Abstract
TLD1433 is the first Ru(II) complex to be tested as a photodynamic therapy agent in a clinical trial. In this contribution we study TLD1433 in the context of structurally-related Ru(II)-imidozo[4,5-f][1,10]phenanthroline (ip) complexes appended with thiophene rings to decipher the unique photophysical properties which are associated with increasing oligothiophene chain length. Substitution of the ip ligand with ter- or quaterthiophene changes the nature of the long-lived triplet state from metal-to-ligand charge-transfer to 3ππ* character. The addition of the third thiophene thus presents a critical juncture which not only determines the photophysics of the complex but most importantly its capacity for 1O2 generation and hence the potential of the complex to be used as a photocytotoxic agent.
Entry for the Table of Contents
A low-lying triplet intraligand state (3IL) determines the properties of the long-lived excited states in a series of Ru(II) complexes. The 3IL state can be accessed by increasing the length of an oligothiophene chain. The 3IL state is extremely efficient at generating 1O2 and thus enhances the potency of the complexes as PDT agents.
Keywords: TLD1433, PDT, long-lived triplet states, singlet oxygen, lifetimes
Introduction
Recent developments highlight Ru(II) polypyridyl complexes with π-expanded ligands as a promising class of new compounds for photodynamic therapy (PDT).[1–11] By extending the pyridyl ligands with organic chromophores, low-lying intraligand (IL) excited states become accessible, and these appear to be crucial to the photophysical function of these systems.[12] Our TLD1433 (Ru-ip-3T in this manuscript, Figure 1) is a compound of this type, having three appended thiophene rings, and has the distinction of being the first Ru(II)-based PDT agent ever to enter a human clinical trial;[1,10,13] Ru-ip-3T is currently being tested in a Phase II PDT trial for non-invasive bladder cancer (ClinicalTrials.gov identifier: NCT03945162).[10]
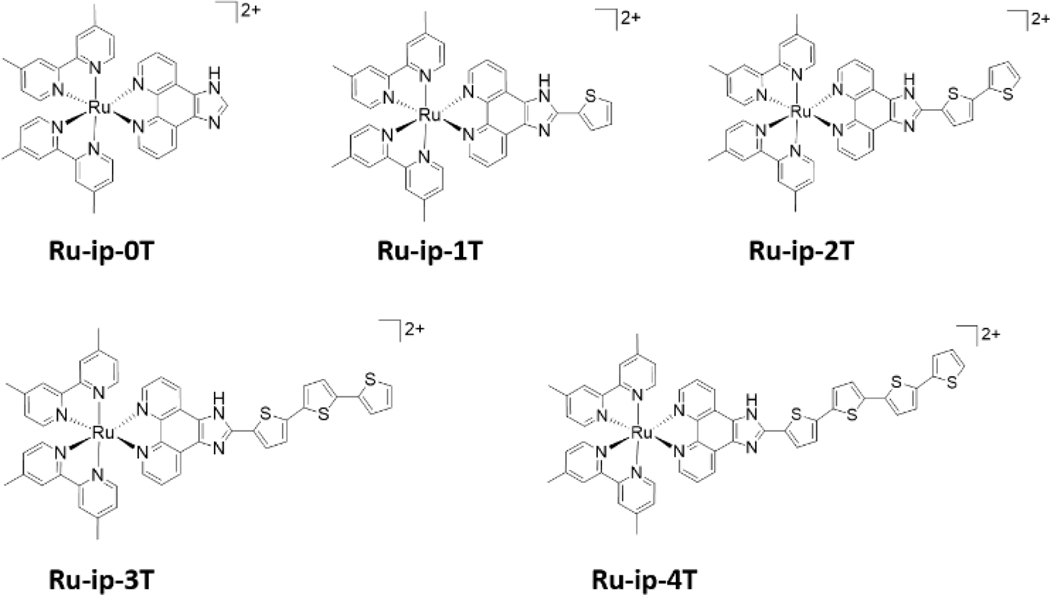
Figure 1.
Chemical structures of Ru-ip-nT complexes.
