Abstract
It was hypothesized that mesenchymal stem cells (MSCs) could provide necessary trophic factors when seeded onto the surfaces of commonly used nerve graft substitutes. We aimed to determine the gene expression of MSCs when influenced by Avance® Nerve Grafts or NeuraGen® Nerve Guides. Human adipose-derived MSCs were cultured and dynamically seeded onto 30 Avance® Nerve Grafts and 30 NeuraGen® Nerve Guides for 12 hours. At six time points after seeding, quantitative polymerase chain reaction analyses were performed for five samples per group. Neurotrophic [nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), pleiotrophin (PTN), growth associated protein 43 (GAP43) and brain-derived neurotrophic factor (BDNF)], myelination [peripheral myelin protein 22 (PMP22) and myelin protein zero (MPZ)], angiogenic [platelet endothelial cell adhesion molecule 1 (PECAM1/CD31) and vascular endothelial cell growth factor alpha (VEGFA)], extracellular matrix (ECM) [collagen type alpha I (COL1A1), collagen type alpha III (COL3A1), Fibulin 1 (FBLN1) and laminin subunit beta 2 (LAMB2)] and cell surface marker cluster of differentiation 96 (CD96) gene expression was quantified. Unseeded Avance® Nerve Grafts and NeuraGen® Nerve Guides were used to evaluate the baseline gene expression, and unseeded MSCs provided the baseline gene expression of MSCs. The interaction of MSCs with the Avance® Nerve Grafts led to a short-term upregulation of neurotrophic (NGF, GDNF and BDNF), myelination (PMP22 and MPZ) and angiogenic genes (CD31 and VEGFA) and a long-term upregulation of BDNF, VEGFA and COL1A1. The interaction between MSCs and the NeuraGen® Nerve Guide led to short term upregulation of neurotrophic (NGF, GDNF and BDNF) myelination (PMP22 and MPZ), angiogenic (CD31 and VEGFA), ECM (COL1A1) and cell surface (CD96) genes and long-term upregulation of neurotrophic (GDNF and BDNF), angiogenic (CD31 and VEGFA), ECM genes (COL1A1, COL3A1, and FBLN1) and cell surface (CD96) genes. Analysis demonstrated MSCs seeded onto NeuraGen® Nerve Guides expressed significantly higher levels of neurotrophic (PTN), angiogenic (VEGFA) and ECM (COL3A1, FBLN1) genes in the long term period compared to MSCs seeded onto Avance® Nerve Grafts. Overall, the interaction between human MSCs and both nerve graft substitutes resulted in a significant upregulation of the expression of numerous genes important for nerve regeneration over time. The in vitro interaction of MSCs with the NeuraGen® Nerve Guide was more pronounced, particularly in the long term period (> 14 days after seeding). These results suggest that MSC-seeding has potential to be applied in a clinical setting, which needs to be confirmed in future in vitro and in vivo research.
Keywords: Avance® Nerve Grafts, dynamic seeding, mesenchymal stem cell, NeuraGen® Nerve Guides, peripheral nerve repair, qPCR
Chinese Library Classification No. R459.9; R364; R741
Introduction
Peripheral nerve injuries result in a major social and economic burden by causing loss of function of target muscles (Landers et al., 2018; Hong et al., 2019; Karsy et al., 2019). In order to restore nerve function when the nerve gap is not suitable for direct end to end coaptation, (sensory) autografts, allografts and artificial guides can be used to bridge the gap. While resulting in optimal recovery rates, autograft nerve options are limited in diameter and length and associated with donor side morbidity (IJpma et al., 2006). Avance® Nerve Grafts and NeuraGen® Nerve Guides are commercially available nerve substitutes, approved for clinical use and are theoretically unlimited in supply. If their clinical outcome would be similar to autografts, these nerve graft substitutes may supplant autograft nerves.
Sensory nerve gaps (< 2.5 cm) can be effectively restored by either nerve conduits or processed nerve allografts, but their application in mixed or motor nerve defects with greater defect length and larger diameters results in varying outcomes (Moore et al., 2009; Brooks et al., 2012; Safa et al., 2019, 2020). In daily clinical practice, processed nerve grafts are described to only lead to good outcomes in mixed/motor nerves with maximal gap lengths of 6 mm and diameters between 3 and 7, but in cases that exceed these dimensions, autograft nerves remain the gold standard by surpassing the results of nerve conduits and allografts (Whitlock et al., 2009; Lin et al., 2013). However, particularly in nerve injuries with large gaps or multiple nerve injuries (i.e. brachial plexus injuries), there are often not enough autologous nerve graft sources to optimally reconstruct the defects (Gordon, 2009).
Seeding of mesenchymal stem cells (MSCs) on nerve graft substitutes may potentially reduce the outcome differences between nerve substitutes and nerve autografts. MSCs can interact with the extracellular matrix (ECM) of the nerve graft substitute to produce trophic factors necessary for tissue regeneration that supplement endogenous trophic sources (Salgado et al., 2010; Cao et al., 2015; Caplan, 2015; Caplan and Hariri, 2015; Mathot et al., 2019). To benefit from these trophic properties, dynamic seeding of MSCs is beneficial as it permits atraumatic introduction of MSCs to the ECM of graft substitutes prior to implantation while preventing damage to both the cells and the graft substitutes.
In peripheral nerve injury, a defined process occurs commencing from Wallerian degeneration to axonal regeneration and muscle reinnervation. This includes a nine step process from injury to regeneration: i) response to stimulus (Schwann cells and neurons change their state and become activated, 3–7 days), ii) regional inflammation (macrophage infiltration, 3–7 days), iii) immune response (7 days), iv) cell proliferation (formation of Bunger bands, 3–7 days), v) cell migration (Schwann cell migration, 7–14 days), vi) axon guidance (7–14 days), vii) myelination (initiated by Schwann Cells, 14 days), viii) extracellular matrix (7–14 days), ix) growth factor activity for axonal regeneration (3–14 days) (Pan et al., 2017).
To determine the exact potential of MSC-seeding in clinical practice and to understand its mode of action within the described regeneration process, it is essential to elucidate the interaction between human MSCs and the ECM of clinically available nerve substitutes. The purpose of this study was to examine this interaction by measuring expression of mRNA biomarkers for myelination, neurotrophic and angiogenic processes, ECM deposition and immune responses in human MSCs as a function of time after dynamic seeding onto Avance® Nerve Grafts and NeuraGen® Nerve Guides.
Materials and Methods
Study design
Human adipose derived MSCs were cultured and seeded onto 30 Avance® Nerve Grafts and 30 NeuraGen® Nerve Guides. At six time points after seeding, quantitative polymerase chain reaction (qPCR) analyses were performed (five samples per group). Five additional unseeded Avance® Nerve Grafts and five unseeded NeuraGen® Nerve Guides provided the baseline gene expression of the nerve substitutes; five samples of unseeded MSCs provided the reference gene expression of MSCs.
Human MSCs
The Mayo Clinical Human Cellular Therapy laboratory (Rochester, MN, USA) provided the human MSCs used in this experiment. These MSCs were obtained from a fat biopsy from a male donor with approval of local institutional review boards (IRB #07-008842). They complied with the criteria defined by the International Society for Cellular Therapy (Dominici et al., 2006). Multi-lineage potential, presence of cell surface markers (CD73, CD90, CD105, CD44, CD14, and CD45) and RNA-sequence transcriptome profiles have all been tested previously (Dudakovic et al., 2014). MSCs were cultured in growth media composed of Advanced MEM (a-MEM, 1×; Gibco by Life TechnologiesTM, Grand Island, NY, USA, Cat# 12492013), 5% platelet lysate (PLTMax®; Mill Creek Life Sciences, Rochester, MN, USA), 1% penicillin/streptomycin (Penicillin-Streptomycin (10.000 U/mL); Gibco by Life Technologies™ Cat# 15140148), 1% GlutaMAX (GlutaMAX™ Supplement 100×; Gibco by Life Technologies, Cat# 35050061) and 0.2% heparin (heparin sodium injection, USP, 1.000 USP units per mL, NovaPlus®, Fresenius Kabi, IL, USA). The MSCs were cultured in an incubator at 37°C (5% CO2), growth medium was changed every 72 hours and cells were split at 80% confluence. Passage 5 MSCs were used in this study (Crespo-Diaz et al., 2011; Mader et al., 2013; Mahmoudifar and Doran, 2013).
