Abstract
With the development of neuroscience, substantial advances have been achieved in peripheral nerve regeneration over the past decades. However, peripheral nerve injury remains a critical public health problem because of the subsequent impairment or absence of sensorimotor function. Uncomfortable complications of peripheral nerve injury, such as chronic pain, can also cause problems for families and society. A number of studies have demonstrated that the proper functioning of the nervous system depends not only on a complete connection from the central nervous system to the surrounding targets at an anatomical level, but also on the continuous bilateral communication between the two. After peripheral nerve injury, the interruption of afferent and efferent signals can cause complex pathophysiological changes, including neurochemical alterations, modifications in the adaptability of excitatory and inhibitory neurons, and the reorganization of somatosensory and motor regions. This review discusses the close relationship between the cerebral cortex and peripheral nerves. We also focus on common therapies for peripheral nerve injury and summarize their potential mechanisms in relation to cortical plasticity. It has been suggested that cortical plasticity may be important for improving functional recovery after peripheral nerve damage. Further understanding of the potential common mechanisms between cortical reorganization and nerve injury will help to elucidate the pathophysiological processes of nerve injury, and may allow for the reduction of adverse consequences during peripheral nerve injury recovery. We also review the role that regulating reorganization mechanisms plays in functional recovery, and conclude with a suggestion to target cortical plasticity along with therapeutic interventions to promote peripheral nerve injury recovery.
Keywords: cortical plasticity, injury, mechanisms, nerve transfer, neurorrhaphy, peripheral nerve, phantom limb pain, recovery, regeneration, treatment
Introduction
Peripheral nerve injury (PNI) affects more than one million people worldwide, and the occurrence of trauma-induced PNI continues to increase (Jiang et al., 2010; Sachanandani et al., 2014). PNI can cause a loss of perception and motor ability to varying degrees, with subsequent chronic dysfunction, and severely reduces quality of life (Modrak et al., 2020). Numerous promising outcomes in terms of local nerve regeneration have been achieved using diverse nerve growth factors, stem cell-derived exosomes, electrical stimulation, and other medical treatments (Quan et al., 2017; Du et al., 2018; Rao et al., 2019a, b). However, the recovery of sensory or motor functions remains limited, even after severed peripheral nerves have been successfully reconnected and a range of therapies have been administered.
Plasticity is a characteristic of neurons that is common in the nervous system. It represents the adaptability of neurons to modify their functions and structures throughout the lifespan in response to various signals from the environment, learning processes, injury, and disease (Navarro et al., 2007; Davis et al., 2011; Colangelo et al., 2019; Sandquist and Sakaguchi, 2019). Reorganization initiated by PNI can be observed in the spinal cord, brainstem, relay nuclei, thalamus, and cortex (Nicolelis et al., 1993; Florence et al., 2000; Mohanty et al., 2015). In the present review, we mainly discuss cortical plasticity and its potential relationship with PNI and regeneration.
A common conception related to plasticity and peripheral nerve regeneration is that an intact peripheral nerve circuit is the only element that limits the recovery of nerve function; however, cerebral cortical reorganization also plays a crucial role in functional recovery (Quraishe et al., 2018; Meyers et al., 2019). Brain imaging techniques have been used to confirm that cortical maps are reorganized following peripheral nerve transection (Lotze et al., 2001; Nordmark and Johansson, 2020). These findings have inspired new strategies aimed at better restoring the performance of injured peripheral nerves, for example by establishing effective connections between the nervous system and target tissues, and by further regulating the ensuing central nervous system functional remodeling (Jiang et al., 2014). In the present study, a literature search of studies in rodents, non-human primates, and humans was performed in the MEDLINE database from 1993 to 2020. The key words/terms were cortical plasticity, peripheral nerve injury, mechanisms, and regeneration. The results were further screened by title and abstract. In addition, an electronic search of the MEDLINE database was performed for methods of regulating cerebral cortical plasticity to promote PNI functional recovery. This search included publications with the following search terms: nerve transfer, neurorrhaphy, phantom limb pain, functional recovery, and treatment. This review describes the plastic changes that occur in the cerebral cortex after PNI. Additionally, it clarifies the regulatory role of cortical reorganization in the process of peripheral nerve injury and regeneration. The aim of this review is to emphasize that the regulation of cortical reorganization is a powerful tool that can be used to promote functional recovery and improve the prognosis of PNI patients.
Cortical Plasticity after Peripheral Nerve Injury
Levels of somatosensory and motor cortical reorganization are strongly associated with the duration and degree of the interruption of peripheral nerve activity. If the nerve injury is reversible—for example, after transient peripheral nerve blockade induced by local anesthetics or ischemia, or mild nerve damage (Sunderland grades 1 and 2) (Figure 1)—the plasticity process will be reversed as signal transmission resumes. However, in the case of transected nerves that are unable to dock accurately because of severe nerve trauma (Sunderland grades 3 and above) or amputation, the involved cortex undergoes a long-term reorganization process (Guo et al., 2012; Jiang et al., 2015).
Figure 1.
