Keywords: brain injury, brain-derived neurotrophic factor, enzyme, hypoxia-ischemia, receptors, recovery, repair
Abstract
Brain-derived neurotrophic factor (BDNF) regulates many neurological functions and plays a vital role during the recovery from central nervous system injuries. However, the changes in BDNF expression and associated factors following hypoxia-ischemia induced neonatal brain damage, and the significance of these changes are not fully understood. In the present study, a rat model of hypoxic-ischemic brain damage was established through the occlusion of the right common carotid artery, followed by 2 hours in a hypoxic-ischemic environment. Rats with hypoxic-ischemic brain damage presented deficits in both sensory and motor functions, and obvious pathological changes could be detected in brain tissues. The mRNA expression levels of BDNF and its processing enzymes and receptors (Furin, matrix metallopeptidase 9, tissue-type plasminogen activator, tyrosine Kinase receptor B, plasminogen activator inhibitor-1, and Sortilin) were upregulated in the ipsilateral hippocampus and cerebral cortex 6 hours after injury; however, the expression levels of these mRNAs were found to be downregulated in the contralateral hippocampus and cerebral cortex. These findings suggest that BDNF and its processing enzymes and receptors may play important roles in the pathogenesis and recovery from neonatal hypoxic-ischemic brain damage. This study was approved by the Animal Ethics Committee of the University of South Australia (approval No. U12-18) on July 30, 2018.
Chinese Library Classification No. R446.1; R741; R363
Introduction
Hypoxic-ischemic (HI) brain damage, which typically results from ischemia and hypoxia in the brain, can impact neuronal development and result in lifelong abnormal function (Sun et al., 2018). Neonatal HI encephalopathy represents a major cause of neonatal morbidity and mortality (Pimentel-Coelho and Mendez-Otero, 2010), often inducing severe asphyxia, seizures, and other severe neurological deficits in children. In addition, HI encephalopathy may also be associated with neurodevelopmental abnormalities, such as learning disorders and mental retardation (Gunn and Thoresen, 2019). Approximately 25% of HI encephalopathy infants have been reported to die during their first year, whereas those who survive present with permanent neurological disabilities (Logitharajah et al., 2009). An increasing number of studies have targeted neonatal HI encephalopathy treatments over the years.
Brain-derived neurotrophic factor (BDNF) has been considered to serve as a potent modulator of synaptic plasticity and is capable of regulating a wide range of brain functions, including motor functions, memory, and learning (Chen et al., 2013; Bae et al., 2020), which could promote the survival and growth of neurons in both the peripheral and central nervous systems (Zuccato et al., 2008; Lopes et al., 2017). BDNF, which is ubiquitously expressed in both the developing and adult mammalian brain, is synthesized in several regions of the hypothalamus and is expressed in almost all cortical regions (Tapia-Arancibia et al., 2004; Yu and Chen, 2011). Previous studies have shown a correlation between the early upregulation of BDNF and its neuroprotective effects after surgery using various ischemic models (Comelli et al., 1992; Rickhag et al., 2007; Madinier et al., 2013). However, studies examining the effects of BDNF expression levels following cerebral HI injury have been very limited. Thus, in this study, an HI injury model was established in neonatal rats to investigate changes in the expression levels of BDNF and its related enzymes and receptors.
Materials and Methods
Animals
Postnatal day 7 Sprague-Dawley (SD) rats (14–16 g, without gender selection) and their mothers were purchased from the Animal Center of the University of South Australia (UNISA) and were accommodated in a warm environment for the following operation. All procedures performed during this study were approved by the Animal Ethics Committee of the University of South Australia (approval No. U12-18) on July 30, 2018, and were in accordance with the Guide for the Care and Use of Laboratory Animals of National Institute of Health.
HI injury model establishment
Seven-day-old neonatal rats were selected and anesthetized by isoflurane (RWD Life Science, Shenzhen, Guangdong Province, China) inhalation (4% for induction, 2% for sustained inhalation anesthesia). Briefly, the midline of the ventral cervical skin was incised, followed by the blunt dissection of parenchyma to expose the right common carotid. Subsequently, the right common carotid was ligated using a monopolar microsurgery electrocoagulator (Chunguang Medical Cosmetology Instrument Co., Ltd., Wuhan, Hubei Province, China). Then, the subcutaneous tissues and skin were sutured, and the animals were returned to their mother for 1 hour before being moved to a hypoxic chamber on their own, containing 8% O2 and 92% N2 (air-flow rate maintained at 1 L/min) for 2 hours. Rats in the sham group underwent the same procedures without ligation of the right carotid artery. Animals were grouped randomly, as shown in Table 1.
Table 1.
Animal grouping
| Experiments | Sample size (rats, N = 54) |
|---|---|
| 2,3,5-Triphenyltetrazolium chloride staining | n = 3/group (sham, HI) |
| Hematoxylin-eosin staining | n = 3/group (sham, HI) |
| Nissl staining | |
| Geotaxis, Righting, and Climbing tests | n = 7/group (sham, 6 h, 15 h, 24 h, 3 d, 7 d) |
| Quantitative real-time polymerase chain reaction | |
| Western blot assay |
Zea-Longa score
The neurological functions of HI group rats were evaluated using the Zea-Longa score. The neurological scores of the rats were recorded at 6, 15, and 24 hours, and 3 and 7 days after HI. The Zea-Longa score was also used to determine that HI injury models were produced successfully. The 5-point Zea-Longa grading criteria are as follows (Longa et al., 1989): 0, no signs of nerve injury; 1, loss of the ability to fully stretch the contralateral forepaw; 2, the animal turns to one side while walking; 3, walking is unstable, the animal falls to one side; and 4, the animal is unable to walk or experiences a loss of consciousness.
Geotaxis test
The geotaxis test was used to assess motor coordination and vestibular sensitivity in neonatal rats 7 days after HI injury (Baharnoori et al., 2012; Ragaeva et al., 2017). The rats were placed in a 45° inclined grid, in a head downwards position, for 5 seconds. The grid provides a surface that can be gripped, allowing for the rodent to reorient itself towards an upwards position. After the rats were released, the time taken to reorient themselves was recorded. Each trial lasted for a maximum of 2 minutes, and each rat performed three trials.
Righting test
The righting test was used to evaluate the ability of newborn rats to roll out of the supine position, 7 days after HI injury (Li et al., 2019). The rats were placed on their backs on a cotton sheet and held in position for 5 seconds. After they were released, the direction in which they rolled to right themselves (left or right) and the time required to right themselves to a prone position was recorded. Each trial lasted for a maximum of 1 minute, and each rat performed three trials.
