Abstract
Spinal cord injury has long been a prominent challenge in the trauma repair process. Spinal cord injury is a research hotspot by virtue of its difficulty to treat and its escalating morbidity. Furthermore, spinal cord injury has a long period of disease progression and leads to complications that exert a lot of mental and economic pressure on patients. There are currently a large number of therapeutic strategies for treating spinal cord injury, which range from pharmacological and surgical methods to cell therapy and rehabilitation training. All of these strategies have positive effects in the course of spinal cord injury treatment. This review mainly discusses the problems regarding stem cell therapy for spinal cord injury, including the characteristics and action modes of all relevant cell types. Induced pluripotent stem cells, which represent a special kind of stem cell population, have gained impetus in cell therapy development because of a range of advantages. Induced pluripotent stem cells can be developed into the precursor cells of each neural cell type at the site of spinal cord injury, and have great potential for application in spinal cord injury therapy.
Keywords: axon regeneration, cell therapy, functional recovery, induced pluripotent stem cell, mesenchymal stem cell, neural cells, neural precursor cell, neural stem cell, remyelination, spinal cord injury, stem cells
Introduction
Spinal cord injury (SCI) causes severe damage because the nerves inside the lesion area are severed, meaning that signals cannot pass to the brain or spinal center, resulting in motor impairments. Worldwide, there are currently more than 27 million people living with chronic motor dysfunction following SCI; 90% are the result of traumatic injury and just 10% are the result of secondary injuries from other diseases (Bradbury and Burnside, 2019). Traumatic SCI can be classified as cervical vertebrae injury, thoracic vertebrae injury, or lumbar vertebrae injury. Of these, cervical vertebrae injuries have a death rate that is two times higher than the other two types, likely because high cervical SCIs occur near the respiratory and heart center of the medulla oblongata (Selvarajah et al., 2014). SCIs have three main causes: accidents, falling, and trauma caused by large objects. In the past, accidents were viewed as the most common cause, but a recent report concluded that falling is at the top of the list of factors that cause SCI (Selvarajah et al., 2014). SCI results in enormous damage to both families and societies. For example, in the USA, each patient with SCI costs between 1.1 and 4.6 million dollars over their lifetime (Ahuja et al., 2017). In addition, other pressures, such as the inability to stand, and urinary or fecal incontinence, are a cause of pain for these patients. All of these challenges can ultimately become physical and psychological problems for patients themselves, as well as for the entire country.
The process of SCI includes several stages: physical contusion, primary injury, secondary injury, and eventually chronic SCI with the maturity of glial scars (Tran et al., 2018). The primary injury begins with an inability to move the body autonomously. With the loss of connections between the injured spinal cord and the higher central nervous system (CNS), blood vessels below the injured plane dilate, and blood pressure drops. Because of the low blood pressure and ischemia, many cells begin to die via necrosis and apoptosis. A series of reactions, involving free radical and excitotoxic neuron generation, iron imbalance, and cell membrane lipid peroxidation, occur in the lesion microenvironment within several hours of the injury, and are accompanied by larger scale expansion of the lesion area. The secondary injury of SCI is thus primed as a more serious stage than the first one. Oligodendrocytes are a type of cell that are located in neuronal tissue, lying side by side between nerve fibers to wrap axons, form insulating myelin sheath structures, and assist the efficient transmission of bioelectrical signals. A decrease in oligodendrocytes and oligodendrocyte precursor cells (OPCs) continues for 3 weeks after SCI, and leads to the complete demyelination of neurons around lesion sites (Paschon et al., 2019). Another phenomenon that occurs in SCI is the secretion by microglia of injury signals, including DNA, RNA, and proteins, which are known as molecular patterns associated with post-traumatic injury (Paschon et al., 2019). This secretion results in the attraction of hypertrophic astrocytes, activated by the inflammatory environment, to the lesion boundary (Bradbury and Burnside, 2019). Over time, an astroglial scar slowly forms under a strong extracellular matrix chondroitin sulfate proteoglycan (CSPG) promoter. If this process progresses, it eventually forms a large function-free cavity, which fails to communicate with the surrounding nerves and results in permanent motor dysfunction (Figure 1).
Figure 1.

Adverse niche for regeneration and the roles of transplanted cells in SCI.
This figure depicts the phenomenon of vascular damage, ischemia, tissue edema, and neuron interruption after SCI. It also shows subsequent second injury, including inflammatory factor/cell aggregation, demyelination, and glial scar and cystic cavity formation. The grafted NSCs/NPCs differentiate into oligodendrocytes, astrocytes, and neurons to play roles in remyelination, the release of neuronal factors, and neural circuit reconstruction, respectively. The grafted MSCs play anti-inflammatory/apoptotic roles, and both transplanted MSCs and OECs provide nutritional support for axonal regeneration. BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; CSPG: chondroitin sulfate proteoglycan; HGF: hepatocyte growth factor; MSC: mesenchymal stem cell; NGF: neural growth factor; NSC/NPC: neural stem/precursor cell; OEC: olfactory ensheathing cell; OPC; oligodendrocyte precursor cell; SCI: spinal cord injury; VEGF: vascular endothelial growth factor.
Currently, SCI remains one of the most challenging complications in the clinic. As well as routine therapeutic strategies, such as pharmacological, surgical, and rehabilitation treatments, cell-based therapies for SCI are also growing rapidly, and progressing from bench to bedside. The number of studies regarding cell therapy for SCI has increased continually from the 1980s, especially in the years after 2000, as observed when we retrieved literature through PubMed using the keywords “spinal cord injury” and “cell therapy” (Figure 2). For decades, the effects of various cell types (including different kinds of stem cells and stem cell-derived oligodendrocytes or neurons) on functional recovery in SCI have been explored. Here, we review the history, progression, limitations, challenges, and perspectives of cell-based therapies in the treatment of SCI, to provide further understanding and references for future cell-based therapeutic alternatives.
Figure 2.

The development process of cell-based SCI therapies.
This figure shows many of the cells covered in the development process of cell-based therapies for SCI. From 1986 to 2006, Schwann cells, OECs, ESCs, NSCs, and MSCs have all been explored for SCI treatment and received more or less satisfactory outcomes. From 2006 to 2019, with the rise and development of IPS technology and IPS-derived cell types have been welcomed. CNS: Central nervous system; IPS: induced pluripotent stem cell; ESC: embryonic stem cell; MSC: mesenchymal stem cell; NSC/NPC: neural stem/precursor cell; OEC: olfactory ensheathing cell; SCI: spinal cord injury.
Search Strategy and Selection Criteria
For the “Cell types involved in SCI” section of this review, a PubMed search for papers published up to 2019 was performed with the following terms: (spinal cord injury [Text Word]) AND (traumatic [Text Word]), which returned 5179 results. For the “Development of cell therapies” section, we searched papers in PubMed using the terms (spinal cord injury [Text Word]) AND (transplantation [Text Word]), and 2510 results were displayed. We then retrieved 62 results using “clinical trial” as the search term selection for the third section of this review, “Clinical trials of cell-based SCI therapies”. For the last section, “IPS technology is a promising alternative treatment for SCI”, we reviewed the 4121 results of a PubMed search for “induced pluripotent stem cell [Title/Abstract]” up to 2019.
