Keywords: bibliometric analysis, citations, CiteSpace, h-index, necroptosis, network analysis, neuroscience, output, VOSviewer, Web of Science
Abstract
There are two types of cell death-apoptosis and necrosis. Apoptosis is cell death regulated by cell signaling pathways, while necrosis has until recently been considered a passive mechanism of cell death caused by environmental pressures. However, recent studies show that necrosis can also be regulated by specific cell signaling pathways. This mode of death, termed necroptosis, has been found to be related to the occurrence and development of many diseases. We used bibliometrics to analyze the global output of literature on necroptosis in the field of neuroscience published in the period 2007–2019 to identify research hotspots and prospects. We included 145 necroptosis-related publications and 2239 references published in the Web of Science during 2007–2019. Visualization analysis revealed that the number of publications related to necroptosis has increased year by year, reaching a peak in 2019. China is the country with the largest number of publications. Key word and literature analyses demonstrated that mitochondrial function change, stroke, ischemia/reperfusion and neuroinflammation are likely the research hotspots and future directions of necroptosis research in the nervous system. The relationship between immune response-related factors, damage-associated molecular patterns, pathogen-associated molecular patterns and necroptosis may become a potential research hotspot in the future. Taken together, our findings suggest that although the inherent limitations of bibliometrics may affect the accuracy of the literature-based prediction of research hotspots, the results obtained from the included publications can provide a reference for the study of necroptosis in the field of neuroscience.
Chinese Library Classification No. R459.9; R363; R364
Introduction
For many years, necrosis was considered accidental and unregulated, until Laster et al. (1988) found that tumor necrosis factor can induce both apoptosis and necrosis. Subsequent studies showed that there is a controllable form of cell necrosis. Degterev et al. (2005) showed that a small-molecule inhibitor, necrostatin-1 (Nec-1), could inhibit necrosis-like cell death, which they termed necroptosis (Degterev et al., 2005). Necroptosis is a form of regulated necrotic cell death in the absence of caspase-8 (Degterev et al., 2005; Newton et al., 2019a, b), and differs from apoptosis in morphological features (Wang et al., 2018a).
The molecular mechanisms of necroptosis began to be clarified in 2000 with the discovery of receptor-interacting serine/threonine kinase 1 (RIPK1), which is a regulator of Fas ligand-induced necroptosis in T cells (Holler et al., 2000). It is known that necroptosis can be triggered by multiple stimuli, including the activation of death receptors (Fas and TNF), toll-like receptors (TLR3 and TLR4), nucleic acid sensors (Z-DNA binding protein 1 (ZBP1), also known as DAI) and adhesion receptors (Vanlangenakker et al., 2012; Shindo et al., 2013; Dowling et al., 2015; Petersen et al., 2015; Arrazola and Court, 2019; Malireddi et al., 2019). Necroptosis signaling can be classified into two pathways—the canonical pathway and the noncanonical pathway. The canonical pathway is initiated by death receptors. The surface of the plasma membrane has tumor necrosis factor (TNF) receptors that can bind to TNF, and this step can activate RIPK1. RIPK1 is an essential adaptor of death receptors and contains a RIP homotypic interaction motif domain (RHIM), which activates RIPK3 via homotypic interaction, which then changes the conformation of mixed lineage kinase domain-like protein (MLKL; Chen et al., 2019; Lim et al., 2019; Baker et al., 2020). Then, phosphorylated MLKL undergoes oligomerization and translocates to the plasma membrane to change permeability, which leads to the disruption of the plasma membrane, and ultimately, cell lysis (Ofengeim and Yuan, 2013; Shan et al., 2018). The noncanonical pathway is triggered by specific pathogen recognition receptors, including TLR3, TLR4, interferon (IFN) and ZBP1. Pathogen recognition receptors contain a RHIM domain, and therefore, they can bind to RIPK3 via the RHIM domain and form a noncanonical necrosome (Huang et al., 2018; Zhang et al., 2020). Phosphorylated RIPK3 can induce the active conformation of MLKL (Moriwaki and Chan, 2017). The downstream mediators of MLKL are unclear at present. However, it is tempting to speculate that the ion channels induce plasma membrane rupture (Ding et al., 2019; Figure 1).
Figure 1.
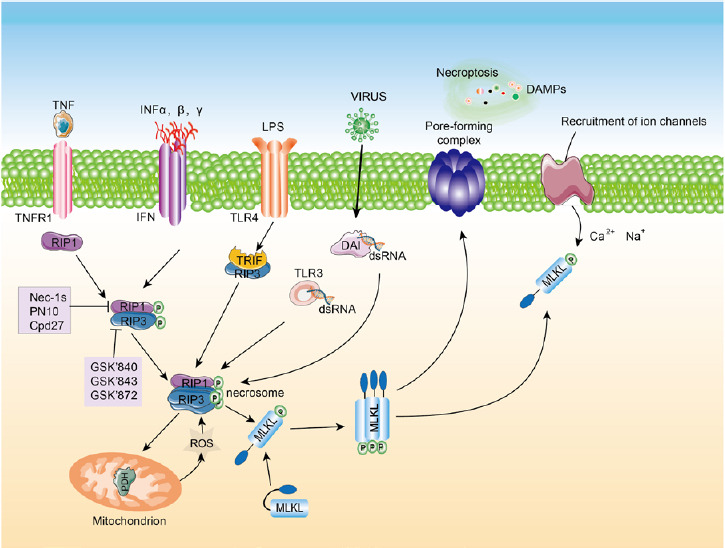
Diagram of the necroptosis signaling pathway. Necroptosis can be initiated by TNF, INF, DAI and viruses.
Activated RIP1 phosphorylates RIP3 and forms a necrosis complex, the necrosome. Activated RIP3 phosphorylates MLKL, inducing oligomerization. The oligomerized MLKL translocates to the plasma membrane to form pores, causing cell death. MLKL: Mixed lineage kinase domain-like protein; RIP: receptor-interacting protein kinase; TLR: Toll-like receptor; TNF: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1.
Our research group has investigated necroptosis in the nervous system for many years, from the occurrence of necroptosis in retinal neurons to a wider field, and we hope to carry out multidimensional and quantitative analysis on the research status of necroptosis within the scope of the nervous system. We found that bibliometric analysis can help us achieve this goal. Bibliometrics is the quantitative analysis of literature using mathematical and statistical methods (Zhang et al., 2017). Bibliometric analysis has emerged as one of the most useful methods to evaluate the scholarly impact, centrality, and quality of publication in a certain field (Ellegaard and Wallin, 2015; Akmal et al., 2020). Evaluation indicators include citation frequency, number of publications, times cited, H-index, impact factor and centrality (Avena and Barbosa, 2017). Benefiting from the development of visualization software, we used VOSviewer and CiteSpace to analyze the publications, which is more objective and rigorous and can provide the co-occurrence network diagram (Synnestvedt et al., 2005; Yeung et al., 2018). In this study, we retrieved and collected the research literature on necroptosis in the nervous system from the Web of Science Core Collection, and used VOSviewer and CiteSpace to analyze the publications. The objective of this bibliometric study was to identify and analyze the global output trend of necroptosis in the neuroscience field. Data analysis was performed to investigate the current hotspots in necroptosis research in the field of the nervous system and to identify future research hotspots. Our analyses will shed new light for investigators to help them plan and manage their scientific work.
Data and Methods
Data strategy and selection criteria
Literature data for this bibliometrics study were retrieved from the Web of Science Core Collection. The Web of Science Core Collection contains several important index types, including Science Citation Index Expanded (SCIE), Social Science Citation Index (SSCI) and Emerging Sources Citation Index (ESCI). To perform a systematic analysis of necroptosis in the field of neuroscience, we chose articles for visualization analysis. The term necroptosis was used in the MeSH (https://www.ncbi.nlm.nih.gov/mesh) search. The word necroptotic was used in some articles. Articles from 2007 to 2019 were selected, the language type was set to English, and type of documents was set to article and review.
