
Keywords: leukoaraiosis, dimethylarginine dimethylaminohydrolase 2, gene polymorphism, allele, asymmetric dimethylarginine, nitric oxide, endothelial dysfunction, cerebrovascular diseases, clinical trial
Abstract
Cerebrovascular endothelial dysfunction is involved in the progression of leukoaraiosis. Asymmetric dimethylarginine is a competitive inhibitor of nitric oxide, which is highly expressed in patients with leukoaraiosis. Dimethylarginine dimethylaminohydrolase (DDAH) is a hydrolytic enzyme that is primarily responsible for eliminating asymmetric dimethylarginine, and it plays a role in the pathogenesis of cardiovascular and cerebrovascular diseases. The DDAH2 subtype is expressed in organs rich in induced nitric oxide synthase, including the heart, the placenta, and the cerebral endothelium during cerebral ischemia, in the stress state, or under neurotoxicity. Overexpression of the DDAH2 gene can inhibit asymmetric dimethylarginine-induced peripheral circulating endothelial cell dysfunction. However, it is unknown whether this polymorphism regulates plasma asymmetric dimethylarginine levels in patients with leukoaraiosis. In this double-blind study, we recruited 46 patients with leukoaraiosis and 46 healthy, matched controls. Plasma asymmetric dimethylarginine levels were determined using enzyme-linked immunoassays. Genomic DNA was isolated from whole blood samples, and polymerase chain reaction, SmaI restriction enzyme digestion, restriction fragment length polymorphisms, and agarose electrophoresis were used to detect DDAH2 (-449 G/C) gene polymorphisms. The results revealed that 95.65% of leukoaraiosis patients had recessive genetic models (GG and CG), while 89.13% of healthy control subjects had dominant genetic models (CC and CG). There was a significant difference in the genotype composition ratio between leukoaraiosis patients and healthy controls (P = 0.0002). The frequency of G alleles in the leukoaraiosis patients (71.74%) was significantly higher than in healthy controls, whereas the frequency of C alleles was lower (χ2= 13.9580, P = 0.0002). Furthermore, asymmetric dimethylarginine concentrations in subjects with the GG genotype were significantly higher than in subjects with the CG and CC genotypes (Kruskal–Wallis H = 24.5955, P < 0.0001). In addition, the GG genotype of DDAH2 (-449 G/C) was more common in patients with leukoaraiosis. These findings suggest that the G allele of DDAH2 (-449 G/C) is a risk factor for leukoaraiosis morbidity and is correlated with high levels of asymmetric dimethylarginine. This study was approved by the Institutional Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University of China (approval No. KY2016-177) on July 28, 2016.
Chinese Library Classification No. R446.9; R742; Q343.1+5
Introduction
Leukoaraiosis (LA) is a cerebral small vessel disease that can lead to cognitive dysfunction, subcortical dementia syndrome, gait instability, and even symptomatic cerebral hemorrhage (Fierini et al., 2017), thus seriously reducing patients’ quality of life. It is generally believed that the main pathogenesis of LA is cerebrovascular endothelial dysfunction (Maccarrone et al., 2017), which results in a series of reactions, such as chronic cerebral ischemia, vascular stenosis, atherosclerosis, and cerebral white matter lesions (Hachinski et al., 1987; Kidwell et al., 2001; Pantoni, 2002; Bian et al., 2019).
Nitric oxide is mainly produced by nitric oxide synthase (NOS) in endothelial cells, and it acts as an important endogenous vasodilator to regulate cerebrovascular systolic and diastolic function (Gunawardena et al., 2019). Asymmetric dimethylarginine (ADMA) is a metabolic byproduct that was first detected in human plasma in the 1970s (Liu et al., 2014) and is essential for protein methylation. ADMA inhibits the production of nitric oxide by competing with NOS (Dovinová et al., 2018), leading to a series of changes in endothelial function and atherosclerosis in patients with hyperlipidemia. Our previous studies revealed that ADMA levels in patients with LA are significantly higher than those in healthy control subjects (Guan et al., 2017), and that high concentrations of ADMA are associated with cognitive dysfunction in LA (Gao et al., 2015).
Why might patients with LA have higher plasma ADMA levels? Some studies have reported that ADMA is mainly (95%) eliminated through degradation by dimethylarginine dimethylaminohydrolase (DDAH), which is a critical factor in regulating plasma ADMA concentration (Gad et al., 2011; Anavi and Tirosh, 2020). The DDAH2 subtype is expressed in organs rich in induced NOS, including the heart, the placenta, and the cerebral endothelium during cerebral ischemia, in the stress state, or under neurotoxicity (Anavi and Tirosh, 2020). Overexpression of the DDAH2 gene can inhibit ADMA-induced peripheral circulating endothelial cell dysfunction (Liu et al., 2012), suggesting that DDAH2 may be involved in the pathogenesis of cardiovascular and cerebrovascular diseases. The core promoter elements of DDAH2 can influence mRNA transcription and protein translation. The -449 G/C (rs805305) polymorphism is located in the core promoter region of DDAH2 (Figure 1) and affects plasma ADMA levels in patients with heart failure and nephropathy (Rector et al., 1996; Dayal and Lentz, 2005; Stühlinger and Stanger, 2005). However, it is unknown whether this polymorphism regulates plasma ADMA levels in patients with LA. Therefore, this study was conducted to explore differences in the -449 G/C polymorphism of DDAH2 in patients with LA, and to investigate the relationship between this polymorphism and ADMA levels.