Cell Types Involved in SCI
Nerve rebuilding depends on sufficient support and nutrients, which are usually offered by the intracellular matrix and astrocyte-mediated nutrient uptake. The best way to promote motor function recovery requires the regeneration of both dead and damaged nerves and cells. However, neural growth factor deficiencies, myelination inhibitors, the glial scar, and the cystic cavity constitute a barrier to neural tissue self-repair that cannot be dealt with using traditional methods (Guo et al., 2019). It is therefore believed that the creation of an appropriate environment for cell-based therapies in SCI treatment is essential to induce cell proliferation, axon sprouting, and remyelination. In cell-based therapies, specific cells are transplanted into the lesion sites, but they often struggle to survive in an unfavorable regenerative microenvironment. These exogenous cells must then accommodate themselves in the new environment and participate in the activity of the existing cells (Ahuja et al., 2017). To make transplanted cells work better, a basic step is to understand the concrete function of the cells that are involved in SCI.
Neurons
Neurons are responsible for passing information to any corner of the body by means of electric signals. This process relies on tight junctions between neurons as well as axonal integrity. In SCI, patients are unable to initiate voluntary movement below the transected section. Unfortunately, it is very difficult to repair the nervous system, especially the CNS (Bradbury and Burnside, 2019). Furthermore, nerve regeneration abilities decrease with age. Anderson et al. (2018) concluded that three factors are partially or totally responsible for the failure of neurons to regenerate in adults: neuronal intrinsic growth ability, the supportive matrix, and chemical attraction. Cooperation between these factors is an important contributor to successful neuronal regeneration. Previous studies have reported that neuronal growth requires the proper impetus; the course of neuronal growth only runs smoothly when negative factors are discarded and positive factors are involved. One group recently reported that V2a interneurons can be induced from pluripotent cells (Butts et al., 2019) and are able to alleviate neurogenic disease and function in the CNS. From this, we can conclude that lost neurons are able to be compensated for by neurons derived from endogenous or exogenous cells with stemness traits. Additionally, many other types of neurons need to be further studied for nerve growth in SCI repair. Neurons are vital for signal transmission; thus, ensuring their quantity and quality is very important.
Astrocytes
Astrocytes normally regulate neurotransmitters and neurovascular dynamics, and maintain the consecutive delivery of stable neural signals together with neurons (Gaudet and Fonken, 2018). However, their fate can be diverted toward repairing the wound after traumatic SCI. To respond to severe contusion conditions, both naïve and active astrocytes quickly proliferate and migrate to the injury environment with the aim of filling the gap, eventually causing an astrocytic scar. This scar is a double-edged sword, making it a hot topic in SCI research. On the one hand, the astrocytic scar limits the amount of toxic factors spreading from the lesion epicenter, thus depressing inflammation expansion and secondary injury; on the other hand, the astrocytic scar shuts out axons and trophic factors, eventually contributing to the failure of damaged neurons to reconnect (Gaudet and Fonken, 2018). Recent reports agree that the advantages of the astrocytic scar are greater than the disadvantages, and suggest that it helps rather than restrains neuronal regrowth (Anderson et al., 2016). The rigid scar is surprisingly thin and is surrounded by a cluster of residual glial cells; these cells are active and can continue with nerve circuit recombination and synapse turnover, depending on their primitive structure and function (O’Shea et al., 2017). What is the role of the glial scar? It has been reported that a kind of extracellular protein deposits on the scar border when astrocytes start to assemble. This protein is CSPG (also known as neuron glial antigen 2; or NG2). CSPG is a nerve growth inhibitor that accumulates on the first day after traumatic SCI and remains there indefinitely (Gaudet and Fonken, 2018). In SCI treatments, it might therefore be useful to target CSPG rather than try to remove the protective glial scar. To do this, we need to understand the mechanisms of CSPG formation and prohibit its initiation (Tran et al., 2018). Interestingly, glial scars may be reversible, because reactive astrocytes in SCI revert to a naïve state after being transplanted to a healthy spine (Hara et al., 2017). That is, the role of astrocytes is reflected in glial scar formation during the wound healing process. We can therefore conclude that in future research, researchers will move away from trying to remove the glial scar or reactive astrocytes, and instead investigate how to make the scar border more penetrable for surrounding cells or extracellular matrix proteins.
Oligodendrocytes and OPCs
An intact neuron cannot function without the myelination of its axons by oligodendrocytes in the CNS and by Schwann cells in the peripheral nervous system (PNS). In the CNS, each oligodendrocyte produces up to 50 myelin sheaths that wrap axons so that neurons can be separated to promote fast action potential propagation. In contrast, in the PNS, each Schwann cell produces just one myelin sheath (Fu et al., 2019). The existence of the myelin sheath is critical for the fluent execution of function. Olfactory ensheathing cells (OECs) are functionally a mix between oligodendrocytes and Schwann cells; they have myelinating and neurotrophic functions, and play an opposite role to astrocytes by inhibiting glial scar formation. Furthermore, OECs are one of the rare renewable cell types in the CNS, and offer an appropriate environment for axon generation and migration, gradually becoming grafted cells for neural regrowth. Oligodendrocytes are the offspring of OPCs; if the former dies when encountering acute SCI, the latter tends to swiftly proliferate to compensate for the marked loss of oligodendrocytes. It is estimated that oligodendrocyte death continues from 15 minutes to 3 weeks after acute SCI, with a toll of up to 93% compared with 50% of OPCs (Gaudet and Fonken, 2018). In addition, damaged oligodendrocytes release large amounts of myelin growth antagonists, such as Nogo, tenascin, myelin-based glycoprotein, and oligodendrocyte-myelin glycoprotein (Willerth and Sakiyama-Elbert, 2008), which pose another obstacle for nervous system self-repair. At the time of secondary injury, the use of 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid, a pharmacological blocker of voltage-dependent anion-selective channel 1 oligomerization, can decrease the oligodendrocyte death rate and lesion size, and increase neuron density and motor function recovery (Paschon et al., 2019). Many researchers have noted that OPCs that are latent in the glial scar border have a potential correlation with CSPG; these OPCs express CSPG protein on the cell surface and are named OPC-CSPGs. The remyelination ability of OPCs is weakened as a result of CSPG attachment (Tran et al., 2018). In the PNS, Schwann cells, with similar abilities to oligodendrocytes, may be useful in SCI treatment. Although their migration capability is limited and they fail to integrate with host astrocytes when transplanted into the body, these limitations can be resolved by the magnetic modification of Schwann cells (Huang et al., 2017). Thus, oligodendrocytes, OPCs, and Schwann cells maintain spinal myelination at all times and help with remyelination after axonal injury. For cell therapies, all three cell types may be appropriate for grafting.
Microglia
In the nervous system, microglia are a family of cells characterized as macrophages in the immune system. These cells normally patrol the nervous system scanning for infections and lesions, and act as guardians of immunity. After SCI, they can foster nerve rebirth; however, they can also become overactivated, leading to cytotoxicity (Gaudet and Fonken, 2018). Spinal lesions lead to blood-spinal imperfections, macrophages and inflammatory molecules receive signs of the aforementioned extracellular injury-associated molecular patterns, and a sequence of intricate reactions then arises. However, we cannot overlook these invasive macrophages, because they are key members of the innate immune system. Taking advantage of internal signaling pathways, they can alter microglia and astrocytes, and lead to further alterations of neuroinflammation in traumatic SCI and some neurodegenerative diseases (e.g., Alzheimer’s disease) (Andreasson et al., 2016). In addition, there is a balance between macrophage subtypes that is disrupted by SCI; SCI affects macrophage excitation, spurring a pro-inflammation class switch that potentiates a prolonged inflammation response (Gensel and Zhang, 2015). The immune response is accelerated by morphological and proteomic alterations of microglia under mutual impacts with astrocytes, which also fosters the secretion of inflammatory factors (Paschon et al., 2019). Hence, a cellular network exists in SCI recovery, and each cell type has a unique identity and cannot be replaced by any other. If we want to treat SCI using cell transplantation, we therefore need to understand the interactions among all neural cells, rather than just eliminating or enriching one cell group.
