Keywords: cells, central nervous system, in vivo, injury, model, neurological function protection, rat, regeneration, repair, spinal cord
Abstract
Cell transplantation is a potential treatment for spinal cord injury. Olfactory ensheathing cells (OECs) play an active role in the repair of spinal cord injury as a result of the dual characteristics of astrocytes and Schwann cells. However, the specific mechanisms of repair remain poorly understood. In the present study, a rat model of spinal cord injury was established by transection of T10. OECs were injected into the site, 1 mm from the spinal cord stump. To a certain extent, OEC transplantation restored locomotor function in the hindlimbs of rats with spinal cord injury, but had no effect on the formation or volume of glial scars. In addition, OEC transplantation reduced the immunopositivity of chondroitin sulfate proteoglycans (neural/glial antigen 2 and neurocan) and glial fibrillary acidic protein at the injury site, and increased the immunopositivity of growth-associated protein 43 and neurofilament. These findings suggest that OEC transplantation can regulate the expression of chondroitin sulfate proteoglycans in the spinal cord, inhibit scar formation caused by the excessive proliferation of glial cells, and increase the numbers of regenerated nerve fibers, thus promoting axonal regeneration after spinal cord injury. The study was approved by the Animal Ethics Committee of the Medical College of Xi’an Jiaotong University, China (approval No. 2018-2048) on September 9, 2018.
Chinese Library Classification No. R456; R741; Q636.1
Introduction
Spinal cord injury (SCI) often leads to severe motor, sensory, and autonomic dysfunction below the injured segment. Current therapeutic strategies for SCI largely focus on reducing secondary injury and improving activities of daily living ability by rehabilitation training; there is no effective way to promote neurological recovery from SCI (Silva et al., 2014).
Intraspinal cell transplantation is regarded as a landmark therapeutic strategy that may provide a new approach for the treatment of SCI. The origins of cells that can be used for transplantation mainly include neural stem cells (Kadoya et al., 2016), Schwann cells (Kanno et al., 2015), bone marrow mesenchymal stem cells (Lin et al., 2014), and olfactory ensheathing cells (OECs) (Khankan et al., 2015; Reshamwala et al., 2020). Of these cell types, OECs are the most suited for transplantation to promote axonal regeneration and remyelination because of their dual characteristics of astrocytes and Schwann cells. Li et al. (1998) reported that OECs isolated from the olfactory bulb of adult rats promoted the regeneration of transected corticospinal axons, which were capable of extending across the injured area. Furthermore, transplanted OECs have been demonstrated to remyelinate, demyelinate, and regenerate axons, as well as promoting functional recovery (Imaizumi et al., 1998; Wang et al., 2020). Chondroitin sulfate proteoglycans (CSPGs) are generally upregulated after SCI, and it is believed that CSPGs inhibit axonal regeneration after SCI (Orr and Gensel, 2018). Glial fibrillary acidic protein (GFAP) is a marker of astrocytes (Schmidt-Kastner and Ingvar, 1994). The expression of GFAP can reflect the degree of reactive gliosis, which is closely related to SCI repair. In this study, we prepared a spinal cord transection model using Sprague-Dawley rats. We then investigated the expression of CSPGs and GFAP after OEC transplantation in this rat model of SCI.
Materials and Methods
Animals
This study was performed with the approval of the Animal Ethics Committee of the Medical College of Xi’an Jiaotong University, China (approval No. 2018-2048) on September 9, 2018. Eighty specific-pathogen-free, healthy adult female Sprague-Dawley rats (weighing 202.68 ± 10.88 g, aged 3 months) were used for animal grouping. Adult male rats (weighing 200–250 g) were used to provide the OECs. All rats were provided by the Experimental Animal Center of Xi’an Jiaotong University Health Science Center (license No. SCXK (Shaan) 2014-003), kept in individual cage under controlled conditions of 22–24°C and relative humidity of 40–60%, with a 12-hour light/dark cycle and free access to food and water. The female rats were randomly divided into the sham (n = 20), SCI (n = 30), and OEC transplantation (n = 30) groups.
SCI model establishment
Each rat was intraperitoneally anesthetized with 40 mg/kg ketamine and 10 mg/kg xylazine, then fastened to a stereotaxic apparatus (DL Naturegene, Beijing, China). In the SCI and OEC transplantation groups, a median skin incision was made at T10 to expose the spinal cord. A laminectomy was performed at T10 and partially at T9 and T11. The exposed spinal cord was completely transected at the level of T10 using a surgical blade (Shanghai Jinmei Medical Equipment Co. Ltd., Shanghai, China), and a preset thread was then smoothly raised (ventral to dorsal) to ensure the complete transection of the spinal cord (Inoue et al., 1998). Rats in the sham group received laminectomy only, without spinal cord transection. Gentle compression with a gelatin sponge was used for hemostasis.