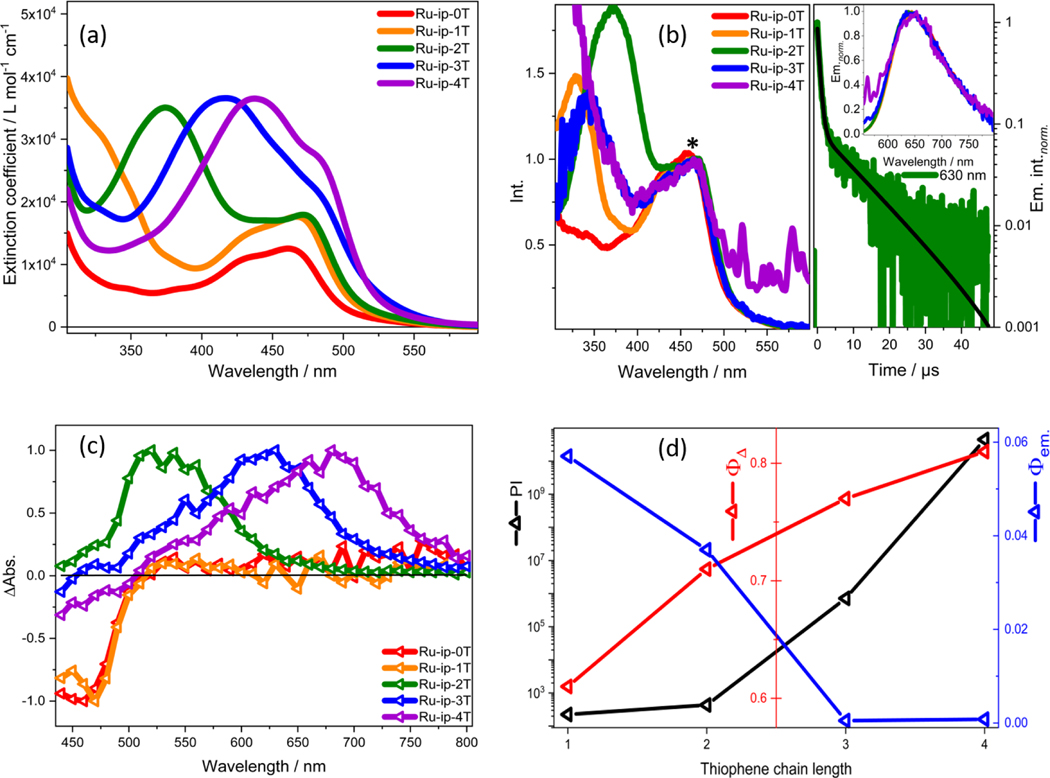
In vitro studies have been previously conducted on the Ru-ip-nT series of compounds, where n indicates the number of appended thiophene rings attached to an imidazo[4,5-f][1,10]phenanthroline (ip) ligand (Figure 1). Visible light illumination of SK-MEL-28 cancer cells treated with the compounds in the series led to increased photocytotoxicity with increasing n. The EC50 values (effective concentration to reduce cell viability by 50%) were 0.72 μM, 0.26 μM, 1.9×10−4 μM and 2.8×10−9 μM for Ru-ip-1T to Ru-ip-4T, respectively (Figure 2d).[1] While these previous findings demonstrate a clear correlation between the length of the thiophene chain and the in vitro phototherapeutic effects, herein we present the key photophysical properties of the compounds in the Ru-ip-nT series that could be responsible for the observed photocytotoxicity.
Figure 2.
(a) Absorption spectra for the Ru-ip-nT complexes in water. (b) Excitation spectra of the Ru-ip-nT complexes in water at 640 nm emission normalized to the MLCT band at 480 nm (*, left); bi-exponential emission decay of Ru-ip-2T with lifetimes of 0.6 μs and 11 μs at 630 nm (right); Inset: Emission spectra of Ru-ip-nT complexes normalized to their respective maxima. (c) Nanosecond TA spectra of Ru-ip-nT complexes in aerated water normalized to their respective maxima at λex.: 410 nm at 300 ns. (d) Relationship between the PI values (black y-axis), singlet-oxygen quantum yield Ф Δ (red y-axis) in aerated MeCN (excitation at 450 nm) and fluorescence quantum yield Ф em. (blue y-axis) in deaerated water (excitation at 450 nm) with increasing thiophene chain length (x-axis).[1] The PI is defined as the ratio of EC50 dark to EC50 visible light values in SK-MEL-28 cells.
