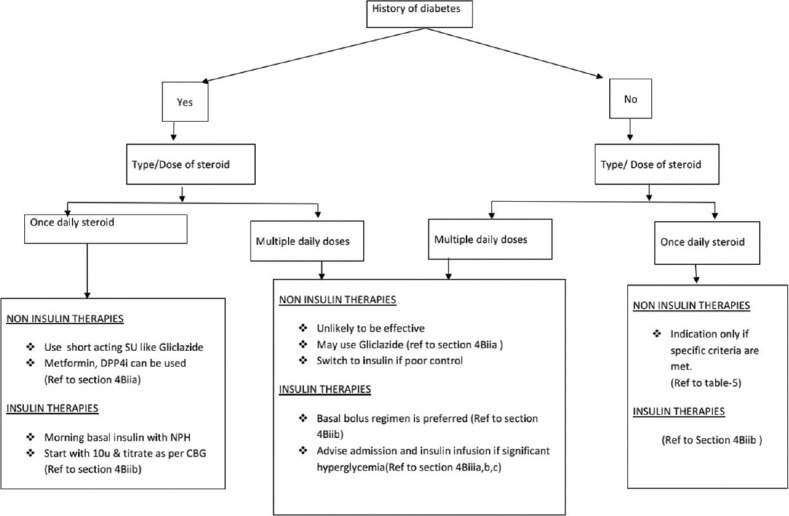
Management of hyperglycemia during home isolation
Table 3.
Steroid types and suggested insulin regimen
| Drug | Peak glycemic effect | Suggested insulin |
|---|---|---|
| Methylprednisolone | 4-6 h after ingestion | NPH |
| Dexamethasone | 8-10 h | Glargine U100/Detemir/Degludec/Glargine U300 |
| NPH twice a day with or without rapid-acting insulin analogs | ||
| Prednisolone | 2-3 h | Rapid-acting insulin analogs/NPH once daily |
Management of hyperglycemia due to steroid use in hospital
Inpatient management of hyperglycemia in COVID patients on GC therapy depends on many factors elaborated in Table 4.
Table 4.
Factors affecting in-hospital management of hyperglycemia
| Various factors |
|---|
| Admission category (emergency/ICU or in ward) |
| Degree and type of hyperglycemia |
| Type, frequency, and route of steroid administration |
| Nutrition and feeding status of the patient |
4Bi) Initial screening and evaluation of hyperglycemia in patients with COVID on GCs admitted to hospital have been depicted in Figure 2.
Figure 2.
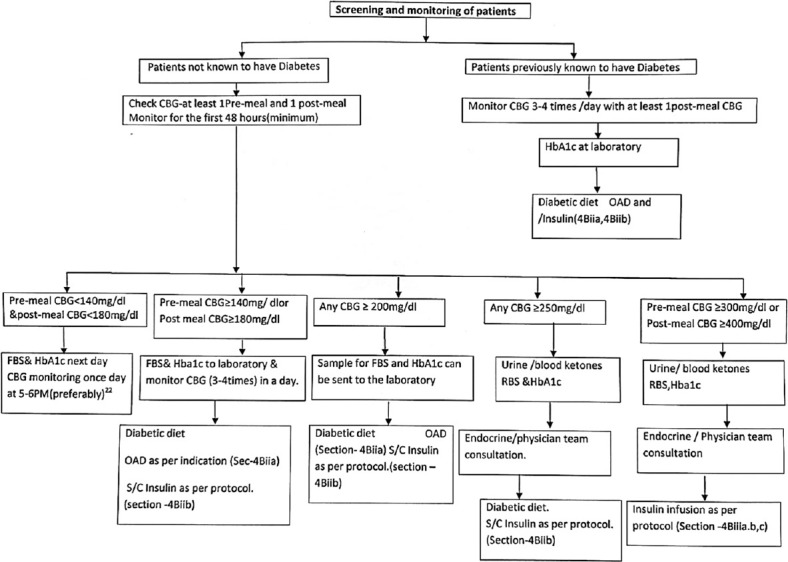
Screening and monitoring of patients on glucocorticoid therapy
4Bii) Management of hyperglycemia in the ward:
Hyperglycemia in admitted patients in ward can be managed by
Oral antidiabetic agents (OADs)
Insulin
Insulin is the preferred drug for all hospitalized patients with COVID on steroids with hyperglycemia.[14] However, in resource-limited setup like India, where the pandemic is huge and there is a scarcity of expert medical care advice, it won't be reasonable to start all patients with insulin or switch all patients on OADs to insulin.[15] Oral glucose-lowering agents can be prescribed in the below-mentioned subset of patients.
