Abstract
The canonical Wnt/β-catenin signaling pathway has been shown to play a major role during embryonic development and maturation of the central nervous system including the retina. It has a significant impact on retinal vessel formation and maturation, as well as on the establishment of synaptic structures and neuronal function in the central nervous system. Mutations in components of the Wnt/β-catenin signaling cascade may lead to severe retinal diseases, while dysregulation of Wnt signaling can contribute to disease progression. Apart from the angiogenic role of Wnt/β-catenin signaling, research in the last decades leads to the theory of a protective effect of Wnt/β-catenin signaling on damaged neurons. In this review, we focus on the neuroprotective properties of the Wnt/β-catenin pathway as well as its downstream signaling in the retina.
Keywords: apoptosis, β-catenin, leukemia inhibitory factor, Müller cells, neurodegeneration, neuroprotection, Norrin, photoreceptors, retina, retinal ganglion cells, Wnt
Introduction
The canonical Wnt/β-catenin signaling pathway is highly conserved among various species and plays an essential role in embryogenesis and adult tissue homeostasis (MacDonald et al., 2009). Over the course of the last decades, a variety of Wnt downstream genes and mutations in Wnt signaling components have been identified. The latter may be involved in cancer development, cardiovascular disorders and bone diseases (Clevers and Nusse, 2012). Furthermore, it is of high importance during vascular morphogenesis in different organs, including the eye. On one hand, mutations in Wnt ligands and receptors that are involved in ocular angiogenesis leading to a decreased expression of Wnt signaling downstream genes can cause genetic diseases such as the Norrie disease and familial exudative vitreoretinopathy – both of them result from failure of retinal vascular development (Selvam et al., 2018). On the other hand, Wnt/β-catenin signaling was observed to be upregulated in proliferative vascular retinal diseases such as proliferative diabetic retinopathy (Chen and Ma, 2017) or choroidal neovascularization in age-related macular degeneration (Tuo et al., 2015). Therefore, a therapeutic approach to modulate Wnt/β-catenin signaling by suppression or promotion of vascular regrowth is of high interest. For example, Wang et al. (2016) showed pro-proliferative effects on retinal vasculature using lithium, an activator of Wnt/β-catenin signaling, in a murine model of familial exudative vitreoretinopathy. In line, in a previous work, we observed retinal vessel regrowth in oxygen-induced retinopathy, a model for the retinopathy of prematurity in mice, when applying the atypical Wnt ligand Norrin (Ohlmann et al., 2010).
Besides its effects on retinal vessel formation and maturation, Wnt/β-catenin signaling plays a crucial role in protection of damaged retinal neurons, which is the focus of the following review article.
Search Strategy
We performed a literature search on Wnt-mediated retinal neuroprotection in PubMed and Google Scholar until June 2020 published in English or German. The key words/terms were neuroprotection, neuronal degeneration, retina, retinal ganglion cells, Norrin, Wnt, (beta)-Catenin, FZD, LRP, Müller glia cells, glaucoma and retinitis pigmentosa.
Canonical Wnt/β-Catenin Signaling Pathway
Wnt/β-catenin signaling is common in mammalian cells and crucial for a variety of fundamental cellular processes, such as cell proliferation and differentiation, stem cell maintenance during embryonic development, and tissue homeostasis in adult organisms (Logan and Nusse, 2004). The Wnt family in mammalian organisms includes 19 secreted Wnt glycolipoproteins that can bind to 10 different frizzled (Fzd) receptors. For activation of the canonical Wnt/β-catenin signaling pathway, a frizzled receptor has to be activated by a ligand and has to dimerize with a co-receptor, for example low-density lipoprotein receptor-related protein (LRP)5 or 6 (He et al., 2004). A major key regulator in canonical Wnt/β-catenin signaling is the transcription factor β-catenin, which is constitutively expressed in cells. Without activation, β-catenin binds to axin and thus forms the β-catenin degradation complex. This complex comprises the casein kinase 1α, glycogen synthase kinase 3β and the adenomatous polyposis coli gene product. In the complex β-catenin is continuously phosphorylated and consecutively ubiquitinated by the beta-transducin repeat containing E3 ubiquitin protein ligase resulting in β-catenin degradation in the proteasome (Figure 1; MacDonald et al., 2009). Activation of Wnt signaling is achieved via β-catenin stabilization. After binding of a Wnt ligand, the Fzd receptor dimerizes with its co-receptor LRP5 or 6 to recruit the cytoplasmatic proteins disheveled and axin to the cell membrane, which subsequently inactivates the β-catenin degradation complex. Eventually, β-catenin accumulates in the cytoplasm, translocates into the nucleus and induces the expression of Wnt specific target genes in association with the transcription factors Lymphoid enhancer-binding factor-1 and T-cell factor-1 (Figure 1; MacDonald et al., 2009).
