Abstract
Decades of research have shown that the concentration of freely dissolved PAH (Cfree) in sediment correlates with PAH bioavailability and toxicity to aquatic organisms. Passive sampling techniques and models have been used for measuring and predicting Cfree, respectively, but these techniques require weeks for analytical chemical measurements and data evaluation. This study evaluated the performance of a portable, field-deployable antibody-based PAH biosensor method that can provide measurements of PAH Cfree within a matter of minutes using a small volume of mechanically-extracted sediment porewater. Four sediments with a wide range of PAHs (ΣPAH 2.4 to 307 mg/kg) derived from petroleum, creosote, and mixed urban sources, were analyzed via three methods: 1) bulk chemistry analysis; 2) ex situ sediment passive sampling; and 3) biosensor analysis of mechanically-extracted sediment porewater. Mean ΣPAH Cfree determined by the biosensor for the four sediments (3.1 to 55 μg/L) were within a factor of 1.1 (on average) compared to values determined by the passive samplers (2.0 to 52 μg/L). All mean values differed by a factor of 3 or less. The biosensor was also useful in identifying sediments that are likely to be non-toxic to benthic invertebrates. In two of the four sediments, biosensor results of 20 and 55 μg/L exceeded a potential risk-based screening level of 10 μg/L, indicating toxicity could not be ruled out. PAH Toxic Units (ΣTU) measured in these two sediments using the passive sampler Cfree results were also greater than the ΣTU threshold of 1 (6.7 and 5.8, respectively), confirming the conclusions reached with the biosensor. In contrast, the other two sediments were identified as non-toxic by both the biosensor (3.1 and 4.3 μg/L) and the passive sampler (ΣTUs of 0.34 and 0.039). These results indicate that the biosensor is a promising tool for rapid screening of sediments potentially-impacted with PAHs.
Keywords: PAH, Sediment, Availability, Passive Sampling, Biosensor
Capsule:
A rapid and field-deployable biosensor tool to measure freely dissolved PAHs (Cfree) in sediment was evaluated, and results indicated it is a promising approach to optimize future investigations.
1. Introduction
Decades of research has shown that measuring freely dissolved PAH (Cfree) in sediment provides a more accurate predictor of bioavailability and toxicity to aquatic organisms compared to measurement of “total” concentrations of PAHs in bulk sediment (Kreitinger et al., 2007; Fernandez et al., 2009; McGrath et al., 2019). Passive sampling methods are considered the most accurate and sensitive methods to measure Cfree and are increasingly being used as primary lines of evidence to support site-specific regulatory-driven decisions and to evaluate active sediment management (USEPA, 2012a; Ghosh et al., 2014; USEPA, 2017a; Kirtay et al., 2018; Fetters et al. 2019).
A recently developed, immunoassay-based “biosensor” method has shown promise as a rapid and portable approach for measuring PAHs in aqueous media, including mechanically-extracted sediment porewater (Spier et al., 2011; Li et al., 2016; Behera et al. 2018). This method detects PAHs using a highly specific monoclonal antibody (Li et al., 2016). Biosensor results are reported as a concentration of the sum of PAHs (ΣPAH) in an aqueous sample (i.e., μg ΣPAH/L). The biosensor has been shown to be more accurate, precise, and quantitative than competitive enzyme-linked immunosorbent assays (ELISA) such as PAH RISc® or RaPID-ELISA (Behera et al., 2018). The analysis can be performed with a small volume (e.g., 20 mL) of mechanically-extracted sediment porewater, enabling its use as a measurement approach for Cfree ΣPAH in sediment. The biosensor can be operated in the field using a battery power source and can generate a result from a liquid sample in approximately 10 minutes (Spier et al., 2011), making it an attractive option for real-time data collection. Using this approach, Hartzell et al. (2018) found that the biosensor porewater results obtained from two sediments spiked with PAHs correlated well with sediment toxicity to the benthic amphipod Leptocheirus plumulosus in the 10-day chronic test (r2 = 0.98), and that lethality was generally observed above a biosensor measurement of 10 μg/L. Additionally, biosensor-derived Cfree ΣPAH LC50 values (24 to 29 μg/L) were more precise (within a factor of 1.2) than LC50 values derived using concentrations in bulk sediment (within a factor of 3.2); however, this is not surprising, as measurements of dose that incorporate availability (i.e., Cfree) are generally more precise and accurate predictors of toxicity than measurements of compounds in bulk sediment.