4Biia) Oral antidiabetic agents
The indications and choice of OADs have been depicted in Table 5 and Table 6.
Table 5.
Indications for starting or continuing OADs (only if all the criteria are met)
| Patients with previous history of type 2 diabetes mellitus | Patients with new-onset hyperglycemia |
|---|---|
| Prior good glycemic control (HbA1c <7%) | Premeal CBG: 140-180 mg/dl or postmeal CBG: 180-250 mg/dl |
| Premeal CBG <140 mg/dl and postmeal CBG <180 mg/dl | Mild COVID symptoms |
| Mild-to-moderate COVID symptoms | No hepatic or renal involvement |
| No hepatic or renal involvement | Tolerating adequate nutrition orally |
| Tolerating adequate nutrition orally | No other contraindications to OADs |
Table 6.
OADs: Considerations
| OADs | Evidence | Use in patients with COVID on steroids |
|---|---|---|
| Metformin | Reduces steroid-induced insulin resistance | Can be used, if indications are met [Table 5] |
| Mortality benefits in hospitalized COVID patients[16] | Risk GI intolerance and lactic acidosis | |
| Sulfonylurea | Modern sulfonylureas like gliclazide[9] and glimepiride[17] can be shown to be effective in glucocorticoid-induced diabetes | Can be used as per indications |
| Hypoglycemia needs to be watched | ||
| Non-sulfonylurea secretagogues (Repaglinide/nateglinide) | Monotherapy with repaglinide/nataglinide or combination with AGI have been shown to be beneficial in glucocorticoid-induced diabetes[18] | Can be used to cover post-prandial glycemic surges, especially postlunch |
| DPP-4 inhibitors | Monotherapy with DPP-4 inhibitors or combination with metformin has shown to be beneficial in glucocorticoid-induced diabetes[18,19] | Due to relative safety, DPP-4 inhibitors can be used as monotherapy or combination with metformin with little side effects and modest efficacy |
| TZD | TZD antagonizes pathways of insulin resistance and improves beta-cell function[20] | Pioglitazone can be effective in managing insulin resistance induced by glucocorticoids |
| Modest action and fluid retention limit its use | ||
| SGLT-2 inhibitors | Can increase the risk of ketosis/euglycemic ketosis | In line with other recommendations, we raise caution on use of SGLT-2 inhibitors due to the risk of ketosis, especially in moderate-to-severe COVID |
| EANITIATE, an ongoing trial comparing efficacy and safety of empagliflozin vs. NPH insulin, will highlight the use of SGLT-2 inhibitors in GID in future[21] | ||
| AGIs | Evidence suggest mild-to-moderate efficacy in reducing blood glucose in glucocorticoid-induced diabetes with the use of acarbose or a combination with repaglinide[18] | Can be beneficial in milder and predominant post-prandial hyperglycemia |
OAD: Oral antidiabetic drugs; DPP-4: dipeptidyl peptidase-4; TZD: thiazolidinediones; AGIs: alfa-glucosidase inhibitors.
4Biib) Insulin
4Biib1) Indications for starting insulin[14]
Individuals with moderate-to-severe COVID on GC therapy
Premeal blood glucose of >180 mg/dl and postmeal blood glucose of >250 mg/dl
Hyperglycemic emergencies (DKA/HHS)
Hemodynamically unstable
Poor and erratic food intake
4Biib2) Initiating and choosing an insulin regimen
Basal insulin-based regimen
Preferred in hospitalized patients with COVID on steroids because it can easily be titrated and can be escalated to basal-plus or basal-bolus regimen. Among the basal insulins, Neutral Protamine Hagedorn (NPH) insulin which has an intermediate duration of action is preferred because of its favorable pharmacokinetic properties matching with the hyperglycemia profile induced by once-daily steroids and also due to its lower cost.[22,23] Grommesh and colleagues[24] in their Randomized Controlled Trial (RCT) have shown that a single subcutaneous starting dose of NPH at a dose of 0.27 units/kg given at the same time as the morning dose of oral prednisolone can effectively control the glycemic excursion.