Development of Cell Therapies
We have summarized some animal trials that have tried cell transplantation for SCI therapy (Table 1). Graft candidates have been tested using diverse cell introduction methods, including in situ injection, intranasal delivery, and cerebrospinal fluid transmission (Satake et al., 2004; Guo et al., 2019), through which implanted cells can survive and transfer to the injured site to execute their functions. In the mouse, rat, dog, pig, and monkey, cell transplantation has been reported to provide a favorable environment for neurogenesis and functional recovery. Current methods used to track progress after cell transplantation include survival time, differentiation ability, expression of neural markers, axon remyelination, neuronal regeneration, and an increase in locomotive Basso-Beattie-Bresnahan scores. In future research, newer and more convincing criteria need to be adopted to provide more precise and reliable information for SCI patients. Next, we summarize the characteristics and action modes of all cell types appropriate for SCI repair.
Table 1.
Cell types tested in animal SCI models
| Cell type | Model | Injury type | Transplantation time after injury | Cell survival time | Results | References |
|---|---|---|---|---|---|---|
| AD-MSC | Rat | Traumatic SCI | 2 wk | 2 mon | Locomotor restoration; cavitation reduction; modulation of microglial and astroglial activation | Mukhamedshina et al. (2019) |
| Pig | 6 wk | 16 wk | Partial somatosensory restoration; cavitation reduction; modulation of astroglial activation | |||
| UC-MSC; BM-MSC | Rat | SCI | 1 wk | 8 wk | Improvement of functional recovery, allodynia, hyperalgesia; alleviation of neuropathic pain | Yousefifard et al. (2016) |
| MSC; NCS | Mice | Thoracic contusive SCI | Immediately | ≥ 4 wk | Motor recovery; modulation of lesion inflammation environment; a slight tissue sparing | Neirinckx et al. (2015) |
| NPC | Rat | Cervical SCI | 2 wk | Not mentioned | Astrogliosis reduction; cervical conduction and physiological improvement; forelimb function improvement | Wilcox et al. (2014) |
| NPC | Rat | Cervical SCI | 1 wk | 8 wk | Respiratory motor recovery | Sandhu et al. (2017) |
| Ips-derived OPCriched NPC/NSC | Mice | Contusive SCI | 9 d | 12 wk | Mature oligodendrocytes differentiation; axonal growth and synapse formation; remyelination; function recovery | Kawabata et al. (2016) |
| GRP | Rat | Cervical semisection | Immediately | ≥ 5 wk | Astrocyte differentiation; recovery of diaphragm electromyography; regeneration of injured axons | Goulao et al. (2019) |
| Astrocyte | Rat; mice | Cervical SCI | Immediately | ≥ 4 wk | Lesion size reduction; diaphragm function preservation | Li et al. (2015) |
| GRP/GDA | Rat | Thoracic SCI | 9 d | ≥ 8 wk | Spared white matter increase; lesion size decrease; anatomical and locomotion recovery | Fan et al. (2013) |
| AST-OPC | Nude rat | Cervical SCI | 1 wk | ≥ 9 mon | Motor behavioral recovery; parenchymal cavitation suppression; no teratoma and tumor formation | Manley et al. (2017) |
| ESC-derived OPC | Rat | Thoracic SCI | 1 wk | 28 d | Motor and sensory recovery; mechanical allodynia relief | Yang et al. (2018) |
| Schwann cell | Rat | Thoracic SCI | Not mentioned | Not mentioned | Improved hindlimb movement; axons elongation | Williams et al. (2015) |
| Rat | Thoracic contusive SCI | 1 wk | Not mentioned | Innate immune cell phenotype alteration | Pearse et al. (2018) | |
| Microglia | Rat | Thoracic SCI | Immediately | ≥ 1 mon | Motor function recovery | Akhmetzyanova et al. (2018) |
| OEC | Rat | Complete thoracic SCI | Immediately | 4 wk | Promote scaffold formation in the lesion site; axon regeneration; neuron preservation | Khankan et al. (2016) |
| OEC | Dog | Thoracolumbar SCI | ≥ 3 mon | ≥ 6 mon | No adverse finding; small improvement in light touch and pin prick sensitivity | Mackay-Sim et al. (2008) |
| IPS-NPCs | Rat | Moderate contusion SCI | 24 h | 2 mon | Mature oligodendrocytes differentiation; a significant increase in the number of myelinated axons; nearly a 5-fold reduction in cavity size and reduced glial scarring | All et al. (2015) |
| IPS-NSCs | Mice | Thoracic SCI | 1 wk | 2 mon | Successful integration; predominant oligodendrocytes differentiation; significant locomotor function improvement | Salewski et al. (2015) |
AD-MSC: Adipose-derived MSC; AST-OPC: astrocyte-restricted OPC; BM-MSC: bone marrow-derived MSC; GDA: glial-restricted precursor-derived astrocyte; GRP: glial-restricted precursor; IPS-NPC: induced pluripotent stem-neural precursor cell; MSC: mesenchymal stem cell; NPC: neural precursor cell; NSC: neural stem cell; OEC: olfactory epithelial cell; SCI: spinal cord injury; UC-MSC: umbilical cord-derived MSC.
Fetal spinal cord tissue
Unlike most cancers, SCI may be not lethal, but it can cause chronic, severe physical disability. At first, routine therapy was the major focus—the idea of transplantation treatment did not appear until scientists became aware of the need to repair the injured nervous system. In 1986, mouse fetal spinal cord tissue was first used as a graft object to transplant into an injured mouse for functional observations (Reier et al., 1986), and a good recovery outcome was achieved. Thus, replacing one damaged tissue with a similar intact counterpart appeared to be a good idea. Subsequently, scientists again implanted embryonic spinal cords into both newborn and adult SCI models. The recovery outcomes indicated that spinal cord transplantation indeed ameliorated lesion-induced functional deficits and decreased the severity of hindquarter lesions (Bregman et al., 1993). However, although the recovery effects of this treatment are clear, it requires fetal spinal cord tissue extraction, which is unfeasible due to ethical considerations and limited source. Nevertheless, based on these ground-breaking transplantation results, cell-based transplantation research began to arise for the treatment of SCI.
Schwann cells and neurons
The ability of Schwann cells to myelinate neurons in the PNS drew attention to their possible use in implantation for SCI repair. Kuhlengel et al. (1990) injected Schwann cells from the PNS into the lesion site of spinal cord models and reported that these surviving Schwann cells packaged neuronal axons and could form basilar membranes. This study demonstrates that Schwann cells can also play an axon ensheathing role in the CNS. Research supporting this conclusion was published the following year by Paino and Bunge (1991). In the same year, embryonic motor neurons were reported to successfully survive and migrate into the host ventral horn to replace depleted neurons (Clowry et al., 1991). These results also indicate that co-transplantation of neurons and Schwann cells after in vitro culturing may improve the performance of Schwann cells. However, the source of these cells is rather limited, because they are highly differentiated and can only be induced from stem cells. Hence, more stem cells with the ability to form functional cells need to be exploited.