Figure 1.
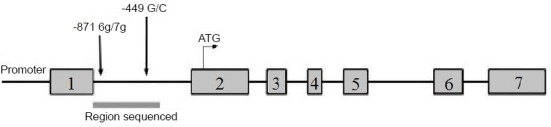
The -449 G/C polymorphism is located in the core promoter region of the DDAH2 gene.
DDAH2: Dimethylarginine dimethylaminohydrolase 2.
Participants and Methods
Participants
Patients were recruited from the Department of Geriatrics and Neurology, whereas the healthy control participants were recruited from the Physical Examination Center of the 2nd Affiliated Hospital of Harbin Medical University. All participants were recruited from January to July 2016, and came from Heilongjiang Province, Jilin Province, Liaoning Province, or the northeastern Inner Mongolia Autonomous Region. All of these regions are in northeastern China and have long winters that last for approximately 5 months. Participants were eligible for inclusion in the double-blind, case-control study if they met the following criteria: a) at least 40 years of age, without a clear history of heart or brain disease; b) underwent brain magnetic resonance imaging (MRI) examination; and c) were conscious and consented to participate in the study. Participants were excluded from the study if they: a) had acute cerebral or myocardial infarction, hemorrhage, transient ischemic attack, or other neurological disorders (epilepsy, trauma, intracranial or extracranial malignancy, schizophrenia, normal pressure hydrocephalus, depressive and anxiety disorder, Parkinson’s disease, or Alzheimer’s disease); b) had high signal abnormalities on MRI scans that could be attributed to long-term radiation exposure, carbon monoxide poisoning, or a history of immune system disease (e.g., multiple sclerosis, vasculitis, or leukodystrophy); c) had severe heart, lung, liver, kidney, or thyroid dysfunction; d) had other malignancies or acute inflammatory disease; e) refused to provide the requested information; or f) had blood relationships with each other, were taking hormones, angiotensin-converting enzyme inhibitors, or statins, or lived in provinces other than the aforementioned regions, which might affect ADMA or DDAH2 levels. All participants signed their informed consent. This study was approved by the Institutional Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University of China (approval No. KY2016-177) on July 28, 2016 (Additional file 1 (106KB, pdf) ).
A double-blind method was adopted in this experiment. All participants were unaware of their grouping. The MRI diagnostic physicians were only responsible for analyzing the MRI scans and were unaware of the grouping, and the testers of plasma ADMA and DDAH2 (-449 G/C) alleles were also unaware of the grouping.
The diagnostic criteria for LA were that the lateral ventricle or centrum semiovale white matter contained diffuse high signals on T2-weighted or fluid-attenuated inversion recovery imaging (T2/FLAIR), observable by MRI (Hachinski et al., 1987). Patients who had normal imaging findings were enrolled as controls. To match the case and control groups, if the numbers of cases were different between the two groups, then cases were excluded using a random number table.
Clinical parameters and biochemical measurements
Our descriptive study collected clinical and demographic data, including age, sex, and history of smoking, hypertension, and diabetes. Blood samples (2 mL) were drawn from all participants’ elbow veins after overnight fasting for at least 8 hours. Serum levels of fasting blood glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, uric acid, and creatinine were determined using an automatic biochemistry analyzer (HCP-7600; Hitachi, Tokyo, Japan) in a clinical laboratory.
Plasma ADMA measurements
The blood samples were centrifuged at 1500 × g for 20 minutes using a 5810R centrifuge (MLX-206; Crystal Technology & Industries, Inc., Dallas, TX, USA). The serum was stored at –80°C for the enzyme-linked immunoassay. According to the kit instructions, serum concentrations of ADMA were determined using enzyme-linked immunoassays (Human ADMA Enzyme-linked Immunoassay Kit; Sinobest Biotechnology Co., Shanghai, China) (Liu et al., 2012). A monoclonal antibody specific to ADMA was precoated onto a microplate. A competitive inhibition reaction was initiated between biotin-labeled ADMA and unlabeled ADMA (standards or samples) with the pre-coated antibody specific to ADMA. After incubation, the unbound conjugate was washed off. Avidin conjugated to horseradish peroxidase was then carefully added to each microplate and incubated. The amount of bound horseradish peroxidase conjugate was inversely proportional to the concentration of ADMA in the sample. After adding the substrate solution, the optical density (OD) value was measured at a single wavelength of 450 nm using an iMark microplate absorbance reader (Bio-Rad, Hercules, CA, USA). The intensity of the resulting color was inversely proportional to the ADMA concentration in the sample.
