Introduction
Chest pain is a common presenting symptom for Emergency Department (ED) visits, second only to abdominal pain in the United States, constituting over 5.5 million visits ED annually.1 Despite this, the number of patients ultimately diagnosed with an acute coronary syndrome (ACS), either ST-elevation myocardial infarction (MI), unstable angina or a non-ST elevation MI, is less than 20%.2
The goals of the evaluation of patients in the ED who present with chest pain include the rapid recognition of conditions that require emergent care, such as ACS (as well as the far less common pulmonary embolism and acute aortic syndromes), and risk stratification for these conditions to allow for rapid treatment and triage decisions. Ideally, if such patients can be identified as being at very low risk of having an ACS (or other serious diagnosis), they can be triaged to home, for further work-up as an out-patient if needed. However, based on the limitations of the standard evaluation strategies, some reports suggest that up to approximately 2% of patients ultimately found to have an ACS are inappropriately discharged home from the ED.2 Again based on the limitations of the standard evaluation, a much larger number of patients are admitted for prolonged observation and additional testing who are ultimately found to not have an ACS. Thus, these observations or admissions could be considered inappropriate, and a suboptimal use of resources.
Patients with a clear diagnosis of ACS based on history, physical exam, initial electrocardiogram (ECG), and/or initial cardiac biomarkers should be rapidly admitted and treated per Guideline recommendations as appropriate.3,4 Conversely, patients with a clear cause of benign non-cardiac chest pain after initial evaluation could be discharged directly from the ED with appropriate treatment and follow-up, if necessary, to avoid unwarranted admission or delay in triage decision. Unfortunately, the initial evaluation of patients who present to the ED with chest pain often fails to provide a firm enough diagnosis to allow a prompt triage decision to be made. While a thorough history should always be obtained, clinical characteristics alone are very often not adequate to make a diagnosis, or predict outcomes.5 The ECG, while a useful diagnostic tool, has similar shortcomings. A completely normal ECG is not only present in a small percentage of ED patients with chest pain, but even in the setting of recent or ongoing chest pain cannot reliably exclude ACS.6,7 Many patients will have some abnormality on ECG at presentation, even if not specific, which limits the ability to interpret ischemia. For example, left bundle-branch block, ventricular paced rhythm, left ventricular hypertrophy, or nonspecific repolarization abnormalities all impact the ability to evaluate for ischemia and infarction.
Joseph Alpert: Chest pain patients are a real challenge for Emergency Department (ED) physicians since they are not familiar with the patient and hence may have a hard time deciding how seriously they should consider the patient’s complaint. This problem is compounded by the very short time that the ED physician has to evaluate the patient given the constant demand for physician decisions in a typically busy ED.
The use of cardiac biomarkers in the evaluation of acute chest pain in the ED is critical. Their use is incorporated into the universal definition of MI,8 and a higher peak value has shown to correlate with increased death rates.9 In unstable angina, however, biomarkers may not become elevated in the absence of cell death. Serial values are often required as well for optimal sensitivity, delaying a diagnosis or triage decision for several hours, although even a delayed elevation in biomarker levels portends a worse prognosis compared to patients with normal values.10
Over the past 3–4 decades, several approaches have been developed in an attempt to improve the rapid evaluation and triage of ED patients with suspected ACS. One approach has been to use “clinical risk scores” derived from clinical data that are readily available in such patients. As an example, one group applied the former Agency for Health Care Policy and Research (AHCPR) clinical risk score to a large population of ED patients seen in Olmstead County11. While there was clear risk stratification value (i.e. high risk scores were associated with higher risk than low risk scores), the major adverse cardiac event rate in the 12% of patients who fell into the low-risk score category was 2.5% at 30 days. While lower than the intermediate- or high-risk score category, this 30 day event rate is not low enough to comfortably allow direct discharge from the ED. Similarly, the Thrombolysis in Myocardial Infarction (TIMI) risk score, derived initially in populations with ACS in clinical trials, has been applied to a more unselected population of almost 4,000 ED chest pain patients12. While there was risk stratification value for 30 day major adverse events, the lowest score of “0” was associated with a 2.1% risk, again not likely low enough to comfortably allow direct ED discharge. Thus, clinical risk scores devised to help determine prognostic and triage decisions cannot be solely relied on given clinically significant event rates in the lowest group, complicated criteria which may lead to incomplete data, and variability depending on which score is used.13–16 Such scores alone may not provide complete enough information to optimally identify very low risk patients.
Joseph Alpert: One problem with clinical risk scores is that they are based on population averages that have been statistically manipulated. This information may well not apply to the individual patient currently under evaluation. Common sense and clinical experience/judgment are needed to make the clinical decision in conjunction with the clinical risk score.
Another approach to optimizing triage in ED chest pain patients has been the development and use of “clinical decision instruments” to augment clinicians’ use of readily available clinical and ECG data. These instruments are developed using known outcome data from large populations, and modeling the baseline clinical information to create predictive tools, providing a probability value for the diagnosis of ACS. Some incorporate automated analysis of the ECG as well. One such instrument - the Acute Cardiac Ischemia Time Insensitive Predictive Instrument (ACI-TIPI) – was validated in large populations and then deployed in a randomized trial in an attempt to improve triage decisions17. While there was no overall effect on triage decisions compared to standard evaluation without the instrument in the whole population, in a subgroup of medical residents whose decisions were not routinely supervised, there was a favorable effect of providing the probability information in that fewer patients were unnecessarily admitted to cardiac intensive care units. Thus, while several such instruments are validated, there has been no definitively favorable effect demonstrated in trials on triage decisions. Moreover, these tools provide a probability estimate along a continuous scale from 0 – 100%, likely more “precise” than the estimates of individual clinicians, but not dichotomous enough to strongly effect decision making.
The use of imaging techniques in the evaluation of patients with chest pain in the ED has increased steadily. Between 1999 and 2008, there was a greater than 4-fold increase in advanced medical imaging, beyond standard x-ray testing, in the ED.1 The goal of the addition of imaging techniques to usual care in the ED is to aid in the early identification of ACS or its absence, and allow risk stratification of all patients. If a normal or “low risk” image is associated with a very high negative predictive value, some very low risk patients may be directly discharged home from the ED, while others will require further inpatient evaluation and/or treatment. An advantage of imaging tests over predictive instruments is that imaging tests may be interpreted in a more dichotomous manner, ie as “normal” or “abnormal” (with varying degrees of abnormality). Conceptually, this may be easier for a clinician to incorporate into his/her data base in order to drive a clinical triage decision.
The ideal candidate for the addition of an imaging test in the ED is one at low or intermediate risk for ACS who cannot adequately be risk stratified by traditional methods such as history, physical exam, ECG, and early biomarkers, such that the evaluating clinician cannot confidently make a discharge decision. This report will review the major imaging modalities that have been studied in this setting.
In the near future, new biomarker assays such as high sensitivity cardiac troponin assays, which are not currently available in the United States, may be more effective than current strategies in reliably and rapidly excluding MI in low risk patients. In a prospective study of a high sensitivity troponin assay, using a very low cutoff in patients who presented to the ED with chest pain excluded MI with a 100% sensitivity.18 Similar results have been found with different assays of high sensitivity troponin, depending on the cutoff for a negative value.19 Studies using an “accelerated diagnostic protocol” which combine a risk score to identify patients (such as a TIMI risk score of 0) and then serial high sensitivity troponin I assays over 2 hours suggest that 10–20% of patients may be identified as very low risk relatively quickly, and require no further testing20. The identification of a very low risk group of patients by new biomarker assays may obviate the use of imaging in this select group.
Rationale for the use of functional and anatomic cardiac imaging
In the setting of myocardial ischemia, oxygen supply through coronary arterial tree is insufficient to meet myocardial demand. Ischemia is not an “all-or-none” phenomenon with regard to functional consequences, but rather exists as degrees of supply/demand mismatch resulting in a stereotypical sequence of functional abnormalities. In the so-called “ischemic cascade”, the earliest manifestation is a perfusion abnormality. As supply/demand mismatch worsens, left ventricular diastolic abnormalities ensue, and then later systolic wall motion abnormalities. Ischemic changes in the ECG and angina are late events.21 The ability to use imaging to observe regional changes in myocardial blood flow (with perfusion imaging), regional variation in systolic function (with echocardiography or cardiac MR imaging), allows for identification of myocardial ischemia in patients even without ECG changes. Visualization of the coronary anatomy (with coronary CT angiography) can allow an indirect assessment of the likelihood that ACS is present by demonstrating the presence or absence of coronary artery disease (CAD).
Rest radionuclide myocardial perfusion imaging
Early reports of rest radionuclide myocardial perfusion imaging (MPI) to assess patients with chest pain in the ED date back to the 1970s. In early studies, thallium-201 planar imaging was performed in patients with unstable angina and suspected MI (Figure 1). The results showed higher sensitivity to detect unstable angina compared to ECG alone, even in the absence of angina.22 Additionally, there were more positive scans in those patients with a complicated course compared to those with a more stable course, providing prognostic data. Thallium-201 scans were subsequently evaluated in triage to the coronary care unit of patients who presented with concern for MI but a non-diagnostic ECG. This study showed a 100% sensitivity of thallium-201 rest MPI to detect acute MI.23 While these very early studies illuminated the potential of non-invasive imaging to assess patients with unstable angina and acute MI, thallium-201 has properties not well suited for this purpose. While the initial uptake reflects myocardial blood flow, the tracer subsequently “redistributes” over time, and thus imaging needs to be completed relatively quickly after injection. This makes using thallium-201 as the perfusion tracer for imaging ED patients challenging.
Figure 1:
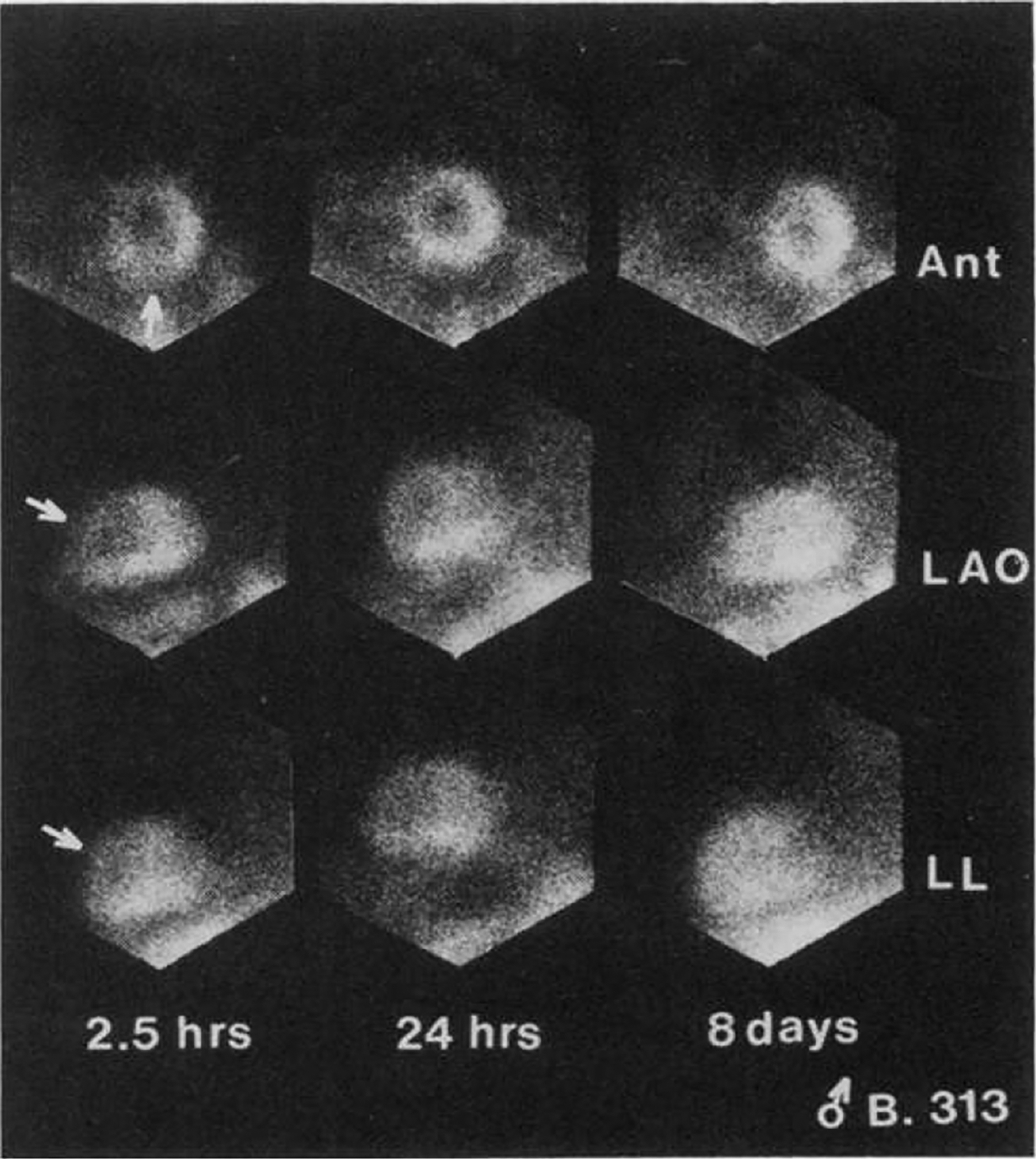
Thallium201 planar imaging after resting injection in a patient with unstable angina. The arrows in the first column point to a perfusion defect in the anterior wall and apex. Images obtained on a separate injection at day 8 (3rd column) show normal perfusion in tose walls, suggesting that the initial defect was a transient perfusion abnormality, consistent with unstable angina. While technically crude by today’s imaging standards, this was among the earliest efforts to noninvasively image myocardial perfusion in the acute ED setting. Reproduced from Wackers et al, N Engl J Med 1976.
However, the subsequent availability of the Tc99m-based agents such as sestamibi and tetrofosmin, which are also taken up initially based on myocardial blood flow but then only minimally redistribute, enabled rest perfusion imaging in this setting to be performed more conveniently. This advance in radiopharmaceuticals, along with the widespread increased availability of single-photon electron computed tomography (SPECT) scintillation cameras helped enable a robust body of literature examining the use of MPI for the evaluation of chest pain in the ED.
Rest only MPI in acute myocardial infarction
Initial studies evaluated the use of rest imaging with Tc99m-sestamibi in patients with myocardial infarction, to assess infarct size. In patients with ST-segment elevation acute MI, rest MPI performed prior to thrombolysis demonstrated areas of hypoperfused myocardium, representing the area-at-risk of infarct, and furthermore, correlated with areas of wall motion abnormalities in those areas where flow was not restored.24 In patients without ECG changes diagnostic of MI, when rest MPI was abnormal, arterial occlusion was seen in the majority of patients, often in the left circumflex territory.25 This work helped to establish rest MPI as a tool to identify the amount of myocardium at risk in acute MI, to detect those patients with MIs that were not evident by the presenting ECG, and most importantly to accurately assess infarct size. Subsequently, the use of rest perfusion imaging and assessment of infarct size using Tc99m-sestamibi became a widely used marker of infarct size in clinical trials of therapeutic agents for patients with myocardial infarction.26
Joseph Alpert: The size of the acute MPI defect reflects abnormal perfusion to necrotic myocardium as well as zones of persistently ischemic but not yet necrotic myocardium. The size of the defect will often decrease over time as ischemia is relieved and the necrotic myocardium is replaced by fibrous tissue.
Rest only MPI in Suspected Acute Coronary Syndromes
Throughout the 1990s, a number of studies established the use of rest MPI in patients presenting to the ED with chest pain to establish or exclude the diagnosis of ACS.27–35 Across these studies, the negative predictive value of rest MPI to exclude myocardial infarction was consistently excellent. Table 1 shows the sensitivity, specificity, positive, and negative predictive values for studies of rest MPI in acute chest pain. Examples of normal and abnormal studies are shown in Figures 2–4.
Table 1.
