Subarachnoid hemorrhage (SAH) is a severe cerebrovascular disease accounting for a significant portion of young patients with stroke with high morbidity and mortality (van Gijn et al., 2007). Secondary brain injury resulting from neuroinflammation is considered to be a key pathological process (Zheng and Wong, 2017). Microglia, the resident immune cells of the brain, are implicated in numerous neurological diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis, stroke, and brain tumors (Colonna and Butovsky, 2017). Recent evidence suggests that microglia-mediated neuroinflammation plays a critical role in injury expansion and brain damage after SAH. In fact, two recent clinical studies have shown evidence of microglia accumulation and activation in the human brain parenchyma and cerebrospinal fluid (CSF) respectively after aneurysmal SAH. Schneider et al. (2015) examined 21 patients that had died within the course of SAH which had not suffered central nervous system (CNS) infection nor from cerebral vasospasm and found that microglia accumulation in human brain autopsy specimens after SAH between days 5 and 15 correlated with mortality rate of patients, and at a microscopic level, microglia accumulation was associated with neuronal apoptosis in a distribution concordant to axonal injury. Coinciding with this cellular inflammatory response, they found signs for axonal injury, displayed by an intraparenchymal accumulation of extracellular amyloid precursor protein; they also identified a significantly higher activity of DNA fragmentation in neurons during the course of experiment (Schneider et al., 2015). In cell counts, an increase of neuronal apoptosis could be detected from day 4 onwards to a peak on day 14 after SAH with a corresponding decline in the absolute number of vital neurons (Schneider et al., 2015). Roa et al. (2020) recruited 13 aneurysmal SAH patients for their study and found that the number of CSF microglia cells (CD45dimCD11b+) progressively increased over time after aneurysmal SAH, in particularly in patients with cerebral vasospasm. In contrast, CSF analysis demonstrated elevated counts of natural killer (NK) cells (CD3–CD161+) and Tc17 cells (CD8+CD161+) during the very acute phase on days 0–1, followed by a rapid reduction of cell numbers (Roa et al., 2020). The time course of microglial activation and accumulation suggested a window of opportunity for pharmacological intervention.
A mouse model of SAH is an effective way to explore the mechanism and treatment target of brain injury in SAH. Our previous work had established a C57BL/6 wild-type mouse model of endovascular perforated (EVP) SAH (Du et al., 2016; Zheng et al., 2020a). In short, the SAH model was established with a filament nylon suture inserted into the external carotid artery, which causes the bifurcation of the internal carotid artery and the middle cerebral artery rupture to mimics the aneurysm rupture in human. The SAH mice showed subarachnoid bleeding around the circle of Willis and presented body weakness, headache-symbolized moaning, and hemiparesis. The average body weight of the SAH mice decreased significantly on day 3 and returned to normal on day 7 after the SAH procedure. In motor and sensory scale test, mice showed a significant neurologic dysfunction during days 1–5 after SAH, which gradually recovered on day 10, and this is similar to the general treatment time window for clinical SAH. After Morris water maze test, the SAH group showed significant deterioration of learning disability and long-term memory dysfunction from day 1 to day 4 after SAH compared with the sham-operated group (Du et al., 2016; Zheng et al., 2020a). The EVP SAH model is an appropriate simulation to aneurysmal bleeding in SAH patients, and this model has a great advantage in reproducibility and consistency.
Our experimental SAH study results showed that microglia accumulated immediately after SAH and they distributed from the cortex adjacent to the perforated site (CAPS) to the remote motor cortex (Zheng et al., 2020b). In the study, EVP mouse model and ionized-binding adaptor molecule 1 (Iba1) immunohistochemistry were employed to detect microglia accumulation under the SAH condition. In the remote sites-M1 cortex and the hippocampus, microglia increased significantly after SAH within the first 3 days, in the calcium, on days 5 and 10, the numbers of Iba-1-positive microglia returned to normal and had no difference between the SAH and sham groups. In CAPS, microglia showed a significantly continuous accumulation compared with the sham group from day 1 to day 10 after SAH (P < 0.001). Accompanying the accumulation of microglia are the changes of microglial morphology. In the present study, microglia demonstrated a ramified shape with multiple processes on days 1 and 3 after SAH and a more rounded shape in the delayed phase. The time course study of microglia allows the capture of the dynamic process of accumulation and morphological changes, thus verifying the activation process of microglia.
