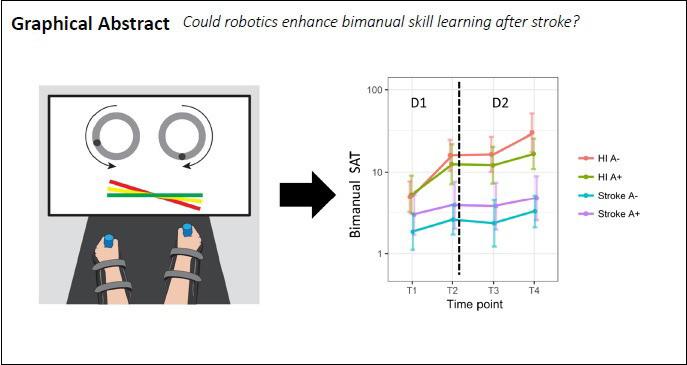
Keywords: bimanual, hemiparesis, motor learning, rehabilitation, robotic, robotic assistance, slacking, stroke retention
Abstract
Using robotic devices might improve recovery post-stroke, but the optimal way to apply robotic assistance has yet to be determined. The current study aimed to investigate whether training under the robotic active-assisted mode improves bimanual motor skill learning (biMSkL) more than training under the active mode in stroke patients. Twenty-six healthy individuals (HI) and 23 chronic hemiparetic stroke patients with a detectable lesion on MRI or CT scan, who demonstrated motor deficits in the upper limb, were randomly allocated to two parallel groups. The protocol included a two-day training on a new bimanual cooperative task, LIFT-THE-TRAY, under either the active or active-assisted modes (where assistance decreased in a pre-determined stepwise fashion) with the bimanual version of the REAplan® robotic device. The hypothesis was that the active-assisted mode would result in greater biMSkL than the active mode. The biMSkL was quantified by a speed-accuracy trade-off (SAT) before (T1) and immediately after (T2) training on days 1 and 2 (T3 and T4). The change in SAT after 2 days of training (T4/T1) indicated that both HI and stroke patients learned and retained the bimanual cooperative task. After 2 days of training, the active-assisted mode did not improve biMSkL more than the active mode (T4/T1) in HI nor stroke patients. Whereas HI generalized the learned bimanual skill to different execution speeds in both the active and active-assisted subgroups, the stroke patients generalized the learned skill only in the active subgroup. Taken together, the active-assisted mode, applied in a pre-determined stepwise decreasing fashion, did not improve biMSkL more than the active mode in HI and stroke subjects. Stroke subjects might benefit more from robotic assistance when applied “as-needed.” This study was approved by the local ethical committee (Comité d’éthique médicale, CHU UCL Namur, Mont-Godinne, Yvoir, Belgium; Internal number: 54/2010, EudraCT number: NUB B039201317382) on July 14, 2016 and was registered with ClinicalTrials.gov (Identifier: NCT03974750) on June 5, 2019.
Chinese Library Classification No. R493; R741
Introduction
The most common deficit after stroke is hemiparesis, i.e., difficulties in controlling contralesional movements (Trombly, 1992). Stroke survivors with a paretic upper limb (UL) have demonstrated deficient coordination in cooperative bimanual tasks (Kantak et al., 2016), which may lead to loss of independence for activities of daily life (ADL). Thus, efficient bilateral UL neurorehabilitation programs are necessary (Veerbeek et al., 2014; Bernhardt et al., 2017; Kantak et al., 2017), based on several premises: (I) since most ADLs are bimanual, neurorehabilitation should involve bimanual exercises. (II) Impairments of the paretic UL cannot predict impairments in bimanual tasks (Lowrey et al., 2014; Kantak et al., 2016) because bimanual tasks are intrinsically more complex. Even if recovering from paretic UL’s impairment is necessary, it likely will not be sufficient to recover bimanual ADL “automatically.” (III): Simultaneous training of both hands/UL may improve the paretic UL function because of their spatial and temporal coupling (Rossini et al., 2003; Carson, 2005). Among bilateral training options (King et al., 2010; Meng et al., 2017; Raghavan et al., 2017), robotics seems promising (Kwakkel et al., 2008; Gassert and Dietz, 2018; Dehem et al., 2019; Duret et al., 2019).
Robotic devices enable repetitive and intensive practice (Lo et al., 2010; Mehrholz et al., 2012; Abdollahi et al., 2018) that can improve post-stroke motor performance and trigger neuroplastic changes (Buma et al., 2013; Han et al., 2013). Robotic devices usually feature several training modalities, among which: (Basteris et al., 2014) (I) The active mode: the subject performs the full movements freely, and the robotic device measures the subject’s performance. (II) The active-assisted mode: the subject initiates the movement, and the robotic device intervenes exclusively under defined conditions (e.g., error). (III) The passive mode: the robotic device performs the movements thoroughly, and the subject is passive (Basteris et al., 2014).
Furthermore, the application of motor learning principles might enhance motor recovery (Kitago and Krakauer, 2013). E.g., the repetition of a motor task leads to lasting improvements in movement accuracy, indicating the acquisition of a motor skill (Willingham, 1998; Shmuelof and Krakauer, 2011; Shmuelof et al., 2012). Learning a motor skill shifts the speed/accuracy trade-off (SAT) and decreases the variability in motor performance between trials (Dayan and Cohen, 2011; Shmuelof et al., 2012). Motor skill learning is also characterized by retention and generalization of the learned motor skill to untrained conditions. Accordingly, several robotic devices have been developed to harness components of motor learning and intensive exercise-based training, including bimanual approaches (Johnson et al., 2000, 2006; Cauraugh and Kim, 2002; Lum et al., 2002, 2004; Hesse et al., 2003a, b; McCombe Waller et al., 2008; Lin et al., 2010; Trlep et al., 2011; Herrnstadt et al., 2015; Grosmaire and Duret, 2017; Abdollahi et al., 2018).
Although the robotic devices led to improved motor performance, whether robotic assistance can improve bimanual coordination remains controversial. Many studies insist on the importance of active patients participation (Reinkensmeyer et al., 2009; Krishnan et al., 2013; Shirzad and Van der Loos, 2016), i.e., favoring minimal assistance from the robotic device such as the active mode. Other studies have shown that assistance during the training may improve paretic UL function (Colombo et al., 2010; Duret and Hutin, 2013), indicating the potential of the active-assisted mode.
In line with this controversy and to characterize the role of the active-assisted mode of a robotic device in bimanual coordination motor learning, we tested the hypotheses that (I) chronic hemiparetic stroke subjects can learn, retain and generalize a challenging bimanual cooperative motor skill and that (II) the active-assisted mode applied in a stepwise decreasing fashion improves early bimanual learning more than the active mode in both healthy individuals (HI) and stroke subjects. For this purpose, we used the bimanual version of the robotic device for UL rehabilitation REAplan® (AXINESIS, Wavre, Belgium) that has been used successfully in previous rehabilitation studies (Gilliaux et al., 2014; Dehem et al., 2017).
Subjects and Methods
Subjects
Twenty-six young, healthy individuals (HI) aged 27.4 ± 3.2 years and 23 chronic stroke patients (aged 63.9 ± 11 years) were enrolled from CHU UCL Namur, Mont-Godinne (Neurology Department, Stroke Unit, Yvoir, Namur, Belgium, 5530) through the local stroke and HI databases after they provided written informed consent (Additional file 1 (243.1KB, pdf) ). The study complied with the Declaration of Helsinki, and procedures were approved on July 14, 2016 (Internal number: 54/2010, EudraCT number: NUB B039201317382) by the local ethical committee (Comité d’éthique médicale, CHU UCL Namur, Mont-Godinne) (Additional file 2 (223.6KB, pdf) ). The study protocol was reported in line with the CONsolidated Standards Of Reporting Trials (CONSORT) 2010 guidelines (Additional Figure 1 (2.1MB, tif) ). For stroke subjects, the inclusion criteria were 1) having a chronic stroke (> 6 months), 2) being between 18-90 years old, 3) showing a lesion on brain imaging, and 4) demonstrating a motor deficit in the UL. The exclusion criteria were 1) problems in understanding or executing commands of the task, 2) alcohol or drug addiction. For HI, the inclusion criteria were 1) being right-handed and 2) being between 18–90 years old. Exclusion criteria were 1) having a history of neurological problems, 2) alcohol or drug addiction (Additional file 3 (153.5KB, pdf) ). Twenty of them had an ischemic stroke, while three had a hemorrhagic stroke (Table 1).