The biosensor method is optimized to detect 3- to 5-ring PAHs, and past studies indicate that biosensor ΣPAH results correlate with traditional gas chromatograph(GC)-based analyses of the sum of the 3- to 5-ring PAHs (ΣPAH3–5) in aqueous samples (water, sediment porewater) containing spiked PAHs and PAHs derived from creosote and multiple industrial sources (Unger et al. 2010; Li et al., 2016; Hartzell et al., 2018). The antibody used in the biosensor analysis does have an affinity for 2-ring PAHs, albeit it is low relative to the 3- to 5- ring PAHs, and it is possible that 2-ring PAHs may contribute to the overall biosensor response signal if present in sufficiently high concentrations (Li et al., 2016). This hypothesis has not been tested, and the ability of the biosensor to respond to 2-ring PAHs is particularly relevant for the use of the biosensor with petroleum-impacted sediments. Unlike creosote and other pyrogenic sources of PAHs common in industrial waterways, petroleum PAH signatures are often enriched with 2-ring PAHs, particularly methylated naphthalenes (Neff et al., 2005). Thus, it is possible that the biosensor may underestimate Cfree ΣPAH results in sediments impacted by petroleum.
The goal of this project was to evaluate the performance of the biosensor in detecting Cfree PAHs in petroleum-impacted sediments. Cfree ΣPAHs measured using the biosensor method and passive sampling were compared using a variety of disparate sediment types containing PAHs from a variety of sources (including petroleum sources) and exhibiting a range of PAHs likely to be encountered in a typical sediment site investigation. To evaluate the potential for the biosensor to serve as a screening approach to identify sediments that are potentially non-toxic to aquatic life (Neff et al., 2005; Hawthorne et al., 2006; McGrath et al., 2019; Simpson et al., 2020), biosensor results were also compared to PAH Toxic Unit (TU) results (USEPA, 2003; USEPA 2012b; USEPA, 2017b; Endo et al. 2020) calculated using passive sampler measurements and the United States Environmental Protection Agency (USEPA) Equilibrium Partitioning (EqP) models (USEPA, 2012b), as applied to concentrations of PAHs and organic carbon content measured in bulk sediment.
2. Materials and Methods
2.1. Test Sediments
Four sediments containing PAHs from a variety of sources were selected for this study: 1) Sediment “C (Petroleum Low)” was obtained from freshwater sediment near primarily petroleum refining sources; 2) Sediment “G2 (Petroleum High)” was obtained from an estuarine sediment near petroleum production (crude oil) sources; 3) Sediment “S (Urban Reference)” was obtained from a freshwater sediment near a variety of urban ambient sources; and 4) Sediment “V1 (Creosote)” was obtained from an estuarine sediment near a known historical creosote wood treatment facility. Sediments were obtained from surficial layers (approximately 30 cm or less below the sediment-water interface) and maintained at 4°C until processing and additional measurements. Additional details on the test sediments and processing are provided in Section 1 of the Supplementary Information.
2.2. Determination of Bulk Sediment PAHs
Following homogenization, aliquots of bulk sediment were analyzed at a commercial analytical laboratory (Eurofins TestAmerica, Knoxville, TN, USA) for parent (un-substituted) and alkylated PAHs via Gas Chromatography/Mass Spectrometry Isotope Dilution methods consistent with United States Environmental Protection Agency (USEPA) Method 8270 (USEPA, 2018), Total Petroleum Hydrocarbons (TPH) analytes TPH Diesel Range, TPH Oil Range, and TPH Gasoline Range via USEPA Method 8015C (USEPA, 2007), and Total Organic Carbon (TOC) and Black Carbon (BC) via the Lloyd Kahn method (USEPA, 1988). 35 PAH analytes were quantified by the USEPA Method 8270 analysis in sediment (Table S1). The analytical list of PAHs targeted in this study is directly comparable to the 34-analyte analytical programs (i.e., “PAH34”) often used to evaluate PAHs in sediment (USEPA, 2012b), as our program provided results for benzo[b]fluoranthene and benzo[k]fluoranthene individually, rather than a summed “benzo[b + k]fluoranthene” analyte in PAH34 programs. Additional details on the analysis of the analytes in the bulk sediment are provided in Section 1 of the Supplementary Information.