The used search strategy was as follows: TS = (necroptosis OR necroptotic) refined by WEB OF SCIENCE CATEGORY (NEUROSCIENCE) AND [excluding] PUBLICATION YEARS: (2020) AND DOCUMENT TYPES: (ARTICLE OR REVIEW) AND LANGUAGES: (ENGLISH) AND WEB OF SCIENCE INDEX: (WOS. SCI), and time span of 2007 to 2019.
A total of 2239 documents were retrieved from the Web of Science Core Collection. After excluding documents published in 2020 or the published year of the documents was not clear, the 2201 documents that remained were used for visualization analysis. A flow chart of the search is given in Figure 2. The search was completed on April 16, 2020.
Figure 2.
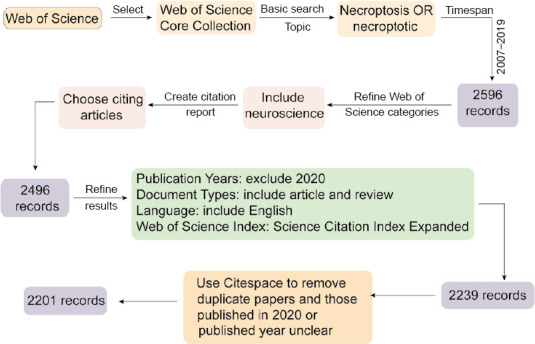
Search flowchart detailing steps in the identification and screening of papers.
Methodology
The retrieval characteristics of necroptosis in the field of neuroscience included the distribution of publication years, countries and regions, organizations, journals, core-authors, keywords and key references. Bibliometric analysis and network visualization were performed with VOSviewer (Version 1.6.14; https://www.vosviewer.com/download#download-vosviewer) and CiteSpace (Version 5.6.R4; https://sourceforge.net/projects/citespace/files/latest/download). Microsoft Excel 2010 was used to assess the distribution of publication years. Gunn map (http://lert.co.nz/map/) online world map was used to evaluate the distribution of countries and regions. Ranking was performed using the Standard Competition Ranking method.
Outcome
We chose the keywords and key references to anticipate the research prospects and hotspots. Keywords and key reference analyses were performed with VOSviewer and CiteSpace. High-frequency terms, including key molecule and primary diseases, were used to anticipate the popular research model and research molecule. VOSviewer analysis method was Linlog/modularity and CiteSpace analysis method was LLR. Silhouette represents network homogeneity. A silhouette above 0.7 indicates that the cluster has a high reality, and above 0.5 suggests that the cluster has a credit.
Results
Distribution of publications by year
There were 1646 (74.78%) articles and 555 (25.22%) reviews among the 2201 publications. The chronological distribution of published documents is shown in Figure 3. From the trend line, the number of documents increased exponentially. The line chart illustrates that the number of documents increased relatively slowly from 2007 (n = 10, 0.45%) to 2017 (n = 257, 11.68%), while the number of documents rose sharply from 2017 (n = 257, 11.68%) to 2019 (n = 592, 26.90%) and reached a peak in 2019. Necroptosis acquired increasing attention in the field of neuroscience worldwide, indicating that it gradually became a research hotspot, even into the future.
Figure 3.
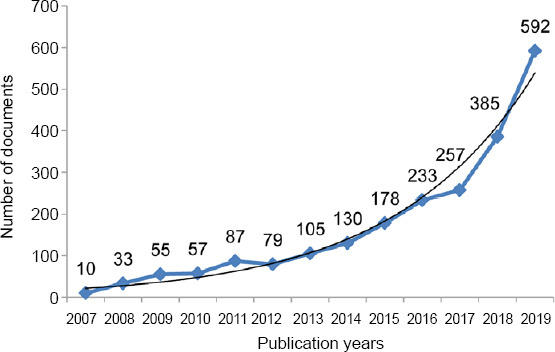
Distribution of publications on necroptosis in the field of neuroscience according to year.
The number of publications increased relatively slowly from 2007 to 2017, while the number of publications rose sharply from 2017 onwards, to a peak in 2019.
Countries and regions
According to the statistical analysis, 2201 documents were published by research groups from 73 countries and regions. Some publications were the result of cooperation among multiple countries and regions, but we classified these into countries and regions. The top 10 most prolific countries and regions are shown in Table 1. The country with the largest number of documents was China (n = 860, 39.07%), followed by the United States (n = 635, 28.85%) and England (n = 100, 4.54%). Although the number of publications from the United States was less than that from China, the citation and centrality were far higher than those from China.
Table 1.
Top 10 most productive countries and regions with publications on necroptosis in the field of neuroscience
| Rank | Country/region | Documents | Citations | Total link strength | Links | Centrality |
|---|---|---|---|---|---|---|
| 1st | China | 860 | 11970 | 199 | 30 | 0.15 |
| 2nd | USA | 635 | 21649 | 369 | 37 | 0.38 |
| 3rd | England | 100 | 3986 | 156 | 32 | 0.09 |
| 4th | Germany | 97 | 4295 | 121 | 27 | 0.08 |
| 5th | Italy | 92 | 2146 | 94 | 34 | 0.15 |
| 6th | Canada | 87 | 2930 | 103 | 31 | 0.07 |
| 7th | Japan | 81 | 2040 | 61 | 27 | 0.09 |
| 8th | Republic of Korea | 80 | 796 | 18 | 7 | 0.06 |
| 9th | Australia | 62 | 2610 | 80 | 31 | 0.13 |
| 10th | India | 61 | 1372 | 23 | 8 | 0.05 |
The top 10 countries and regions with the strongest citation bursts are shown in Figure 4. India had the highest burst strength of 6.1423. The duration of burst began in 2010 and ended in 2012, indicating that there were many researchers studying necroptosis in India during 2010 to 2012. Canada had the lowest strength of 2.2056. The study of necroptosis exploded in 2013 and then ended that year.
Figure 4.
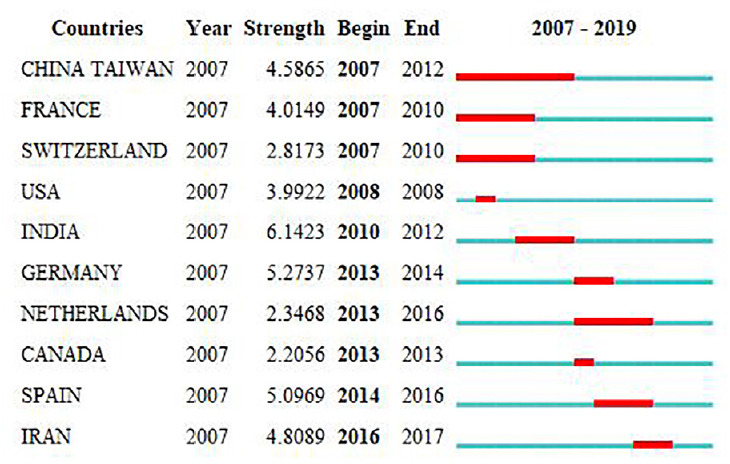
Top 10 countries/regions with the strongest citation bursts.
A strong citation burst indicates that a variable undergoes a great change in a short period of time. Red bars indicate the duration of bursts.