Performance Characteristics of Resting Myocardial Perfusion Imaging for Detection of Relevant Diagnostic Endpoints in the ED Setting
| Author/Reference | Patients | Sensitivity | Specificity | NPV | Endpoint |
|---|---|---|---|---|---|
| Wackers et al. 23 | 203 | 100 | 63 | 100 | MI |
| Bilodeau et al. 27 | 45 | 96 | 76 | 94 | CAD (by angiography) |
| Varetto et al. 28 | 64 | 100 | 67 | 100 | MI |
| 100 | 92 | 100 | CAD | ||
| Hilton et al. 29 | 102 | 100 | 78 | 99 | MI |
| 94 | 83 | 99 | All Events | ||
| Tatum et al. 30 | 438 | 100 | 78 | 100 | MI |
| 82 | 83 | 98 | MI, revasc | ||
| Kontos et al. 31 | 532 | 93 | 71 | 99 | MI |
| 81 | 76 | 95 | MI, revasc | ||
| Heller et al. 32 | 357 | 90 | 60 | 99 | MI |
| Duca et al. 33 | 75 | 100 | 73 | 100 | MI |
| 73 | 93 | 81 | CAD | ||
| Kosnik et al. 34 | 69 | 71 | 92 | 97 | MI, revasc, or cardiac death |
| Kontos et al. 35 | 620 | 92 | 67 | 99 | MI |
| Udelson et al. 36 | 1215 | 96 | NR | 99 | MI |
| Schaeffer et al. 36a | 479 | 77 | 92 | 99 | MI |
Figure 2:
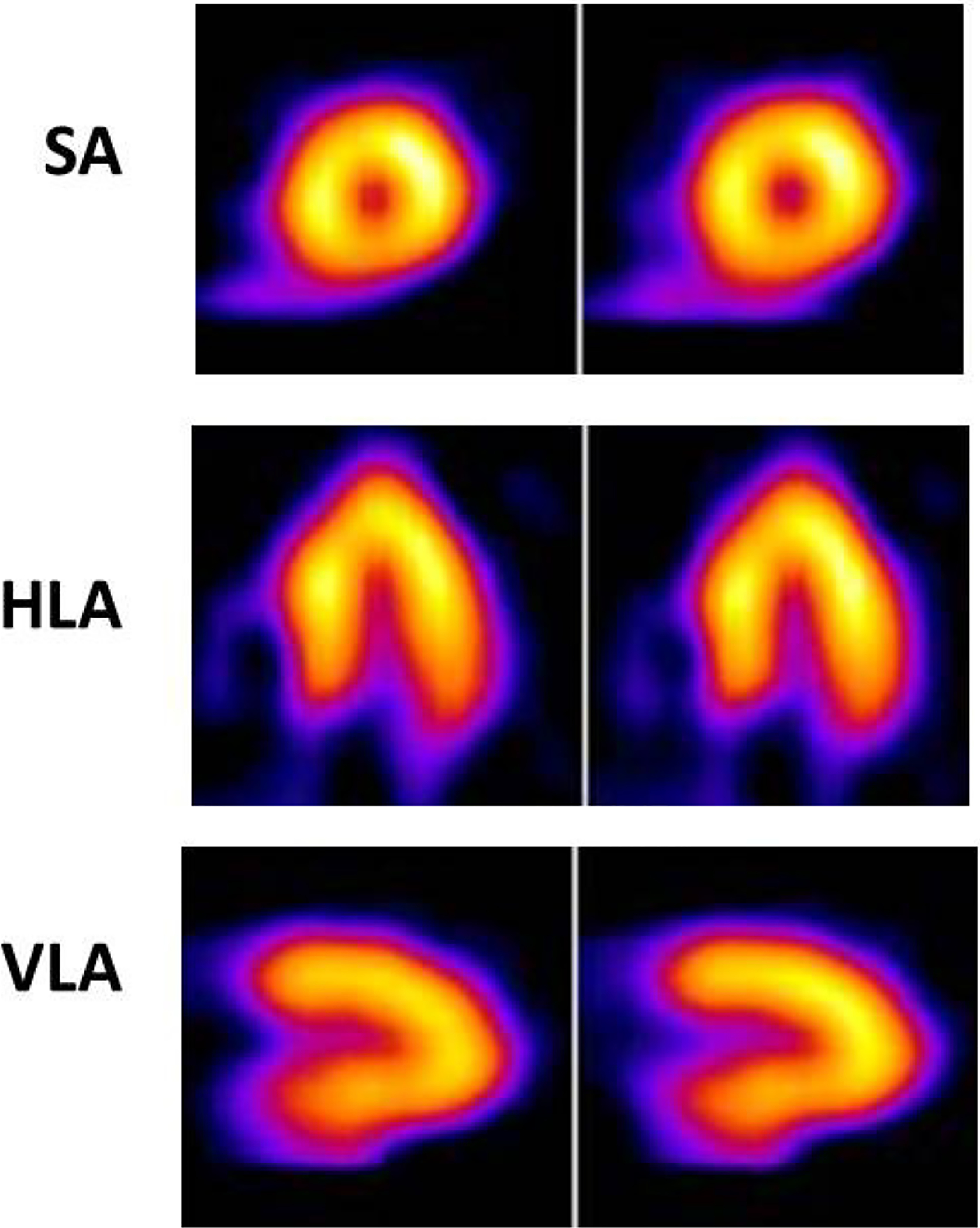
Normal myocardial perfusion at rest is seen following injection of a Tc99m perfusion tracer in the ED in a patient with cheat pain but a low-to-intermediate likelihood of ACS, in the short-axis (SA), horizontal long-axis (HLA) as well as in the vertical long-axis (VLA) tomograms. The results suggest a very low probability of ACS.
Figure 4:
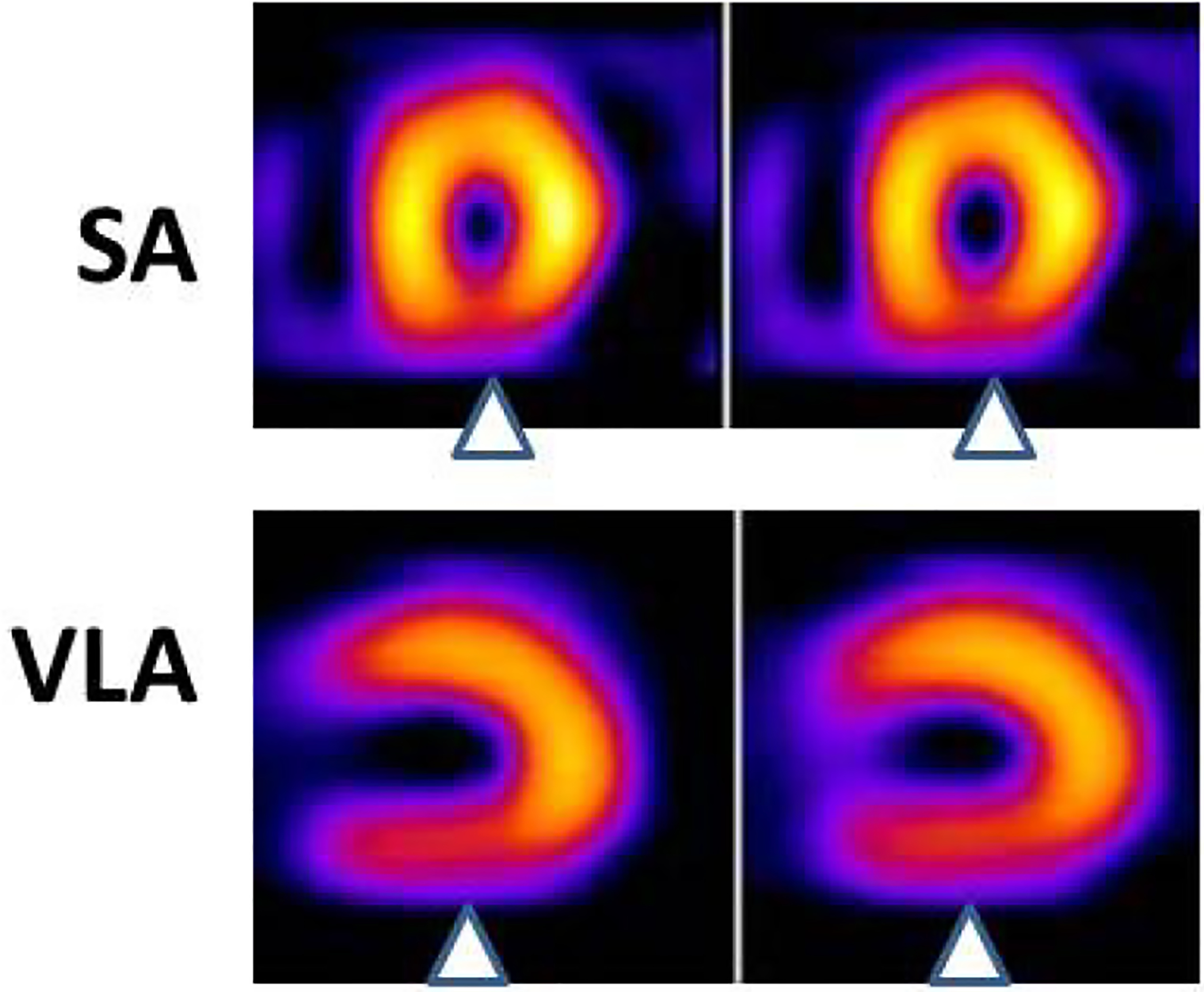
In the short-axis (SA) view, an inferior wall perfusion defect is seen (arrowheads). This finding is confirmed in the vertical long-axis (VLA) tomograms (arrowheads). This finding is consistent with a perfusion defect at rest in the inferior wall, likely secondary to an acute coronary syndrome.
In one of the earliest studies of the contemporary era using Tc99m-agents, Bilodeau and colleagues studied hospitalized patients with suspected ACS. Tc-99m-sestamibi was injected at the time of symptoms, and an ECG was obtained. The sensitivity of the rest SPECT perfusion image was significantly higher than the ECG recorded during symptoms at detecting the presence of a coronary stenosis on subsequent angiography. These data suggest that rest perfusion imaging could indeed identify an acute abnormality in myocardial blood flow, and do so with higher sensitivity than resting ECG, even during symptoms.
Abnormal rest myocardial perfusion scans in this setting are also associated with cardiovascular outcomes beyond what occurs in the ED.28–30,32,36 In the first study to examine this association, Varetto and colleagues reported in a relatively small group of patients who had resting SPECT MPI in the ED, and then were followed over time. The finding of greatest interest was that among patients with suspected ACS who had normal resting perfusion scans, there were no adverse cardiovascular events (death, MI, need for revascularization) either in the short-term or during one year of follow-up. While the number of patients in this report was modest, the data suggested that a normal perfusion study identified patients at very low risk of having ACS as well as subsequent events, suggesting that ED direct discharge could be possible.
Subsequently, in a larger study30, almost 1,200 ED patients with suspected ACS were assigned to 1 of 5 categories of risk for ACS based on clinical characteristics of chest pain, ECG findings, and history. Patients in the two highest categories of risk, as well as those identified as having chest pain of a non-cardiac cause (the lowest risk category) did not undergo MPI. Only those patients with ECGs that were non-diagnostic for ischemia or infarction, and were identified based on clinical history to have possible or probable unstable angina (low to moderate risk) had perfusion scans performed. Consistent with other similar studies, the sensitivity for MI was 100% (95% confidence interval [CI], 64% to 100%), and negative predictive value for MI or revascularization over 1 year of follow-up was 97% (95% CI, 95% to 98%). No patients with a normal perfusion scan suffered death or MI in the 12 months follow-up, compared to 11% with MI and 8% who died with an abnormal perfusion scan. Including revascularization, the total event rate was 0.9% in patients with a normal resting scan and 42% in those with abnormal findings. These data added great weight to the concept that a normal perfusion study identified a very low risk group, potentially eligible for early discharge, and that an abnormal rest perfusion study identified a very high likelihood of ACS, patients who could be triaged to more rapid treatment rather than awaiting serial biomarker studies followed by a stress test.
Joseph Alpert: These studies demonstrate that MPI is very sensitive, that is there are few to no false negatives. However, there are still a substantial number of false positive results based on subsequent coronary angiographic findings.
Throughout the 1990’s, data continued to accumulate regarding the implications of rest SPECT MPI in the ED setting. Heller et al reported data from a multicenter observational study showing that the SPECT MPI results were the strongest predictor of MI when compared with clinical and ECG data in a multiple logistic regression analysis, and that there is an incremental increase in adverse outcomes based on the degree of abnormality of rest MPI scans.27
Thus, numerous reports from different institutions and countries, some single center and some multicenter, in a total of approximately 1,800 patients, had reported the relation between scan findings and outcomes when rest SPECT MPI was performed in the ED setting in low-to-intermediate risk patents with suspected ACS. Recent data have shown consistent results even in developing countries.37 Given the consistent association of very low event rates with normal myocardial perfusion scans, the implication was that rest MPI could be a useful tool in the triage decision in the ED. Patients with a normal rest MPI study could in theory be safely discharged home from the ED, with further follow up as indicated, while those with an abnormal study would best be triaged rapidly to admission and ACS treatment.
Joseph Alpert: Despite these reassuring numbers, it is still frequently difficult for ED physicians to send a patient with atypical chest discomfort home without further evaluation.
Randomized trials of SPECT MPI in the ED
Traditional assessment of the value of imaging modalities has involved calculation of performance characteristics such as sensitivity and specificity in relation to a truth or gold standard (usually in this case stenosis > 50% or 70% on invasive angiography), and later, analysis of the relation between imaging findings and outcomes during follow-up (prognostic value). The initial years of reports on MPI in the ED setting followed this course. However, to critically assess the true value of any test, as assessment should be made of the effect on clinical decision making when using the test vs. when not using the test. Evaluation of the role of imaging modalities in assessing patients with chest pain in the ED has been an area in which many randomized trials have been performed and reported, an example of how imaging should ultimately be evaluated in all clinical syndromes.
The first such trial to be reported was that of a small number of patients (n=46) with chest pain in EDs, who were randomized to an evaluation strategy guided by the results of rest Tc99m-tetrofosmin imaging vs. evaluation by a conventional strategy not incorporating imaging38. Further testing such as catheterization or stress testing was protocol-defined i.e. not completely at the discretion of the clinicians. The results showed shorter length of stay and lower costs in the imaging guided group, with similar rates of events both in-hospital and out to 30 days of follow-up. This was an important step forward in setting directions for study. However, the results were driven by the protocol directed care, so whether clinicians in real life settings would come to similar results is uncertain.
This study and the observational literature that preceded it are examples of “efficacy” analyses, that is, how tests perform when evaluated by experts in the field under highly controlled conditions. This is in fact how much of the literature in the cardiovascular imaging field is performed. A higher level of evidence is provided by studies of “effectiveness”, which reflect how tests work in a more real life setting, and clinicians are not directed in their decisions by protocol.
The Emergency Room Assessment of Sestamibi for the Evaluation of Chest Pain (ERASE Chest Pain) multicenter trial was specifically designed as an effectiveness trial, to test whether providing results of rest MPI to ED clinicians for patients with low-to-intermediate likelihood of ACS would improve clinical decision making defined as the appropriateness of an admitting decision36. An appropriate admission was defined as admission of a patient who was ultimately found to have a final diagnosis of ACS (blindly adjudicated), while an unnecessary admission was defined as admission of a patient who was ultimately found to have a final diagnosis of “not ACS”. Approximately 2,500 patients at 7 centers were randomized to one of 2 evaluation strategies – one strategy involved a rest SPECT MPI study, with the scan read right away and results provided to the ED clinician, who incorporated those results with other available information to make a decision to admit or discharge from the ED. The alternative strategy was the standard of care evaluation of such patients in each ED, without imaging. The hypothesis, based on the strong observational literature, which was provided to the ED clinicians, was the incorporation of the imaging results would reduce unnecessary admissions without compromising appropriate admissions. It is of interest to note that in such a trial, the effect of a test on decision making is being tested, rather than the performance of the test itself for identifying disease or non-disease, as effectiveness trials take place once those characteristics have been reasonably established.
The results supported the hypothesis, in that among the patients randomized to the imaging strategy who ultimately were found to not have ACS, unnecessary admissions were significantly reduced (relative risk 0.84; 95% CI, 0.77–0.92), while there was no change in appropriate admission for those with ACS.
Joseph Alpert: These findings demonstrate a 16% reduction in unnecessary admissions to the hospital of patients with chest discomfort.
The results of this large multicenter randomized effectiveness trial represented strong evidence that incorporating rest MPI in this setting can improve triage decisions.
Issues in Image Interpretation
The clinician familiar with the interpretation of MPI knows that all scans cannot be clearly divided between normal and abnormal. A normal scan (Figure 2) shows homogenous uptake of radiotracer in all coronary distributions, in several reconstructions. An abnormal scan (Figures 3 and 4) demonstrates a relative decrease in radiotracer uptake in one or more coronary distributions. Equivocal scans are not an uncommon finding.