2.3. Determination of Cfree PAHs
Cfree PAHs in sediment was measured ex situ using 250-mL aliquots of the four test sediments. Two approaches were used. The biosensor approach was conducted with an aliquot of sediment porewater (approximately 20 mL per sample) obtained via centrifugation of a portion of the 250-mL sediment sample in 50 mL Teflon® centrifuge tubes for 15 min at 3500 g. Sufficient porewater volume for the V1 sediment was not obtained via this approach, so a porewater sample was obtained via centrifugation of a sediment slurry that had been tumbled for 28 d (see Section 2 of the Supplementary Information). The resulting porewater supernatant from each sediment was decanted and temporarily stored in 20-mL glass scintillation vials. If fine particulates were observed to be present in the porewater samples, samples were filtered through a 0.45-μm Teflon® syringe filter. It should be noted that the centrifugation and filtration of porewater for use in the biosensor is likely insufficient to remove potentially unavailable PAHs that may be associated with colloidal matter and dissolved organic carbon. The same day of porewater collection and filtration, the samples were analyzed with a KinExA Inline instrument (Sapidyne Instruments, Boise ID) to determine the ΣPAH using the antibody-based biosensor method, as described in detail in Li et al. (2016). This method utilizes the monoclonal antibody 2G8 that has high affinity for parent and alkylated PAH compounds with 3 to 5 rings; the antibody has a lower affinity for 2-ring PAHs. The KinExA Inline instrument utilized an automated sample-handling program and was calibrated for ΣPAH concentration with phenanthrene standards (ICN Pharmaceuticals) ranging from 0.1 to 2.5 mg/L that were created daily in deionized water by serial dilution from a stock solution. Samples with a detector response outside the calibration curve were diluted with deionized water to bring the instrument response into the most linear portion of the concentration range. Additional procedures for the biosensor are described in more detail in Spier et al. (2011) and Li et al. (2016). Multiple biosensor Cfree ΣPAH measurements were made on each sediment (Table S3); the mean value of the multiple measurements were used for subsequent data analysis.
The second approach for ex situ Cfree determination used commercially-available equilibrium passive samplers (SP3™, https://www.siremlab.com/). The SP3™ sampler design consisted of a 3 centimeter (cm) × 3 cm piece of 13 micrometer (μm) thick low-density polyethylene (PE) housed in a stainless-steel mesh envelope that was solvent cleaned and spiked with Performance Reference Compounds (PRCs) consisting of rare PCBs congeners. Passive samplers were added to 500-mL amber glass jars containing a slurry composed of an approximate 250-mL aliquot of wet test sediment and 250-mL volume of ultrapure water spiked with 1 mL of a 25-g/L sodium azide solution to inhibit microbial transformation (Jonker et al., 2020). The jars (four per test sediment) were maintained at an average temperature of 20°C and rotated end-over-end for 28 d to facilitate uptake of Cfree PAHs by the passive sampler. After the exposure period, the passive samplers were removed from the jars and packaged individually for storage at 4°C until they could be processed, weighed, and solvent-extracted prior to analysis of the solvent to determine the concentration of 35 PAH analytes (Table S1) in the PE via standard methods at a commercial analytical laboratory. The resulting data were then used to calculate Cfree for the 35 individual PAH analytes. Samplers exposed to the S (Urban Reference) sediment indicated that many of the PAH analytes were undetectable, so two of the four solvent extracts were combined, concentrated further under inert gas to enable lower detection limits to be achieved, and reanalyzed (see Section 2 of the Supplementary Information). Methods for passive sampler preparation, deployment, storage, extraction, analysis, and estimation of Cfree values are detailed further in Section 2 of the Supplementary Information, and were consistent with passive sampling practices recommended by USEPA (2017a).
Cfree of the 35 PAHs were also predicted using USEPA Equilibrium Partitioning (EqP) modeling (USEPA, 2012b) with the concentration of PAHs in bulk sediment, TOC and BC contents of the bulk sediment, and model constants (i.e. TOC and BC partitioning coefficients). Two predictions of Cfree of each PAH for each of the four test sediments were calculated. The first predicted Cfree value was calculated using the 1-carbon model, which assumes partitioning between TOC and sediment porewater; the second value was calculated using 2-carbon model, which assumes partitioning among TOC, BC, and sediment porewater. Additional details on the modeling, as well as EqP predictions, are provided in Section 3 of the Supplementary Information.
3. Results and Discussion
3.1. Cfree ΣPAH Values
In terms of providing an estimate of an inclusive measurement to reflect a sum of freely-dissolved PAHs within a particular sediment, the biosensor indicated comparable results to Cfree ΣPAH measured by the passive sampler and predicted by EqP modeling (Figure 1, Table S6), although, as discussed below, these approaches do not align perfectly in terms of the analytes they quantify. Mean Cfree ΣPAH values between the biosensor and passive sampler for the four test sediments agreed within a factor of 3 or less. On average, mean (SD) biosensor Cfree ΣPAH values were 1.1 (0.53) times higher than mean Cfree ΣPAH measured by the passive sampler. Cfree ΣPAH results were sufficiently precise to indicate statistically-significant differences (t-test, P < 0.05; see Section 4 of the Supplementary Information) between the biosensor and passive sampler in two of the three test sediments in which multiple measurements with both approaches were available (sediments C, G2, and V1). However, it is not uncommon to obtain statistically-detectable differences between measurements using two different aliquots of sediment by two different laboratories, even with standardized chemical measurements (Quevauviller, 1998; Turle et al., 2007). Thus, while statistically different, the correspondence of biosensor and passive sampler results are close enough to be considered functionally indifferent given the intended goal of the biosensor as a predictive or screening tool.
Fig. 1.