DNA isolation, genetic variant selection, and genotyping
Genomic DNA was isolated from whole blood samples using the QIAamp DNA Blood Mini Kit (GenStar Biosolutions Co., Ltd., Beijing, China) in accordance with the manufacturer’s instructions. DNA was extracted from 200 µL of whole blood using the spin columns provided. The isolated DNA was stored at –20°C and the optical density values at 260 nm (OD260 nm) and 280 nm (OD280 nm) were determined. The concentration and purity of the extracted DNA were calculated according to standard formulas, and the OD260/OD280 was between 1.7 and 1.9, indicating that the DNA purity of the extracted samples was high. The primers for the DDAH2 -449C/G (rs805305) polymorphism were: forward: 5′-CTA CCC ACC TCT CTC CCC TCT CTC-3′ and reverse: 5′-CAC TCT ACC CAG CAC CCT CAG AG-3′. The polymerase chain reaction (PCR) conditions consisted of one cycle of 5 minutes at 94°C; 35 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C; followed by 10 minutes at 72°C. A GeneAmp PCR 2720 (Applied Biosystems, Foster City, CA, USA) was used. The amplification was performed using a 20 µL reaction volume containing 5 µL of isolated DNA, 1 µL of each upstream and downstream primer, 10 µL of 2× Taq Mix (GenStar Biosolutions Co., Ltd., Beijing, China), and 3 µL of double-distilled H2O. The primer concentration was 10 µM. The amplified fragment was the target band (330 bp). The electrophoresis gel consisted of 0.5 g of heated agarose and 40 mL of 0.5× Tris–borate–ethylenediaminetetraacetic acid. After the gel cooled to 50°C, 10 µL of ethidium bromide was added to form an electrophoresis mold. A well comb was inserted into the mold and removed after 30 minutes. Next, 10 µL of the PCR product or DNA marker was added to the gel and the electrophoretic reaction was initiated (100 V, 60 mA, 60–80 minutes). Finally, the PCR product was detected using a gel imaging system. Gene Tools software (Gene Tools, LLC, Philomath, OR, USA) was used to identify single nucleotide polymorphisms (SNPs). The position of the nucleotide sequence was based on the reference sequence obtained from the National Center for Biotechnology Information (NCBI) SNP database (http://www.ncbi.nlm.nih.gov/SNP/).
The PCR products were digested with SmaI restriction enzymes for 10–14 hours in a water bath set to 30°C, and the products were detected using agarose electrophoresis. The length of the DDAH2 rs805305 (-449 G/C) fragment obtained from PCR was 330 bp. There were two products from the Sma I enzyme digestion: two bands at 103 and 228 bp for gene locus C, or one band at 330 bp for gene locus G. Thus, the CC genotype had two bands, at 103 and 228 bp; the GG genotype had only one band, at 330 bp; and the CG genotype had three bands, at 103, 228, and 330 bp (Xuan et al., 2016).
Statistical analysis
According to the formula for calculating the sample size of simple random samples, N = Z2 [P (1 – P)] / δ2, with confidence of 95% and error of not more than 4%, the estimated sample size should be 625. Considering losses from follow-up and halfway withdrawal from the study, it is appropriate to increase the sample size by 20%, so the ideal final sample size is approximately 750. Analyses were performed using SPSS software version 19.0 (IBM, Armonk, NY, USA). All data were double checked. Student’s t-tests and one-way analysis of variance were performed for values with a normal distribution, which were expressed as the mean ± standard deviation (SD). Values with a non-normal distribution were expressed as the median and quartile spacing (P25, P75) and compared using the rank-sum test. The distributions of the categorical variables were expressed as frequencies and percentages, and comparisons were performed using the least significant difference test with the chi-squared test. The association between ADMA and the risk of LA was estimated by calculating the odds ratio and its 95% confidence interval. Multivariate unconditional logistic regression was used to estimate odds ratios and 95% confidence intervals after adjusting for age; sex; history of hypertension, diabetes, and smoking; fasting blood glucose; total cholesterol; fasting blood glucose; high-density lipoprotein cholesterol; low-density lipoprotein cholesterol; uric acid; and creatinine. The Hardy–Weinberg equilibrium was calculated using a Q test with one degree of freedom (Xuan et al., 2016). We examined the differences in ADMA concentrations between the three genotypes (GG, CC, and CG) using the Kruskal–Wallis H test. If there was statistical significance, the Nemenyi test was used for comparisons between any two genotypes. All reported P values were two-sided, and P < 0.05 was considered statistically significant.
Results
Clinical characteristics
The selection and inclusion of patients and controls are shown in Figure 2. The characteristics of the 46 LA patients and 46 control participants are summarized in Table 1. Although there were no differences in sex between the two groups, the LA patients were older (P < 0.0001) and had a higher prevalence of hypertension (P = 0.0101) than the controls. There were no differences between the two groups in their smoking or diabetes history (P > 0.05). Thus, the basic profiles between the two groups were matched as closely as possible.
Figure 2.
