Abstract
The degree of nerve regeneration after peripheral nerve injury can be altered by the microenvironment at the site of injury. Stem cells and vascularity are postulated to be a part of a complex pathway that enhances peripheral nerve regeneration; however, their interaction remains unexplored. This review aims to summarize current knowledge on this interaction, including various mechanisms through which trophic factors are promoted by stem cells and angiogenesis. Angiogenesis after nerve injury is stimulated by hypoxia, mediated by vascular endothelial growth factor, resulting in the growth of pre-existing vessels into new areas. Modulation of distinct signaling pathways in stem cells can promote angiogenesis by the secretion of various angiogenic factors. Simultaneously, the importance of stem cells in peripheral nerve regeneration relies on their ability to promote myelin formation and their capacity to be influenced by the microenvironment to differentiate into Schwann-like cells. Stem cells can be acquired through various sources that correlate to their differentiation potential, including embryonic stem cells, neural stem cells, and mesenchymal stem cells. Each source of stem cells serves its particular differentiation potential and properties associated with the promotion of revascularization and nerve regeneration. Exosomes are a subtype of extracellular vesicles released from cell types and play an important role in cell-to-cell communication. Exosomes hold promise for future transplantation applications, as these vesicles contain fewer membrane-bound proteins, resulting in lower immunogenicity. This review presents pre-clinical and clinical studies that focus on selecting the ideal type of stem cell and optimizing stem cell delivery methods for potential translation to clinical practice. Future studies integrating stem cell-based therapies with the promotion of angiogenesis may elucidate the synergistic pathways and ultimately enhance nerve regeneration.
Keywords: angiogenesis, exosomes, nerve graft, nerve regeneration, peripheral nerve injury, revascularization, Schwann cells, stem cells, stem cell delivery, vascularity
Introduction
Patients with peripheral nerve injuries (PNI) can face severe disability resulting in sensory loss, motor deficits, and neuropathic pain. These deficits may result in a devastating impact on a patient’s quality of life (Sullivan et al., 2016). Despite advancements in microsurgical techniques and basic and translational research, surgical reconstruction of PNIs continues to have unsatisfactory clinical outcomes, particularly for reconstructions of major mixed motor and sensory nerves (Lundborg, 2000; Khalifian et al., 2015). When end-to-end tension-free neurorrhaphy is not possible, the current gold standard remains reconstruction with autologous cabled nerve graft interposition after excision of the injured nerve stumps. Harvest of autologous nerves faces associated drawbacks, such as permanent donor site morbidity with loss of sensation in the distribution of the harvested nerve (Sunderland, 1952; Terzis et al., 1975; Ray and Mackinnon, 2010; De la Rosa et al., 2018). Attempts to create a commercially available nerve graft substitute have resulted in a variety of bioabsorbable synthetic conduits or decellularized human allograft nerves. Their clinical efficacy has yet to equal or surpass autologous nerve grafts, especially for defects greater than three centimeters (Kornfeld et al., 2019).
Engineering of a synthetic substrate for nerve regeneration to mimic the ultrastructure of autologous nerves has been extremely challenging. The use of human allograft fresh nerves for reconstruction requires systemic immunosuppression to prevent graft rejection and is associated with side effects, such as severe opportunistic infections (Bulatova et al., 2011; Saffari et al., 2019). An alternative to fresh human allograft nerves or to engineering a nerve graft is to decellularize and process human allograft nerve. Decellularized allografts serve as a temporary scaffold for regenerating nerve fibers and do not require systemic immunosuppression due to diminished graft rejection potential. Decellularized allografts provide the essential ultrastructural elements and may be pretreated with irradiation, cold preservation, trophic factors, or seeded with Schwann cells or stem cells, to advance outcomes after peripheral nerve reconstruction (De la Rosa et al., 2018; Patel et al., 2018). Stem cell-based therapy may offer a suitable treatment with several regenerative benefits to restore neuronal function, including supporting remyelination and revascularization of the affected organ (Jiang et al., 2017). Specifically, stem cells that have been differentiated into Schwann-like cells, mimicking the function of the original facilitators of axonal regeneration, may enhance neuron survival to improve functional outcomes (Keilhoff et al., 2006; De la Rosa et al., 2018). Growth factors secreted by stem cells may enhance angiogenesis, the sprouting of new capillaries from preexisting ones, to promote revascularization (Risau, 1997; Rissanen et al., 2004; Caplan, 2015; Mathot et al., 2019).
The exact interaction between stem cells and vascularity remains unexplored and is postulated to be part of a complex pathway that enhances peripheral nerve regeneration. This review will provide an in-depth perspective of currently available stem cell-based therapy applications in PNIs and discusses the interaction between stem cells and vascularity in peripheral nerve regeneration. Furthermore, sources of stem cells, methods of delivery to the injury site, relevant pre-clinical studies and clinical trials, and future applications will be reviewed.
Search Strategy and Selection Criteria
Literature research was performed using PubMed, Web of Science, MEDLINE and Google Scholar databases, using the following search terms: angiogenesis, vascularity, nerve regeneration, nerve transplantation, nerve graft, neural stem cells, mesenchymal stem cells, exosomes and various combinations of the above terms. Available English studies discussing stem cells and vascularity in peripheral nerve injuries in both animals and humans were included until July 2020.
Interaction of Stem Cells, Vascularity and Nerve Regeneration
The interaction of stem cells, vascularity, and axon regeneration is complex (Figure 1). The paracrine property of stem cells is much broader than previously was appreciated. When stimulated by surrounding tissues, stem cells have the ability to affect vascularity via cell-to-cell communication, differentiation, and release of angiogenic factors, such as vascular endothelial growth factor (VEGF) (Rehman et al., 2004; Hong et al., 2010; Watt et al., 2013). On the other hand, stem cells demonstrate the capacity to stimulate the upregulation of neurotrophic factors to enhance nerve regeneration as a response to nerve injury (Kondo et al., 2009; Hong et al., 2010; Rbia et al., 2018; Mathot et al., 2019). After nerve injury, angiogenesis is stimulated by hypoxia, usually mediated by VEGF, resulting in the growth of pre-existing vessels into new areas. It is postulated that these newly modeled vessel tracks precede the repair of damaged nerves (Carmeliet et al., 1996; Risau, 1997; Rissanen et al., 2004; Saffari et al., 2020a). The therapeutic potency of stem cells in nerve regeneration and vascularity is influenced by its microenvironment. The interaction of the environment, stem cells, and vascularity with respect to peripheral nerve regeneration will be discussed per causal order below.
Figure 1.