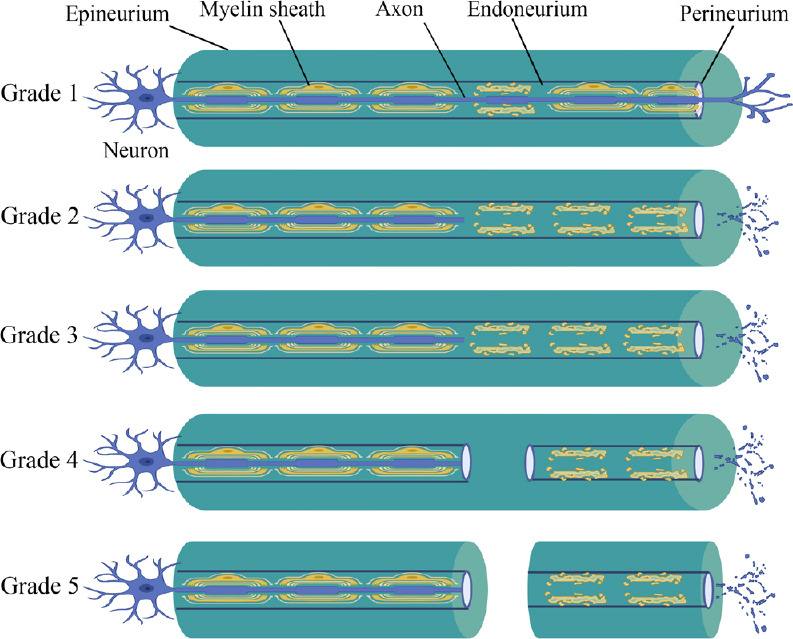
Sunderland’s classification of nerve injuries.
Grade 1: The continuity of nerve fibers remains intact, without Wallerian degeneration. Grade 2: The continuity of the axon and its myelin sheath is interrupted. The endoneurial tube remains intact, and the distal end of the injured nerve shows Wallerian degeneration. Grade 3: Nerve fibers (including axons and the endoneurium) are interrupted. The perineurium remains intact. The distal end of the injured nerve shows Wallerian degeneration. The possibility of self-recovery remains. Grade 4: Only the epineurium remains intact. The distal end of the injured nerve shows Wallerian degeneration. Grade 5: The entire nerve is completely interrupted. The distal end of the injured nerve shows Wallerian degeneration. Figure 1 was created with BioRender. com.
There is growing evidence to suggest that the removal of sensory stimulation can result in conspicuous rearrangements of cortical morphology (Chen et al., 2002; Socolovsky et al., 2017). In early primate experiments investigating the reorganization of the somatosensory cortex, researchers reported a marked loss of activity in the relevant cortical district after disconnecting the dorsal rootlets of the index finger and thumb. In addition, the boundaries of the cortical skin receptive field corresponding to the severed nerve became blurred, and stimulation of the adjacent area also caused a response (Darian-Smith and Brown, 2000). Moreover, evidence of plasticity in the somatosensory cortex has been found in a rat carpal tunnel syndrome model. A dynamic plastic process occurred in the cerebral cortex of these carpal tunnel syndrome rats, which was similar to the results of primate experiments. Functional magnetic resonance imaging studies have demonstrated that the sensory area of the affected limb expands in the early stage and narrows in the later stage after injury. At the early stage, the brains of rats with median nerve entrapment attempt to compensate for sensory loss by enlarging the median nerve-involved regions of the sensorimotor cortex, as well as the related brain regions of sensorimotor networks. At the later stage, activation in the same area is markedly decreased in carpal tunnel syndrome rats. This result reflects the maladaptive process of the brain that is caused by peripheral nerve blockade (Bao et al., 2018). A series of changes in the central sensory cortex may reflect an abnormal state of supercompensation of the cerebral cortex after the interruption of input signals. With the support of human brain imaging technology, researchers have revealed that visual stimulation in deaf people can activate regions of the temporal cortex related to hearing function. This activation state is positively correlated with the duration of deafness (Que et al., 2018). Brain imaging techniques can thus provide valuable temporal and spatial information about dysfunctional areas. Such results imply that the cerebral cortex may not stabilize in the short-term after injury, and can become further altered after a longer time.
Paralysis caused by transection of the peripheral nerve is caused by the interruption of output signals from the motor cortex to the denervated muscles. In previous studies, researchers have observed that the motor cortex also launches plasticity processes after motor nerve injury. In early rodent experiments, researchers discovered that the corresponding motor cortex regions of denervated muscles lose their activation characteristics when vibrissal nerves are damaged. Moreover, the involved areas fail to produce similar muscle activation even after receiving electrical stimulation. However, after a few hours, the forearm and eyelid produce motor responses to the same stimulation of the same area. Similarly, in patients with forearm amputations, stimulation of the motor cortex region that previously innervated the forearm muscles can cause muscle movement in the shoulders (Sanes and Donoghue, 2000; Tomov et al., 2002). Thus, like in the sensory cortex, reorganization of the bilateral motor cortex can also occur after unilateral PNI. A recent study revealed that in a unilateral whisker deprivation mouse model, the bilateral cortex is recruited to reorganize the response to sensations from the unaffected peripheral area or to control its movement (Petrus et al., 2019). These results indicate that reorganization of the motor cortex occurs at the cortical boundary of the innervated area, and that the reorganized area regains control of the surrounding tissue.
Cortical plasticity is a cross-species phenomenon. Consistent with observations in animal experiments, cerebral cortex reorganization also occurs after PNI in humans. PNI and limb immobilization can induce a decrease in cortical thickness in the affected primary motor and somatosensory areas (Langer et al., 2012). Additionally, after limb amputation, local cortical reorganization can be clearly detected in the primary sensorimotor cortex (Makin et al., 2015). An alteration of cortical activity in the local area that involved the injured peripheral nerve can promote the remodeling of both the local region and the network of the sensorimotor system. A study of patients with brachial plexus injury reported that when subjects were asked to imagine performing unilateral gestures, the activation of cortical innervation on the side of the brachial plexus injury was significantly lower than that of the normal group. Moreover, the supplementary motor area was almost not activated in the patients with brachial plexus injury. However, activation patterns were similar when comparing between healthy participants and the normal hands of patients with brachial plexus injury (Lu et al., 2016). In addition, the local cortical area of a patient who underwent limb transplantation and successful rehabilitation was able to become a canonical structure from an abnormal state (Hernandez-Castillo et al., 2018). Furthermore, several studies have reported that good clinical results after advanced surgical re-innervation techniques also depend on peripheral nerve regeneration ability and the adaptation capacity of cortical plasticity (Dahlin et al., 2017; Sturma et al., 2018).