Olfactory ensheathing cells
OECs are currently popular in cell transplantation because of their links with nerve cells. For example, they promote neurite growth without visible graft-related complications (Ahuja et al., 2017). Research relating to SCI treatment using OECs began in 1995, when Doucette recognized that OECs expressed many phenotypic features resembling astrocytes and Schwann cells. In addition, OECs survived to facilitate axonal growth after spinal cord implantation, thus demonstrating the promising therapeutic potential of OECs (Doucette, 1995). In support of this idea, OECs were reported to regenerate the inactive rat tail accompanied by the growth of lesioned axons after being introduced to an acute SCI section (Li et al., 1997). Furthermore, the use of biological tracer technology revealed that OECs with delayed transplantation, at 8 weeks post injury, settled and induced cortical axon regeneration and traveled approximately 10 mm, crossing the transplant bridge (Feron et al., 2005). Therefore, for migration and proliferation, OECs transplanted at both acute and chronic time points can promote neuronal and axonal regrowth. This indicates a relatively large time window for cell implantation, and dispels any misgivings that the acute phase is too transient for cell preparation.
Embryonic stem cells
Embryonic stem cells (ESCs) are popular in the regenerative medicine community for their properties of self-renewal, rapid proliferation, and multi-differentiation. The tendency of OECs to differentiate into nervous system cells was confirmed as early as 1999, with the discovery of oligodendrocyte and astrocyte precursors in OEC medium (Brustle et al., 1999). These precursor cells had successful intercellular communication and could myelinate neurons, which initiated research into ESC transplantation for SCI treatment. The first project appraising the functional recovery promotion of ESCs was performed by McDonald et al. (1999), who reported oligodendrocyte formation at the site of the ESC graft. Nevertheless, ESC grafts will not achieve clinical use until their latent oncogenesis can be completely eliminated. One way to overcome this barrier may be to guide ESCs toward oligodendrocyte or oligosphere formation in vitro, because these pre-differentiated cell types show identical myelin regeneration functions to ESCs when implanted into the lesion site (Liu et al., 2000). Trials in mice indicated that, when implanted at ~10 days after SCI, ESCs could differentiate into astrocytes, oligodendrocytes, and neurons over several weeks, and improved locomotor scores during migration away from the injection site. However, these consequences did not occur if transplantation was delayed up to 10 months after SCI (Keirstead et al., 2005). Therefore, although cell grafts can be performed in both the acute and chronic phases, recovery outcomes remain time-dependent. Furthermore, the appearance of factor-secreting ESCs can markedly improve therapeutic effects. They offer a variety of neural growth and trophic factors, chemokines, and specific proteins to assist the robust growth of neural cells (Chen et al., 2005). Importantly, human-derived ESCs have been demonstrated to be safe (Shroff and Barthakur, 2015), and are effective in the adult SCI rat. For human-derived ESC implantation, the successful differentiation and enhancement of locomotor performance, combined with a lack of toxicity, neurodynia, tumors, or other adverse observations, supports the initiation of phase I complete SCI clinical trials (Manley et al., 2017). Nonetheless, the source of human-derived ESCs is confined to newly formed embryos aborted by pregnant women, and animals must be killed to extract embryos for animal-derived ESCs. These cell acquisition methods have important ethical considerations, meaning that it is difficult for ESCs to be used clinically for human application.
Neural stem and precursor cells
Neural precursor cells (NPCs) and neural stem cells (NSCs) are currently the two cells with the most potential for SCI therapy because they can differentiate into all cell types in the nervous system, including astrocytes, oligodendrocytes, and OPCs. This intrinsic neural lineage differentiation trait makes them potential candidates for CNS cell transplantation. It was commonly believed that NSCs are a cell type that cannot be regenerated and that their number gradually decreases with age in the CNS; this contributed to the traditional idea of exogenous graft targeting for neural regeneration after CNS injury. However, Johansson et al. (1999) reported that endogenous ependymal cells, which were later demonstrated to be NPCs, could foster axon regeneration by self-proliferation and nestin expression. Remarkably, this phenomenon only appears after SCI, and is not present under normal conditions (Namiki and Tator, 1999). This discovery resulted in the idea of NSC and NPC transplantation. During implanted NSC differentiation in vivo, these cells mainly become astrocytes (in contrast to rare neurons and oligodendrocytes) after survival, which may be an adverse reaction in the case of axon regeneration (Vroemen et al., 2003). This tendency was able to be reversed by the use of valproic acid, a histone deacetylase inhibitor, so that the differentiation balance was inclined to neurons rather than astrocytes (Zhu et al., 2018). Moreover, other methods can be used in combination to enhance the functional efficiency. For instance, NPCs co-grafted with fibroblasts lead to significantly enhanced functional outcomes, because fibroblasts provide a mesenchymal platform for cystic cavity restoration and NPC adhesion and differentiation (Pfeifer et al., 2004). Moreover, Suzuki et al. (2017) reported that 1 week of chondroitinase ABC administration contributed to marked improvements in grafted NPC survival and differentiation. To rule out the tumorigenesis potential of NSCs and NPCs, they are generally transplanted into immunodeficient animals for long-term observation. In most contused cervical or lumbar animal spinal cords, grafted NSCs/NPCs can survive for 2–8 months or more without tumor detection, and differentiate into three cell lines (astrocytes, oligodendrocytes, and neurons), reduce cell apoptosis, and increase function as measured by the Basso-Beattie-Bresnahan score (Jin et al., 2016; Sankavaram et al., 2019; Zhao et al., 2019). Some human NPCs/NSCs have also been used in rat, pig, and monkey SCI models in preclinical trials. These produced neural-specific markers and improved locomotion, indicating that clinical trials should be conducted (Rosenzweig et al., 2018; Kutikov et al., 2019). The infiltration of numerous inflammatory factors, oxidative products, pro-apoptotic factors, and many other hostile surrounding elements all contribute to acute transplantation being ineffective, while a longer time in the chronic state leads to an amplified cystic cavity and glial scar, so a 9-day delayed operation appears to be the most appropriate (Okano, 2002). Before investigating further therapeutic improvements and possible human applications, efforts must be made to elaborate the underlying mechanisms of NSC/NPC activation, sustainability, and subsequent mitosis, migration, and maturation.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) have a reputation for versatility and have been successful in recent cell research. Moreover, they have exhibited many benefits in clinical application. Their immune modulation properties have been used to alleviate immunological rejection and simultaneously regulate the immune microenvironment, and their multiple lineage potencies have been used to induce many kinds of cells for cancer therapy (Podesta et al., 2019). MSCs have also received much scientific attention for their reported anti-inflammation/apoptosis and cytokine-releasing behaviors in the injured spinal cord. In 2000, adult rat-/human-derived MSCs were found to express nestin and tropomyosin receptor kinase A and present neuron-like phenotypes during more than 20 passages in vitro (Woodbury et al., 2000). This finding indicates that MSCs can break germ layer commitment to develop a neural cell fate. In accordance with this idea, in the same year, researchers transplanted MSCs into the CNS to treat middle cerebral artery occlusion and reported positive results (Chen et al., 2000). Together, these findings suggest that MSCs are promising cell candidates for SCI transplantation therapy. Unlike many other stem cell types, they have extensive sources, such as bone marrow, umbilical cord, and adipose tissue. Moreover, their acquisition methods are also simple and ethical and the culture process is easy. Experiments using rat SCI models have indicated that MSCs can display weak NeuN immunoreactivity at 5 weeks, and can also establish a nerve fiber-permeable bridge across debris, which possibly occurs via a cue from the internal lesion microenvironment (Hofstetter et al., 2002). However, MSCs do not appear to transdifferentiate into true neural cells in vivo, although they can migrate to the spinal cord and survive for a long time (Castro et al., 2002; Jendelova et al., 2004). In contrast, when MSCs are cultured in medium and cAMP is added, they are easily diverted to become neural cell progenitors (Deng et al., 2001). In brief, the aim for MSC transplantation is not only to induce them to become functional neural cells, but also to take advantage of their role in environment modulation to promote axonal growth, secrete chemokines and growth factors, and suppress the adverse impacts of inflammation (Parr et al., 2007). Furthermore, MSCs are a self-derived and immune rejection-preventable implantation choice. MSCs are also advantageous in that they evade ethical and moral issues, and are able to overcome the difficult cellular access problems of the previously listed stem cell types. They also avoid the lineage limitation problems of terminally differentiated cells. A recent study reported that MSC-derived exosomes can freely penetrate the blood-brain barrier equipped with phosphatase and tensin homolog (PTEN) small interfering RNA (siRNA) to silence PTEN, an intrinsic inhibitor of axonal growth (Guo et al., 2019). MSCs may be able to cure complete SCI, relying on a neurite sprouting phenomenon in vitro and a functional recovery phenomenon in vivo. The proper graft time is also important with regards to the beneficial conditions for implanted cells in the later SCI period—after inflammation, oxidative reactions, and the release of lyases (Hofstetter et al., 2002).