OEC transplantation
As previously reported, OECs were isolated from the olfactory bulb of adult male rats, and then cultured and purified by using different attachment rates (Yang et al., 2002). After spinal cord transection, the OECs were transplanted immediately into animals in the OEC transplantation group. The intersection between the plane and the midline, 1 mm from the transected end of the spinal cord, was defined as the needle-entry point. The insertion was conducted at a 45° angle to the spinal cord. The OECs (0.5 µL, 5000 cells at each depth) were administered at four injection sites, each with a different depth (1.75, 1.25, 1, and 0.5 mm). In total, 20,000 cells were injected at the transected end of the spinal cord in each rat. The injections were performed at 0.1 µL/minute, for 5 minutes, at each injection site. Rats in the sham group received no specific treatment. All animals urinated passively 2–3 times daily, and received anti-infection and anti-hemorrhoid treatments.
Locomotor evaluation
Locomotor evaluation was performed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995). The rats were placed in a round, flat, non-skid plastic plate that was 7 cm in height and 90 cm in diameter and were allowed to move freely. The observation time was 4 minutes, and each rat’s behaviors were scored from 0 (no spontaneous locomotor activity) to 21 (normal movement: coordinated gait, with parallel paw placement). A higher BBB score indicates better neurological function. Hindlimb motor function was scored by two testers who were familiar with the BBB scale, but were not involved with any other procedures in the study.
Immunohistochemical staining
At 1, 2, 4, 6, and 8 weeks after SCI, 2–3 rats from each group were anesthetized and transcardially perfused with 200 mL normal saline and 200 mL 4% paraformaldehyde. Spinal cord segments, 0.5 cm apart, were excised from the rostral and caudal sites and further fixed in 4% paraformaldehyde for 24 hours. The segments were then trimmed, embedded in paraffin, and cut into 10-µm sections. After deparaffinization and rehydration, routine immunohistochemistry was performed. Neural/glial antigen 2 (NG2), neurocan, GFAP, growth-associated protein 43 (GAP-43), and neurofilament (NF) proteins were detected using an SP kit (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The sections were blocked in phosphate-buffered saline with 1% bovine serum albumin and 0.3% Triton X-100 for 1 hour at room temperature, and they were then incubated with primary antibody overnight at 4°C. Next, the sections were washed and incubated with biotin-labeled goat anti-rabbit IgG (1:400; Cat# SP-9000D; Zhongshan Jinqiao Biotechnology Co., Ltd.) at room temperature for 1 hour. Rabbit anti-GAP-43 antibody (1:100) was purchased from Bioss (Cat# bs-0154R; Beijing, China), rabbit anti-NG2 antibody (1:200) was purchased from Abcam (Cat# ab83178; Cambridge, MA, USA), rabbit anti-neurocan antibody (1:200) was purchased from Millipore (Cat# ABT1382; Billerica, MA, USA), and rabbit anti-GFAP (1:100 Cat# BA0056) and -NF (1:100; Cat# A00279) antibodies were purchased from Boster (Wuhan, China). Photographs were taken using a light microscope (Leica Microsystems Inc., Buffalo Grove, IL, USA), analyzed using the LeicaQ500IW image analysis system (Leica Microsystems Inc.), and quantified using ImageJ v1.48 (National Institutes of Health, Bethesda, MD, USA).
Hematoxylin-eosin staining
Sections of injured spinal cords from rats at 3 days and at 1, 2, 4, and 8 weeks after SCI were placed into xylene and then rehydrated through alcohol and water. Next, the sections were stained with hematoxylin for 3–5 minutes, before being differentiated with 1% acid alcohol for 5 minutes. After washing with water, the sections were stained with 1% eosin Y for 10 minutes, and then dehydrated through alcohol and xylene. Finally, the sections were observed under a light microscope.
Silver staining
Silver staining was used to detect nerve fibers in the injured spinal cord at 8 weeks after SCI. For silver staining, the sections were placed into xylene and then rehydrated through alcohol and water. The silver solution (1% silver nitrate in an aqueous solution) as preheated to 60°C, and the sections were incubated in the silver solution for 3 hours. After a quick wash with distilled water, the sections were placed into the reducing solution at 45°C for 1 hour and were then washed several times with distilled water. Finally, the sections were observed under a light microscope.
Picric acid staining
A picric acid dyeing kit (Cat# SBJ-0294S) was purchased from Nanjing SenBeiJia Biological Technology Co., Ltd. (Nanjing, China) and used according to the manufacturer’s instructions. Using tissue from rats at 8 weeks after SCI, the sections were routinely dewaxed and rehydrated to water. Sections were stained with sirius red for 1 hour, and then rinsed with running water to remove dye from the surface of the sections. Next, nuclei were stained with Mayer’s hematoxylin solution for 8–10 minutes. Sections were then rinsed with running water for 10 minutes, before being routinely dehydrated, cleared, and coverslipped.
Statistical analysis
Experimental data are expressed as the mean ± standard deviation (SD), and were statistically analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The unpaired t-test was used to compare differences between two groups, while the one-way analysis of variance was used to assess groups containing multiple comparisons. The significance level was set as P < 0.05.