The scope of brain plasticity involves local, long-distance, intra-hemispheric, and trans-hemispheric processes, and can usually be regarded as a remodeling process that adapts to stimuli. However, the compensatory mechanism that recruits the deprived cerebral cortex to process adjacent complete sensory information may be a beneficial adaptive change, or may conversely lead to maladaptive changes (Lee and Whitt, 2015; Lent and Tovar-Moll, 2015; Bahia et al., 2019).
Cortical Plasticity and Phantom Limb Pain
According to clinical reports, most patients with severe PNI or amputation suffer from phantom limb pain (PLP) (Shankar et al., 2015; Kuffler, 2018). In recent years, basic and clinical medical research has initially revealed a close relationship between PLP and cortical reorganization (Diers et al., 2015). Two research (Karl et al., 2001; Raffin et al., 2016) reports have noted that when upper limb amputees perform phantom movements, neurons in the somatosensory cortex corresponding to the elbow and mouth are activated. The reorganization of primary sensory and motor cortices after PNI is related to phantom limb pain. Researchers have discovered that the associated cortex after PNI is invaded by representations of the body parts adjacent to the missing limb. There is a positive correlation between the severity of phantom limb pain and the degree of neuronal reorganization; that is, a higher degree of reorganization in the deprived cortex relates to a higher PLP score. These results indicate that the severity of PLP is positively correlated with the degree of somatosensory and motor cortical reorganization.
Research has shown that the reversible ability of cortical plasticity may be used as a potential treatment for PLP (Lefaucheur et al., 2008). In a within‐participants, double‐blind, sham‐controlled study, Kikkert et al. (2019) demonstrated that PLP outcomes were able to be relieved with noninvasive brain stimulation interventions. They revealed that both short- and long-term pain relief was associated with increased activity in the brain regions related to pain caused by the interventional stimulation. In another such study, patients with PLP caused by brachial plexus avulsion underwent brain machine interface training of a neuroprosthetic (robotic) hand using real-time magnetoencephalography signals. Brain machine interface training was able to enhance PLP, while less brain machine interface training helped to reduce pain. These outcomes suggest a direct link between sensorimotor cortical plasticity and PLP resulting from severe PNI, but the authors also hypothesized that an ideal rehabilitation method might alleviate pain by providing an intact nerve circuit (Yanagisawa et al., 2016). The application of central plasticity in the treatment of PNI should be based on preventing abnormal increases or decreases in central areas by regulating sensory input and motor output signals.
Mechanisms of Cortical Reorganization
The reorganization of the extensive central nervous network, including the cortex, thalamus, brainstem, and spinal cord, after PNI is considered to be a progressive injury-related adaptation process. The mechanisms involve many changes at the tissue, cellular, and molecular levels. However, the exact mechanisms of reorganization remain unclear.
Imbalance of excitatory and inhibitory neurons
In the cortical neural network, excitatory pyramidal neurons and inhibitory interneurons form local neural circuits through synaptic structures. These circuits are the structural basis of the excitation–inhibition balance in the cortex. Increased inhibition in the visual cortex after visual deprivation can be regulated through either the long-term depression of excitatory intracortical synapses (Rittenhouse et al., 1999) or the potentiation of inhibitory synapses (Maffei et al., 2006). It is generally believed that action potentials emitted by excitatory neurons are transmitted along axons to presynaptic membranes, and excitatory postsynaptic potentials then activate inhibitory neurons through synaptic transmission. If they reach a certain threshold, inhibitory neurons produce inhibitory postsynaptic potentials on the excitatory neurons innervated by them, thereby inhibiting excitatory neurons. Alterations of neurotransmitter levels have a significant impact on the reorganization process. One study has found that a combination of excitatory neurons and inhibitory neurons can regulate the excitability and dynamic range of neural circuits (Benali et al., 2008). The process of neuronal remodeling is promoted by an imbalance between the two kinds of neurons, induced by nerve damage (Jones et al., 2002). The results of histology, immunostaining, and brain imaging techniques indicate that, after sensory deprivation, the cortex is more likely to be remodeled with increased inhibitory interneuron activity, whereas the activity of excitatory pyramidal neurons has less of an impact on cortical plasticity (Pelled et al., 2009).
Long-term plasticity
The long-term reorganization of the cerebral cortex may involve more stable functional or structural mechanisms, including long-term potentiation and long-term depression. PNI contributes to chronic pain and causes synapses to respond with long-term potentiation (Chen et al., 2014; Bliss et al., 2016). However, some researchers believe that whisker-deprived rats have attenuated excitatory layer IV input of L2/3 pyramidal cells in the primary somatosensory cortex, and that this loss of somatosensory input causes long-term depression (Allen et al., 2003; Bender et al., 2006). The Ca2+ influx is regulated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate receptors, which in turn affect long-term cortical plasticity through synaptic plasticity (Mohanty et al., 2015). Signs of new axon and dendrite formation after sensory deprivation have also been found in the cerebral cortex (Bahia et al., 2019).