Other cell types
Preclinical trials of cell therapies using astrocytes and microglia are scarce; they are often used as co-grafting objects to protect cells and proteins associated with axon myelination and growth, provide neurotrophic factors and molecules for tissue repair, and modulate the immune environment (Nicaise et al., 2015). Hematopoietic stem cells are a kind of stem cell that differs from the neural lineage. However, in spite of this discrepancy, hematopoietic stem cells can still divert to neural cells and foster functional recovery after being transplanted into SCI mice (Koshizuka et al., 2004). Although research into this method is rare, it is indeed an interesting phenomenon, and further suggests that we need to explore the mechanisms involved in grafted cell behaviors in the injured host. Moreover, fibroblasts as mesenchymal cells can play an indispensable role in SCI treatment if they undergo gene modification. Pizzi and Crowe (2006) reported that matrix metalloproteinase-3-overexpressing fibroblasts might degrade extracellular CSPGs to help with neuron junctions, but this function depends on their co-transplantation with neural cells, because fibroblast-only transplantation is ineffective. Many studies have been conducted using genetically engineered cell options. Genetically altered cells can excrete large quantities of neurotrophic factors, such as neurotrophin 3, nerve growth factor, basic fibroblast growth factor, brain-derived neurotrophic factor, and myelin gene regulator factor, which have benefits for myelination, axon regeneration, and the recovery of locomotor function (Grill et al., 1997; Pizzi and Crowe, 2006). These findings indicate that simultaneous implantation might be a promising option, given that one cell type can supply a growth platform and sufficient nutrients for another cell type (Willerth and Sakiyama-Elbert, 2008). Cooperation between two different lineage cells may therefore create good therapeutic outcomes. However, there are a large number of harmful features opposed to normal axon elongation, neuron self-repair, and neural network shaping between implanted cells and local neurons. Therefore, in forthcoming studies, proper cell candidates, antagonists of axonal growth inhibitors, and other related strategies are expected to be discussed. For example, the use of antibodies and gene knockouts are promising means of removing undesired elements.
Clinical Trials of Cell-Based Spinal Cord Injury Therapies
A recent review has summarized recent clinical trials of stem cell-based therapies for SCI patients (Silvestro et al., 2020), and concluded that NSCs and MSCs have been the main focus and have yielded relatively satisfactory results. Here, we have also summarized several clinical trials for each possible cell type that is involved in clinical trials (Table 2). The first instance of treating SCI patients with human ESC implantation, after other therapies had been trialed, was performed in 2016 (Shroff, 2016). Despite the favorable outcomes, reported safety, advantages of rapid proliferation, and convenient genetic manipulation and induction, transforming ESCs for universal clinical application remains a huge ethical and moral challenge. Both autologous and exogenous MSC grafts have been performed in various kinds of human SCI patients, and have been traced for at least 6 months to confirm that there are no transplantation-related adverse effects. The involved female or male human receptors led to different effects on sensitivity, neurogenic bowel/bladder dysfunction, or sexual damage (Vaquero et al., 2016, 2018a, b). However, limited therapeutic consequences were reported in a phase III clinical trial, in which only 2 of the 16 patients had obvious neurological status improvement without any uncomfortable feelings about transplantation (Oh et al., 2016). In contrast, other positive trials were sufficient to suggest the safety, feasibility, and practicability of MSC implantation in the human spinal cord. In 2008, Erik Curtis reported the first instance of spinal cord-derived autologous NSC treatment, in which two of the four patients with T2–12 SCI displayed one to two levels of neurological improvement without any adverse events after 1.5–2.5 years (Curtis et al., 2018). Interestingly, the implanted autologous hematopoietic stem cells and Schwann cells all survived and infused into the injured tissue. Hematopoietic stem cells affect motor evoked potentials or somatosensory evoked potentials, whereas Schwann cells improve slight neuropathic pain or muscle spasticity (Frolov and Bryukhovetskiy, 2012; Anderson et al., 2017). Previous studies have demonstrated that there are no or very few side effects of human cell transplantation, and cell therapy to treat SCI is very feasible. In future clinical trials, further adverse effects and more safety data are expected to be reported. Simultaneously, the development of optimal personalized injections will need to cover cell dosage, delivery methods, injection times, and auxiliary medicine according to each patient’s condition. Thus, understanding the latent restorative mechanisms of diverse cell therapies is of critical significance.
Table 2.
Cell types applied in SCI clinic trials
| Cell type and source, design type of studies | Patient age (yr) | SCI type and level | Transplantation time after injury | Cell survival time | Results | References |
|---|---|---|---|---|---|---|
| BM-MSC; autologous; phase I | 18–65 | Chronic traumatic thoracic or lumbar SCI (A) | > 6 mon | ≥ 6 mon | Gain low limbs motor function; sacral sparing; urologic function improvement; low-intensity pain at the lesion site | Mendonca et al. (2014) |
| 28–62 | Cervical, dorsal or dorsolumbar (A,B,C) | 13.65±14.79 yr | Not mentioned | Variety clinical improvement in sensitivity, motor power, spasms, spasticity, neuropathic pain, sexual function and sphincter dysfunction | Vaquero et al. (2018a) | |
| UC-MSC; xenogenous; phase II | 19–57 | Thoracolumbar (A) | 12–50 mon | Not mentioned | Significant and stable improvement in movement, self-care ability, muscular tension | Cheng et al. (2014) |
| NSC/NPC; xenogenous; phase I/IIa | 18–60 | Sensor&motor complete and sensor incomplete cervical SCI (A,B) | Four groups: acute (< 1 wk), early subacute (1–8 wk), late subacute (9 wk–6 mon), and chronic (> 6 mon) | 1 yr | Improvements of increased motor scores, motor levels recovery, responses to electrophysiological | Shin et al. (2015) |
| NSC; xenogenous; phase I | 25–35 | Complete thoracic SCI (A) | 1 yr | 18–27 mon | Neurological improvement; high-density human axonal sprouting | Curtis et al. (2018) |
| OEC; autologous; phase II | 18–55 | Thoracic SCI (A) | 6 mon–3 yr | 3 yr | Significant and stable improvement in movement, self-care ability, and muscular tension; only one patient presented radiating neuralgia | Cheng et al. (2014) |
BM-MSC: Bone marrow-derived MSC; MSC: mesenchymal stem cell; NPC: neural precursor cell; NSC: neural stem cell; OEC: olfactory epithelial cell; SCI: spinal cord injury; UC-MSC: umbilical cord-derived MSC.