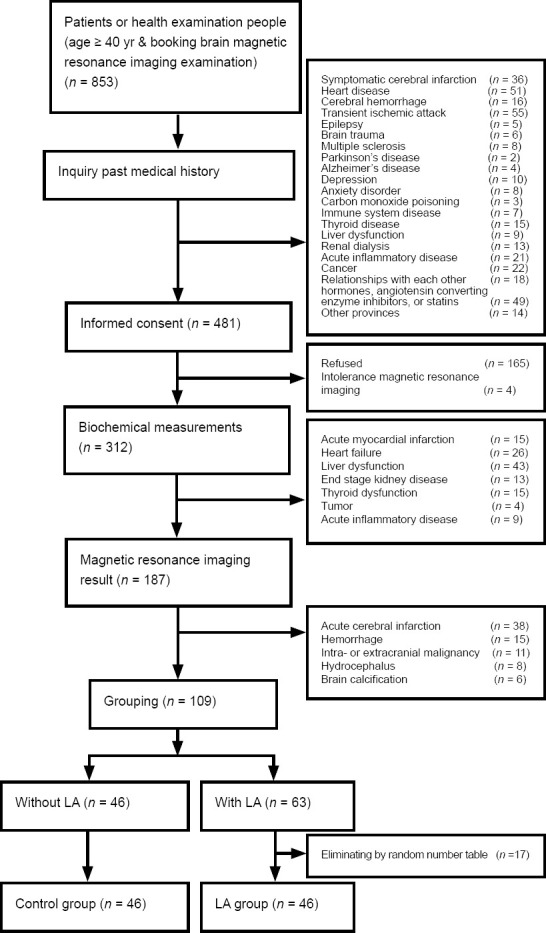
Selection of the LA and control groups.
LA: Leukoaraiosis.
Table 1.
General characteristics of the control and leukoaraiosis groups
| Characteristics | Control (n = 46) | Leukoaraiosis (n = 46) | P-value |
|---|---|---|---|
| Gender (male/female)& | 25/21 | 23/23 | 0.6767 |
| Age (yr)* | 58.50±3.85 | 69.00±5.46 | 0.0002 |
| Smoking# | 10 (22) | 14 (30) | 0.3440 |
| Hypertension# | 11 (24) | 23 (50) | 0.0109 |
| Diabetes mellitus# | 11 (24) | 7 (15) | 0.4224 |
&Categorical variables are expressed as the numbers of male and female participants. The P-values of the categorical variables were calculated using the chi-squared test. *Continuous variables are expressed as the mean ± SD. The P-values of the continuous variables were calculated using the unpaired t-test. #Categorical variables are expressed as the number (percentage). The P-values of the categorical variables were calculated using the chi-squared test.
Biochemical parameters
The biochemical parameters for all participants at baseline are summarized in Table 2. LA patients had higher ADMA levels than the control subjects (P < 0.05). There were no differences between the two groups in fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, uric acid, or creatinine.]
Table 2.
Correlations of biochemical parameters in the control and leukoaraiosis groups
| Factor | OR (95% CI) | P-value |
|---|---|---|
| Fasting blood glucose | 0.701 (0.495, 0.992) | 0.0449 |
| Total cholesterol | 0.991 (0.644, 1.526) | 0.9675 |
| High-density lipoprotein cholesterol | 0.490 (0.170, 1.409) | 0.1857 |
| Low-density lipoprotein cholesterol | 0.794 (0.468, 1.348) | 0.3935 |
| Uric acid | 1.003 (0.998, 1.008) | 0.1910 |
| Creatinine | 1.020 (0.995, 1.045) | 0.1190 |
| Asymmetric dimethylarginine | 970.375 (36.538, 25673.1249) | < 0.0001 |
P-values of the discontinuous variables were calculated using univariate logistic regression analysis. CI: Confidence interval; OR: odds ratio.
Next, LA morbidity was used as the dependent variable. The most significant parameters and the regression equation were determined using multivariate logistic stepwise regression analysis. Age and ADMA exhibited statistical significance as independent variables (Table 3).
Table 3.
Correlation of leukoaraiosis morbidity
| Factor | Wald χ2 | OR (95% CI) | P-value |
|---|---|---|---|
| Age (yr) | 4.9577 | 1.136 (1.015, 1.271) | 0.0260 |
| Asymmetric dimethylarginine (μM) | 16.8971 | 970.375 (36.538, 25673.1249) | < 0.0001 |
Discontinuous variables were calculated using multivariable logistic regression analysis. CI: Confidence interval; OR: odds ratio.
Gene polymorphisms of DDAH2
The rs805305 (-449 G/C) SNP of DDAH2 was genotyped in all participants. The genotypes of the LA patients and control subjects are summarized in Table 4. In the LA group, the GG and CG genotypes were the most common, each accounting for 48% of all patients. In contrast, the GG genotype was the least common in the control group, accounting for just 11% of the control participants. The difference in genotype composition ratio between the two groups was statistically significant (P = 0.0002).
Table 4.
Genotype frequencies [n (%)] for the rs805305 (-449 G/C) polymorphism of DDAH2 in control and leukoaraiosis subjects
| Group | CC | CG | GG | χ2 | P-value |
|---|---|---|---|---|---|
| Control (n = 46) | 10 (22) | 31 (68) | 5 (11) | 17.5653 | 0.0002 |
| Leukoaraiosis (n = 46) | 2 (4) | 22 (48) | 22 (48) |
Discontinuous variables are expressed as the number (percentage). The P-values of the categorical variables were calculated using the chi-squared test. DDAH: Dimethylarginine dimethylaminohydrolase.
Allele frequency of DDAH2 (-449 G/C) in LA patients
The frequency distributions of the C and G alleles were 55% and 45%, respectively, which was consistent with the Hardy–Weinberg equilibrium (P > 0.05). Compared with the results from the control group, the LA group had a higher frequency of the G allele and a lower frequency of the C allele. The difference in allele frequency between the two groups was statistically significant (P = 0.0002; Table 5). The sensitivity and specificity of the G allele for LA were 72% and 55%, respectively.