Climbing test
The climbing test was performed 7 days after HI. Rats were placed on a pad with a 45° slope, on which a line was drawn. The time taken for rats to climb across the line, with their heads facing downwards, was recorded by the researchers (Ennaceur et al., 2017). Each rat performed three independent trials.
Tissue collection
For morphological analysis, rats in the sham [n = 3 for 2,3,5-triphenyltetrazolium chloride (TTC) staining, n = 3 for hematoxylin-eosin (HE) and Nissl staining] and HI (n = 3 for TTC staining, n = 3 for HE and Nissl staining) groups (Table 1) were euthanized 24 hours after HI, under deep anesthesia using 4% isoflurane (sustained inhalation anesthesia) for 2 minutes. After the perfusion of 0.9% normal saline, followed by 4% paraformaldehyde, the brain was harvested and incubated in 4% paraformaldehyde for more than 72 hours. After paraffin embedding, 5 µm brain sections were prepared for HE and Nissl staining, and 2 mm sections were prepared for TTC staining.
For quantitative real-time polymerase chain reaction (qRT-PCR) and western blot assays, neonatal rats were sacrificed at 6 (n = 7), 15 (n = 7), and 24 hours (n = 7), and 3 (n = 7) and 7 days (n = 7) after surgery (Table 1), under deep anesthesia conditions induced by continuous inhalation of 4% isoflurane for 2 minutes, followed by perfusion with 0.9% normal saline. The cortex was removed and stored at –80°C. In accordance with previously described coordinates (Paxinos and Watson, 1998), two incisions, approximately 0.7 mm deep, were made to the cerebral cortex at the ends of both hemispheres and 1.5–2 mm away from the ends of both hemispheres, along the ventral side of the brain to expose the hippocampus. Then, the hippocampus was separated from the surrounding tissues along the dorsal side, towards the ventral side. The entire ipsilateral and contralateral hippocampus were collected in a 1.5 mL centrifuge tube and stored for the later use.
TTC staining
To evaluate brain damage following HI injury in neonatal rats, TTC staining was performed to observe the infarction of brain tissues. Whole brains from rats in the sham and HI groups were quickly removed, 16 hours after rats were deeply anesthetized with 4% isoflurane, and brain tissues were subsequently removed (operating on ice) and placed in a refrigerator (Haier Group, Qingdao, Shandong Province, China) at –20°C for 10 minutes, before being cut into five coronal pieces (2 mm each). Afterward, the sections were incubated in 0.5% TTC solution (Servicebio, Wuhan, Hubei Province, China) at 37°C for 30 minutes. The sections were then imaged using a digital camera (Huawei, Shenzhen, Guangdong Province, China). The infarction ratio (%) was analyzed using ImageJ software (National Institute of Health, Bethesda, MD, USA) and was calculated as follows: (contralateral area – ipsilateral non-infarction area)/contralateral area × 100.
HE staining
The harvested cortical and hippocampal tissues were embedded in paraffin after being transparentized with xylene and dehydrated and were cut into 5 µm sections. After dewaxing and hydrating, the sections were soaked in hematoxylin for 1 minute, which resulted in tissues turning red. The tissues were then rinsed with running water for 1 minute. Then, the sections were incubated in 1% hydrochloric acid ethanol for 30 seconds and again dehydrated and transparentized. The morphological changes in each section (0.3 mm2/field) were observed by a light microscope (CX40; Shunyu, Ningbo, Zhejiang Province, China).
Nissl staining
Paraffin-embedded tissues, after being sectioned and deparaffinized, were then stained with 30 µL of 1% cresyl violet solution (Solarbio, Beijing, China) in a wet incubator for 9 minutes. After washing with distilled water, the Nissl differentiation solution was added to the sections for 2 minutes. Subsequently, 95% ethanol was added for swift differentiation until Nissl bodies were purple, and the other tissues were colorless. Finally, specimens were dehydrated by absolute ethyl alcohol, transparentized by xylene, and sealed with neutral gum. Images of the damaged cortex and hippocampal CA2 (0.3 mm2) were taken using a light microscope (Leica) at 40× and 200× magnification.
qRT-PCR
Cortical and hippocampal tissues were collected, and total RNA was extracted using RNAiso plus (Takara Bio Inc., Otsu, Japan). The concentration and purity of total RNA were determined using a microplate reader (BioTek, Shanghai, China), which was then reverse transcribed into complementary DNA using the Revert Aid™ First Strand cDNA Synthesis System (Invitrogen, Carlsbad, CA, USA). The primers were designed for the detected factors using Primer 5.0 software (Premier, San Francisco, CA, USA), as shown in Table 2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Quantitative PCR reactions were performed using the QuantiNova SYBR Green PCR Kit (QIAGEN, Louisville, KY, USA) according to the manufacturer’s instructions. The expression level of each gene was normalized to that for GAPDH using the 2–ΔΔCt method (Phan et al., 2018).
Table 2.
Primer sequences used for quantitative real-time polymerase chain reaction
| Gene | Forward sequences | Reverse sequences |
|---|---|---|
| BDNF | 5’-GGT GTC GTA AAG TTC CAC CA-3’ | 5’-GCC AAG TTG CCT TGT CCG T-3’ |
| MMP-9 | 5’-CCC CCT ACT GCT GGT CCT-3’ | 5’-TTG GCT TCC TCC GTG ATT-3’ |
| Furin | 5’-GGC AAC CAG AAT GAG AAG CA-3’ | 5’-ACA GCC CGT AGC CAT AGG AA-3’ |
| tPA | 5’-CTT TGT GGA GTG GCG TTC A-3’ | 5’-CCC CAT TTT CTG CTG TGC T-3’ |
| Sortilin | 5’-CAA ATG GGG ACC AAA CAA-3’ | 5’-AGA GGC GAA GAG GAA ACG-3’ |
| TrkB | 5’-AAC CTC ACT GTG CAT TTT G-3’ | 5’-GTT GCC TCA CAG TGA ATG-3’ |
| PAI-1 | 5’-GCA CTA CAA AAG GTC AAG A-3’ | 5’-AAC CAC AAA GAG AAA GGA T-3’ |
| GAPDH | 5’-CCT CAA GAT TGT CAG CAA T-3’ | 5’-CCA TCC ACA GTC TTC TGA GT-3’ |
BDNF: Brain-derived neurotrophic factor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MMP-9: matrix metallopeptidase 9; PAI-1: plasminogen activator inhibitor 1; tPA: plasminogen activator, tissue type.