Figure 3:
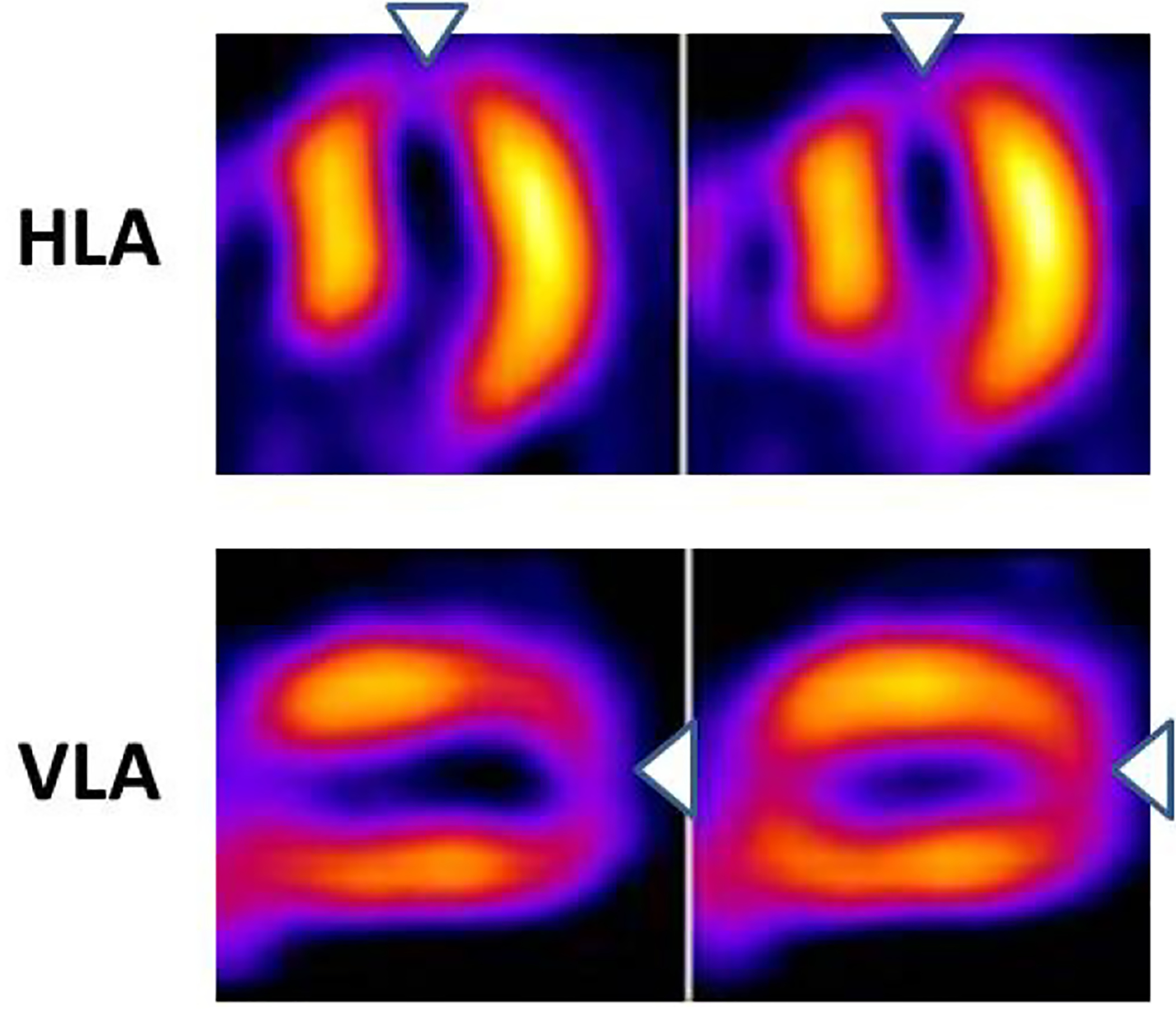
In the horizontal long-axis (HLA) as well as in the vertical long-axis (VLA) tomographic views, an apical perfusion defect is demonstrated (arrowheads) suggestive of resting ischemia or infarct. Catheterization showed a severe stenosis of the left anterior descending coronary artery.
Joseph Alpert: Patients with equivocal scans will almost certainly need hospital admission for further evaluation, for example, coronary angiography.
Patient motion, body habitus, breast shadow, diaphragmatic attenuation, interference from gastrointestinal tract uptake of radiotracer, and delayed acquisition of imaging can all result in limitations in interpretation. Corrective measures, such as repeat imaging in the prone position in patients with inferior defects when diaphragmatic attenuation artifact is suspected, should be performed to minimize false positive results (Figure 5). Care should be taken to maintain optimal sensitivity and negative predictive value of this modality by erring on the side of reporting indeterminate findings as such.
Figure 5:
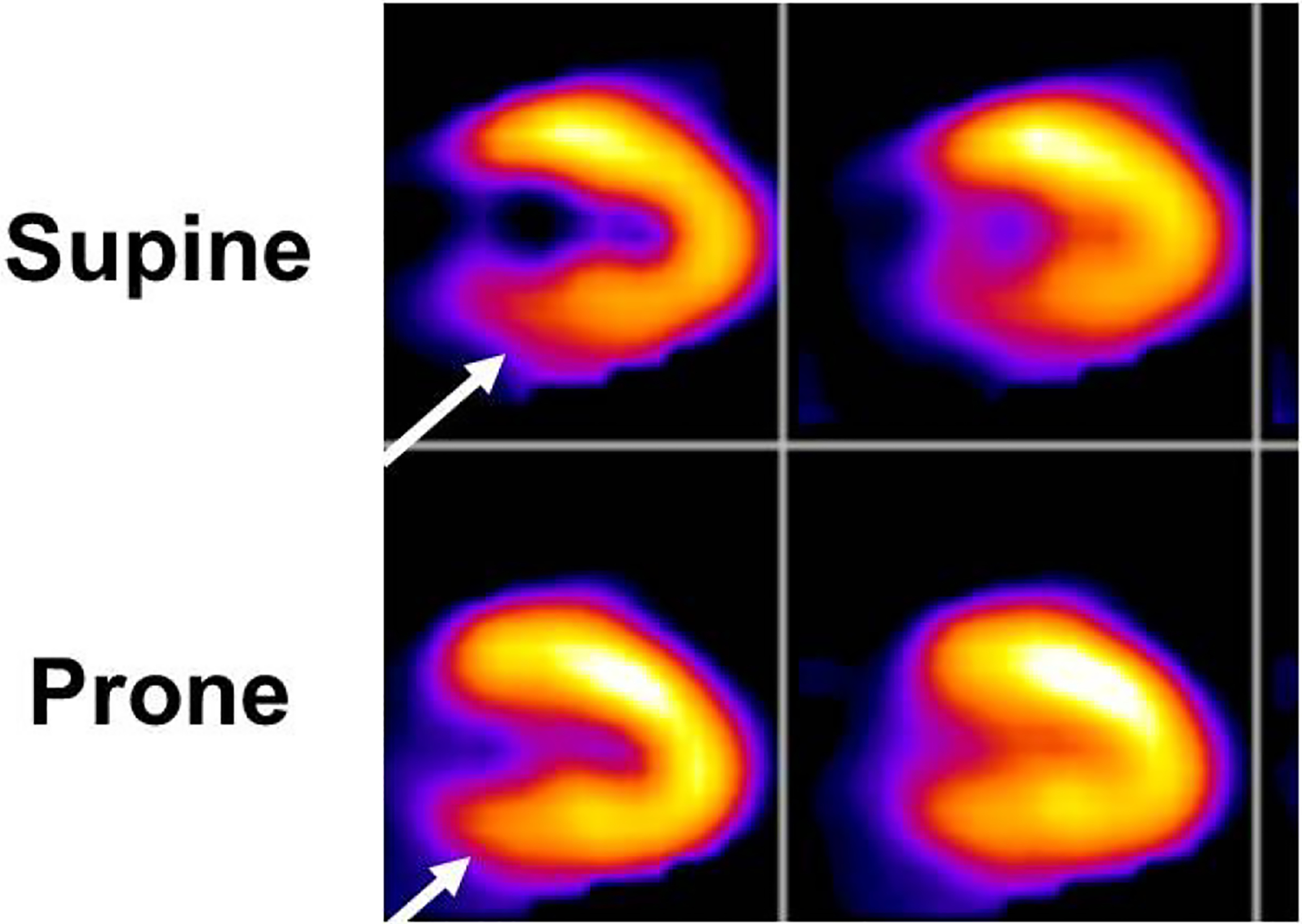
An example of the use of prone imaging to clarify the status of the inferobasal wall. In the images obtained from the usual supine position of the patient (top row), there is an apparent inferobasal abnormality (arrow). However, this could also represent attenuation by the diaphragm. Imaging was repeated in the prone position, which is thought to Position the heart further away from the diaphragm. The resultant images (bottom row) show normal homogeneous perfusion of the inferobasal wall (arrow) and the study was interpreted as normal.
Gated SPECT using Tc-99m radiotracers has the added benefit of allowing the evaluation of left ventricular function and regional wall motion. In one large single-center study of patients who presented with chest pain and underwent gated MPI, severely abnormal left ventricular function (<35%) was the strongest independent predictor of 1 year mortality after multivariate analysis.39 Mild to moderate left ventricular dysfunction (35%−50%) was also an independent risk factor for death. Given the generally low risk nature of this type of population, such a finding is unusual, but when present is highly important.
The interpretation of rest MPI studies in patients with a prior history of myocardial infarction can be limited for assessment of a new ACS, unless a prior perfusion study is available for comparison. In the absence of a prior scan, a defect seen may represent an area of acute infarct, ongoing ischemia, or an area of prior infarct. The availability of this information should be considered prior to undertaking an evaluation with MPI, as abnormal findings may not contribute to acute management or triage decision-making.
Timing of Radiotracer Injection
The optimal timing of radiotracer injection is while the presenting symptom of chest pain is ongoing; however, myocardial perfusion abnormalities may persist for several hours beyond resolution of symptoms. In an experimental model of ischemia performed in the cardiac catheterization laboratory during coronary balloon inflation for stent deployment, Tc-99m sestamibi was injected at baseline, and in certain subjects at an additional time point ranging from 1 to 3 hours later. Another group was injected with radiotracer only at 15 minutes after coronary balloon occlusion. All patients were injected with radiotracer and imaged again 24–48 hours after the initial injection. In comparison to baseline imaging, where all scans displayed a perfusion defect, 70%, 13%, and 19% of subjects had a visible defect at 15 minutes, 1–3 hours, and 24–48 hours, respectively.40 In a clinical study that included patients who had radiotracer injected up to 6 hours after the resolution of symptoms, there was no difference seen in the sensitivity of rest MPI for any endpoint studied, including MI, revascularization, or significant coronary artery disease (defined as ≥70% stenosis in a major coronary artery or its branches or ≥50% stenosis of left main coronary artery) from images acquired after an injection earlier vs. later with that time period.31 The timing of when exactly the sensitivity of perfusion imaging drops (i.e., when does perfusion return to normal after symptoms subside) is not known.
Appropriate Use Criteria and Guidelines
Most recent appropriate use criteria for the use of radionuclide imaging supported by a number of different societies including the American College of Cardiology, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, and the Society of Nuclear Medicine, among others, rate the use of rest only MPI in the setting of acute chest pain as appropriate, given ACS is suspected, the initial ECG is non-diagnostic or normal, initial troponin is negative, and pain is ongoing or recent.41
Joseph Alpert: It is important to remember that MPI and other stress tests are most useful when patients have a lower or intermediate likelihood of having ACS. Patients who are deemed high risk for ACS should be admitted to the hospital for further evaluation, often involving coronary angiography.
Future Directions
One significant barrier to overcome is the need for ongoing or recent symptoms for rest MPI to have its highest sensitivity. At the cellular and molecular level, ischemic myocardium switches from the metabolism of fatty acids to the use of glucose for production of adenosine triphosphate. Even if normal myocardial perfusion is restored, the switch back to fatty acid metabolism is delayed for many hours. This so-called “ischemic memory” can be exploited to identify metabolic abnormalities reflective of the prior ischemic event even many hours after symptoms have resolved. β-methyl-p-[123I]-iodophenyl-pentadecanoic acid (BMIPP) is a fatty acid that is taken up in the myocardium and not significantly metabolized. Imaging of this agent, even up to 30 hours after the resolution of chest pain symptoms, allows for delayed detection of ischemic myocardium, which appears as a defect reflecting the reduced regional fatty acid metabolism.42 In a study of ED patients with chest pain that could have resolved within the previous 30 hours, the addition of imaging with BMIPP added to initial clinical information increases the sensitivity to detect ACS from 43% to 81% (Figure 6).43 The identification of patients with ACS more than a day after resolution of symptoms with a single imaging test would allow for the rapid evaluation of a much larger percentage of patients presenting to the ED for complaints of chest pain. At the time of this report, BMIPP has not been approved by the Food and Drug Administration for this purpose in the United States.
Figure 6:
Imaging of fatty acid metabolism with BMIPP. In a patient injected with the tracer many hours after resolution of symptoms, a defect is visualized in the anterior wall and apex. This is consistent with regional reduction of fatty acid metabolism many hours after an episode of ischemia, at which time the myocardial metabolism switched from predominantly fatty acid to glucose metabolism. This persisted for hours after blood flow was restored, so-called “ischemic memory”. Adapted from reference 43.
Joseph Alpert: This interesting new approach might be useful in determining if a patient had had an episode of myocardial ischemia that had recently resolved. However, it is unlikely that this test would be able to differentiate between myocardial ischemia that was the result of ACS and myocardial ischemia that was the result of vigorous exercise.
Positron emission tomography has better resolution compared to SPECT, there is less attenuation of the higher energy emitted photons, and its use allows for quantification of myocardial blood flow. The potential of its use in the detection of ACS is attractive in concept. However, deployment of this technology for use in the ED setting is currently limited by the relatively limited availability of imaging equipment and radiotracers. As the use of position emission tomography for myocardial perfusion potentially becomes more widespread, this modality holds promise for advances in the imaging of patients with acute chest pain.
Echocardiography
A major strength of resting 2-dimensional (2-D) echocardiography in the evaluation of acute chest pain is the ability to identify several causes of pain beyond ACS. Visualization for presence of a pericardial effusion, dissection of the ascending aorta, valvular heart disease, and right ventricular dilation and dysfunction in pulmonary embolism is achieved with a single study. The availability of echocardiography is widespread, although a skilled operator is needed to acquire images and experience is required for expert interpretation of images. The equipment is reasonably portable, and imaging can be performed directly at the bedside in the ED. Evaluation of suspected ACS by rest 2-D echocardiography is based on that concept that in the “ischemic cascade” a perfusion abnormality of a certain severity will result in an abnormality of regional wall motion and myocardial thickening, and possibly if extensive enough an impact on left ventricular ejection fraction (Figure 7).
Figure 7:
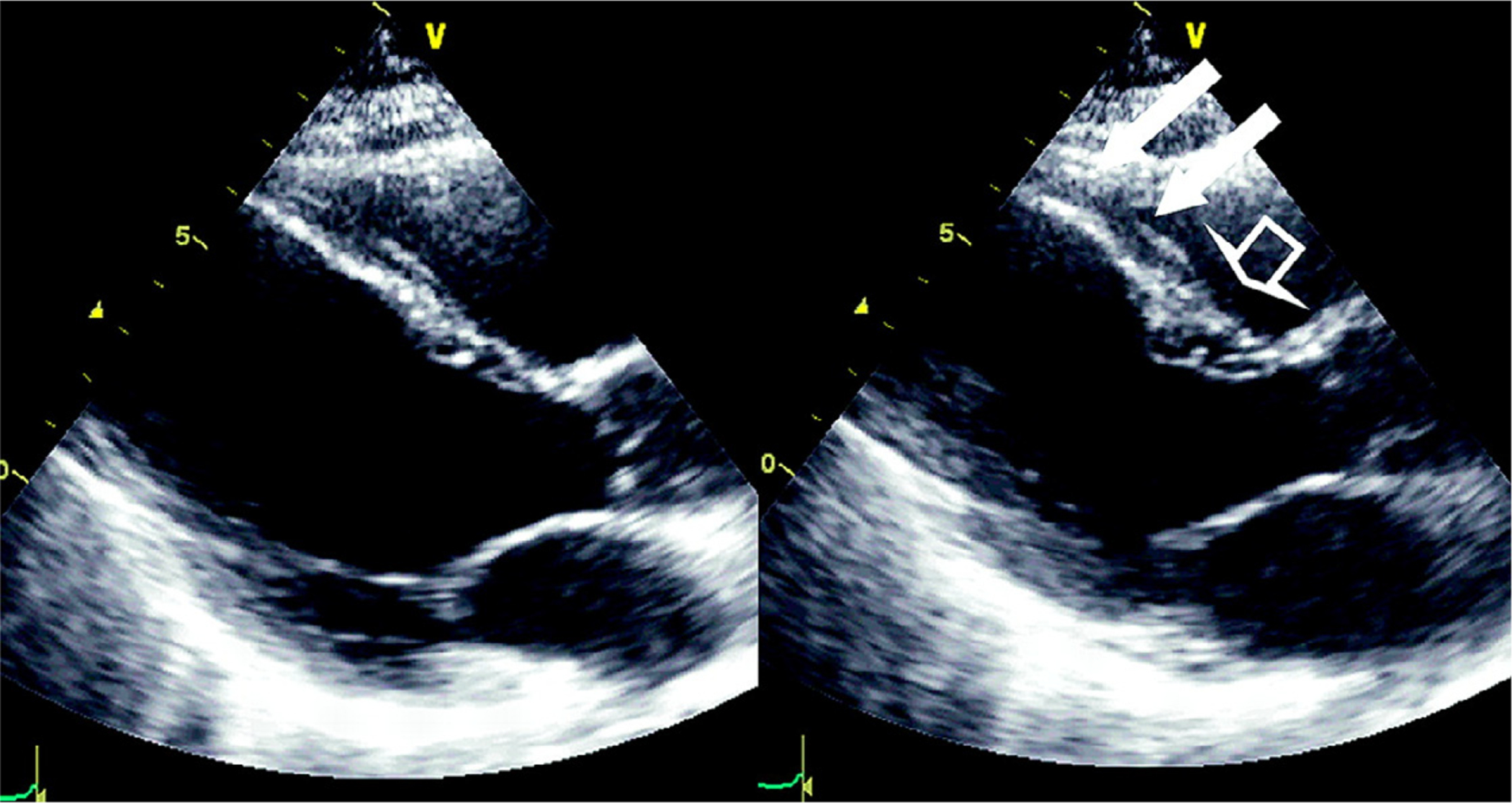
Echocardiography to detect a region of abnormal wall motion at rest, from end diastole (Left panel) to end-systole (right panel). In the end-systolic frame, the white arrows highlight a dyskinetic segment of the distal anterior septum, contrasted with the normally thickening basal septum (open arrow). This finding could represent resting ischemia or infarct when seen in an ED patient with ongoing symptoms. Reproduced from Flachskampf, Daniel. Cardiac imaging in the patient with chest pain: echocardiography. Heart 2010
Joseph Alpert: One distinct advantage of echocardiographic stress testing is that patients do not receive any ionizing radiation.