Table 5.
Allele frequencies [n (%)] for the rs805305 (-449 G/C) polymorphism of DDAH2 in control and leukoaraiosis subjects
| Group | C | G | χ2 | P-value |
|---|---|---|---|---|
| Control (n = 46) | 51 (55) | 41 (45) | 13.9580 | 0.0002 |
| Leukoaraiosis (n = 46) | 26 (28) | 66 (72) |
Discontinuous variables are expressed as the number (percentage). The P-values of the categorical variables were calculated using the chi-squared test. DDAH: Dimethylarginine dimethylaminohydrolase.
DDAH2 gene polymorphism and ADMA concentration
ADMA concentration levels were highest in GG homozygotes [1.61 (1.18, 1.93) µM] and lowest in CC homozygotes [0.34 (0.27, 0.67) µM]. ADMA was detectable at intermediate levels in heterozygotes [0.47 (0.29, 1.37) µM] (Figure 3). The ADMA concentrations in the three genotypes were significantly different according to the Kruskal–Wallis H test (H = 24.5955, P < 0.0001). Furthermore, the least significant difference test was used to compare ADMA concentrations between the three genotypes. ADMA concentration levels in the GG group were significantly higher than in the CG and CC groups (both P < 0.05).
Figure 3.
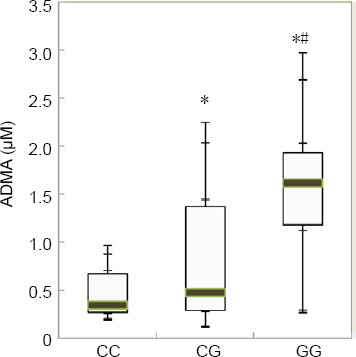
Comparison of ADMA concentrations in different DDAH2 (-449 G/C) genotypes.
P-values of the discontinuous variables were calculated using the Nemenyi test. *P < 0.05, vs. CC; #P < 0.05, vs. CG. ADMA: Asymmetric dimethylarginine; DDAH2: dimethylarginine dimethylaminohydrolase 2.
Discussion
With the growing popularity of imaging methods, including computed tomography and MRI, LA diagnoses have increased substantially, especially in the older population. However, specific blood markers for the diagnosis of LA are still lacking. Previous studies have reported that higher concentrations of the endogenous NOS inhibitor, ADMA, are associated with the incidence of LA (Janatuinen et al., 2003; Tsikas et al., 2018).
Numerous studies have reported that lipids such as creatinine, uric acid, and urea nitrogen are associated with ADMA concentrations. ADMA can be excreted in the urine and may accumulate in the bodies of patients with renal insufficiency. Similarly, plasma creatinine, uric acid, and urea nitrogen also accumulate in the body. In addition, hyperlipidemia is considered to be a risk factor for atherosclerosis (Tarnoki et al., 2012). Several studies have confirmed that ADMA concentrations are correlated with insulin resistance in diabetes and diabetic complications, especially vascular complications (Namiranian et al., 2005; Marra et al., 2013). In the present study, the blood glucose levels in the LA group matched those in the control group. In addition, individuals with severe diabetic complications were excluded from both groups to avoid interference from diabetes-induced serious vascular lesions.
The removal of ADMA mainly occurs through degradation by DDAH, and it is then eliminated via glomerular filtration. There are two known isoforms of DDAH, which have similar molecular structures but different substrate-binding domains. The DDAH2 gene, located on chromosome 6p21.3, is expressed in induced NOS-rich organs, including the heart, placenta, and immune system, and is a key determinant of plasma ADMA concentrations (Yokoro et al., 2017). There are individual differences in DDAH2 expression, which may be related to gene polymorphisms. A DDAH2 gene mutation (6G/7G) in the core promoter element -871 during coding transcription can affect subsequent mRNA transcription and protein translation, thus inhibiting protein expression and function. DDAH2 activity is higher at sites that express 7G than at sites that express 6G, and its activity has a strong effect on ADMA metabolism (Yokoro et al., 2017). In the present study, the highest ADMA concentrations occurred with the GG genotype of DDAH2 (-449 G/C), followed by CG and CC, indicating that polymorphisms in DDAH2 (-449 G/C) affect plasma ADMA concentrations. This is consistent with previous results regarding ADMA levels and DDAH2 (-449 G/C) polymorphisms in patients with heart or kidney failure (Zoungas et al., 2006; Marra et al., 2013).
The G allele affects DDAH2 mRNA transcription and protein translation, reducing the hydrolysis of ADMA and increasing plasma ADMA concentrations. DDAH2 (-449 G/C) gene polymorphisms are risk factors for multiple diseases, including chronic kidney disease, hypertension, and coronary heart disease (Bode-Böger et al., 2003; Zoungas et al., 2006; Xu et al., 2012; Marra et al., 2013; Hallmark et al., 2019). To the best of our knowledge, there are no previous reports of LA and DDAH2 gene polymorphisms. In the current study, there was a significant difference in DDAH2 (-449 G/C) genotype between the control and LA groups. Genotypes in the control group were mostly CG and CC (67.39% and 21.74%, respectively), while GG was the least common. The prevalence of LA was associated with DDAH2 (-449 G/C) gene polymorphisms; the G allele may be a susceptibility factor for LA.