Results
General postoperative conditions of SCI rats with OEC transplantation
There were four postoperative deaths in the OEC transplantation group and five postoperative deaths in the SCI group; there was no significant difference between the two groups (P = 0.43). The causes of death were mainly urinary retention, hematuria, and urinary tract infections at the early stage, and hemorrhoids at the late stage. No deaths occurred in the sham group. A loss of body mass occurred postoperatively in all rats. After spinal cord transection at the level of T9, the SCI rats suffered urinary retention and fecal incontinence, although spontaneous defecation recovered gradually. The rats dragged their hindlimbs when crawling and had typical signs of denervation—ulcers and hemorrhoids—at the knee joints. OEC transplantation triggered neurotrophic changes in the denervated knee and ankle joints, and the symptoms of denervation gradually improved from 6 weeks postoperatively. With the recovery of muscle strength in the quadriceps, dragging of the hindlimbs was also markedly improved. In contrast, rats in the SCI group had no improvements in the symptoms of denervation, while rats in the sham group had no abnormal changes.
OEC transplantation improves locomotor function in SCI rats
Within the first 4 weeks postoperatively, the mean BBB score was 0 in both the OEC transplantation group and the SCI group; this was significantly lower than that in the sham group (P < 0.01). Compared with the SCI group, BBB scores were significantly higher at 6 and 8 weeks after OEC transplantation (P < 0.01; Table 1).
Table 1.
Basso, Beattie, and Bresnahan locomotor function scores after OEC transplantation in rats with SCI
| Time after surgery (wk) | Sham group | SCI group | OECs transplantation group |
|---|---|---|---|
| 0 | 19.8±0.14 | 0 | 0 |
| 2 | 20.6±0.19 | 0 | 0 |
| 4 | 20.7±0.12 | 0 | 0 |
| 6 | 20.9±0.10 | 0.41±0.01 | 2.98±0.15* |
| 8 | 20.2±0.13 | 0.42±0.13 | 4.45±0.12* |
Data are expressed as the mean ± SD. *P < 0.05, vs. SCI group (unpaired t-test). OEC: Olfactory ensheathing cell; SCI: spinal cord injury.
OEC transplantation reduces pathological injuries of the spinal cord in SCI rats
Hematoxylin-eosin staining results
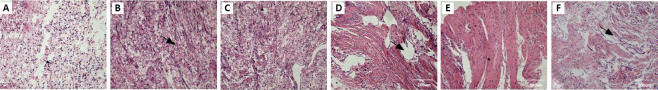
At 1 and 3 days after SCI, hematoxylin-eosin staining revealed fragmented tissue, scattered spinal cord residue, and erythrocyte infiltration at the injured area in the SCI group. There was also splinter hemorrhage, neuronal reduction, degeneration, edema, and necrosis, as well as inflammatory cell infiltration. At 1 week after SCI, the gray and white matter structures in the injured center were completely destroyed. This was accompanied by the production of cystic cavities, voids, and a large number of inflammatory cells, which were mainly macrophages. At 2 weeks after SCI, axonal degeneration was detected in the white matter and was immediately followed by void formation. Gray matter was severely damaged, with most neurons degenerated or lost, and cystic degeneration of the spinal cord was observed, with the formation of cystic cavities. Furthermore, hyperplasia was observed in glial cells. At 4 weeks after SCI, the injured area had a disordered structure, and the fibers were observed to have distorted and inconsistent paths. Voids often formed in the injured segment in the presence of very large cystic cavities. Axons were regenerated in the injured area, which was full of glial scar tissue. In the OEC transplantation group, there was better repair than in the SCI group. At 8 weeks after SCI, nerve fibers were clearly regenerated in the OEC transplantation group, but had a disordered arrangement. Distorted matrix fibers contained a large number of cells, with some infiltrated lymphoid cells. In the SCI group, few cells were observed in the injured area, and the tissue structure was disordered. Few nerve fibers with distorted structures were detected, and there was a large amount of glial scar tissue. The injured spinal cord had cystic degeneration and a large number of void cavities of varying sizes. In addition, neuroglia were observed at the edges of cystic cavities (Figure 1).
Figure 1.
Histological changes in the injured spinal cord of SCI rats with OEC transplantation (hematoxylin-eosin staining, original magnification 200×, scale bars: 200 μm).
(A–E) Hematoxylin-eosin staining in the SCI group at 3 days and at 1, 2, 4, and 8 weeks after SCI, respectively. After SCI, the spinal cord was completely destroyed (arrows in A and B), which was accompanied by the production of cystic cavities (arrow in C), voids (arrow in D) and glia scar (arrow in E). (F) Hematoxylin-eosin staining in the OEC transplantation group at 8 weeks after SCI. The nerve fibers in the OEC transplantation group were distorted, with a large number of cells and lymphoid cell infiltration. Compared with the OEC transplantation group, there were few cells in the damaged area of the SCI group, the tissue structure was disordered (arrows), and a large amount of glial scar tissue had formed. OEC: Olfactory ensheathing cell; SCI: spinal cord injury.