Organizations
According to VOSviewer analysis, 2201 documents were published by 2126 different organizations, and 80 met the threshold. After excluding disjointed organizations, the remaining 76 organizations were used for the visualization map. We listed the top 10 prolific organizations in Table 2, and the most prolific organization was Soochow University (n = 70, 3.18%), followed by Zhejiang University (n = 52, 2.36%) and Central South University (n = 49, 2.23%). Among the top 10 organizations, seven are Chinese organizations and the remaining three are American organizations. However, the sum total of the citations of the seven Chinese organizations (n = 4126) was lower than that of Harvard University (n = 4387). The co-occurrence relations are shown in Figure 5. It is worth noting that the organizations with the highest citations included Harvard University, Ghent University, and University of California San Diego. Most were from the United States, and some were from Belgium, such as Ghent University. All evidence indicates that the United States still dominates necroptosis research in the field of neuroscience. Furthermore, the node in yellow indicates that the organization’s average publishing year is 2018, and therefore, Harvard Medical University, which is represented by a yellow node, published a higher number of documents than any other organization, suggesting that it might be an emerging research organization.
Table 2.
Top 10 most productive organizations
| Rank | Organizations | Country | Documents | Citations | Total link strength |
|---|---|---|---|---|---|
| 1st | Soochow University | China | 70 | 1394 | 34 |
| 2nd | Zhejiang University | China | 52 | 641 | 23 |
| 3rd | Central South University | China | 49 | 419 | 10 |
| 4th | Harvard University | USA | 41 | 4387 | 31 |
| 4th | Shanghai Jiao Tong University | China | 41 | 390 | 23 |
| 6th | Fudan University | China | 33 | 453 | 30 |
| 7th | Harvard Medical School | USA | 32 | 609 | 29 |
| 8th | Southern Medical University | China | 29 | 337 | 15 |
| 8th | Johns Hopkins University | USA | 29 | 853 | 12 |
| 10th | Nanjing Medical University | China | 27 | 492 | 17 |
Figure 5.
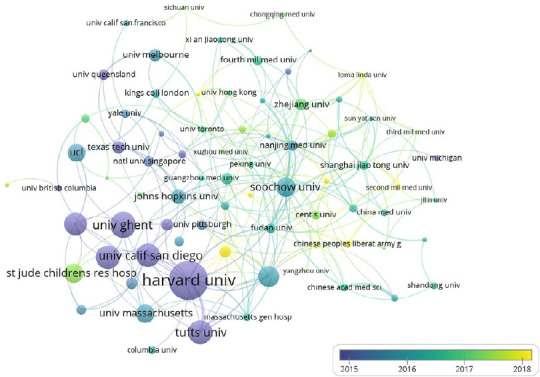
Organizations with co-occurrence relations shown as an overlay graph plotted with VOSviewer 1.6.14.
The analysis method was Linlog/modularity. The weight was citations. Scores are the average published year. The thickness of lines indicates the strength of the relationship. The color indicates the average published year.
Journals
It is helpful to identify core journals by analyzing the distribution of publication sources. Based on data analysis, the documents related to necroptosis in the field of neuroscience published from 2007 to 2019 are mainly distributed in 680 different journals. As shown in Table 3, the most prolific journal was Cell Death & Disease and International Journal of Molecular Sciences, which had 40 documents each. The range of 2019 impact factors, given in Table 3, is 2.274 to 6.304. The 2019 impact factor of Cell Death & Disease was the highest, and that of Neuroscience Letters was the lowest. Judging from the number of publications and the impact factor of journals, Cell Death and Disease might be the most influential journal.
Table 3.
Top 12 largest number of publications
| Rank | Journals | Documents | 2019 impact factor |
|---|---|---|---|
| 1st | Cell Death & Disease | 40 | 6.304 |
| 1st | International Journal of Molecular Sciences | 40 | 4.556 |
| 3rd | Brain Research | 38 | 2.733 |
| 4th | Molecular Neurobiology | 37 | 4.500 |
| 4th | PLoS One | 37 | 2.740 |
| 6th | Neuroscience Letter | 34 | 2.274 |
| 7th | Neurochemistry International | 33 | 3.881 |
| 7th | Frontiers in Cellular Neuroscience | 33 | 3.921 |
| 9th | Neural Regeneration Research | 31 | 3.171 |
| 10th | Neurochemical Research | 29 | 3.038 |
| 10th | Scientific Reports | 29 | 3.998 |
| 12th | Frontiers in Neuroscience | 26 | 3.707 |
Authors
A total of 11,293 authors were found in 2201 documents. It is beneficial for probing the distribution of documents by analyzing core authors. The evaluation criteria of core authors included the number of published documents, the total citations and H-index. Table 4 lists core authors of documents regarding necroptosis in the field of neuroscience from 2007 to 2019. Yuan JY ranked the first, both in the number of documents (n = 20) and total citations (n = 3335), indicating that Yuan JY is the most influential investigator of necroptosis in the field of neuroscience. Kreomer Guido had the highest H-index of 194. The core authors primarily were from the United States, Belgium, France and China, suggesting that the dominant authors were from prolific countries and organizations. Figure 6 shows the co-authorship relations of authors. A total of 11,293 authors were found, and 229 met the threshold. After excluding unconnected authors, the remaining 137 authors were included for the visualization map. From the visualization map, we can observe that influential authors such as Yuan JY and Kreomer Guido collaborated more closely. In addition, their average published year was primarily 2013, indicating that they were the first to pay attention to this research topic. Furthermore, there are many emerging groups engaged in necroptosis research, indicating that necroptosis is still a hotspot.
Table 4.
Core authors of publications of necroptosis in the field of neuroscience from 2007 to 2019
| Authors | Organizations | Documents | Citations | H-index |
|---|---|---|---|---|
| Yuan Junying | Harvard Medical School (USA) | 20 | 3335 | 81 |
| Vandenabeele Peter | Ghent University (Belgium) | 11 | 3079 | 101 |
| Vanden Berghe Tom | Ghent University (Belgium) | 8 | 2487 | 46 |
| Degterev Alexei | Tufts University (USA) | 15 | 2197 | 35 |
| Kreomer Guido | Sorbonne University (France) | 9 | 1866 | 194 |
| Green Douglas R | St Jude Children’s Research Hospital (USA) | 6 | 838 | 155 |
| Xu Xingshun | Soochow University (China) | 13 | 495 | 16 |
| Maiese Kenneth | Cellular & Mol Signaling | 17 | 423 | 61 |
| Xiong Kun | Central South University (China) | 20 | 438 | 13 |
Figure 6.
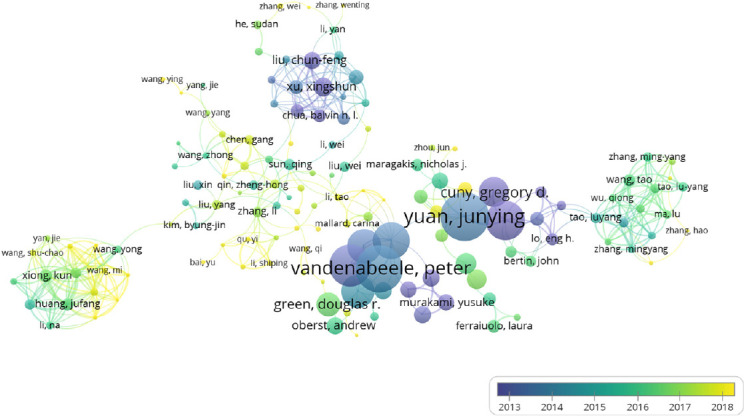
Overlay visualization of co-authorship analysis of authors.
The analysis method was Linlog/modularity. The weight was citations. Scores are the average published year. The thickness of the line indicates the strength of the relationship. The color of the circle represents the average published year.
Keywords
A total of 9311 keywords were retrieved from 2201 documents, and 97 met the threshold. The network visualization map shows the co-occurrence relations of keywords (Figure 7). The size of the circle indicates the occurrence of keywords. As shown in Figure 7, the high-frequency keywords are apoptosis, oxidative stress, and inflammation. The average published year of these keywords, including cerebral ischemia, neuroprotection and stroke, is 2014. However, in the last 2 years, an increasing number of researchers have given attention to spinal cord injury, microglial autophagy and mitophagy, and neuroprotection by melatonin in necroptosis, indicating that the molecular mechanisms of necroptosis in the mitochondria will be the focus of future research.