The origin of accumulated microglia or active microglia is the basis for understanding the neuroinflammatory response after SAH, but it remains controversial. Recently, our study involving transgenic mice provides strong evidence of the origin of microglia. In the study by Zheng et al. (2020b), Cx3cr1GFP/GFPCcr2RFP/RFP transgenic mice were used to investigate the dynamics of microglia reaction in the EVP SAH model. Ccr2 is the receptor of CCL2 that is highly expressed in peripheral macrophage and was used to monitor the entry of peripheral macrophage into the central nervous system. Cx3cr1 is a kind of stably expressed marker both in microglia and macrophage. The distribution of Cx3cr1 specific microglia was visualized by the co-expressed green fluorescent protein (GFP). Then our study found that on day 5 after SAH, Ccr2 expression was low and Cx3cr1 was highly expressed (Ccr2–/Cx3cr1+), which considers the resident microglia in the majority of immune cell groups in the brain. At the same time, a small group of Ccr2+/Cx3cr1+ cells were being observed as well, which are considered as the macrophages leaked from the peripheral circulation. In addition, co-immunofluorescence with CD68, a specific marker of activated microglia/macrophage, indicated that most of the activated CNS immune cells originated from the resident microglia pool. This suggested that the accumulating Iba1-positive cells mostly originated from the resident microglia pool and pointed to continuing local neuroinflammation rather than the peripheral immune reaction (Zheng et al., 2020b).
Microglia co-express many macrophage-related markers (such as CD11b), however, the lineage relationship clearly indicates that microglia are separate cell types from the macrophages (Ginhoux et al., 2010). Microglia are a kind of plastic cells, highly dynamic, and can be rapidly activated in response to inflammatory factors in brain injury or degeneration conditions. Microglia generally have two polarization directions: one is from a resting state to the M1-classical activation connected with pro-inflammatory responses, and the other is M2-alternative activation connected with anti-inflammatory effect (Zheng and Wong, 2017). In the study by Zheng et al. (2020b), a dynamic polarization of microglia has also been observed which was from M1 to M2 phenotype, accompanied by the morphological change from ramified to amoeboid shapes. On days 1 and 3 after SAH, microglia mainly appeared a ramified shape (high expression of CD16/32, indicative of M1 phenotype). Later microglia of spindle shape with fewer processes in this distinct phase were named bipolar microglia in the delayed phase (the bipolar microglia expressed both M1 and M2 markers). On days 5 and 10 after SAH, the morphology of microglia transformed to amoeboid shape (high expression of CD206, indicative of M2 phenotype), which means an activated status. The polarization is determined by immunofluorescence (IF) consistent with the real time-polymerase chain reaction (PCR) results, and microglia showed the early M1-predominant and delayed M2-predominant polarization after SAH. In summary, microglia have the abilities of both accumulation and polarization after SAH in our EVP model. This is coincident with the results found in postmortem SAH patients. The time course polarization of microglia after SAH also provides a possible intervention window for the treatment of SAH (Zheng et al., 2020b).