Results and Discussion
STEADY STATE ABSORPTION AND EMISSION SPECTROSCOPY
The electronic absorption spectra of the compounds within the Ru-ip-nT series (Figure 2a) are characterized by IL transitions localized to the dmb and phen portion of the ip ligand below 300 nm as well as a broad metal-to-ligand charge transfer (MLCT) band in the 420–550 nm range, consistent with the spectrum of the parent [Ru(dmb)3]2+ complex.[14,15] An additional band centered near 370 nm is visible in Ru-ip-2T that shifts to longer wavelengths in Ru-ip-3T and Ru-ip-4T. This band corresponds to the ππ* 1IL transition associated with the oligothiophene.[16] These oligothiophene-localized transitions have been computed to have substantial charge transfer character and are best described as intraligand charge transfer (ILCT) states, but are referred to herein more generally as IL.[17,18]
The steady-state emission spectra of the complexes show a single structureless emission band centered at 625 nm (Figure 2b), suggesting a similar emissive 3MLCT state for all complexes in the Ru-ip-nT family.[19] The emission quantum yields (Φem.) in deaerated water (five freeze pump thaw cycles under a nitrogen atmosphere) drop with increasing n, from 6% for Ru-ip-1T to below 0.1% for Ru-ip-3T (Table 1). Emission from Ru-ip-4T is barely detectable (Figure 2d). The emission quantum yields for Ru-ip-3T and Ru-ip-4T are extremely small and do not notably increase upon deaeration of the solvent. This suggests that collisional deactivation by 3O2 is not the prime quenching process of the 3MLCT state in the complexes.
Table 1.
Excited state lifetimes and quantum yields for emission and singlet oxygen production for Ru-ip-nTunder different conditions.
| Complex | τem.[c] | τTA.[c] | Ф em.[c] | Ф Δ[d] | |||
|---|---|---|---|---|---|---|---|
| Aerated (μs) | Deaerated (μs) | Aerated (μs) | Deaerated (μs) | Aerated | Deaerated | ||
| Ru-ip-0T | 0.4 | 0.7 | 0.4 | 0.7 | 0.038 | 0.05 | 0.68 |
| Ru-ip-1T | 0.4 | 0.8 | 0.4 | 0.7 | 0.044 | 0.06 | 0.61 |
| Ru-ip-2T | 0.3; 1[a] | 0.6; 11[a] | 1.4 | 14 | 0.006 | 0.04 | 0.71 |
| Ru-ip-3T | 0.3 | 0.6 | 1.4 | 48 | -[b] | 4.8×10−4 | 0.77 |
| Ru-ip-4T | 0.3 | 0.6 | 1.4 | 29 | -[b] | 8.1×10−4 | 0.81 |
τem. : emission lifetime; τTA.: recovery lifetime; em.: quantum yield of emission; Δ: singlet oxygen quantum yield. Singlet oxygen was determined spectroscopically by monitoring its emission centered at 1275 nm in aerated MeCN. [Ru(bpy)3][PF6]2 is used as the reference obtaining em.[27] and Δ.[28]
A biexponential decay of the respective signal is observed.
Emission is too low to obtain a reasonable estimate of the emission quantum yield.
Solvent used is H2O.
Solvent used is MeCN.
The excitation spectra recorded for Ru-ip-0T, Ru-ip-1T and Ru-ip-2T using the emission signal at 640 nm resemble the respective absorption spectra (Figure 2a, b), suggesting that the emissive 3MLCT state is populated regardless of whether the initially populated states are 1MLCT or 1IL (i.e., ππ*). The absence of excitation signals that would correspond to broad 1IL transitions in the UV-vis spectra at 420 nm and 440 nm for Ru-ip-3T and Ru-ip-4T, respectively, indicates that excitation of the 1IL state does not populate the emissive 3MLCT state.