Figure 7.
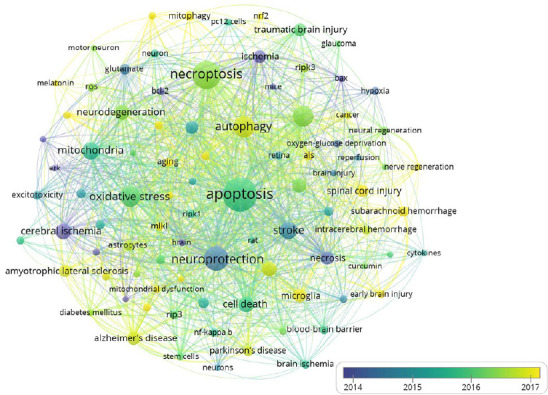
Co-occurrence analysis of keywords.
The analysis method was Linlog/modularity. The weight was occurrence. The color of the circle represents the average published year.
Figure 8 shows the top 15 keywords with the strongest citation bursts. Cerebral ischemia had the highest burst strength of 29.8706. The burst in cerebral ischemia research in 2007 continued to 2013, suggesting that cerebral ischemia is a hotspot. In addition, ischemia had occurred five times in the top 15 keywords with the strongest citation bursts. All of this evidence suggests that ischemia is a hotspot, and many scholars have devoted themselves to researching it.
Figure 8.
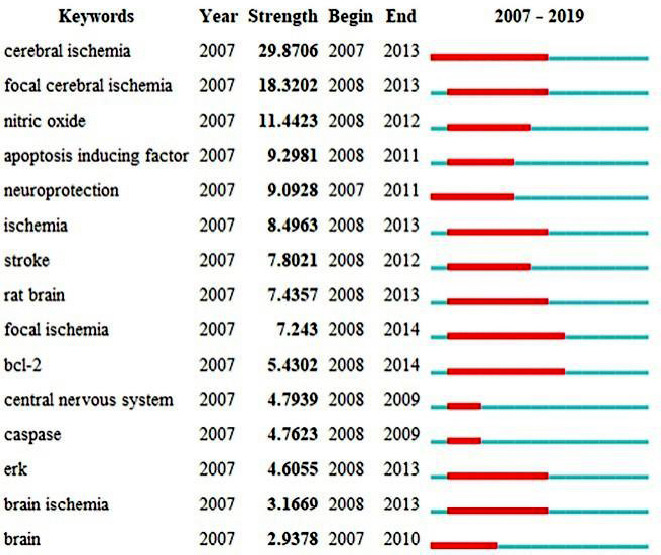
Top 15 keywords with the strongest citation bursts.
A strong citation burst indicates that a variable undergoes a great change in a short period of time. Red bars indicate the durations of the bursts.
According to statistical analysis of the keywords, numerous molecules participate in the progress of necroptosis, and these molecules often suggest that some pathways and receptors also play a role in necroptosis, and different molecules or pathways also occur in different cell types. We listed the major molecules, pathways, receptors and cell types in Table 5. Table 6 shows the diseases and pathological states involved in the study of necroptosis in the nervous system.
Table 5.
Top 10 key molecules, pathways, cell types and receptor types involved in necroptosis in the nervous system
| Rank | Key molecules | Occurrence | Pathways | Occurrence | Cell types | Occurrence | Receptor types | Occurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | Rips | 295 | Pi3k/AKT pathway | 14 | Astrocyte | 104 | Toll-like receptor | 46 |
| 2 | NF-κB | 265 | JNK pathway | 13 | Stem cells | 95 | NMDA receptor | 35 |
| 3 | Nitric oxide | 117 | Cell death pathway | 10 | Endothelial cells | 91 | Cb receptor (cb1/cb2) | 20 |
| 4 | TNFα | 80 | WNT pathway | 8 | Motor neurons | 68 | Glutamate receptor | 20 |
| 5 | Bcl-2/Beclin-1 | 79 | AMPK pathway | 7 | Retinal ganglion cells | 42 | Chemokine receptor | 10 |
| 6 | Caspases | 73 | ERK pathway | 6 | Cancer cells | 40 | Hormone receptor | 9 |
| 7 | Necrostatin-1 | 73 | MAPK pathways | 6 | Pc12 cells | 36 | Acetylcholine receptor | 9 |
| 8 | Cyclosporine | 65 | Mitochondrial pathway | 6 | Glial-cells | 35 | AMPA receptor | 8 |
| 9 | Glutamate | 65 | AKT pathway | 5 | Hippocampal neurons | 33 | Sigma 1 receptor | 7 |
| 10 | AMPK | 48 | NF-κb pathway | 5 | Sh-sy5y cells | 31 | TNF receptor | 7 |
AKT: Protein kinase B; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor; AMPK: adenosine 5' -monophosphate (AMP)-activated protein kinase; ERK: extracellular regulated protein kinases; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; NF-κb: nuclear factor kappa-B; NMDA: N-methyl-D-aspartic acid receptor; Rips: receptor interacting protein family; TNFα: tumor necrosis factor; WNT: wingless/integrated.
Table 6.
The diseases and pathologies involved in the study of necroptosis
| Pathology | Occurrence | Diseases | Occurrence |
|---|---|---|---|
| Ischemia | 796 | Stroke | 330 |
| Oxidative stress | 461 | Amyotrophic lateral sclerosis | 209 |
| Ischemia-reperfusion | 187 | Other brain injury | 195 |
| Neurodegeneration | 166 | Alzheimer’s disease | 176 |
| Motor neuron death | 77 | Traumatic brain injury | 130 |
| Artery occlusion | 56 | Optic nerve diseases | 127 |
| DNA damage | 34 | Nervous cancer | 109 |
| Axonal degeneration | 27 | Parkinson’s disease | 88 |
| Brain edema | 11 | Cognition dysfunction | 85 |
| Cerebral vasospasm | 9 | Spinal cord injury | 54 |
| Axonal regeneration | 7 | Subarachnoid hemorrhage | 53 |
| White matter injury | 7 | Multiple sclerosis | 30 |
| Anoxia | 5 | Huntington’s disease | 27 |
Citations
According to the citation analysis of documents, which reflects the number of times the documents were cited, we listed the top 10 highly cited documents in Table 7. The range of the number of citations was 431 to 1225. “Molecular mechanism of necroptosis: an ordered cellular explosion” ranked the first, which was published by Peter Vandenabeele in 2010 and was cited 1225 times. “Identification of RIP 1 kinase as a specific cellular target of necrostatins” ranked the second, and was published by Alexei Degterev in 2008. “Regulated necrosis: the expanding network of non-apoptotic cell death pathways”, ranked the third, and was cited 706 times. “Necroptosis as an alternative form of programmed cell death” ranked the last, with 431 citations among the 10 documents. It is worth noting that “Decoding ALS: from genes to mechanism” ranked sixth, but it was cited 500 times in the past 4 years. This review was likely cited so many times because it summarized the impact of the dysfunction in RNA metabolism and protein homeostasis and endoplasmic reticulum stress in ALS.
Table 7.