Microglia are the main source of immune cell response to brain injury, and the time course level of cytokines, and inflammatory factors related to microglial polarization after SAH were investigated in a previous study (Zheng et al., 2020b). We found that the M1 polarization was associated with a proinflammatory response, and M2 polarization was associated with anti-inflammatory response. On day 1 after SAH, the M1 microglia related markers interleukin (IL) 6 and tumor necrosis factor-alpha (TNF-α) increased remarkably in three brain regions CAPS, M1 cortex, and the hippocampus. The turning point of the increasing trend of M1 is the third day after SAH, and then the M1 microglia level starts to decrease. The M2 markers started to increase also on day 3 after SAH. The M2 related genes and anti-inflammatory responses (IL-4 and transforming growth factor-beta (TGF-β)) upregulated in the late phase after SAH in the whole brain. The expression profile of inflammatory cytokines was identical to the dynamics of microglial polarization. Based on a previous study (Zheng et al., 2020b), we found that function-related microglial polarization advanced along with the morphological transformation from ramified shape to amoeboid shape. In response to SAH, rest microglia were activated to M1 phenotype related to pro-inflammation in the early phase and subsequently transited to the M2 phenotype (Zheng et al., 2020b). Similarly, in SAH clinical studies, Gris et al., (2019) found that IL-6 levels were rapidly increased following SAH both in SAH patients and experimental SAH. Other inflammatory factors such as intercellular adhesion molecule 1, basic fibroblast growth factor (bFGF) and IL-7 change over time and the variations were different between 13 SAH patients with different prognoses. It proves the similarity of systematic inflammation between SAH patients and SAH mouse models. In addition, there are some different changes in cytokines and inflammatory factors in patients with different prognoses, and this needs further exploration in large cohort clinical studies.
Microglia as the immune cells in the brain have been newly recognized and found closely related to SAH neuroinflammation in recent years. Some recent study found that signal transducer and activator of transcription 3 (STAT3) signal pathway plays an important role in the microglia-mediated inflammation. With the STAT3 gene expression enrich the analysis of rat SAH sequencing data, Samraj et al. (2014) found that accumulation of unphosphorylated STAT3 in the cerebral arteries has a pivotal role in modulating the genes that are involved in the late cerebral ischemia and related pathogenesis after SAH. Using TSG-6 knockdown rat SAH model, Li et al. (2018) found that in TSG-6 knockdown SAH rats with detrimental effects of microglia accompanied with more neurological deficits, rh-TSG-6 significantly ameliorated brain injury, decreased proinflammatory mediators, and skewed microglia towards a more anti-inflammatory property 24 hours after SAH. The anti-inflammatory effects of rh-TSG-6 were associated with microglia phenotypic shift by regulating the level of suppressor of cytokine signaling 3 (SOCS3)/STAT3 axis. These findings suggest that modulating microglia polarization, thus controling or reducing neuroinflammation after SAH could be a potential target to halt the pathological process related to neuroinflammation in SAH, and STAT3 may be a potential target for SAH treatment.
Microglia, as CNS immune cells, can interact with other cells to respond to physiological conditions; microglia can also act as phagocytes to scavenge plaques, neurofibrillary tangles, and cell debris in the CNS (Lan et al., 2017). CX3C chemokine receptor 1 is highly expressed on microglia while CX3C motif chemokine ligand 1 is expressed on neurons (Lan et al., 2017). Microglia can modulate neuronal apoptosis through TLR4-MyD88, mTOR, and HMGB1 related signal pathway (Hanafy, 2013; You et al., 2016; Ieong et al., 2018). Microglia can assist the clearance of hematoma by the tissue plasminogen activator in microglia Zheng et al. (2017). Evidence also shows that the movement of microglia towards injury in the CNS can be triggered by the extracellular ATP released from the damaged tissue and surrounding astrocytes (Davalos et al., 2005). Thus, the connection of microglia with other cells and other functions of microglia in SAH caused inflammation should be concerned and further clarified in future study (Figure 1).
Figure 1.
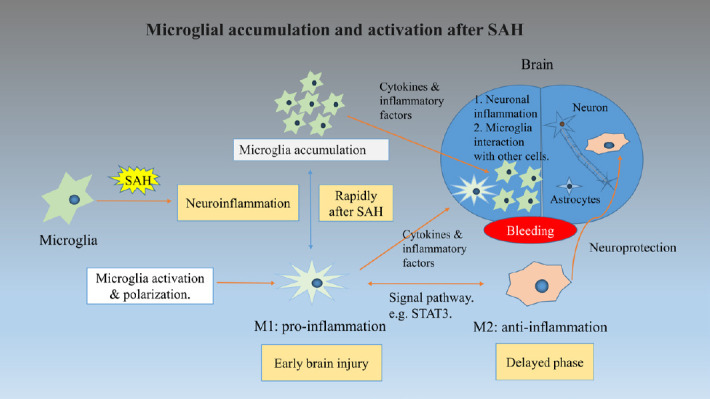
This figure shows the process of microglia accumulation and activation under subarachnoid hemorrhage (SAH) conditions.