In addition to absorption and emission characteristics, the singlet oxygen quantum yields (ΦΔ) were also measured. Values for ΦΔ increase from 0.61 for Ru-ip-1T to 0.81 for Ru-ip-4T (Table 1). This trend is consistent with the trend for photocytotoxicity as reflected in the PI values that increase with n (see Figure 2d). This positive correlation between singlet oxygen quantum yields and photocytotoxicity suggests that singlet oxygen is involved in their mode of action.[20–22] Enhanced sensitivity toward oxygen is normally reflected as a drastic decrease in triplet excited state lifetime in the presence of oxygen.[8] The relative changes of excited state lifetimes for Ru-ip-nT upon exposure to oxygen will be discussed in the next section.
TRANSIENT ABSORPTION AND EMISSION LIFETIMES
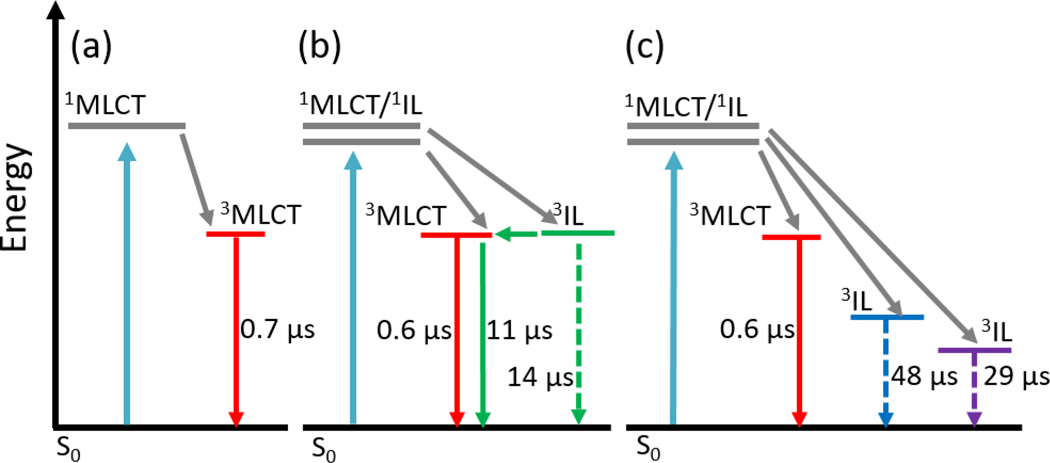
The photophysical properties of the longer-lived excited states, from which singlet oxygen sensitization occurs, were probed by transient absorption (TA) spectroscopy yielding excited-state absorption spectra and characteristic time-constants for ground state recovery (τTA) and emission decay (τem). Table 1 summarizes these key characteristics of the long-lived excited states in aerated or deaerated water. The emission and TA lifetimes of Ru-ip-0T and Ru-ip-1T are the same, 0.4 μs, indicative of a single excited state being depopulated (Figure 3a). The fact that deaeration lengthens the lifetime by only two-fold suggests that this state is only weakly quenched by oxygen. Ru-ip-2T behaves somewhat differently than Ru-ip-0T and Ru-ip-1T. In aerated aqueous solutions, τem and τTA for Ru-ip-2T are 1 μs and 1.4 μs, respectively. In deaerated solutions, however, the excited state(s) was much longer lived, and the emission decay was biexponential (τem=0.6; 11 μs, τTA=14 μs). An excited state lifetime that is ten-fold (or more) longer than the typical 1 μs lifetime of 3MLCT states in Ru(II) polypyridyl complexes and is very sensitive to O2 is consistent with the involvement of an 3IL state.[23,24] The second thiophene lowers the energy of this state sufficiently that the emissive 3MLCT and non-emissive 3IL states are in energetic proximity (Figure 3b). The biexponential emission decay, where the longer component matches the decay of the nonemissive state by TA, suggests that the shorter 0.6-μs component is due to prompt 3MLCT emission and the longer 11-μs lifetime corresponds to delayed emission from the 3MLCT state resulting from population of the 3MLCT from the nearly isoenergetic 3IL state (Figure 3b).[24]
Figure 3.
Jablonski diagrams depicting the photophysical models that describe the nanosecond-microsecond excited state dynamics of Ru-ip-nT complexes with 410 nm excitation in deaerated water: (a) Ru-ip-0T, Ru-ip-1T; (b) Ru-ip-2T; (c) Ru-ip-3T, Ru-ip-4T. [Note: grey arrows represent processes occurring faster than the investigated timescale.]