Silver staining results
At 8 weeks after SCI, there were regenerated nerve fibers in glial scar tissue in the OEC transplantation group. There were fewer nerve fibers in the SCI group than in the sham group, and the nerve fibers had a more disordered arrangement. The numbers of nerve fibers in the injured area were much higher in the OEC transplantation group than in the SCI group (P < 0.05; Figure 2).
Figure 2.
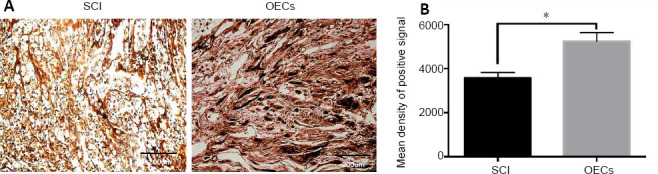
Nerve fibers in the injured spinal cord of SCI rats with OEC transplantation at 8 weeks after injury.
(A) Representative images of spinal cord sections with silver staining (original magnification 200×, scale bars: 200 µm). Nerve fibers were stained brown–yellow (arrows) in cross sections of the transected ends of the spinal cord. (B) Quantitative results of the positive silver staining signals. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). OEC: Olfactory ensheathing cell; SCI: spinal cord injury.
Picric acid staining results
In the SCI group, picric acid staining revealed fragmented tissue and erythrocyte infiltration in the injured area. In addition, the axons were fragmented, and there was a large amount of axonal debris. At 8 weeks after SCI, many glial scars had formed at the transverse end of the lesion in both the OEC transplantation group and the SCI group; there was no significant difference in glial scar area between the two groups (P > 0.05; Figure 3).
Figure 3.
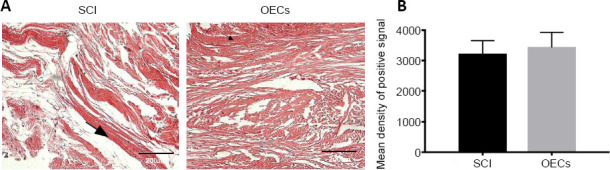
Glial scars in the injured spinal cord of SCI rats with OEC transplantation at 8 weeks after injury.
(A) Picric acid staining in the injured area (original magnification 200×, scale bars: 200 µm). At 8 weeks after SCI, a large number of glial scars were observed at the transected ends of cross-sectional injuries in both the OEC transplantation group and the SCI group. Arrows indicate the glial scars. (B) Quantitative analysis of picric acid staining results. There was no significant difference in picric acid staining between the two groups. Data are expressed as the mean ± SD (n = 6), and were analyzed by unpaired t-test. OEC: Olfactory ensheathing cell; SCI: spinal cord injury.
OEC transplantation inhibits NG2, neurocan, and GFAP immunopositivity in the injured spinal cord of SCI rats
NG2 immunopositivity was significantly lower in the OEC transplantation group than in the SCI group at 2 and 4 weeks after SCI (both P < 0.05; Figure 4).
Figure 4.
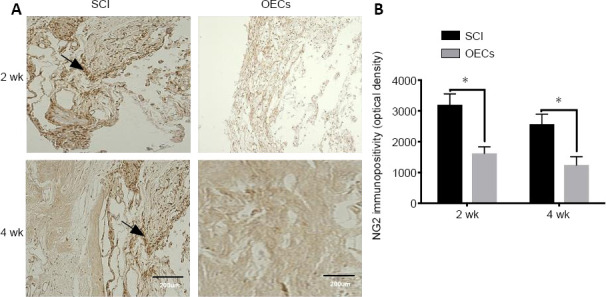
Effects of OEC transplantation on NG2 immunopositivity in the injured spinal cord of rats.
(A) NG2 immunopositivity in the injured area was detected using immunohistochemistry (original magnification 200×; scale bars: 200 µm). NG2 was expressed in the injured area in both the SCI group and the OEC transplantation group. (B) Quantitative analysis of NG2 immunopositivity. The expression of NG2 (arrows) in the OEC transplantation group was significantly lower than that in the SCI group at 2 and 4 weeks after SCI. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). NG2: Neural/glial antigen 2; OEC: olfactory ensheathing cell; SCI: spinal cord injury.
Neuron immunopositivity in the OEC transplantation group was significantly lower than that in the SCI group (P < 0.05) at each time point, although it was higher than that in the sham group (P < 0.05; Figure 5).
Figure 5.
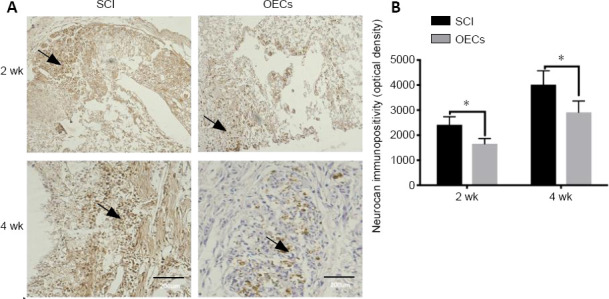
Effects of OEC transplantation on neurocan immunopositivity in the injured spinal cord of rats.