Echocardiography in Acute MI
The ability to detect acute MI by wall motion abnormality on 2-D echocardiography is dependent on the quality of the imaging and the presence and extent of wall motion abnormality. In a study performed to evaluate the detection of MI in 180 patients who presented with ongoing or recent chest pain or shortness of breath, the presence of regional wall motion abnormality outperformed abnormal ECG findings in making the diagnosis of acute MI.44 Thirty of the 180 patients had biomarker confirmed MI, 27 of whom had a resting wall motion abnormality on 2-D echocardiogram. In comparison, only 9 patients had evidence of ST segment elevation on ECG. The remaining 21 ECGs were either uninterpretable, normal, or showed nonspecific changes. . Additionally, the presence of regional wall motion abnormalities on 2-D echocardiography was a better predictor of in-hospital complications (arrhythmia, cardiogenic shock, or persistent angina) than either physical examination or the initial ECG. All thirteen patients who had in-hospital complications had regional wall motion abnormalities on echo, but only 4 had ST segment elevation on ECG and no physical exam findings were helpful in identifying these patients. However, 60 of 140 patients who did not have an acute MI but had a technically adequate 2-D echocardiogram had regional wall motion abnormalities. An additional 22 had global left ventricular dysfunction. These changes may have been due to ischemia without myocardial necrosis or prior MI. Overall, rest 2-D echocardiography has a sensitivity of 93% for the diagnosis of acute MI, but a lower specificity.45
Joseph Alpert: Regional left ventricular dysfunction can also be seen on occasion in patients with cardiomyopathy or serious illness such as sepsis. Rarely, patients with acute alcoholic cardiomyopathy will demonstrate regional wall motion abnormalities.
Similar to rest MPI, echocardiography cannot reliably establish old versus new areas of infarct, or distinguish old infarct from a wall motion abnormality associated with a new region of ischemia. Changes of increased echogenicity and thinning of wall segments may be seen with fibrosis after remodeling or thinning of previously infracted wall, but this is not reliable enough to differentiate old from new regions of wall motion abnormalities. Therefore, unless prior studies are available for comparison in patients who have a documented history of myocardial infarction, a wall motion abnormality seen on a study performed in the setting of acute chest pain may represent either ongoing ischemia or infarction, or simply an area of old infarct.
Echocardiography in ACS
When examining 2-D echocardiography in the detection of unstable angina and myocardial ischemia, both the sensitivity and specificity are somewhat lower than the data reported in studies of clear acute MI, with an average sensitivity of 88% reported.46–48 Furthermore, because of the possibility that regional wall motion abnormalities may resolve in patients relatively soon after resolution of angina, but not AMI, many studies evaluating the use of 2-D echocardiography for the detection of ACS have been performed with symptoms ongoing.48 The presence of ongoing symptoms is an important determinant of sensitivity. In a study of 46 patients evaluated after presentation for chest pain without a diagnostic ECG or biomarkers, the sensitivity of 2-D echocardiography to identify MI or significant coronary artery disease was 88% in patients who were imaged during symptoms. In the group of patients that were imaged after resolution of chest pain, the sensitivity dropped to 64%.47 Conversely, in another study of 260 patients who had echocardiography performed within 4 hours of arriving to the ED for a suspicion of ACS, the sensitivity of echocardiography for predicting cardiac events was substantially higher than ECG. In this particular study, the sensitivity of echo for detecting cardiac events remained high, despite the delay in image acquisition, at 91%.46 Because there is no clear timeframe during which regional systolic wall motion abnormalities will resolve after resolution of myocardial ischemia, the highest yield of 2-D echocardiography in the evaluation of ACS is with chest pain ongoing.
Joseph Alpert: Or when chest discomfort has recently resolved.
Portable Cardiac Ultrasound
Although equipment used to perform echocardiography is relatively portable, there is an interest in making the technology even more portable with handheld devices that can be used rapidly in the initial evaluation of patients arriving to the ED with chest pain. Some smaller series have reported similar sensitivity and specificity of these handheld ultrasound devices to traditional 2-D echocardiography detect regional wall motion abnormalities, even when performed by operators without extended formal training.49,50
Joseph Alpert: Experience in utilizing this new technology is essential if false positive results are to be avoided. As the late professor Judah Folkman of the Harvard Medical School often stated: “The only substitute for brilliance is experience.”
Emergency room providers often use ultrasound in their initial evaluation, including for those patients with chest pain. The so called focused cardiac ultrasound examination is intended to rapidly identify pericardial effusion, assess global systolic function, discover significant left or right ventricular enlargement, and assess intravascular volume through identification of the diameter and degree of collapse of the inferior vena cava. Additionally, certain procedures such as pericardiocentesis and transvenous pacing wire can be guided with the focused cardiac ultrasound exam. The American Society of Echocardiography consensus statement reports the exam is not intended to replace a comprehensive echocardiogram, and the majority of providers who perform the test will not be vigorously trained in the acquisition and interpretation of ultrasound imaging to identify regional wall motion abnormalities.51 As of yet, there are no strong data to support the use of handheld ultrasound in the initial evaluation of suspected ACS, without concomitant high suspicion of dissection or pericardial effusion.
Echocardiographic Imaging with Contrast
Echocardiographic contrast consists of gas microbubbles that are encapsulated, and create a nonlinear vibration from contact with the ultrasound wave emitted from the transducer. In the left ventricle, this provides a contrast to the surrounding myocardium, and allows for more optimal delineation of the cavity borders. The use of contrast echocardiography for opacification of the left ventricular cavity is safe in the setting of ACS.52 Improved identification of the endocardial border allows for enhanced assessment of regional wall motion abnormalities and overall left ventricular function, especially when imaging is technically difficult in the case of large body habitus, lung disease, or if patient discomfort or distress precludes ideal positioning.
Beyond the use of contrast for left ventricular cavity opacification, it has also been investigated for evaluation of myocardial perfusion. The gas microbubbles of echocardiographic contrast also enter the myocardial circulation. The bubbles are fragile, and if a strong ultrasound pulse is generated, they will burst. Careful imaging of the myocardium in the cycles after the ultrasound pulse will demonstrate new contrast agent entering the myocardial microvasculature (Figure 8). This can be visualized and analyzed based on the time to reperfuse, and correlates with myocardial blood flow to various segments.53
Figure 8:
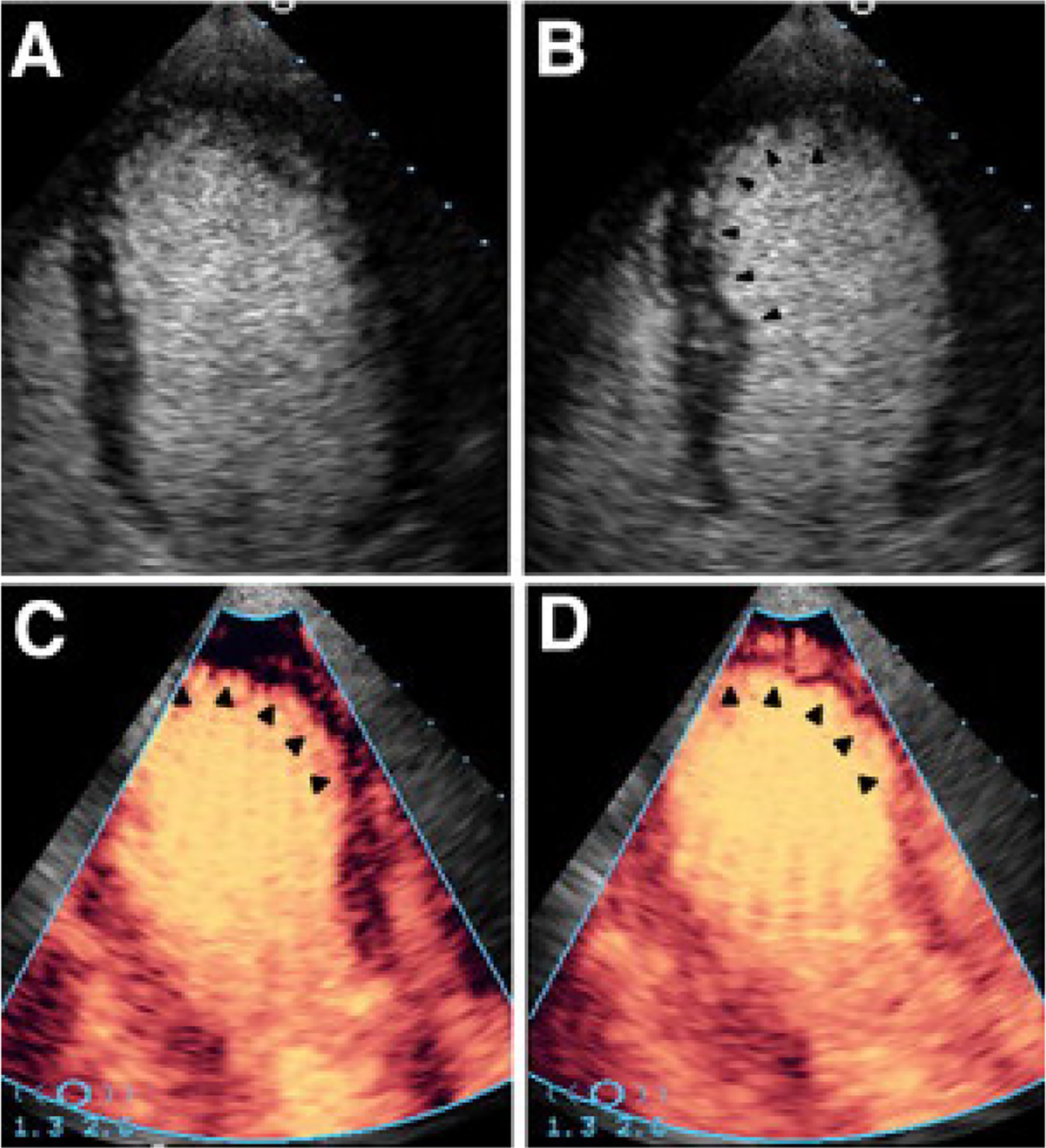
Contrast enhanced echocardiography in the evaluation of acute chest pain. An apical 4-chamber view of the left ventricle at (A) end diastole when compared to (B) end systole shows absence of myocardial contraction in the distal anterior septum and apex (arrowheads). These akinetic segments likely represent ongoing ischemia or infarction in the territory of the left anterior descending coronary artery. The remainder of the visualized segments contract normally. Panel (C) demonstrates contrast perfusion imaging. In this apical 3-chamber view, after an initial high energy ultrasound pulse is delivered, contrast is seen reentering all myocardial segments except those where a wall motion abnormality was seen. (D) After several cardiac cycles, only a small subendocardial defect remains with contrast reperfusing the remainder of the visualized myocardium. Although abnormal, the majority of the perfusion in the territory of the left anterior descending artery is preserved. Reproduced from Wei, Utility Contrast Echocardiography in the Emergency Department. J Am Coll Cardiol Img 2010
Although not FDA approved for the indication of assessing myocardial perfusion, myocardial contrast echocardiography has been extensively studied, and the data suggest that its use is safe and may provide useful and simultaneous data regarding myocardial perfusion and wall motion.
The perfusion and wall motion data derived from contrast perfusion echocardiography in the setting of ACS correlate with radionuclide MPI. A study by Kontos and colleagues54 investigated contrast echocardiography for wall motion analysis and myocardial perfusion in patients with possible ACS who underwent rest SPECT imaging for chest pain. Sixty patients were studied, and both wall motion and perfusion with echocardiographic contrast showed a >80% agreement with SPECT imaging. When results of the two imaging modalities were discordant, contrast echocardiography was abnormal and SPECT was normal in this small cohort. The destruction of bubbles causing an apparent perfusion defect may have led to the relatively increased number of abnormal contrast echo studies, particularly given that more studies were abnormal in the anterior wall and apex, i.e. those segments closest to the ultrasound transducer.
A larger study of 203 patients also evaluated both contrast perfusion echocardiography and SPECT imaging in patients who presented with suspected ACS without ST elevation on initial ECG.55 The concordance between contrast echocardiography and SPECT was again near 80%, with more abnormal contrast echo studies than SPECT. Thirty-eight patients had adverse events defined as MI, revascularization, or cardiac related death, all within 48 hours. Of these, 30 had regional wall motion abnormalities identified and 29 had abnormal perfusion with contrast echo. When compared to clinical, demographic, and ECG data, contrast echo provided 17% incremental information regarding adverse events. Across studies, the methods of administration of echocardiographic contrast, image acquisition and interpretation are still being refined. Further development and standardization of techniques will be required before the widespread routine use of myocardial contrast echocardiography. To date, myocardial perfusion imaging using contrast echocardiographic agents has not been approved for use for regulatory purposes in the United States.
Appropriate Use Criteria and Guidelines
The 2011 appropriate use criteria for echocardiography rate the evaluation of acute chest pain with suspected MI and nondiagnostic ECG when a resting echocardiogram can be performed during pain as appropriate.56 In the absence of pain, but with other features of an ischemic equivalent or positive biomarkers, the use of echocardiography is similarly appropriate.
Joseph Alpert: Which imaging test should be chosen in a particular institution depends on who at that particular institution is most experienced in performing and interpreting a specific test as noted below by the authors.
Stress Testing With or Without Imaging in the Evaluation of ED Chest Pain Patients
The incorporation of stress testing, with or without the imaging modalities described, can be of benefit in the correct clinical setting. Patients who present to the ED with ongoing chest pain and have a negative rest MPI study have a very low likelihood of ACS and a very low adverse event rate and can be safely discharged from the ED with appropriate follow up. However, many ED and chest pain unit protocols call for evaluation with a stress test to assess for provocable ischemia prior to discharge, following negative serial biomarkers.
Typically, stress testing is undertaken in low-to -intermediate risk patients after two negative sets of cardiac biomarkers have been drawn at 6 to 8 hour intervals to exclude ACS before inducing further ischemia.57 However, there is literature suggesting that in carefully selected patients at apparent very low risk on the basis of clinical factors available very early after presentation, immediate ECG stress testing without the need to await serial biomarkers may be safe and effective. In a study by Amsterdam and colleagues, 1,000 patients with low risk chest pain as assessed by such clinical variables underwent immediate symptom limited exercise stress testing with ECG. Almost two-thirds of the tests were negative, but rates of death, MI, revascularization or diagnosis of coronary artery disease was significantly higher in patients with an abnormal or nondiagnostic test compared to a normal study (29%, 13%, and 0.3%, respectively).58 Despite immediate testing without serial, or even initial, biomarker testing there were no adverse events of stress testing. Four patients were ultimately discovered to have evolving ACS at the time of stress test, which in theory and practice can be avoided with point of care biomarker testing. There have not been extensive publications on this approach beyond the authors’ initial reports.