Nerve allografts and guides
Avance® Nerve Grafts are human nerve allografts that have been decellularized and irradiated to obtain non-immunogenic, sterile human nerves with remaining ultrastructure. The NeuraGen® Nerve Guides are composed of purified bovine type I collagen and are empty conduits that do not contain any ultrastructure in the central portion of the guide (Meek and Coert, 2008). Both the Avance® Nerve Graft and the NeuraGen® Nerve Guide have been approved by the USA Food and Drug Administration for human clinical use since 2007 and 2001, respectively.
A total of 35 Avance® Nerve Grafts and 35 NeuraGen® Nerve Guides of 15 mm in length were voluntarily provided by Axogen® (AxoGen, Inc., Alachua, FL, USA) and Integra (Integra LifeSciences Corporation, Princeton, NJ, USA) respectively. Five samples of each group were used to determine the baseline gene expression and 30 of each group were used to study the interaction with MSCs over time.
MSC seeding
To seed MSCs on nerve substitutes in a non-traumatic manner, a previously described seeding strategy was applied (Dietz et al., 2017; Rbia et al., 2018). Previous studies have demonstrated a 66% and 94% seeding efficiency for the Avance® Nerve Grafts and the NeuraGen® Nerve Guides, respectively (Mathot et al., 2020b). Prior to seeding, the Avance® Nerve Grafts and the NeuraGen® Nerve Guides were soaked in α-MEM to restore the salt balance and to remove any harmful detergents. The nerve substitutes were placed in conical tubes containing 1 × 106 MSCs in 10 mL growth medium. The conical tubes were rotated for 12 consecutive hours on a bioreactor (Revolver™ Adjustable lab rotator, Labnet International, Edison, NJ, USA) placed in a 37°C incubator.
Quantitative PCR analysis
Quantitative PCR analysis was performed on five duplicates per group before seeding (n = 5 per group, baseline gene expression) and at six time points: directly after seeding, 1, 3, 7, 14 and 21 days (n = 5 per group per time point) after seeding. Between seeding and qPCR analysis, the samples were placed in wells containing growth media. To determine the baseline gene expression of the MSCs, qPCR analysis was performed on five samples of MSCs only.
At each time point, the seeded nerve substitutes were removed from the wells, placed in Qiazol Lysis Reagent (Qiagen, Valencia, CA, USA) and frozen at –80°C. The seeded nerve substitutes were minced with a sterile needle to ensure the DNA of the MSCs was dissolved in the fluid. Ribonucleic acid (RNA) extraction took place according to the manufacturer’s protocol (Direct-zol™ RNA MiniPrep Kit; Zymo Research, Irvine, CA, USA). RNA concentration was measured with the NanoDrop™ 2000/2000c Spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Subsequently, complementary deoxyribonucleic acid (cDNA) was obtained (SuperScript III, Invitrogen, Carlsbad, CA, USA; 3 minutes at 65°C, 90 minutes at 37°C and 5 minutes at 95°C) and diluted to establish a concentration of 10 ng/µL. The obtained cDNA was combined with the selected primers (all manufactured by Sigma-Aldrich, St. Louis, MO, USA, Table 1) and real time-qPCR master mix (Qiagen, Valencia, CA, USA) and amplified by real time-qPCR using a thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under the following parameters: 15 minutes at 95°C, followed by 50 cycles of 95°C for 20 seconds, 60°C for 35 seconds and 72°C for 35 seconds. This provided the mRNA expression levels of the investigated genes.
Table 1.
mRNA primer sequences
| Gene ID | Definition | Biology | Forward primer (5’–3’) | Reverse primer (5’–3’) |
|---|---|---|---|---|
| NGF | Nerve growth factor | Neurotrophic marker | ATA CAG GCG GAA CCA CAC TCA G | ATA CAG GCG GAA CCA CAC TCA G |
| GDNF | Glial cell line-derived neurotrophic factor | Neurotrophic marker | CAC CAG ATA AAC AAA TGG CAG TGC | CAC CAG ATA AAC AAA TGG CAG TGC |
| PTN | Pleiotrophin | Neurotrophic marker | ACT GGA AGT CTG AAG CGA GC | CTT CTT CTT AGA TTC TGC TTG AGG T |
| GAP43 | Growth associated protein 43 | Neurotrophic marker | GTC CAC TTT CCT CTC TAT TTC | TGT TCA TTC CAT CAC ATT GA |
| BDNF | Brain-derived neurotrophic factor | Neurotrophic marker | AGA GGC TTG ACA TCA TTG GCT G | CAA AGG CAC TTG ACT ACT GAG CAT C |
| PMP22 | Peripheral myelin protein 22 | Myelination marker | GTT AAA GGG AAC GCC AGG A | AGT TTC TGC AGC CCA AAG GA |
| MPZ | Myelin protein zero | Myelination marker | GAG GAG GCT CAG TGC TAT GG | GCC CGC TAA CCG CTA TTT CT |
| VEGFA | Vascular endothelial cell growth factor alpha | Angiogenic marker | ATC TGC ATG GTG ATG TTG GA | GGG CAG AAT CAT CAC GAA G |
| PECAM/CD31 | Platelet endothelial cell adhesion molecule 1 | Angiogenic marker | AAC AGT GTT GAC ATG AAG AGC C | AAC AGT GTT GAC ATG AAG AGC C |
| COL1A1 | Collagen type alpha I | Extracellular matrix protein | GTA ACA GCG GTG AAC CTG G | CCT CGC TTT CCT TCC TCT CC |
| COL3A1 | Collagen type alpha III | Extracellular matrix protein | TTG AAG GAG GAT GTT CCC ATC T | ACA GAC ACA TAT TTG GCA TGG TT |
| FBLN1 | Fibulin 1 | Extracellular matrix protein | AGA GCT GCG AGT ACA GCC T | CGA CAT CCA AAT CTC CGG TCT |
| LAMB2 | Laminin beta 2 subunit | Extracellular matrix protein | ACA CGC AAG CGA GTG TAT GA | AAT CAC AGG GCA GGC ATT CA |
| CD96 | Cluster of differentiation 96 | Cell surface marker/ immunoglobulin | AGA TTG TGT GAT GAA GGA CAT GG | AGA TTG TGT GAT GAA GGA CAT GG |
| AKT1 | Threonine kinase 1 | Household gene | ATG GCG CTG AGA TTG TGT CA | CCC GGT ACA CCA CGT TCT TC |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Household gene | CCC GGT ACA CCA CGT TCT TC | TGT GGT CAT GAG TCC TTC CA |
Analysis of mRNA biomarkers
In relation to the described Wallerian degeneration and axon regeneration process, the neurotrophic effects of the interaction between MSCs and nerve substitutes were measured by assessing a panel of mRNA biomarkers, including nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), pleiotrophin (PTN), growth associated protein 43 (GAP43) and brain-derived neurotrophic factor (BDNF). The expression of myelination marker genes peripheral myelin protein 22 (PMP22) and myelin protein zero (MPZ) were measured. The angiogenic potential of the seeded MSCs was measured by the gene expression of platelet endothelial cell adhesion molecule 1 (PECAM1/CD31) and vascular endothelial cell growth factor alpha (VEGFA). Establishment of the ECM was assessed by monitoring expression of collagen type alpha I (COL1A1), collagen type alpha III (COL3A1), Fibulin 1 (FBLN1) and laminin subunit beta 2 (LAMB2). Immunoglobulin expression was determined by quantification of cluster of differentiation 96 (CD96), a cell surface marker.