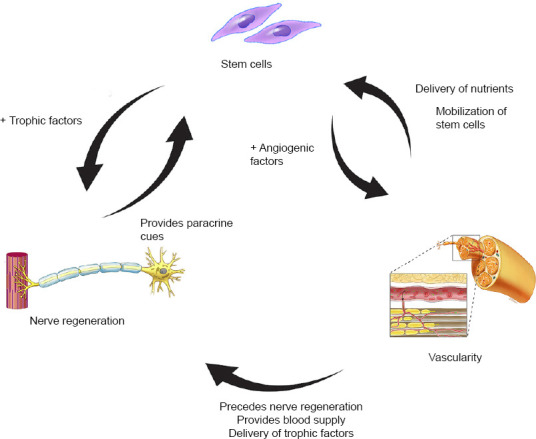
Schematic drawing of interaction between stem cells, vascularity, and nerve regeneration.
After a nerve injury, paracrine cues are provided to stem cells to produce trophic and angiogenic factors that enhance nerve regeneration and angiogenesis, respectively. Blood supply mobilizes stem cells and delivers nutrients and trophic factors to the site of injury to improve nerve regeneration. Blood supply is not only important for the survivability of stem cells but also precedes nerve regeneration after nerve trauma. Copyrighted and used with permission from the Mayo Foundation for Medical Education and Research; all rights reserved.
Stem cells and revascularization of nerve
Blood vessels have been postulated to be a systemic source of stem cells in regenerating hematopoietic cells secondary to their vascular origin. Embryologically, hematopoietic stem cells emerge closely in the vicinity of vascular endothelial cells (Tavian et al., 2005; Chen et al., 2012). The subendothelial zone, located in the tunica intima of blood vessels, has been specifically implicated as a source of endothelial progenitor cells (Zengin et al., 2006). Evidence has demonstrated that other structural layers also serve as niches for stem cells that are mostly quiescent and are activated in response to injury (Zhang et al., 2018). The close vicinity of these progenitor cells to the circulation allows for their mobilization to the region of interest to facilitate a number of processes including tissue regeneration (Fakoya, 2017). Differentiation of mesenchymal stem cells (MSCs) into Schwann-like cells enhances the secretion of various angiogenic factors, including angiopoietin-1 and VEGF-A, resulting in enhanced angiogenic potency and neurite outgrowth (Kingham et al., 2014; Mathot et al., 2019). MSCs differentiated into Schwann-like cells specifically, have been shown to increase revascularization of nerve allografts after in vivo reconstruction of sciatic nerve defects in rats (Mathot et al., 2020a).
Revascularization of nerve and neural regeneration
Following PNI, axons and myelin degenerate distally to the injury site by interactions of Schwann cells and macrophages in a process known as Wallerian degeneration (Waller, 1851; Cheng et al., 2017). During regeneration, blood vessels precede axonal extension and serve as tracks for Schwann cells to migrate and guide axonal growth, suggesting interdependence between neurite outgrowth and vascularity (Hobson et al., 1997; Hobson, Green et al. 2000). VEGF is central to the control of angiogenesis and critical in the process of maturation and stabilization of vessels (Connolly et al., 1989; Akhavani et al., 2008). In addition to stimulating the outgrowth of Schwann cells and blood vessels, VEGF also enhances axonal outgrowth from dorsal root ganglia (Sondell et al., 1999). It has been proposed that VEGF improves hematopoietic stem cell survival by an internal autocrine loop mechanism (Sondell et al., 1999; Gerber et al., 2002). This mechanism implies that it is not accessible for extracellular inhibitors, such as antibodies, to block this loop and proposes that the VEGF-dependent loop is solely generated in stem cells and not in endothelial cells (Gerber et al., 2002).
VEGF had become the focus of numerous basic science studies and was used to augment nerve grafts. However, it was found that the angiogenic effect of VEGF did not translate into enhanced motor recovery (Lee et al., 2016), suggesting that the role of vascularity in nerve regeneration does not depend on one element solely, but is broader and more complex (Saffari et al., 2020a). Recent research investigated the effect of an adipofascial vascularized flap on nerve revascularization in nerve allografts using novel microcomputed imaging. These results suggest that revascularization patterns follow longitudinal inosculation, the growth of host vessels from nerve coaptation ends, occurring primarily from proximal, rather than from both nerve ends, as previously believed (Chalfoun et al., 2003; Saffari et al., 2020b). Organized longitudinally running vessels provide modeled vessel tracks to precede the repair of damaged nerves (Carmeliet et al., 1996; Risau, 1997; Rissanen et al., 2004; Saffari et al., 2020a).
Stem cells and nerve regeneration
The importance of stem cells in peripheral nerve regeneration relies on their ability to enhance neurotrophic factors, promote myelin formation, and their capacity to be influenced by the microenvironment to differentiate into Schwann-like cells (Jiang et al., 2017). Schwann cells are essential within the context of peripheral nerve regeneration after trauma and are crucial during Wallerian degeneration, however, difficult to transplant (Salzer and Bunge, 1980; De la Rosa et al., 2018). The acquisition of autologous Schwann cells requires the harvest of large segments of healthy nerve tissue, resulting in donor site morbidities. Additionally, proliferation of Schwann cells in vitro is associated with an extensive culturing and expansion time period. As a result of these limitations, research has been directed towards the use of MSCs, which are easily accessible, and can be differentiated into Schwann-like cells (De la Rosa et al., 2018). Schwann-like cells are found to express neurotrophic factors and to interact through multiple pathways to support the repair of injured nerves (Tomita et al., 2013; Mathot et al., 2019). These factors and pathways include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and myelin related growth factors. BDNF is upregulated in motor neurons and increases myelin thickness in regenerating nerves while promoting remyelination and axonal sprouting (Gordon, 2009; De la Rosa et al., 2018). In myelin sheath formation, a number of myelin proteins are induced such as myelin basic protein, peripheral myelin protein-22, and pleiotrophin. Pleiotrophin is involved in myelinated axon regeneration specifically and is increasingly expressed in differentiated MSCs in comparison to undifferentiated MSCs (Mi et al., 2007; Mathot et al., 2020b). The level of growth factors in the microenvironment influences the sequential growth factor production by the transplanted stem cells, emphasizing their paracrine feedback properties.
Only 5% of all types of stem cells can spontaneously differentiate into Schwann cells; however, pre-differentiation towards the desired phenotype in vitro via chemical induction or transfection with growth factors has been shown to be a more effective method to increase the number of Schwann-like cells (Cuevas et al., 2002; Dore et al., 2009; Jiang et al., 2017). Drawbacks of pre-differentiation include the need for additional preparation time and higher costs, making this process less favorable for clinical translation (Mathot et al., 2019). More investigation is needed to confirm the optimal dosage of transplanted stem cells, delivery methods, in vivo survivability, and interaction mechanisms with their environment prior to conducting clinical studies.