Top 10 highly cited documents of necroptosis in the field of neuroscience
| Rank | Title | First author | Journals | Publication year | Total citations |
|---|---|---|---|---|---|
| 1st | Molecular mechanisms of necroptosis: an ordered cellular explosion | Peter Vandenabeele | Nature Reviews Molecular Cell Biology | 2010 | 1225 |
| 2nd | Identification of RIP 1 kinase as a specific cellular target of necrostatins | Alexei Degterev | Nature Chemical Biology | 2008 | 1049 |
| 3rd | Regulated necrosis: the expanding network of nonapoptotic cell death pathways | Tom Vanden Berghe | Nature Reviews Molecular Cell Biology | 2014 | 706 |
| 4th | Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway | Junichi Hitomi | Cell | 2008 | 628 |
| 5th | Necroptosis: the release of damage-associated molecular patterns and its physiological relevance | Agnieszka Kaczmarek | Immunity | 2013 | 548 |
| 6th | Decoding ALS: from genes to mechanism | J. Paul Taylor | Nature | 2016 | 500 |
| 7th | Neuroprotection for ischemic stroke: past, present and future | Myron D Ginsberg | Neuropharmacology | 2008 | 478 |
| 8th | Necroptosis | Andress Linkermann | New England Journal of Medicine | 2014 | 467 |
| 9th | Essential versus accessory aspects of cell death: recommendation of the NCCD 2015 | L Galluzzi | Cell Death and Differentiation | 2015 | 449 |
| 10th | Necroptosis as an alternative form of programmed cell death | Dana E Christofferson | Current Opinion in Cell Biology | 2010 | 431 |
In addition to analyzing citations, co-citation analysis is also an important method of evaluating core references. As shown in Figure 9, the top highly-cited references were Mehta et al. (2007), Sun et al. (2012), Zhang et al. (2009), Cho et al. (2009), Degterev et al. (2008), He et al. (2009), Re et al. (2014), Vandenabeele et al. (2010) and Rosenbaum et al. (2010). Degterev et al. (2005) had the highest centrality, indicating that it was the most influential in necroptosis in the field of neuroscience. This is likely because this publication was the first to demonstrate that Nec-1 could inhibit regulated necrosis, which they termed necroptosis for the first time.
Figure 9.
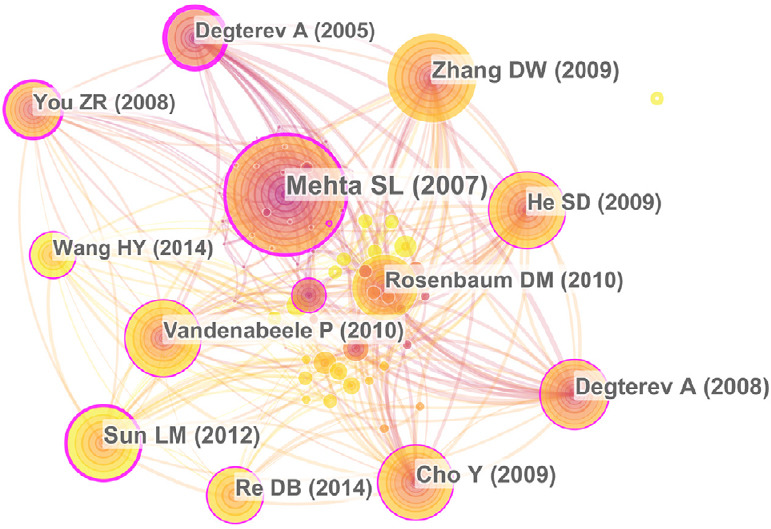
Co-citation analysis of references.
The size of the circle indicates the record of documents cited. The purple part of the circle indicates the centrality of the documents.
Discussion
During the preceding 12 years covered by this study, the number of annual publications increased gradually and reached a peak in 2019. The curve suggests that an increasing number of researchers became interested in necroptosis, indicating that it continues to be a research hotspot, and that publications related to necroptosis might continue to increase over the next few years. Moreover, a great majority of publications were original articles, and only a few were reviews, according to the bibliometrics, suggesting that there is a continuing need for novel investigation at this stage (Paunkov et al., 2019). However, there are still many unresolved domains in the field of necroptosis, such as clinical diseases, the upstream and downstream molecular mechanisms of necroptosis, and how to halt or slow the onset of diseases by blocking necroptosis signaling pathways (Molnar et al., 2019). The dominant countries, organizations and journals are in the West, and these countries will be in a leading position in the study of necroptosis for many years into the future.
According to our study, the core authors were from the most outstanding/productive organizations, such as Yuan JY, who worked at Harvard University in the past and is now at Harvard Medical School. Yuan JY published 235 documents from 1990 to 2020. Yuan’s laboratory recently found that non-cleavable variants of RIPK1 led to an autoinflammatory response (Tao et al., 2020), and that casein kinases 1γ1 and 3 promote TNFα-induced necroptosis through RIPK3 (Lee et al., 2019). In addition, they found that RIPK1 activated necroptosis, and that RIPK3 deficiency and TAK1 loss could transform necroptosis into apoptosis (Naito et al., 2020). She recently published a review, “Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases”, which became a highly cited and hot paper in the Web of Science database just a few months ago. They cooperated with other teams from China, Canada, Republic of Korea and Germany, suggesting that international collaboration is beneficial to producing high-quality papers. Vandenabeele, Peter and Vanden Berghe, Tom, at Ghent University in Belgium, published numerous documents and had high total citations. Therefore, Belgium has occupied a place in the field of necroptosis. Xiong’s laboratory from the Central South University has engaged in necroptosis research over the last few years (Huang et al., 2013; Ding et al., 2015; Chen et al., 2016; Liao et al., 2017; Shang et al., 2017; Wang et al., 2020). They are the first group to link necroptosis with methamphetamine. They found that necroptosis participated in methamphetamine-induced neurotoxicity (Xiong et al., 2016; Yang et al., 2018; Guo et al., 2020). However, from the analysis results, we can see that the main problem of Xiong’s team is the lack of international communication and cooperation with other teams or institutions, which makes their research results similar to an islet, with few links with others. Moreover, the team of Tao Luyang, a group of emerging scholars, began to study necroptosis, indicating that necroptosis still garners the attention of investigators.
Taken together, 9311 keywords were retrieved from all of the documents. Stroke was the most frequent nervous system disease, indicating that stroke interests more investigators. Furthermore, Degterev et al. (2005) found that Nec-1 can specifically suppress RIPK1, providing a huge contribution to ischemia/reperfusion injury research, and contributing to the cerebral ischemia and necroptosis clusters boom in 2007. Even at present, focus on ischemia/reperfusion is still very high among investigators studying diseases and pathologies associated with necroptosis. Ischemia, ischemia/reperfusion and stroke, and brain injury rank high. The number of studies on diseases and pathologies ranked high is greater than those on other types, and the number of citations is also high. From the perspective of bibliometrics, this phenomenon shows that these molecules or diseases are frequently studied as research subjects. The number of existing papers is high, and the future growth trend may tend to slow. However, new research avenues on these molecules or diseases may continue to be discovered, resulting in a second spurt of research. In contrast, diseases or pathologies that are ranked lower, such as motor neuron injury, ocular nerve disease and DNA damage, may have sufficient development room for more scholars to engage in.