Table 1.
Demographic characteristics of stroke patients
| No. | Sex | Age (yr) | Time since stroke (yr) | Stroke localization | Type of stroke | Paretic hand | Dominant hand | mRS | NIHSS | ABILHAND Score | B&B | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paretic hand (T1) | Non-paretic hand (T1) | Paretic hand (T4) | Non-paretic hand (T4) | |||||||||||
| P1 | F | 52 | 0.5–1 | C | Ischemic | Right | Right | 3 | 4 | –3.46 | 1.3 | 68.7 | 1.3 | 67.3 |
| P2 | F | 28 | 1–3 | C | Ischemic | Right | Right | 2 | 4 | –3.41 | 0 | 74.7 | 0 | 78.7 |
| P3 | F | 76 | >3 | SC | Hemorrhagic | Left | Left | 4 | 3 | –6.07 | 0 | 46 | 0 | 48.7 |
| P4 | M | 78 | >3 | SC | Hemorrhagic | Right | Right | 4 | 5 | –3.83 | 0 | 80.7 | 0 | 81.3 |
| P5 | F | 63 | >3 | SC | Ischemic | Right | Right | 4 | 4 | –1.46 | 0 | 53.7 | 0 | 60.3 |
| P6 | M | 73 | >3 | SC | Ischemic | Right | Right | 2 | 2 | 0.91 | 0 | 76.7 | 0.3 | 81 |
| P7 | F | 62 | >3 | C | Ischemic | Left | Right | 3 | 1 | –3.46 | 0 | 64.7 | 0 | 65 |
| P8 | M | 50 | >3 | C | Ischemic | Right | Right | 3 | 4 | 2.96 | 41.7 | 71.3 | 41.7 | 75.3 |
| P9 | F | 53 | >3 | C | Ischemic | Left | Right | 2 | 6 | 3.71 | 40 | 47.7 | 43 | 52.7 |
| P10 | M | 68 | >3 | SC | Ischemic | Left | Right | 2 | 4 | –2.16 | 0 | 57 | 0 | 58 |
| P11 | F | 50 | 1–3 | C | Ischemic | Left | Right | 2 | 6 | –6.07 | 0 | 61.3 | 0 | 65 |
| P12 | M | 84 | 1–3 | C | Ischemic | Left | Right | 3 | 4 | –1.03 | 27.7 | 41.3 | 28 | 41.3 |
| P13 | F | 62 | >3 | C | Hemorrhagic | Left | Right | 3 | 6 | –6.01 | 0 | 53.3 | 0 | 68.3 |
| P14 | M | 67 | >3 | SC | Ischemic | Left | Right | 3 | 4 | 0.67 | 18 | 43.7 | 15 | 51.3 |
| P15 | M | 77 | >3 | SC | Ischemic | Right | Right | 2 | 4 | –6.07 | 0 | 38 | 43 | 0 |
| P16 | M | 83 | >3 | C | Ischemic | Left | Right | 3 | 7 | –2.7 | 5 | 56 | 3 | 61.7 |
| P17 | F | 66 | 1–3 | SC | Ischemic | Left | Right | 3 | 4 | 2.71 | 57.7 | 68 | 60.7 | 71.7 |
| P18 | M | 85 | 1–3 | C | Ischemic | Right | Right | 3 | 4 | 3.46 | 0 | 53 | 0 | 55 |
| P19 | F | 71 | >3 | C | Ischemic | Right | Right | 3 | 6 | 0.008 | 0 | 61.7 | 0 | 68.7 |
| P20 | M | 63 | >3 | SC | Ischemic | Right | Right | 4 | 5 | 0.55 | 21.7 | 50 | 17 | 57.3 |
| P21 | F | 57 | >3 | C | Ischemic | Right | Right | 3 | 8 | –4.45 | 0 | 44.7 | 0 | 55 |
| P22 | M | 32 | >3 | C | Ischemic | Right | Right | 2 | 4 | –1.98 | 0 | 56.7 | 0 | 59 |
| P23 | M | 69 | 1–3 | SC | Ischemic | Left | Right | 3 | 2 | 1.19 | 23.7 | 41.3 | 25 | 46.3 |
| Mean± SD | F:11/M:12 | 63.9±11 | C:13/SC:10 | I:20/H:3 | R:11/L:12 | R:22/L:1 | 2.69±0.5 | 4.4±1.2 | –1.56 | 10.3±13.7 | 57±10 | 12.1±15.3 | 59.5±11.3 | |
T1: Before training on day 2. T4: after training on day 2. ABILHAND score (logit); B&B: Box & Blocks test (n of transferred blocks); C: cortical; F: female; M: male; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; SC: sub-cortical.
The National Institutes of Health Stroke Scale (NIHSS) was used to estimate the level of neurological impairments in stroke subjects, and their activity limitation was evaluated with the modified Rankin Scale (mRS) (van Swieten et al., 1988) and the ABILHAND questionnaire (Penta et al., 2001) that assesses specifically residual bimanual capacity. The Box & Blocks test (B&B) (Mathiowetz et al., 1985; Desrosiers et al., 1993) was used to measure the baseline residual unimanual dexterity as well as potential generalization (Rossini et al., 2003; Carson, 2005) from bimanual training to unimanual task performance. The primary outcome measure was the change in SAT (see the Data analysis section).
Bimanual motor skill learning task: LIFT-THE-TRAY
The bimanual task involved circular, in-phase mirror movements of the ULs with 90° of the phase difference between their starting points (Figure 1). The robotic arms of the REAplan® (AXINESIS, Wavre, Belgium) simulated two virtual pulleys, each pulley was attached to one end of a virtual horizontal tray displayed on the screen and was controlled by one hand through the corresponding REAplan® handle. Simultaneous, coordinated, 90° out-of-phase (as if one virtual rope was shorter than the other one), rotating movements of both ULs on the two pulleys lifted the tray toward the top of the screen. The participants were told that different interactions with the REAplan® could be experienced during the different parts of the study. They were instructed to go as fast (speed constraint) and as accurately as possible during each block of training. Accuracy was defined as keeping the tray horizontal: with ideally coordinated bimanual movements, the tray should be lifted with zero-degree inclination compared to the horizontal (x) axis. Real-time feedback of accuracy was provided as a color code reflecting the angular deviation from the horizontal axis. As long as bimanual movements were perfectly coordinated or slightly de-synchronized (i.e., tray angle between 0° and 5° compared to the horizontal axis), the tray remained green. In the case of a moderate bimanual de-synchronization (between 6° and 19°), it turned yellow. If large bimanual de-synchronizations occurred (between 20° and 30°), the tray turned red. The maximum angle of de-synchronization allowed by the virtual wheels was set to 30° (Additional file 4 (149.5KB, pdf) ).
Figure 1.