Plasticity can be conducted by synaptic activities that regulate protein synthesis and degradation after PNI (Bingol et al., 2010; Jarome and Helmstetter, 2014). Cortical plasticity is regulated by the synthesis and degradation rates of synaptic proteins affected by nociceptive signal stimulation from surrounding receptors (Ko et al., 2020). Neurotrophic factors, including nerve growth factor, brain-derived neurotrophic factor, neurotrophin 3, and neurotrophin 4, are important signaling molecules that take part in the cortical reorganization process. These factors are not only involved in controlling changes in synaptic structures and the efficiency of signal transmission, but they also regulate dynamic changes in the brain’s neuronal network (Gibon and Barker, 2017). For example, neurotrophins play a pivotal role in glutamatergic neurotransmission and the γ-aminobutyric acidergic transmission system, which affect the process of cortical plasticity (Kim et al., 2017; Meis et al., 2019).
In summary, cerebral cortex modification is supported by changes in neural circuits. Different internal environments activate distinct areas of neuromodulatory systems and affect the excitatory–inhibitory balance of the cerebral cortex to facilitate long-term cortical plasticity.
Effects of Nerve Repair on Remodeling
Nerve function can be restored faster and better if the continuity of the severed peripheral nerve is repaired as soon as possible. Multiple therapies are suitable for PNI with different gap defects. At present, nerve neurorrhaphy is a common option in the treatment of small or unclear nerve defects. Nerve lengthening or small-segment nerve transplantation is typically applied for short- or middle-distance peripheral nerve defects (Griffin et al., 2013). Furthermore, nerve transposition repair is thought to be able to cover long-segment peripheral nerve defects, such as brachial plexus injuries (Ali et al., 2015). Here, we briefly discuss the potential impact of different repair methods on the cortex.
Neurorrhaphy and autografts
Ideally, the corresponding cortical region will be reset as a normal structure if the transected peripheral nerve achieves perfect nerve regeneration. Generally, this situation only occurs in Sunderland grades 1–2 (axons can regenerate in their original endometrial sheaths after slight crush injuries).
After direct neurorrhaphy or autograft repair, there is a high rate of regenerative nerve misdirection (de Ruiter et al., 2008). Misdirection of regenerating peripheral nerves, which results in an exception occurring in bidirectional signal transduction between the target tissue and the cortex, can lead to somatosensory cortex reorganization and skin area reorganization (Lundborg, 2003; Nordmark and Johansson, 2020). If this occurs, the previous cortical area, which has been explicitly defined, will disappear and be replaced by a discontinuous and incomplete texture in the reconstructed cortical area. Our previous studies have demonstrated that, compared with ordinary nerve neurorrhaphy, the use of chitosan conduits to repair small gap peripheral nerve defects have a better effect in correcting the direction of nerve regeneration (Zhang et al., 2013; Yu et al., 2016; Wang et al., 2018). This finding implies that bridging small gap defects with chitosan conduits may have a potential role in the study of cortical remodeling as well as for treating small gap peripheral nerve defects.
Nerve transposition
After transferring healthy C7 roots to repair the contralateral injured median nerve, the cortices of rodents undergo reorganization. At 3, 5, and 7 months after the nerve transfer, intracortical microstimulation of the primary motor cortex was performed to construct a motor cortex response map. Results demonstrated that the injured limb was able to be moved by stimulating the motor cortex of the contralateral hemisphere. At 5 months after transfer, the injured limb was able to be moved by stimulating the ipsilateral motor cortex. Moreover, stimulation of the bilateral cortices elicited motion of the injured limb at 7 months after the surgery. Notably, the injured forelimb representation area was identified in the contralateral motor cortex at 10 months after the repair. The results of this experiment indicate that the transfer of functional plasticity between the two hemispheres is time dependent (Jiang et al., 2010). In addition, transhemispheric functional reorganization occurs based on the plasticity of the central nervous system, especially via the corpus callosum, which can cause extensive functional transformation between the two hemispheres (Lou et al., 2006). Sokki et al. (2012) noted synkinetic movements of elbow flexion during inhalation in patients in the early stages after successful intercostal–musculocutaneous nerve transfer. After a period of recovery, this uncoordinated phenomenon gradually faded and these patients were then able to autonomously flex their elbows without interfering with their respiratory activity. In addition, functional magnetic resonance imaging results from this study revealed that the cortical activation of the original intercostal muscle motor area was transferred to the elbow flexion area. These results demonstrate that cortical reorganization occurs after intercostal–musculocutaneous nerve transfer (Sokki et al., 2012). Together, these findings indicate that cortical plasticity participates in both the pathophysiology and the recovery process of PNI. Thus, functional recovery after PNI requires an understanding of not only the promotion of peripheral nerve regeneration, but also the role of brain reorganization at this stage.
Cortical Plasticity in the Treatment of Peripheral Nerve Injury
For better rehabilitation after PNI, it is necessary to carry out comprehensive therapy focused on the mutual influences of PNI and the cerebral cortex.
Sensory reeducation
The progressive process of brain reorganization, which occurs through cognitive learning techniques (for example, visualization and verbalization) and alternate senses (such as vision, hearing, and graded tactile stimuli) is called sensory reeducation (SR). This technique aims to improve the functional use of the affected limb through maintaining and/or restoring reorganizational sensory areas (Jerosch-Herold, 2011). SR mainly includes two stages. In phase I, within 24 hours after denervation, the corresponding sensory cortical area begins to shrink, while the surrounding cortical areas expand and occupy the area represented by the injured nerve. The aim of SR is to provide another sensory input to the sensory cortex before the regenerated nerve fibers have reached the surrounding target. This is also known as cross-modal sensory substitution technology. In this phase, SR keeps the initial cortical map by using other sensory techniques and sensations, including touch observation, mirror visual feedback technology (audio-visual interaction), and the sensory glove system (auditory interaction) (Rosén and Lundborg, 2007). Phase II, or classical SR, begins when the initial regeneration is confirmed by a positive Tinel’s sign and touch threshold test. The process of phase II is a combination of vision, memory, and learning sensory signals that stimulate the corresponding cortical area from multiple aspects, thereby affecting the process of brain reorganization. In a prospective study of patients who had undergone long-term median nerve microsurgical repair, sensory function in the hands of patients treated with SR was better than that of patients without SR treatment (Rosén et al., 2003; Antonopoulos et al., 2019).