Stem Cell Sources
Stem cells can be categorized as embryonic stem cells or adult stem cells according to their development stage, which relates to their differentiation potential. An overview of all stem cell sources is provided in Additional Table 1.
Additional Table 1.
Overview of sources of stem cells, showing its origin, location of harvest, properties, and mechanisms
| Source | Origin | Location of harvest | Properties/ Mechanism | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Embryonic stem cells | Pluripotent stem cells | Blastocyst-stage embryo | Stimulate myelination, exert neurotrophic factors | - Unlimited source of cells - Superior differentiation potential - Homogenous - Long-term proliferation capacity |
- Ethical dilemma - Immunogenicity - Carcinogenic potential (e.g., teratoma) - Differentiation to specialized cell lines remains challenging |
| Neural stem cells | Multipotent stem cells | Sub-ventricular zone, hippocampus | Replace Schwann cells | - Highly migratory cells that give rise to neurons, astrocytes, and oligodendrocytes - Contributing extensively to remyelination |
- Difficulty harvesting - Evokes neural-immune reactions after transplantation - Neuroblastoma formation - Directed differentiation to specialized cell lines remains challenging |
| Mesenchymal stem cells | Multipotent stem cells | Bone-marrow, adipose tissue, peripheral blood, amniotic fluid, umbilical cord, tendon and ligaments, hair follicle, synovial membranes, olfactory mucosa, dental pulp and fetal tissue | Stimulate myelination, exert neurotrophic factors | - Dependent on sub-type | - Dependent on sub-type |
| Bone marrow-derived stem cells | Multipotent stem cells | Bone marrow | Stimulate myelination, exert neurotrophic factors | - Not restricted by ethical concerns - Lack of immune recognition and have immunosuppressive action, resulting in allogenic transplantation without induced immune rejection |
- Painful procurement procedure necessitating anesthesia - Inferior proliferation capacity and differentiation potential - Low fraction of stem cells available for use |
| Adipose-derived stem cells | Multipotent stem cells | Adipose tissue | Stimulate myelination, exert neurotrophic factors, reduce inflammation | - Minimally invasive harvesting - A higher proportion, superior proliferation, stem cell fraction and differentiation potential compared to bone marrowderived stem cells - Valid Schwann cell alternative, one of the optimal choices for pre clinical studies, aid angiogenesis |
- Differentiation potential exists towards adipocytes |
| Fetal derived stem cells | Multipotent stem cells | Amniotic membrane, amniotic fluid, umbilical cord cells, umbilical cord blood, and Wharton’s jelly. | Augmented blood perfusion, enhanced intraneural vascularity | - Easily obtained - Less immunoreactivity |
- Require storage of autologous cells after harvest |
| Skin derived precursors | Multipotent cells | Dermis | Replace Schwann cell myelination | - Easy to harvest | - Prolonged periods of cell expansion - Accessible - Highly similar to neural crest cells - Durable proliferative ability |
| Muscle-derived stem/progenitor cells | Progenitor cells | Skeletal muscle | Exert neurotrophic factors | - Highly accessible - Long-term proliferation - Multipotent differentiation |
- Limited scientific support |
| Hair follicle stem cells | Multipotent stem cells | Hair follicle | Replace Schwann cell myelination, exert neurotrophic factors | - Abundant and accessible source, multipotent differentiation - Differentiation into pure human Schwann cell population |
- Cannot be stored for long periods, difficult to isolate - Prolonged periods of cell expansion |
| Dental pulp stem cells | Multipotent stem cells | Exfoliated deciduous teeth | Replace Schwann cell myelination, exert neurotrophic factors | - Stronger proliferation, greater clonogenicity, and larger population compared to bone marrow-derived stem cells - Most convenient source of multipotent stem cells |
- Require controlled storage of autologous cells after harvest - Limited scientific support |
| Induced pluripotent stem cells | Pluripotent stem cells | Dermis, blood | Replace Schwann cell myelination, exert neurotrophic factors | - No ethical and immunosuppressive restrictions - Inducible from easily obtainable somatic cells |
- Differentiation occurs with reduced efficiency and increased variability - Higher risk at tumorigenicity - Chromosomal aberrations - Epigenetic memory from original somatic cells |
| Exosomes | Membrane nanovesicles | In almost all biological body fluids including blood, urine, breast milk, ascites, and saliva | Transmit genetic material, neurotrophic factors, and proteins | - Could eliminate the risks associated to mesenchymal stem cells transplantation - Lower immunogenicity - Cross biological barriers - Easier stored and available in the long term while maintaining quality |
- No standardized techniques for isolation, quantification, and purification - No ethical safety assessment or guidance. - Limited scientific support. |
The advantages and disadvantages of each mentioned stem cell source are mentioned in the last two columns (Fairbairn et al., 2015; Jiang et al., 2017; Dong et al., 2019).
Embryonic stem cells
Thomson et al. (1998) described the isolation of pluripotent cell lines from the inner cell mass of human blastocyst-stage embryos. Following this discovery, stem cells have been harvested from several fetal as well as adult tissues (Figure 2). Embryonic stem cells (ESCs) are undifferentiated cells, capable of self-renewal and have superior differentiation potential and long-term proliferation capacity compared to adult stem cells. ESCs have the ability of in vivo myelination since they can differentiate into neurons and glial cells of the central and peripheral nervous system. In humans, the use of ESCs in neural tissue engineering is primarily inhibited by the ethical controversy of the use and destruction of a human embryo. Additional limitations include ESC’s immunogenicity and carcinogenic potential including teratoma formation which limits clinical translation. Differentiation of ESCs into specialized neural cell lines remains challenging and protocols have only been established for a few cell lines (Cui et al., 2008; Fairbairn et al., 2015; De la Rosa et al., 2018; Jones et al., 2018; Yi et al., 2020).
Figure 2.
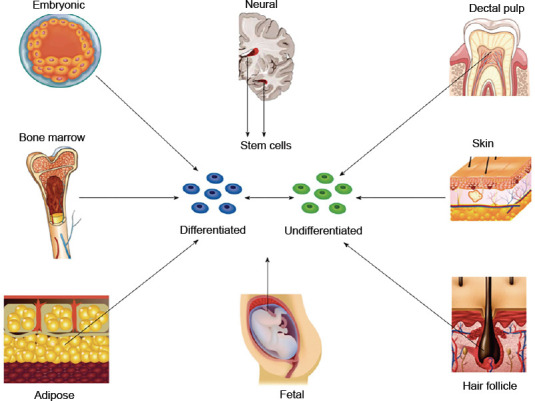
Schematic overview of different sources of stem cells.