For Ru-ip-3T and Ru-ip-4T, the aerated and deaerated emission lifetimes were monoexponential and relatively short (τem≈0.4 μs) as observed for Ru-ip-0T and Ru-ip-1T, indicating that the emissive 3MLCT state cannot be populated from the 3IL when n=3 or 4. Ru-ip-3T and Ru-ip-4T are barely emissive, with emission quantum yields too small to be calculated in the aerated solutions and vanishingly small in deaerated conditions. These observations point to the fact that a significant fraction of the Ru-ip-3T and Ru-ip-4T excited states must deactivate via non-emissive 3IL states. The aerated TA lifetimes for Ru-ip-3T and Ru-ip-4T were identical to that of Ru-ip-2T, consistent with efficient quenching of the 3IL states by O2 and corroborated by all three being very good 1O2 sensitizers. The values for τTA in deaerated water were considerably longer, 48 μs and 29 μs for Ru-ip-3T and Ru-ip-4T, respectively. This is attributed to the 3IL state of both Ru-ip-3T and Ru-ip-4T being sufficiently lower in energy than the emissive 3MLCT state (Figure 3c), which remains unchanged throughout the series (evidenced by a constant emission maximum). The triplet is thereby trapped in the longer-lived 3IL state, unable to populate the 3MLCT, which is significantly uphill in energy. The 3IL relaxes much slower due to the reduced intersystem crossing rate in organic chromophores compared to transition metal complexes, in which the heavy metal ion increases spin-orbit coupling. The lifetime of the 3IL state for Ru-ip-4T may be shorter than the corresponding lifetime for Ru-ip-3T due to the energy gap law.[25] However, additional relaxation pathways cannot be excluded without further investigation.
The change in the nature of the long-lived photobiologically active excited states is also supported by the spectral shape of the ns TA spectra within the Ru-ip-nT series. The TA spectra of Ru-ip-0T and Ru-ip-1T show a ground-state bleach below 510 nm plus a very weak, unstructured excited-state absorption extending from 510 to 800 nm (Figure 2c). This signature is typical for the 3MLCT states of Ru(II) polypyridyl complexes that lack π-extended ligands and thus low-lying 3IL states.[26] The TA spectra change substantially for Ru-ip-2T through Ru-ip-4T, which have very strong and much more structured excited state absorptions with maxima at 550, 630, and 680 nm, respectively. This systematic red-shift of the excited state absorption with increasing n, by over 100 nm, is characteristic of oligothiophene-based 3IL states.[5,17] Thus, the ns TA spectra indicate that the character of the long-lived state changes from 3MLCT for Ru-ip-0T and Ru-ip-1T to predominantly 3IL with additional thiophene rings.
Conclusion
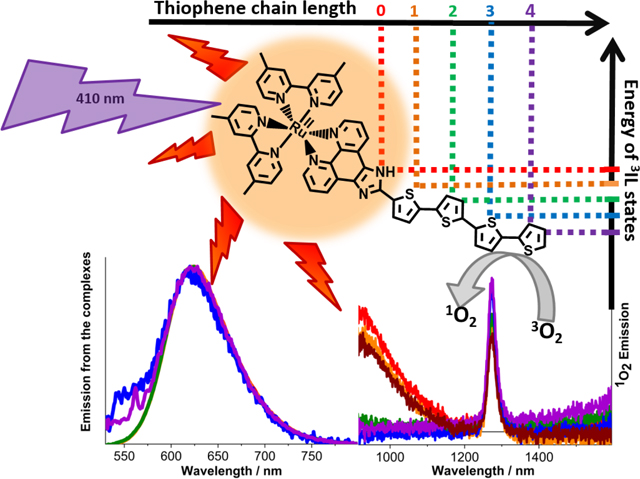
This study highlights the photophysical properties of the long-lived excited states in a series of Ru(II) complexes, which underlie their previously reported photocytotoxicity. The results show that the spectroscopic signatures of the long-lived excited states and the biological activity in this series of complexes are determined by the energy of the non-emissive 3IL state relative to the energy of the emissive 3MLCT state. This energy depends on the length of the oligothiophene chain and determines the TA absorption and emission lifetimes, emission quantum yields and light EC50 values. In Ru-ip-0T and Ru-ip-1T the energy of the 3IL is too high and the state does not contribute to the ns-/μs-photophysics and photobiology of the complexes. Ru-ip-2T represents the situation where the 3MLCT and 3IL states are close in energy. In this case, the 3IL state serves as an excited state reservoir for populating the 3MLCT state and results in delayed 3MLCT emission. In Ru-ip-3T and Ru-ip-4T the 3IL state is the lowest-lying triplet and plays a predominant role in the excited state relaxation. The oxygen-sensitive 3IL state appears to be responsible for the increased photocytotoxicity of these complexes. This is manifested in a sharp increase in the photocytotoxicity and efficiency of singlet oxygen sensitization upon increasing the length of the oligothiophene chain.