Figure 1.
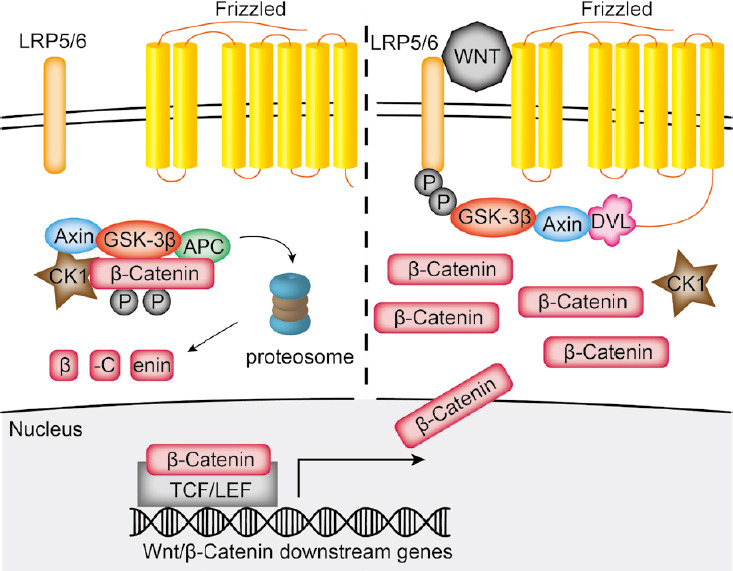
Schematic drawing of the canonical Wnt signaling pathway.
In the non-activated state, constitutively expressed β-catenin is phosphorylated and degraded via the proteasome. After binding of a Wnt ligand, the frizzled receptor will dimerize with its co-receptor LRP5 or 6, which leads to recruitment of disheveled (DVL) and axin to the membrane and subsequent inactivation of the β-catenin degradation complex. Thus, β-catenin can translocate in the nucleus and induce the expression of specific target gene. APC: Adenomatous polyposis coli; CK-1: casein kinase-1; GSK-3β: glycogen synthase kinase 3β; LEF: lymphoid enhancer-binding factor-1; LRP: low-density lipoprotein receptor-related protein; TCF: T-cell factor-1.
The intensity of the Wnt/β-catenin signaling pathway can be modified at many levels. This includes an altered expression of secreted proteins that are antagonists to the Wnt ligand, such as secreted Frizzled-related proteins (sFrp) and Wnt inhibitory proteins. Both are able to bind Wnt and thus inhibit interactions between the Wnt proteins and Fzd receptors (Bovolenta et al., 2008). Further endogenous Wnt antagonists comprise proteins of the Dickkopf (Dkk) and the WISE/Sclerostin family, which are antagonists of the Wnt/β-catenin signaling pathway via interaction with the LRP5/6 receptor (Glinka et al., 1998). Both Dkk1 and Sclerostin interfere with the LRP5 or 6 co-receptors and hence block the dimerization between Fzd and LRP receptor (Semenov et al., 2005; Ellwanger et al., 2008).
Wnt/β-Catenin Signaling in Retinal Pathology
First hints of protective effects of canonical Wnt/β-catenin signaling in the retina were obtained in patients suffering from retinitis pigmentosa, which had an increased expression of sFrp-1, -2, -3 and -5 when compared to normal retinae (Jones et al., 2000a, b). Since sFrp-2 has a cysteine-rich domain like the putative Wnt-binding site of frizzled receptors, its extracellular binding of Wnt proteins could lead to decreased Wnt/β-catenin signaling. In turn, an enhancement of the photoreceptor damage in retinitis pigmentosa could occur. However, in rd1 mice, which suffer from early onset retinal degeneration, an enhanced expression of Dkk-3, an activator of Wnt/β-catenin signaling (Nakamura et al., 2007), Wnt-5a, Wnt-5b and Wnt-10a was observed (Hackam et al., 2004; Yi et al., 2007), thus strongly suggesting that Wnt/β-catenin signaling could play a role in endogenous mechanisms to attenuate photoreceptor loss.