Western blot assay
The ipsilateral cortex and hippocampus from rats were collected and lysed with radioimmunoprecipitation assay buffer containing a proteinase inhibitor cocktail (Roche Life Science, Shanghai, China) on ice for 30 minutes. The lysates were centrifuged at 12,000 r/min for 10 minutes at 4°C. Protein concentrations were determined by bicinchoninic acid protein quantification kit (Beyotime, Shanghai, China). The protein samples were boiled and denatured with a loading buffer (Biosharp, Shanghai, China), and 40 µg of protein was run at 120 V for 90 minutes. The protein in the gel was transferred to a polyvinylidene difluoride membrane using 200 mA current. The polyvinylidene difluoride membrane was then blocked with 5% skim milk, which was prepared by dissolving 2.5 g skim milk powder in 50 mL phosphate buffered saline with Tween-20. After blocking for 90 minutes at room temperature, membranes were further incubated with a primary antibody against BDNF (rabbit; 1:500; Cat# bs-0248R; BIOSS, Beijing, China) at 4°C overnight. The membranes were then incubated with secondary antibodies for 2 hours at room temperature (anti-rabbit; 1:100; Cat# Ba0565-1; Boster, Wuhan, Hubei Province, China). β-Actin was used as an internal control. Finally, the membranes were developed, and the bands were visualized using an enhanced chemiluminescent solution (Smart Life Sciences, Changzhou, Jiangsu Province, China). The signals were quantitatively analyzed using ImageJ software. The results were expressed as an optical density ratio between the protein of interest and β-actin.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL, USA), and results are expressed as the mean ± standard deviation (SD). The data between two groups were analyzed by two-tailed Student’s t-test for the comparison of neuronal counts between the sham and HI groups, in addition to comparisons of the behavioral evaluations between the two groups. A repeated-measures analysis of variance was used to analyze qRT-PCR data. A one-way analysis of variance was used for western blot analysis. Differences were considered significant when P < 0.05.
Results
HI injury induces brain damage and neurological dysfunction
TTC staining showed clear cerebral infarctions on the right sides of the brains in the HI group compared with those in the sham group (Figure 1A). The infarction rate of the HI group was significantly higher than that of the sham group (P < 0.001; Figure 1B). HE staining was examined to observe morphological variations in neurons in neonatal rats after HI injury. More cell cavities were observed in the HI group compared with the sham group, and the cell nuclei were compressed into the sides in the cortex in the HI group (Figure 1C). Nissl staining further confirmed HI-induced neuronal loss, as indicated by a significant decrease in total neurons in the cortex (P < 0.001) and the hippocampus (P < 0.001), as well as an increased number of dead neurons in the cortex (P < 0.01; Figure 1D, F, and G) and hippocampus (P < 0.001; Figure 1E, F, and G). Zea-Longa scores were assessed in rats after the operation. The Zea-Longa scores of rats in the HI group increased, peaking at 6 hours after the operation and then gradually decreasing, but remained higher than those in the sham group at each time point (P < 0.001; Figure 1H). To further verify the balance and muscle tone of rats after HI brain damage, geotaxis, righting, and climbing assessments were performed 7 days after the HI insult. The geotaxis results showed that the time required for HI rats to turn themselves was longer than the time required for sham rats (P < 0.01; Figure 1I). The righting test results also demonstrated that rats in the HI group took longer to turn over onto their feet than those in the sham group (P < 0.001; Figure 1J). The climbing test revealed that rats in the HI group spent more time crossing the assigned line than the sham group (P < 0.01; Figure 1K). These results revealed that the HI injury procedure induced neurological functional impairments and hypomotility.
Figure 1.
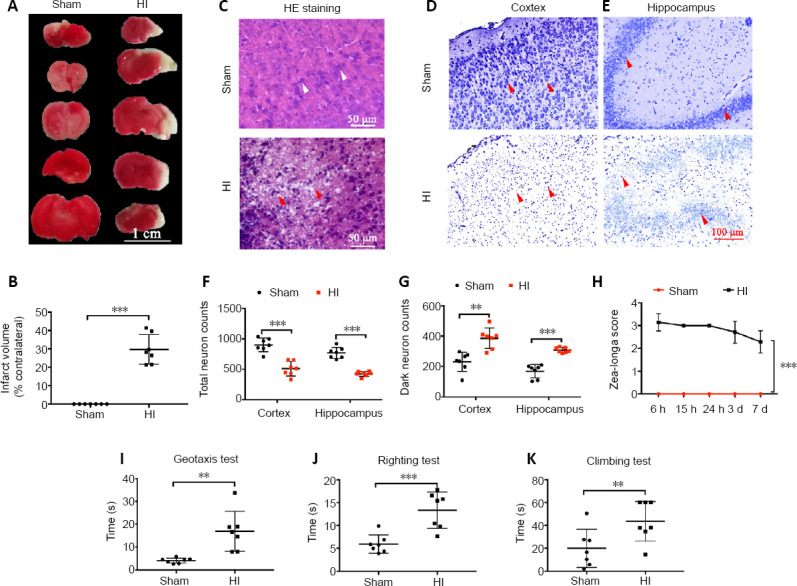
Effects of HI injury on brain damage and neurological dysfunction.
(A) 2,3,5-Triphenyltetrazolium chloride-stained images of brains from rats in the sham and HI groups at 24 hours post-HI injury. The non-ischemic tissue appears red, and the ischemic tissue appears white. Brain tissues in the HI group were obviously infarcted, whereas those in the sham group did not exhibit changes. (B) Quantitative evaluation of the infarction rate. (C) The morphology of cells in the cortex by hematoxylin-eosin staining. The number of vacuoles in the HI group increased relative to the sham group. The white arrows represent normal cells, and the red arrows represent vacuoles. (D, E) The morphology of neurons in the cortex (D) and hippocampus (E) by Nissl staining. The number of dead neurons in the HI group was greater than that in the sham group. Scale bars: 1 cm in A, 50 µm in C, 100 µm in D. (F, G) Quantitative results of total neurons and dark neurons (dead neurons) in the cortex and hippocampus, as detected by Nissl staining. (H) Quantitative evaluation of the Zea-Longa scores. (I–K) Quantitative evaluations of geotaxis (I), righting (J), and climbing test (K) results 7 days after HI insult. Data are expressed as the mean ± SD (n = 7/group). **P < 0.01, ***P < 0.001 (two-tailed Student’s t-test). HI: Hypoxia-ischemia.