Basal insulin analogs like glargine U100/detemir/degludec/glargine U300 can be used as part of basal-plus or basal-bolus insulin regimen, when long-acting steroids or multiple doses of steroids are used.[23]
Bolus insulin
Rapid-acting insulin analogs are preferred over human regular insulin to control postmeal hyperglycemia as they are associated with less hypoglycemia and better flexibility.[23]
Premix insulin
Ease of initiation of premix insulin offers compliance; may not be ideal in comparison to basal-based insulin regimen in hospitalized patients on steroids.[23]
Predominant postmeal hyperglycemia by steroids can be addressed by once-, twice-, or thrice-a-day injections with premix analogs and preferably use of high mix (lispro mi × 50 or aspart mi × 50) before breakfast and lunchtime and low mix (aspart mi × 30 or lispro mi × 25) before dinner time.
Sliding scale insulin should be discouraged[23]
4Biib3) The initiation, monitoring, and titration of insulin in the ward have been depicted in Figure 3.
Figure 3.
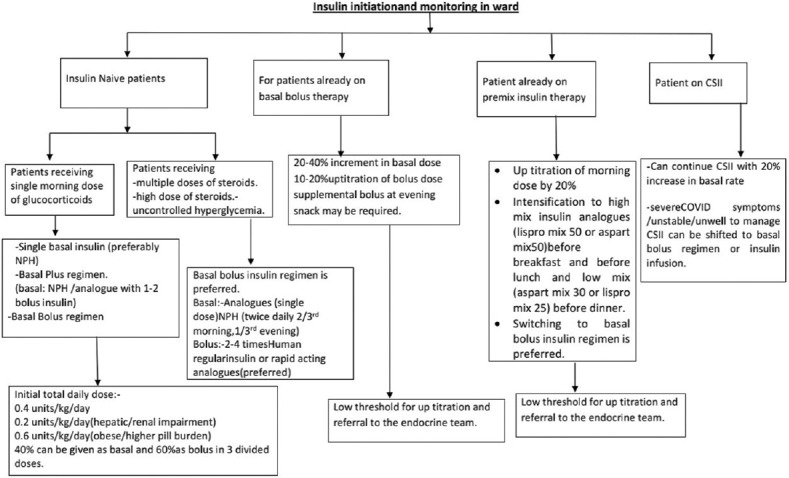
Insulin initiation and glucose monitoring amongst in-hospital patients
4Biii) Management in ICU
Intravenous insulin infusion is the preferred modality of insulin delivery in patients who are admitted to ICU and are on steroids.[22] There are several published insulin protocols available (both paper-driven and computerized). The exact protocol is probably less important than its presence in an institution, adaptation to the individual hospital, simplicity of use, bedside availability, implementation by staff, and periodic validation and review of effectiveness.
4Biiia) Indications for insulin infusion[14]
The indications for insulin infusion in ICU for COVID-19patients on steroids are highlighted in Table 7.
Table 7.
Indications for insulin infusion
| Hyperglycemic emergencies such as DKA or HHS |
| Severe hyperglycemia (premeal glucose values of >300 mg/dl and/or postmeal values of >400 mg/dl) |
| Critically ill with sepsis or on inotropes or on ventilators |
| A lower threshold for starting insulin infusion in ICU is if capillary glucose is >180 mg/dl on repeated occasions |
| Uncontrolled hyperglycemia despite the appropriate use of a basal-bolus insulin regimen |
| Patients with erratic diet and frequent vomiting in ICU |
DKA: Diabetic ketoacidosis; HHS: hyperglycemic hyperosmolar state.
4Biiib) Insulin infusion preparation:
Insulin infusions can be provided at a strength of 1 unit of regular insulin/1 ml of normal saline (0.9% sodium chloride solution), preferably in an infusion pump or gravity-assisted pediatric infusion set. Unused insulin solution should be discarded after 24 h of preparation.[25,26]
Initial priming should be done by flushing the tubing with 50 ml of the prepared solution before starting the insulin infusion.[25]
4Biiic) Infusion rate and titration
A simple formula to calculate initial insulin infusion rate (units/h) is CBG level (mg/dl)/100[25] or it can be initiated at a dose of 0.05–0.10 units/kg/h.
Insulin infusion rate can be titrated taking into account several factors like existing blood glucose level, magnitude of blood glucose change in the previous hour, desired blood glucose target, insulin sensitivity, and the expected intake of a major or minor meal.[14]
Initial fall of CBG should be 50–75 mg/dl/h. If the rate of fall of capillary glucose is <50 mg/dl or >75 mg/dl, an increase or decrease in infusion rate should be considered.