Protective Effects of Wnt/β-Catenin Signaling on Retinal Neurons in vitro
Protective effects of enhanced Wnt/β-catenin signaling on retinal neurons can be mediated directly or indirectly via other cell types, which in turn enhance their expression of neuroprotective factors.
Direct protective effects of enhanced retinal Wnt/β-catenin signaling were observed for the first time in primary retinal culture. After treatment with hydrogen peroxide, the additional activation of the Wnt/β-catenin pathways with SB216763 and Wnt-3a led to an enhanced survival of retinal neurons (Yi et al., 2007). In line, the overexpression of Wnt14 in retinal precursor cells (R28 cells) leads to a significantly reduced cell loss following serum deprivation or treatment with glutamate when compared to control cells without Wnt14 expression (Mizukami et al., 2009). As a potential mechanism of the protective effect of Wnt14, a reduced activity of caspase-3 was detected (Mizukami et al., 2009).
In the retina, particular effort has been made to analyze the direct protective effects of Wnt/β-catenin signaling on retinal ganglion cells, which are located in the inner retina and project their axons to the brain. In immortalized retinal ganglion cells (RGC)-5, the incubation with Wnt3a induced a substantial activation of canonical Wnt/β-catenin signaling, which was detected by immunohistochemistry and top-flash luciferase reporter assay measuring transcriptional activity of this pathway (Fragoso et al., 2011). Following treatment of RGC-5 cells with an elevated pressure, the additional incubation of the cells with Wnt3a led to a modest but significant reduction of apoptotic cells (Fragoso et al., 2011). The protective effect of Wnt3a could be blocked, at least in part, by Dkk-1, strongly suggesting that Wnt3a mediates its protective properties via canonical Wnt/β-catenin signaling (Fragoso et al., 2011). Since in conditioned cell culture medium of RGC-5 cells, the concentration of brain-derived neurotrophic factor (BDNF), neurotrophin-3 and nerve growth factor was increased after treatment with elevated pressure and Wnt3a (Fragoso et al., 2011), it is most likely that Wnt3a mediates its protective effects via these factors in an autocrine manner. In line, following treatment of staurosporine-differentiated RGC-5 cells in serum-derivated cell culture medium with Norrin, an atypical activator of Wnt/β-catenin signaling, a moderate increase of cell survival was observed (Seitz et al., 2010). However, the protective effect of Norrin on RGC-5 cells could not be blocked by Dkk-1 (Seitz et al., 2010), suggesting that non-canonical Wnt signaling or additional protective pathways might be involved. Intriguingly, RGC-5 cells themselves are able to enhance their expression of Wnt3a. In RGC-5 cells treated with anoxia and glucose deprivation, astragaloside, a component of Astragalus membranaceus, inhibits apoptosis via induction of miR21, which in turn rises Wnt3a expression and subsequently activates Wnt/β-catenin signaling (Bao et al., 2019). The protective effects of astragaloside were substantially blocked when Dkk-1 was added (Bao et al., 2019), thus strongly suggesting that astragaloside mediates, at least in part, its protective properties via the Wnt/β-catenin pathway.
Protective Effects of Wnt/β-Catenin Signaling on Retinal Neurons after Acute Damage
To investigate the protective effects of the Wnt/β-catenin pathway and the underlying signaling network in the retina, various animal models inducing acute damage as well as chronic degeneration of RGC or photoreceptors have been established.
In mice after intravitreal injection of N-methyl-D-aspartic acid (NMDA) to induce an acute excitotoxic damage of RGC, the additional treatment with Norrin prevents apoptosis of these cells (Seitz et al., 2010). The Norrin-mediated effects were accompanied by increased retinal β-catenin levels and could be blocked by the additional intravitreal injection of Dkk-1, an inhibitor of canonical Wnt/β-catenin signaling (Seitz et al., 2010). Further on, in RNA from retinae, which were treated with NMDA and Norrin, an increased expression of leukemia inducible factor (Lif), endothelin-2 (Edn2), fibroblast growth factor-2 (Fgf2), Bdnf, lens epithelium-derived growth factor and ciliary neurotrophic factor was detected (Figure 2); Seitz et al., 2010). In addition, after acute excitotoxic damage the additional activation of Wnt/β-catenin signaling further enhances the gliosis reaction of Müller cells (Seitz et al., 2010), suggesting that Müller cells might be the source of the enhanced expression of protective factors. In line, activation of Wnt/β-catenin signaling in cultured Müller cells by Norrin induced the expression of protective factors which in turn protects differentiated RGC-5 cells from degeneration (Seitz et al., 2010).