Both glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and AKT serine/threonine kinase 1 (AKT1) were used as reference genes. Expression of the AKT1 gene was determined to be more stable and considered the most optimal housekeeping gene based on BestKeeper analysis and therefore was used as reference gene in the analysis (Pfaffl et al., 2004). The 2–ΔΔCT method was used to calculate the mRNA levels which were expressed as a ratio of AKT1 (Livak and Schmittgen, 2001; Dudakovic et al., 2014; Jesuraj et al., 2014). Primer sequences for the genes that were analyzed are displayed in Table 2.
Table 2.
Summary of interaction-induced relative changes in gene expression of human mesenchymal stem cells on the short and long term
| Gene ID | Biology | Short term (0–24 h) | Long term (14–21 d) | ||||
|---|---|---|---|---|---|---|---|
| Avance® | NeuraGen® | Significance | Avance® | NeuraGen® | Significance | ||
| NGF | Neurotrophic marker | ↑↑ | ↑↑ | ***/** | ↓↓ | ↓↓ | –/– |
| GDNF | Neurotrophic marker | ↑↑ | ↑↑ | –/– | ≈ | ↑↑ | –/ – |
| PTN | Neurotrophic marker | ↓↓ | ↓↓ | –/– | ↓↓ | ≈ | */*** |
| GAP43 | Neurotrophic marker | ↓↓ | ↓↓ | –/– | ↓ | ↓ | –/– |
| BDNF | Neurotrophic marker | ↑ | ↑↑ | –/– | ↑↑ | ↑↑ | */– |
| PMP22 | Myelination marker | ↑ | ↑ | –/– | ≈ | ≈ | –/– |
| MPZ | Myelination marker | ↑ | ↑ | –/– | ↑ | ↑ | –/– |
| VEGFA | Angiogenic marker | ↑↑ | ↑↑↑ | */– | ↑↑ | ↑↑↑ | –/* |
| PECAM/CD31 | Angiogenic marker | ↑ | ↑ | –/– | ↓ | ↑ | –/– |
| COL1A1 | Extracellular matrix protein | ≈ | ↑ | **/– | ↑ | ↑ | –/– |
| COL3A1 | Extracellular matrix protein | ≈ | ↑ | –/– | ≈ | ↑ | –/*** |
| FBLN1 | Extracellular matrix protein | ↓ | ≈ | –/– | ↓ | ↑ | */*** |
| LAMB2 | Extracellular matrix protein | ↓ | ↓ | –/– | ↓ | ↓ | –/– |
| CD96 | Cell surface marker/immunoglobulin | ↑ | ↑ | –/– | ≈ | ↑ | –/– |
The signs under significance display whether there were significant differences between the groups at the first two (directly and 24 hours after seeding, separated by a forward slash) and the final two (14 days and 21 days after seeding, separated by a forward slash) time points. ↑↑↑ = extreme enhancement; ↑↑ = moderate enhancement; ↓↓ = moderate reduction; ↑ = slight enhancement; ↓ = slight reduction; ≈ = no enhancement, no reduction; – = no significant difference. *P < 0.05, **P < 0.01, ***P < 0.001. BDNF: Brain-derived neurotrophic factor; CD96: cluster of differentiation 96; COL1A1: collagen type alpha I; COL3A1: collagen type alpha III; ECM: extracellular matrix; FBLN1: Fibulin 1; GAP43: growth associated protein 43; GDNF: glial cell line-derived neurotrophic factor; LAMB2: laminin subunit beta 2; MPZ: myelin protein zero; NGF: nerve growth factor; PECAM1/CD31: platelet endothelial cell adhesion molecule 1; PMP22: peripheral myelin protein 22; PTN: pleiotrophin; VEGFA: vascular endothelial cell growth factor alpha.
Statistical analysis
The mRNA levels of the seeded MSCs on both nerve substitutes were expressed as a ratio of the average mRNA levels of unseeded MSCs (= reference group) which resulted in a gene expression ratio. Gene expression ratios of MSCs seeded onto Avance® Nerve Grafts were compared to gene expression ratios of MSCs seeded onto NeuraGen® Nerve Guides over time and were analyzed using two-way repeated measures analysis of variance with post hoc Bonferroni correction. This analysis provided insight in the effects of the type of nerve substitute and the time passed after seeding. All results are expressed as the mean ± SEM. A P-value of < 0.05 was considered statistically significant.
Results
Control experiments with nerve substitutes
The presence of RNA was examined in unseeded Avance® Nerve Grafts (n = 5) and NeuraGen® Nerve Guides (n = 5) and no RNA was detected.
Neurotrophic gene expression
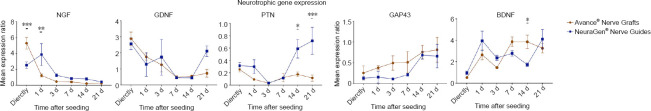
Gene expressions of five neurotrophic factors (NGF, GDNF, PTN, GAP43 and BDNF) were measured by qPCR to assess whether nerve substitutes can induce neurotrophic factors in MSCs (Figure 1).
Figure 1.
Gene expression curves of human MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides concerning neurotrophic genes NGF, GDNF, PTN, GAP43 and BDNF at six time points after seeding.
The curves demonstrate the mRNA levels of the seeded MSCs on both nerve substitutes as a ratio of the average mRNA levels of unseeded MSCs (= reference group). n = 5 per group per timepoint. Comparisons between groups (MSCs seeded onto Avance® Nerve Grafts versus MSCs seeded onto NeuraGen® Nerve Guides) over time were obtained using two-way repeated measures analysis of variance with post hoc Bonferroni correction. Significant differences between groups are indicated by an *. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars = Standard error of the mean. BDNF: Brain-derived neurotrophic factor; GAP43: growth associated protein 43; GDNF: glial cell line-derived neurotrophic factor; MSC: mesenchymal stem cell; NGF: nerve growth factor; PTN: pleiotrophin.
NGF
NGF is endogenously produced by cells like neurons and Schwann cells and is crucial for neuroplasticity since it promotes neuron maturation and can induce cell repair and apoptosis (Aarao et al., 2018; Manni et al., 2013). The factor time solely had a significant effect on NGF expression (P < 0.001), while the type of nerve substitute did not (P = 0.228). Directly after seeding, MSCs on Avance® Nerve Grafts expressed significantly increased levels of NGF compared to MSCs on NeuraGen® Nerve Guides (5.304 ± 0.731 vs. 2.530 ± 0.419, P < 0.001), while MSCs on NeuraGen® Nerve Guides expressed significantly increased NGF expression ratio 1 day after seeding (1.226 ± 0.187 vs. 3.868 ± 1.379, P = 0.002). During 1–3 days after seeding, the NGF expression of MSCs in both groups was comparable to the expression in unseeded MSCs. Thus, NGF expression is transiently elevated in MSCs seeded onto nerve substitutes shortly after seeding and subsides over time.
GDNF
GDNF is described to have a crucial role in neuronal migration, proliferation and synaptogenesis (Yamada et al., 2004; Paratcha and Ledda, 2008). Enhanced GDNF delivery has demonstrated to result in earlier regeneration after nerve crush injuries (Magill et al., 2010) and its expression decreases coincidentally with the ingrowth of regenerating axons (Yamada et al., 2004). The GDNF expression ratio of seeded MSCs was significantly affected by culture duration after seeding (P < 0.001); the expression slowly diminished over time with the exception of GDNF expression after 21 days in MSCs seeded onto Avance® Nerve Grafts. No significant differences were found in GDNF expression between both groups. The GDNF expression curves of both groups approximated the unseeded MSC expression from 1 day onwards.
PTN
PTN expression was measured as it is involved in neurite outgrowth and synaptogenesis after peripheral nerve injury (Blondet et al., 2005; Jin et al., 2009). The expression ratio of PTN was modulated in MSCs by the type of nerve substitute (P < 0.001) and by the time after seeding (P = 0.001). PTN expression decreased up to 7 days after seeding for both groups, after which particularly the PTN expression of MSCs seeded on NeuraGen® Nerve Guides increased again up to 21 days after seeding. The MSCs on the NeuraGen® Nerve Guide expressed a significant higher PTN ratio than MSCs on Avance® Nerve Grafts after 14 (0.590 ± 0.151 vs. 0.174 ± 0.037, P = 0.011) and 21 days (0.718 ± 0.29 vs. 0.116 ± 0.050, P < 0.001). Although the PTN expression of MSCs on NeuraGen® Nerve Guides approximated the baseline gene expression of unseeded MSCs after 21 days, measures at all the other time points demonstrated downregulation of the baseline expression.