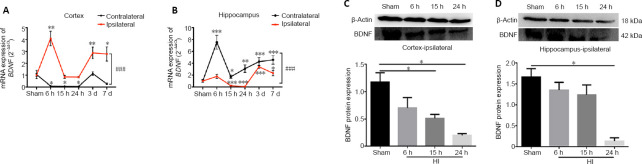
Changes in the mRNA and protein expression levels of BDNF in the cortex and hippocampus of HI rats
BDNF mRNA levels in the ipsilateral cortex increased in rats in the HI group compared with those in the sham group 6 hours after surgery (P < 0.01), but the levels were reduced at 15 and 24 hours relative to that at 6 hours, followed by a gradual increase over the next two days (P < 0.01). The mRNA expression levels of BDNF in the contralateral cortex decreased 6 hours after HI injury and remained at a low levels compared with the levels in the ipsilateral hemisphere at the same time points (P < 0.001; Figure 2A). The mRNA expression level of BDNF in the ipsilateral hippocampus was increased at 6 hours and 3 days in the HI group compared with that in the sham group (P < 0.001). The mRNA levels of BDNF in the contralateral hippocampus were higher than those in the ipsilateral side at each time point (P < 0.001; Figure 2B). The protein expression levels of BDNF in the ipsilateral cortex exhibited marked reductions at 15 and 24 hours compared with the same time points in the sham group (P < 0.05; Figure 2C), whereas the protein levels in the ipsilateral hippocampus were significantly diminished at 24 hours relative to those in the sham group (P < 0.05; Figure 2D).
Figure 2.
Changes in the mRNA and protein expression levels of BDNF in the cortex and hippocampus after HI injury.
(A, B) BDNF mRNA expression levels in the contralateral and ipsilateral cortex (A) and hippocampus (B), as detected by quantitative real-time polymerase chain reaction. The BDNF mRNA expression level was expressed relative to the level in the sham group. (C, D) BDNF protein expression level in the ipsilateral cortex (C) and hippocampus (D), as detected by western blot assay. The BDNF protein expression was expressed as the optical density relative to the optical density of β-actin. Data are expressed as the mean ± SD (n = 7/group), analyzed by repeated-measures analysis of variance (A, B) or one-way analysis of variance (C, D). *P < 0.05, **P < 0.01, ***P < 0.001, vs. sham group; ###P < 0.001. BDNF: Brain-derived neurotrophic factor; HI: hypoxia-ischemia.
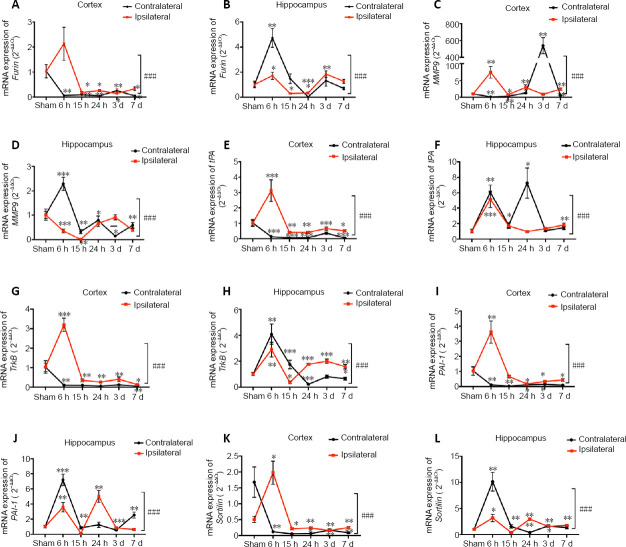
Changes in the mRNA expression level of BDNF-related enzymes and receptors in the cortex and hippocampus of HI rats
The mRNA expression level of Furin [a precursor of the BDNF converting enzyme (Zhu et al., 2018)] in the ipsilateral cortex of rats in the HI group increased at 6 hours compared with the sham rats, then decreased at 15 hours and was sustained at a low level for 7 days relative to that observed at 6 hours (P < 0.05; Figure 3A). Furin mRNA expression in the ipsilateral hippocampus of rats in the HI group was elevated at 6 hours and 3 days compared with that in the sham group (P < 0.05) and was lower than the expression levels observed in the contralateral hemisphere of rats in the HI group within 24 hours (P < 0.001; Figure 3B). Similarly, the mRNA expression level of matrix metallopeptidase 9 [MMP-9, an extracellular converting enzyme (Cai et al., 2017)] in the ipsilateral cortex was significantly increased at 6 and 24 hours (P < 0.01; Figure 3C), compared with those in the sham group, whereas the mRNA expression levels of MMP-9 in the ipsilateral hippocampus were notably depressed at 6 (P < 0.001) and 15 hours (P < 0.01) compared with those in the sham group (Figure 3D). The mRNA expression levels of Furin and MMP-9 were both increased at 6 hours in the contralateral hippocampus compared with those in the sham group (P < 0.01 for Furin and P < 0.001 for MMP-9).
Figure 3.
Changes in the mRNA expression levels of brain-derived neurotrophic factor-related enzymes and receptors in the cortex and hippocampus after HI injury.
(A, B) Furin mRNA expression in the contralateral and ipsilateral cortex (A) and hippocampus (B). (C, D) MMP-9 mRNA expression in the contralateral and ipsilateral cortex (C) and hippocampus (D). (E, F) tPA mRNA expression in the contralateral and ipsilateral cortex (E) and hippocampus (F). (G, H) TrkB mRNA expression in the contralateral and ipsilateral cortex (G) and hippocampus (H). (I, J) PAI-1 mRNA expression in the contralateral and ipsilateral cortex (I) and hippocampus (J). (K, L) Sortilin mRNA expression in the contralateral and ipsilateral cortex (K) and hippocampus (L). Data are expressed as the mean ± SD (n = 7/group), analyzed by repeated-measures analysis of variance. *P < 0.05, **P < 0.01, ***P < 0.001, vs. sham group; ###P < 0.001. HI: Hypoxia-ischemia; MMP-9: matrix metallopeptidase 9; PAI-1: plasminogen activator inhibitor 1; tPA: plasminogen activator, tissue type; TrkB: neurotrophic receptor tyrosine kinase 2.