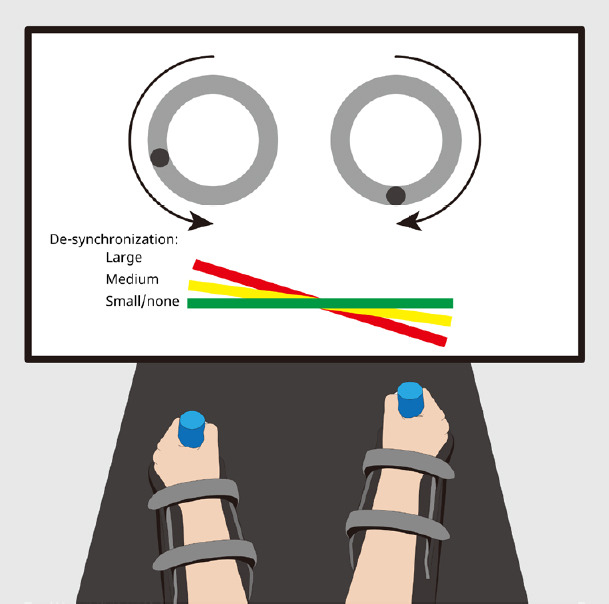
The bimanual cooperative task “LIFT-THE-TRAY.
” The bimanual task was implemented on the bimanual version of the REAplan®. The subjects were instructed to lift the tray as quickly and accurately (i.e., keeping the tray horizontal) as possible by turning two virtual pulleys (illustrated by the light grey circles) through cyclical, in-phase movements. There was a 90-degree phase difference between the starting points of the virtual pulleys, illustrated by the dark grey dots. The forearms were supported by gutters, and the robotic handles were adapted to the residual grasp of the stroke subjects. The color of the tray provided real-time feedback of the tray orientation’s accuracy (angle from the horizontal line 0°–5°: green, 6°–19°: yellow, 20°–30°: red, maximal deviation allowed: 30°).
REAplan®
The current study is the first one carried out with the bimanual version of the REAplan® (AXINESIS, Wavre, Belgium), an end-effector rehabilitation robotic device. Participants made movements in the horizontal plane while holding the two robotic handles. Customized gloves helped the stroke subjects with manual impairments to grasp the handles; the forearms were supported by two gutters to ensure stabilization (Figure 1). A large screen located in front of the subject provided online visual feedback. Position sensors of the REAplan® sampled the position of the handles at 80 Hz and recorded it on the controlling PC. Lateral robotic assistance was present during all exercises and for all subjects: it kept each handle on a circular trajectory, giving to the subject the illusion of controlling two “hard” pulleys through fixed forces. Additionally, longitudinal robotic assistance was provided exclusively during the active-assisted mode as an interaction to keep fixed the distance corresponding to the 90° out-of-phase difference between the two pulleys.
Study protocol
The study was a single-blind (i.e. only the subjects were blind to the experimental conditions), parallel-group, randomized clinical trial (RCT, ClinicalTrials.gov ID: NCT02308852). The subjects trained with either the active-assisted or the active robotic modes to learn a complex bimanual motor skill. For stroke subjects, the randomization was performed with minimization software (http://www.saghaei.net/qminim/). Pre-specified randomization criteria (Table 1) were encoded in the software, which then determined whether the stroke subject’s allocation to the active-assisted or active subgroups. The HI was allocated to the active-assisted or active subgroups in a pseudo-randomized fashion based on inclusion order. In HI, the active-assisted and active subgroups included twelve and fourteen subjects, respectively. Twelve and eleven stroke patients were randomized into the active-assisted and active subgroups, respectively (Figure 2 and Additional Figure 1 (2.1MB, tif) ).
Figure 2.
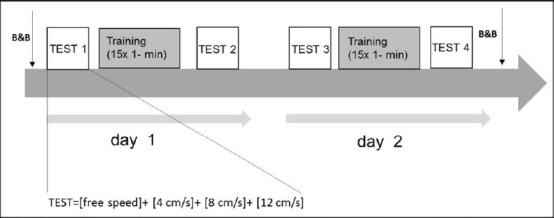
Study protocol.
The protocol consisted of two consecutive days. D1 started with a series of TEST at free speed and with three imposed speeds (4, 8, and 12 cm/s). Then, the subjects trained for 15 minutes with the active-assisted mode or active mode, according to the randomization. After a short pause, training was followed by another, identical, series of TEST. D2 was identical to D1. Before starting TEST 1 and after finishing TEST 4, unimanual hand dexterity was evaluated using the Box & Blocks test (B&B), only in stroke subjects.
HI and stroke participants followed an identical protocol over two consecutive days (Figure 2). Each day (D1, D2) started with four series of exercises called TEST (further explanations below), followed by 20-min of training with either the active or active-assisted robotic modes (parallel-group design) and finished with another identical series of TEST. On D1 and D2, the protocol was identical. Thus, altogether there were four TESTs: T1, the TEST on D1 before training (baseline); T2 on D1 after training; T3 on D2 before training (assessing overnight retention), and finally T4 at on D2 after training. Each TEST included four series of exercises. Each exercise (20 seconds) was repeated five times, separated by a 50-second pause. When the tray reached the top of the screen, it re-appeared at the bottom of the screen with the same tilt angle. In the first series of exercises (free speed), the subjects were asked to perform the task as fast and as accurately as possible, exactly as during the training blocks. Then, they practiced three series of exercises during which a phantom tray ascended on the REAplan® screen with fixed, imposed speeds (i.e., 4, 8, and 12 cm/s, respectively). The participants were instructed to synchronize the ascent of their tray with that of the phantom as much as possible (fixed speed constraint) while attempting to keep the tray horizontal (accuracy constraint). The exercises with imposed speeds aimed to look for a generalization of the bimanual skill. For the stroke subjects, the B&B (Desrosiers et al., 1994) was performed twice, before T1 and after T4 (mean of three trials for each hand, starting with the non-paretic hand), to asses potential generalization of bimanual skill training to a unimanual task.
After the T1 and T3 periods, the participants received 20 minutes of training at the free speed that comprised of 15 blocks of 1 minute separated by 25 seconds of between-block pause. In the active-assisted group, they started the first block with 100% longitudinal assistance helping them to keep the distance between the two handles constant (i.e., imposing optimal bimanual synchronization). Thus, no effort was needed to keep the tray horizontal during this block. During the next 14 blocks, the longitudinal assistance decreased in an imposed, stepwise fashion: 93%, 86%, 79%, 71%, 64%, 57%, 50%, 43%, 36%, 29%, 21%, 14%, 7% and finally 0%. In the active group, the participants performed the 15 training blocks without longitudinal assistance.
Data analysis
The REAplan® sampled the following data at 80 Hz that were stored on a PC for the offline analysis:
Speed: the speed of the tray, in cm/s
Error: the angle of the tray compared to the horizontal axis, in degree
SAT was calculated as: SAT = speed/error, in arbitrary units (a.u.)
The software recorded the exercise number and the percentage of longitudinal assistance provided during each block for the active-assisted mode. Longitudinal assistance was always 0% in the active group.
The data were processed using customized Matlab® (2015b, The MathWorks, Inc, Natick, MA, USA) routines; the mean of the SAT was calculated for each 20-second block of the exercises during the TESTs and each 1-minute block during training.
The primary outcome measure was the change in SAT in exercises with free speed and the three imposed speeds (4, 8, and 12 cm/s) in time (at T2, T3 and T4 compared to T1) in the active vs. active-assisted subgroups, for stroke patients and HI.
Statistical analysis
This is a first-time study with this robot and task, so no pilot data were available to compute a power analysis. The sample size (HI n = 26, stroke n = 23) is “custom” in the field for such experiments. The R software (The R Foundation, Boston, MA, USA, https://www.r-project.org/) including the nlme and ggplot2 packages was used to generate generalized linear mixed models (GLMMs). The distribution of SAT values was asymmetrical, so statistical tests were performed on log-transformed values, and a logarithmic scale was used in the Figures displaying the results. Besides, since a logarithmic scale was used, the geometric mean was calculated using the following formula:
The geometric mean of SAT (Ti): n√(SAT1× SAT2× … × SATn)
where: i = 1,2,3,4; and n = number of the HI or stroke participants
For each group (stroke subjects and HI) and each type of exercise (free speed, 4, 8, and 12 cm/s), we used a generalized linear mixed model (GLMM) with and a random intercept for each subject. The ln (SAT) was the dependent variable, and the independent fixed variables were: time (T1, T2, T3, and T4, considered as a categorical variable), intervention (active or active-assisted mode), and the [time X intervention] interaction. Fixed effect coefficients (β) were then used to compute the fold change (FC) = Reβ.