Figure 2.
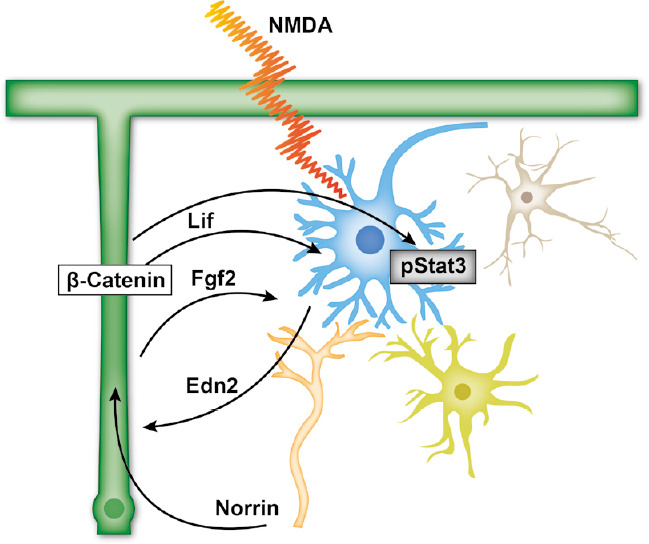
Schematic drawing of the Wnt/β-catenin-Lif-Stat3 axis.
After retinal damage, Wnt3a or Norrin promotes Wnt/β-catenin signaling, which induces the expression of Lif. In turn, Lif further enhances Müller cell gliosis that induces the expression of several other protective factors and activates Stat3 signaling. Edn2: Endothelin-2; Fgf2: fibroblast growth factor-2; Lif: leukemia inducible factor; NMDA: N-methyl-D-aspartic acid.
For mediating of the Wnt/β-catenin induced neuroprotective effects Lif appears to be a central mediator. Following acute excitotoxic damage of RGC in Lif deficient mice the additional treatment of Norrin had no effect on the survival of RGC (Kassumeh et al., 2020). Further on, the significant increase of Fgf2 and Edn2 expression observed in Norrin-treated wild-type mice was blocked in Lif deficient mice (Kassumeh et al., 2020). Overall, following acute excitotoxic damage of RGC, Norrin enhances the expression of Lif via Wnt/β-catenin signaling, which in turn most likely mediates protective effects on RGC directly or indirectly via the induction of protective factors such as Fgf2 or Edn2 (Figure 2).
Activated Wnt/β-catenin signaling not only mediates protective effects on RGC after acute damage, but also on photoreceptors after light-induced damage. In transgenic mice with an overexpression of Norrin in the retinal pigment epithelium (RPE) to activate retinal Wnt/β-catenin signaling, less TUNEL positive cells and more nuclei in the outer nuclear layer were detected following light-induced damage when compared to wild-type littermates, an effect that could be blocked by Dkk-1 (Braunger et al., 2013). Further on, after damage of photoreceptors in these transgenic mice, the expression of brain-derived neurotrophic factor and phosphorylation of Akt, a powerful molecule to protect various neurons, was increased (Braunger et al., 2013). Since in transgenic mice with an overexpression of Norrin in the RPE, the expression of glial fibrillary acidic protein (GFAP), a common marker for Müller cell gliosis, is increased, it is tempting to speculate that after light-induced damage of photoreceptors, retinal Wnt/β-catenin signaling induces the expression of BDNF in Müller cells, which in turn leads to an enhanced activation of Akt. Homologous results were obtained in rats with an N-methyl-N-nitrosourea (MNU)-mediated degeneration of photoreceptors after activation of the Wnt/β-catenin pathway by lithium chloride (LiCl), which inhibits the glycogen synthase kinase 3β but also activates Akt signaling (Chalecka-Franaszek and Chuang, 1999; Ryves and Harwood, 2001). Following activation of Wnt/β-catenin signaling in combination with MNU treatment, a significant improvement of the electroretinogram and an increased survival of photoreceptors were detected when compared to MNU only injected animals (Wang et al., 2017). In this animal model LiCl enhances both Wnt/β-catenin and Akt signaling (Wang et al., 2017), which both presumably mediate the protective effect of LiCl.