The mRNA expression levels of plasminogen activator, tissue type [tPA, a precursor of BDNF converting enzyme (Rahman et al., 2018)] in the ipsilateral cortex were significantly increased at 6 hours compared with sham group (P < 0.001), followed by a gradual reduction and sustained lower levels at 15 hours and 24 days (P < 0.01; Figure 3E) relative to levels at 6 hours. In the hippocampus, the tPA mRNA expression level in the ipsilateral hemisphere was markedly elevated at 6 hours compared with that in the sham group (P < 0.01; Figure 3F). Similarly, the mRNA expression level of tropomyosin receptor kinase B [TrkB, a mature BDNF signaling receptor (Sheng et al., 2018)] in the ipsilateral cortex was significantly increased at 6 hours compared with that in the sham group (P < 0.001; Figure 3G), and the TrkB mRNA expression levels in the ipsilateral hippocampus were significantly increased at 6 (P < 0.001) and 24 hours (P < 0.001) and 3 days (P < 0.001; Figure 3H) compared with those in the contralateral hemisphere at the same time points. The mRNA expression levels of tPA and TrkB were notably augmented at 6 hours in the contralateral hippocampus compared with the levels in the ipsilateral hemisphere at the same time point (P < 0.01 for tPA and P < 0.001 for TrkB). The mRNA expression level of plasminogen activator inhibitor 1 [PAI-1, a tPA inhibitor (Thomas et al., 2016)] in the ipsilateral cortex showed an obvious increase at 6 hours in comparison with the sham group (P < 0.05), followed by a gradual reduction and sustained lower levels at 24 hours (P < 0.05) and 3 days (P < 0.05; Figure 3I) relative to those at 6 hours. In the hippocampus, the PAI-1 mRNA expression levels in the ipsilateral hemisphere were significantly increased at 6 and 24 hours compared with those of the sham group (both P < 0.01; Figure 3J). Similarly, the mRNA expression of Sortilin [a precursor of a BDNF signaling co-receptor (Vaegter et al., 2011) in the ipsilateral cortex was significantly increased at 6 hours compared with that in the sham group (P < 0.01; Figure 3K), whereas the mRNA expression levels in the ipsilateral hippocampus were notably decreased at 6 hours (P < 0.001) compared with those in the contralateral hemisphere at the same time point (Figure 3L). The mRNA expression levels of PAI-1 and Sortilin were both increased at 6 hours in the contralateral hippocampus relative to those in the sham group (P < 0.01 for PAI-1 and P < 0.001 for Sortilin). Summarized mRNA expression level changes and differences in the expression levels of BDNF and its related factors between samples from the HI and sham groups at different time points can be found in Table 3 and Additional Table 1.
Table 3.
Changes in the mRNA expression levels of BDNF and its related enzymes and receptors in the cortex and hippocampus after hypoxia-ischemia injury
| Gene | Position | 6 h | 15 h | 24 h | 3 d | 7 d |
|---|---|---|---|---|---|---|
| BDNF | Contralateral cortex | ↓ | ↓ | ↓ | No change | ↓ |
| Ipsilateral cortex | ↑ | No change | No change | ↑ | ↑ | |
| Contralateral hippocampus | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Ipsilateral hippocampus | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Furin | Contralateral cortex | ↓ | ↓ | ↓ | ↓ | ↓ |
| Ipsilateral cortex | No change | ↓ | ↓ | ↓ | ↓ | |
| Contralateral hippocampus | ↑ | No change | ↓ | No change | No change | |
| Ipsilateral hippocampus | ↑ | ↓ | ↓ | ↑ | No change | |
| MMP-9 | Contralateral cortex | ↓ | ↓ | ↑ | ↑ | ↓ |
| Ipsilateral cortex | ↓ | ↓ | No change | No change | ↓ | |
| Contralateral hippocampus | ↑ | ↓ | No change | ↓ | No change | |
| Ipsilateral hippocampus | ↓ | ↓ | ↓ | No change | ↓ | |
| tPA | Contralateral cortex | ↓ | ↓ | ↓ | ↓ | ↓ |
| Ipsilateral cortex | ↑ | No change | ↓ | ↓ | No change | |
| Contralateral hippocampus | ↑ | ↑ | ↑ | No change | No change | |
| Ipsilateral hippocampus | ↑ | No change | No change | No change | ↑ | |
| Sortilin | Contralateral cortex | ↓ | ↓ | ↓ | ↓ | ↓ |
| Ipsilateral cortex | ↑ | ↓ | ↓ | ↓ | ↓ | |
| Contralateral hippocampus | ↑ | No change | ↓ | ↑ | No change | |
| Ipsilateral hippocampus | ↑ | No change | ↑ | ↑ | ↑ | |
| TrkB | Contralateral cortex | ↓ | ↓ | ↓ | ↓ | ↓ |
| Ipsilateral cortex | ↑ | No change | No change | No change | ↓ | |
| Contralateral hippocampus | ↑ | ↑ | ↓ | No change | ↓ | |
| Ipsilateral hippocampus | ↑ | ↓ | ↑ | ↑ | ↑ | |
| PAI-1 | Contralateral cortex | ↑ | ↓ | No change | No change | No change |
| Ipsilateral cortex | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Contralateral hippocampus | No change | No change | ↑ | No change | ↑ | |
| Ipsilateral hippocampus | ↑ | No change | ↑ | ↑ | No change |
BDNF: Brain-derived neurotrophic factor; MMP-9: matrix metallopeptidase 9; PAI-1: plasminogen activator inhibitor 1; tPA: plasminogen activator, tissue type.
Additional Table 1.