Induced Pluripotent Stem Cell Technology Is a Promising Alternative Treatment for Spinal Cord Injury
Induced pluripotent stem cell (IPS) technology, also called cell reprogramming, came about through the efforts of Shinya Yamanaka in 2006 and John B. Gurdon in 1962 (Gurdon, 1962; Takahashi and Yamanaka, 2006). Both of their contributions suggest that mature cells can be reprogrammed to become pluripotent. As its name implies, IPS technology aims to alter cell fate and is of great historical significance, setting a milestone in both biological and medical fields. With IPS technology, cell therapy gained new hope. The reprogramming system of IPS can be developed using retroviral vectors, Sendai virus, episomal DNA, or chemical reprogramming. Chemical reprogramming has recently captured attention because it boasts a range of the laudable characteristics of chemical molecules: it penetrates well and is immunogenicity-free, adjustable, economical, easy to synthesize and conserve, and reversible (Hou et al., 2013). More importantly, it generates a non-carcinogenic XEN-like (extraembryonic endoderm-like) cell state that expands rapidly in vitro without destroying genomic integrity or stability (Li et al., 2017). Regardless of the path that is used for reprogramming, the rationale of cell reprogramming may be 1) the double alteration of gene expression procedures for one somatic cell (Li et al., 2017); and 2) that differentiated cells are in a temporary stable situation that is overturned once homeostasis is destroyed in the case of injury, disorders, or natural aging (Obokata et al., 2014). Until now, IPS has been used to successfully treat SCI, diabetes, sickle cell anemia, Parkinson’s disease, and thrombocytopenia in rodent disease models, and retinal pigment epithelium, leukemia, thrombocytopenia, and transmissible melanoma in human patients. IPS technology is currently evolving and may be used for a human SCI treatment protocol in the near future.
Although pre-existing cell therapies targeting chronic SCI have achieved various positive outcomes, they are unsatisfactory because many questions remain about source restriction, individual incompatibility, short survival times, and failure to integrate into the host nervous system, among others. With the emergence and maturation of IPS technology and its human application, many of these challenges have been overcome. The differentiation potential of IPSs means that they enjoy popularity in the medical field, because they can grow into any kind of functional cells according to patient needs. In parallel, IPSs bypass the ethical controversies associated with ESCs and nuclear transplantation, and avoid immune rejection problems because of their autonomous derivation from each patient’s own somatic cells. By using IPS technology to treat SCI, we may be able to rebuild the self-repair functions of absent cells in the nerve system via homologous cell reprogramming and differentiation. Moreover, the chronic development process of SCI and the long preparation required for IPS treatments are a good match. Autologous IPS-derived cells are able to escape from the host immune system and survive for a long time, meaning that they can play stable roles in the nervous system. The first instance of SCI therapy with human IPSs was performed in a mouse model, in 2011. The grafted hIPS-derived neurospheres survived, migrated, communicated with host neurons, and differentiated into astrocytes/oligodendrocytes/neurons for up to 112 days. They also presented outcomes of angiogenesis and neurite growth, and increased myelination and motor function recovery without tumorigenesis (Nori et al., 2011), which suggests the feasibility of applying IPSs to treat human SCI. Thus, to treat a patient with SCI, we can obtain somatic cells from every convenient tissue of the patient, induce them to IPSs via the Oct3/4, Sox2, Klf4, and c-Myc (OSKM) method or chemical reprogramming, and differentiate them into neural-specific cells to participate in spinal cord repair (Figure 3).
Figure 3.
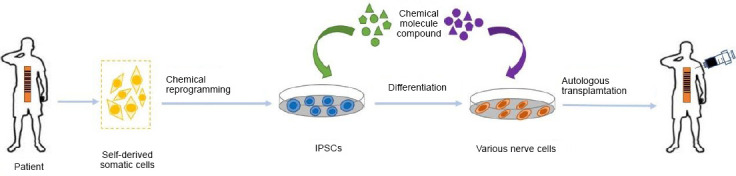
Application of IPS technology for SCI therapy using autologous somatic cells.
During IPS-based SCI therapy, somatic cell collection, IPS formation, cell differentiation, and autologous cell transplantation are performed in order. IPS: Induced pluripotent stem cell; SCI: spinal cord injury.
There are several limitations of IPSs for clinical application, such as a high risk of tumor germination and a low induction efficiency because of residual somatic cell epigenetic memory (Shi et al., 2017; Keefe and Li, 2020; Mao et al., 2020). Fortunately, the tumor formation limitation can be resolved using γ-secretase inhibitor, quality checks, pre-differentiation, and suicide gene introduction (Fatima et al., 2019; Nagoshi et al., 2019). A recommended solution for the low induction efficiency involves stepping away from current autologous grafts toward allografts, which rely on the formation of a cell bank. A cell bank was accomplished for the first time by Taylor et al. (2012), on the basis of major histocompatibility complex (MHC) matching between the receiver and exogenous stem cells. This idea involves extracting disease-free somatic cells for IPS generation and storing them in a cell bank, without losing stemness, for future direct use. Thus, the establishment of an IPS-derived cell bank underlies an IPS-derived NSC/NPC bank, which is expected to provide possibilities for immediate use and increase success rates of recovery (Okano and Yamanaka, 2014). In this way, we would be able to use IPSs from the same origin in a large number of patients with SCI, thus boosting IPS efficiency. Nevertheless, optimal transplantation times; differences between experimental and clinical stem cells caused by culture conditions, donor standards, and receptor types; and cell action modes should be taken into consideration (Takahashi, 2018).
Conclusions and Perspectives
SCI is an increasingly intractable problem worldwide. Given that many difficulties remain to be resolved with cell therapies, we believe that IPS technology should be used as an SCI therapy. Based on previous animal results, the further human introduction of IPS, and all phases of clinical tests being passed, the preclinical data needs to be summarized to create a safe and efficient protocol. The use of both biological scaffolds and physical training is likely to improve functional outcomes accompanied by the establishment of innervation networks, and more than 10 times the number of anti-inflammatory cells have been reported in treatment group compared with the control group (Lin et al., 2019). The combined transplantation of easily degradable biological scaffolds and stem cells may enhance the effects of therapies as a result of more appropriate growth environments. In the future, a more comprehensive therapy should be created, because neither traditional therapies nor IPS-derived cell implantation can individually lead to functional recovery. Current medical developments involve selecting the most suitable cell type for transplantation in accordance with each patient’s injury degree and site, and formulating a patient-specific therapeutic schedule. Additionally, patient mindset is of critical importance; if a good treatment effect is desired, frequent psychological counseling is essential. A cure for SCI is expected in the near future using cell therapies and IPS technology.
Additional file: Open peer review report 1 (87.5KB, pdf) .