Impact of Müller Cells for Mediating the Neuroprotective Effects of Wnt/β-Catenin Signaling on Retinal Neurons
Following damage of retinal neurons, the gliosis reaction of Müller cells plays a central role for retinal maintenance but also degeneration (Bringmann et al., 2009). Depending on environmental conditions as well as on the nature of damaged neurons, Müller cells can mediate protective but also devastating signals promoting survival or apoptosis of several retinal neurons (Bringmann et al., 2009). Different pathways have been shown to modify Müller cell gliosis in the retina such as Lif and Edn2 signaling (Joly et al., 2009). Intriguingly, following induced Müller cell disruption in transgenic mice, an enhanced retinal Wnt/β-catenin signaling was observed (Zhu et al., 2018). As a potential signaling mechanism, a decreased expression of the Wnt/β-catenin inhibitors sFrp-3, Dkk-1 and -3 as well as of components of the β-catenin degradation complex was assumed (Zhu et al., 2018). In line, in wild-type mice, an acute damage of RGC with NMDA led to increased levels of β-catenin (Seitz et al., 2010; Boesl et al., 2020).
To investigate whether endogenous Wnt/β-catenin signaling in Müller cells mediates protective effects on retinal neurons and gliosis reaction of Müller cells, mice with an inducible deletion of the β-catenin gene were treated with NMDA to induce an excitotoxic damage of RGC. Following treatment of mice with a β-catenin deficiency in Müller cells with NMDA, more apoptotic cells in the RGC layer and less RGC axons in optic nerves were detected when compared to wild-type littermates (Boesl et al., 2020). Further on, in the retina of these mice, the mRNA expression of Lif and Fgf2 was blocked (Boesl et al., 2020) strongly suggesting that endogenous Wnt/β-catenin signaling in Müller cells mediated protective effects on damaged retinal neurons via an enhanced expression of neuroprotective factors (Figure 2). Intriguingly, in the retina of mice with a β-catenin deficiency in Müller cells the expression of Gfap mRNA, a common marker for Müller cell gliosis, was significantly increased when compared to wild-type controls. The additional treatment with NMDA did not lead to a further increased Gfap expression (Boesl et al., 2020) corroborating the essential role of the Wnt/β-catenin pathway for the maintenance of Müller cell homeostasis and gliosis reaction. Overall, to protect damaged retinal neurons, one key mechanism of the Wnt/β-catenin pathway is an enhanced gliosis reaction of Müller cells, which in turn leads to an enhanced expression of various neuroprotective factors. Even though the expression of specific factors in Müller cells seems to depend on the nature of the damage and/or environmental conditions, it is tempting to speculate that the canonical Wnt/β-catenin signaling in Müller cells activates specific signaling networks to protect retinal neurons.
Protective Effects of Wnt/β-Catenin Signaling on Retinal Neurons Suffering from a Chronic Degeneration
In humans and mice suffering from a retinal degeneration, an altered expression of Wnts and sFrp has been observed indicating a role of Wnt/β-catenin signaling to modify apoptotic processes in retinal neurons.