Experimental Section
All samples were dissolved in the respective solvent and measured in a 1 cm quartz cell. All solvents were deaerated by freeze pump thaw cycles for five times using nitrogen as inert gas. For measuring em. and Δ samples with an OD of about 0.05 were used. Quantum yields were calculated according to the the equation where and are the quantum yields of the sample and a reference, respecitively. and are the integrated emission intensity of the sample and the references, while ODr and ODs are the optical densities of the sample and the reference at the excitation wavelength. ηr and ηs refer to the refractive indices of the media in the sample and the reference. Since the same medium is used for measuring the sample and the reference equals 1.
UV-vis absorption measurements utilized a Jasco V-670 spectrophotometer and emission measurements were carried out on a FLS980 spectrophotometer (Edinburgh Instruments). 1O2 emission was detected using a FLS980 spectrophotometer equipped with a NIR detector with parameters upon excitation at 450 nm.
Nanosecond transient absorption measurements used a 10 Hz Nd:YAG laser (Surelite) combined with an OPO for excitation. The Transient data was recorded by a commercial detection system (Pascher Instruments AB) with a time resolution of 10 ns. The OD of the samples at the excitation wavelength was ~0.25 in a 1-cm cuvette. The integrity of the samples after nanosecond measurements were checked by measuring absorption spectra before and after the measurement. No degradation was observed. Time resolved emission measurements utilized time correlation single photon counting (TCSPC, Becker & Hickl GmbH) upon excitation at 390 nm.
Acknowledgements
We are grateful to the German Science Foundation (grant No. 395358570), the Carl Zeiss Foundation. We also thank the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (Award R01CA222227) for partial support of this work. The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
S. A. M. has a potential research conflict of interest due to a financial interest with Theralase Technologies, Inc. and PhotoDynamic, Inc. A management plan has been created to preserve objectivity in research in accordance with UTA policy.
References
- [1].Monro S, Colón KL, Yin H, Roque J, Konda P, Gujar S, Thummel RP, Lilge L, Cameron CG, McFarland SA, Chem. Rev 2019, 119, 797–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stephenson M, Reichardt C, Pinto M, Wa M, Sainuddin T, Shi G, Yin H, Monro S, Sampson E, Dietzek B, Mcfarland SA, J. Phys. Chem. A 2014, 10507–10521. [DOI] [PubMed] [Google Scholar]
- [3].Chakrabortty S, Agrawalla BK, Stumper A, Vegi NM, Fischer S, Reichardt C, Kögler M, Dietzek B, Feuring-Buske M, Buske C, Rau S, Weil T, J. Am. Chem. Soc 2017, 139, 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Howerton BS, Heidary DK, Glazer EC, J. Am. Chem. Soc 2012, 134, 8324–8327. [DOI] [PubMed] [Google Scholar]
- [5].Ghosh G, Colón KL, Fuller A, Sainuddin T, Bradner E, McCain J, Monro SMA, Yin H, Hetu MW, Cameron CG, McFarland SA, Inorg. Chem 2018, 57, 7694–7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sainuddin T, McCain J, Pinto M, Yin H, Gibson J, Hetu M, McFarland SA, Inorg. Chem 2016, 55, 83–95. [DOI] [PubMed] [Google Scholar]