(A) Neurocan immunopositivity in the injured area was detected using immunohistochemistry (original magnification, 200×; scale bars: 200 µm). Arrows indicate neurocan-positive cells. (B) Quantitative analysis of neurocan immunopositivity. Compared with the SCI group, neurocan immunopositivity in the OEC transplantation group was significantly lower at 2 and 4 weeks after SCI. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). OEC: Olfactory ensheathing cell; SCI: spinal cord injury.
In the SCI group, GFAP immunopositivity was low at 1 week after SCI, and the area of GFAP-positive signal in the injured center was significantly increased at 2 weeks. Neurites extended from glial cells and interconnected to form reticular structures. Swollen glial cells were mainly located around the cystic cavity. Compared with the SCI group, there was less GFAP-positive signal in the OEC transplantation group (P < 0.05). However, there was no significant difference in the area of glial scar formation between the OEC transplantation group and the SCI group (Figure 6).
Figure 6.
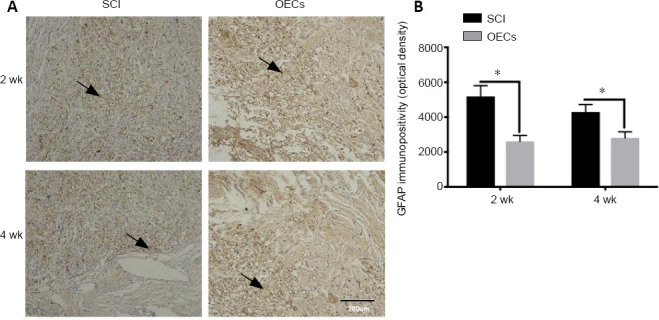
Effects of OEC transplantation on GFAP immunopositivity in the injured spinal cord of rats.
(A) GFAP immunopositivity in the injured area was detected using immunohistochemistry (original magnification 200×; scale bar: 200 µm). GFAP was highly expressed in both the SCI group and the OEC transplantation group. Arrows indicate GFAP-positive cells. (B) Quantitative analysis of GFAP immunopositivity. GFAP immunopositivity in the OEC transplantation group was significantly lower than that in the SCI group at 2 and 4 weeks after SCI. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). GFAP: Glial fibrillary acidic protein; OEC: olfactory ensheathing cell; SCI: spinal cord injury.
OEC transplantation increases GAP-43 and NF immunopositivity in the injured spinal cord of SCI rats
GAP-43 immunopositivity was significantly higher in the OEC transplantation group compared with the SCI group at both 2 and 4 weeks after SCI (both P < 0.05; Figure 7).
Figure 7.
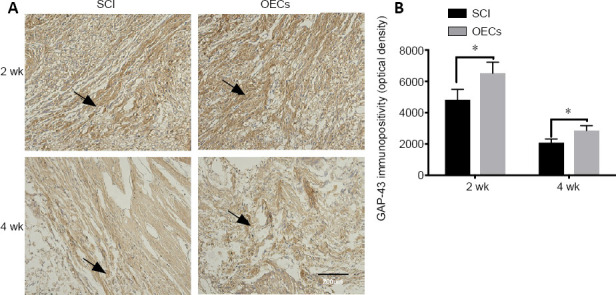
Effects of OEC transplantation on GAP-43 immunopositivity in the injured spinal cord of rats.
(A) GAP-43 immunopositivity in the injured area was detected using immunohistochemistry (original magnification 200×; scale bar: 200 µm). Arrows indicate GAP-43-positive cells. (B) Quantitative analysis of GAP-43 immunopositivity. Compared with the SCI group, GAP-43 immunopositivity in the OEC transplantation group was significantly higher at 2 and 4 weeks after SCI. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). GAP-43: Growth-associated protein 43; OEC: olfactory ensheathing cell; SCI, spinal cord injury.
In the OEC transplantation group, NF-positive fibers formed a network distribution at 2–4 weeks. There was significantly higher NF immunopositivity in the OEC transplantation group compared with the SCI group at both 2 and 4 weeks after SCI (both P < 0.05; Figure 8).
Figure 8.
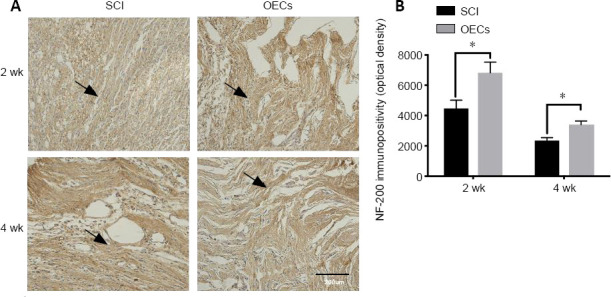
Effects of OEC transplantation on NF-200 immunopositivity in the injured spinal cord of rats.