The most critical necroptosis-related molecules are RIP1, RIP3 and MLKL, among which MLKL oligomerization is the executor of necroptosis. This is basically consistent with our results of keyword extraction and analysis. RIPs have the highest word frequency, which indicates that the number of publications on these molecules is the highest. However, with in-depth study of necroptosis, investigators have discovered additional molecules related to necroptosis. For example, Yuan et al. (2019) found that TAM promotes necroptosis by regulating MLKL oligomerization. TAM kinases are known for their anti-apoptotic and anti-inflammatory roles, but Najafov et al. (2019) found that they are also necessary for necroptosis, and that knocking out TAM kinases can attenuate necroptosis. The linear ubiquitination of RIPK1 can inhibit apoptosis and necroptosis, and thereby plays a critical role in cell survival. Mice with knockout of the RIPK1 gene die from caspase-8-mediated apoptosis and RIP3-mediated necroptosis (Dillon et al., 2014; Kaiser et al., 2014; Rickard et al., 2014). It is currently known that RIPK1 can promote cell survival through two mechanisms. One is by promoting cFLIP recruitment of caspase-8, causing caspase-8 inactivation (Oberst et al., 2011). The other is promoting the activation of NF-κB and pro-survival molecules, including cFLIP, A20, cIAP2 and Bcl2 family members (Micheau and Tschopp, 2003). In addition, RIPK1 can be de-ubiquitinated by zinc finger protein A20 and CYLD, causing RIPK1 downregulation and failure to activate NF-κB signaling (Ea et al., 2006). This is consistent with our keyword frequency analysis results. The statistical analyses of key molecules, pathways and receptors all indicate the importance of the NF-κB pathway in necroptosis. The ubiquitination of RIPK1 is essential for TNF activation by the NF-κB signaling pathway (Draber et al., 2015). Furthermore, the heat shock protein (HSP90)/CDC37 co-chaperone complex increases the stability of the necrosome (Li et al., 2015; Zhao et al., 2016; Wang et al., 2018b). In contrast, HSP70 enhances the stability of a necroptosis antagonist. HSP70 promotes MLKL polymerization to activate necroptosis (Johnston and Wang, 2020).
The statistical analysis of keywords suggests that we need to regard the statistical results of word frequency of keywords as a net structure. As shown in Figure 9, in the statistical results of these keywords representing the core content of the article, words are related to each other. For example, the word frequency of glial cells ranks high, suggesting that the role of glial cells in necroptosis is one of the research hotspots. Diseases related to necroptosis, including stroke and brain injury, are also ranked high in terms of frequency. Neuroinflammation is an important pathological state in these diseases (Yuan et al., 2019), and the important cells involved in the process of neuroinflammation are glial cells (Yang and Zhou, 2019). According to the statistical results of key molecules, the word frequencies of NO and TNF-α also ranked high, indicating that they are mainly related to glial cells in the nervous system (Yang and Zhou, 2019). The results of these reticular structures, from the perspective of bibliometrics, suggest that, on one hand, the molecules, pathways, receptors and diseases involved in the top ranked words may be one of the research hotspots, and there are many published research results, which may inhibit the growth of new directions in the future. On the other hand, the number of research results represented by the lower ranked words is relatively small, which may therefore have explosive growth potential. These results can be used as a reference when investigators select their starting point in necroptosis research.
Necroptosis as a form of necrosis has attracted increasing attention by investigators. Future research directions may focus on noncanonical pathways and subcellular structures such as mitochondria. Phosphorylated RIP3 can activate MLKL, causing MLKL oligomerization and insertion into the membrane. In addition, phosphorylated RIP3 can activate PGAM5, a mitochondrial serine/threonine protein phosphatase, which is a well-recognized major factor controlling necroptosis via multiple mechanisms (Al-Lamki et al., 2016; Couto et al., 2017). PGAM5 is able to influence mitochondrial fission by phosphorylating Drp-1, and can influence mitophagy by regulating FUNDC1 phosphorylation (Wang et al., 2012; Chen et al., 2014). Furthermore, activated PGAM5 can regulate CypD to control mPTP opening (Zhou et al., 2018).
Numerous studies have shown that inflammation also plays an important role in the process of cell death (Pasparakis and Vandenabeele, 2015; Martin et al., 2019). For example, in immune-mediated diseases, the complex of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) plays an important role in the immune response, and this complex may be related to cell death processes (Pisetsky, 2011). DAMP, as the carrier of PAMP (Hernández-Pedro et al., 2016), plays a promoting role in inflammation (Pandolfi et al., 2016), and inflammatory factors (interleukin-1, TNFα, etc.) produced in the process of neuroinflammation participate in necroptosis (Deepa et al., 2018), suggesting that the relationship between the immune response and its associated molecules, DAMP and PAMP, and necroptosis may become a potential research hotspot in the future.
Necroptosis is not only involved in CNS diseases, but also in other pathologies (Choi et al., 2019). Numerous studies have demonstrated that necroptosis is involved in diseases of other systems, including the digestive system, circulatory system, immune system, and genital system (Zhe-Wei et al., 2018; Liu et al., 2019; Mulay et al., 2019; Ruan et al., 2019; Saeed et al., 2019). Nec-1, the top RIPK1 inhibitor in keyword analysis, can inhibit necroptosis in a variety of disease models and reduce the mortality rate of diseases (Shen et al., 2019).
According to our analysis results, it only takes about 10 years to develop programmed death in the nervous system. The collaboration between authors is not great. As seen in Figure 6, all the co-operators appear in the form of “islands”. The center of these “islands” is a core author (the author with strong influence). The center of each “island” is surrounded by the team members of the core author. However, there are few connections between these islands. This indicates that the collaboration between core authors (or core teams) in the same research field is relatively low. Perhaps in the future, with the continuous research on programmed death and the strengthening of cooperation among authors, teams, insitutions, and countries, analysis and discussion regarding collaboration can be further carried out.
This study is the first bibliometric study using visual analysis software to analyze publications of necroptosis in the global field of neuroscience. The publications increased year by year, especially from 2017 to 2019. In the future, the amount of literature will keep increasing continuously. The leading countries were China and USA. The most influential author is Yuan Junying. Furthermore, the research prospects and hotspots might be the detailed mechanisms by which MLKL permeabilizes the membrane and the signaling pathway by which RIP3 activates downstream molecules in the mitochondria. Overall, this study provides insight into the trends and characteristics of necroptosis in the field of neuroscience, and should provide a helpful reference for further in-depth research.
Study limitations: Although this is the first bibliometric study of necroptosis in the field of neuroscience, there are some limitations, as follows: (1) The search was conducted on April 16, 2020 and included all documents up to December 31, 2019, but the Web of Science Core Collection would have been still open for documents related to 2019, and this part was omitted; (2) Only publications with the terms “necroptosis” or “necroptotic” in the title, abstract and keywords were retrieved. However, papers with these terms within the main body of the text were not retrieved for analysis; (3) Each article can only have 3–10 keywords. Because of this limitation, the author could only identify the core content with a keyword if it was present as a keyword in the publication, although it may appear elsewhere in the paper, resulting in incomplete extraction; (4) Because the search was limited to the Web of Science Core Collection indexed journals, a few documents not included in the Web of Science Core Collection were missed. These limitations have also been reported in other bibliometric studies (Wang et al., 2017; Azer and Azer, 2019; Wang et al., 2019).
Additional file: Open peer review report 1 (82KB, pdf) .
Footnotes
P-Reviewer: Deepa SS; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Song LP; T-Editor: Jia Y
Conflicts of interest: None declared.
Financial support: This work was supported by the National Natural Science Foundation of China, Nos. 81772134, 81971891, and 81571939 (to KX); the Key Research and Development Program of Hunan Province of China, No. 2018SK2091 (to KX); Hunan Provincial Innovation Foundation For Postgraduate, No. CX20200116 (to WTY); Wu JiePing Medical Foundation of the Minister of Health of China, No. 320.6750.14118 (to KX); Foundation of Science and Technology of Hunan Province of China, No. 2018JJ2552 (to YC); the Project of Graduate Independent Exploration and Innovation Plan of Central South University of China, No. 2020zzts218 (to WTY). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Sathyaseelan S. Deepa, University of Oklahoma Health Sciences Center, USA.
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 81772134, 81971891, and 81571939 (to KX); the Key Research and Development Program of Hunan Province of China, No. 2018SK2091 (to KX); Hunan Provincial Innovation Foundation For Postgraduate, No. CX20200116 (to WTY); Wu JiePing Medical Foundation of the Minister of Health of China, No. 320.6750.14118 (to KX); Foundation of Science and Technology of Hunan Province of China, No. 2018JJ2552 (to YC); the Project of Graduate Independent Exploration and Innovation Plan of Central South University of China, No. 2020zzts218 (to WTY).