Vagus nerve stimulation
Vagus nerve stimulation (VNS) is a neurostimulation therapy. The electrical stimulation of the vagus nerve is considered an effective treatment for enhancing recovery in multiple neurological disorders, including stroke, traumatic brain injury, and spinal cord injury (Hays, 2016; Darrow et al., 2020). Cooperating with sensory, motor, or cognitive events during rehabilitation, temporary bursts of VNS at appropriate times can strengthen cortical remodeling and retain permanent functional improvements. Meyers et al. (Meyers et al., 2019) established complete transection models of the median and ulnar nerves in rats, and then used nerve conduits to repair the severed nerves. Promising results were obtained after 6 weeks of closed-loop VNS treatment in this rat model of nerve injury. The closed-loop VNS was able to control the injured neural circuit by accurately timing the release of neuromodulators (acetylcholine), and effectively reversed the maladaptive expansion of the cortical circuit without significantly impacting on the peripheral nerve or muscle after nerve transection (Meyers et al., 2019). These findings suggest that closed-loop VNS can be considered an easy-to-implement therapy, and suitable operations that regulate cortical plasticity may be beneficial for restoring sensorimotor function.
Local nerve blockade
Previous studies have reported that blocking the local nerves of normal hands causes reorganization in the motor cortex of the involved nerves, representing the expansion in the motor cortex of upper limb muscles in the adjacent parts. Hence, it is envisaged that using local nerve blockade to regulate the representative area of the cortex adjacent to damaged innervated tissue may promote the functional recovery of the affected hand (Weiss et al., 2004; Björkman et al., 2005). In a further study, patients with median nerve injury or ulnar nerve injury underwent 2 weeks of local skin anesthesia of the forearm while performing hand sensory recovery training. After 6 weeks, the sensory function of the experimental group was significantly better than that of the control group (Walbruch and Kalliainen, 2015). The reason for this effect is likely related to cortical plasticity mechanisms, which manifest as the expansion and contraction of brain regions.
Action observation with peripheral nerve stimulation
Neurophysiological experiments have demonstrated that the excitability of the motor cortex is activated when observing actions performed by another individual or when thinking about simulated movements (Rizzolatti and Craighero, 2004). However, this effect can disappear quickly if follow-up training is not carried out in time. According to reports, the combination of action observation and behavior replication has a positive effect on retaining information (Bisio et al., 2015). Furthermore, action observation combined with peripheral nerve stimulation shortens the time interval between observation and execution, and simultaneously enhances and consolidates the activation of motor cortex excitability through afferent feedback from peripheral nerves, thereby improving the long-term excitability of the cerebral cortex. Clinical trials have demonstrated that action observation with peripheral nerve stimulation effectively induces lasting plasticity of the cortical area by acting as a reorganization mechanism, similar to long-term potentiation (Bisio et al., 2017). Action observation with peripheral nerve stimulation supports the effectiveness of increasing the excitability of cortical areas, and may be used as a potential method for promoting functional recovery in patients with PNI.
Concluding Remarks
Pathological activity in disturbed neural circuits after PNI can impair both normal function and the rehabilitation process. In addition to repairing the integrity of neural circuits, the precise control of cortical remodeling may be a promising factor for repairing PNI and obtaining better rehabilitation results (Figure 2).
Figure 2.
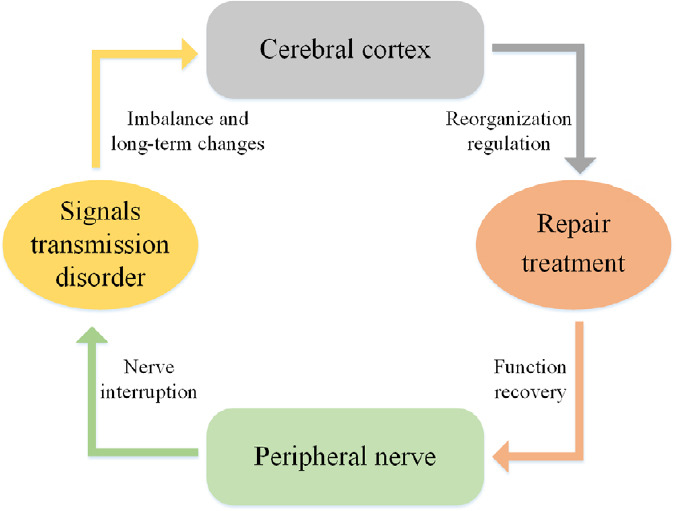
Schematic of the interactions between cortical plasticity and peripheral nerve injury.
Injuries to the peripheral nerve can cause the interruption of signal transmission. The cerebral cortex then undergoes reorganization because of the signal transmission disorder. Regulating the reorganization of the brain affects both the recovery of peripheral nerves and the sensorimotor functions of corresponding target organs.
Looking to the future, the optimization of nerve repair technology should make use of neuroplasticity, which is an intrinsic characteristic of the nervous system. The mechanisms of cortical remodeling and PNI need to be further clarified, and may provide new ideas for studying the correlation of nerve injury. On this basis, it may be possible to create multiple combination therapies (through the joint action of the brain, spinal cord, peripheral nerves, and target organs) to further enhance this synergistic effect. Multiple combination therapies show great potential for comprehensive treatment because of the inseparable neurological connections at all levels.