GAP43
GAP43 expression increases after axotomy, eventually leading to enhanced axon density in regenerating nerve fibers (Ceber et al., 2015). The GAP43 expression of seeded MSCs was significantly affected by the time after seeding (P = 0.008) and the type of nerve substitute (P = 0.046). The GAP43 expression of MSCs on both nerve substitutes increased over time, of which the expression of MSCs on Avance® Nerve Grafts most evenly increased over time. There were no significant differences in GAP43 expression between both nerve substitutes. All measured expression ratios were below 1, implicating downregulation of the baseline GAP43 expression.
BDNF
BDNF induces neuronal cell survival and differentiation and accelerates axonal outgrowth (Vogelin et al., 2006), but also is involved in synapse formation (Sendtner et al., 1992; Zagrebelsky and Korte, 2014). The BDNF expression ratio of seeded MSCs was significantly affected by the time passed after seeding (P < 0.001). The BDNF expression in MSCs seeded on Avance® Nerve Grafts (3.846 ± 0.636) was significantly higher after 14 days compared to the expression in MSCs on NeuraGen® Nerve Guides (1.720 ± 0.164; P = 0.020). The expression ratio in both groups approximated 1 directly after seeding, and increased up to 4 (i.e., a fourfold of the baseline BDNF expression in unseeded MSCs) on various time points in later phases (1 and 21 days after seeding for the NeuraGen® Nerve Guide, 7, 14 and 21 days after seeding for the Avance® Nerve Grafts).
Myelination gene expression
PMP22
The PMP22 gene is mainly expressed in Schwann cells and encodes a relatively minor but crucial component of the myelin sheeth (Scherer and Wrabetz, 2008). Duplication or deletion of PMP22 leads to demyelination and axon loss, resulting in common demyelinating neuropathies (Hui-Chou et al., 2011; Taioli et al., 2011). PMP22 expression ratio was significantly affected by the time after seeding (P = 0.0.26), but not by the type of nerve substitute (P = 0.542) on which the MSCs were seeded. The expression ratio started high for both groups (1.90 ± 0.231 for Avance® Nerve Grafts, 1.78 ± 0.663 for NeuraGen® Nerve Guides) and decreased to a level comparable to unseeded MSCs after 21 days (0.716 ± 0.278 for Avance® Nerve Grafts, 0.928 ± 0.102 for NeuraGen® Nerve Guides). There were no significant differences between groups over time (Figure 2).
Figure 2.
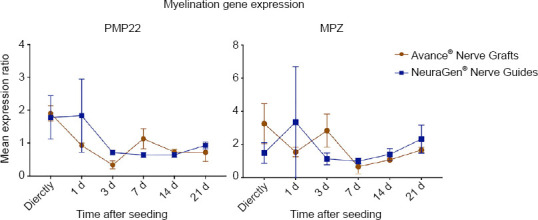
Gene expression curves of MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides concerning myelination genes PMP22 and MPZ at six different time points after seeding.
The curves demonstrate the mRNA levels of the seeded MSCs on both nerve substitutes as a ratio of the average mRNA levels of unseeded MSCs (= reference group). n = 5 per group per timepoint. Comparisons between groups (MSCs seeded onto Avance® Nerve Grafts versus MSCs seeded onto NeuraGen® Nerve Guides) over time were obtained using two-way repeated measures analysis of variance with post hoc Bonferroni correction. Error bars = Standard error of the mean. MSC: Mesenchymal stem cell; MPZ: myelin protein zero; PMP22: peripheral myelin protein 22.
MPZ
MPZ is also expressed by Schwann cells and is the main protein of (compact) myelin. Reduced expression of MPZ results in instable myelination, leading to neuropathies and axonal loss. MPZ is therefore pivotal in successful nerve regeneration (Scherer and Wrabetz, 2008). The MPZ gene expression ratio was not significantly affected by type of nerve substitute (P = 0.936), nor by the time passed after seeding (P = 0.650). Compared to the baseline MPZ expression in unseeded MSCs, the expression ratio in both groups was increased both at the beginning (up to 3 days after seeding) and at the end (21 days) of the experiment. There were no significant differences in MPZ expression over time between groups (Figure 2).
Angiogenic gene expression
VEGFA
Neoangiogenesis occurs from 2 days onwards after nerve injury and facilitates the delivery of trophic factors at the nerve stump and guides Schwann cells in the right direction (Cutting and McCarthy, 1983; Parrinello et al., 2010; Caillaud et al., 2019). Enhanced expression and production of VEGFA induces local angiogenesis, playing an important role in the peripheral nerve regeneration process (Nishida et al., 2018). The type of nerve substitute had no significant effect on the VEGFA expression of seeded MSCs (P = 0.107). Time passing did have a significant effect on the VEGFA expression of seeded MSCs (P < 0.001). The VEGFA expression in both groups was high (4 to 12 fold of the baseline expression) in the first 24 hours after seeding, equal to unseeded MSCs around 3 to 7 days after seeding and then increased again (3 to 8 fold of the baseline expression) up to 21 days after seeding. The VEGFA expression was significantly higher in the MSCs seeded on NeuraGen® Nerve Guides directly after seeding (11.006 ± 0.33 vs. 6.698 ± 1.502, P = 0.020) and 21 days after seeding (8.690 ± 2.062 vs. 4.118 ± 1.847, P = 0.011; Figure 3).
Figure 3.
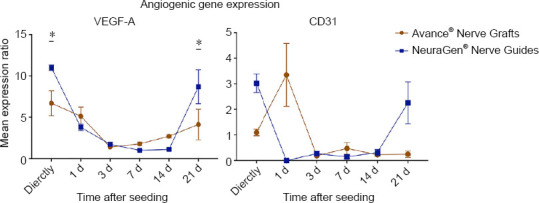
Gene expression curves of MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides concerning angiogenic genes VEGFA and CD31 at six different time points after seeding.
The curves demonstrate the mRNA levels of the seeded MSCs on both nerve substitutes as a ratio of the average mRNA levels of unseeded MSCs (= reference group). n = 5 per group per timepoint. Comparisons between groups (MSCs seeded onto Avance® Nerve Grafts versus MSCs seeded onto NeuraGen® Nerve Guides) over time were obtained using two-way repeated measures analysis of variance with post hoc Bonferroni correction. Significant differences between groups are indicated by an *. *P < 0.05. Error bars = Standard error of the mean. MSC: Mesenchymal stem cell; VEGFA: vascular endothelial cell growth factor alpha.
PECAM-1/CD31
CD31 regulates endothelial cell adhesion, being crucial in the process of angiogenesis (DeLisser et al., 1997). The factor time significantly influenced the CD31 expression ratio (P < 0.001), but the type of nerve substitute did not have a significant impact (P = 0.77). When comparing groups over time, MSCs seeded onto NeuraGen® Nerve Guides had a significant higher CD31 expression ratio compared to MSCs seeded onto Avance® Nerve Grafts directly after seeding (3.016 ± 0.364 vs. 1.096 ± 0.13, P = 0.025) and 21 days after seeding (2.254 ± 0.815 vs. 0.244 ± 0.123, P = 0.017). The expression in the NeuraGen® Nerve Guide group was significantly lower than in the Avance® Nerve Graft group 1 day after seeding (P < 0.001). With the exception of directly, 1 and 21 days after seeding, all other time points demonstrated significant downregulation of the baseline CD31 expression of unseeded MSCs (Figure 3).