In stroke patients, we counted the number of transferred blocks on the B&B for both paretic and non-paretic hand at T1 and T4. We then calculated the change from T1 to T4 separately for each hand with a 95% confidence interval (CI).
Results
Bimanual motor skill learning
Both the HI (n = 26) and chronic stroke subjects (n = 23) learned the bimanual cooperative task, as evidenced by the improvement of the SAT in the exercises with free speed over the 2 days (Table 2 and Figure 3). In HI, between T1 and T4, the SAT improved as shown by an FC of 6 and 3 in the active and active-assisted subgroups, respectively. In stroke subjects, the FC between T1 and T4 was 1.8 and 1.6, respectively.
Table 2.
Change in speed/accuracy trade-off (SAT) in healthy individuals (HI) and stroke subjects
| Participants | Speed | Robotic assistance | Measure | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|---|
| Healthy | 4 cm/s | A– | GM (GSD) | 76.63 (1.16) | 87.19 (1.04) | 88.89 (1.04) | 88.33 (1.05) |
| A+ | GM (GSD) | 64.79 (1.44) | 83.30 (1.12) | 88.43 (1.05) | 87.27 (1.06) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.14 [1.02–1.26] | 1.16 [1.04–1.29] | 1.15 [1.04–1.28] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.29 [1.15–1.44] | 1.36 [1.22–1.53] | 1.35 [1.20–1.51] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 1.13 [0.97–1.32] | 1.18 [1.01–1.37] | 1.17 [1.00–1.36] | ||
| 8 cm/s | A– | GM (GSD) | 72.41 (1.16) | 83.70 (1.03) | 86.79 (1.04) | 86.39 (1.05) | |
| A+ | GM (GSD) | 67.16 (1.18) | 80.16 (1.07) | 84.66 (1.06) | 84.42 (1.06) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.16 [1.10–1.22] | 1.20 [1.14–1.26] | 1.19 [1.13–1.26] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.19 [1.13–1.26] | 1.26 [1.19–1.33] | 1.26 [1.19–1.33] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 1.03 [0.96–1.12] | 1.05 [0.97–1.14] | 1.05 [0.98–1.14] | ||
| 12 cm/s | A– | GM (GSD) | 70.37 (1.17) | 81.04 (1.02) | 83.63 (1.04) | 84.53 (1.05) | |
| A+ | GM (GSD) | 63.38 (1.24) | 75.48 (1.09) | 80.58 (1.06) | 82.49 (1.06) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.15 [1.08–1.22] | 1.19 [1.12–1.26] | 1.20 [1.13–1.28] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.19 [1.11–1.27] | 1.27 [1.19–1.36] | 1.30 [1.22–1.39] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 1.03 [0.95–1.13] | 1.07 [0.98–1.17] | 1.08 [0.99–1.19] | ||
| Free | A– | GM (GSD) | 5.00 (1.55) | 16.12 (1.55) | 16.50 (1.63) | 30.22 (1.71) | |
| A+ | GM (GSD) | 5.32 (1.71) | 12.52 (1.76) | 12.16 (1.68) | 16.74 (1.53) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 3.23 [2.64–3.95] | 3.30 [2.70–4.04] | 6.05 [4.94–7.40] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 2.35 [1.89–2.93] | 2.29 [1.84–2.84] | 3.15 [2.53–3.91] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 0.73 [0.54–0.98] | 0.69 [0.51–0.93] | 0.52 [0.39–0.70] | ||
| Stroke | 4 cm/s | A– | GM (GSD) | 22.69 (1.65) | 29.98 (1.56) | 30.96 (1.84) | 30.34 (1.84) |
| A+ | GM (GSD) | 17.13 (1.82) | 19.52 (2.56) | 21.00 (2.59) | 21.64 (2.60) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.32 [0.96–1.81] | 1.36 [0.99–1.87] | 1.34 [0.97–1.84] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.14 [0.85–1.52] | 1.23 [0.92–1.64] | 1.26 [0.95–1.69] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 0.86 [0.56–1.33] | 0.90 [0.58–1.38] | 0.94 [0.61–1.45] | ||
| 8 cm/s | A– | GM (GSD) | 17.66 (1.71) | 24.76 (1.97) | 27.95 (1.79) | 30.30 (1.74) | |
| A+ | GM (GSD) | 16.41 (1.96) | 17.16 (2.11) | 22.77 (2.16) | 20.79 (2.47) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.40 [1.02–1.92] | 1.58 [1.16–2.17] | 1.72 [1.25–2.35] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.05 [0.79–1.39] | 1.39 [1.04–1.85] | 1.27 [0.95–1.69] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 0.75 [0.49–1.14] | 0.88 [0.57–1.34] | 0.74 [0.48–1.13] | ||
| 12 cm/s | A– | GM (GSD) | 14.95 (1.76) | 19.95 (1.80) | 24.84 (1.92) | 25.76 (1.90) | |
| A+ | GM (GSD) | 19.86 (1.57) | 15.58 (2.48) | 20.66 (2.25) | 19.07 (2.55) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.33 [0.98–1.81] | 1.66 [1.22–2.25] | 1.72 [1.27–2.34] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 0.78 [0.59–1.04] | 1.04 [0.79–1.37] | 0.96 [0.73–1.27] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 0.59 [0.39–0.89] | 0.63 [0.41–0.95] | 0.56 [0.37–0.84] | ||
| Free | A– | GM (GSD) | 1.85 (1.66) | 2.60 (1.52) | 2.36 (1.94) | 3.29 (1.57) | |
| A+ | GM (GSD) | 2.98 (1.75) | 3.92 (1.95) | 3.82 (1.95) | 4.77 (1.87) | ||
| A– | FC [95%CI] | 1.00 [ref.] | 1.41 [1.09–1.82] | 1.28 [0.99–1.65] | 1.78 [1.38–2.30] | ||
| A+ | FC [95%CI] | 1.00 [ref.] | 1.32 [1.03–1.69] | 1.28 [1.00–1.64] | 1.60 [1.25–2.05] | ||
| A+/A– | FC [95%CI] | 1.00 [ref.] | 0.94 [0.65–1.34] | 1.00 [0.70–1.43] | 0.90 [0.63–1.28] |
The biMSkL was quantified by a speed-accuracy trade-off before (T1) and immediately after (T2) training on days 1 and 2 (T3 and T4). At T1, the geometric mean (GM) and geometric standard deviation (GSD) are given. The fold change (FC, mean and [95 % CI]) of SAT in HI and stroke subjects at T2, T3 and T4 relative to their baseline (T1) in the active (A–) and the active-assisted (A+) subgroups, and the comparison between them (A+/A–), were calculated with a generalized linear mixed model for each speed. a.u.: Arbitrary units.
Figure 3.