- [7].Sainuddin T, Pinto M, Yin H, Hetu M, Colpitts J, McFarland SA, J. Inorg. Biochem 2016, 158, 45–54. [DOI] [PubMed] [Google Scholar]
- [8].Lincoln R, Kohler L, Monro S, Yin H, Stephenson M, Zong R, Chouai A, Dorsey C, Hennigar R, Thummel RP, McFarland SA, J. Am. Chem. Soc 2013, 135, 17161–17175. [DOI] [PubMed] [Google Scholar]
- [9].Reichardt C, Pinto M, Wächtler M, Stephenson M, Kupfer S, Sainuddin T, Guthmuller J, McFarland SA, Dietzek B, J. Phys. Chem. A 2015, 119, 3986–3994. [DOI] [PubMed] [Google Scholar]
- [10].Shi G, Monro S, Hennigar R, Colpitts J, Fong J, Kasimova K, Yin H, DeCoste R, Spencer C, Chamberlain L, Mandel A, Lilge L, McFarland SA, Coord. Chem. Rev 2015, 282–283, 127–138. [Google Scholar]
- [11].Yin H, Stephenson M, Gibson J, Sampson E, Shi G, Sainuddin T, Monro S, McFarland SA, Inorg. Chem 2014, 53, 4548–4559. [DOI] [PubMed] [Google Scholar]
- [12].Albani BA, Peña B, Leed NA, De Paula NABG, Pavani C, Baptista MS, Dunbar KR, Turro C, J. Am. Chem. Soc 2014, 136, 17095–17101. [DOI] [PubMed] [Google Scholar]
- [13].McFarland SA, Mandel A, Dumoulin-White R, Gasser G, Curr. Opin. Chem. Biol 2020, 56, 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tamaki Y, Tokuda K, Yamazaki Y, Saito D, Ueda Y, Ishitani O, Front. Chem 2019, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Damrauer NH, McCusker JK, J. Phys. Chem. A 1999, 103, 8440–8446. [Google Scholar]
- [16].Grebner D, Helbig M, Rentsch S, J. Phys. Chem 1995, 99, 16991–16998. [Google Scholar]
- [17].Roque JA III, Barrett PC, Cole HD, Lifshits LM, Bradner E, Shi G, von Dohlen D, Kim S, Russo N, Deep G, Cameron CG, Alberto ME, McFarland SA, Inorg. Chem 2020, 59, 16341–16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roque JA III, Barrett PC, Cole HD, Lifshits LM, Shi G, Monro S, Von Dohlen D, Kim S, Russo N, Deep G, Cameron CG, Alberto ME, McFarland SA, Chem. Sci 2020, 11, 9784–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Curtright AE, McCusker JK, J. Phys. Chem. A 1999, 103, 7032–7041. [Google Scholar]
- [20].Lifshits LM, Roque III JA, Konda P, Monro S, Cole HD, von Dohlen D, Kim S, Deep G, Thummel RP, Cameron CG, Gujar S, McFarland SA, Chem. Sci 2020, 11740–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reichardt C, Monro S, Sobotta FH, Colón KL, Sainuddin T, Stephenson M, Sampson E, Roque J, Yin H, Brendel JC, Cameron CG, McFarland S, Dietzek B, Inorg. Chem 2019, 58, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].MacDonald IJ, Dougherty TJ, J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar]
- [23].Wang XY, Del Guerzo A, Schmehl RH, J. Photochem. Photobiol. C Photochem. Rev 2004, 5, 55–77. [Google Scholar]
- [24].McClenaghan ND, Leydet Y, Maubert B, Indelli MT, Campagna S, Coord. Chem. Rev 2005, 249, 1336–1350. [Google Scholar]
- [25].Englman R, Jortner J, Mol. Phys 1970, 18, 285–287. [Google Scholar]
- [26].Peuntinger K, Pilz TD, Staehle R, Schaub M, Kaufhold S, Petermann L, Wunderlin M, Görls H, Heinemann FW, Li J, Drewello T, Vos JG, Guldi DM, Rau S, Dalt. Trans 2014, 43, 13683–13695. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki K, Kobayashi A, Kaneko S, Takehira K, Yoshihara T, Ishida H, Shiina Y, Oishi S, Tobita S, Phys. Chem. Chem. Phys 2009, 11, 9850–9860. [DOI] [PubMed] [Google Scholar]
- [28].Abdel-Shafi AA, Beer PD, Mortimer RJ, Wilkinson F, Helv. Chim. Acta 2001, 84, 2784–2795. [Google Scholar]