To investigate whether enhanced Wnt/β-catenin signaling can protect retinal neurons against a chronic degeneration of photoreceptors, Wnt/β-catenin signaling was enhanced via injection of Wnt3a or an adenoviral overexpression of β-catenin in the retina of rd10 mice. The phenotype of rd10 mice is characterized by a chronic loss of photoreceptors and sclerotic vessels similar to the phenotype of Retinitis pigmentosa in humans (Chang et al., 2002). Following Wnt3a injection or adenoviral overexpression of β-catenin, an enhanced Wnt/β-catenin signaling in the retina was detected leading to an improved ERG and increased survival of photoreceptors (Patel et al., 2015). The protective effects of enhanced Wnt/β-catenin signaling were mediated via an enhanced phosphorylation of Stat3, which is a well-known neuroprotective signaling mechanism downstream of Lif (Figure 2; Patel et al., 2015). Further on, protective effects of enhanced Wnt/β-catenin signaling were also observed in DBA/2J mice suffering from a chronic degeneration of RGC. In this mouse model, Wnt/β-catenin signaling was increased by a transgenic overexpression of Norrin in the inner retina (Leopold et al., 2017). In DBA/2J mice, the intraocular pressure increases from an age of 6 months on with the result of a significant loss of RGC in 1-year-old mice (John et al., 1998). In transgenic mice with a retinal overexpression of Norrin an enhanced Wnt/β-catenin signaling in the inner retina was detected with the result of approximately 30% more RGC axons in optic nerves in 1-year-old animals when compared to wild-type controls (Leopold et al., 2017). However, in the retina of transgenic mice, only a weak increase of Gfap, Fgf2, Edn2 or Bdnf mRNA levels and no signal for Lif were detected, while an enhanced expression of insulin-like growth factor (Igf)1 was observed, which in turn led to an increased activation of Akt signaling in the inner retina (Leopold et al., 2017). Overall, these results strongly suggest that in the context of a chronic degeneration of RGCs, Müller cell gliosis could only play a minor role and Wnt/β-catenin signaling activates the Akt pathway via the induction of Igf1 to mediate its protective effects. Intriguingly, the Norrin-mediated activation of Wnt/β-catenin signaling was not only restricted to the retina but was also observed in the trabecular meshwork, which led to a lower intraocular pressure (Leopold et al., 2017).
Therapeutic Relevance and Translational Potential
In the retina, neurodegenerative processes play an important role in the pathogenesis of various ocular diseases such as glaucoma or retinitis pigmentosa.
Glaucoma is a multifactorial disease. One key factor in its pathogenesis is an increased intraocular pressure. As a result, a progressive damage of retinal ganglion cells occurs that subsequently leads to optic nerve degeneration (Russo et al., 2016). Consecutively, patients may experience visual field defects, and eventually complete loss of vision. Besides the intraocular pressure, a genetic component is undeniable, as can be seen in primary congenital glaucoma (Siggs et al., 2019). However, to date only in 5% of all primary open angle glaucoma cases specific mutated gene loci could be identified. As research proceeds, more mutations are to be found (Shiga et al., 2018). Still, the development of a causal gene therapy is unlikely until major genes responsible for glaucoma are finally revealed.
Retinitis pigmentosa is a retinal dystrophy caused by a step-by-step loss of photoreceptors with subsequent loss of RPE cells. Typically, patients describe night blindness in early disease stages, followed by a decrease of peripheral vision resulting in the so-called “tunnel vision” (Mrejen et al., 2017). Over the course of the last years, more than 150 different mutations in the rhodopsin gene have been identified to cause retinitis pigmentosa. Establishing a gene therapy for every single mutation seems unlikely in the near future.
As it is still a long way towards causal treatments for the aforementioned or other neurodegenerative ocular diseases, pharmacological neuroprotection of retinal ganglion cells and photoreceptors resulting in a prolonged cell survival would be game-changing. A major advantage of utilizing the canonical Wnt/β-catenin signaling pathway is its multilateral approach via modulation of different subsequent signaling pathways.
However, one has to keep in mind that inappropriate activation of Wnt/β-catenin signaling may be procarcinogenic, most commonly promoting colon cancer via dysfunction of the adenomatous polyposis coli protein, which is part of the β-catenin degradation complex (Clevers and Nusse, 2012). Nevertheless, most of the ocular tissue is of post-mitotic, neural origin and therefore unlikely to undergo malign transformation. In addition, as part of the blood-ocular barrier, the retina is delimitated from the surrounding blood vessels via a tight blood-retinal barrier. Thus, intravitreally administered pharmacological agents, such as ranibizumab, which is regularly used in the treatment of age-related macular degeneration typically show only minor systemic concentrations with a fast clearance (Avery et al., 2017).
To conclude, pharmacological neuroprotection via enhancement of Wnt/β-catenin signaling intravitreally and ideally specifically targeting retinal ganglion cells, photoreceptors or Müller cells would be a promising novel approach in the treatment of severe neurodegenerative diseases of the retina.
Conclusion and Future Perspective
In summary, activation of the Wnt/β-catenin pathway in damaged retinae activates a protective signaling network that involves both direct as well as indirect mechanisms. For the indirect mechanisms, the Wnt/β-catenin-mediated enhanced Müller cell gliosis appears to play a central role, since the activation or deletion of Wnt/β-catenin in Müller cells leads to increased or decreased expression of neuroprotective factors, which in turn activate several signaling pathways. Further on, the Lif -Stat3 axis, seems to play a major role in these pathways, since Lif signaling is required for Müller cell gliosis but also mediates protective effects on damaged retinal neurons (Patel et al., 2015; Kassumeh et al., 2020). However, several reports indicate that for Wnt/β-catenin-mediated protection of retinal neurons, various downstream signaling pathways will be activated depending on the nature of damage and the type of retinal neuron. Whether there is a common superordinate signaling coordinating the different downstream pathways has to be analyzed in prospective studies.