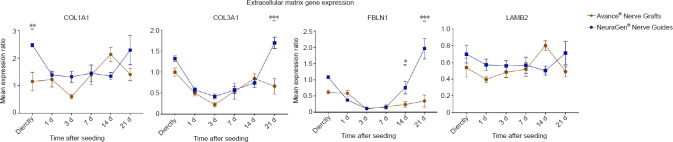
Extracellular matrix gene expression
COL1A1
The COL1A1 gene encodes for the main component of type 1 collagen. This ECM-component regulates Schwann cell proliferation and differentiation, but overexpression of COL1A1 could impede axon sprouting by inducing scarring (Koopmans et al., 2009). The time passed after seeding (P = 0.014) and the type of nerve substitute (P = 0.033) both had a significant effect on the COL1A1 gene expression ratio. Directly after seeding, MSCs on NeuraGen® Nerve Guides (2.468 ± 0.064) had a significant higher COL1A1 expression ratio than MSCs on Avance® Nerve Grafts (1.152 ± 0.332; P = 0.008). Aside from increased COL1A1 expression directly (NeuraGen® Nerve Guide), 14 (Avance® Nerve Graft) and 21 days (NeuraGen® Nerve Guides) after seeding, the COL1A1 expression in the NeuraGen® Nerve Guide group was comparable to the baseline COL1A1 expression of unseeded MSCs (Figure 4).
Figure 4.
Gene expression curves of MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides concerning extracellular matrix genes COL1A1, COL3A1, FBLN1 and LAMB2 at six different time points after seeding.
The curves demonstrate the mRNA levels of the seeded MSCs on both nerve substitutes as a ratio of the average mRNA levels of unseeded MSCs (= reference group). n = 5 per group per timepoint. Comparisons between groups (MSCs seeded onto Avance® Nerve Grafts versus MSCs seeded onto NeuraGen® Nerve Guides) over time were obtained using two-way repeated measures analysis of variance with post hoc Bonferroni correction. Significant differences between groups are indicated by an *. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars = Standard error of the mean. COL1A1: Collagen type alpha I; COL3A1: collagen type alpha III; FBLN1: Fibulin 1; LAMB2: laminin subunit beta 2; MSC: mesenchymal stem cell.
COL3A1
Just like type 1, collagenase type 3 is a fibril forming collagen and an ECM component in the peripheral nervous system, regulating Schwann cell differentiation and axonal guidance (Hubert et al., 2009; Koopmans et al., 2009). The COL3A1 gene expression ratio was significantly affected by time after seeding (P < 0.001) and the type of nerve substitute (P = 0.014). The expression curve of both groups was u-shaped and approximated a ratio of 1 (i.e., comparable to baseline COL3A1 expression), with a significant higher expression ratio of MSCs seeded onto NeuraGen® Nerve Guides after 21 days (1.696 ± 0.132 vs. 0.664 ± 0.192, P < 0.001; Figure 4).
FBLN1
FBLN-1 expression was assessed as it is a component of the perineurium of peripheral nerves and fulfills a axonal guiding and supporting function (Miosge et al., 1996). Time after seeding (P < 0.001) and type of nerve substitute (P = 0.002) both significantly affected the FBLN-1 expression ratio. When comparing groups over time, the MSCs seeded onto NeuraGen® Nerve Guides had a significantly higher FBLN-1 expression ratio after 14 (0.760 ± 0.193 vs. 0.236 ± 0.079, P = 0.037) and 21 days (1.962 ± 0.318 vs. 0.340 ± 0.183, P < 0.001). The expression in the Avance® Nerve Graft group remained low over time, while the expression in the NeuraGen® Nerve Guide group seemed to arise from 7 days onwards, demonstrating significant upregulation of the baseline FBLN1 expression (Figure 4).
LAMB2
LAMB2 is a basis laminin protein that is essential in proper synaptogenesis at neuromuscular junctions (Maselli et al., 2012). There was no significant effect of either the time passed after seeding (P = 0.247) nor the type of nerve substitute (P = 0.354). No significant differences between groups occurred over time, while the expression in both groups remained lower than the expression in unseeded MSCs (Figure 4).
Immunoglobulin expression
CD96
CD96 is an immunoglobulin family member that interferes in the adhesive interactions between cells, in the late phase immune response (Georgiev et al., 2018). Only the factor time significantly affected the CD96 expression rate (P = 0.011). 21 days after seeding, the expression in MSCs seeded onto NeuraGen® Nerve Guides (2.226 ± 0.443) was significantly higher than the expression in MSCs seeded onto Avance® Nerve Grafts (1.326 ± 0.097, P = 0.030). The expression curve of the NeuraGen® Nerve Guide group seemed to increase on the long term, while the expression curve of the Avance® Nerve Graft group remained stable on the long term. The obtained CD96 expression demonstrated a slight upregulation in comparison to baseline gene expression of unseeded MSCs at most time points (Figure 5).
Figure 5.
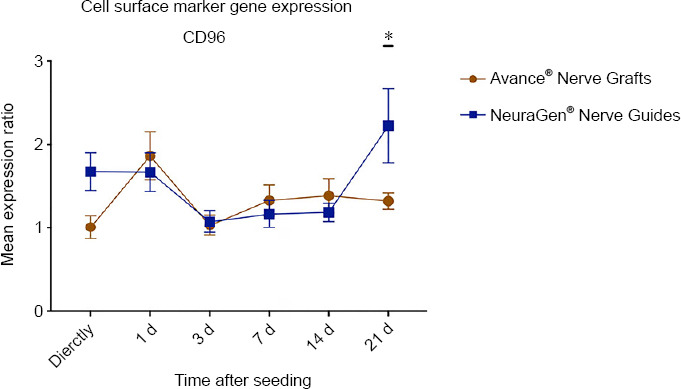
Gene expression curve of MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides concerning the immunoglobulin marker CD96 at six different time points after seeding.
The curves demonstrate the mRNA levels of the seeded MSCs on both nerve substitutes as a ratio of the average mRNA levels of unseeded MSCs (= reference group). n = 5 per group per timepoint. Comparisons between groups (MSCs seeded onto Avance® Nerve Grafts versus MSCs seeded onto NeuraGen® Nerve Guides) over time were obtained using two-way repeated measures analysis of variance with post hoc Bonferroni correction. Significant differences between groups are indicated by an *. *P < 0.05. Error bars = Standard error of the mean. CD96: Cluster of differentiation 96; MSC: mesenchymal stem cell.
A summary of the described gene expression trends and differences is displayed in Table 2.
Discussion
Secondary to their regenerative ability, MSCs are of broad biomedical relevance (Koga et al., 2008; Hare et al., 2012, 2017; Mushtaq et al., 2014; Shen et al., 2015; Sutton et al., 2016, 2017). While differentiation of MSCs into specific cell types (i.e., differentiation into Schwann type cells) has been described as one mode of action (Orbay et al., 2011; Tomita et al., 2013), secretion of essential trophic factors is a functionally different and possible more plausible explanation for their regenerative capacities (Ma et al., 2014; Caplan, 2015; Caplan and Hariri, 2015; Castro-Manrreza and Montesinos, 2015). This putative tissue-specific trophic expression of MSCs occurs in response to cues from their microenvironment (Liu et al., 2013; Kingham et al., 2014; Mathot et al., 2019).
In this study, gene expression profiles of human MSCs seeded onto Avance® Nerve Grafts and NeuraGen® Nerve Guides were examined over time to provide mechanistic insight in the interaction of MSCs seeded on nerve substitutes. Expression curves of a select panel of prominent neurotrophic, myelination, angiogenic, ECM and cell surface marker/immunoglobulin genes have been analyzed.
The displayed gene expressions of seeded MSCs are all expressed as a ratio of the gene expression in unseeded MSCs, the reference group. Considering a gene expression ratio of 1.0 meaning that the interaction between MSCs and the nerve substitutes did not result in changes in gene expression, one could argue that the interaction of MSCs with the Avance® Nerve Grafts leads to a clear upregulation in the first hours to days after seeding of neurotrophic genes NGF, GDNF and BDNF, myelination markers PMP22, MPZ and angiogenic genes CD31 and VEGFA. In the long term, the interaction between MSCs and the Avance® Nerve Grafts causes an upregulation of BDNF, VEGFA and COL1A1. The interaction between MSCs and the NeuraGen® Nerve Guide led on the short term to an upregulation of neurotrophic genes NGF, GDNF and BDNF, myelination markers PMP22 and MPZ, angiogenic genes CD31 and VEGFA, ECM gene COL1A1 and cell surface marker CD96. In the long term, the expression of neurotrophic genes GDNF and BDNF, angiogenic genes CD31 and VEGFA, ECM genes COL1A1, COL3A1 and FBLN1 and cell surface marker CD96 were all upregulated by the interaction between the MSCs and the NeuraGen® Nerve Guide. Genes of which the expression was downregulated for both groups were PTN, GAP43 and LAMB2.