Embryonic stem cells are obtained from the inner cell mass of the blastocyst and therefore require destruction of the embryo. Nerve stem cells are harvested from the subventricular layer of the lateral ventricle and the subgranular layer of the hippocampus. Bone marrow-derived stem cells are harvested from the marrow cavity of long bones. Adipose-derived stem cells are derived from subcutaneous fat and are abundantly available following commonly performed procedures such as liposuction. Skin derived precursors are harvested from the dermis and represent a related population of cells harvested from hair follicles. Fetal tissue provides populations of cells from amniotic membrane, amniotic fluid, umbilical cord blood, umbilical cord tissue, and Wharton’s jelly. Dental pulp stem cells can be harvested from deciduous teeth. Copyrighted and used with permission of the World Journal of Stem Cells; all rights reserved. Reprinted with permission from Fairbairn et al. (2015).
Neural stem cells
Neural stem cells (NSCs) are naturally capable of differentiating into neurons or glial cells and play a role during neurogenesis in the development of the brain and spinal cord, which almost exclusively occurs during embryogenesis. In human adults, NSCs are located in the subventricular zone and hippocampus and have a limited role in the regeneration of central nervous system injuries (Paspala et al., 2011; Fairbairn et al., 2015; Matarredona and Pastor, 2019). While several basic science studies suggest enhancement of nerve regeneration following NSC administration to the injury site after both acute and chronic PNI (Heine et al., 2004; Fairbairn et al., 2015; Wang et al., 2017), commercially available NSCs have been reported to be associated with the formation of neuroblastoma after implantation (Johnson et al., 2008). Additional limitations to application in humans include the technical difficulties associated with cell harvest and the need to direct differentiation of specialized neural cell lines. The application of NSCs could greatly benefit clinical practice if these limitations can be overcome (Wang et al., 2017).
Mesenchymal stem cells
The multipotent MSCs can be derived from the bone marrow and a wide range of non-marrow sources, including adipose tissue, peripheral blood, amniotic fluid, umbilical cord, tendon and ligaments, hair follicle, synovial membranes, olfactory mucosa, dental pulp and fetal tissue (Maltman et al., 2011). Their potentials for differentiation, ease of isolation, and immunomodulation have contributed to their considerable application in tissue regeneration. MSCs have the ability to differentiate into all mesoderm lineages: adipose tissue, bone, muscle, and cartilage (Pittenger et al., 1999) and serve as targets for genetic modification. Depending on the appropriate stimuli and environmental conditions, MSCs have shown to express plasticity and transdifferentiation and can be differentiated into non-mesenchymal lineages, such as neurons, astrocytes, Schwann-cell like cells and myelinating cells of the peripheral nervous system to enhance nerve regeneration (Tohill et al., 2004; Fairbairn et al., 2015; Jiang et al., 2017; De la Rosa et al., 2018). Bone marrow-derived and adipose-derived MSCs are the most commonly used stem cells for PNI.
Bone marrow-derived mesenchymal stem cells
Bone marrow-derived mesenchymal stem cells (BMSCs) can differentiate into non-mesodermal lineages such as neurons, astrocytes, and Schwann-like cells under appropriate environmental conditions (Tohill et al., 2004). Consequently, BMSCs may enhance neurite outgrowth and express neurotrophic factors (NGF, BDNF, glial cell line-derived neurotrophic factor, and ciliary neurotrophic factor) as well as extracellular matrix components including collagen, fibronectin, and laminin. Administration of BMSCs to nerves contributes to enhanced angiogenesis (Chen et al., 2007; Yang et al., 2011; Wei et al., 2012; Fan et al., 2014; Yi et al., 2020). A number of studies in rodent models have found that BMSC application to conduits and acellular nerve grafts resulted in superior functional outcomes in nerve regeneration compared to untreated grafts (Cui et al., 2008; Fairbairn et al., 2015). BMSCs demonstrated dose-dependent enhancement of the extent of myelination, thickness of myelin sheath, and axonal thickness in a rat sciatic nerve model (Raheja et al., 2012). When cultured, BMSCs lack immune recognition and have immunosuppressive action, which may overcome induced immune rejection after allogenic transplantation (Aggarwal and Pittenger, 2005; Yi et al., 2020). Although BMSCs are more easily harvested than ESCs and NSCs and have minimal ethical use issues, BMSCs have inferior proliferation capacity and differentiation potential. The harvesting procedure is invasive and painful for donors and the quantity of stem cells obtained is lower compared to other sources (Fairbairn et al., 2015; Jiang et al., 2017).
Adipose-derived mesenchymal stem cells
Adipose-derived mesenchymal stem cells (ADSCs) can easily be harvested from abundant adipose tissue with minimally invasive procedures such as liposuction. Compared to BMSCs, ADSCs show superior stem cell fraction, proliferation, and differentiation potential. ADSCs can be differentiated into a Schwann cell-like phenotype, sharing functional and morphological characteristics, and similar effectivity levels as autologous Schwann cells. It is postulated that ADSCs facilitate endogenous Schwann cell recruitment by expressing growth factors such as NGF, VEGF, and BDNF leading to a long-lasting therapeutic effect promoting nerve regeneration and protection, which outlasts the life span of ADSC (Kingham et al., 2007; Erba et al., 2010; Fairbairn et al., 2015). Interestingly, ADSCs are reported to support angiogenesis by direct differentiation into vascular endothelium, as well as by associated paracrine effects (Chen et al., 2011; Nie et al., 2011). Difficulties include the unfavorable differentiation potential towards adipocytes (Faroni et al., 2016). Nonetheless, ADSCs are currently the most practical source of stem cells to obtain and have been and continue to be translated to clinical use (Fairbairn et al., 2015).
Other types of MSCs including fetal derived stem cells, skin-derived precursors, muscle-derived stem/progenitor cells, hair follicle stem cells and dental pulp stem cells, as well as induced pluripotential stem cells are detailed in Additional Table 1 (Fairbairn et al., 2015; Jiang et al., 2017). Currently, inherent disadvantages associated with MSC-based therapy still exist: instability of the cellular phenotype, high cost, ethical issues, and difficulties of cellular origin (Dong et al., 2019). These difficulties may be overcome by the application of exosomes.