The choice of which stress test to use after negative serial biomarkers depends on several factors, including patient characteristics, availability of different modalities, and expertise of the center. Patients who are able to adequately exercise and have an ECG suitable for interpretation (absence of left bundle branch block, ventricular pacing, left ventricular hypertrophy, ≥1 mm ST segment depression, or use of digoxin) should undergo an exercise treadmill ECG test. In addition to the previously mentioned study, several others have confirmed a high negative predictive value of 98–100% without any adverse events from the test.59–61Exercise ECG testing is completed more rapidly and engenders lower costs than stress testing with imaging.
Stress Radionuclide MPI
In patients unable to exercise, or those with an uninterpretable ECG, the addition of an imaging modality to stress is warranted. One study for the evaluation of chest pain in the ED reported a protocol that used a multi-step process including history and physical, 2-hour biomarker levels, serial ECGs, and stress MPI for select patients based on risk category. The sensitivity and specificity for the diagnosis of ACS at 30 days for those patients who underwent stress testing was 99% and 87%, respectively.62 The protocol studied was successful in showing the excellent performance of stress MPI, and its safety even at 2 hours after presentation in select patients.
More recently, a randomized trial of incorporating stress MPI into the evaluation pathway was published.63 Following a negative observation period involving serial ECG monitoring and serial biomarkers, 1,508 patients were randomized to the use of stress MPI in the ED or to complete a clinical evaluation. Overall, fewer patients who had stress imaging performed were admitted (18.5% vs 10.2%). However, event rates were low in both group, and most patients were able to exercise and had an interpretable ECG. The predictive value of exercise ECG was very similar to stress MPI, so while effective, the additional costs of imaging should be considered in such a situation under clinical conditions.
Stress Echocardiography
Stress echocardiography avoids radiation concerns associated with stress radionuclide MPI, however the acquisition of the testing is more labor-intensive and operator dependent. For patients unable to exercise, dobutamine stress echocardiography can be performed.
A study of 377 patients who underwent dobutamine stress echocardiography with a normal or nondiagnostic ECG and following negative serial biomarker levels at 6 hours examined the ability of early ED testing to predict outcomes.64 Testing was not possible in 23 of 404 patients initially screened into the study because of poor acoustic windows, a proportion similar to the general population. With dobutamine stress testing, 39 patients tested were unable to complete the protocol due to intolerable side effects such as arrhythmia, severe hypertension, or hypotension. The overall event rate including death, MI, rehospitalization, or revascularization, was 31% in patients with a positive stress echocardiogram and 4% in patients with a negative study. The negative predictive value was 96%, slightly lower than that reported for in studies of radionuclide imaging.
In addition to the need of a skilled sonographer to acquire images for stress echocardiograms, an experienced reader must be available to interpret the study – a barrier to the use of these studies during off-hours. A study designed to test the utility of transmitting images to an off-site cardiologist reported the feasibility of this process to minimize hospital admissions for chest pain.65 163 patients with nondiagnostic ECG, negative serial cardiac biomarkers, and a normal resting echo underwent dobutamine stress echocardiograms supervised by a trained nurse. Despite a high (54.7%) rate of mild side effects, there were no adverse effects of testing. The negative predictive value of a normal study was 98.5%. In the final stage of the study, where a negative result was used to discharge patients home directly from the ED, 72% of patients who would have otherwise been admitted for further observation were discharged after negative dobutamine stress test.
There is some suggestion that dobutamine stress echo may be a cost-effective strategy when compared to exercise treadmill testing alone.66 Nucifora and colleagues reported on 190 patients with chest pain, serial negative biomarkers and nondiagnostic ECG results who were randomized to undergo either dobutamine stress echocardiography or exercise ECG testing. Patients with negative results on either test were discharged from the ED and followed for events. There was a higher event rate in patients who were discharged after negative exercise ECG testing compared to dobutamine stress echocardiography (11% vs. 0%, p=0.004). Costs were lower in the dobutamine echocardiography group at both 1 and 2-month follow up compared to exercise ECG testing ($1,026 ± $253 vs $1,329 ± $1,288 at 1-month, p=0.03 at 1 month and $1,029 ± $253 vs $1,684 ± $2,149, p=0.005 at 2-months). Lower costs in the dobutamine stress echo group were thought to be due to shorter length of stay, and less need for follow up testing for indeterminate results which are more likely with exercise ECG testing alone.
Appropriate Use Criteria and Guidelines
The 2011 American College of Cardiology/American Heart Association guideline recommendations for the management of patients with unstable angina/Non-ST elevation MI present a class I recommendation that in patients with suspected ACS in whom ischemic heart disease is present or suspected, if the follow-up 12 lead ECG and cardiac biomarkers measurements are normal, a stress test (exercise or pharmacological) to provoke ischemia should be performed in the ED, in a chest pain unit, or on an outpatient basis in a timely fashion as an alternative to inpatient admission. Also, patients with possible ACS and negative cardiac biomarkers who are unable to exercise or who have an abnormal resting ECG should undergo a pharmacological stress test with imaging.3
The 2009 appropriate use criteria for cardiac radionuclide imaging rate the use of stress MPI in the setting of possible ACS with a 1) normal or nondiagnostic ECG; 2) either low or high clinical risk based on TIMI score; and 3) either negative, borderline, equivocal, or minimally elevated troponin all as appropriate.41
Joseph Alpert: Optimal selection and best practices for all of these tests are often achieved when institutions have dedicated and approved chest pain units situated in or near the ED.
The 2008 appropriate use criteria for stress echocardiography rate the use of stress echocardiography for the indication of acute chest pain appropriate in the setting of an intermediate pre-test probability of coronary artery disease, and an ECG without dynamic ST changes when serial cardiac enzymes are negative.67
The Society statements do not advocate the use of one type of stress test above another. This decision should be based on clinical suspicion, patient characteristics, and expertise of the particular center.
Cardiac Computed Tomography Angiography (CCTA)
CCTA for the detection of coronary artery disease
A significant coronary stenosis can be detected by invasive coronary angiography in the majority of patients with acute coronary syndrome (>80%).68.69 Furthermore, the occurrence of ACS is rare in the absence of coronary atherosclerosis.
CCTA has evolved into a robust and reliable technique for detection and assessment of coronary stenosis and atherosclerotic plaque. A wealth of single and multicenter trials have established CCTA as a noninvasive diagnostic test with excellent sensitivity (97.2%, 95% CI, 96.2% to 98.0%) and very good specificity (87.4%, 95% CI, 84.5% to 89.8%) for the detection of >50% coronary artery stenosis as compared to the gold standard invasive coronary angiography.70–75 The major strength of CCTA is its high negative predictive value (typically approaching 99%) and thus CCTA permits confident exclusion of significant coronary stenosis. In addition, CCTA is highly sensitive (90%, 95% CI: 83% to 94%) and specific (92%, 95% CI: 90% to 93%) to detect calcified and noncalcified coronary atherosclerotic plaque as compared to the gold standard intravascular ultrasound.76–79 CCTA is also highly reproducible for detection of coronary plaque and stenosis (kappa: 0.85 to 0.93).80–83
Observational single-center experiences with CCTA in evaluation of patients with acute chest pain
The ability to rapidly image the coronary arteries with a noninvasive technique with strong performance characteristics is a potentially very attractive option in the setting of evaluating patients with suspected ACS in the ED. With substantial technical developments and wide availability, CCTA has evolved into a viable alternative to standard of care management in patients presenting to the ED with acute chest pain. Multiple single center studies have demonstrated that the exclusion of a significant coronary stenosis by CCTA nearly excludes ACS and thus may potentially allow for earlier discharge than stress test based management (Figure 9).84–95
Figure 9:
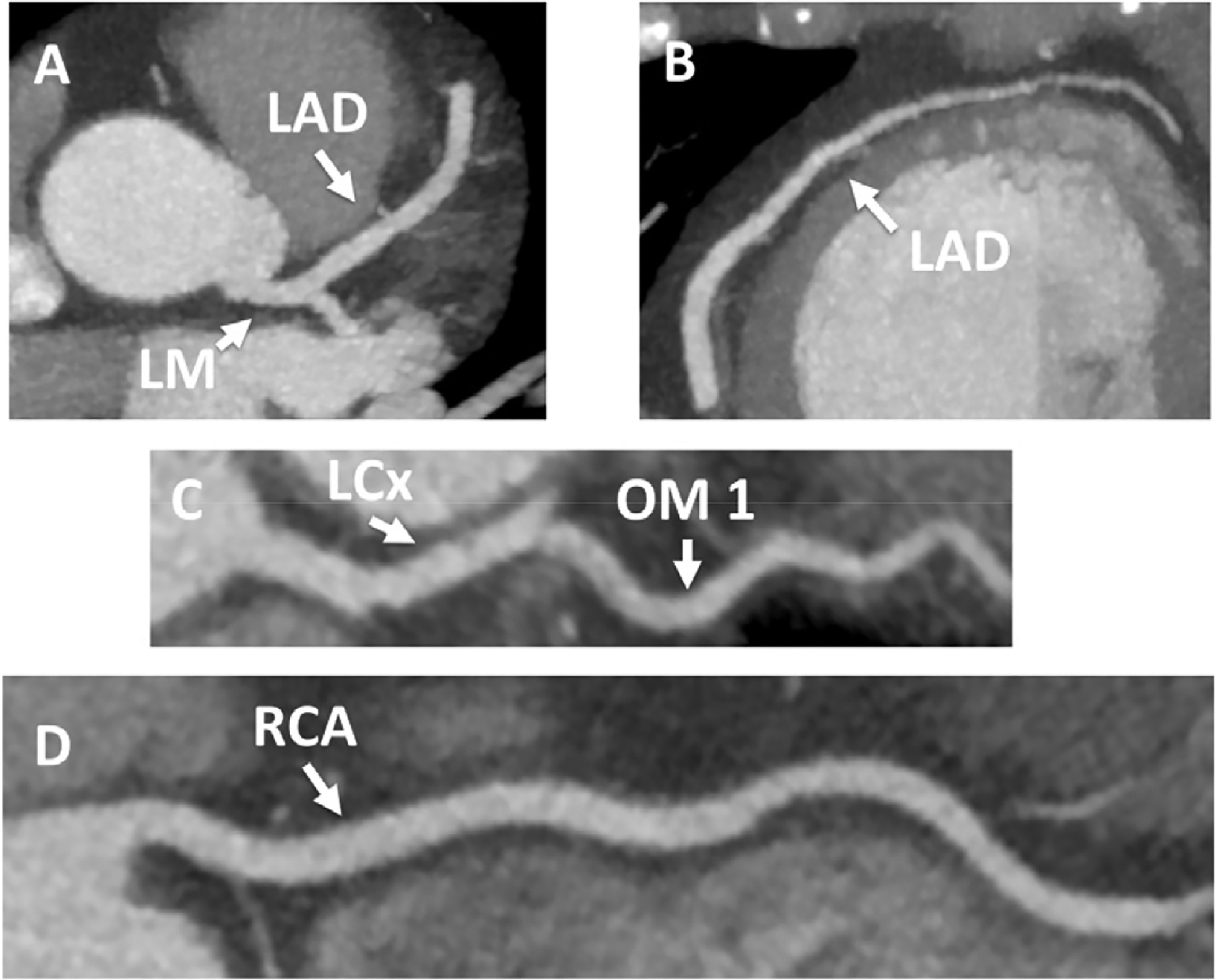
Coronary Computed Tomography Angiography with Normal Coronary Arteries. A 56-year-old woman presented to the ED with an episode of substernal pressure that had occurred at work in the office. She had normal electrocardiogram and negative troponin on presentation. She underwent coronary computed tomography angiography (CCTA), which demonstrated normal coronary arteries (Panel A maximum intensity projection image of the left main [LM] and left anterior descending [LAD] coronary artery, Panel B maximum intensity projection image of the mid and distal LAD, Panel C curved multiplanar reformatted image of the proximal left circumflex [LCx] coronary artery and the first obtuse marginal branch [OM1], Panel D curved multiplanar reformatted image of the right coronary artery [RCA]). She was discharged from the emergency department after CCTA.
In 2007, Rubinshtein et al. studied 58 patients with acute chest pain, negative biomarkers and electrocardiograms and demonstrated that CCTA based assessment of obstructive CAD had a very good diagnostic accuracy (sensitivity 100%, specificity 92%, positive predictive value 87%, and negative predictive values 100%) for ACS.84 They suggested that CCTA might allow for a safe and early discharge, since no major adverse cardiovascular events occurred among those who were directly discharged from the ED based on CCTA results.
Those initial observations from a small study were confirmed and extended in the prospective observational cohort ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial published in 2009.89 The ROMICAT trial had a unique design. The trial included 368 patients with acute chest pain from the ED with an initial inconclusive assessment, who underwent CCTA. Care providers were blinded to the CCTA results, and therefore the diagnostic performance of CCTA for ACS and its association with other test findings could be studied in a truly unbiased fashion. CCTA detected no evidence of CAD in approximately half of patients and approximately 20% patients had obstructive CAD. Absence of any CAD (stenosis or coronary plaque) by CCTA accurately predicted the absence of ACS (negative predictive value 100%). The presence of obstructive CAD (>50% luminal narrowing) was associated with 77% sensitivity and 87% specificity for ACS. Not surprisingly, the presence and extent of coronary plaque and stenosis were superior in their discriminative capacity for ACS, as compared to clinical risk scores such as TIMI or Goldman.96
Joseph Alpert: Similar to other imaging modalities employed in the evaluation of patients with chest discomfort in the ED, CCTA is particularly helpful when it is normal.
Takakuwa et al. performed a meta-analysis of observational studies that had evaluated CCTA in populations of acute chest pain patients in the ED.97 They pooled the results of 9 studies with the total number of 1559 patients (42% women, mean age 52 years, low-to-intermediate likelihood of ACS). The detection of significant stenosis by CCTA had the pooled sensitivity of 93.3% (95% CI 88.3% −96.6%), specificity of 89.9% (95% CI 88.3% - 91.3%), positive predictive value of 48.1% (95% CI 42.5% - 53.8%), and negative predictive value of 99.3% (95% CI 98.7% - 99.6%) for detection of major cardiovascular events at 30 days.
However, the studies demonstrated that the mere detection of obstructive CAD by CCTA does not equate to a diagnosis of ACS. In the ROMICAT trial only 20 out of 34 patients with obstructive CAD were clinically diagnosed with ACS.89 In the study by Hollander et al. only 7 out of 54 patients with obstructive CAD by CCTA had a stenosis confirmed by invasive coronary angiography (i.e., underwent invasive angiography on clinical grounds) or a major cardiovascular event within 30 days.24 The imperfect specificity of CCTA detected stenosis combined with the low prevalence of ACS in the studied acute chest pain populations (2–8%) resulted in positive predictive values in the range of 35 to 50%. This is not unexpected, as CCTA is a technique that is imaging coronary anatomy in a precise way with contemporary equipment and technology, but the presence of a coronary stenosis does not necessarily mean that the preceding symptoms or clinical syndrome was related to the stenosis. Therefore, approaches that would improve specificity and positive predictive value of CCTA for ACS are desirable. There are several such techniques in active development that would interrogate the potential impact of flow abnormalities. The new methods for the analysis of CCTA datasets such as assessment of global and regional left ventricular (LV) function98–100, evaluation of myocardial perfusion,99,101–103, coronary plaque analysis104–106 and non-invasive fractional flow reserve107,108 have the potential to improve accuracy and efficiency of CCTA in patients with acute chest pain (Figures 10 and 11).