The accumulation of microglia and polarization of M1 microglia responses rapidly in the early injury stage of SAH. Then the cytokines and inflammatory factors interact with microglia, caused neuronal inflammation and other brain cell function changes even cell death. M2 microglia emerge in the delayed phase of SAH and act as neuroprotection function. The polarization between M1 and M2 can be modulated through a specific signaling pathway.
In summary, based on recent researches, we recapitulate some key points about microglia accumulation and activation in recent preclinical and clinical studies of SAH. Our research pointed out that neuroinflammation after SAH is mediated by microglia from resident microglia pool. The functional changes of microglia precede morphological changes in the SAH pathophysiology process. Meanwhile, our data and other clinical researches showed that the accumulation and activation of microglia were strongly linked to neuroapoptosis, inflammatory reaction, and neurobehavioral impairment, which are associated with SAH. This provides a sound theoretical basis for future translational research to explore pharmacological interventions, for example, modulating the signal pathway (STAT3) to regulate microglia activation, reduce inflammatory reaction, and protect the brain after SAH.
Footnotes
C-Editors: Zhao M, Song LP; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Colonna M, Butovsky O. Microglia Function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 3.Du GJ, Lu G, Zheng ZY, Poon WS, Wong KC. Endovascular perforation murine model of subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;121:83–88. doi: 10.1007/978-3-319-18497-5_14. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gris T, Laplante P, Thebault P, Cayrol R, Najjar A, Joannette-Pilon B, Brillant-Marquis F, Magro E, English SW, Lapointe R, Bojanowski M, Francoeur CL, Cailhier JF. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation. 2019;16:253. doi: 10.1186/s12974-019-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10:83. doi: 10.1186/1742-2094-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieong C, Sun H, Wang Q, Ma J. Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates inflammative effect after acute subarachnoid hemorrhage in rat model. J Clin Neurosci. 2018;47:278–284. doi: 10.1016/j.jocn.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–433. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, Li X, Zhang X, He X, Duan C. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15:231. doi: 10.1186/s12974-018-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roa JA, Sarkar D, Zanaty M, Ishii D, Lu Y, Karandikar NJ, Hasan DM, Ortega SB, Samaniego EA. Preliminary results in the analysis of the immune response after aneurysmal subarachnoid hemorrhage. Sci Rep. 2020;10:11809. doi: 10.1038/s41598-020-68861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samraj AK, Müller AH, Grell AS, Edvinsson L. Role of unphosphorylated transcription factor STAT3 in late cerebral ischemia after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2014;34:759–763. doi: 10.1038/jcbfm.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider UC, Davids AM, Brandenburg S, Müller A, Elke A, Magrini S, Atangana E, Turkowski K, Finger T, Gutenberg A, Gehlhaar C, Brück W, Heppner FL, Vajkoczy P. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol. 2015;130:215–231. doi: 10.1007/s00401-015-1440-1. [DOI] [PubMed] [Google Scholar]
- 13.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 14.You W, Wang Z, Li H, Shen H, Xu X, Jia G, Chen G. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J Neurol Sci. 2016;367:224–231. doi: 10.1016/j.jns.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Zheng VZ, Wong GKC. Neuroinflammation responses after subarachnoid hemorrhage: A review. J Clin Neurosci. 2017;42:7–11. doi: 10.1016/j.jocn.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Zheng ZV, Lam PK, Poon WS, Wong KCG. The time course of cognitive deficits in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2020a;127:121–125. doi: 10.1007/978-3-030-04615-6_18. [DOI] [PubMed] [Google Scholar]
- 17.Zheng ZV, Lyu H, Lam SYE, Lam PK, Poon WS, Wong GKC. The dynamics of microglial polarization reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl Stroke Res. 2020b;11:433–449. doi: 10.1007/s12975-019-00728-5. [DOI] [PubMed] [Google Scholar]