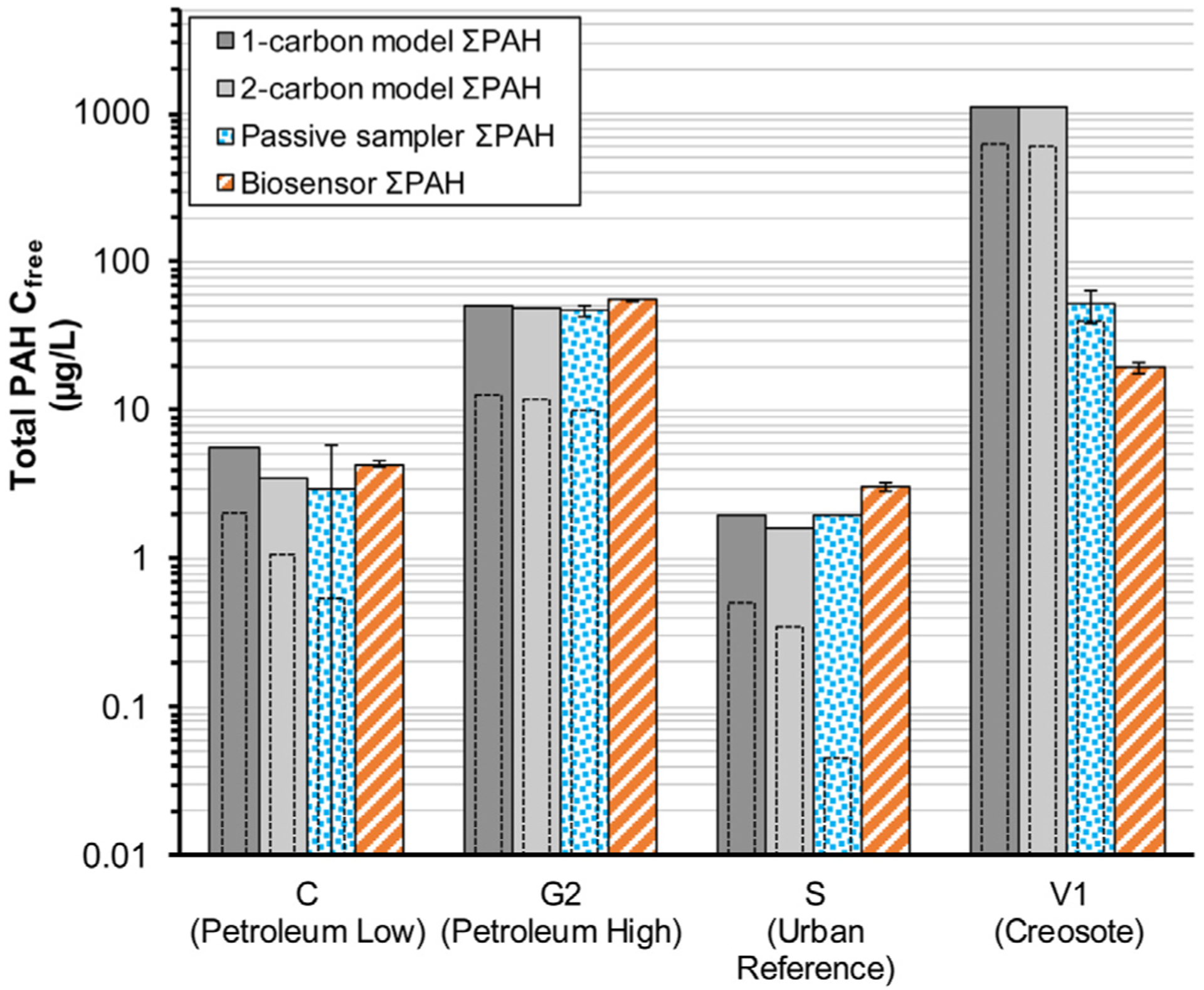
Sum of the total detected Cfree PAHs (ΣPAH) predicted by 1-carbon and 2-carbon EqP models and measured via passive samplers and the biosensor in the four test sediments. In cases where multiple measurements are available, values are mean (SD). The nested, dashed-line columns represent the Cfree ΣPAH3–5 predicted EqP models and measured via passive samplers.