(A) NF-200 immunopositivity in the injured area was detected using immunohistochemistry (original magnification 200×; scale bar: 200 µm). Arrows indicate NF-200-positive cells. (B) Quantitative analysis of NF-200 immunopositivity. NF-200 immunopositivity in the OEC transplantation group was significantly higher than that in the SCI group at 2 and 4 weeks after SCI. Data are expressed as the mean ± SD (n = 6). *P < 0.05 (unpaired t-test). NF-200: Neurofilament 200; OEC: olfactory ensheathing cell transplantation group; SCI: spinal cord injury.
Discussion
Previous studies have demonstrated that glial activation is a very active process after central nervous system injury, triggering the proliferation of glial cells and the formation of glial scars (Karimi-Abdolrezaee and Billakanti, 2012; Liu et al., 2019). Glial scars can mechanically inhibit axonal regeneration. Moreover, extracellular matrices secreted by activated glial cells can function as a chemical barrier that hinders early axonal regeneration following SCI. CSPGs, as the most important component of glia scar (Orr and Gensel, 2018), are composed of proteoglycans and chondroitin sulfates. Proteoglycans are the core structure of CSPGs. As an inhibitory extracellular matrix, CSPGs include a large class of complex molecules that can be divided into five types according to their different chondroitin sulfates (GAG chains): NG2, neurocan, aggrecan, brevican, and versican. Neurocan is the main molecule of CSPGs. CSPGs are widely distributed in the central nervous system and contribute to the inhibition of axonal regeneration, no matter whether they function as inhibitory molecules targeting neurons during development or participate in the formation of neuronal networks in adult individuals (Fawcett and Asher, 1999; Rhodes and Fawcett, 2004). Dynamic changes in CSPG expression after SCI have been previously reported. NG2 and neurocan immunolabeling increase at the injury site at 1 day after SCI and peak after 2 weeks. Neurocan and versican increase significantly at 4 days after SCI, and brevican expression persists stably for 2 months. Phosphacan immunolabeling decreases significantly at the injured site immediately after SCI, but later returns to normal and peaks after 2 months (Brittis et al., 1992). Furthermore, the combined use of GFAP immunohistochemistry and in situ hybridization has indicated that astrocytes become a source of neurocan following SCI, and that all GSPGs are highly expressed in the first 2 months following injury (Lemons et al., 1999). Moreover, a comparative study on the immune response of CSPGs in the normal (no surgery) and injured (laminectomy) spinal cord demonstrated that CSPG expression increases significantly at 4 days after injury, while both GFAP and CSPGs have restricted expression in terms of density and space at 40 days after SCI (Jones et al., 2003). These findings indicate that CSPGs inhibit axonal growth after SCI, where the GAG chain is believed to be involved in the inhibition by CSPGs of axonal growth after SCI? (Lemons et al., 1999). In the present study, early gliosis of glial cells was observed postoperatively, and was especially clear at 2 weeks after SCI. However, OEC transplantation immediately after SCI significantly reduced the levels of gliosis, and 2 weeks after SCI there was a lower expression of GFAP in the OEC transplantation group compared with the SCI group. This finding indicates that OEC transplantation may reduce the viability of reactive glial cells. In an adult rat model of hemi-transection or complete transection of the spinal cord, astrocytes proliferating in a nearby region of the SCI were unable to function as a mechanical barrier to completely inhibit nerve fiber regeneration, although the interconnected cell processes intertwined to form a loose network structure (You and Su, 2004). To date, a variety of chemicals that inhibit axon growth, such as CSPGs, have been isolated from the glial scar parenchyma. These chemicals may form a chemical barrier to axonal regeneration that is superior to the mechanical barrier in inhibiting the regeneration of the central nervous system. Recently, many enzymes have been applied in animal models of injuries. For example, chondroitinase ABC is a bacterial extracellular enzyme that specifically digests the GAG chain of CSPGs while retaining its protein core, but does not degrade proteoglycans in other extracellular matrices.
The ability of OECs to control the direction of axonal regeneration has been confirmed, and OECs are able to form a “glial bridge” which transverses scar tissue and longitudinally extends along axons (Pérez-Bouza et al., 1998). Shen et al. (2002) cut the rat spinal cord at the level of T10, and immunohistochemical staining with anti-myelin basic protein and anti-nerve growth factor receptor indicated numerous regenerated axons at 10 weeks after OEC transplantation. Furthermore, in a model of spinal cord transection (Ramón-Cueto et al., 1998), transplanted OECs promoted long-term axonal regeneration, and injured spinal cord axons regenerated through glial scars and grew into the transected end of the spinal cord at a length of 3 cm. In this previous study, the paraplegic rats recovered the autonomous activities of their hind limbs, which were able to fully extend and support their body weight. Moreover, their sensitivity to light touch and proprioception also recovered to some extent. In animal models of SCI, OECs transplanted into the injured site in adult rats can integrate with the host’s nerve tissue to help regenerated axons to transverse inhibitory barriers and glial scars, and extend to corresponding targets. OEC transplantation immediately following SCI can therefore inhibit glial cell proliferation. Our findings indicate that transplanted OECs mainly exert their effects by inhibiting the formation of chemical barriers.