The mutation frequency of the G locus in DDAH2 (-449 G/C) in the LA group was 72%, which is 5–10% higher than that reported in the general population in Hunan and Guangdong provinces, as well as in other regions in southern China (He, 2009; Xu et al., 2012).
There are several potential explanations for this finding. First, the random selection of LA subjects in the present study was limited by our strict standards, and there may therefore be sampling bias. Second, the subjects selected in our study mainly lived in Heilongjiang, Jilin, Liaoning, and northeastern Inner Mongolia. These four regions are in northeastern China, where winters last a long time (approximately 5 months) and the climate is cold and dry. Studies have shown that cold environments may cause gene mutations as a result of adaptations to the environment (Kim et al., 2015; Łaczmański et al., 2015; Leipold et al., 2015; Tao et al., 2018). We therefore speculate that environmental factors may influence gene mutations at the G locus and increase mutation rates. Cold environmental factors may also affect the mRNA involved in protein translation after transcription (Kim et al., 2015; Leipold et al., 2015), resulting in changes in protein function and increased ADMA concentrations (Zoungas et al., 2006). Some studies have also suggested that dietary factors are associated with genetic mutations (Tanner et al., 2012; Hallmark et al., 2019). A high-salt diet is prevalent in northeastern China, and preserved foods are eaten more frequently than in southern and southeastern China. A high frequency of G allele mutations as a result of diet and other factors may influence ADMA concentrations during protein translation. However, based on the results of this study, there is no clear experimental evidence for a relationship between mutation rates at the G locus and environmental factors. Further experimental studies are therefore needed to compare population genotypes in northern and southern China.
Nucleosomes, the basic unit of chromatin composed of core DNA, account for 75% of the eukaryotic genome and control protein-binding sites in gene-coding transcription. The presence or absence of nucleosomes near functional gene sites, or the different positions of nucleosomes, can affect gene expression regulation for a variety of reasons (Segal et al., 2006). In a large number of consecutive repeated sequences, nucleosomes are located in preferential sequence regions, which may depend on the A + T and G + C contents. A CpG island, which is a DNA region that has high GC content, has high transcriptional activity (Jones and Takai, 2001). In mammals, CpG islands are often associated with gene promoters (Metushi et al., 2016) that have multiple transcription start sites without common core promoter elements, such as the TATA box. Acetylation and methylation often occur on gene promoters with active transcription (Corsini and Bortolini, 2013; Adalsteinsson and Ferguson-Smith, 2014). In the present study, the ADMA hydrolase DDAH2 rs805305 (-449 G/C) allele was located in the promoter CG sequence, which may be the active transcription start site. After gene mutation occurs, the frequency of G alleles increases, causing an increase in the transcription of the resting gene promoter containing the A/T gene locus. This results in an increase in the expression of DDAH2 proteins; however, the expressed proteins are less functional, leading to higher blood ADMA in LA patients.
In our study, the exclusion criteria were relatively strict. The subjects had no relationship to one another, and the control group was selected so that the history of the patients matched as closely as possible between the two groups. The sample size was therefore relatively small, and results from a larger study are required to confirm these preliminary findings.
In summary, we demonstrated that the GG genotype of DDAH2 (-449 G/C) is more common in LA patients than in healthy controls. We also revealed that the G allele is a risk allele that is positively associated with LA in northeastern China, and may increase the levels of ADMA in LA patients. Mutations from the wild-type C to the G allele may alter the function of DDAH2 and reduce the hydrolysis of ADMA, thereby competitively reducing nitric oxide, leading to endothelial vasodilatory dysfunction and, indirectly, to the formation of cerebrovascular lesions. This may result in reduced white matter perfusion and facilitate the development of LA. Therefore, the detection of gene polymorphisms in DDAH2 may be beneficial for the early detection of LA-susceptible individuals, as well as for early interventions to delay the development of LA.
Additional files:
Additional file 1 (106KB, pdf) : Hospital ethics approval (Chinese).
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner B, Yu J, Song CP; T-Editor: Jia Y
Conflicts of interest: We have no conflicts of interest to this work.
Financial support: This study was supported by the Foundation of Harbin Science Technology Bureau of China, No. 2014RFQGJ042 (to YF), Harbin Medical University Scientific Research Innovation Fund of China, No. 2016LCZX06 (to QG), and the Natural Science Foundation of Heilongjiang of China, No. H2016027 (to QG). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: This study was approved by the Institutional Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University of China (approval No. KY2016-177) on July 28, 2016.
Declaration of participant consent: The authors certify that they have obtained the consent forms from participants. In the form, participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published.
Reporting statement: The writing and editing of the article were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the epidemiologist of the 2nd Affiliated Hospital of Harbin Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Data will be available immediately following publication, with no end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Foundation of Harbin Science Technology Bureau of China, No. 2014RFQGJ042 (to YF), Harbin Medical University Scientific Research Innovation Fund of China, No. 2016LCZX06 (to QG), and the Natural Science Foundation of Heilongjiang of China, No. H2016027 (to QG).