Exosomes
Recent research has proven that the therapeutic effect of MSCs is highly likely to be accredited to the indirect regeneration of endogenous Schwann cells through cellular paracrine mechanisms, which are partially thought to be mediated by MSC exosomes (Sowa et al., 2016). MSC exosomes are a subtype of extracellular vesicles released from all cell types, but particularly from stem cells. MSC exosomes are located in nearly all biological body fluids including blood, urine, breast milk, ascites, and saliva (Kourembanas, 2015). These particles maintain cell-to-cell communication by delivering proteins, lipids, DNA, mRNA, and micro ribonucleic acids miRNAs (miRNA), and other subtypes of RNA, which regulate cell biological behavior and can be used to mediate intercellular communication (Corrado et al., 2013; Li et al., 2017; Blanc and Vidal, 2018; Rezaie et al., 2018). MSC exosomes are found to increase axonal regeneration and promote local angiogenesis by transmitting a number of genetic materials, neurotrophic factors and proteins to axons and thereby restoring the homeostasis of the microenvironment (Dong et al., 2019). Furthermore, MSC exosomes have been proven to promote the proliferation of Schwann cells and reduce their apoptosis rate by upregulating the pro-apoptotic Bax mRNA expression resulting in increased regeneration (Liu et al., 2020). It is postulated that MSC exosomes are mediators of communication with vascular endothelial cells resulting in enhancement of the plasticity of blood vessels following PNI (Gong et al., 2017). MSC exosome-based therapy provides many benefits including the decrease of associated risks with transplantation as exosomes contain fewer membrane-bound proteins. These characteristics present MSC exosomes with lower immunogenicity, the ability to cross biological barriers, and to be more stable over time (Qing et al., 2018; Dong et al., 2019). The primary limitation of exosomes application is the limited scientific support and lack of standardized techniques for its isolation, quantification and purification. The efficacy of injected MSC exosomes and the effect of MSC exosomes travelling through the body remains unknown and to be explored (Qi et al., 2020).
Additionally, no guidelines for ethical safety assessment exists yet. MSC exosomes have great potential to be utilized for future therapeutic strategies in clinical practice following PNI if these challenges can be addressed (Dong et al., 2019).
Mode of Stem Cell Delivery
Stem cells can be delivered to the nerve reconstruction site by a variety of methods (Table 1). The selection of the delivery method depends on the intended mechanism of action of the stem cells. The process of microinjection (either intra-neural, around the nerve, intramuscular or intravenous) decreases the viability of stem cells due to the traumatic damage secondary to the pressure build-up in the syringe and flow through the needle during injection. Furthermore, intra-neural microinjection of stem cells is associated with damage of the nerve ultrastructure with unpredictable cell distributions, obstruction of axonal ingrowth, and leakage of cells (Lévesque et al., 2009; Fairbairn et al., 2015; Wang et al., 2015; Jiang et al., 2017; Mathot et al., 2019). Dynamic seeding has been a successful technique in delivering stem cells and results in an efficient and uniform distribution of stem cells. When cells are dynamically seeded on the surface of a decellularized nerve allograft, these cells remain viable on the nerve surface allowing for interaction with the nerve ultrastructure and resulting in the upregulation of neurotrophic factors (Rbia et al., 2018; Mathot et al., 2019). According to Mathot et al. (2019), an efficiency of at least 1 × 106 MSCs on a 10-mm nerve graft is needed to generate noticeable outcomes. Induced differentiation of MSCs may have an effect on the final efficiency, as delivery methods have only been tested on undifferentiated MSCs, however, its effect remains unknown. Controversy remains on the optimal method of delivery and requires further evaluation.
Table 1.
Overview of delivery methods of stem cells, showing its efficiency, advantages, and disadvantages
| Methods | Efficiency | Advantages | Limitations |
|---|---|---|---|
| Intravenous injection | 100% | - Does not induce nerve damage and cell-leakage - Focuses on the trophic function of stem cells |
- Entrapment stem cells in capillaries - The desired amount at the recipient site may not suffice - Reduction stem cell viability after needle passage due to pressure build-up |
| Intramuscular injection | 100% | - Does not induce nerve damage and cell-leakage - Significantly improves functional recovery and neuroconduction velocity compared to intravenous injection - Enhances nerve regeneration |
- Reduction stem cell viability after needle passage due to pressure build-up, low number of stem cells at nerve regeneration site |
| Dynamic seeding on nerve grafts or conduits | 89.20% | - Does not harm stem cells or nerve infrastructure - More successful than static seeding, uniform distribution of adhered stem cells - Upregulation neurotrophic factors - Very promising method |
- Stem cells do not migrate from the delivery site - Can only be used when the nerve gap is bridged with a nerve graft or conduit |
| Intra-neural microinjection | 10–40% | - Delivers a high quantity of cells directly to the site of the inner and middle nerve zones | - Traumatic to stem cells as well as intra-neural architecture - Unpredictable cell distribution, reduction stem cell viability after needle passage due to pressure build-up - Leakage cells - May obstruct axonal ingrowth |
| Microinjection around nerve | 10–40% | - Does not induce nerve damage and cell-leakage - Outcomes comparable to intra-neural microinjection |
- Reduction stem cell viability after needle passage due to pressure build-up |
| Cell encapsulation | Unknown | - Overcomes side effects of transplantation (e.g., teratoma formation, tumors) - Prevents in vivo migration of cells - Enhances the formation of multicellular aggregates |
- A high volume of micro-particles needed to reach a beneficial number of cell release |
| Hydrogel | Unknown | - Similarity to extracellular matrix - Can be processed under mild conditions - Minimally invasive delivery - Degradation can be designed to coincide with angiogenesis and revascularization - Potentially clinical translatable |
- Research is still in the early stage - Degradation hydrogel may affect stem cell survivability |
| 3-D printing | Unknown | - Includes the personalized complex features of the nerve structures | - Research is still in the early stages - Technical challenges to realize the formation of the desired nerve structure |
The Regenerative Potential of Cell-Based Therapy
The regenerative potential of cell-based therapy following nerve injury is particularly relevant for large diameter and long nerve defects (Faroni et al., 2015). In rodent models, extensive research using stem cells in peripheral nerve repair suggests that the application of stem cells enhances functional motor outcomes. It has been shown that stem cells elevate expression of neurotrophic factors, angiogenic growth factors and contribute to angiogenesis (Zhao et al., 2011; Fan et al., 2014; Mathot et al., 2020b; Yi et al., 2020). The exact survivability of stem cells in vivo, however, is difficult to investigate and remains largely unknown. When MSCs were dynamically seeded on nerve allografts, in vivo survivability up to 29 days was found using luciferase-based bioluminescence imaging (Rbia et al., 2019). This suggests that survivability of stem cells may be hampered by rejection, inflammation, or migration. Larger animal models are also investigating augmentation of nerve repair outcomes by the provision of BMSCs to nerve grafts (Ding et al., 2010; Muheremu et al., 2017). Tissue-engineered nerve grafts enhanced with autologous BMSCs have been used to repair 50 mm-long median nerve defects in rhesus monkeys. After 1 year, histological and morphometric analyses of regenerative nerves found results comparable to autograft repair. Blood samples and histopathological examination of nerve found no immune rejection, confirming that tissue-engineered nerve grafts augmented with BMSCs were safe to use in monkeys (Hu et al., 2013). Extensive investigations of safety in preceding pre-clinical trials have provided the data necessary to proceeding evaluation of MSCs in clinical trials (Pal et al., 2009).