In the retina other superordinate signaling pathways are also supposed to mediate protective effects on damaged neurons. One of those is the sonic hedgehog signaling pathway, which is known for its role in the embryonic development and cancer biology. Intriguingly, a crosstalk between sonic hedgehog signaling and the Wnt/β-catenin pathway has been described before (McNeill et al., 2013). Since sonic hedgehog signaling mediates neuroprotective effects in the brain (Liu et al., 2018), it is tempting to speculate if sonic hedgehog but also other signaling pathways could be part of the neuroprotective Wnt/β-catenin signaling network in the retina. In addition, it could be worthwhile to test if components of the Wnt/β-catenin signaling network can be used to develop treatment strategies for patients suffering from a chronic degeneration of retina neurons.
Footnotes
Co-Editors: Zhao M, Wang L; T-Editor: Jia Y
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This work was supported by the Deutsche Forschungsgemeinschaft (OH 214/4-3; FOR 1075, TP7).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Co-Editors: Zhao M, Wang L; T-Editor: Jia Y
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (OH 214/4-3; FOR 1075, TP7).
References
- 1.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Maia M, Van Everen S, Le K, Hanley WD. Systemic pharmacokinetics and pharmacodynamics of intravitreal Aflibercept, Bevacizumab, and Ranibizumab. Retina. 2017;37:1847–1858. doi: 10.1097/IAE.0000000000001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao H, Sun D, Qi P, Jiang S. Astragaloside protects oxygen and glucose deprivation induced injury by regulation of microRNA-21 in retinal ganglion cell line RGC-5. Biomed Pharmacother. 2019;109:1826–1833. doi: 10.1016/j.biopha.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Boesl F, Drexler K, Muller B, Seitz R, Weber GR, Priglinger SG, Fuchshofer R, Tamm ER, Ohlmann A. Endogenous Wnt/beta-catenin signaling in Muller cells protects retinal ganglion cells from excitotoxic damage. Mol Vis. 2020;26:135–149. [PMC free article] [PubMed] [Google Scholar]
- 4.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 5.Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis. 2013;50:1–12. doi: 10.1016/j.nbd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Ma JX. Canonical Wnt signaling in diabetic retinopathy. Vision Res. 2017;139:47–58. doi: 10.1016/j.visres.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragoso MA, Yi H, Nakamura RE, Hackam AS. The Wnt signaling pathway protects retinal ganglion cell 5 (RGC-5) cells from elevated pressure. Cell Mol Neurobiol. 2011;31:163–173. doi: 10.1007/s10571-010-9603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 14.Hackam AS, Strom R, Liu D, Qian J, Wang C, Otteson D, Gunatilaka T, Farkas RH, Chowers I, Kageyama M, Leveillard T, Sahel JA, Campochiaro PA, Parmigiani G, Zack DJ. Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci. 2004;45:2929–2942. doi: 10.1167/iovs.03-1184. [DOI] [PubMed] [Google Scholar]
- 15.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 16.John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 17.Joly S, Samardzija M, Wenzel A, Thiersch M, Grimm C. Nonessential role of beta3 and beta5 integrin subunits for efficient clearance of cellular debris after light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2009;50:1423–1432. doi: 10.1167/iovs.08-2432. [DOI] [PubMed] [Google Scholar]
- 18.Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Altered expression of secreted frizzled-related protein-2 in retinitis pigmentosa retinas. Invest Ophthalmol Vis Sci. 2000a;41(6):1297–1301. [PubMed] [Google Scholar]
- 19.Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000b;11:3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kassumeh S, Leopold S, Fuchshofer R, Thomas CN, Priglinger SG, Tamm ER, Ohlmann A. Norrin protects retinal ganglion cells from excitotoxic damage via the induction of leukemia inhibitory factor. Cells. 2020;9:227. doi: 10.3390/cells9020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopold SA, Zeilbeck LF, Weber G, Seitz R, Bosl MR, Jagle H, Fuchshofer R, Tamm ER, Ohlmann A. Norrin protects optic nerve axons from degeneration in a mouse model of glaucoma. Sci Rep. 2017;7:14274. doi: 10.1038/s41598-017-14423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Zhao B, Xiong X, Xia Z. The neuroprotective roles of sonic hedgehog signaling pathway in ischemic stroke. Neurochem Res. 2018;43:2199–2211. doi: 10.1007/s11064-018-2645-1. [DOI] [PubMed] [Google Scholar]