References
- 1.Akmal M, Hasnain N, Rehan A, Iqbal U, Hashmi S, Fatima K, Farooq MZ, Khosa F, Siddiqi J, Khan MK. Glioblastome multiforme: a bibliometric analysis. World Neurosurgery. 2020;136:270–282. doi: 10.1016/j.wneu.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lamki RS, Lu W, Manalo P, Wang J, Warren AY, Tolkovsky AM, Pober JS, Bradley JR. Tubular epithelial cells in renal clear cell carcinoma express high RIPK1/3 and show increased susceptibility to TNF receptor 1-induced necroptosis. Cell Death Dis 7. 2016:e2287. doi: 10.1038/cddis.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrazola MS, Court FA. Compartmentalized necroptosis activation in excitotoxicity-induced axonal degeneration: a novel mechanism implicated in neurodegenerative disease pathology. Neural Regen Res. 2019;14:1385–1386. doi: 10.4103/1673-5374.253520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avena MJ, Barbosa DA. Bibliometric indicators of the nursing journals according to the index databases. Revista Da Escola De Enfermagem Da Usp. 2017;51 doi: 10.1590/s1980-220x2017014603262. doi: 101590/S1980-220X2017014603262. [DOI] [PubMed] [Google Scholar]
- 5.Azer SA, Azer S. Top-cited articles in medical professionalism: a bibliometric analysis versus altmetric scores. BMJ Open 9. 2019:e029433. doi: 10.1136/bmjopen-2019-029433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker M, Shanmugam N, Pham CLL, Strange M, Steain M, Sunde M. RHIM-based protein:protein interactions in microbial defence against programmed cell death by necroptosis. Semin Cell Dev Biol. 2020;99:86–95. doi: 10.1016/j.semcdb.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, Duan L, Wang X, Liu L, Liu X, Shen Y, Zhu Y, Chen Q. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Kos R, Garssen J, Redegeld F. Molecular insights into the mechanism of necroptosis: the necrosome as a potential therapeutic target. Cells. 2019;8 doi: 10.3390/cells8121486. doi: 103390/cells8121486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Yan J, Deng HX, Long LL, Hu YJ, Wang M, Shang L, Chen D, Huang JF, Xiong K. Inhibition of calpain on oxygen glucose deprivation-induced RGC-5 necroptosis. J Huazhong Univ Sci Technolog Med Sci. 2016;36:639–645. doi: 10.1007/s11596-016-1639-y. [DOI] [PubMed] [Google Scholar]
- 10.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019 doi: 10.1172/jci.insight.128834. doi: 10.1172/jci.insight.128834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couto JA, Ayturk UM, Konczyk DJ, Goss JA, Huang AY, Hann S, Reeve JL, Liang MG, Bischoff J, Warman ML, Greene AK. A somatic GNA11 mutation is associated with extremity capillary malformation and overgrowth. Angiogenesis. 2017;20:303–306. doi: 10.1007/s10456-016-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepa SS, Unnikrishnan A, Matyi S, Hadad N, Richardson A. Necroptosis increases with age and is reduced by dietary restriction. Aging Cell 17. 2018:e12770. doi: 10.1111/acel.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 16.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding W, Shang L, Huang JF, Li N, Chen D, Xue LX, Xiong K. Receptor interacting protein 3-induced RGC-5 cell necroptosis following oxygen glucose deprivation. BMC Neurosci. 2015;16:49. doi: 10.1186/s12868-015-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y, He C, Lu S, Wang X, Wang C, Wang L, Zhang J, Piao M, Chi G, Luo Y, Sai K, Ge P. MLKL contributes to shikonin-induced glioma cell necroptosis via promotion of chromatinolysis. Cancer Lett. 2019;467:58–71. doi: 10.1016/j.canlet.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Dowling JP, Nair A, Zhang J. A novel function of RIP1 in postnatal development and immune homeostasis by protecting against RIP3-dependent necroptosis and FADD-mediated apoptosis. Front Cell Dev Biol. 2015;3:12. doi: 10.3389/fcell.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, Rieser E, Martino L, Rittinger K, Walczak H. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13:2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJJ. Activation of IKK by TNF alpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: How great is the impact. Scientometrics. 2015;105:1809–1831. doi: 10.1007/s11192-015-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo LM, Wang Z, Li SP, Wang M, Yan WT, Liu FX, Wang CD, Zhang XD, Chen D, Yan J, Xiong K. RIP3/MLKL-mediated neuronal necroptosis induced by methamphetamine at 39 degrees C. Neural Regen Res. 2020;15:865–874. doi: 10.4103/1673-5374.268902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Hernández-Pedro N, Magana-Maldonado R, Ramiro AS, Pérez-De la Cruz V, Rangel-López E, Sotelo J, Pineda B. PAMP-DAMPs interactions mediates development and progression of multiple sclerosis. Front Biosci (Schol Ed) 2016;8:13–28. doi: 10.2741/s443. [DOI] [PubMed] [Google Scholar]
- 26.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 27.Huang JF, Shang L, Zhang MQ, Wang H, Chen D, Tong JB, Huang H, Yan XX, Zeng LP, Xiong K. Differential neuronal expression of receptor interacting protein 3 in rat retina: involvement in ischemic stress response. BMC Neurosci. 2013;14:16. doi: 10.1186/1471-2202-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Zhou T, Sun X, Zheng Y, Cheng B, Li M, Liu X, He C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018;25:180–189. doi: 10.1038/cdd.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, Geng J, Amin P, DeWitt JP, Mookhtiar AK, Florez M, Ouchida AT, Fan JB, Pasparakis M, Kelliher MA, Ravits J, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–608. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston AN, Wang Z. HSP70 promotes MLKL polymerization and necroptosis. Mol Cell Oncol. 2020 doi: 10.1080/23723556.2020.1791561. doi: 101080/2372355620201791561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- 34.Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW, Park EJ, Chun KH, Song J. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun. 2012;3:978. doi: 10.1038/ncomms1981. [DOI] [PubMed] [Google Scholar]
- 35.Lee SY, Kim H, Li CM, Kang J, Najafov A, Jung M, Kang S, Wang S, Yuan J, Jung YK. Casein kinase-1gamma1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis. 2019;10:923. doi: 10.1038/s41419-019-2146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A. 2015;112:5017–5022. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao L, Shang L, Li N, Wang S, Wang M, Huang Y, Chen D, Huang J, Xiong K. Mixed lineage kinase domain-like protein induces RGC-5 necroptosis following elevated hydrostatic pressure. Acta Biochim Biophys Sin (Shanghai) 2017;49:879–889. doi: 10.1093/abbs/gmx088. [DOI] [PubMed] [Google Scholar]
- 38.Lim J, Park H, Heisler J, Maculins T, Roose-Girma M, Xu M, McKenzie B, van Lookeren Campagne M, Newton K, Murthy A. Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife. 2019 doi: 10.7554/eLife.44452. doi: 107554/eLife44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Liu T, Lei T, Zhang D, Du S, Girani L, Qi D, Lin C, Tong R, Wang Y. RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review) Int J Mol Med. 2019;44:771–786. doi: 10.3892/ijmm.2019.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception. Neural Regen Res. 2019;14:939–947. doi: 10.4103/1673-5374.250566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 44.Molnar T, Mazlo A, Tslaf V, Szollosi AG, Emri G, Koncz G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019;10:860. doi: 10.1038/s41419-019-2094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriwaki K, Chan FK. The inflammatory signal adaptor RIPK3: functions beyond necroptosis. Int Rev Cell Mol Biol. 2017;328:253–275. doi: 10.1016/bs.ircmb.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrice JR, Gregory-Evans CY, Shaw CA. Necroptosis in amyotrophic lateral sclerosis and other neurological disorders. Biochim Biophys Acta Mol Basis Dis. 2017;1863:347–353. doi: 10.1016/j.bbadis.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Mulay SR, Honarpisheh MM, Foresto-Neto O, Shi C, Desai J, Zhao ZB, Marschner JA, Popper B, Buhl EM, Boor P, Linkermann A, Liapis H, Bilyy R, Herrmann M, Romagnani P, Belevich I, Jokitalo E, Becker JU, Anders HJ. Mitochondria permeability transition versus necroptosis in oxalate-induced AKI. J Am Soc Nephrol. 2019;30:1857–1869. doi: 10.1681/ASN.2018121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naito MG, Xu D, Amin P, Lee J, Wang H, Li W, Kelliher M, Pasparakis M, Yuan J. Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc Natl Acad Sci U S A. 2020;117:4959–4970. doi: 10.1073/pnas.1916427117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, Li Y, Lu Q, Yuan J. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol Cell. 2019;75:457–468e4. doi: 10.1016/j.molcel.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, Komuves L, Webster JD, Dixit VM. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019a;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 51.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, Roose-Girma M, Modrusan Z, Sagolla MS, Webster JD, Dixit VM. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019b;575:679–682. doi: 10.1038/s41586-019-1752-8. [DOI] [PubMed] [Google Scholar]