The described steps of Wallerian degeneration and axon regeneration in which the evaluated genes are hypothetically involved are illustrated in Figure 6. It displays the proposed mechanism of MSC-seeding; MSCs are seeded on the outer surface, gene expression of the MSCs is changed by the ECM, resulting in production of trophic factors that are involved in Wallerian degeneration and axon regeneration inside the nerve substitutes.
Figure 6.
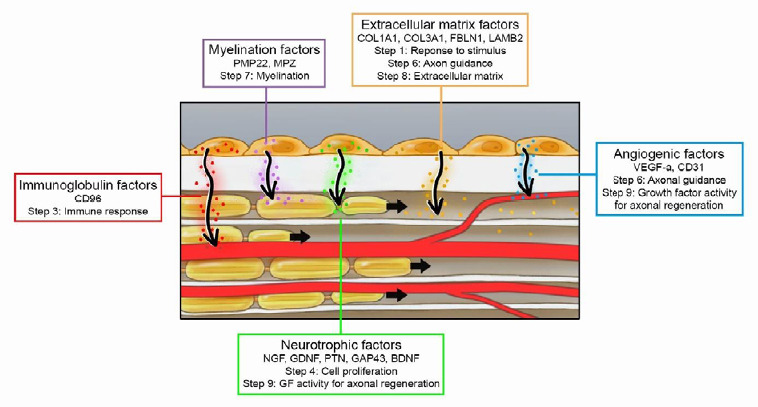
Proposed mechanism of MSC-seeding on nerve substitutes.
The interaction between MSCs and the nerve substitute results in changes in gene expression profiles, leading to production of trophic factors that are involved in Wallerian degeneration and axon regeneration. BDNF: Brain-derived neurotrophic factor; CD96: cluster of differentiation 96; COL1A1: collagen type alpha I; COL3A1: collagen type alpha III; ECM: extracellular matrix; FBLN1: Fibulin 1; GAP43: growth associated protein 43; GDNF: glial cell line-derived neurotrophic factor; GF: growth factor; LAMB2: laminin subunit beta 2; MPZ: myelin protein zero; MSC: mesenchymal stem cell; NGF: nerve growth factor; PECAM1/CD31: platelet endothelial cell adhesion molecule 1; PMP22: peripheral myelin protein 22; PTN: pleiotrophin; VEGFA: vascular endothelial cell growth factor alpha.
As described, NGF, GDNF, PTN, GAP43 and BDNF play a part in the stimulation of axonal outgrowth and the proliferation of neurons and Schwann cells (steps 4 and 9, Figure 6; Boyd and Gordon, 2003; Hoyng et al., 2014; Zhang and Rosen, 2018). Previous in vivo research in a rat model demonstrated that particularly NGF is expressed in a significant higher manner in nerve autografts than in decellularized allografts (Rbia et al., 2020). In our study, NGF and GDNF expression was enhanced in the first 0–24 hours after seeding and the GDNF expression after 21 days seemed to increase again in the NeuraGen® Nerve Guide group. BDNF expression was enhanced in both groups among the entire follow-up period (up to 21 days), but PTN and GAP43 expressions did not increase in comparison to unseeded MSCs (ratio < 1.0). The long-term low expression of PTN and GAP43 and the enhanced expression of BDNF after seeding was correspondingly described in in vitro research using human nerve allografts that were processed with elastase and stored at 4°C. In the study of Rbia and colleagues enhanced BDNF expression also led to enhanced levels of BDNF growth factor production. In contradiction to our findings, NGF and GDNF were not enhanced in that particular study, which might be due to differences in the ECM as a result of the different decellularization process (Rbia et al., 2019). Our results suggests that the interaction between MSCs and the ECM of nerve substitutes stimulates neural proliferation or may enhance neural outgrowth, particularly by upregulation of NGF, GDNF and BDNF.
MPZ and PMP22 are mainly expressed in Schwann cells, which initiate axon myelination, occurring approximately 2 weeks after injury (step 7; Figure 6) (Pan et al., 2017). The short-term (first 24 hours) enhanced expression of PMP22 and MPZ demonstrated in this study corresponds to previous in vitro research using the same seeding strategy on different nerve allografts (Rbia et al., 2019). Since transdifferentiation into Schwann-like cells is unlikely to have occurred in the described time-span, the elevated level of PMP22 might be subscribed to its role in the development of intercellular junctions (Roux et al., 2004). The PMP22 and MPZ expression was not significantly altered on the long term (from 7 days onwards) by the interaction with the nerve substitutes in the current study; this could be due to the absence of Schwann cells in this in vitro setting. Previously, rat autograft nerves did not express significantly different levels of PMP22 and MPZ in vivo than unseeded processed allografts, which could insinuate that these genes are not pivotal for improving nerve regeneration in processed nerve allografts to a level equal to autografts (Rbia et al., 2020).
VEGFA functions particularly in axon regeneration and guidance (steps 6 and 9, Figure 6) by stimulating formation of blood vessels and enhancing Schwann cell and neuron survival (Hobson et al., 2000; Hoyng et al., 2014). In vivo, rat autograft nerves previously demonstrated to express significantly higher levels of VEGFA than unseeded processed nerve allografts (Rbia et al., 2020) The demonstrated upregulation of VEGFA expression in this study in the first 24 hours and from two weeks onwards after seeding is in accordance with the described nerve regeneration cascade and with previous in vivo research, supporting the role that MSCs can play in revascularization (Rbia et al., 2020). CD31 is a platelet endothelial cell adhesion molecule (Pecam1) that is required for the motility and organization of endothelial cells, essential for angiogenesis (step 9, Figure 6) (Cao et al., 2002). Autografts do not express significantly different levels of CD31 in vivo than processed nerve allografts (Rbia et al., 2020). Our data describes enhanced CD31 expression directly after seeding that diminishes after 1 to 3 days after seeding.
ECM components derived from genes like COL1A1, COL3A1, FBLN1 and LAMB2 are essential for creating a pro-regenerative environment in the early stages after nerve injury and facilitate reinnervation in later stages by guiding the growth cone in the right direction (Figure 6) (Allodi et al., 2012; Pan et al., 2017). Although autografts previously demonstrated a trend of enhanced expression of these ECM markers in vivo compared to unseeded allografts, none of the differences were statistically significant (Rbia et al., 2020). In the current study, MSCs seeded onto NeuraGen® Nerve Guides demonstrated a U-shaped expression of COL1A1, COL3A1 and FBLN1, corresponding to the described cascade and previous in vitro studies (Rbia et al., 2019; Mathot et al., 2020a). Considering the absence of detectable RNA levels of unseeded Avance® Nerve Grafts and NeuraGen® Nerve Guides, the influence of the material components of the nerve substitutes on itself on the ECM gene expression is estimated as negligibly small.
CD96 is a membrane protein that is involved in the late phase immune response by interfering in adhesive interactions between cells (step 3, Figure 6), which potentially explains why its expression remains more or less stable over time (Georgiev et al., 2018).
Study limitations
When studying the demonstrated expression curves, some inconsistent expression ratios can be identified. Measures were taken to minimize the vulnerabilities during the obtainment of the mRNA levels like using five replicates per time point, a stable reference gene and experienced researchers. Besides, not all these inconsistent ratios differ significantly from the measures before and after that specific time point. Studying the demonstrated expression curves, we identified four general trends; a linear decline (NGF, GDNF, and CD31), a linear increase (PTN, GAP43, and BDNF), a stable curve (PMP22, MPZ, COL1A1, LAMB2, and CD96) and a U-shaped curve (VEGFA, COL3A1, and FBLN1). We believe that those trends are more reliable and therefore a more important finding than the individual expression ratios at each of the time points.