Clinical stem cell application is novel and has been applied to several fields including treatment for cardiovascular diseases with promising results (Hong et al., 2010). Several clinical trials investigating central nervous system diseases, including spinal cord injury and traumatic brain injury, have proven the safety of MSC application. Ongoing trials suggest that MSCs prepare the environment of injury for axonal ingrowth and stimulate angiogenesis. While this is promising, more studies are needed to assess the time and route of administration to obtain more consistent data (Badyra et al., 2020). In the field of PNI, most clinical trials related to stem cell treatment focus on hemifacial spasm, burn wound healing and diabetic peripheral neuropathy (De la Rosa et al., 2018) and are still in the early phases. Although the application of Schwann cells is not desired due to the aforementioned limitations, the first FDA-regulated dose phase I trial on human Schwann cell transplantation in spinal cord injuries has found promising results with no adverse effects (Anderson et al., 2017). Currently, little pre-clinical or clinical research has addressed the interaction of stem cells and vascularity in nerve regeneration, which creates an opportunity for elucidating its synergistic pathways in future research.
Future Applications
Future applications integrating stem cell-based therapies with the promotion of angiogenesis are needed to enhance nerve regeneration through multiple pathways. Feasibility with respect to cost and time efficiency is needed for translation to clinical practice. Current research developing future applications includes prevascularized stem cell nerve conduits, three-dimensional printing, and hydrogel scaffolding (Fakoya, 2017; Du and Jia, 2019; Fan et al., 2020).
Fan et al. (2020) have developed a novel prevascularized nerve conduit based on a MSC sheet for treating spinal cord injuries, resulting in enhanced nerve regeneration and revascularization. Other novel research has focused on three-dimensional printing in order to fabricate a nerve guidance conduit with stem cells that reproduces the complex nerve features of a patient’s long nerve defect, such as branching nerve networks and intrinsic chemical mechanisms that steer regenerating motor and sensory axons along correct anatomical pathways. Three-dimensional printing may provide the desired personalized dimensions and structures for nerve regeneration and cell organization (Du and Jia, 2019).
Another novel future application focuses on promising stem cell delivery methods, which are still in early stages of research, such as stem cell encapsulation delivered in a hydrogel scaffold (Fakoya, 2017; Allbright et al., 2018). Hydrogels constructed of natural biomaterials, including collagen and fibrin, as well as synthetic biomaterials such as poly(lactic-co-glycolic acid) and polyethylene glycol, may act as carriers for delivery of stem cells or growth factors. The use of hydrogel as a scaffold is attractive due to its high water content mimicking an extracellular matrix as well as its ease of delivery. Hydrogel degradation can be designed to respond to tissue proteases and to deliver stem cells or other growth factors in a timely manner to coincide with the processes of angiogenesis. In vascular tissue engineering, poly(lactic-co-glycolic acid) hydrogels have been designed to ensure a controlled release of angiogenic factors such as VEGF for an extended release time. Basic criteria for the use of hydrogel include effective cell adhesion to the gel matrix, sufficient stem cell survival in the hydrogel, safety for the micro-environment, and mechanical stability (Fakoya, 2017; Maiti and Díaz, 2018). The use of prefabricated conduits or encapsulated cells locally delivered in a hydrogel is feasible, has strong potential to enhance survival of transplanted cells and may be less immunogenic when combined with exosomes.
As tissues consist of multiple cell types, potential synergistic therapeutic benefit may occur when multiple stem cell types are administered simultaneously. By combining the properties and secretion of a mixture of neuroregenerative as well as vascular growth factors, cytokines and miRNA, regenerative capacities may be enhanced. Currently, limited data supporting combined cell type administration exist, however, may be promising for future applications (Williams et al., 2013; Avolio et al., 2015). The ideal type of stem cell for translation needs to be easily accessible and harvested, proliferate rapidly without carcinogenic consequences, and immune-compatible. Locally delivered cell-based therapy is expected to increasingly take part in enhancing outcomes after nerve reconstruction in the next decades.
Conclusions
Improved understanding of the interaction of stem cells and vascularity will provide therapeutic targets to improve outcomes after peripheral nerve reconstruction. The degree of nerve regeneration after PNI is particularly dependent on the local environment of injury and may be altered to promote functional recovery. The microenvironment may be modulated by stem cells and angiogenesis. This topic of interest is complex and involves the secretion of trophic factors that enhance regeneration and revascularization. Revascularization of nerve is suggested to enhance nerve regeneration by organized longitudinally running vessels that provide modeled vessel tracks to precede the repair of damaged nerves. The effect of stem cells, on the other hand, is dependent on their differentiation potential and stimulation by paracrine cues, which are provided by the environment during nerve regeneration. This review discusses the synergistic pathways of stem cells and vascularity and their interaction with nerve following PNI. Despite advancements in well-designed pre-clinical studies, translation of stem cells to clinical practice is currently impeded by ethical issues, risk of tumorigenesis, unknown side-effects and technical challenges. Future research may be shifted towards the use of exosomes, to overcome difficulties associated with the harvest and culture of stem cells. Local modulation of nerve environment is still evolving, and the importance of stem cell and vascularity-based therapies is expected to take a larger part in treatment options of PNIs.
Additional files:
Additional Table 1: Overview of sources of stem cells, showing its origin, location of harvest, properties, and mechanisms.
Acknowledgments:
We would like to thank Dr. Fairbairn and colleagues, and the World Journal of Stem Cells for granting us the permission to re-print Figure 2.
Footnotes
P-Reviewer: Al-Sharea A; C-Editors: Zhao M, Wang L; T-Editor: Jia Y
Conflicts of interest: The authors have no conflict of interest to disclose.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Annas Al-Sharea, Baker IDI Heart and Diabetes Institute, Melbourne, Vic, Australia.