Footnotes
P-Reviewer: Urbanchek MG; C-Editor: Zhao M; S-Editors: Qiu Y, Li CH; L-Editors: Gardner B, Stow A, Qiu Y, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This project was supported by the National Key Research and Development Program of China, No. 2017YFA0104304 (to BW), 2017YFA0205400 (to PPS), and 2017YFA0506000 (to PPS); the National Natural Science Foundation of China, No. 81571213 (to BW); the Nanjing Medical Science and Technique Development Foundation of China, No. QRX17006 (to BW); the Nanjing Medical Science and Innovation Platform, No. ZDX16005 (to BW) and the Innovation and Entrepreneurship Plan of Jiangsu Province (2019) (to BW).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Melanie G. Urbanchek, University of Michigan, USA.
Funding: This project was supported by the National Key Research and Development Program of China, No. 2017YFA0104304 (to BW), 2017YFA0205400 (to PPS), and 2017YFA0506000 (to PPS); the National Natural Science Foundation of China, No. 81571213 (to BW); the Nanjing Medical Science and Technique Development Foundation of China, No. QRX17006 (to BW); the Nanjing Medical Science and Innovation Platform, No. ZDX16005 (to BW) and the Innovation and Entrepreneurship Plan of Jiangsu Province (2019) (to BW).
References
- 1.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9-S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 2.Akhmetzyanova ER, Mukhamedshina YO, Zhuravleva MN, Galieva LR, Kostennikov AA, Garanina EE, Rizvanov AA. Transplantation of microglia in the area of spinal cord injury in an acute period increases tissue sparing, but not functional recovery. Front Cell Neurosci. 2018;12:507. doi: 10.3389/fncel.2018.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.All AH, Gharibani P, Gupta S, Bazley FA, Pashai N, Chou BK, Shah S, Resar LM, Cheng L, Gearhart JD, Kerr CL. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One 10. 2015:e0116933. doi: 10.1371/journal.pone.0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerstrom-Noga E, Wood P, Levi AD. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, Monsonego A, O’Banion MK, Pekny M, Puschmann T, Russek-Blum N, Sandusky LA, Selenica ML, Takata K, Teeling J, Town T, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016;138:653–693. doi: 10.1111/jnc.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. doi: 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993;123:3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- 9.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 10.Butts JC, Iyer N, White N, Thompson R, Sakiyama-Elbert S, McDevitt TC. V2a interneuron differentiation from mouse and human pluripotent stem cells. Nat Protoc. 2019;14:3033–3058. doi: 10.1038/s41596-019-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–716. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Bernreuther C, Dihne M, Schachner M. Cell adhesion molecule l1-transfected embryonic stem cells with enhanced survival support regrowth of corticospinal tract axons in mice after spinal cord injury. J Neurotrauma. 2005;22:896–906. doi: 10.1089/neu.2005.22.896. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clowry G, Sieradzan K, Vrbova G. Transplants of embryonic motoneurones to adult spinal cord: survival and innervation abilities. Trends Neurosci. 1991;14:355–357. doi: 10.1016/0166-2236(91)90162-n. [DOI] [PubMed] [Google Scholar]
- 16.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 18.Doucette R. Olfactory ensheathing cells: potential for glial cell transplantation into areas of CNS injury. Histol Histopathol. 1995;10:503–507. [PubMed] [Google Scholar]
- 19.Fan C, Zheng Y, Cheng X, Qi X, Bu P, Luo X, Kim DH, Cao Q. Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int J Biol Sci. 2013;9:78–93. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatima A, Gutiérrez-Garcia R, Vilchez D. Induced pluripotent stem cells from Huntington’s disease patients: a promising approach to define and correct disease-related alterations. Neural Regen Res. 2019;14:769–770. doi: 10.4103/1673-5374.249223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 22.Frolov AA, Bryukhovetskiy AS. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transplant 21 Suppl. 2012;1:S49–55. doi: 10.3727/096368912x633761. [DOI] [PubMed] [Google Scholar]
- 23.Fu MM, McAlear TS, Nguyen H, Oses-Prieto JA, Valenzuela A, Shi RD, Perrino JJ, Huang TT, Burlingame AL, Bechstedt S, Barres BA. The Golgi outpost protein TPPP nucleates microtubules and is critical for myelination. Cell. 2019;179:132–146. doi: 10.1016/j.cell.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet AD, Fonken LK. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics. 2018;15:554–577. doi: 10.1007/s13311-018-0630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Goulao M, Ghosh B, Urban MW, Sahu M, Mercogliano C, Charsar BA, Komaravolu S, Block CG, Smith GM, Wright MC, Lepore AC. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia. 2019;67:452–466. doi: 10.1002/glia.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 29.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 30.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, Okada S. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 31.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Xia B, Liu Z, Cao Q, Huang J, Luo Z. Superparamagnetic iron oxide nanoparticle-mediated forces enhance the migration of schwann cells across the astrocyte-Schwann cell boundary in vitro. Front Cell Neurosci. 2017;11:83. doi: 10.3389/fncel.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jendelova P, Herynek V, Urdzikova L, Glogarova K, Kroupova J, Andersson B, Bryja V, Burian M, Hajek M, Sykova E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Bouyer J, Shumsky JS, Haas C, Fischer I. Transplantation of neural progenitor cells in chronic spinal cord injury. Neuroscience. 2016;320:69–82. doi: 10.1016/j.neuroscience.2016.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 37.Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M, Shibata S, Kohyama J, Iwanami A, Toyama Y, Matsumoto M, Nakamura M, Okano H. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keefe F, Li M. Pluripotent stem cell derived inhibitory interneurons - principles and applications in health and disease. Neural Regen Res. 2020;15:251–252. doi: 10.4103/1673-5374.265547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khankan RR, Griffis KG, Haggerty-Skeans JR, Zhong H, Roy RR, Edgerton VR, Phelps PE. Olfactory ensheathing cell transplantation after a complete spinal cord transection mediates neuroprotective and immunomodulatory mechanisms to facilitate regeneration. J Neurosci. 2016;36:6269–6286. doi: 10.1523/JNEUROSCI.0085-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koshizuka S, Okada S, Okawa A, Koda M, Murasawa M, Hashimoto M, Kamada T, Yoshinaga K, Murakami M, Moriya H, Yamazaki M. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2004;63:64–72. doi: 10.1093/jnen/63.1.64. [DOI] [PubMed] [Google Scholar]
- 42.Kutikov AB, Moore SW, Layer RT, Podell PE, Sridhar N, Santamaria AJ, Aimetti AA, Hofstetter CP, Ulich TR, Guest JD. Method and apparatus for the automated delivery of continuous neural stem cell trails into the spinal cord of small and large animals. Neurosurgery. 2019;85:560–573. doi: 10.1093/neuros/nyy379. [DOI] [PubMed] [Google Scholar]
- 43.Li K, Javed E, Scura D, Hala TJ, Seetharam S, Falnikar A, Richard JP, Chorath A, Maragakis NJ, Wright MC, Lepore AC. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp Neurol. 2015;271:479–492. doi: 10.1016/j.expneurol.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Liu D, Ma Y, Du X, Jing J, Wang L, Xie B, Sun D, Sun S, Jin X, Zhang X, Zhao T, Guan J, Yi Z, Lai W, Zheng P, Huang Z, Chang Y, Chai Z, Xu J, et al. Direct reprogramming of fibroblasts via a chemically induced XEN-like state. Cell Stem Cell. 2017;21:264–273. doi: 10.1016/j.stem.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Anopas D, Milbreta U, Lin PH, Chin JS, Zhang N, Wee SK, Tow A, Ang WT, Chew SY. Regenerative rehabilitation: exploring the synergistic effects of rehabilitation and implantation of a bio-functional scaffold in enhancing nerve regeneration. Biomater Sci. 2019;7:5150–5160. doi: 10.1039/c9bm01095e. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PA, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manley NC, Priest CA, Denham J, Wirth ED, 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med. 2017;6:1917–1929. doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao Q, Pang YH, Lu YX, Liang XL. miRNA-148a promotes the differentiation of human induced pluripotent stem cells into cardiomyocyte-like cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:2978–2984. [Google Scholar]