- 52.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 54.Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38:1017–1028. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 56.Paunkov A, Chartoumpekis DV, Ziros PG, Sykiotis GP. A bibliometric review of the Keap1/Nrf2 pathway and its related antioxidant compounds. Antioxidants (Basel) 2019 doi: 10.3390/antiox8090353. doi: 103390/antiox8090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22:1846–1857. doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pisetsky D. Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med Wkly 141. 2011:w13256. doi: 10.4414/smw.2011.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray CA, Pickup DJ. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217:384–391. doi: 10.1006/viro.1996.0128. [DOI] [PubMed] [Google Scholar]
- 60.Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, Phani S, Ikiz B, Hoffmann L, Koolen M, Nagata T, Papadimitriou D, Nagy P, Mitsumoto H, Kariya S, Wichterle H, Henderson CE, Przedborski S. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–1008. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rehorova M, Vargova I, Forostyak S, Vackova I, Turnovcova K, Kupcova Skalnikova H, Vodicka P, Kubinova S, Sykova E, Jendelova P. A Combination of intrathecal and intramuscular application of human mesenchymal stem cells partly reduces the activation of necroptosis in the spinal cord of SOD1(G93A) Rats. Stem Cells Transl Med. 2019;8:535–547. doi: 10.1002/sctm.18-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruan ZH, Xu ZX, Zhou XY, Zhang X, Shang L. Implications of necroptosis for cardiovascular diseases. Curr Med Sci. 2019;39:513–522. doi: 10.1007/s11596-019-2067-6. [DOI] [PubMed] [Google Scholar]
- 65.Saeed WK, Jun DW, Jang K, Koh DH. Necroptosis signaling in liver diseases: an update. Pharmacol Res. 2019;148:104439. doi: 10.1016/j.phrs.2019.104439. [DOI] [PubMed] [Google Scholar]
- 66.Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev. 2018;32:327–340. doi: 10.1101/gad.312561.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shang L, Ding W, Li N, Liao L, Chen D, Huang J, Xiong K. The effects and regulatory mechanism of RIP3 on RGC-5 necroptosis following elevated hydrostatic pressure. Acta Biochim Biophys Sin (Shanghai) 2017;49:128–137. doi: 10.1093/abbs/gmw130. [DOI] [PubMed] [Google Scholar]
- 68.Shao L, Yu S, Ji W, Li H, Gao Y. The contribution of necroptosis in neurodegenerative diseases. Neurochem Res. 2017;42:2117–2126. doi: 10.1007/s11064-017-2249-1. [DOI] [PubMed] [Google Scholar]
- 69.Shen B, Mei M, Pu Y, Zhang H, Liu H, Tang M, Pan Q, He Y, Wu X, Zhao H. Necrostatin-1 attenuates renal ischemia and reperfusion injury via meditation of HIF-1 alpha/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids. 2019;17:701–713. doi: 10.1016/j.omtn.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shindo R, Kakehashi H, Okumura K, Kumagai Y, Nakano H. Critical contribution of oxidative stress to TNF alpha-induced necroptosis downstream of RIPK1 activation. Biochem Biophys Res Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 71.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 72.Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 73.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, Pan H, Bai R, Zhang J, Wang Y, Lee PY, Ying W, Zhou Q, Hou J, Wang W, Sun B, Yang M, Liu D, Fang R, Han H, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577:109–114. doi: 10.1038/s41586-019-1830-y. [DOI] [PubMed] [Google Scholar]
- 74.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 75.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M, Wan H, Wang S, Liao L, Huang Y, Guo L, Liu F, Shang L, Huang J, Ji D, Xia X, Jiang B, Chen D, Xiong K. RSK3 mediates necroptosis by regulating phosphorylation of RIP3 in rat retinal ganglion cells. J Anat. 2020;237:29–47. doi: 10.1111/joa.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang XQ, Peng MS, Weng LM, Zheng YL, Zhang ZJ, Chen PJ. Bibliometric study of the comorbidity of pain and depression research. Neural Plast. 2019;2019:1657498. doi: 10.1155/2019/1657498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Wang Q, Wei X, Shao J, Zhao J, Zhang Z, Chen Z, Bai Y, Wang N, Wang Y, Li M, Zhai X. Global scientific trends on exosome research during 2007-2016: a bibliometric analysis. Oncotarget. 2017;8:48460–48470. doi: 10.18632/oncotarget.17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Guo LM, Wang SC, Chen D, Yan J, Liu FX, Huang JF, Xiong K. Progress in studies of necroptosis and its relationship to disease processes. Pathol Res Prac. 2018a;214:1749–1757. doi: 10.1016/j.prp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Guo LM, Wang Y, Zhou HK, Wang SC, Chen D, Huang JF, Xiong K. Inhibition of HSP90alpha protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J Cell Physiol. 2018b;233:4864–4884. doi: 10.1002/jcp.26294. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 82.Xiong K, Liao H, Long L, Ding Y, Huang J, Yan J. Necroptosis contributes to methamphetamine-induced cytotoxicity in rat cortical neurons. Toxicol In Vitro. 2016;35:163–168. doi: 10.1016/j.tiv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. 2019;67:1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- 84.Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, He J, Liao L, Xiong K, Yi CX, Yan J. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front Mol Neurosci. 2018;11:186. doi: 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeung AWK, Tzvetkov NT, Atanasov AG. When neuroscience meets pharmacology: a neuropharmacology literature analysis. Front Neurosci. 2018;12:852. doi: 10.3389/fnins.2018.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 88.Zhang S, Mao G, Crittenden J, Liu X, Du H. Groundwater remediation from the past to the future: a bibliometric analysis. Water Res. 2017;119:114–125. doi: 10.1016/j.watres.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 89.Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, Shubina M, Ragan KB, Ishizuka T, Crawford JC, Tummers B, Rodriguez DA, Xue J, Peri S, Kaiser WJ, Lopez CB, Xu Y, Upton JW, Thomas PG, Green DR, Balachandran S. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell. 2020;180:1115–1129e1113. doi: 10.1016/j.cell.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, Hou JJ, Cui YM, Jia XL, Zhang SQ. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 7. 2016:e2089. doi: 10.1038/cddis.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhe-Wei S, Li-Sha G, Yue-Chun L. The role of necroptosis in cardiovascular disease. Front Pharmacol. 2018;9:721. doi: 10.3389/fphar.2018.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res. 2018 doi: 10.1111/jpi.12503. doi: 10.1111/jpi.12503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