Results from the present study indicate that OEC transplantation has little effect on glial scar size and formation. The decreased expression of NG2, neurocan, and GFAP after OEC transplantation indicates that OECs may inhibit the response of reactive astrocytes and reduce the inhibition of axons by CSPGs at the injured site, thus promoting axonal regeneration. Similar results have been observed in the white matter of the brain after transplantation of OECs and Schwann cells (Lakatos et al., 2003). Generally, OECs coexist with astrocytes in the glomerular layer of the olfactory bulb, and do not cause astrocyte reactivity. Compared with Schwann cells, transplanted OECs migrate more extensively in the injured area (Ramón-Cueto and Nieto-Sampedro, 1994), trigger less response to glial cells, and help more regenerated axons through the graft–host interface. Moreover, CSPGs are at lower levels when they are in contact with OECs compared with Schwann cells, meaning that OECs are more conducive to axonal regeneration. Additionally, OECs can eliminate or reduce chemical barriers to axonal growth in the extracellular matrix by inhibiting the expression of CSPGs, such as NG2 and neurocan, and GFAP.
GAP-43 is a 43-kDa growth-associated and nerve-specific protein that is inhibited in the mature central nervous system, thus ensuring normal physiological activity. GAP-43 can be induced when damaged axons are regenerated and extended. Its products mainly occur on the surface of the neuronal growth cone membrane and allow growth by accelerating the expansion of the cytoplasmic membrane at the base of growth cones. GAP-43 also promotes neuronal growth and synaptic remodeling, and increases as much as 20–100-fold during axonal regeneration (Tolner et al., 2003). Studies have shown that endogenous nerve growth factors not only increase the expression of GAP-43 after SCI, but also allow GAP-43 to release calmodulin to participate in nerve regeneration. GAP-43 is delivered to axons immediately after synthesis in neurons, is positioned on the cytoplasmic side of the axolemma, and is involved in the formation of the growth cone membrane (Meiri and Gordon-Weeks, 1990), which plays an important role in axon growth and regeneration. High expression of GAP-43 is also considered to be a typical feature of nerve growth and regeneration. GAP-43 is mainly distributed in neuronal axons, regenerated Schwann cells, and glial cells, and is considered a molecular marker of neuronal axon growth and plasticity (Hassiotis et al., 2002). Curtis et al. (1993) reported an increase in the expression of GAP-43 in cell bodies and axons around the lesion after SCI. Furthermore, Chaisuksunt et al. (2000) confirmed that axonal regeneration by most neurons in the central and peripheral nervous systems is accompanied by the upregulation of GAP-43 mRNA; this upregulation is deemed a prerequisite for axonal regeneration. Schmitt et al. (1999) suggested that the upregulation of GAP-43 is not infinite after injury, although levels increase considerably in the early stages of injury. With the progression of damage repair, GAP-43 expression is continuously reduced, and recovers when compensated synaptic connections are re-established.
Findings from the present study indicate that OEC transplantation promotes GAP-43 upregulation, and suggest that OECs can secrete a specific variety of neurotrophic factors, including nerve growth factor, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, ciliary neurotrophic factor, neurotrophin-3, and neurotrophin-4 (Wewetzer et al., 2001; Woodhall et al., 2001). It may be that OECs secrete neurotrophic factors that alter the local microenvironment after SCI, and then upregulate the expression of GAP-43 mRNA. The upregulation of GAP-43 mRNA triggers the synthesis of GAP-43 protein and promotes axonal extension and regeneration, thereby effectively improving central nervous system repair.
The possible mechanism of OECs changing chondroitin sulfate proteoglycans will be further studied in the future. In conclusion, transplanted OECs can inhibit the formation of scars as physical and chemical barriers caused by the excessive proliferation of glial cells. They also eliminate chemical barriers that hinder axonal regeneration, increase the numbers of regenerative nerve fibers, and promote axonal regeneration for spinal cord repair.
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner B, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: None declared.
Financial support: This study was supported by Shaanxi Provincial Key Research and Development Plan in 2018, No. 2018SF-124 (to GYW). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: This study was approved by the Animal Ethics Committee of the Medical College of Xi’an Jiaotong University, China (approval No. 2018-2048) on September 9, 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by Shaanxi Provincial Key Research and Development Plan in 2018, No. 2018SF-124 (to GYW) and National Key Research and Development Project of the People’s Republic of China, No. 2018YFE0114200 (to XJH).