Agreement between the 1-carbon and 2-carbon EqP model Cfree ΣPAH predictions and the values measured by the biosensor was good for three of the four test sediments. For test sediments C, G2, and S, mean (SD) Cfree ΣPAH values indicated by the biosensor were a factor of 1.1 (0.4) and 1.4 (0.4) times higher than the 1-carbon and 2-carbon EqP model predictions, respectively (Figure 1, Table S6). A similar level of agreement for these three sediments was found between the Cfree ΣPAH values indicated by the passive sampler and the EqP models. The exception was found in the case of the creosote (V1) sediment, as the 1-carbon and 2-carbon EqP model Cfree ΣPAH predictions were approximately 60 times higher than mean Cfree ΣPAH values indicated by the biosensor and approximately 20 times higher than values measured via passive sampling. Many researchers have observed that EqP models often tend to overestimate Cfree of hydrophobic organic compounds (Hawthorne et al., 2007; Arp et al., 2009; Fernandez et al., 2009; McDonough et al 2010; Gschwend et al., 2011; Brennan and Johnson, 2018; McGrath et al., 2019; Endo et al., 2020). The inability of the EqP model to accurately predict Cfree in the V1 sediment further indicates the importance of accurate Cfree measurement approaches.
Overall, the relative level of agreement observed between the biosensor and passive sampler approaches is notable, considering the approaches rely on completely different methods to quantify and detect Cfree PAHs. The PAHs in the test sediments varied in PAH signature (reflecting different sources and environmental setting, as detailed in Table S2 and Supplementary Information Section 5), exhibited Cfree ΣPAH ranging over two orders of magnitude (Figure 1), and featured detectable concentrations of all 35 PAHs measured (Table S4). The relatively good agreement among methods indicates the potential utility of the rapid biosensor method to serve as a powerful screening tool for use in a diverse array of sediment and PAH characteristics likely to be encountered by typical sediment site investigations.
It is acknowledged that the Cfree approaches evaluated in this investigation may not fully quantify all freely-dissolved PAHs that may be present. As noted in the introduction, the biosensor is optimized to detect 3- to 5-ring PAHs, and there is uncertainty with regards to its ability to fully and accurately quantify 2-ring PAHs that may comprise a large proportion of the PAHs found in petroleum-impacted sediment. Passive sampler results indicated that 2-ring PAHs represented a significant proportion (22% to 98%) of the total detected Cfree (Figure 2), especially for the sediments with PAHs primarily derived from petroleum sources (e.g., sediments C and G2). Despite the large proportion of 2-ring PAHs in the petroleum-impacted C and G2 sediments, Cfree ΣPAH values as measured by the biosensor and passive sampler were comparable, with the biosensor values 1.2 to 1.5 higher than passive sampler values (Figure 1). If the biosensor only responded to 3- to 5-ring PAHs, it would be expected that the Cfree ΣPAH3–5 in the C and G2 sediments, as measured by the passive sampler and predicted by the EqP models, would be in closer agreement with the Cfree ΣPAH values indicated by the biosensor. This was not the case, as passive sampler and EqP Cfree ΣPAH3–5 values were a factor of 2 to 8 times lower than Cfree ΣPAH values indicated by the biosensor (Figure 1). It is also likely that the GC-based measurements used for the analysis of passive samplers and bulk sediment do not fully quantify the hundreds of environmentally relevant PAHs that may be present in PAH-impacted sediment (Neff et al., 2005). For example, the biosensor may detect a wider array of alkylated PAHs compared to GC-based methods (Li et al., 2016), and this may explain the discrepancy between passive sampler and EqP Cfree ΣPAH3–5 values and Cfree ΣPAH values indicated by the biosensor (if one assumes the biosensor primarily detects 3- to 5-ring PAHs). Sediments with PAHs derived from petroleum are more likely to exhibit higher amounts of alkylated PAHs compared to sediments with creosote-derived PAHs. For example, 81–96% (on average) of the Cfree PAH measured by passive samplers in sediments C and G2 were comprised of alkylated PAHs (Table S4). Thus, it is possible that all alkylated PAHs are not fully or directly quantified in the analysis of the passive sampler, and this may explain the difference in Cfree ΣPAH (biosensor) and ΣPAH3–5 (passive sampler) for these two sediments. Additional experimentation with expanded GC analytical capability and controlled experiments with single compound PAHs would be useful in evaluating biosensor PAH responses.
Fig. 2.

Mean percentage of total detected Cfree PAHs (ΣPAH) comprised of 2-, 3-, 4-, and 5-ring PAHs, as measured by passive samplers. 6-ring PAHs comprised less than 0.04% of Cfree ΣPAH, and are not included in the graph.