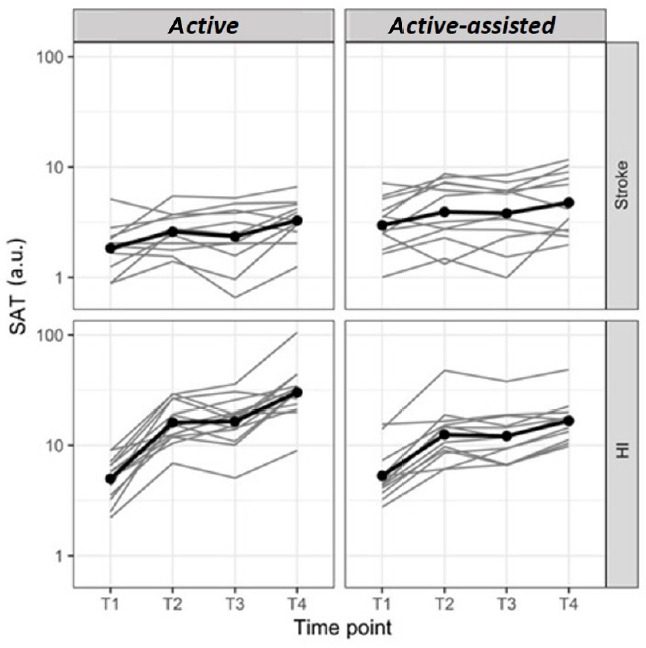
Evolution of SAT in free speed TESTs on D1 and D2.
The bimanual SAT was expressed in arbitrary units (a.u.) and displayed on a logarithmic scale at four time points: on day 1 before (T1, baseline) and after training (T2), on day 2 before (T3) and after training (T4), in stroke subjects and healthy individuals (HI) in both the active and active-assisted subgroups. Thin grey lines: individuals, thick black lines: mean of the groups. Both the HI and chronic stroke subjects improved on LIFT-THE-TRAY on D1, retained overall the improvements overnight, and kept improving on D2, showing learning of the bimanual cooperative task. SAT: Speed/Accuracy trade-off.
In HI, between T1 and T2 (i.e., on D1), the SAT improved with an FC of 3 and 2 in the active and active-assisted subgroups, respectively. In stroke subjects, the FC was 1.4 and 1.3, respectively. Overall, there was no offline (overnight) improvement from T2 to T3, and both the HI and stroke subjects kept improving on D2 from T3 to T4 (Table 2 and Figure 3).
Generalization
In HI, for both active and active-assisted subgroups, we observed generalization at all three imposed speeds (4, 8, and 12 cm/s) at the three time points (i.e., T2, T3 & T4) compared to T1 (Table 2 and Figure 4). In stroke subjects, for the imposed speed of 8 cm/s, we observed a generalization in the active subgroup with an FC of 1.40 from T1 to T2, and with an FC of 1.72 from T1 to T4. For the imposed speed of 12 cm/s, generalization was observed in the active subgroup only from T1 to T4, with an FC of 1.72. No generalization was observed at the imposed speed of 4 cm/s. In the active-assisted subgroup, generalization was detected exclusively for 8 cm/s from T1 to T3, with an FC of 1.39.
Figure 4.
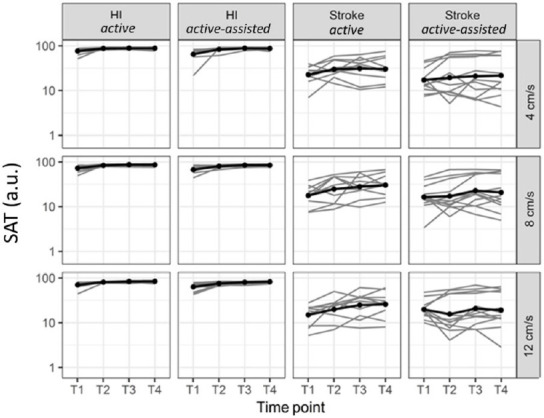
Generalization: SAT evolution from T1 to T4 (imposed speeds: 4, 8, and 12 cm/s).
The bimanual SAT was expressed in arbitrary units (a.u.) and displayed on a logarithmic scale at four time points: on day 1 before (T1, baseline) and after training (T2), on day 2 before (T3) and after training (T4), in stroke subjects and healthy individuals (HI) in both the active and active-assisted subgroups. Thin lines: individuals, thick lines: mean of the groups. In healthy individuals (HI), for both active and active-assisted subgroups, generalization was found at all three imposed speeds and time points. SAT: Speed/Accuracy trade-off.
B&B results
In stroke subjects, the number of transferred blocks in unimanual B&B indicated no generalization to this unimanual task from T1 to T4 in the paretic or non-paretic hand in the active-assisted or active subgroups (Additional Figure 2 (1.4MB, tif) ).
Effect of the active-assisted mode on bimanual motor skill learning
In stroke subjects, the active-assisted mode did not improve bimanual motor skill learning (biMSkL) compared to the active mode (FC [95% CI]: 0.90 [0.63–1.28] at T4). In HI, the active-assisted mode was even found to halve biMSkL compared to the active mode (0.52 [0.39–0.70] at T4). Furthermore, the active-assisted mode did not improve generalization at imposed speeds compared to the active mode in HI nor stroke subjects (Table 2). Finally, the active-assisted mode did not result in better generalization in B&B when compared with the active mode in the paretic hand (–0.02 [–15.0–15.0]) or in non-paretic hand (0.4 [–14.5–15.4]) Additional Figure 2 (1.4MB, tif) and Table 1).
Discussion
In this study, both HI and chronic hemiparetic stroke subjects learned and retained a new bimanual cooperative skill with the bimanual version of the REAplan® rehabilitation robot. HI, generalized the bimanual skill learned under an SAT approach to the exercises with imposed speeds, in both the active and active-assisted subgroups. Whereas the stroke subjects in the active subgroup generalized the bimanual skill to the two fastest imposed speeds (8 and 12 cm/s), there was no generalization at the slowest speed (4 cm/s), nor for the stroke subjects in the active-assisted subgroup at any imposed speed.
In contradiction with our hypothesis, the pre-defined, stepwise decrease of robotic assistance in the active-assisted mode did not improve bimanual cooperative skill learning, retention, or generalization in HI or stroke subjects compared to the active mode. In HI, bimanual cooperative motor learning even deteriorated in the active-assisted subgroup compared to the active subgroup. Chemuturi and colleagues (Chemuturi et al., 2013) showed that during a unimanual reaching task, when the active-assisted mode of a robotic device was adjusted to the individual’s need (i.e., “assistance-as-needed”), it improved motor performance in HI. Accordingly, one possible explanation for the lack of effect of active-assisted mode in our study might be that the robotic assistance was applied as a fixed, stepwise, decrease rather than assistance-as-needed. Theoretically, because the robotic assistance decreased at each training block, the HI should have progressively relied less on it. However, the active-assisted mode might have induced slacking, i.e., naturalistic trends to reduce efforts when the error is small and thus to rely “too heavily” on robotic assistance (Kahn et al., 2006a; Reinkensmeyer et al., 2012) Another explanation could be that the active-assisted mode might improve biMSkL in HI only when the task is very complex (Li et al., 2013). Our bimanual task, LIFT-THE-TRAY, might have been too easy for HI (e.g., with a potential ceiling effect). Finally, although review papers have demonstrated that robot-assisted therapies are beneficial for UL neurorehabilitation after stroke (Kwakkel et al., 2008; Gassert and Dietz, 2018), the effect of the active-assisted mode on motor skill learning in HI requires more studies to compare this model with other robotic modes.