- 51.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 52.Mendonca MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukhamedshina Y, Shulman I, Ogurcov S, Kostennikov A, Zakirova E, Akhmetzyanova E, Rogozhin A, Masgutova G, James V, Masgutov R, Lavrov I, Rizvanov A. Mesenchymal stem cell therapy for spinal cord contusion: a comparative study on small and large animal models. Biomolecules. 2019;9:811. doi: 10.3390/biom9120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagoshi N, Tsuji O, Nakamura M, Okano H. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen Ther. 2019;11:75–80. doi: 10.1016/j.reth.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Namiki J, Tator CH. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol. 1999;58:489–498. doi: 10.1097/00005072-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, Franzen R, Wislet S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015;6:211. doi: 10.1186/s13287-015-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicaise C, Mitrecic D, Falnikar A, Lepore AC. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J Stem Cells. 2015;7:380–398. doi: 10.4252/wjsc.v7.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obokata H, Wakayama T, Sasai Y, Kojima K, Vacanti MP, Niwa H, Yamato M, Vacanti CA. Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature. 2014;505:641–647. doi: 10.1038/nature12968. [DOI] [PubMed] [Google Scholar]
- 61.Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78:436–447. doi: 10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 62.Okano H. Stem cell biology of the central nervous system. J Neurosci Res. 2002;69:698–707. doi: 10.1002/jnr.10343. [DOI] [PubMed] [Google Scholar]
- 63.Okano H, Yamanaka S. iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain. 2014;7:22. doi: 10.1186/1756-6606-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paino CL, Bunge MB. Induction of axon growth into Schwann cell implants grafted into lesioned adult rat spinal cord. Exp Neurol. 1991;114:254–257. doi: 10.1016/0014-4886(91)90043-c. [DOI] [PubMed] [Google Scholar]
- 65.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 66.Paschon V, Morena BC, Correia FF, Beltrame GR, Dos Santos GB, Cristante AF, Kihara AH. VDAC1 is essential for neurite maintenance and the inhibition of its oligomerization protects spinal cord from demyelination and facilitates locomotor function recovery after spinal cord injury. Sci Rep. 2019;9:14063. doi: 10.1038/s41598-019-50506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearse DD, Bastidas J, Izabel SS, Ghosh M. Schwann cell transplantation subdues the pro-inflammatory innate immune cell response after spinal cord injury. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeifer K, Vroemen M, Blesch A, Weidner N. Adult neural progenitor cells provide a permissive guiding substrate for corticospinal axon growth following spinal cord injury. Eur J Neurosci. 2004;20:1695–1704. doi: 10.1111/j.1460-9568.2004.03657.x. [DOI] [PubMed] [Google Scholar]
- 69.Pizzi MA, Crowe MJ. Transplantation of fibroblasts that overexpress matrix metalloproteinase-3 into the site of spinal cord injury in rats. J Neurotrauma. 2006;23:1750–1765. doi: 10.1089/neu.2006.23.1750. [DOI] [PubMed] [Google Scholar]
- 70.Podesta MA, Remuzzi G, Casiraghi F. Mesenchymal stromal cells for transplant tolerance. Front Immunol. 2019;10:1287. doi: 10.3389/fimmu.2019.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- 72.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4:743–754. doi: 10.5966/sctm.2014-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandhu MS, Ross HH, Lee KZ, Ormerod BK, Reier PJ, Fuller DD. Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output after high cervical spinal cord injury. Exp Neurol. 2017;287:205–215. doi: 10.1016/j.expneurol.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sankavaram SR, Hakim R, Covacu R, Frostell A, Neumann S, Svensson M, Brundin L. Adult neural progenitor cells transplanted into spinal cord injury differentiate into oligodendrocytes, enhance myelination, and contribute to recovery. Stem Cell Reports. 2019;12:950–966. doi: 10.1016/j.stemcr.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976) 2004;29:1971–1979. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 77.Selvarajah S, Hammond ER, Haider AH, Abularrage CJ, Becker D, Dhiman N, Hyder O, Gupta D, Black JH, 3rd, Schneider EB. The burden of acute traumatic spinal cord injury among adults in the united states: an update. J Neurotrauma. 2014;31:228–238. doi: 10.1089/neu.2013.3098. [DOI] [PubMed] [Google Scholar]
- 78.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, Park KI. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shroff G. Human embryonic stem cell therapy in chronic spinal cord injury: a retrospective study. Clin Transl Sci. 2016;9:168–175. doi: 10.1111/cts.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shroff G, Barthakur JK. Safety of human embryonic stem cells in patients with terminal/incurable conditions-a retrospective analysis. Ann Neurosci. 2015;22:132–138. doi: 10.5214/ans.0972.7531.220303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int J Mol Sci. 2020;21:659. doi: 10.3390/ijms21020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi J. Stem cells and regenerative medicine for neural repair. Curr Opin Biotechnol. 2018;52:102–108. doi: 10.1016/j.copbio.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 85.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina L Neurological Cell Therapy G. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18:1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, Tapiador N, Sevilla M, Vazquez D, Carballido J, Fernandez C, Rodriguez-Boto G, Ovejero M Neurological Cell Therapy Group from Puerta de Hierro-Majadahonda H. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy. 2018a;20:806–819. doi: 10.1016/j.jcyt.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 88.Vaquero J, Zurita M, Rico MA, Aguayo C, Fernandez C, Rodriguez-Boto G, Marin E, Tapiador N, Sevilla M, Carballido J, Vazquez D, Garcia-Olmo D, Guadalajara H, Leon M, Valverde I. Neurological Cell Therapy Group From Puerta De Hierro-Majadahonda H (2018b) Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. 20:796–805. doi: 10.1016/j.jcyt.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 90.Wilcox JT, Satkunendrarajah K, Zuccato JA, Nassiri F, Fehlings MG. Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury. Stem Cells Transl Med. 2014;3:1148–1159. doi: 10.5966/sctm.2014-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Willerth SM, Sakiyama-Elbert SE. Cell therapy for spinal cord regeneration. Adv Drug Deliv Rev. 2008;60:263–276. doi: 10.1016/j.addr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams RR, Henao M, Pearse DD, Bunge MB. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 2015;24:115–131. doi: 10.3727/096368913X674657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Xiong LL, Wang YC, He X, Jiang L, Fu SJ, Han XF, Liu J, Wang TH. Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered microRNA expression. Mol Med Rep. 2018;17:771–782. doi: 10.3892/mmr.2017.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther. 2016;7:36. doi: 10.1186/s13287-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao XM, He XY, Liu J, Xu Y, Xu FF, Tan YX, Zhang ZB, Wang TH. Neural stem cell transplantation improves locomotor function in spinal cord transection rats associated with nerve regeneration and IGF-1 R expression. Cell Transplant. 2019;28:1197–1211. doi: 10.1177/0963689719860128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y, Uezono N, Yasui T, Nakashima K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn. 2018;247:75–84. doi: 10.1002/dvdy.24558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