Figure 10:
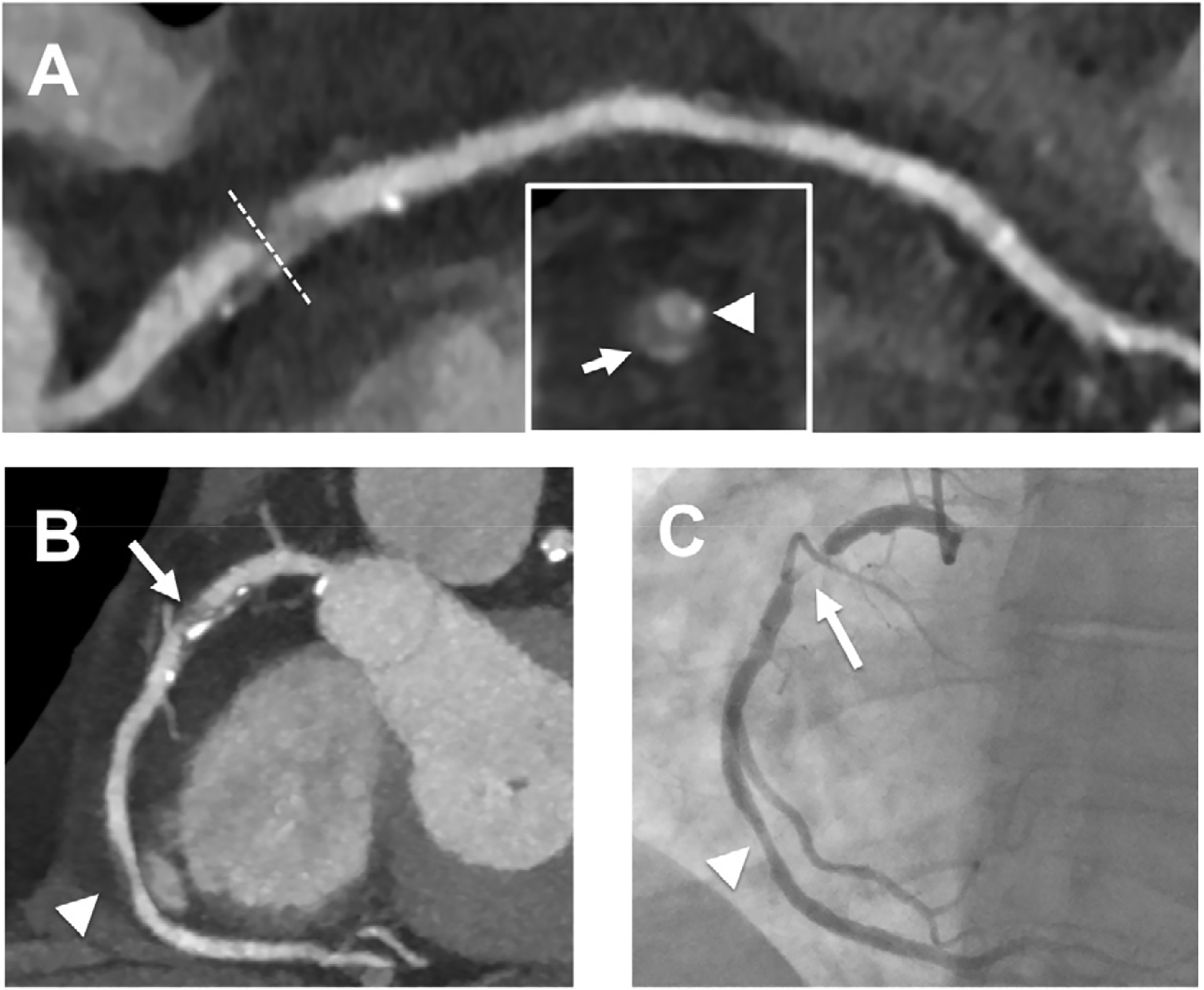
CCTA Demonstrating Severe Coronary Stenosis and High-risk Plaque Features in a Patient with Unstable Angina Pectoris. A 53-year-old woman with a history of multiple risk factors presented to the ED with the chief complaint of occasional exertional chest pain during the past 3 weeks. The curved multiplanar image of the right coronary artery (Panel A) showed a severe stenosis (>70%, dotted line) in the proximal segment caused by partially calcified coronary plaque. The plaque was characterized by the presence of low CT attenuation plaque component (<30 HU) and positive remodeling. The image insert showed a cross-section of the artery with spotty calcium (arrowhead) next to the lumen and the presence of the napkin-ring sign (central low CT attenuation plaque surrounded by peripheral rim of higher CT attenuation plaque; arrow). These plaque features have been described as high-risk plaque features and were associated with acute coronary syndrome. Maximum intensity projection image of the right coronary artery confirmed severe stenosis (>70%) in the proximal segment (arrow) and mild stenosis (<50%) in the distal third of the mid segment (arrowhead). These findings were confirmed by invasive coronary angiography (Panel C). The patient was diagnosed with unstable angina and underwent percutaneous coronary intervention with a drug-eluting stent.
Figure 11:
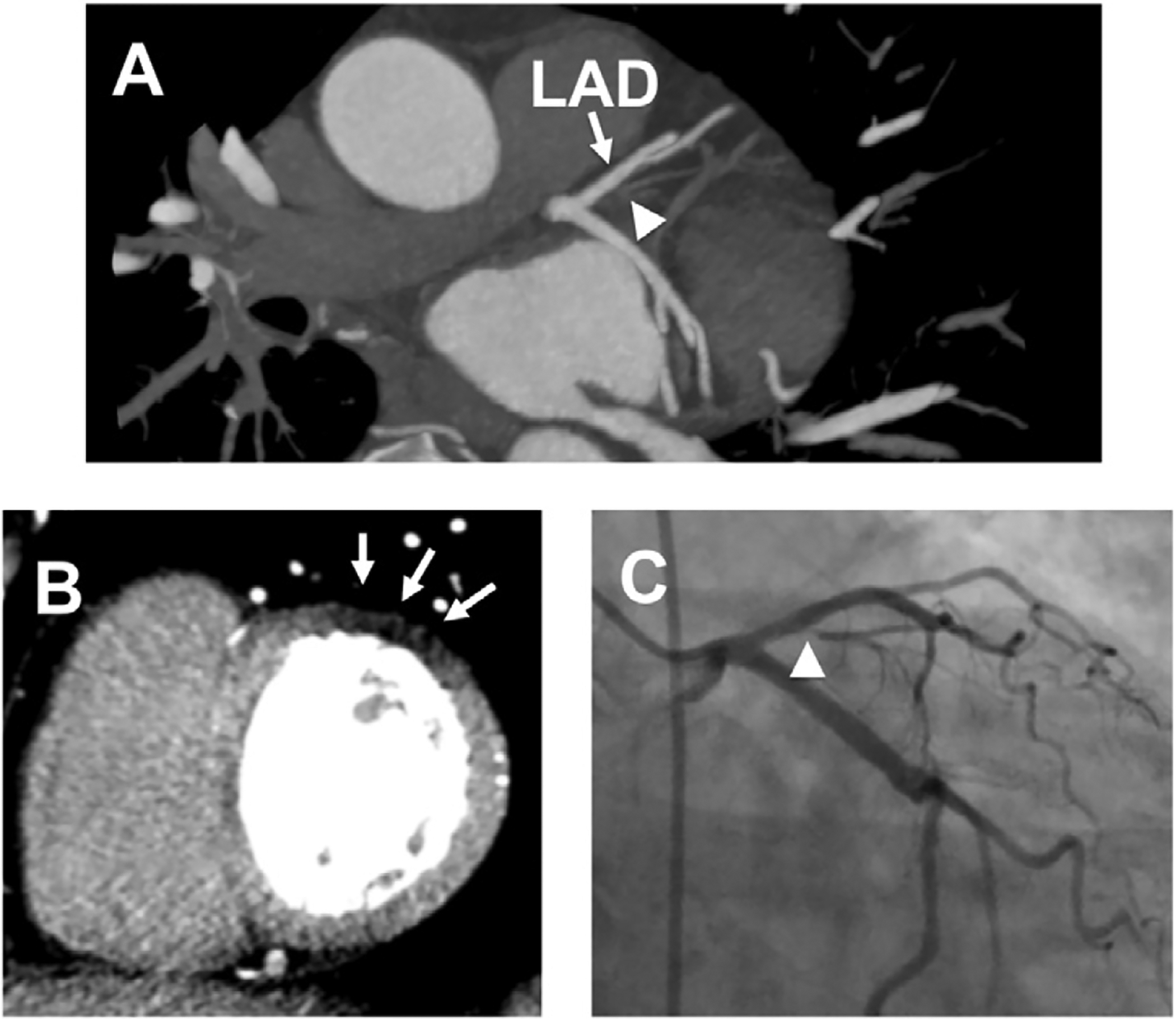
Significant Coronary Stenosis with Corresponding First-past Myocardial Perfusion Defect on CCTA. A 51-year-old man presented to the ED after a 20-minute episode of substernal chest pain. He was chest pain free in the ED and his initial electrocardiogram and troponin T were negative. He underwent a CCTA that showed subtotal to total occlusion of the small first diagonal branch (Panel A, arrowhead, LAD left anterior descending coronary artery). This finding correlated with the first-pass CT perfusion defect (arrows) in the basal anterolateral segment of the left ventricle (Panel B). The second and third troponin T were positive. He underwent invasive coronary angiography that demonstrated severe stenosis in the proximal first diagonal branch (Panel C, arrow). No intervention was performed due to the small caliber of the vessel. (Courtesy of Dr. Brian Ghoshhajra.)
Joseph Alpert: Many patients, particularly older individuals, will have asymptomatic coronary arterial stenosis. In such patients, identification of coronary artery disease is not proof that the patient’s chest discomfort was the result of myocardial ischemia. This may be another example of the famous adage, “true, true, and unrelated”, i.e., true that the patient has chest discomfort, true that the patient has atherosclerotic coronary arterial lesions, but these two findings are unrelated.
Thus, similar to the evolutionary trajectory of rest SPECT MPI in this clinical setting, the CCTA literature grew to a point suggesting that the performance characteristics for detecting CAD or ACS were potentially very useful for improving triage in the ED. The data however were from studies of efficacy, that is, from generally expert centers with the CCTA information not driving patient care. As with the SPECT MPI evolution, the next step was randomized trials to assess the effectiveness of incorporating the CCTA information on actual care.
Randomized trials of CCTA vs. standard of care in the evaluation of patients with acute chest pain
The first study that randomized acute chest pain patients in the ED to CCTA-based strategy vs. standard of care strategy was published by Goldstein et al. in 2007.85 The study was performed in one academic medical center, and 197 patients were randomized to CCTA vs. standard of care, the latter with a radionuclide myocardial perfusion imaging stress test. The primary outcome was safety. Both groups had 100% safety defined as the lack of test complications and major cardiovascular events at 6 months. However, the event rates were extremely low (no patients had myocardial infarction or death during the index hospitalization or during 6-month follow-up) suggesting a very low risk population at baseline. The additional analyses showed that CCTA shortened time to diagnosis (3.4 hours vs. 15.0 hours) with lower cost of care ($1586 vs. $1872). Patients in the CCTA arm underwent more additional testing (invasive coronary angiography (11.1% vs. 3.1%) and additional stress testing after indeterminate CCTA (24%).
This study was an important step forward, but did illustrate some of the limitations of using CCTA. Over 40% of potentially eligible patients were excluded due to an inability to have a CCTA study done, secondary to pulmonary disease making safety of beta-blockade questionable, contrast allergy or atrial fibrillation.
Joseph Alpert: An elevated value for serum creatinine is a risk factor for the development of contast induced renal insufficiency and physicians must assess the risk/benefit of proceeding with CCTA which requires the administration of angiographic contrast solution.
The very low event rate in this population was in part driven by the study design, in that patients were randomized only after serial ECGs and biomarkers were negative for ACS, leaving a very low risk group to be studied. Ideally, an imaging modality would be deployed earlier in the evaluation process.
The initial experience was followed by the recent publication of three multicenter randomized trials that compared CCTA to the standard evaluation in acute chest pain patients in the ED.86, 92, 109 These trials enrolled a total of more than 3,000 patients with low to intermediate likelihood of ACS.
Goldstein et al published the results of the CT-STAT trial86 In this multicenter trial, 699 patients were randomized to have a CTA study or a stress myocardial perfusion imaging test, after serial ECGs and biomarker studies ruled out an acute MI. The primary endpoint was time to diagnosis, defined as the time from enrollment to the time the test results were reported to the ED clinician. The use of CCTA was associated with a 54% reduction in this time interval (median 2.9 h vs. 6.3 hr, p < 0.0001), which to some degree was driven by the known time it takes to perform these distinct tests. Costs were lower in the CCTA group. There was no difference in the very low event rate observed in either group. The study was not designed to assess the effect of the imaging modalities on triage decisions.
Litt et al92 reported a multicenter trial enrolling 1,370 low-to-intermediate risk for ACS patients randomized 2:1 to incorporate CCTA into their evaluation strategy or to undergo a more standard of acre evaluation strategy. The primary endpoint was a safety endpoint, that was defined as the absence of MI or cardiac death during the 30 days after randomization among the patients randomized to CCTA who had normal coronaries. The trial was powered to demonstrate that the upper bound of the 95% confidence interval for this major cardiac event rate in such patients would not exceed 1%. The clinical implication would be that such patients are safe to directly discharge from an ED. The trial was positive in that regard, as among those whose CTA scans showed normal coronaries, there were no cardiac deaths of MIs, and the upper bound of the 95% confidence interval for this event rate was 0.57%, which satisfied the pre-specified criterion of being <1%. From a study design point of interest, the primary endpoint of the trial only involved a subgroup of one of the randomization groups.
Secondary end points involved comparisons of clinical care between the randomization groups. The group randomized to CTA had more direct discharges from the ED, and shorter length of stay.92
Hoffmann et al performed a multicenter Rule-Out Myocardial Infarction with Computed Tomography (ROMICAT)-II study.109 Among the three reported randomized trials of CTA in the ED setting, this study was the only one specifically designed as an effectiveness trial to test the impact of the CTA results on clinical decision making as the primary endpoint, with length-of-stay as the metric. One thousand patients at 9 centers with low-to-intermediate likelihood of ACS and an initial negative troponin were randomized to an evaluation strategy incorporating CTA results, or to a more standard of care strategy without CTA. The prevalence of ACS at final diagnosis was 8%, higher than in the other randomized trials. The trial was positive, in that length of stay was shorter in the group randomized to the CTA strategy (Figure 12). In addition secondary endpoints demonstrated that there were far more direct discharges from the ED in the CTA group, very likely driven by the knowledge of normal coronary arteries, given the high negative predictive value of CTA.
Figure 12:
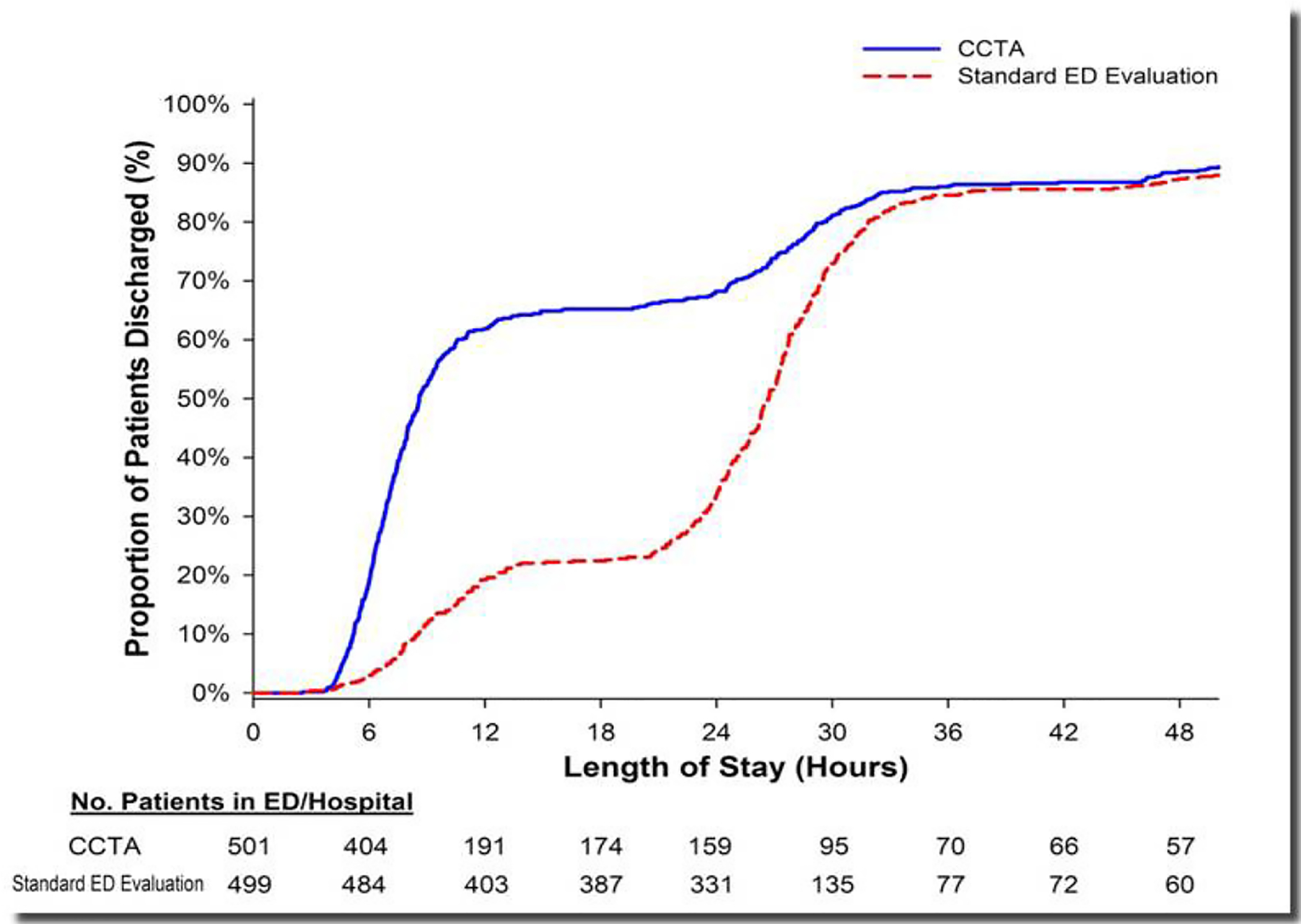
Cumulative distribution graph of length-of-stay results fromt eh ROMICAT-II randomized trial. The x-axis depicts the hours of length-of-stay, the y-axis the cumulative proportion of patients discharged at the varying lengths of stay. For tha patients randomized to the CCTA strategy (blue line), more were discharged earlier from the ED, with approximately 50% being discharged by 8 hours after presentation. In contrast, among the group randomized to the usual care strategy (red dashed line), approximately 50% patients were not discharged until 26 hours after presentation. Reproduced from reference 109.