The in vitro setting of our study does not provide the required micro-environmental signals that are essential to mimic the described regeneration cascade. Studying the effects on gene expression that is solely caused by the interaction between MSCs and nerve substitutes does demonstrate the potential of MSCs to interfere in the previously mentioned cascade steps when dynamically seeded on the outer surface of clinically available nerve graft substitutes. It is recognized that corroborating the mRNA expression changes to protein expression changes could have contributed to the described findings. However, measuring protein levels is a costly technique, vulnerable to flaws and the absence of environmental regenerative signals in vitro would have resulted in outcomes that cannot per definition be related to in vivo protein expression and would still need translation to an in vivo model. Therefore, this study is used to demonstrate that interaction between MSCs and the nerve substitutes occurs, effects a wide range of genes and that it lasts on the long term, while limiting the costs and still preventing unnecessary sacrifices of extra animals in the future.
Although it was hypothesized that the biological composition of the Avance® Nerve Grafts (i.e., neural tissue) would lead to more expression of neurotrophic genes in MSCs, analysis demonstrated that the MSCs seeded onto NeuraGen® Nerve Guides expressed higher levels of neurotrophic (GDNF and PTN), angiogenic (CD31 and VEGFA), ECM (COL3A1 and FBLN1) and immunoglobulin (CD96) genes. Higher seeding efficiency and a different composition of the guide may have resulted in improved sustainability of the graft/MSCs in vitro and better cell proliferation in the long-term, leading to the described enhanced gene expressions. Most neurotrophic factors mediate other processes that are not involved in nerve regeneration which could explain enhanced gene expression levels in the absence of any neural material in the NeuraGen® Nerve Guide group (Gordon, 2009) .
While our data suggest that nerve autograft substitutes could benefit from the addition of MSCs, future studies are necessary to determine gene expression patterns and the resulting trophic factor production of MSCs in the presence of injured nerve tissue. Furthermore, the in vivo effects on functional outcomes of the described interactions need to be correlated and compared to determine the clinical relevance of our findings.
Conclusion
When human MSCs are dynamically seeded onto the surfaces of Avance® Nerve Grafts and NeuraGen® Nerve Guides, their interactions with the ECM of these nerve substitutes result in a change and mostly an upregulation of the expression of numerous genes important for nerve regeneration over time. The in vitro interaction of MSCs with the NeuraGen® Nerve Guide is greater than the Avance® Nerve Grafts, particularly in the long-term (> 14 days after seeding). Future studies should focus on translation to an in vivo model to confirm the potential of the described techniques and mRNA expression changes for clinical application.
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: None of the authors has any conflicts of interest.
Financial support: The study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (No. R01NS102360). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional review board statement: The study was approved by local institutional review boards (IRB #07-008842).
Author statement: The article had been presented at American Society for Peripheral Nerve (ASPN) 2019 Annual meeting, February 2, Palm Desert, California, USA; oral presentation.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (No. R01NS102360). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Aarão TLS, de Sousa JR, Falcão ASC, Falcão LFM, Quaresma JAS. Nerve growth factor and pathogenesis of leprosy: review and update. Front Immunol. 2018;9:939. doi: 10.3389/fimmu.2018.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Blondet B, Carpentier G, Lafdil F, Courty J. Pleiotrophin cellular localization in nerve regeneration after peripheral nerve injury. J Histochem Cytochem. 2005;53:971–977. doi: 10.1369/jhc.4A6574.2005. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE, Wisotsky SM, Cho MS, Wilson C, Cooper EO, Ingari JV, Safa B, Parrett BM, Buncke GM. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. doi: 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- 6.Caillaud M, Richard L, Vallat JM, Desmouliere A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res. 2019;14:24–33. doi: 10.4103/1673-5374.243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao F, Liu T, Xu Y, Xu D, Feng S. Culture and properties of adipose-derived mesenchymal stem cells: characteristics in vitro and immunosuppression in vivo. Int J Clin Exp Pathol. 2015;8:7694–7709. [PMC free article] [PubMed] [Google Scholar]
- 8.Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015;2015:628767. doi: 10.1155/2015/628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI, Hariri R. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem Cells Translat Med. 2015;4:695–701. doi: 10.5966/sctm.2014-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceber M, Sener U, Mihmanli A, Kilic U, Topcu B, Karakas M. The relationship between changes in the expression of growth associated protein-43 and functional recovery of the injured inferior alveolar nerve following transection without repair in adult rats. J Craniomaxillofac Surg. 2015;43:1906–1913. doi: 10.1016/j.jcms.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Cutting CB, McCarthy JG. Comparison of residual osseous mass between vascularized and nonvascularized onlay bone transfers. Plas Reconstr Surg. 1983;72:672–675. doi: 10.1097/00006534-198311000-00016. [DOI] [PubMed] [Google Scholar]
- 14.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, Dave M, Friton J, Nair A, Camilleri ET, Dudakovic A, van Wijnen AJ, Faubion WA. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2017;153:59–62e2. doi: 10.1053/j.gastro.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115:1816–1828. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgiev H, Ravens I, Papadogianni G, Bernhardt G. Coming of age: CD96 emerges as modulator of immune responses. Front Immunol. 2018;9:1072. doi: 10.3389/fimmu.2018.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 20.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197(Pt 4(Pt 4)):591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong TS, Tian A, Sachar R, Ray WZ, Brogan DM, Dy CJ. Indirect Cost of Traumatic Brachial Plexus Injuries in the United States. J Bone Joint Surg Am 101. 2019:e80. doi: 10.2106/JBJS.18.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJ, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014;261:578–593. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Hubert T, Grimal S, Carroll P, Fichard-Carroll A. Collagens in the developing and diseased nervous system. Cell Mol Life Sci. 2009;66:1223–1238. doi: 10.1007/s00018-008-8561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui-Chou HG, Hashemi SS, Hoke A, Dellon AL. Clinical implications of peripheral myelin protein 22 for nerve compression and neural regeneration: a review. J Reconstr Microsurg. 2011;27:67–74. doi: 10.1055/s-0030-1267832. [DOI] [PubMed] [Google Scholar]
- 27.IJpma FF, Nicolai JP, Meek MF. Sural nerve donor-site morbidity: thirty-four years of follow-up. Ann Plast Surg. 2006;57:391–395. doi: 10.1097/01.sap.0000221963.66229.b6. [DOI] [PubMed] [Google Scholar]
- 28.Jesuraj NJ, Santosa KB, Macewan MR, Moore AM, Kasukurthi R, Ray WZ, Flagg ER, Hunter DA, Borschel GH, Johnson PJ, Mackinnon SE, Sakiyama-Elbert SE. Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve. 2014;49:267–276. doi: 10.1002/mus.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, Jianghai C, Juan L, Hao K. Pleiotrophin and peripheral nerve injury. Neurosurg Rev. 2009;32:387–393. doi: 10.1007/s10143-009-0202-8. [DOI] [PubMed] [Google Scholar]
- 30.Karsy M, Watkins R, Jensen MR, Guan J, Brock AA, Mahan MA. Trends and cost analysis of upper extremity nerve injury using the national (nationwide) inpatient sample. World Neurosurg. 2019;123:e488-500. doi: 10.1016/j.wneu.2018.11.192. [DOI] [PubMed] [Google Scholar]