Footnotes
P-Reviewer: Murali K; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner B, Yu J, Song CP; T-Editor: Jia Y
Conflicts of interest: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support: This work was supported by the Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education of China, No. BMU2020XY005-03; National Natural Science Foundation of China, No. 31771322; Beijing Science & Technology New Star Cross Project of China, No. 201819; Major R & D Program of National Ministry of Science and Technology of China, No. 2018YFB1105504; a grant from National Center for Trauma Medicine, Beijing, China, No. BMU2020XY005-01 (all to PXZ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Kumarasamy Murali, Indian Institute of Technology, India.
Funding: This work was supported by the Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education of China, No. BMU2020XY005-03; National Natural Science Foundation of China, No. 31771322; Beijing Science & Technology New Star Cross Project of China, No. 201819; Major R & D Program of National Ministry of Science and Technology of China, No. 2018YFB1105504; a grant from National Center for Trauma Medicine, Beijing, China, No. BMU2020XY005-01 (all to PXZ).
References
- 1.Ali ZS, Heuer GG, Faught RW, Kaneriya SH, Sheikh UA, Syed IS, Stein SC, Zager EL. Upper brachial plexus injury in adults: comparative effectiveness of different repair techniques. J Neurosurg. 2015;122:195–201. doi: 10.3171/2014.9.JNS132823. [DOI] [PubMed] [Google Scholar]
- 2.Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- 3.Antonopoulos DK, Mavrogenis AF, Megaloikonomos PD, Mitsiokapa E, Georgoudis G, Vottis CT, Antonopoulos GK, Papagelopoulos PJ, Pneumatikos S, Spyridonos SG. Similar 2-point discrimination and stereognosia but better locognosia at long term with an independent home-based sensory reeducation program vs no reeducation after low-median nerve transection and repair. J Hand Ther. 2019;32:305–312. doi: 10.1016/j.jht.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Bahia CP, Vianna-Barbosa RJ, Tovar-Moll F, Lent R. Terminal arbors of callosal axons undergo plastic changes in early-amputated rats. Cereb Cortex. 2019;29:1460–1472. doi: 10.1093/cercor/bhy043. [DOI] [PubMed] [Google Scholar]
- 5.Bao BB, Qu DQ, Zhu HY, Gao T, Zheng XY. Brain remodeling after chronic median nerve compression in a rat model. Neural Regen Res. 2018;13:704–708. doi: 10.4103/1673-5374.230298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benali A, Weiler E, Benali Y, Dinse HR, Eysel UT. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J Neurosci. 2008;28:12284–12293. doi: 10.1523/JNEUROSCI.1952-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Bisio A, Avanzino L, Biggio M, Ruggeri P, Bove M. Motor training and the combination of action observation and peripheral nerve stimulation reciprocally interfere with the plastic changes induced in primary motor cortex excitability. Neuroscience. 2017;348:33–40. doi: 10.1016/j.neuroscience.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Bisio A, Avanzino L, Gueugneau N, Pozzo T, Ruggeri P, Bove M. Observing and perceiving: A combined approach to induce plasticity in human motor cortex. Clin Neurophysiol. 2015;126:1212–1220. doi: 10.1016/j.clinph.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Björkman A, Rosén B, Lundborg G. Enhanced function in nerve-injured hands after contralateral deafferentation. Neuroreport. 2005;16:517–519. doi: 10.1097/00001756-200504040-00020. [DOI] [PubMed] [Google Scholar]
- 12.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Wang W, Dong YL, Zhang MM, Wang J, Koga K, Liao YH, Li JL, Budisantoso T, Shigemoto R, Itakura M, Huganir RL, Li YQ, Zhuo M. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol Brain. 2014;7:76. doi: 10.1186/s13041-014-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colangelo AM, Cirillo G, Alberghina L, Papa M, Westerhoff HV. Neural plasticity and adult neurogenesis: the deep biology perspective. Neural Regen Res. 2019;14:201–205. doi: 10.4103/1673-5374.244775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlin LB, Andersson G, Backman C, Svensson H, Björkman A. Rehabilitation, using guided cerebral plasticity, of a brachial plexus injury treated with intercostal and phrenic nerve transfers. Front Neurol. 2017;8:72. doi: 10.3389/fneur.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darian-Smith C, Brown S. Functional changes at periphery and cortex following dorsal root lesions in adult monkeys. Nat Neurosci. 2000;3:476–481. doi: 10.1038/74852. [DOI] [PubMed] [Google Scholar]
- 18.Darrow MJ, Torres M, Sosa MJ, Danaphongse TT, Haider Z, Rennaker RL, Kilgard MP, Hays SA. Vagus nerve stimulation paired with rehabilitative training enhances motor recovery after bilateral spinal cord injury to cervical forelimb motor pools. Neurorehabil Neural Repair. 2020;34:200–209. doi: 10.1177/1545968319895480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis KD, Taylor KS, Anastakis DJ. Nerve injury triggers changes in the brain. Neuroscientist. 2011;17:407–422. doi: 10.1177/1073858410389185. [DOI] [PubMed] [Google Scholar]
- 20.de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diers M, Kamping S, Kirsch P, Rance M, Bekrater-Bodmann R, Foell J, Trojan J, Fuchs X, Bach F, Maaß H, Cakmak H, Flor H. Illusion-related brain activations: a new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 2015;1594:173–182. doi: 10.1016/j.brainres.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Zhen G, Chen H, Zhang S, Qing L, Yang X, Lee G, Mao HQ, Jia X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials. 2018;181:347–359. doi: 10.1016/j.biomaterials.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–3159. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- 24.Gibon J, Barker PA. Neurotrophins and proneurotrophins: focus on synaptic activity and plasticity in the brain. Neuroscientist. 2017;23:587–604. doi: 10.1177/1073858417697037. [DOI] [PubMed] [Google Scholar]