3.2. Biosensor Results and PAH Toxic Units
Ultimately, a key role for Cfree measurements or predictions within the context of a sediment investigation is to predict the likelihood of adverse effects on biota in a particular sediment or resolve the contribution of PAHs (relative to other sediment characteristics) in sediments in which adverse effects are observed with sediment toxicity tests or other biological evaluations. Cfree measurements with passive samplers or Cfree EqP predictions can be evaluated with respect to the potential toxicity of PAHs to aquatic life using the PAH Toxic Unit (TU) approach (USEPA, 2003; Hawthorne et al., 2006; Hawthorne et al., 2007; Kreitinger et al., 2007; McDonough et al., 2010; Arp et al., 2011; USEPA 2012b; USEPA, 2017b; Brennan and Johnson, 2018; McGrath et al., 2019; Endo et al. 2020; Simpson et al., 2020). This approach calculates PAH TU values via dividing the concentrations of individual PAHs (or other organic compounds) in an aqueous medium by their respective toxic thresholds (no-observed effect concentrations, effective concentration 20%, etc.). Summation of the TU values generates a single value (ΣTU). For the USEPA (2003, 2012b) PAH TU approach, ΣTU values less than one are considered to indicate sediments in which PAH-derived effects are not expected, whereas ΣTU values above one indicates that the absence of potential toxicity cannot be assumed without additional evaluation. The precise ΣTU (above a value of one) at which adverse effects become apparent is unclear. For example, various studies have indicated that for PAH-impacted sediments tested with laboratory benthic organisms, relatively consistent reductions in survival are observed at ΣTU values of approximately 10 or higher (Kreitinger et al., 2007; McDonough et al 2010; Arp et al., 2011; McGrath et al., 2019). It should be noted that this approach is specific to PAHs, and it does not evaluate the potential for toxicity derived from other chemicals that may be present.
Mean (SD) PAH ΣTUs, as calculated using the passive sampler Cfree measurements (Section 7 of the Supplementary Information) were 0.34 (0.04), 5.8 (0.49), 0.039, and 6.7 (1.3) for the petroleum low (C), petroleum high (G2), and urban reference (S), and creosote (V1) sediments, respectively (Table S7). 1-carbon and 2-carbon model predicted PAH ΣTU values for three of the four test sediments were within a factor of 3 of PAH ΣTU values based on those measured with passive samplers, with the exception of the creosote (V1) sediment (Figure 3). The passive sampler, 1-carbon model, and 2-carbon model were all in agreement with regards to which sediments exceeded the ΣTU threshold of 1 (petroleum high (G2) and creosote (V1)). The biosensor indicated mean values Cfree ΣPAH of 20 and 55 μg/L for petroleum high (G2) and creosote (V1) sediments, respectively (Table S6). Cfree ΣPAH for these two sediments exceed a previously noted 10 μg/L biosensor threshold, at which the onset of mortality was observed in 10-d Leptocheirus plumulosus sediment toxicity tests (Hartzell et al., 2018). In contrast, the biosensor indicated mean values Cfree ΣPAH of 3.1 to 4.3 μg/L for the other sediments (petroleum low (C) and urban reference (S)). Passive sampler PAH ΣTUs were 0.34 and 0.039 for the C and S sediments, respectively (Table S7), and EqP model-predicted PAH ΣTU values were below 1 for these sediments (Table S8); thus, all three approaches indicated that the C and S sediments would not be expected to be toxic. Overall, the passive sampler, biosensor, and EqP model results agreed regarding the identification of the two test sediments for which PAH-derived toxicity was not expected; however, additional toxicity testing on a wider-range of PAH-contaminated sediments is needed to validate the 10 μg/L biosensor threshold as an appropriate toxicity screening threshold for site assessment.
Fig. 3.

Mean Cfree total PAH Toxic Units (PAH ΣTU) measured with passive samplers compared to 1-carbon (a) and 2-carbon (b) EqP model predictions of Cfree PAH ΣTU. Points between the diagonal dashed lines indicate agreement within a factor of 3 or less between measured and predicted values.
The agreement between the passive sampler PAH ΣTUs values and biosensor results (with respect to a hypothetical 10 μg/L biosensor threshold) is less likely to be affected by the potential discrepancies in the two methods with regards to detecting 2-ring PAHs. For example, an average of 60% (range 44% to 85%) of the mean PAH ΣTUs indicated by the passive sampler were derived from the 3- to 5-ring PAHs (Figure 4) that are likely to be most accurately quantified by the biosensor. Thus, if most of the contribution to toxic potential is due to 3- to 5-ring PAHs (as in these sediments), biosensor and passive sampler results would be expected to agree with respect to identifying non-toxic sediments.
Fig. 4.

Percentage of mean Cfree total PAH Toxic Units (PAH ΣTU) derived from 2-, 3-, 4-, and 5-ring PAH TUs, as measured by passive samplers. 6-ring PAH TUs comprised less than 1% of Cfree PAH ΣTU, and are not included in the graph.
Conclusions
The Cfree PAH results from the four test sediments, ranging over two orders of magnitude and derived from different sources (petroleum, creosote, and mixed urban sources), indicated that the biosensor is a promising tool for evaluating the availability and potential toxicity of PAHs to aquatic life at sediment sites. The biosensor offers flexible field- or laboratory-based rapid assessment capabilities for generating real-time data that can be used to prioritize sediment for additional evaluations such as Cfree determination with passive samplers, toxicological testing, or other ecological evaluations. These capabilities are particularly attractive for evaluating sediments in remote locations and during a variety of investigation and remediation monitoring phases of typical sediment sites. This application would benefit from additional field demonstration with higher sample numbers and types of sediments, ideally involving organism toxicity testing to evaluate the ability of the biosensor to serve as a screening tool for identifying potential sediment toxicity due to PAHs. Standardization and commercialization of a sediment porewater biosensor method would also be worthwhile so that this approach can be widely applied by sediment assessment practitioners.