References
- 1.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- 3.Chaisuksunt V, Zhang Y, Anderson PN, Campbell G, Vaudano E, Schachner M, Lieberman AR. Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-jun and growth-associated protein-43. Neuroscience. 2000;100:87–108. doi: 10.1016/s0306-4522(00)00254-2. [DOI] [PubMed] [Google Scholar]
- 4.Curtis R, Green D, Lindsay RM, Wilkin GP. Up-regulation of GAP-43 and growth of axons in rat spinal cord after compression injury. J Neurocytol. 1993;22:51–64. doi: 10.1007/BF01183975. [DOI] [PubMed] [Google Scholar]
- 5.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 6.Hassiotis M, Ashwell KW, Marotte LR, Lensing-Höhn S, Mai JK. GAP-43 Immunoreactivity in the brain of the developing and adult wallaby (Macropus eugenii) Anat Embryol (Berl) 2002;206:97–118. doi: 10.1007/s00429-002-0278-1. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue T, Kawaguchi S, Kurisu K. Spontaneous regeneration of the pyramidal tract after transection in young rats. Neurosci Lett. 1998;247:151–154. doi: 10.1016/s0304-3940(98)00297-3. [DOI] [PubMed] [Google Scholar]
- 9.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 10.Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, Takashima Y, Biane J, Conner J, Zhang SC, Tuszynski MH. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB. Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci. 2015;26:121–128. doi: 10.1515/revneuro-2014-0068. [DOI] [PubMed] [Google Scholar]
- 12.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 13.Khankan RR, Wanner IB, Phelps PE. Olfactory ensheathing cell-neurite alignment enhances neurite outgrowth in scar-like cultures. Exp Neurol. 2015;269:93–101. doi: 10.1016/j.expneurol.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakatos A, Barnett SC, Franklin RJ. Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than Schwann cells following transplantation into adult CNS white matter. Exp Neurol. 2003;184:237–246. doi: 10.1016/s0014-4886(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 15.Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W, Li M, Li Y, Sun X, Li X, Yang F, Huang Y, Wang X. Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci. 2014;35:449–457. doi: 10.1007/s10072-013-1490-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res. 2019;14:1352–1363. doi: 10.4103/1673-5374.253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meiri KF, Gordon-Weeks PR. GAP-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction. J Neurosci. 1990;10:256–266. doi: 10.1523/JNEUROSCI.10-01-00256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Bouza A, Wigley CB, Nacimiento W, Noth J, Brook GA. Spontaneous orientation of transplanted olfactory glia influences axonal regeneration. Neuroreport. 1998;9:2971–2975. doi: 10.1097/00001756-199809140-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ramón-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 23.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reshamwala R, Shah M, Belt L, Ekberg JAK, St John JA. Reliable cell purification and determination of cell purity: crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair. Neural Regen Res. 2020;15:2016–2026. doi: 10.4103/1673-5374.282218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS. J Anat. 2004;204:33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt-Kastner R, Ingvar M. Loss of immunoreactivity for glial fibrillary acidic protein (GFAP) in astrocytes as a marker for profound tissue damage in substantia nigra and basal cortical areas after status epilepticus induced by pilocarpine in rat. Glia. 1994;12:165–172. doi: 10.1002/glia.440120302. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt AB, Breuer S, Voell M, Schwaiger FW, Spitzer C, Pech K, Brook GA, Noth J, Kreutzberg GW, Nacimiento W. GAP-43 (B-50) and C-Jun are up-regulated in axotomized neurons of Clarke’s nucleus after spinal cord injury in the adult rat. Neurobiol Dis. 1999;6:122–130. doi: 10.1006/nbdi.1998.0231. [DOI] [PubMed] [Google Scholar]
- 28.Shen H, Tang Y, Wu Y, Chen Y, Cheng Z. Influences of olfactory ensheathing cells transplantation on axonal regeneration in spinal cord of adult rats. Chin J Traumatol. 2002;5:136–141. [PubMed] [Google Scholar]
- 29.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Tolner EA, van Vliet EA, Holtmaat AJ, Aronica E, Witter MP, da Silva FH, Gorter JA. GAP-43 mRNA and protein expression in the hippocampal and parahippocampal region during the course of epileptogenesis in rats. Eur J Neurosci. 2003;17:2369–2380. doi: 10.1046/j.1460-9568.2003.02687.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang GY, Cheng ZJ, Yang BH, Li HP, He XJ. Olfactory ensheathing cell transplantation promotes the ultrastructure repair at the lesion site of rat models of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:699–703. [Google Scholar]
- 32.Wewetzer K, Grothe C, Claus P. In vitro expression and regulation of ciliary neurotrophic factor and its alpha receptor subunit in neonatal rat olfactory ensheathing cells. Neurosci Lett. 2001;306:165–168. doi: 10.1016/s0304-3940(01)01891-2. [DOI] [PubMed] [Google Scholar]
- 33.Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, You SW, Wang CT, Ju G. In vitro culture, purification and morphological properites of olfactory ensheathing cells. Jiepou Kexue Jinzhan. 2002;8:101–105. [Google Scholar]
- 35.You SW, Su GH. Exploring new medicines to enhance neural regeneration of the central nervous system. Zhongguo Chufangyao. 2004:30–33. [Google Scholar]