- 25.Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Chambers AR, Darrow KN, Hancock KE, Shinn-Cunningham BG, Polley DB. Robustness of cortical topography across fields, laminae, anesthetic states, and neurophysiological signal types. J Neurosci. 2012;32:9159–9172. doi: 10.1523/JNEUROSCI.0065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays SA. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics. 2016;13:382–394. doi: 10.1007/s13311-015-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Castillo CR, Diedrichsen J, Aguilar-Castañeda E, Iglesias M. Decoupling between the hand territory and the default mode network after bilateral arm transplantation: four-year follow-up case study. Brain Imaging Behav. 2018;12:296–302. doi: 10.1007/s11682-017-9683-1. [DOI] [PubMed] [Google Scholar]
- 29.Jarome TJ, Helmstetter FJ. Protein degradation and protein synthesis in long-term memory formation. Front Mol Neurosci. 2014;7:61. doi: 10.3389/fnmol.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerosch-Herold C. Sensory relearning in peripheral nerve disorders of the hand: a web-based survey and delphi consensus method. J Hand Ther. 2011;24:292-298; quiz 299. doi: 10.1016/j.jht.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Jiang BG, Feng Yin X, Xun Zhang P, Han N, Kou YH. Hypothesis of peripheral nerve regeneration induced by terminal effectors. Artif Cells Nanomed Biotechnol. 2014;42:92–94. doi: 10.3109/21691401.2013.785955. [DOI] [PubMed] [Google Scholar]
- 32.Jiang G, Yin X, Li C, Li L, Zhao L, Evans AC, Jiang T, Wu J, Wang J. The plasticity of brain gray matter and white matter following lower limb amputation. Neural Plast. 2015;2015:823185. doi: 10.1155/2015/823185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang S, Li ZY, Hua XY, Xu WD, Xu JG, Gu YD. Reorganization in motor cortex after brachial plexus avulsion injury and repair with the contralateral C7 root transfer in rats. Microsurgery. 2010;30:314–320. doi: 10.1002/micr.20747. [DOI] [PubMed] [Google Scholar]
- 34.Jones EG, Woods TM, Manger PR. Adaptive responses of monkey somatosensory cortex to peripheral and central deafferentation. Neuroscience. 2002;111:775–797. doi: 10.1016/s0306-4522(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 35.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609–3618. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikkert S, Mezue M, O’Shea J, Henderson Slater D, Johansen-Berg H, Tracey I, Makin TR. Neural basis of induced phantom limb pain relief. Ann Neurol. 2019;85:59–73. doi: 10.1002/ana.25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Lee S, Kang S, Kim SH, Kim JC, Yang M, Moon C. Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural Regen Res. 2017;12:1733–1741. doi: 10.4103/1673-5374.217353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko HG, Park DI, Lee JH, Turck CW, Kaang BK. Proteomic analysis of synaptic protein turnover in the anterior cingulate cortex after nerve injury. Mol Brain. 2020;13:19. doi: 10.1186/s13041-020-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuffler DP. Coping with Phantom Limb Pain. Mol Neurobiol. 2018;55:70–84. doi: 10.1007/s12035-017-0718-9. [DOI] [PubMed] [Google Scholar]
- 40.Langer N, Hänggi J, Müller NA, Simmen HP, Jäncke L. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- 41.Lee HK, Whitt JL. Cross-modal synaptic plasticity in adult primary sensory cortices. Curr Opin Neurobiol. 2015;35:119–126. doi: 10.1016/j.conb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, Nitsche M, Paulus W. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1:337–344. doi: 10.1016/j.brs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Lent R, Tovar-Moll F. How can development and plasticity contribute to understanding evolution of the human brain. Front Hum Neurosci. 2015;9:208. doi: 10.3389/fnhum.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124:2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- 45.Lou L, Shou T, Li Z, Li W, Gu Y. Transhemispheric functional reorganization of the motor cortex induced by the peripheral contralateral nerve transfer to the injured arm. Neuroscience. 2006;138:1225–1231. doi: 10.1016/j.neuroscience.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 46.Lu YC, Liu HQ, Hua XY, Shen YD, Xu WD, Xu JG, Gu YD. Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury. Neural Regen Res. 2016;11:670–675. doi: 10.4103/1673-5374.180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundborg G, Richard P. Bunge memorial lecture. Nerve injury and repair--a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209–226. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 48.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 49.Makin TR, Filippini N, Duff EP, Henderson Slater D, Tracey I, Johansen-Berg H. Network-level reorganisation of functional connectivity following arm amputation. Neuroimage. 2015;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meis S, Endres T, Munsch T, Lessmann V. Impact of chronic BDNF depletion on GABAergic synaptic transmission in the lateral amygdala. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20174310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyers EC, Kasliwal N, Solorzano BR, Lai E, Bendale G, Berry A, Ganzer PD, Romero-Ortega M, Rennaker RL, 2nd, Kilgard MP, Hays SA. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun. 2019;10:5782. doi: 10.1038/s41467-019-13695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination: Potential therapeutic strategies. J Neurosci Res. 2020;98:780–795. doi: 10.1002/jnr.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohanty CB, Bhat D, Devi BI. Role of central plasticity in the outcome of peripheral nerve regeneration. Neurosurgery. 2015;77:418–423. doi: 10.1227/NEU.