In stroke subjects, bimanual priming rehabilitation with active-passive bimanual movement therapy (APBT) (Stinear and Byblow, 2004) and bimanual motor training with Bilateral Upper Limb Trainer (Sampson et al., 2012) result in improvements of UL performances and evidenced by the improvements in UL Fugl Meyer Assessment (FMA-UL). Given that “conventional” bilateral arm training approaches have shown promising results in neurorehabilitation (Cauraugh and Kim, 2002; McCombe Waller et al., 2008; Lin et al., 2010), several robotic devices such as the Bi-Manu-track (Hesse et al., 2003a), mirror-image motion enabler (Johnson et al., 2000, 2006; Lum et al., 2004), and the bimanual wearable robotic device (Herrnstadt et al., 2015) were designed to offer bilateral training. E.g., three weeks of 15-minute additional training with the active-assisted mode of the Bi-Manu-Track reduced spasticity in 8 out of 12 stroke subjects (Hesse et al., 2003b). However, the improvements in motor control scores (impairment) did not translate into functional task improvements (activity limitation). Using the mirror-image motion enabler, the effect of bimanual robot-assisted training with conventional training in 27 chronic stroke subjects were compared (Lum et al., 2002). Compared to conventional therapy, twenty-four sessions of bilateral training with the mirror-image motion enabler resulted in larger improvements in the UL Fugl Meyer Assessment and in the reaching magnitude. Using a Haptic Master Robot, Trlep et al. (2011) trained four hemiparetic chronic stroke subjects on a bimanual tracking task requiring coordination between the hands, which improved bimanual motor performance as well as force production in the paretic UL. On the other hand, motor training, both with and without robotics, resulted in the same improvements of UL function in stroke subjects quantified with the Arm Motor Ability Test and UL Fugl Meyer Assessment (McCabe et al., 2015). Along the same line, in chronic stroke subjects, twenty-four sessions of reaching training with either active or active-assisted mode resulted in similar improvements in UL’ velocity and range of motions (Kahn et al., 2006b), raising the question of whether training with robotic devices results in greater improvements of UL function compared to non-robotic interventions. In line with this concern, review papers have reported no effect (Norouzi-Gheidari et al., 2012; Rodgers et al., 2019) or a small effect (Kwakkel et al., 2008; Ferreira et al., 2018) of robot-assisted therapy on motor control recovery, possibly because of defective methods or insufficient length of treatment (Ferreira et al., 2018). Therefore, the potentially superior effect of training with robotic devices compared to “conventional” neurorehabilitation is still controversial and requires further studies.
In our study, the active-assisted mode, applied in an imposed, stepwise, decreasing manner did not improve biMSkL in chronic hemiparetic stroke subjects compared to the active mode. Krishnan and colleagues demonstrated that twelve sessions of gait training with minimal robotic assistance improved the speed of walking as well as the lower extremity FMA scores in a chronic stroke subject more significantly compared to a full robotic assistance condition (Krishnan et al., 2013). Training involving the active participation of stroke subjects may thus be more beneficial in reducing functional impairments (Krishnan et al., 2013). In line with this study, it has been suggested that in the context of motor recovery, tasks need to be minimally assisted (Emken et al., 2007; Casadio et al., 2009), and “assistance as needed” might be beneficial for stroke subjects (Casadio et al., 2009), in order to avoid slacking (Kahn et al., 2006a; Reinkensmeyer et al., 2012). In contrast with “assistance as needed,” in our study, the level of robotic assistance in the active-assisted mode decreased steadily block after block in an imposed manner, irrespective of the individual’s need, which failed to show effectiveness. Hence, the optimum strategy for using robotic active-assisted modes in stroke subjects might be “assistance as needed” rather than imposed levels of (stepwise decreasing) assistance.
Finally, despite the retention of the bimanual cooperative skill, the 2-day training with the active or active-assisted modes did not generalize to improved unimanual hand dexterity in chronic stroke subjects, as demonstrated by the stability of the B&B scores. In a previous study, we demonstrated that 30 minutes of training with another complex bimanual cooperative task resulted in significant improvements of hand dexterity quantified by the B&B scores (Doost et al., 2019). Several elements might explain this difference. First, the setups were quite different (REAplan® robotic device versus a custom-made system with two computer mice). Second, the tasks were also different, with cyclical, out-of-phase, rotating cooperative movements in the LIFT-THE-TRAY task versus very asymmetrical cooperative movements in the bimanual CIRCUIT task involving the navigation of a (bimanually controlled) cursor within a complex circuit displayed on the screen (Doost et al., 2019). It is also possible that more extended training with LIFT-THE-TRAY is necessary to improve hand dexterity in stroke subjects.
In conclusion, both HI and chronic hemiparetic stroke subjects learned and retained a complex bimanual cooperative motor task implemented in the bimanual version of the REAplan® neurorehabilitation robot. The generalization to different imposed speeds occurred in HI training with either the active or the active-assisted mode. However, in stroke subjects, generalization occurred exclusively -and inconstantly- under the active mode. Finally, the active-assisted mode did not improve bimanual cooperative motor skill learning in stroke subjects nor HI compared to the active mode. Further studies with longer training sessions, with long term retention testing, and with the robotic assistance applied “as needed” customized to the stroke subject’s need and their level of recovery rather than a fixed, stepwise schedule are necessary to investigate whether the active-assisted mode applied “as needed” can improve biMSkL more than the active mode in HI and stroke subjects.
Additional files:
Additional file 1 (243.1KB, pdf) : Model consent form (French).
Additional file 2 (223.6KB, pdf) : Ethical approval documentation (French).
Additional file 3 (153.5KB, pdf) : Stroke subjects screening.
Stroke subjects screening
Additional file 4 (149.5KB, pdf) : Supplementary information about the bimanual motor skill learning task, LIFT-THE-TRAY.
Supplementary information about the bimanual motor skill learning task, LIFT-THE-TRAY
Additional Figure 1 (2.1MB, tif) : CONSORT flowchart.
CONSORT flowchart.
The Stroke database of the CHU UCL Namur (Mont-Godinne) was used for screening stroke subjects that correspond to the inclusion and exclusion criteria. Healthy individuals (HI) were recruited from the hospital staff and students.
Additional Figure 2 (1.4MB, tif) : Box and Blocks Test (B&B).
Box and Blocks Test (B&B).
The number of transferred blocks at T1 and T4 for each stroke subjects in both the active and active-assisted conditions. Evolution in both conditions were similar for the non-paretic hand (NPH), showing a non-significant trend for improvement. There was no change for the paretic hand (PH), patients in the active-assisted (ASSIST) subgroup had higher T1 and T4 values. Thin grey lines: individuals, thick black lines: mean of the groups. T1: Baseline.T4: after training on day 2.
Additional Table 1: Evolution of The Box & Blocks test scores.
Additional Table 1.
Evolution of the Box & Blocks test scores
| Hand | Assist. | T1 | T4 | Progression (n) | 95% CI |
|---|---|---|---|---|---|
| A- | 56.8 ±10.9 | 61.0 ±10.9 | 4.2 | (-6.6-15.0) | |
| NPH | A+ | 57.1 ±13.8 | 61.8 ±12.7 | 4.6 | (-5.7-15.0) |
| [A+] - [A-] | 0.4 | (-14.5-15.4) | |||
| A- | 6.4 ± 13.7 | 6.3 ± 13.9 | -0.1 | (-10.9-10.8) | |
| PH | A+ | 13.9 ±19.5 | 13.8 ±20.3 | -0.1 | (-10.4-10.3) |
| [A+] - [A-] | 0.0 | (-15.0-15.0) |
Increase in the number of transferred blocks (Progression, n) in the active (A-) and active-assisted (A+) conditions in the non-paretic hand (NPH) and paretic hand (PH). CI: 95% CI. In stroke patients, we counted the number of transferred blocks on the Box & Blocks test for both paretic and non-paretic hand at T1 and T4. We then calculated the change from T1 to T4 separately for each hand with a 95% confidence interval (CI). T1: Baseline.T4: after training on day 2.
Acknowledgments:
We are grateful to the Fondation Baron Albert Frère and the Fondation Louvain that enabled the implementation and development of the bimanual version of the neurorehabilitation robot REAplan® (AXINESIS, Wavre, Belgium) in the Stroke Unit of the CHU UCL Namur (site Mont-Godinne). We thank Pr Renaud Ronsse (UCLouvain, Institute of Mechanics, Materials and Civil Engineering (IMMC)/Louvain Bionics) for fruitful discussions on bimanual robotic tasks and Adrien Denis (UCLouvain, Louvain Bionics) for software development on the LIFT-THE-TRAY serious game. We thank Matt Pilgrim for his help in the English editing of the manuscript.
Footnotes
C-Editors; Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflict of interest.