Supplementary Material
Acknowledgments
We acknowledge the financial support of Chevron Energy Technology Company for funding the majority of this study. Portions of the biosensor work conducted at VIMS were supported by NIEHS-SRP grant RO1ES024245. The authors wish to thank Dr. Lance Fontenot and Dr. Alice Wang (Geosyntec Consultants) for thoughtful reviews on an initial draft of the manuscript, for Dr. Fontenot’s generous donation of test sediment. We also thank G. Vadas and M.A. Vogelbein for assistance with sample preparation and biosensor analyses.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.envadv.2021.100032.
References
- Arp HP, Breedveld GD, Cornelissen G, 2009. Estimating the in situ sediment-porewater distribution of PAHs and chlorinated aromatic hydrocarbons in anthropogenic impacted sediments. Environ. Sci. Technol 43, 5576–5595. [DOI] [PubMed] [Google Scholar]
- Arp HPH, Azzolina NA, Cornelissen G, Hawthorne SB, 2011. Predicting pore water EPA-34 PAH concentrations and toxicity in pyrogenic-impacted sediments using pyrene content. Environ. Sci. Technol 45, 5139–5146. [DOI] [PubMed] [Google Scholar]
- Behera BK, Das A, Sarkar DJ, Weerathunge P, Parida PK, Das BK, Thavamani P, Ramanthan R, Bansal V, 2018. Polycyclic aromatic hydrocarbons (PAHs) in inland aquatic ecosystems: Perils and remedies through biosensors and bioremediation. Environ. Pollut 241, 212–233. [DOI] [PubMed] [Google Scholar]
- Brennan AA, Johnson NW, 2018. The utility of solid-phase microextraction in evaluating polycyclic aromatic hydrocarbon bioavailability during habitat restoration with dredged material at moderately contaminated sites. Integr. Environ. Assess. Manag 14, 212–223. [DOI] [PubMed] [Google Scholar]
- Endo S, Yoshimura M, Kumata H, Uchida M, Yabuki Y, Nakata H, 2020. Reduced bioavailability of polycyclic aromatic hydrocarbons (PAHs) in sediments impacted by carbon manufacturing plant effluent: Evaluation by ex situ passive sampling method. Environ. Pollut 256, 114448. [DOI] [PubMed] [Google Scholar]
- Fetters K, Grover M, Rosen G, Kirtay V, Chadwick B, Conder J, Paris Sacks V, Magar V, 2019. Demonstration and validation of enhanced monitored natural recovery at a pesticide contaminated sediment site. J. Soils Sediments 20, 204–219. [Google Scholar]
- Fernandez LA, Macfarlane JK, Tcaciuc AP, Gschwend PM, 2009. Measurement of freely dissolved PAH concentrations in sediment beds using passive sampling with low-density polyethylene strips. Environ. Sci. Technol 43, 1430–1436. [DOI] [PubMed] [Google Scholar]
- Ghosh U, Kane Driscoll S, Burgess RM, Jonker MTO, Reible D, Gobas F, Choi Y, Apitz SE, Maruya KA, Gala WR, Mortimer M, Beegan C, 2014. Passive sampling methods for contaminated sediments: practical guidance for selection, calibration, and implementation. Integr. Environ. Assess. Manag 10, 210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend PM, MacFarlane JK, Reible DD, Lu X, Hawthorne SB, Nakles DV, Thompson T, 2011. Comparison of polymeric samplers for accurately assessing PCBs in pore waters. Environ. Toxicol. Chem 6, 1288–1296. [DOI] [PubMed] [Google Scholar]
- Hartzell SE, Unger MA, Vadas GG, Yonkos L, 2018. Evaluating porewater PAH-related toxicity at a contaminated sediment site using a spiked field-sediment approach. Environ. Chem. and Toxicol 37, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne SB, Miller DJ, Kreitinger JP, 2006. Measurement of total polycyclic aromatic hydrocarbon concentrations in sediments and toxic units used for estimating risk to benthic invertebrates at manufactured gas plant sites. Environ. Toxicol. Chem 25, 287–296. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Azzolina NA, Neuhauser EF, Kreitinger JP, 2007. Predicting bioavailability of sediment polycyclic aromatic hydrocarbons to Hyalella azteca using equilibrium partitioning, supercritical fluid extraction, and pore water concentrations. Environ. Sci. Technol 41, 6297–6304. [DOI] [PubMed] [Google Scholar]