The results of these 3 studies are summarized in Table 2. Patients enrolled in the studies had negative initial serum troponin and non-ischemic electrocardiograms. The average age of patients was 50 years and both men and women were well represented. Both academic medical centers and non-academic hospitals were included in the studies. In all three trials, an important outcome of interest – the length of stay or time to diagnosis – was significantly shorter in the CCTA arm. This was accomplished safely, without an increase in major adverse cardiovascular events during a 28-day follow-up. Moreover, the ROMICAT II trial and the study by Litt et al. also showed a quadrupling of direct discharges from the emergency department as compared to standard of care (50% vs. 12%).92,109 Probably because of the high sensitivity and negative predictive value of CCTA for coronary artery disease (CAD). Similar to previous observational studies, there was an increase in additional testing and invasive procedures in CCTA arms of these studies (Figure 13). This likely is driven by the knowledge that some CAD is present, and the reflexive decision that catheterization is needed. Hulten et al. in their meta-analysis estimated the increase in invasive coronary angiography and percutaneous coronary interventions (PCI) after CCTA of 21 and 20 per 1,000 CCTA scans; respectively.110 There are no reliable data to indicate whether the improved detection of CAD and subsequent PCI in this acute setting will improve long-term health outcomes. This is a challenging point to address, as by definition the patients participating in these studies are fairly low risk patients, the vast majority of whom do not have CAD at all.
Table 2.
Randomized controlled multicenter trials with coronary CT angiography as a diagnostic intervention in patients with acute chest pain. The workup in the emergency department (ED) using coronary CT angiography (CCTA)was compared either with a workup strategy requiring nuclear myocardial perfusion imaging(MPI)or with a traditional standard of care (SOC) workup strategy
| Population | Randomization | Outcomes | Observed difference (CCTA vs control) | |
|---|---|---|---|---|
| Goldstein19 (CT-STAT) | Negative troponin | CCTA, n = 361 | Prevalence of ACS | 1.2% vs 2.7% |
| Nondiagnostic ECG | MPI, n = 338 | MACE during follow-up | 0.8% vs 0.4% | |
| Age: 50 ± 10 y | Direct ED discharges* | 73% vs 81%† | ||
| Women: 54% | Time to diagnosis* | 2.9 vs 6.2 h | ||
| Number of centers: 16 | Invasive coronary angiography | 7% vs 6% | ||
| Coronary revascularization | 4% vs 2% | |||
| ED cost* | $2137 vs $3458 | |||
| Radiation dose* | 12 vs 13 mSv | |||
| Litt25 | Negative troponin | CCTA, n = 929 | Prevalence of ACS | 4% vs 2% |
| Nondiagnostic ECG | SOC, n = 463 | MACE during follow-up | 3% vs 1% | |
| Age: 49 ± 10 y | Direct ED discharges* | 50% vs 23% | ||
| Women: 53% | Length of stay* | 18.0 vs 24.8 h | ||
| Number of centers: 5 | Invasive coronary angiography | 5% vs 4% | ||
| Coronary revascularization | 3% vs 1% | |||
| ED cost | not available | |||
| Radiation dose | not available | |||
| Hoffmann42 (ROMICAT II) | Negative troponin | CCTA, n = 501 | Prevalence of ACS | 9% vs 6% |
| Nondiagnostic ECG | SOC, n = 499 | MACE during follow-up | 0.4% vs 1.2% | |
| Age: 54 ± 8 y | Direct ED discharges* | 47% vs 12% | ||
| Women: 47% | Length of stay* | 23.2 vs 30.8 h | ||
| Number of centers: 9 | Invasive coronary angiography | 11% vs 7% | ||
| Coronary revascularization | 7% vs 4% | |||
| ED cost | $2101 vs $2566 | |||
| Radiation dose* | 14 vs 5 mSv |
CT-STAT, CT-systematic triage of acute chest pain patients to treatment; MACE, major adverse cardiac events
Significant difference (p < 0.05).
Estimated from present data.
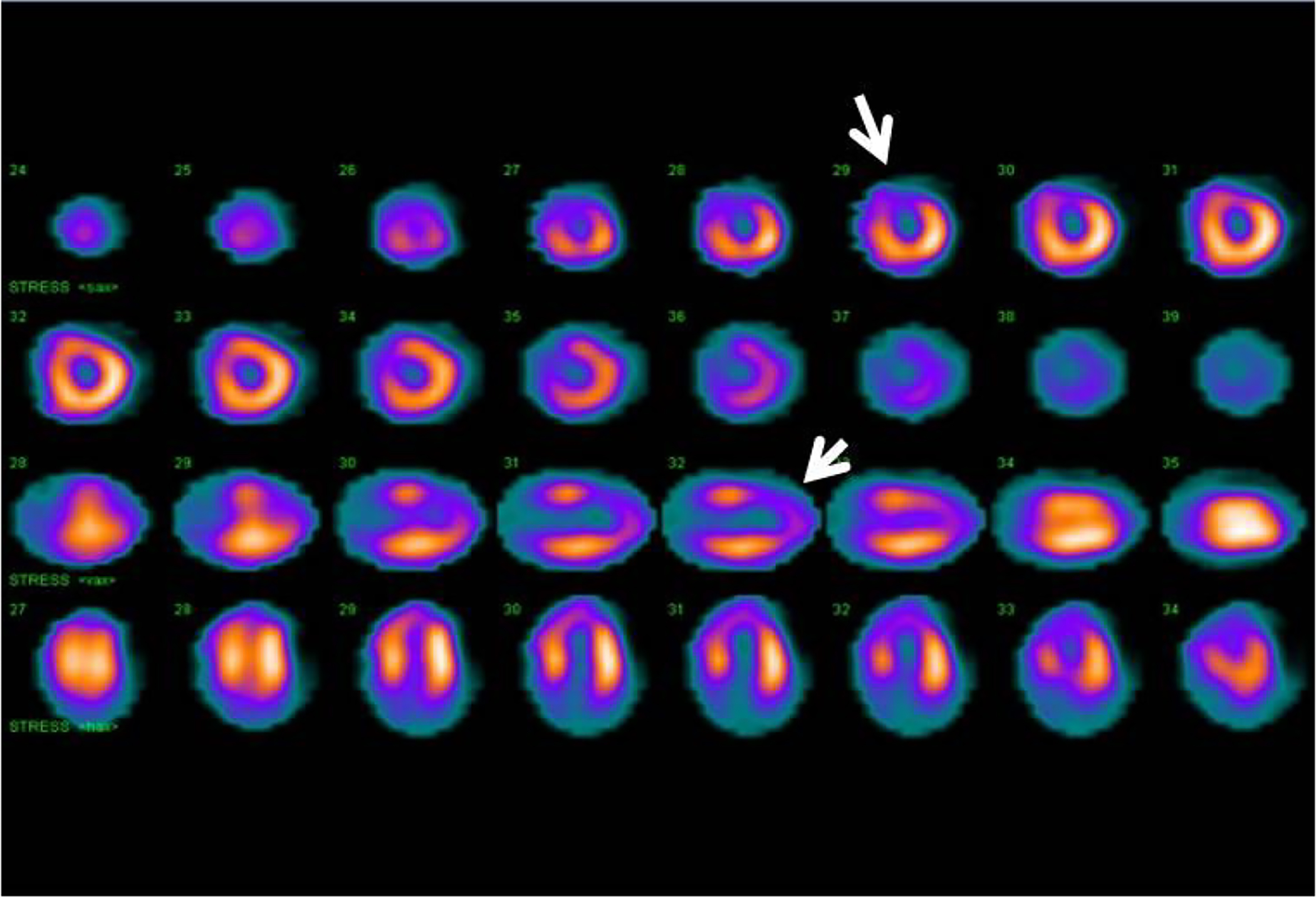
Figure 13:
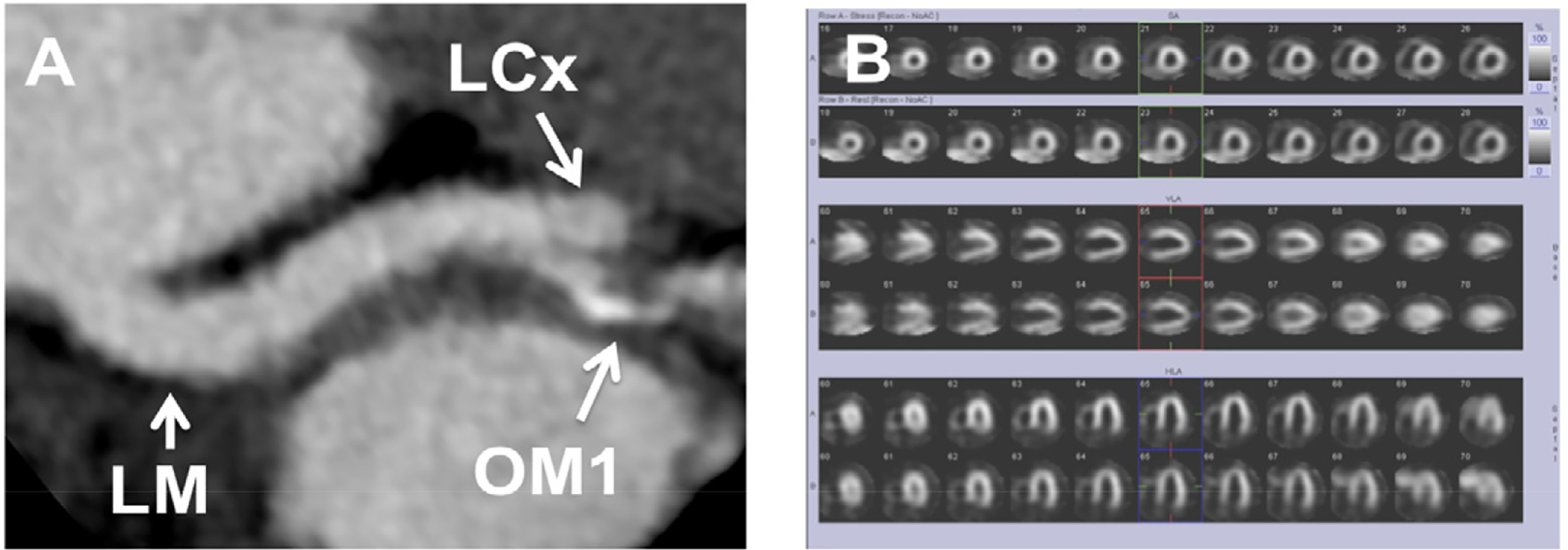
CCTA Suggestive of Severe Coronary Stenosis with Negative Nuclear Myocardial Perfusion Imaging Exercise Stress Test. A 52-year-old man presented to the ED with chest pain. At time of admission, electrocardiogram was normal and troponin T was negative. The patient underwent CCTA, which showed apparently severe luminal narrowing in the proximal obtuse marginal branch (Panel A, LM left main coronary artery, LCx left circumflex coronary artery, OM1 first obtuse marginal branch). Evaluation of CCTA was limited due to the presence of calcium. There were no significant stenoses in the left anterior descending and right coronary artery. The patient underwent further evaluation by stress nuclear myocardial perfusion imaging. The patient had excellent exercise capacity, the stress electrocardiogram was negative for ischemia, and the myocardial perfusion images had no evidence of ischemia or infarction (Panel B top rows- stress, bottom rows- rest). The patient was discharged and was doing well after 1-month follow-up. This illustrates the fact that CCTA may identify anatomic CAD that may not be physiologically significant.
Despite the consistent increase in subsequent testing after CCTA, the overall cost of the index hospitalization was not significantly higher in those randomized to CCTA in these studies, as the greater rate of direct discharges likely balances the excess testing tha occurs in some patients. The estimated radiation exposure in the CCTA group was higher (13.9 mSv) as compared to the standard of care (4.7 mSv) including patients undergoing only exercise treadmill stress test or stress echocardiography in the ROMICAT II trial109, but was similar in a direct comparison of CCTA (11.2 mSv) and myocardial stress perfusion imaging (12.8 mSv) in the CT-STAT trial.86 In summary, the studies demonstrated that early CCTA permits accurate and safe direct emergency department discharge of 50% of all acute chest pain patients.
Joseph Alpert: Clearly, utilization of this approach would result in major cost savings for the healthcare system.
The data from the randomized trials are conceptually consistent in suggesting that the use of CCTA in this setting can allow much earlier and direct discharge form the ED, particularly when normal coronaries are demonstrated. The higher rate of catheterization and revascularization suggested by the meat-analysis of these studies highlights the challenges in clinical decision making however, when a coronary stenosis that may or may not be at all involved in an ACS clinical syndrome is now illuminated by imaging. Going forward, more refined analysis of the potential role of an imaged plaque or stenosis in the clinical syndrome may allow better selection of those who will truly benefit from an invasive catheterization.
Joseph Alpert: There are currently a number of studies underway comparing the various imaging modalities for assessing the likelihood that a particular patient has an ACS. One such trial is the multicentered PROMISE trial which should produce helpful results in the near future.
Appropriate Use Criteria and Guidelines
The current guideline recommendations (ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography) include a section on the use of CCTA in acute chest pain.111 The guidelines considered CCTA in patients with acute chest pain, negative electrocardiogram and biomarkers, and low or intermediate likelihood of obstructive CAD as appropriate. The most recent AHA/ACC guidelines for the management of patients with non-ST-segment-elevation myocardial infarction and unstable angina pectoris do not provide a specific recommendation for the use of CCTA in patients with acute chest pain and suspected ACS.112 However, they include CCTA in the section on future directions and mention that CCTA has undergone favorable initial evaluation for assessment of the low-to-intermediate- risk chest pain patient. Of note, these guidelines did not consider the results of more recent randomized trials. Based on the experience from large multicenter randomized trials, the available evidence suggests that CCTA use in the setting of low and low-to-intermediate risk of ACS in acute chest pain patients in the ED may have a favorable impact on patient management, largely through speeding the direct discharge of those without CAD. Approximately half of patients studied in the three randomized trials had no evidence of CAD on CCTA. This type of patient, with no CAD on CCTA, can be safely discharged after CCTA reducing the duration of the hospital stay and cost in this subgroup. Further, patients with minimal non-obstructive CAD are also candidates for early discharge with arrangement of outpatient follow-up. On the other side of the spectrum are patients with a definite coronary stenosis. These patients require admission to the hospital, further evaluation and guideline directed therapies. An area of uncertainty involves patients with non-diagnostic CCTA or evidence of non-negligible coronary plaque, but no significant stenosis. These patients usually require observation with serial biomarkers and electrocardiograms and often a functional stress test for evaluation of ischemia. A suggested guide on the deployment of CCTA for use in ED patients with low-to-intermediate likelihood of ACS given potential findings on CCTA is provided in Figure 14.