Financial support: The work of YV was supported by the following grants: Fonds de la Recherche Scientifique – FNRS 1.R.506.16, 1.R.506.18 & 1.R.506.20, Fonds de la Recherche Scientifique Médicale (FRSM) 3.4.525.08.F, Fonds Spécial de Recherche (FSR) from the UCLouvain, Fondation Van Goethem-Brichant, and Fondation Mont-Godinne. The work of MYD was supported by the following grants: FRNS-FRIA n° F3/5/5-MCF/ROI/BC-19727 and F3/5/5-MCF/XH/FC-17514, and Fondation Mont-Godinne 2018. The work of AR was supported by grants from the Fondation Mont-Godinne 2015-2016, Fonds Spécial de Recherche (FSR) of the UCLouvain 2016-2018, and Fondation Roi Baudouin/Fonds Amélie 2018-2019.
Institutional review board statement: The study complied with the Declaration of Helsinki, and procedures were approved on July 14, 2016 (Internal number: 54/2010, EudraCT number: NUB B039201317382) by the local ethical committee (Comité d’éthique médicale, CHU UCL Namur, Mont-Godinne, Yvoir, Belgium).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the CONsolidated Standards Of Reporting Trials (CONSORT) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of the CHU UCL Namur, Mont-Godinne (UCLouvain) in Belgium.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Behavioral data and Matlab® codes may be shared upon reasonable request to the authors.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The work of YV was supported by the following grants: Fonds de la Recherche Scientifique – FNRS 1.R.506.16, 1.R.506.18 & 1.R.506.20, Fonds de la Recherche Scientifique Médicale (FRSM) 3.4.525.08.F, Fonds Spécial de Recherche (FSR) from the UCLouvain, Fondation Van Goethem-Brichant, and Fondation Mont-Godinne. The work of MYD was supported by the following grants: FRNS-FRIA n° F3/5/5-MCF/ROI/BC-19727 and F3/5/5-MCF/XH/FC-17514, and Fondation Mont-Godinne 2018. The work of AR was supported by grants from the Fondation Mont-Godinne 2015-2016, Fonds Spécial de Recherche (FSR) of the UCLouvain 2016-2018, and Fondation Roi Baudouin/Fonds Amélie 2018-2019.
References
- 1.Abdollahi F, Corrigan M, Lazzaro EDC, Kenyon RV, Patton JL. Error-augmented bimanual therapy for stroke survivors. NeuroRehabilitation. 2018;43:51–61. doi: 10.3233/NRE-182413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basteris A, Nijenhuis SM, Stienen AH, Buurke JH, Prange GB, Amirabdollahian F. Training modalities in robot-mediated upper limb rehabilitation in stroke: a framework for classification based on a systematic review. J Neuroeng Rehabil. 2014;11:111. doi: 10.1186/1743-0003-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt J, Godecke E, Johnson L, Langhorne P. Early rehabilitation after stroke. Curr Opin Neurol. 2017;30:48–54. doi: 10.1097/WCO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 4.Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restor Neurol Neurosci. 2013;31:707–722. doi: 10.3233/RNN-130332. [DOI] [PubMed] [Google Scholar]
- 5.Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Casadio M, Giannoni P, Morasso P, Sanguineti V, Squeri V, Vergaro E. 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Minneapolis, MN, USA: IEEE; 2009. Training stroke patients with continuous tracking movements: Evaluating the improvement of voluntary control; pp. 5961–5964. [DOI] [PubMed] [Google Scholar]
- 7.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: Electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 8.Chemuturi R, Amirabdollahian F, Dautenhahn K. Adaptive training algorithm for robot-assisted upper-arm rehabilitation, applicable to individualised and therapeutic human-robot interaction. J Neuroeng Rehabil. 2013;10:102. doi: 10.1186/1743-0003-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo R, Sterpi I, Mazzone A, Delconte C, Minuco G, Pisano F. Measuring changes of movement dynamics during robot-aided neurorehabilitation of stroke patients. IEEE Trans Neural Syst Rehabil Eng. 2010;18:75–85. doi: 10.1109/TNSRE.2009.2028831. [DOI] [PubMed] [Google Scholar]
- 10.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehem S, Gilliaux M, Lejeune T, Detrembleur C, Galinski D, Sapin J, Vanderwegen M, Stoquart G. Assessment of upper limb spasticity in stroke patients using the robotic device REAplan. J Rehabil Med. 2017;49:565–571. doi: 10.2340/16501977-2248. [DOI] [PubMed] [Google Scholar]
- 12.Dehem S, Gilliaux M, Stoquart G, Detrembleur C, Jacquemin G, Palumbo S, Frederick A, Lejeune T. Effectiveness of upper-limb robotic-assisted therapy in the early rehabilitation phase after stroke: A single-blind, randomised, controlled trial. Ann Phys Rehabil Med. 2019;62:313–320. doi: 10.1016/j.rehab.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers J, Bravo G, Hébert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75:751–755. [PubMed] [Google Scholar]
- 14.Desrosiers J, Hébert R, Dutil E, Bravo G, Kane R, Sinacore J. Development and reliability of an upper extremity function test for the elderly: the TEMPA. Can J Occup Ther. 1993;60:9–16. [Google Scholar]
- 15.Doost MY, Orban de Xivry JJ, Herman B, Vanthournhout L, Riga A, Bihin B, Jamart J, Laloux P, Raymackers JM, Vandermeeren Y. Learning a bimanual cooperative skill in chronic stroke under noninvasive brain stimulation: a randomized controlled trial. Neurorehabil Neural Repair. 2019;33:486–498. doi: 10.1177/1545968319847963. [DOI] [PubMed] [Google Scholar]
- 16.Duret C, Grosmaire AG, Krebs HI. Robot-assisted therapy in upper extremity hemiparesis: overview of an evidence-based approach. Front Neurol. 2019;10:412. doi: 10.3389/fneur.2019.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duret C, Hutin E. Effects of prolonged robot-assisted training on upper limb motor recovery in subacute stroke. NeuroRehabilitation. 2013;33:41–48. doi: 10.3233/NRE-130926. [DOI] [PubMed] [Google Scholar]
- 18.Emken JL, Benitez R, Sideris A, Bobrow JE, Reinkensmeyer DJ. Motor adaptation as a greedy optimization of error and effort. J Neurophysiol. 2007;97:3997–4006. doi: 10.1152/jn.01095.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira FMRM, Chaves MEA, Oliveira VC, Van Petten AMVN, Vimieiro CBS. Effectiveness of robot therapy on body function and structure in people with limited upper limb function: A systematic review and meta-analysis. PLoS One 13. 2018:e0200330. doi: 10.1371/journal.pone.0200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil. 2018;15:46. doi: 10.1186/s12984-018-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliaux M, Lejeune T, Detrembleur C, Sapin J, Dehez B, Selves C, Stoquart G. Using the robotic device REAplan as a valid, reliable, and sensitive tool to quantify upper limb impairments in stroke patients. J Rehabil Med. 2014;46:117–125. doi: 10.2340/16501977-1245. [DOI] [PubMed] [Google Scholar]
- 22.Grosmaire AG, Duret C. Does assist-as-needed upper limb robotic therapy promote participation in repetitive activity-based motor training in sub-acute stroke patients with severe paresis. NeuroRehabilitation. 2017;41:31–39. doi: 10.3233/NRE-171454. [DOI] [PubMed] [Google Scholar]
- 23.Han C, Wang Q, Meng P, Qi M. Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: a randomized controlled trial. Clin Rehabil. 2013;27:75–81. doi: 10.1177/0269215512447223. [DOI] [PubMed] [Google Scholar]
- 24.Herrnstadt G, Alavi N, Randhawa BK, Boyd LA, Menon C. Bimanual elbow robotic orthoses: Preliminary investigations on an impairment force-feedback rehabilitation method. Front Hum Neurosci. 2015;9:169. doi: 10.3389/fnhum.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003a;16:705–710. doi: 10.1097/01.wco.0000102630.16692.38. [DOI] [PubMed] [Google Scholar]