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 54.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- 56.Nordmark PF, Johansson RS. Disinhibition of human primary somatosensory cortex after median nerve transection and reinnervation. Front Hum Neurosci. 2020;14:166. doi: 10.3389/fnhum.2020.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelled G, Bergstrom DA, Tierney PL, Conroy RS, Chuang KH, Yu D, Leopold DA, Walters JR, Koretsky AP. Ipsilateral cortical fMRI responses after peripheral nerve damage in rats reflect increased interneuron activity. Proc Natl Acad Sci U S A. 2009;106:14114–14119. doi: 10.1073/pnas.0903153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrus E, Saar G, Ma Z, Dodd S, Isaac JTR, Koretsky AP. Interhemispheric plasticity is mediated by maximal potentiation of callosal inputs. Proc Natl Acad Sci U S A. 2019;116:6391–6396. doi: 10.1073/pnas.1810132116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quan Q, Chang B, Liu RX, Sun X, Wang Y, Lu SB, Peng J, Zhao Q. Peripheral nerve injury and regeneration:application and progress of novel nerve scaffolds. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:962–968. [Google Scholar]
- 60.Que M, Jiang X, Yi C, Gui P, Jiang Y, Zhou YD, Wang L. Language and sensory neural plasticity in the superior temporal cortex of the deaf. Neural Plast. 2018;2018:9456891. doi: 10.1155/2018/9456891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quraishe S, Forbes LH, Andrews MR. The extracellular environment of the CNS: Influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast. 2018;2018:2952386. doi: 10.1155/2018/2952386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raffin E, Richard N, Giraux P, Reilly KT. Primary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limb. Neuroimage. 2016;130:134–144. doi: 10.1016/j.neuroimage.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 63.Rao F, Yuan Z, Zhang D, Yu F, Li M, Li D, Jiang B, Wen Y, Zhang P. Small-molecule SB216763-loaded microspheres repair peripheral nerve injury in small gap tubulization. Front Neurosci. 2019a;13:489. doi: 10.3389/fnins.2019.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao F, Zhang D, Fang T, Lu C, Wang B, Ding X, Wei S, Zhang Y, Pi W, Xu H, Wang Y, Jiang B, Zhang P. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells Int. 2019b;2019:2546367. doi: 10.1155/2019/2546367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 66.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 67.Rosén B, Lundborg G. Enhanced sensory recovery after median nerve repair using cortical audio-tactile interaction. A randomised multicentre study. J Hand Surg Eur. 2007;32:31–37. doi: 10.1016/j.jhsb.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Rosén B, Balkeniu C, Lundborg G. Sensory re-education today and tomorrow: a review of evolving concepts. Br J Hand Ther. 2003;8:48–56. [Google Scholar]
- 69.Sachanandani NF, Pothula A, Tung TH. Nerve gaps. Plast Reconstr Surg. 2014;133:313–319. doi: 10.1097/01.prs.0000436856.55398.0f. [DOI] [PubMed] [Google Scholar]
- 70.Sandquist EJ, Sakaguchi DS. Adult neural stem cell plasticity. Neural Regen Res. 2019;14:256–257. doi: 10.4103/1673-5374.244785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 72.Shankar H, Hansen J, Thomas K. Phantom pain in a patient with brachial plexus avulsion injury. Pain Med. 2015;16:777–781. doi: 10.1111/pme.12635. [DOI] [PubMed] [Google Scholar]
- 73.Socolovsky M, Malessy M, Lopez D, Guedes F, Flores L. Current concepts in plasticity and nerve transfers: relationship between surgical techniques and outcomes. Neurosurg Focus. 2017;42:E13. doi: 10.3171/2016.12.FOCUS16431. [DOI] [PubMed] [Google Scholar]
- 74.Sokki AM, Bhat DI, Devi BI. Cortical reorganization following neurotization: a diffusion tensor imaging and functional magnetic resonance imaging study. Neurosurgery. 2012;70:1305–1311. doi: 10.1227/NEU.0b013e318241017d. [DOI] [PubMed] [Google Scholar]
- 75.Sturma A, Hruby LA, Prahm C, Mayer JA, Aszmann OC. Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: protocols for clinical application. Front Neurosci. 2018;12:906. doi: 10.3389/fnins.2018.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomov TL, Guntinas-Lichius O, Grosheva M, Streppel M, Schraermeyer U, Neiss WF, Angelov DN. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Exp Neurol. 2002;178:207–218. doi: 10.1006/exnr.2002.8040. [DOI] [PubMed] [Google Scholar]
- 77.Walbruch B, Kalliainen L. The optimization of peripheral nerve recovery using cortical reorganization techniques: A retrospective study of wrist level nerve repairs. J Hand Ther. 2015;28:341–346. doi: 10.1016/j.jht.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Fan J, Yang X, Zhang W, Zhang P, Jiang B. The neural regeneration effect of chitin biological absorbable tubes bridging sciatic nerve defects with sural nerve grafts. Am J Transl Res. 2018;10:2362–2371. [PMC free article] [PubMed] [Google Scholar]
- 79.Weiss T, Miltner WH, Liepert J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci. 2004;20:3413–3423. doi: 10.1111/j.1460-9568.2004.03790.x. [DOI] [PubMed] [Google Scholar]
- 80.Yanagisawa T, Fukuma R, Seymour B, Hosomi K, Kishima H, Shimizu T, Yokoi H, Hirata M, Yoshimine T, Kamitani Y, Saitoh Y. Induced sensorimotor brain plasticity controls pain in phantom limb patients. Nat Commun. 2016;7:13209. doi: 10.1038/ncomms13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu Y, Zhang P, Han N, Kou Y, Yin X, Jiang B. Collateral development and spinal motor reorganization after nerve injury and repair. Am J Transl Res. 2016;8:2897–2911. [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang P, Han N, Wang T, Xue F, Kou Y, Wang Y, Yin X, Lu L, Tian G, Gong X, Chen S, Dang Y, Peng J, Jiang B. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013;10:171–175. doi: 10.7150/ijms.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]