- Jonker MTO, Burgess RM, Ghosh U, Gschwend PM, Hale SE, Lohmann R, Lydy MJ, Maruya KA, Reible D, Smedes F, 2020. Ex situ determination of freely dissolved concentrations of hydrophobic organic chemicals in sediments and soils: basis for interpreting toxicity and assessing bioavailability, risks and remediation necessity. Nat. Protoc 15, 1800–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtay V, Conder J, Rosen G, Magar V, Grover M, Arblaster J, Fetters K, Chadwick B, 2018. Performance of an in situ activated carbon treatment to reduce PCB availability in an active harbor. Environ. Toxicol. Chem 37, 1767–1777. [DOI] [PubMed] [Google Scholar]
- Kreitinger JP, Neuhauser EF, Doherty FG, Hawthorne SB, 2007. Greatly reduced bioavailability and toxicity of polycyclic aromatic hydrocarbons to Hyalella azteca in sediments from manufactured-gas plant sites. Environ. Toxicol. Chem 26, 1146–1157. [DOI] [PubMed] [Google Scholar]
- Li X, Kaattari SL, Vogelbein MA, Vadas GG, Unger MA, 2016. A highly sensitive monoclonal antibody based biosensor for quantifying 3–5 ring polycyclic aromatic hydrocarbons (PAHs) in aqueous environmental samples. Sens. Bio-sens. Res 7, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KM, Azzolina NA, Hawthorne SB, Nakles DV, Neuhauser EF, 2010. An evaluation of the ability of chemical measurements to predict polycyclic aromatic hydrocarbon-contaminated sediment toxicity to Hyalella azteca. Environ. Toxicol. Chem 29, 1545–1550. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Joshua N, Bess AS, Parkerton TF, 2019. Review of polycyclic aromatic hydrocarbons (PAHs) sediment quality guidelines for the protection of benthic life. Integr. Environ. Assess. Manag 15, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff JM, Stout SA, Gunster DG, 2005. Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: Identifying sources and ecological hazard. Integr. Environ. Assess. Manag 1, 22–33. [DOI] [PubMed] [Google Scholar]
- Quevauviller P, 1998. Operationally defined extraction procedures for soil and sediment analysis: I. Standardization. Trends Anal. Chem 17, 289–298. [Google Scholar]
- Simpson SS, Spadaro DA, Batley GE, Irvine IA, Synnot RN, 2020. Remediation criteria for gasworks-impacted sediments: assessing the effects of legacy hydrocarbons and more recent metal contamination. Sci. Tot. Environ 737, 139725. [DOI] [PubMed] [Google Scholar]
- Spier CS, Vadas GG, Kaattari SL, Unger MA, 2011. Near-real-time, on-site, quantitative analysis of PAHs in the aqueous environment using an antibody-based biosensor. Environ. Toxicol. Chem 30, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Turle R, Nason T, Malle H, Fowlie P, 2007. Development and implementation of the CCME reference method for the Canada-wide standard for petroleum hydrocarbons (PHC) in soil: a case study. Anal. Bioanal. Chem 387, 957–964. [DOI] [PubMed] [Google Scholar]
- Unger MA, Spier C, Bromage ES, 2010. Validation of a Real-Time, Field Deployable Biosensor, for the Detection and Quantification of Polycyclic Aromatic Hydrocarbons (PAHs) in Aquatic Systems. Final Report Submitted to The NOAA/UNH Cooperative Institute for Coastal and Estuarine Environmental Technology (CICEET) May. [Google Scholar]
- USEPA, 1988. Determination of Total Organic Carbon in Sediment (Lloyd Kahn Method) July 27.
- USEPA, 2003. Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: PAH Mixtures EPA-600-R-02–013.
- USEPA. 2007. Method 8015C, nonhalogenated organics by gas chromatography. Revision 3, February.
- USEPA, 2012a. Guidelines for Using Passive Samplers to Monitor Organic Contaminants at Superfund Sediment Sites. U.S. Environmental Protection Agency, Washington, DC: EPA/600/R-11/115. [Google Scholar]
- USEPA, 2012b. Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Procedures for the Determination of the Freely Dissolved Interstitial Water Concentrations of Nonionic Organics EPA/600/R-02/012.
- USEPA., 2017a. Laboratory, Field, and Analytical Procedures for Using Passive Sampling in the Evaluation of Contaminated Sediments: User’s Manual EPA/600/R-16/357.
- USEPA., 2017b. Developing Sediment Remediation Goals at Superfund Sites Based on Pore Water for the Protection of Benthic Organisms from Direct Toxicity to Nonionic Organic Contaminants EPA/600/R 15/289.
- USEPA, 2018. Method 8270E, Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry SW-846 Update VI, June.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