- 26.Hesse S, Schulte-Tigges G, Konrad M, Bardeleben A, Werner C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch Phys Med Rehabil. 2003b;84:915–920. doi: 10.1016/s0003-9993(02)04954-7. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MJ, Van Der Loos HFM, Burgar CG, Leifer LJ. Stanford, CA: 2000. Driver’s SEAT: simulation environment for arm therapy. ICORR ’99: International Conference on Rehabilitation Robotics. [Google Scholar]
- 28.Johnson MJ, Wisneski KJ, Anderson J, Nathan D, Smith RO. The First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, 2006. BioRob 2006. Pisa, Italy: IEEE; 2006. Development of ADLER: The Activities of Daily Living Exercise Robot; pp. 881–886. [Google Scholar]
- 29.Kahn LE, Lum PS, Rymer WZ, Reinkensmeyer DJ. Robot-assisted movement training for the stroke-impaired arm: Does it matter what the robot does. J Rehabil Res Dev. 2006a;43:619–629. doi: 10.1682/jrrd.2005.03.0056. [DOI] [PubMed] [Google Scholar]
- 30.Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study. J Neuroeng Rehabil. 2006b;3:12. doi: 10.1186/1743-0003-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantak S, Jax S, Wittenberg G. Bimanual coordination: A missing piece of arm rehabilitation after stroke. Restor Neurol Neurosci. 2017;35:347–364. doi: 10.3233/RNN-170737. [DOI] [PubMed] [Google Scholar]
- 32.Kantak S, McGrath R, Zahedi N. Goal conceptualization and symmetry of arm movements affect bimanual coordination in individuals after stroke. Neurosci Lett. 2016;626:86–93. doi: 10.1016/j.neulet.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 33.King M, Hijmans J, Sampson M, Satherley J, Hale L. Proceedings of the 4th International Convention on Rehabilitation Engineering & Assistive Technology. 2010. Bilateral movement training with computer games for stroke rehabilitation. [Google Scholar]
- 34.Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handb Clin Neurol. 2013;110:93–103. doi: 10.1016/B978-0-444-52901-5.00008-3. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan C, Kotsapouikis D, Dhaher YY, Rymer WZ. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Arch Phys Med Rehabil. 2013;94:1202–1206. doi: 10.1016/j.apmr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22:111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Inoue Y, Liu T, Sun L. Validation of bimanual-coordinated training supported by a new upper-limb rehabilitation robot: a near-infrared spectroscopy study. Disabil Rehabil Assist Technol. 2013;8:38–48. doi: 10.3109/17483107.2012.671439. [DOI] [PubMed] [Google Scholar]
- 38.Lin K, Chen Y, Chen C, Wu C, Chang Y. The effects of bilateral arm training on motor control and functional performance in chronic stroke: a randomized controlled study. Neurorehabil Neural Repair. 2010;24:42–51. doi: 10.1177/1545968309345268. [DOI] [PubMed] [Google Scholar]
- 39.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT, Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowrey CR, Jackson CPT, Bagg SD, Dukelow SP, Scott SH. A Novel Robotic Task for Assessing Impairments in Bimanual Coordination Post-Stroke. Int J Phys Med Rehabil. 2014;s3:1–10. [Google Scholar]
- 41.Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post-stroke hemiparesis. IEEE Trans Neural Syst Rehabil Eng. 2004;12:186–194. doi: 10.1109/TNSRE.2004.827225. [DOI] [PubMed] [Google Scholar]
- 42.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 43.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 44.McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:981–990. doi: 10.1016/j.apmr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 45.McCombe Waller S, Liu W, Whitall J. Temporal and spatial control following bilateral versus unilateral training. Hum Mov Sci. 2008;27:749–758. doi: 10.1016/j.humov.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robotassisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. In: Mehrholz J, editor. Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd; 2012. p. CD006876. [DOI] [PubMed] [Google Scholar]
- 47.Meng G, Meng X, Tan Y, Yu J, Jin A, Zhao Y, Liu X. Short-term efficacy of hand-arm bimanual intensive training on upper arm function in acute stroke patients: a randomized controlled trial. Front Neurol. 2017;8:726. doi: 10.3389/fneur.2017.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehabil Res Dev. 2012;49:479–496. doi: 10.1682/jrrd.2010.10.0210. [DOI] [PubMed] [Google Scholar]
- 49.Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32:1627–1634. doi: 10.1161/01.str.32.7.1627. [DOI] [PubMed] [Google Scholar]
- 50.Raghavan P, Aluru V, Milani S, Thai P, Geller D, Bilaloglu S, Lu Y, Weisz DJ. Coupled bimanual training using a non-powered device for individuals with severe hemiparesis: a pilot study. Int J Phys Med Rehabil. 2017;5:404. doi: 10.4172/2329-9096.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinkensmeyer DJ, Wolbrecht ET, Chan V, Chou C, Cramer SC, Bobrow JE. Comparison of three-dimensional, assist-as-needed robotic arm/hand movement training provided with Pneu-WREX to conventional tabletop therapy after chronic stroke. Am J Phys Med Rehabil. 2012;91(11 Suppl 3):S232–241. doi: 10.1097/PHM.0b013e31826bce79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, Aird L, Alvarado N, Andole S, Cohen DL, Dawson J, Fernandez-Garcia C, Finch T, Ford GA, Francis R, Hogg S, Hughes N, Price CI, Ternent L, Turner DL, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394:51–62. doi: 10.1016/S0140-6736(19)31055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 54.Sampson M, Shau Y, James king M. Bilateral upper limb trainer with virtual reality for post-stroke rehabilitation: case series report. Disabil Rehabil Assist Technol. 2012;7:55–62. doi: 10.3109/17483107.2011.562959. [DOI] [PubMed] [Google Scholar]
- 55.Shirzad N, Van der Loos HFM. Evaluating the user experience of exercising reaching motions with a robot that predicts desired movement difficulty. J Mot Behav. 2016;48:31–46. doi: 10.1080/00222895.2015.1035430. [DOI] [PubMed] [Google Scholar]
- 56.Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning. Neuron. 2011;72:469–476. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned. Change and invariance at the levels of task success and trajectory control. J Neurophysiol. 2012;108:578–594. doi: 10.1152/jn.00856.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21:124–131. doi: 10.1097/00004691-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Trlep M, Mihelj M, Puh U, Munih M. Rehabilitation robot with patient-cooperative control for bimanual training of hemiparetic subjects. Adv Robot. 2011;25:1949–1968. [Google Scholar]
- 60.Trombly CA. Deficits of reaching in subjects with left hemiparesis: a pilot study. Am J Occup Ther. 1992;46:887–897. doi: 10.5014/ajot.46.10.887. [DOI] [PubMed] [Google Scholar]
- 61.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 62.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke. A systematic review and meta-analysis. PLoS One. 2014;9:e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willingham D. A neuropsychological theory of motor skill learning* 1. Psychol Rev. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stroke subjects screening
Supplementary information about the bimanual motor skill learning task, LIFT-THE-TRAY
CONSORT flowchart.
The Stroke database of the CHU UCL Namur (Mont-Godinne) was used for screening stroke subjects that correspond to the inclusion and exclusion criteria. Healthy individuals (HI) were recruited from the hospital staff and students.
Box and Blocks Test (B&B).
The number of transferred blocks at T1 and T4 for each stroke subjects in both the active and active-assisted conditions. Evolution in both conditions were similar for the non-paretic hand (NPH), showing a non-significant trend for improvement. There was no change for the paretic hand (PH), patients in the active-assisted (ASSIST) subgroup had higher T1 and T4 values. Thin grey lines: individuals, thick black lines: mean of the groups. T1: Baseline.T4: after training on day 2.