Repeated measurement design analysis of variance of mRNA expression levels of BDNF and its-related enzymes and receptors in the cortex and hippocampus after hypoxia-ischemia
| Variables | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| BDNF in cortex | |||||
| Intervene | 1 | 55.786 | 55.786 | 1357.966 | <0.001 |
| Intergroup error | 12 | 0.493 | 0.041 | ||
| Time | 2.961 | 37.208 | 12.567 | 67.914 | <0.001 |
| Time × Intervene | 2.961 | 37.550 | 12.682 | 68.538 | <0.001 |
| Repeated measurement error | 35.531 | 6.574 | 0.185 | ||
| BDNF in hippocampus | |||||
| Intervene | 1 | 105.018 | 105.018 | 408.284 | <0.001 |
| Intergroup error | 12 | 3.087 | 0.257 | ||
| Time | 2.056 | 177.547 | 86.363 | 128.322 | <0.001 |
| Time × Intervene | 2.056 | 71.447 | 34.754 | 51.638 | <0.001 |
| Repeated measurement error | 24.670 | 16.603 | .673 | ||
| Furin in cortex | |||||
| Intervene | 1 | 3.715 | 3.715 | 75.125 | <0.001 |
| Intergroup error | 12 | 0.593 | 0.049 | ||
| Time | 1.585 | 15.022 | 9.480 | 61.647 | <0.001 |
| Time × Intervene | 1.585 | 11.761 | 7.422 | 48.265 | <0.001 |
| Repeated measurement error | 19.015 | 2.924 | 0.154 | ||
| Furin in hippocampus | |||||
| Intervene | 1 | 4.518 | 4.518 | 25.177 | <0.001 |
| Intergroup error | 12 | 2.153 | 0.179 | ||
| Time | 2.120 | 75.233 | 35.484 | 186.055 | <0.001 |
| Time × Intervene | 2.120 | 34.712 | 16.372 | 85.844 | <0.001 |
| Repeated measurement error | 25.442 | 4.852 | 0.191 | ||
| MMP-9 in cortex | |||||
| Intervene | 1 | 161366.853 | 161366.853 | 216.546 | <0.001 |
| Intergroup error | 12 | 8942.235 | 745.186 | ||
| Time | 1.001 | 836216.193 | 835450.050 | 223.008 | <0.001 |
| Time × Intervene | 1.001 | 849030.666 | 848252.782 | 226.425 | <0.001 |
| Repeated measurement error | 12.011 | 44996.629 | 3746.284 | ||
| MMP-9 in hippocampus | |||||
| Intervene | 1 | 1.947 | 1.947 | 59.656 | <0.001 |
| Intergroup error | 12 | 0.392 | 0.033 | ||
| Time | 3.046 | 11.729 | 3.851 | 143.417 | <0.001 |
| Time × Intervene | 3.046 | 13.792 | 4.528 | 168.641 | <0.001 |
| Repeated measurement error | 36.553 | 0.981 | 0.027 | ||
| PAI-1 in cortex | |||||
| Intervene | 1 | 11.457 | 11.457 | 242.113 | <0.001 |
| Intergroup error | 12 | 0.568 | 0.047 | ||
| Time | 1.349 | 22.308 | 16.535 | 84.569 | <0.001 |
| Time × Intervene | 1.586 | 21.615 | 13.632 | 81.940 | <0.001 |
| Repeated measurement error | 16.190 | 3.165 | 0.196 | ||
| PAI-1 in hippocampus | |||||
| Intervene | 1 | 2.854 | 2.854 | 26.247 | <0.001 |
| Intergroup error | 12 | 1.305 | 0.109 | ||
| Time | 1.795 | 250.344 | 139.495 | 286.018 | <0.001 |
| Time × Intervene | 1.795 | 107.396 | 59.842 | 122.699 | <0.001 |
| Repeated measurement error | 21.536 | 10.503 | 0.488 | ||
| Sortilin in cortex | |||||
| Intervene | 1 | 0.81 | 0.810 | 23.975 | <0.001 |
| Intergroup error | 12 | 0.405 | 0.034 | ||
| Time | 1.925 | 15.732 | 8.172 | 101.121 | <0.001 |
| Time × Intervene | 1.925 | 16.371 | 8.504 | 105.228 | <0.001 |
| Repeated measurement error | 23.101 | 1.867 | 0.081 | ||
| Sortilin in hippocampus | |||||
| Intervene | 1 | 15.721 | 15.721 | 49.677 | <0.001 |
| Intergroup error | 12 | 3.798 | 0.316 | ||
| Time | 1.169 | 333.997 | 285.594 | 193.870 | <0.001 |
| Time × Intervene | 1.169 | 182.769 | 156.282 | 106.089 | <0.001 |
| Repeated measurement error | 14.034 | 20.673 | 1.473 | ||
| TPA in cortex | |||||
| Intervene | 1 | 12.793 | 12.793 | 204.557 | <0.001 |
| Intergroup error | 12 | 0.75 | 0.063 | ||
| Time | 1.577 | 31.791 | 20.153 | 107.549 | <0.001 |
| Time × Intervene | 1.577 | 31.82 | 20.172 | <0.001 | |
| Repeated measurement error | 18.93 | 3.547 | 0.187 | ||
| tPA in hippocampus | |||||
| Intervene | 1 | 26.223 | 26.223 | 57.468 | <0.001 |
| Intergroup error | 12 | 5.476 | 0.456 | ||
| Time | 1.406 | 244.098 | 173.587 | 90.627 | <0.001 |
| Time × Intervene | 1.406 | 117.113 | 83.283 | 43.481 | <0.001 |
| Repeated measurement error | 16.874 | 32.321 | 1.915 | ||
| TrkB in cortex | |||||
| Intervene | 1 | 8.998 | 8.998 | 428.852 | <0.001 |
| Intergroup error | 12 | 0.252 | 0.021 | ||
| Time | 1.96 | 27.859 | 14.213 | 227.932 | <0.001 |
| Time × Intervene | 1.96 | 25.224 | 12.869 | 206.374 | <0.001 |
| Repeated measurement error | 23.521 | 1.467 | 0.062 | ||
| TrkB in hippocampus | |||||
| Intervene | 1 | 0.677 | 0.677 | 5.503 | 0.037 |
| Intergroup error | 12 | 1.476 | 0.123 | ||
| Time | 1.337 | 67.601 | 50.556 | 147.812 | <0.001 |
| Time × Intervene | 1.337 | 27.225 | 20.361 | 59.529 | <0.001 |
| Repeated measurement error | 16.046 | 5.488 | 0.342 |
BDNF: Brain-derived neurotrophic factor; DF: degree of freedom; MMP-9: matrix metallopeptidase 9; MS: mean square; PAI-1: plasminogen activator inhibitor 1; SS: sum of squares; tPA: plasminogen activator, tissue type.
Discussion
In our study, we successfully established an HI injury model and verified HI-induced pathological brain damage and behavioral deficits in neonatal rats. After investigating the expression changes in the mRNA and protein levels of BDNF and its related enzymes and receptors in the cortex and hippocampus, we determined that BDNF and its related factors may play potential roles in the pathogenesis and recovery from HI injury.
After HI insult, pathological changes and neuronal damage can be observed in rats (Zhang et al., 2018). The HI injury has previously been shown to have a harmful influence on the developing brain and has been associated with persistent motor, sensory, and cognitive impairments (Millar et al., 2017; Sanches et al., 2019; Jiang et al., 2020). The results of the behavioral experiments performed in this study showed that rats in the HI group displayed worse performance on the behavioral tests than the sham rats, indicating that the HI insult deteriorated the motor coordination and vestibular sensitivity of neonatal rats. The results of this study demonstrated altered neuronal morphologies and diminished neurons counts in the cortex and hippocampus of rats in the HI group, suggesting that aggravated neurological dysfunction may be attributed to HI-induced neuronal damage. Thus, the molecular alterations that occur within damaged neurons must be clarified.