References
- 1.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 2.Akhavani MA, Sivakumar B, Paleolog EM, Kang N. Angiogenesis and plastic surgery. J Plast Reconstr Aesthet Surg. 2008;61:1425–1437. doi: 10.1016/j.bjps.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Allbright KO, Bliley JM, Havis E, Kim DY, Dibernardo GA, Grybowski D, Waldner M, James IB, Sivak WN, Rubin JP, Marra KG. Delivery of adipose‐derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve. 2018;58:251–260. doi: 10.1002/mus.26094. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerström-Noga E, Wood P, Levi AD. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 5.Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, Reni C, Alvino VV, Rowlinson J, Livi U, Cesselli D, Angelini G, Emanueli C, Beltrami AP, Madeddu P. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81-94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]
- 6.Badyra B, Sułkowski M, Milczarek O, Majka M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl Med. 2020;9:1174–1189. doi: 10.1002/sctm.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9:95–106. doi: 10.1080/21541248.2016.1264352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulatova N, Yousef AM, Al-Khayyat G, Qosa H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr Drug Saf. 2011;6:3–11. doi: 10.2174/157488611794480043. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015;2015:628767. doi: 10.1155/2015/628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 11.Chalfoun C, Scholz T, Cole MD, Steward E, Vanderkam V, Evans GR. Primary nerve grafting: A study of revascularization. Microsurgery. 2003;23:60–65. doi: 10.1002/micr.10082. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–453. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen CW, Corselli M, Péault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Translational Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Q, Wang YX, Yu J, Yi S. Critical signaling pathways during Wallerian degeneration of peripheral nerve. Neural Regen Res. 2017;12:995–1002. doi: 10.4103/1673-5374.208596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 2013;14:5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, González-Corrochano R, Diaz-González D, Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24:634–638. doi: 10.1179/016164102101200564. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356–1365. doi: 10.1634/stemcells.2007-0333. [DOI] [PubMed] [Google Scholar]
- 20.De la Rosa MB, Kozik EM, Sakaguchi DS. Adult stem cell-based strategies for peripheral nerve regeneration. Adv Exp Med Biol. 2018;1119:41–71. doi: 10.1007/5584_2018_254. [DOI] [PubMed] [Google Scholar]
- 21.Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X. Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A. 2010;16:3779–3790. doi: 10.1089/ten.TEA.2010.0299. [DOI] [PubMed] [Google Scholar]
- 22.Dong R, Liu Y, Yang Y, Wang H, Xu Y, Zhang Z. MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. Biomed Res Int. 2019;2019:6458237. doi: 10.1155/2019/6458237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dore JJ, DeWitt JC, Setty N, Donald MD, Joo E, Chesarone MA, Birren SJ. Multiple signaling pathways converge to regulate bone-morphogenetic-protein-dependent glial gene expression. Dev Neurosci. 2009;31:473–486. doi: 10.1159/000210187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Jia X. Engineering nerve guidance conduits with three-dimenisonal bioprinting technology for long gap peripheral nerve regeneration. Neural Regen Res. 2019;14:2073–2074. doi: 10.4103/1673-5374.262580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811-817. doi: 10.1016/j.bjps.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J Stem Cells. 2015;7:11–26. doi: 10.4252/wjsc.v7.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakoya AO. New delivery systems of stem cells for vascular regeneration in ischemia. Front Cardiovasc Med. 2017;4:7. doi: 10.3389/fcvm.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan L, Yu Z, Li J, Dang X, Wang K. Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC Musculoskelet Disord. 2014;15:165. doi: 10.1186/1471-2474-15-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Z, Liao X, Tian Y, Xuzhuzi X, Nie Y. A prevascularized nerve conduit based on a stem cell sheet effectively promotes the repair of transected spinal cord injury. Acta biomater. 2020;101:304–313. doi: 10.1016/j.actbio.2019.10.042. [DOI] [PubMed] [Google Scholar]
- 30.Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Faroni A, Smith RJ, Lu L, Reid AJ. Human Schwann‐like cells derived from adipose‐derived mesenchymal stem cells rapidly de‐differentiate in the absence of stimulating medium. Eur J Neurosci. 2016;43:417–430. doi: 10.1111/ejn.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 33.Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–45212. doi: 10.18632/oncotarget.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 35.Heine W, Conant K, Griffin JW, Höke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189:231–240. doi: 10.1016/j.expneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Hobson MI, Brown R, Green CJ, Terenghi G. Inter-relationships between angiogenesis and nerve regeneration: a histochemical study. Br J Plast Surg. 1997;50:125–131. doi: 10.1016/s0007-1226(97)91325-4. [DOI] [PubMed] [Google Scholar]
- 37.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 39.Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, Yang Y, Ding F, Gu X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34:100–111. doi: 10.1016/j.biomaterials.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. Int J Mol Sci. 2017;18:94. doi: 10.3390/ijms18010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson TS, O’Neill AC, Motarjem PM, Nazzal J, Randolph M, Winograd JM. Tumor formation following murine neural precursor cell transplantation in a rat peripheral nerve injury model. J Reconstr Microsurg. 2008;24:545–550. doi: 10.1055/s-0028-1088228. [DOI] [PubMed] [Google Scholar]
- 42.Jones I, Novikova LN, Novikov LN, Renardy M, Ullrich A, Wiberg M, Carlsson L, Kingham PJ. Regenerative effects of human embryonic stem cell‐derived neural crest cells for treatment of peripheral nerve injury. J Tissue Eng Regen Med. 2018;12:e2099-2109. doi: 10.1002/term.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keilhoff G, Goihl A, Langnäse K, Fansa H, Wolf G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur J Cell Biol. 2006;85:11–24. doi: 10.1016/j.ejcb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Khalifian S, Sarhane KA, Tammia M, Ibrahim Z, Mao HQ, Cooney DS, Shores JT, Lee WP, Brandacher G. Stem cell-based approaches to improve nerve regeneration: potential implications for reconstructive transplantation. Arch Immunol Ther Exp (Warsz) 2015;63:15–30. doi: 10.1007/s00005-014-0323-9. [DOI] [PubMed] [Google Scholar]
- 45.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 47.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 48.Kornfeld T, Vogt PM, Radtke C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med Wochenschr. 2019;169:240–251. doi: 10.1007/s10354-018-0675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 50.Lee JY, Giusti G, Friedrich PF, Bishop AT, Shin AY. Effect of vascular endothelial growth factor administration on nerve regeneration after autologous nerve grafting. J Reconstr Microsurg. 2016;32:183–188. doi: 10.1055/s-0035-1563709. [DOI] [PubMed] [Google Scholar]
- 51.Lévesque MF, Neuman T, Rezak M. Therapeutic microinjection of autologous adult human neural stem cells and differentiated neurons for Parkinson’s disease: five-year post-operative outcome. Open Stem Cell J. 2009;1:20–29. [Google Scholar]