An institutional protocol for management of hypoglycemia should be in place.
Both intravenous insulin and use of steroids in patients with COVID can be associated with hypokalemia. Therefore, serum potassium should be monitored daily or more frequently if necessary.
4Biiid) Target CBG level
CBG level should be tried to be kept at a range of 140–180 mg/dl for majority of patients.[10]
4Biiid) Switching from insulin infusion to basal-bolus insulin regimen Indications
BG levels are controlled on insulin infusion.
Patient is orally accepting or on RT feeds.
Hemodynamically stable.
Steps to switch
Insulin infusion should not be stopped abruptly and infusion should be overlapped with subcutaneous insulin for 60–120 min.[14,26]
Calculate the total daily dose (TDD) based on insulin infusion requirements for the last 24 h. TDD is 80% of the total daily insulin requirement on IV infusion in the last 24 h. Fifty percent of the TDD can be given as basal and rest 50% can be given as bolus divided into three equal parts.[14,26]
4Biv) In-hospital glycemic monitoring and targets
Point-of-care monitoring of blood glucose is to be done preferably with capillary method.
In cases of severe COVID in ICU patients with hypotension, shock, and use of vasopressors, venous blood sampling should be used instead.
4Biva) For patients who are on insulin infusion
CBG monitoring should be done every 1–2 hourly. Can be extended to 4 hourly, where insulin requirement is low, glucose values are stable and in target range.[26]
4Bivb) For patients on subcutaneous insulin therapy
Seven-point profile of three premeals and three postmeals with 3 am capillary glucose monitoring is ideal.[10]
In the resource-limited setup, a 3-point CBG monitoring is acceptable.
4Bivc) Glycemic targets
ICU: a target glucose range of 140–180 mg/dl should be ideal.[10]
Wards: pre-prandial CBG <140 mg/dl and post-prandial CBG <180 mg/dl.[10]
The targets can be changed depending on the presence of comorbidities, intake of food, risk of hypoglycemia, and resource husbandry.
DISCHARGE ADVICE
Steroid tapering
The patients are usually discharged with tapering doses of steroids and advised about the strict glycemic control to hasten the recovery process.
It is important to educate the patient and family members about the dynamic changes in the requirement of the glucose-reducing agents during tapering of steroids.
A detailed discharge summary should be given coupled with a glucose-monitoring chart and the follow-up consultation could be either physical or virtual.
Insulin resistance reduces with reducing dose of steroids and may persist even for few weeks after stopping the steroids. It is essential to monitor for hyperglycemia during this phase and be dynamic in managing the glucose fluctuations.
Patients using insulin are advised to maintain the glucose log and adjust the insulin dose in consultation with the health-care practitioners.
Normoglycemia may be observed in many patients after stopping of steroids. The definite test for development of diabetes is done after 12 weeks of complete stoppage of steroids.
All patients should be educated about the symptoms of dysglycemia and check the CBG frequently to identify the problem early.
The patients should receive education about the diet, lifestyle modification, and hypoglycemia.
Steroid withdrawal syndrome is seen in few patients with rapid tapering and the manifestations are typical to that of adrenal insufficiency. It is important to educate the patient about the same and intervene with a gradual tapering schedule.[27]
Ancillary therapies
The use of GCs may result in few systemic consequences in patients with COVID-19 and it is important to minimize the same by ancillary therapies.[28]
The important short-term systemic consequences and therapies to minimize the morbidity are summarized in Table 8.
Table 8.
Glucocorticoid and their effects on other systems
| System | Consequences | Monitoring | Therapy |
|---|---|---|---|
| Endocrine | Adrenal suppression | Morning cortisol | Gradual tapering |
| HPA axis evaluation | |||
| Cardiovascular | Hypertension | ABPM, Edema | Uptitrate the antihypertensive agents |
| GIT | Gastritis, peptic ulcer disease | UGIE | Proton pump inhibitors |
| Infections | Mucormycosis | CT scan, PNS, black growth in nose | Amphotericin-B |
Follow-up
A close follow-up during the initial phase after steroid use is essential to identify the adrenal insufficiency or a life-threatening infection like mucormycosis.
The patients should be screened for a definitive diagnosis of diabetes after a gap of at least 12 weeks after complete cessation of steroids.