BDNF has biological effects and has been shown to play a pivotal role in reducing the death of cells surrounding lesions, repairing central nervous system damage, and promoting nerve regeneration (Koshimizu et al., 2009; Numakawa et al., 2010a, b). In this study, we found the early upregulation of BDNF and its related enzymes and receptors in the ipsilateral cortex of HI injury model rats as early as 6 hours after HI induction. The early post-HI injury alterations of these factors might result in the neuronal damage observed in our models. We believe that the early upregulation of BDNF and its enzymes and receptors may be associated with the infarcted tissues, and the elevated levels went through the injuring processes within 6 hours after HI insult.
The gene expression levels of BDNF converting enzyme precursors, including Furin, MMP-9, and tPA, were significantly upregulated 6 hours after HI injury in HI injury model rats compared with sham rats. The early upregulation of BDNF converting enzyme precursors may be occurring in the peri-infarcted areas, where mature BDNF is generated to facilitate neuronal survival (Rahman et al., 2018). Furin, a member of the pro-protein convertase family, is a key cutting enzyme in the endocrine pathway of BDNF-expressing cells, and BDNF precursor protein can be cut by Furin to form the bioactive, mature form of BDNF (Zhu et al., 2018). However, Furin also enhances the post-ischemic activation of MMP-2, which exerts damaging effects by weakening the blood-brain barrier and causing oxidative DNA damage in the brain (McMahon et al., 2005). BDNF is synthesized as a precursor molecule that undergoes proteolytic cleavage by MMP-9 and tPA (Mizoguchi et al., 2011). MMP-9 is involved in the brain injury process starting at an early stage and plays a pivotal role in the recovery of cerebral ischemic damage; however, MMP-9 also appears to contributes to the recovery of brain injury during later stages (Cai et al., 2015, 2017; Savard et al., 2015). The upregulation of MMP-9 expression in the infarcted and peri-infarcted areas could increase the infarction region, damaging the blood-brain barrier after ischemia (Planas et al., 2001). Thus, both beneficial and harmful effects could be induced by MMP-9 and Furin upregulation after injury, suggesting that sensorimotor impairments observed in neonatal rats after HI might be relevant to the reduced Furin and MMP-9 expression levels observed after 7 days. Evidence suggests that tPA upregulation could induce the conversion of early upregulated precursor BDNF into mature BDNF in the infarcted area to slow the injury process (Rahman et al., 2018), and the tPA-BDNF axis has been shown to play a critical role in cognitive functions (Obiang et al., 2011). We also noticed that PAI-1 was upregulated in the ipsilateral cortex and hippocampus of rats in the HI group 6 hours after HI injury. PAI-1 is known for inhibitory effects against tPA, which impairs the BDNF precursor cleavage required to generate mature BDNF (Thomas et al., 2016). Therefore, our results indicated that PAI-1 upregulation might suppress the proteolytic conversion of precursor BDNF into mature BDNF protein.
The BDNF-TrkB pathway in astrocytes and neurons has been demonstrated to play a protective role in the brain, especially in the cortex and striatum (Sheng et al., 2018). Similarly, the expression levels of TrkB were upregulated 6 hours after HI injury in the ipsilateral cortex and ipsilateral hippocampus of rats HI in the HI group compared with rats in the sham group. Previous studies indicated that increased BDNF levels induced the transient and continuous activation of TrkB receptors (Guo et al., 2018). Our findings suggested the significant upregulation of Sortilin in the ipsilateral cortex of rats in the HI group compared with rats in the sham group 6 hours after HI induction, after which Sortilin expression levels diminished and remained at lower levels. Sortilin promotes the intracellular trafficking of neurotrophins and induces neuronal damage when interacting with proneurotrophins (Vaegter et al., 2011). The later downregulation of Sortilin in the ipsilateral cortex observed in this study might promote post-HI injury neuronal plasticity through other neurotrophins.
Collectively, the interaction between BDNF and its related enzymes and receptors might play an important role in reducing neuronal damage and enhancing nerve regeneration, contributing to the pathogenesis and recovery from neonatal HI injury. However, the time-dependent change in protein levels for BDNF-related enzymes and receptors were not examined in this study, and the further interactive relationships among these factors were not investigated. A more in-depth exploration of the underlying mechanisms remains necessary in future studies.
Additional file:
Additional Table 1: Repeated measurement design analysis of variance of mRNA expression levels of BDNF and its-related enzymes and receptors in the cortex and hippocampus after hypoxia-ischemia.
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Giles L, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 82001604 (to LLX); the Joint Subject of Southwest Medical University and Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University of China, No. 2018XYLH-004 (to LLX); the National Construction Project of Regional Chinese Medicine Treatment Centre of China, No. 2018205 (to XB); the National Construction Project of the Second Clinical Research Base of Chinese Medicine of China, No. 2018131 (to XB). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study was approved by the Animal Ethics Committee of the University of South Australia (approval No. U12-18) on July 30, 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 82001604 (to LLX); the Joint Subject of Southwest Medical University and Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University of China, No. 2018XYLH-004 (to LLX); the National Construction Project of Regional Chinese Medicine Treatment Centre of China, No. 2018205 (to XB); the National Construction Project of the Second Clinical Research Base of Chinese Medicine of China, No. 2018131 (to XB).