- 31.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 33.Koopmans G, Hasse B, Sinis N. Chapter 19: The role of collagen in peripheral nerve repair. Int Rev Neurobiol. 2009;87:363–379. doi: 10.1016/S0074-7742(09)87019-0. [DOI] [PubMed] [Google Scholar]
- 34.Landers ZA, Jethanandani R, Lee SK, Mancuso CA, Seehaus M, Wolfe SW. The psychological impact of adult traumatic brachial plexus injury. J Hand Surg Am. 2018;43:950e1–950e6. doi: 10.1016/j.jhsa.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29:331–348. doi: 10.1016/j.hcl.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X, Deng W. A new method for Schwann-like cell differentiation of adipose derived stem cells. Neurosci Lett. 2013;551:79–83. doi: 10.1016/j.neulet.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death and Diff. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mader EK, Butler G, Dowdy SC, Mariani A, Knutson KL, Federspiel MJ, Russell SJ, Galanis E, Dietz AB, Peng KW. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magill CK, Moore AM, Yan Y, Tong AY, MacEwan MR, Yee A, Hayashi A, Hunter DA, Ray WZ, Johnson PJ, Parsadanian A, Myckatyn TM, Mackinnon SE. The differential effects of pathway- versus target-derived glial cell line-derived neurotrophic factor on peripheral nerve regeneration. J Neurosurg. 2010;113:102–109. doi: 10.3171/2009.10.JNS091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmoudifar N, Doran PM. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnol Prog. 2013;29:176–185. doi: 10.1002/btpr.1663. [DOI] [PubMed] [Google Scholar]
- 42.Manni L, Rocco ML, Bianchi P, Soligo M, Guaragna M, Barbaro SP, Aloe L. Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors. 2013;31:115–122. doi: 10.3109/08977194.2013.804073. [DOI] [PubMed] [Google Scholar]
- 43.Maselli RA, Arredondo J, Ferns MJ, Wollmann RL. Synaptic basal lamina-associated congenital myasthenic syndromes. Ann N Y Acad Sci. 2012;1275:36–48. doi: 10.1111/j.1749-6632.2012.06807.x. [DOI] [PubMed] [Google Scholar]
- 44.Mathot F, Rbia N, Thaler R, Bishop AT, Van Wijnen AJ, Shin AY. Gene expression profiles of differentiated and undifferentiated adipose derived mesenchymal stem cells dynamically seeded onto a processed nerve allograft. Gene. 2020a;724:144151. doi: 10.1016/j.gene.2019.144151. [DOI] [PubMed] [Google Scholar]
- 45.Mathot F, Rbia N, Thaler R, Bishop AT, van Wijnen AJ, Shin AY. Introducing human adipose-derived mesenchymal stem cells to Avance, nerve grafts and NeuraGen nerve guides. J Plast Reconstr Aesthet Surg. 2020b;73:1473–1481. doi: 10.1016/j.bjps.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathot F, Shin AY, Van Wijnen AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17–23. doi: 10.1016/j.gene.2019.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meek MF, Coert JH. US Food and Drug Administration /Conformit Europe- approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg. 2008;60:466–472. [PubMed] [Google Scholar]
- 48.Miosge N, Gotz W, Sasaki T, Chu ML, Timpl R, Herken R. The extracellular matrix proteins fibulin-1 and fibulin-2 in the early human embryo. Histochem J. 1996;28:109–116. doi: 10.1007/BF02331415. [DOI] [PubMed] [Google Scholar]
- 49.Moore AM, Kasukurthi R, Magill CK, Farhadi HF, Borschel GH, Mackinnon SE. Limitations of conduits in peripheral nerve repairs. Hand. 2009;4:180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM. Rationale and design of the percutaneous stem cell injection delivery effects on neomyogenesis in dilated cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Translat Res. 2014;7:769–780. doi: 10.1007/s12265-014-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida Y, Yamada Y, Kanemaru H, Ohazama A, Maeda T, Seo K. Vascularization via activation of VEGF-VEGFR signaling is essential for peripheral nerve regeneration. Biomed Res. 2018;39:287–294. doi: 10.2220/biomedres.39.287. [DOI] [PubMed] [Google Scholar]
- 52.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012;65:657–664. doi: 10.1016/j.bjps.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Pan B, Liu Y, Yan JY, Wang Y, Yao X, Zhou HX, Lu L, Kong XH, Feng SQ. Gene expression analysis at multiple time-points identifies key genes for nerve regeneration. Muscle Nerve. 2017;55:373–383. doi: 10.1002/mus.25225. [DOI] [PubMed] [Google Scholar]
- 54.Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 57.Rbia N, Bulstra LF, Bishop AT, van Wijnen AJ, Shin AY. A Simple Dynamic Strategy to Deliver Stem Cells to Decellularized Nerve Allografts. Plast Reconstr Surg. 2018;142:402–413. doi: 10.1097/PRS.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rbia N, Bulstra LF, Friedrich PF, Bishop AT, Nijhuis THJ, Shin AY. Gene expression and growth factor analysis in early nerve regeneration following segmental nerve defect reconstruction with a mesenchymal stromal cell-enhanced decellularized nerve allograft. Plast Reconstr Surg Glob Open 8. 2020:e2579. doi: 10.1097/GOX.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rbia N, Bulstra LF, Lewallen EA, Hovius SER, van Wijnen AJ, Shin AY. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: An in vitro analysis of the gene expression and growth factors produced. J Plast Reconstr Aesthet Surg. 2019;72:1316–1325. doi: 10.1016/j.bjps.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Roux KJ, Amici SA, Notterpek L. The temporospatial expression of peripheral myelin protein 22 at the developing blood-nerve and blood-brain barriers. J Compar Neurol. 2004;474:578–588. doi: 10.1002/cne.20154. [DOI] [PubMed] [Google Scholar]
- 61.Safa B, Jain S, Desai MJ, Greenberg JA, Niacaris TR, Nydick JA, Leversedge FJ, Megee DM, Zoldos J, Rinker BD, McKee DM, MacKay BJ, Ingari JV, Nesti LJ, Cho M, Valerio IL, Kao DS, El-Sheikh Y, Weber RV, Shores JT, et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery. 2020 doi: 10.1002/micr.30574. doi: 101002/micr30574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safa B, Shores JT, Ingari JV, Weber RV, Cho M, Zoldos J, Niacaras TR, Nesti LJ, Thayer WP, Buncke GM. Recovery of motor function after mixed and motor nerve repair with processed nerve allograft. Plast Reconstr Surg Glob Open 7. 2019:e2163. doi: 10.1097/GOX.0000000000002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 64.Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–1589. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 66.Shen H, Wang Y, Zhang Z, Yang J, Hu S, Shen Z. Mesenchymal stem cells for cardiac regenerative therapy: optimization of cell differentiation strategy. Stem Cells Int. 2015;2015:524756. doi: 10.1155/2015/524756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutton MT, Fletcher D, Episalla N, Auster L, Kaur S, Gwin MC, Folz M, Velasquez D, Roy V, van Heeckeren R, Lennon DP, Caplan AI, Bonfield TL. Mesenchymal stem cell soluble mediators and cystic fibrosis. J Stem Cell Res Ther. 2017;7:400. doi: 10.4172/2157-7633.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutton MT, Fletcher D, Ghosh SK, Weinberg A, van Heeckeren R, Kaur S, Sadeghi Z, Hijaz A, Reese J, Lazarus HM, Lennon DP, Caplan AI, Bonfield TL. Antimicrobial properties of mesenchymal stem cells: therapeutic potential for cystic fibrosis infection, and treatment. Stem Cells Int. 2016;2016:5303048. doi: 10.1155/2016/5303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taioli F, Cabrini I, Cavallaro T, Acler M, Fabrizi GM. Inherited demyelinating neuropathies with micromutations of peripheral myelin protein 22 gene. Brain. 2011;134:608–617. doi: 10.1093/brain/awq374. [DOI] [PubMed] [Google Scholar]
- 70.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 71.Vögelin E, Baker JM, Gates J, Dixit V, Constantinescu MA, Jones NF. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp Neurol. 2006;199:348–353. doi: 10.1016/j.expneurol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 72.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 73.Yamada Y, Shimizu K, Nitta A, Soumiya H, Fukumitsu H, Furukawa S. Axonal regrowth downregulates the synthesis of glial cell line-derived neurotrophic factor in the lesioned rat sciatic nerve. Neurosci Lett. 2004;364:11–15. doi: 10.1016/j.neulet.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 74.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 75.Zhang R, Rosen JM. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen Res. 2018;13:757–763. doi: 10.4103/1673-5374.232457. [DOI] [PMC free article] [PubMed] [Google Scholar]