References
- 1.Adalsteinsson BT, Ferguson-Smith AC. Epigenetic control of the genome-lessons from genomic imprinting. Genes. 2014;5:635–655. doi: 10.3390/genes5030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anavi S, Tirosh O. iNOS as a metabolic enzyme under stress conditions. Free Radic Biol Med. 2020;146:16–35. doi: 10.1016/j.freeradbiomed.2019.10.411. [DOI] [PubMed] [Google Scholar]
- 3.Bian Y, Wang JC, Sun F, Sun ZY, Lin YJ, Liu Y, Zhao B, Liu L, Luo XG. Assessment of cerebrovascular reserve impairment using the breath-holding index in patients with leukoaraiosis. Neural Regen Res. 2019;14:1412–1418. doi: 10.4103/1673-5374.251332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 5.Corsini A, Bortolini M. Drug-induced liver injury: the role of drug metabolism and transport. J Clin Pharmacol. 2013;53:463–474. doi: 10.1002/jcph.23. [DOI] [PubMed] [Google Scholar]
- 6.Dayal S, Lentz SR. ADMA and hyperhomocysteinemia. Vasc Med 10 Suppl. 2005;1:S27–33. doi: 10.1191/1358863x05vm599oa. [DOI] [PubMed] [Google Scholar]
- 7.Dovinová I, Hrabárová E, Jansen E, Kvandová M, Majzúnová M, Berenyiová A, Barančík M. ADMA, homocysteine and redox status improvement affected by 7-nitroindazole in spontaneously hypertensive rats. Biomed Pharmacother. 2018;106:1478–1483. doi: 10.1016/j.biopha.2018.07.096. [DOI] [PubMed] [Google Scholar]
- 8.Fierini F, Poggesi A, Pantoni L. Leukoaraiosis as an outcome predictor in the acute and subacute phases of stroke. Expert Rev Neurother. 2017;17:963–975. doi: 10.1080/14737175.2017.1371013. [DOI] [PubMed] [Google Scholar]
- 9.Gad MZ, Hassanein SI, Abdel-Maksoud SM, Shaban GM, Abou-Aisha K. Association of DDAH2 gene polymorphism with cardiovascular disease in Egyptian patients. J Genet. 2011;90:161–163. doi: 10.1007/s12041-011-0043-4. [DOI] [PubMed] [Google Scholar]
- 10.Gao Q, Fan Y, Mu LY, Ma L, Song ZQ, Zhang YN. S100B and ADMA in cerebral small vessel disease and cognitive dysfunction. J Neurol Sci. 2015;354:27–32. doi: 10.1016/j.jns.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Guan J, Yan C, Gao Q, Li J, Wang L, Hong M, Zheng X, Song Z, Li M, Liu M, Fan Y, Ma L. Analysis of risk factors in patients with leukoaraiosis. Medicine (Baltimore) 96. 2017:e6153. doi: 10.1097/MD.0000000000006153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawardena D, Raju R, Münch G. Hydrogen peroxide mediates pro-inflammatory cell-to-cell signaling: a new therapeutic target for inflammation. Neural Regen Res. 2019;14:1430–1437. doi: 10.4103/1673-5374.253529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 14.Hallmark B, Karafet TM, Hsieh P, Osipova LP, Watkins JC, Hammer MF. Genomic evidence of local adaptation to climate and diet in indigenous Siberians. Mol Biol Evol. 2019;36:315–327. doi: 10.1093/molbev/msy211. [DOI] [PubMed] [Google Scholar]
- 15.He Y. Changsha: Central South University; 2009. Clinical research on the relationship between DDAH gene polymorphisms regulating ADMA and chronic kidney disease. [Google Scholar]
- 16.Janatuinen T, Laakso J, Laaksonen R, Vesalainen R, Nuutila P, Lehtimäki T, Raitakari OT, Knuuti J. Plasma asymmetric dimethylarginine modifies the effect of pravastatin on myocardial blood flow in young adults. Vasc Med. 2003;8:185–189. doi: 10.1191/1358863x03vm490oa. [DOI] [PubMed] [Google Scholar]
- 17.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 18.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging. 2001;11:229–235. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim HI, Lee HJ, Cho CH, Kang SG, Yoon HK, Park YM, Lee SH, Moon JH, Song HM, Lee E, Kim L. Association of CLOCK, ARNTL, and NPAS2 gene polymorphisms and seasonal variations in mood and behavior. Chronobiol Int. 2015;32:785–791. doi: 10.3109/07420528.2015.1049613. [DOI] [PubMed] [Google Scholar]
- 20.Łaczmański Ł, Jakubik M, Bednarek-Tupikowska G, Rymaszewska J, Słoka N, Lwow F. Vitamin D receptor gene polymorphisms in Alzheimer’s disease patients. Exp Gerontol. 2015;69:142–147. doi: 10.1016/j.exger.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Leipold E, Hanson-Kahn A, Frick M, Gong P, Bernstein JA, Voigt M, Katona I, Oliver Goral R, Altmüller J, Nürnberg P, Weis J, Hübner CA, Heinemann SH, Kurth I. Cold-aggravated pain in humans caused by a hyperactive NaV1.9 channel mutant. Nat Commun. 2015;6:10049. doi: 10.1038/ncomms10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu LH, Guo Z, Feng M, Wu ZZ, He ZM, Xiong Y. Protection of DDAH2 overexpression against homocysteine-induced impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells. Cell Physiol Biochem. 2012;30:1413–1422. doi: 10.1159/000343329. [DOI] [PubMed] [Google Scholar]