- 52.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu CY, Yin G, Sun YD, Lin YF, Xie Z, English AW, Li QF, Lin HD. Effect of exosomes from adipose‐derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther. 2020;26:189–196. doi: 10.1111/cns.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25:391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- 55.Maiti B, Díaz Díaz D. 3D printed polymeric hydrogels for nerve regeneration. Polymers (Basel) 2018;10:1041. doi: 10.3390/polym10091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Matarredona ER, Pastor AM. Neural stem cells of the subventricular zone as the origin of human glioblastoma stem cells. Therapeutic Implications. Front Oncol. 2019;9:779. doi: 10.3389/fonc.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathot F, Rbia N, Bishop AT, Hovius SER, Shin AY. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery. 2020a;40:585–592. doi: 10.1002/micr.30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathot F, Rbia N, Thaler R, Bishop AT, Van Wijnen AJ, Shin AY. Gene expression profiles of differentiated and undifferentiated adipose derived mesenchymal stem cells dynamically seeded onto a processed nerve allograft. Gene. 2020b;724:144151. doi: 10.1016/j.gene.2019.144151. [DOI] [PubMed] [Google Scholar]
- 60.Mathot F, Shin AY, Van Wijnen AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17–23. doi: 10.1016/j.gene.2019.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mi R, Chen W, Höke A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. Proc Natl Acad Sci U S A. 2007;10:4664–4669. doi: 10.1073/pnas.0603243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muheremu A, Chen L, Wang X, Wei Y, Gong K, Ao Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci Rep. 2017;7:44002. doi: 10.1038/srep44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–216. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 64.Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 65.Paspala SA, Murthy TV, Mahaboob VS, Habeeb MA. Pluripotent stem cells-a review of the current status in neural regeneration. Neurol India. 2011;59:558–565. doi: 10.4103/0028-3886.84338. [DOI] [PubMed] [Google Scholar]
- 66.Patel NP, Lyon KA, Huang JH. An update-tissue engineered nerve grafts for the repair of peripheral nerve injuries. Neural Regen Res. 2018;13:764–774. doi: 10.4103/1673-5374.232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 68.Qi J, Liu Q, Reisdorf RL, Boroumand S, Behfar A, Moran SL, Amadio PC, Gingery A, Zhao C. Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J Orthop Res. 2020;38:1845–1855. doi: 10.1002/jor.24587. [DOI] [PubMed] [Google Scholar]
- 69.Qing L, Chen H, Tang J, Jia X. Exosomes and their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair. 2018;32:765–776. doi: 10.1177/1545968318798955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raheja A, Suri V, Suri A, Sarkar C, Srivastava A, Mohanty S, Jain KG, Sharma MC, Mallick HN, Yadav PK, Kalaivani M, Pandey RM. Dose-dependent facilitation of peripheral nerve regeneration by bone marrow-derived mononuclear cells: a randomized controlled study. J Neurosurg. 2012;117:1170–1181. doi: 10.3171/2012.8.JNS111446. [DOI] [PubMed] [Google Scholar]
- 71.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rbia N, Bulstra LF, Bishop AT, van Wijnen AJ, Shin AY. A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plast Reconstr Surg. 2018;142:402–413. doi: 10.1097/PRS.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rbia N, Bulstra LF, Thaler R, Hovius SER, van Wijnen AJ, Shin AY. In vivo survival of mesenchymal stromal cell-enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. J Hand Surg Am. 2019;44:514e1–514e11. doi: 10.1016/j.jhsa.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 75.Rezaie J, Ajezi S, Avci ÇB, Karimipour M, Geranmayeh MH, Nourazarian A, Sokullu E, Rezabakhsh A, Rahbarghazi R. Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol. 2018;55:3372–3393. doi: 10.1007/s12035-017-0582-7. [DOI] [PubMed] [Google Scholar]
- 76.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 77.Rissanen TT, Rutanen J, Ylä-Herttuala S. Gene transfer for therapeutic vascular growth in myocardial and peripheral ischemia. Adv Genet. 2004;52:117–164. doi: 10.1016/S0065-2660(04)52004-7. [DOI] [PubMed] [Google Scholar]
- 78.Saffari TM, Bedar M, Zuidam JM, Shin AY, Baan CC, Hesselink DA, Hundepool CA. Exploring the neuroregenerative potential of tacrolimus. Expert Rev Clin Pharmacol. 2019;12:1047–1057. doi: 10.1080/17512433.2019.1675507. [DOI] [PubMed] [Google Scholar]
- 79.Saffari TM, Bedar M, Hundepool CA, Bishop AT, Shin AY. The role of vascularization in nerve regeneration of nerve graft. Neural Regen Res. 2020a;15:1573–1579. doi: 10.4103/1673-5374.276327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saffari TM, Mathot F, Friedrich PF, Bishop AT, Shin AY. Revascularization patterns of nerve allografts in a rat sciatic nerve defect model. J Plast Reconstr Aesthet Surg. 2020b;73:460–468. doi: 10.1016/j.bjps.2019.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salzer JL, Bunge RP. Studies of Schwann cell proliferation I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84:739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 83.Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, Tabata Y, Mazda O. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plast Reconstr Surg. 2016;137:318e-330e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016;17:2101. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sunderland S. Factors influencing the course of regeneration and the quality of the recovery after nerve suture. Brain. 1952;75:19–54. doi: 10.1093/brain/75.1.19. [DOI] [PubMed] [Google Scholar]
- 86.Tavian M, Zheng B, Oberlin E, Crisan M, Sun B, Huard J, Peault B. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- 87.Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975;56:166–170. [PubMed] [Google Scholar]
- 88.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 89.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 90.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 91.Waller A. Experiments on the section of the glosso-pharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Edinb Med Surg J. 1851;76:369–376. [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C, Lu CF, Peng J, Hu CD, Wang Y. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen Res. 2017;12:2106–2112. doi: 10.4103/1673-5374.221171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Li ZW, Luo M, Li YJ, Zhang KQ Biological conduits combining bone marrow mesenchymal stem cells and extracellular matrix to treat long-segment sciatic nerve defects. Biological conduits combining bone marrow mesenchymal stem cells and extracellular matrix to treat long-segment sciatic nerve defects. Neural Regen Res. 2015;10:965–971. doi: 10.4103/1673-5374.158362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Y, Yuan X, Ding F, Yao D, Gu Y, Liu J, Gu X. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17:2231–2244. doi: 10.1089/ten.TEA.2010.0633. [DOI] [PubMed] [Google Scholar]
- 98.Yi S, Zhang Y, Gu X, Huang L, Zhang K, Qian T, Gu X. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020;8:tkaa002. doi: 10.1093/burnst/tkaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Issa Bhaloo S, Chen T, Zhou B, Xu Q. Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res. 2018;122:1608–1624. doi: 10.1161/CIRCRESAHA.118.313058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Z, Wang Y, Peng J, Ren Z, Zhan S, Liu Y, Zhao B, Zhao Q, Zhang L, Guo Q, Xu W, Lu S. Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31:388–394. doi: 10.1002/micr.20882. [DOI] [PubMed] [Google Scholar]