References
- 1.Bae SH, Yoo MR, Kim YY, Hong IK, Kim MH, Lee SH, Kim DY. Brain-derived neurotrophic factor mediates macrophage migration inhibitory factor to protect neurons against oxygen-glucose deprivation. Neural Regen Res. 2020;15:1483–1489. doi: 10.4103/1673-5374.274340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull. 2012;38:444–456. doi: 10.1093/schbul/sbq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai H, Mu Z, Jiang Z, Wang Y, Yang GY, Zhang Z. Hypoxia-controlled matrix metalloproteinase-9 hyperexpression promotes behavioral recovery after ischemia. Neurosci Bull. 2015;31:550–560. doi: 10.1007/s12264-015-1533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Ma Y, Jiang L, Mu Z, Jiang Z, Chen X, Wang Y, Yang GY, Zhang Z. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther. 2017;25:1448–1459. doi: 10.1016/j.ymthe.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen A, Xiong LJ, Tong Y, Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep. 2013;1:167–176. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comelli MC, Seren MS, Guidolin D, Manev RM, Favaron M, Rimland JM, Canella R, Negro A, Manev H. Photochemical stroke and brain-derived neurotrophic factor (BDNF) mRNA expression. Neuroreport. 1992;3:473–476. doi: 10.1097/00001756-199206000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Ennaceur A, Hussain MD, Abuhamdah RM, Mostafa RM, Chazot PL. Slope climbing challenges, fear of heights, anxiety and time of the day. Behav Brain Res. 2017;316:169–182. doi: 10.1016/j.bbr.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Gunn AJ, Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb Clin Neurol. 2019;162:217–237. doi: 10.1016/B978-0-444-64029-1.00010-2. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, Nagappan G, Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev Neurobiol. 2018;78:647–659. doi: 10.1002/dneu.22592. [DOI] [PubMed] [Google Scholar]
- 10.Jiang LJ, Xu ZX, Wu MF, Dong GQ, Zhang LL, Gao JY, Feng CX, Feng X. Resatorvid protects against hypoxic-ischemic brain damage in neonatal rats. Neural Regen Res. 2020;15:1316–1325. doi: 10.4103/1673-5374.272615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XM, Rong H, Qian JY, Dong MX, Niu YC. Effect of maternal exposure to Curcumae Rhizoma during pregnancy on neurodevelopment and apoptosis mechanism in offspring. Zhongguo Zhong Yao Za Zhi. 2019;44:541–545. doi: 10.19540/j.cnki.cjcmm.20181012.004. [DOI] [PubMed] [Google Scholar]
- 13.Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66:222–229. doi: 10.1203/PDR.0b013e3181a9ef34. [DOI] [PubMed] [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Lopes CDF, Gonçalves NP, Gomes CP, Saraiva MJ, Pêgo AP. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials. 2017;121:83–96. doi: 10.1016/j.biomaterials.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Madinier A, Bertrand N, Rodier M, Quirié A, Mossiat C, Prigent-Tessier A, Marie C, Garnier P. Ipsilateral versus contralateral spontaneous post-stroke neuroplastic changes: involvement of BDNF. Neuroscience. 2013;231:169–181. doi: 10.1016/j.neuroscience.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 17.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 18.Millar LJ, Shi L, Hoerder-Suabedissen A, Molnár Z. Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front Cell Neurosci. 2017;11:78. doi: 10.3389/fncel.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, Sawada M, Sato J, Yamada K. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31:12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010a;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 21.Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H. Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem. 2010b;1:133–143. doi: 10.4331/wjbc.v1.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obiang P, Maubert E, Bardou I, Nicole O, Launay S, Bezin L, Vivien D, Agin V. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 2011;96:121–129. doi: 10.1016/j.nlm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain atlas in stereotaxic coordinates. San Diego: Academic; 1998. [Google Scholar]
- 24.Phan HD, Li J, Poi M, Nakanishi K. Quantification of miRNAs co-immunoprecipitated with argonaute proteins using SYBR green-based qRT-PCR. Methods Mol Biol. 2018;1680:29–40. doi: 10.1007/978-1-4939-7339-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19:299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- 26.Planas AM, Solé S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 27.Ragaeva DS, Tikhonova MA, Petrova OM, Igonina TN, Rozkova IN, Brusentsev EY, Amstislavskaya TG, Amstislavsky SY. Neonatal reflexes and behavior in hypertensive rats of ISIAH strain. Physiol Behav. 2017;175:22–30. doi: 10.1016/j.physbeh.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Rahman M, Luo H, Sims NR, Bobrovskaya L, Zhou XF. Investigation of mature BDNF and proBDNF signaling in a rat photothrombotic ischemic model. Neurochem Res. 2018;43:637–649. doi: 10.1007/s11064-017-2464-9. [DOI] [PubMed] [Google Scholar]
- 29.Rickhag M, Teilum M, Wieloch T. Rapid and long-term induction of effector immediate early genes (BDNF, Neuritin and Arc) in peri-infarct cortex and dentate gyrus after ischemic injury in rat brain. Brain Res. 2007;1151:203–210. doi: 10.1016/j.brainres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Sanches EF, van de Looij Y, Toulotte A, Sizonenko SV, Lei H. Mild neonatal brain hypoxia-ischemia in very immature rats causes long-term behavioral and cerebellar abnormalities at adulthood. Front Physiol. 2019;10:634. doi: 10.3389/fphys.2019.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savard A, Brochu ME, Chevin M, Guiraut C, Grbic D, Sébire G. Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia. J Neuroinflammation. 2015;12:111. doi: 10.1186/s12974-015-0330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng S, Huang J, Ren Y, Zhi F, Tian X, Wen G, Ding G, Xia TC, Hua F, Xia Y. Neuroprotection against hypoxic/ischemic injury: δ-opioid receptors and BDNF-TrkB pathway. Cell Physiol Biochem. 2018;47:302–315. doi: 10.1159/000489808. [DOI] [PubMed] [Google Scholar]
- 33.Sun LQ, Guo GL, Zhang S, Yang LL. Effects of microRNA-592-5p on hippocampal neuron injury following hypoxic-ischemic brain damage in neonatal mice - involvement of PGD2/DP and PTGDR. Cell Physiol Biochem. 2018;45:458–473. doi: 10.1159/000486923. [DOI] [PubMed] [Google Scholar]
- 34.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Thomas AX, Cruz Del Angel Y, Gonzalez MI, Carrel AJ, Carlsen J, Lam PM, Hempstead BL, Russek SJ, Brooks-Kayal AR. Rapid increases in proBDNF after pilocarpine-induced status epilepticus in mice are associated with reduced proBDNF cleavage machinery. eNeuro. 2016;3:ENEURO0020–00152016. doi: 10.1523/ENEURO.0020-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, Lewin GR, Willnow TE, Chao MV, Nykjaer A. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci. 2011;14:54–61. doi: 10.1038/nn.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH. Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immun. 2018;70:179–193. doi: 10.1016/j.bbi.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu B, Zhao L, Luo D, Xu D, Tan T, Dong Z, Tang Y, Min Z, Deng X, Sun F, Yan Z, Chen G. Furin promotes dendritic morphogenesis and learning and memory in transgenic mice. Cell Mol Life Sci. 2018;75:2473–2488. doi: 10.1007/s00018-017-2742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225–238. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]