- 23.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeill B, Mazerolle C, Bassett EA, Mears AJ, Ringuette R, Lagali P, Picketts DJ, Paes K, Rice D, Wallace VA. Hedgehog regulates Norrie disease protein to drive neural progenitor self-renewal. Hum Mol Genet. 2013;22:1005–1016. doi: 10.1093/hmg/dds505. [DOI] [PubMed] [Google Scholar]
- 26.Mizukami M, Souchelnytskyi N, Kiuchi Y, Kanamoto T. Wnt14 inhibits death of retinal precursor cells. Exp Eye Res. 2009;89:462–468. doi: 10.1016/j.exer.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Mrejen S, Audo I, Bonnel S, Sahel JA. Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2017;58:191–201. doi: 10.1159/000455281. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura RE, Hunter DD, Yi H, Brunken WJ, Hackam AS. Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol. 2007;8:52. doi: 10.1186/1471-2121-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlmann A, Seitz R, Braunger B, Seitz D, Bosl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci. 2010;30:183–193. doi: 10.1523/JNEUROSCI.3210-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, Lee T, Hackam AS. Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology. 2015;91:1–12. doi: 10.1016/j.neuropharm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur J Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 32.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 33.Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvam S, Kumar T, Fruttiger M. Retinal vasculature development in health and disease. Prog Retin Eye Res. 2018;63:1–19. doi: 10.1016/j.preteyeres.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 36.Shiga Y, Akiyama M, Nishiguchi KM, Sato K, Shimozawa N, Takahashi A, Momozawa Y, Hirata M, Matsuda K, Yamaji T, Iwasaki M, Tsugane S, Oze I, Mikami H, Naito M, Wakai K, Yoshikawa M, Miyake M, Yamashiro K Japan Glaucoma Society Omics Group (JGS-OG) Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Hum Mol Genet. 2018;27:1486–1496. doi: 10.1093/hmg/ddy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siggs OM, Souzeau E, Pasutto F, Dubowsky A, Smith JEH, Taranath D, Pater J, Rait JL, Narita A, Mauri L, Del Longo A, Reis A, Chappell A, Kearns LS, Staffieri SE, Elder JE, Ruddle JB, Hewitt AW, Burdon KP, Mackey DA, et al. Prevalence of FOXC1 variants in individuals with a suspected diagnosis of primary congenital glaucoma. JAMA Ophthalmol. 2019;137:348–355. doi: 10.1001/jamaophthalmol.2018.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu F, Qian H, Shen D, Penalva R, Xu H, Ma JX, Chan CC. Wnt signaling in age-related macular degeneration: human macular tissue and mouse model. J Transl Med. 2015;13:330. doi: 10.1186/s12967-015-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Hu C, Yang X, Du F, Feng Y, Li H, Zhu C, Yu X. Inhibition of GSK-3beta activation protects SD rat retina against N-Methyl-N-Nitrosourea-induced degeneration by modulating the Wnt/beta-catenin signaling pathway. J Mol Neurosci. 2017;63:233–242. doi: 10.1007/s12031-017-0973-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Liu CH, Sun Y, Gong Y, Favazza TL, Morss PC, Saba NJ, Fredrick TW, He X, Akula JD, Chen J. Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am J Pathol. 2016;186:2588–2600. doi: 10.1016/j.ajpath.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi H, Nakamura RE, Mohamed O, Dufort D, Hackam AS. Characterization of Wnt signaling during photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2007;48:5733–5741. doi: 10.1167/iovs.07-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L, Shen W, Zhang T, Wang Y, Bahrami B, Zhou F, Gillies MC. Characterization of canonical Wnt signalling changes after induced disruption of Muller cell in murine retina. Exp Eye Res. 2018;175:173–180. doi: 10.1016/j.exer.2018.06.016. [DOI] [PubMed] [Google Scholar]