Patient education is important to explain the higher risk of contracting diabetes and the need for regular monitoring.
The suggested follow-up after stopping steroid completely is given in Figure 4.
Figure 4.
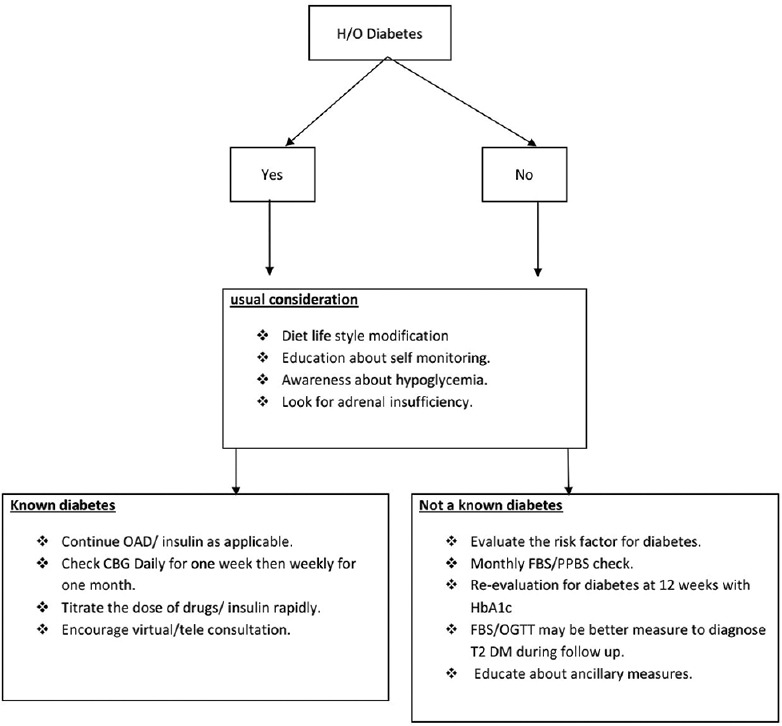
Follow-up protocol after withdrawal of steroids
Telemedicine for hypergycemia and steroid
Telemedicine facilities and virtual consultations should be encouraged to reinforce blood glucose monitoring and awareness of danger signs including emergency alerts (for DKA, HHS, mucormycosis, adrenal insufficiency) at periodic intervals. The exchange of reliable information for the diagnosis and treatment of hyperglycemia due to steroid use for COVID-19 during domiciliary care will be helpful to optimize outcomes for the patients.[29,30]
CONCLUSION
An appropriate diagnosis and management of hyperglycemia in home isolation, in hospital, and postdischarge is the need of the hour for optimizing outcomes in COVID-19 patients on steroid therapy. This position statement provides expert recommendations for the diagnosis and management of steroid-associated hyperglycemia amongst in-hospital and domiciliary COVID-19 patients based on practical experience and limited published studies in resource-constrained settings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Al-Rifai RH, Saha S. Diabetes prevalence and mortality in COVID-19 patients: A systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;31:1–12. doi: 10.1007/s40200-021-00779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. CORONADO investigators.Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020;63:1500–15. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–50. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A, Prattichizzo F. Pharmacological management of COVID-19 in type 2 diabetes? J Diabetes Complications. 2021;17:107927. doi: 10.1016/j.jdiacomp.2021.107927. doi: 10.1016/j.jdiacomp. 2021.107927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta Y, Goyal A, Kubihal S, Golla KK, Tandon N. A guidance on diagnosis and management of hyperglycemia at COVID care facilities in India. Diabetes Metab Syndr. 2021;15:407–13. doi: 10.1016/j.dsx.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A Meta-analysis. JAMA. 2020;324:1330–41. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med. 2020;383:789–90. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts A, James J, Dhatariya K Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperglycemia and steroid (glucocorticoid) therapy: A guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018;35:1011–7. doi: 10.1111/dme.13675. [DOI] [PubMed] [Google Scholar]
- 10.Moghissi ES, Korytkowski MT, Dinardo MM, Einhorn D, Richard H, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469–74. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 12.Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99:277–80. doi: 10.1016/j.diabres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: Defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119–30. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Guidance on Diabetes Management at COVID-19 Patient Management Facility. Government of India Ministry of Health and Family Welfare. [Dated 26th August, 2020]. Available from: https://www.mohfw.gov.in/pdf/Clinical Guidance on Diabetes Management at COVID19 Patient Management Facility.pdf .