- 23.Liu SJ, Zhong Y, You XY, Liu WH, Li AQ, Liu SM. Insulin-like growth factor 1 opposes the effects of C-reactive protein on endothelial cell activation. Mol Cell Biochem. 2014;385:199–205. doi: 10.1007/s11010-013-1828-y. [DOI] [PubMed] [Google Scholar]
- 24.Maccarrone M, Ulivi L, Giannini N, Montano V, Ghiadoni L, Bruno RM, Bonuccelli U, Mancuso M. Endothelium and oxidative stress: The Pandora’s box of cerebral (and non-only) small vessel disease. Curr Mol Med. 2017;17:169–180. doi: 10.2174/1566524017666170822114739. [DOI] [PubMed] [Google Scholar]
- 25.Marra M, Marchegiani F, Ceriello A, Sirolla C, Boemi M, Franceschi C, Spazzafumo L, Testa I, Bonfigli AR, Cucchi M, Testa R. Chronic renal impairment and DDAH2-1151 A/C polymorphism determine ADMA levels in type 2 diabetic subjects. Nephrol Dial Transplant. 2013;28:964–971. doi: 10.1093/ndt/gfs516. [DOI] [PubMed] [Google Scholar]
- 26.Metushi I, Uetrecht J, Phillips E. Mechanism of isoniazid-induced hepatotoxicity: then and now. Br J Clin Pharmacol. 2016;81:1030–1036. doi: 10.1111/bcp.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namiranian K, Mittermayer F, Artwohl M, Pleiner J, Schaller G, Mayer BX, Bayerle-Eder M, Roden M, Baumgartner-Parzer S, Wolzt M. Free fatty acids do not acutely increase asymmetrical dimethylarginine concentrations. Horm Metab Res. 2005;37:768–772. doi: 10.1055/s-2005-921100. [DOI] [PubMed] [Google Scholar]
- 28.Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc Dis 13 Suppl. 2002;2:7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- 29.Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 30.Segal E, Fondufe-Mittendorf Y, Chen L, Thåström A, Field Y, Moore IK, Wang JP, Widom J. A genomic code for nucleosome positioning. Nature. 2006;442:772–778. doi: 10.1038/nature04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stühlinger MC, Stanger O. Asymmetric dimethyl-L-arginine (ADMA): a possible link between homocyst(e)ine and endothelial dysfunction. Curr Drug Metab. 2005;6:3–14. doi: 10.2174/1389200052997393. [DOI] [PubMed] [Google Scholar]
- 32.Tanner SM, Sturm AC, Baack EC, Liyanarachchi S, de la Chapelle A. Inherited cobalamin malabsorption. Mutations in three genes reveal functional and ethnic patterns. Orphanet J Rare Dis. 2012;7:56. doi: 10.1186/1750-1172-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao N, Ge H, Wu W, An H, Liu J, Xu X. Association of glucocorticoid receptor gene polymorphism and occupational stress with hypertension in desert petroleum workers in Xinjiang, China. BMC Med Genet. 2018;19:213. doi: 10.1186/s12881-018-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarnoki AD, Baracchini C, Tarnoki DL, Lucatelli P, Boatta E, Zini C, Fanelli F, Molnar AA, Meneghetti G, Stazi MA, Medda E, Cotichini R, Nisticò L, Fagnani C, Osztovits J, Jermendy G, Preda I, Kiss RG, Metneki J, Horvath T, et al. Evidence for a strong genetic influence on carotid plaque characteristics: an international twin study. Stroke. 2012;43:3168–3172. doi: 10.1161/STROKEAHA.112.666016. [DOI] [PubMed] [Google Scholar]
- 35.Tsikas D, Bollenbach A, Hanff E, Kayacelebi AA. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes. Cardiovasc Diabetol. 2018;17:1. doi: 10.1186/s12933-017-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu AG, Xu RM, Lu CQ, Li DD, Xu QF, Guo J, Fu X, Zhao W, Yao MY. Association study of dimethylarginine dimethylaminohydrolase 2 gene polymorphisms and coronary heart disease. Mol Med Rep. 2012;6:1103–1106. doi: 10.3892/mmr.2012.1038. [DOI] [PubMed] [Google Scholar]
- 37.Xuan C, Xu LQ, Tian QW, Li H, Wang Q, He GW, Lun LM. Dimethylarginine dimethylaminohydrolase 2 (DDAH 2) gene polymorphism, asymmetric dimethylarginine (ADMA) concentrations, and risk of coronary artery disease: A case-control study. Sci Rep. 2016;6:33934. doi: 10.1038/srep33934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoro M, Nakayama Y, Yamagishi SI, Ando R, Sugiyama M, Ito S, Yano J, Taguchi K, Kaida Y, Saigusa D, Kimoto M, Abe T, Ueda S, Fukami K. Asymmetric dimethylarginine contributes to the impaired response to erythropoietin in CKD-anemia. J Am Soc Nephrol. 2017;28:2670–2680. doi: 10.1681/ASN.2016111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