- 15.Goyal A, Gupta S, Gupta Y, Tandon N. Proposed guidelines for screening of hyperglycemia in patients hospitalized with COVID-19 in low resource settings Diabetes Metab Syndr. 2020;14:753–6. doi: 10.1016/j.dsx.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34–41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasayama S, Tanaka T, Hashimoto K, Koga M, Kawase I. Efficacy of glimepiride for the treatment of diabetes occurring during glucocorticoid therapy. Diabetes Care. 2002;25:2359–60. doi: 10.2337/diacare.25.12.2359. [DOI] [PubMed] [Google Scholar]
- 18.Klarskov CK, Holm Schultz H, Wilbek Fabricius T, Persson F, Pedersen-Bjergaard U, Lommer Kristensen P. Oral treatment of glucocorticoid-induced diabetes mellitus: A systematic review. Int J Clin Pract. 2020;74:e13529. doi: 10.1111/ijcp.13529. [DOI] [PubMed] [Google Scholar]
- 19.Perez A, Jansen-Chaparro S, Saigi I, Bernal-Lopez MR, Miñambres I, Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6:9–20. doi: 10.1111/1753-0407.12090. [DOI] [PubMed] [Google Scholar]
- 20.Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Res Clin Pract. 2002;58:87–96. doi: 10.1016/s0168-8227(02)00127-4. [DOI] [PubMed] [Google Scholar]
- 21.Klarskov CK, Holm Schultz H, Persson F, Møller Christensen T, Almdal TP, Snorgaard O, et al. Study rationale and design of the EANITIATE study (EmpAgliflozin compared to NPH Insulin for sTeroId diAbeTEs)-a randomized, controlled, multicenter trial of safety and efficacy of treatment with empagliflozin compared with NPH-insulin in patients with newly onset diabetes following initiation of glucocorticoid treatment. BMC Endocr Disord. 2020;20:86. doi: 10.1186/s12902-020-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayman G, Lumb AN, Kennon B, Cottrell C, Nagi D, Page E, et al. Dexamethasone therapy in COVID-19 patients: Implications and guidance for the management of blood glucose in people with and without diabetes. Diabet Med. 2021;38:e14378. doi: 10.1111/dme.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khazai NB, Hamdy O. Inpatient diabetes management in the twenty-first century. Endocrinol Metab Clin North Am. 2016;45:875–94. doi: 10.1016/j.ecl.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Grommesh B, Lausch MJ, Vannelli AJ, Mullen DM, Bergenstal RM, Richter SA, et al. Hospital insulin protocol aims for glucose control in glucocorticoid-induced hyperglycemia. Endocr Pract. 2016;22:180–9. doi: 10.4158/EP15818.OR. [DOI] [PubMed] [Google Scholar]
- 25.Shetty S, Inzucchi SE, Goldberg PA, Cooper D, Siegel MD, Honiden S. Adapting to the new consensus guidelines for managing hyperglycemia during critical illness: The updated Yale insulin infusion protocol. Endocr Pract. 2012;18:363–70. doi: 10.4158/EP11260.OR. [DOI] [PubMed] [Google Scholar]
- 26.Kelly JL. Continuous insulin infusion: When, where, and how? Diabetes Spectr. 2014;27:218–23. doi: 10.2337/diaspect.27.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: How to detect and manage them. Cleve Clin J Med. 2011;78:748–56. doi: 10.3949/ccjm.78a.10180. [DOI] [PubMed] [Google Scholar]
- 28.Patt H, Bandgar T, Lila A, Shah N. Management issues with exogenous steroid therapy. Indian J Endocr Metab. 2013;17:S612–7. doi: 10.4103/2230-8210.123548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastogi A, Hiteshi P, Bhansali A. Improved glycemic control amongst people with long-standing diabetes during COVID-19 lockdown: A prospective, observational, nested cohort study. Int J Diabetes Dev Ctries. 2020;21:1–6. doi: 10.1007/s13410-020-00880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastogi A, Hiteshi P, Bhansali A, Jude EB. Virtual triage and outcomes of diabetic foot complications during Covid-19 pandemic: A retro-prospective, observational cohort study. PLoS One. 2021;16:e0251143. doi: 10.1371/journal.pone.0251143. [DOI] [PMC free article] [PubMed] [Google Scholar]