Figure 14:
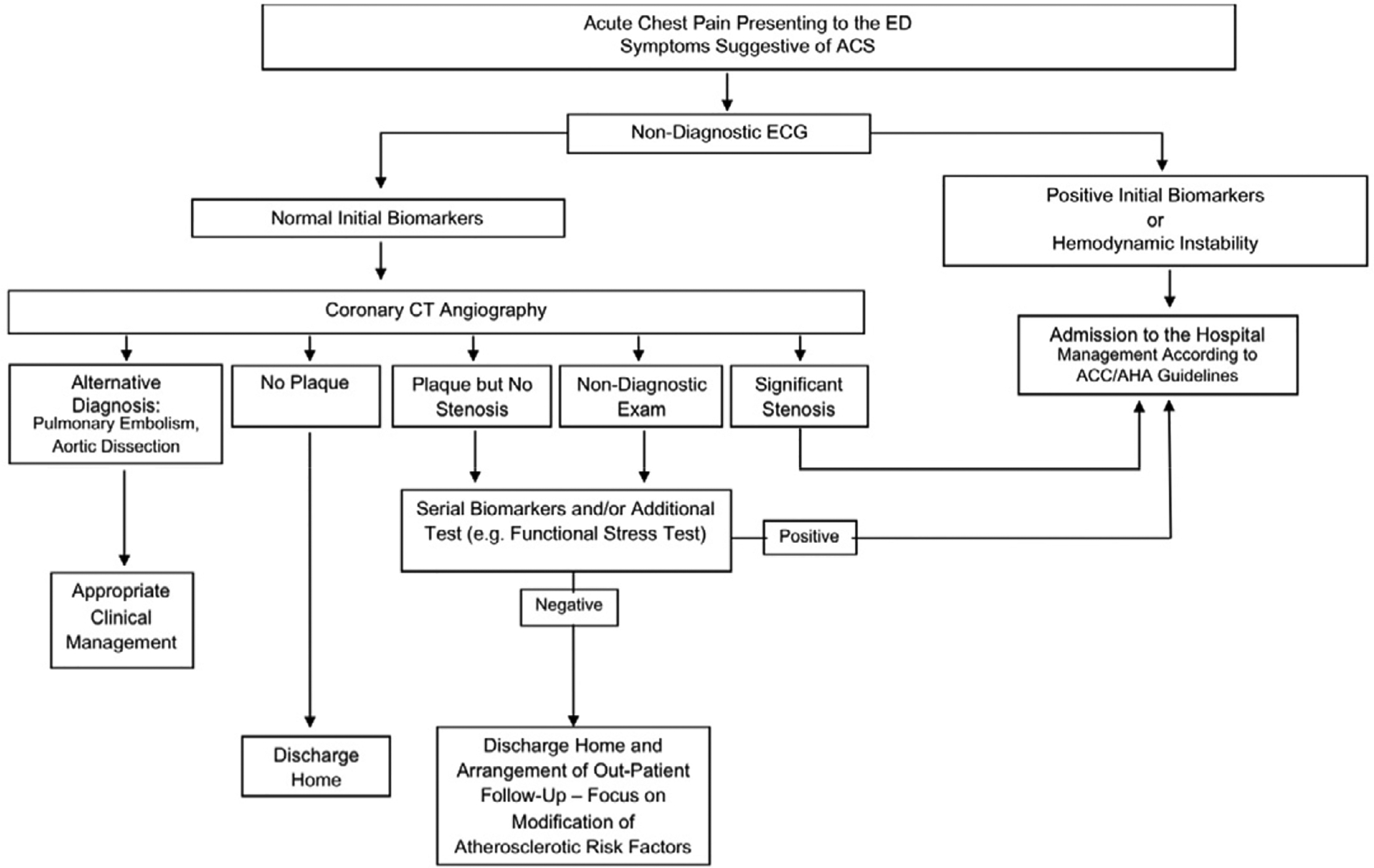
Proposed Algorithm for Evaluation and Management of Patients Suspected of Having ACS Using Early CCTA
Cardiac Magnetic Resonance Imaging
While much of the literature on the use of cardiac imaging techniques in ED patients with chest pain and suspected ACS has involved radionuclide imaging of myocardial perfusion, echocardiographic imaging of regional function or perfusion using contrast agents, or CCTA imaging of the coronary arteries, there has been a slowly evolving literature on the use of cardiac magnetic resonance (CMR) imaging for this clinical purpose. The strengths of CMR imaging are particularly attractive in this setting. These strengths lie in the ability to comprehensively evaluate cardiac morphology, function, perfusion, tissue characterization and potentially anatomic coronary artery visualization without radiation exposure. However, CMR imaging is complex and requires substantial technical expertise to ensure high-quality diagnostic examinations. While MR technology is widely available and often used for evaluation of patients in the ED setting for orthopedic or neurologic issues among others, the specific expertise to perform and interpret CMR imaging is not as widely available.
In the setting of acute chest pain, superb tissue characterization of the myocardium presents the most attractive finding. The key attributes of characterization of myocardial tissue include the ability to differentiate infarcted tissue (using the late gadolinium enhancement technique) and ischemic myocardium (rest perfusion defect without evidence of infarct) from normal myocardium, to diagnose myocardial infarction (MI) before troponin elevation 113,114, to differentiate between new and old infarcts115, and determine prognosis116. This ability makes CMR attractive in patients with known CAD or prior MI, a group of patients that may not benefit as much from CCTA or ECG or perfusion imaging based stress testing. This is also founded in observations that stress perfusion MRI is superior to SPECT117,118 and echocardiography119 in the detection of significant CAD. It should be noted however, that although these studies have recently been multicenter, they generally are performed at centers with substantial expertise in CMR imaging, and how the data generalize to centers without such experience and expertise is uncertain.
Joseph Alpert: I remain concerned about placing a patient with a potential ACS in the confines of an MRI tunnel particularly at night or on weekends when staffing levels are at their minimum. Further experience will demonstrate whether or not I am justified in this concern.
Observational Studies of CMR Imaging in Patients with Suspected ACS in the ED
Analogous to the trajectory of all of the other imaging modalities in this clinical setting, the initial reports focused on performance of the technique to identify those patients with ACS and those without. In patients with chest pain with suspicion of an acute coronary syndrome, the potential usefulness of CMR imaging was initially demonstrated by Kwong et al.113 In the study of 161 patients, the final prevalence of ACS was 16%. An abnormal CMR study by qualitative analysis was defined by either a regional wall motion abnormality or an area of late gadolinium enhancement. A regional perfusion abnormality was considered abnormal if also accompanied by either a regional wall motion abnormality or an area of late gadolinium enhancement. The data showed that CMR imaging had a sensitivity of 84% and a specificity of 85% in detecting ACS, data that were more favorable in one of those characteristics than various ECG criteria, the TIMI risk score, and the troponin assay available at the time (Figure 15). These important data suggested for the first time that performing CMR imaging in such patients was feasible, and the patients were away from the ED for approximately 60 minutes to complete the test.1 It is important to note that these represent efficacy data, as the investigators reporting these data are highly expert.
Figure 15:
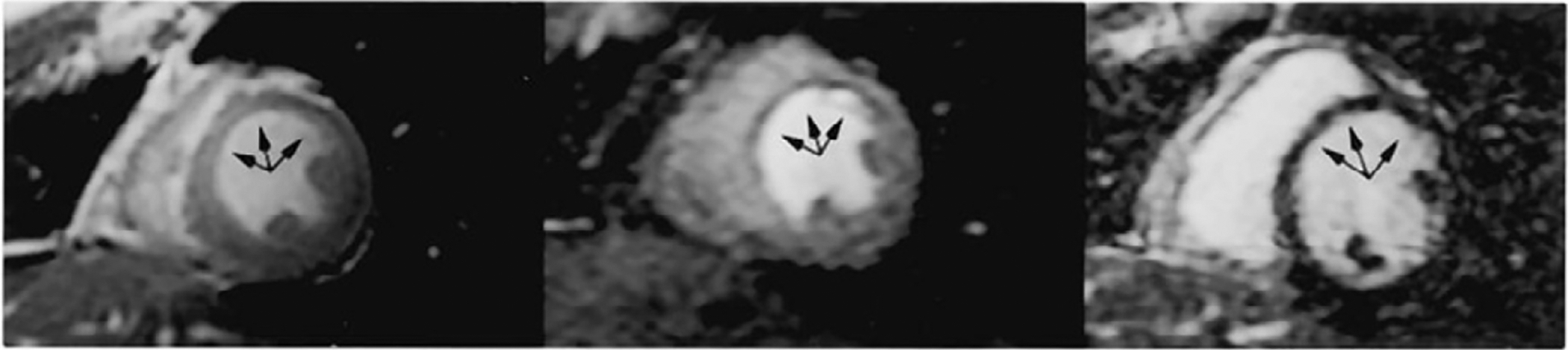
Use of cardiac magnetic resonance (CMR) imaging for patients with suspected ACS in the ED setting. In the left panel, an end-systolic frame from cine-CMR shows absence of thickening of the anteroseptal segment (arrows), consistent with a regional wall motion abnormality. The middle panel is an image of myocardial perfusion, and the darker band in the anteroseptum subendocardium (arrows), represents a subendocardial perfusion abnormality. In the right panel, there is no evidence of late gadolinium enhancement in this area (arrows), suggesting absence of infarct. These imaging data are consistent with a resting perfusion abnormality resulting in abnormal wall motion, and an ACS. Catheterization revealed a 95% stenosis of the left anterior descending coronary artery. Reproduced from reference 113.
Subsequently, Cury et al reported on the incorporation of T2-weighted CMR imaging into the acquisition protocol. This technique allows imaging of myocardial edema, which was hypothesized to further improves the specificity of CMR for the detection of ACS, by allowing differentiation of acute abnormalities of regional perfusion or infarction from those with chronic infarcts.114 These investigators reported on 62 patients with suspected ACS, 20% of whom had a final diagnosis of ACS. They found in this modest sample size that the addition of T2-weighted imaging of edema and analysis of left ventricular wall thickness increased the specificity, positive predictive value, and overall accuracy to detect or rule out ACS compared with the more standard CMR protocol analyzing regional function, perfusion, and delayed-enhancement (Figure 16). This study illustrated the potential for a very comprehensive assessment of the patient by CMR imaging, beyond what other modalities can accomplish.
Figure 16:
Use of T2 weighted imaging to assess myocardial edema associated with regional ischemia. Panel A is the T2 weighted image of a patient with suspected ACS. There is a region of T2 hyperintensity in the anterior wall consistent with myocardial edema (arrow). Panel B demonstrates a resting perfusion abnormality in the same territory (arrow), while Panel C shows absence of delayed hyperenhancement after gadolinium contrast. The findings are suggestive of resting ischemia. Subsequent stress testing was positive, and catheterization revealed a 90% stenosis in the proximal left anterior descending coronary artery as seen in Panel D.
In a long-term follow-up study of patients who presented to an ED and within 72 hours underwent a comprehensive CMR study that also included stress adenosine perfusion imaging, Ingkanisorn and colleagues reported that the stress CMR perfusion imaging identified patients who were found to have CAD or an event over a year of follow-up with sensitivity and specificity of 100% and 93% respectively.120 Among those with a normal stress and rest CMR perfusion study, there were no events and none a diagnosis made of CAD during the year of follow-up. These data suggest that incorporating stress perfusion imaging into the CMR analysis adds value to the diagnostic and prognostic power, in expert hands. However, despite these encouraging results, the large number of patients with acute chest pain in the ED and the administrative, technical and logistical challenges of performing cardiac MR (perhaps even with adenosine stress) have slowed the deployment of more routine use of CMR imaging in this situation.
Randomized Trials Incorporating CMR Imaging
Again similar to the evolutionary examples of imaging in this area set by the SPECT studies, there have been randomized trials attempting to better define the value of incorporating CMR imaging into the assessment of patients with chest pain in the ED. Miller, Hundley and colleagues have reported a series of single center studies exploring this issue in depth, with stress CMR imaging performed as part of an observation unit (OU) protocol. In the initial publication, 110 patients who were thought to be at intermediate or high probability for ACS but without obvious ischemic ECG changes or initial biomarker positivity were randomized to stress CMR imaging incorporated into an OU versus standard care as an inpatient. The primary outcome of this initial study was direct hospital cost, including both hospital and professional costs. The CMR OU strategy had reduced median costs, and 79% were managed in the OU setting without full admission.121 These investigators then followed the patients out to 1 year to assess cumulative costs (and to rule out that the initially lower costs could have been due to deferral of testing).122 They found that over 1 year, costs continued to be lower in the group initially randomized to a CMR OU strategy. The results appeared to be driven in this group by fewer cardiac-related ED visits, hospitalizations, and catheterizations, possibly due to the perceived more definitive diagnostic information provided initially by CMR.
A subsequent trial from the same investigators randomized 105 patients felt to be at intermediate likelihood for ACS to an OU strategy with stress CMR imaging, or to usual care as provided by the patients’ cardiologists and internists. This latter strategy is more in keeping with standard of care for such patients, compared to the authors’ previous studies where inpatient care was the comparator. The group randomized to the CMR strategy had shorter length of stay, as well as reduced rate of revascularization, hospital readmissions, and recurrent cardiac testing over 90 days of follow-up.123
Another trial from the same authors examined the OU stress CMR strategy compared to a stress testing modality selected by the patients’ clinicians, which was stress echocardiography in the majority of cases.124 A difference from their prior studies was that this trial enrolled lower likelihood patients. The results showed no differences between the randomization groups in length of stay, referral for catheterization, admitting decision, or 30 day incidence of ACS. However, costs were lower in the clinician directed group. The authors concluded that in lower risk patients with suspected ACS, “…the ability of a physician to select a cardiac stress imaging modality (including echocardiography, CMR, or radionuclide testing) was more cost-effective than a pathway that mandates a CMR stress test.”124
This interesting series of studies suggests the potential for CMR imaging to be incorporated into an evaluation strategy pathway for ED patients with suspected ACS. Unlike almost all of the trial involving rest SPECT perfusion imaging or CCTA, the CMR studies examined patients at intermediate or higher likelihood of ACS, based in part on the stronger capability of CMR to differentiate ischemia from prior infarct. However when lower risk patients were studied, more “standard” tests appeared to be less costly and similarly effective. The studies do help to direct where CMR may be best deployed.
It is also important to note that all of these randomized trials were single center studies that emanated from the same institution and group of investigators, who are highly expert at the acquisition and interpretation of CMR imaging studies. Further investigation will be needed to assess the true generalizability of their findings.
Identifying Alternative Etiologies for Apparent ACS
An intriguing and unique aspect of CMR imaging is use for the characterization of patients who present with acute chest pain, positive troponin indicative of myocardial necrosis, but have no obstructive CAD at catheterization, specifically, myocardial infarction with angiographically normal or near-normal coronary arteries. Asomull125 evaluated troponin-positive patients with cine imaging and T2-weighted imaging for detection of inflammation, and late gadolinium enhancement imaging for detection of infarction/fibrosis. Remarkably, a cause for troponin elevation was established in 65% of patients. The most common underlying cause was myocarditis (50%) and in 35% of patients, myocardial infarction or significant myocardial fibrosis could be excluded. Hence, CMR imaging may be a valuable adjunct to conventional investigations in a diagnostically challenging and important group of patients with troponin-positive chest pain and unobstructed coronary arteries. These results have been supported by other studies suggesting that late gadolinium enhancement patterns of active myocarditis can be found in more than 50% of patients with positive troponin but without significant CAD126. Overall, assessment of edema and late gadolinium enhancement are the hallmarks of these investigations.
Appropriate Use Criteria and Guidelines
The 2006 ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR appropriateness criteria for cardiac computed tomography and CMR imaging list the use of vasodilator or dobutamine stress CMR imaging as of “uncertain” appropriateness in patients in the aftermath of acute chest pain127, which in contemporary nomenclature would be considered as “may be appropriate”. It is important to note that virtually all of the literature on the use of CMR in this setting has appeared after that document was prepared. Updated Appropriate Use Criteria for testing modalities potentially used in the evaluation of patients with suspected ACS in the ED setting are forthcoming.
Summary and Future Directions
In reviewing the evidence underlying recommendations contained in ACC/AHA Guidelines, Tricoci and colleagues reported that only a small minority of recommendations concerning cardiovascular imaging tests are supported by a high level evidence base128, that is, level of evidence “A” in such Guidelines. The area of assessing the value of imaging tests for patients with suspected ACS in the ED is an exception, as there are numerous randomized trials rigorously evaluating the various modalities.
In the design of virtually all of these trials, patients are randomized to a strategy where all undergo the imaging test of interest, which is compared to a control strategy of usual care or an alternative imaging strategy. However, a key aspect for future studies will be to determine the optimal and most efficient deployment of imaging, as patients at very low risk for ACS are unlikely to benefit from an imaging strategy in a cost-effective manner. The existing clinical risk scores11,12 may not identify a low enough risk profile for such a purpose.
A promising approach involves the use of the evolving assays for high-sensitivity troponins. As noted above, at the time of this writing these assays are not yet available in the United States. Studies published to date from outside the US suggest that the combination of a clinically very low risk patient along with an undetectable high sensitivity troponin value within a short time after ED presentation identifies a group with very low probability for ACS20, in whom imaging is not likely to add value. In these studies this low risk finding is seen in 10–15% of patients, and in concept such patients could be discharged directly without further testing for out-patient follow-up.
The data on the use of high-sensitivity troponins also begins to suggest that serial analysis of the markers over time may not be routinely necessary. In a very large cohort (n=14,636) of ED chest pain patients seen at one ED in Sweden, 21% (n=8,907) had undetectable levels of an initial high-sensitivity troponin accompanied by a non-ischemic initial ECG. Among these patients, the 30-day risk of acute MI was 0.44%, translating into a negative predictive value of 99.8% (95% CI, 99.7–99.9%)129. Future studies will likely incorporate these approaches in an attempt to identify the group of patients most likely (and least likely) to benefit from imaging.
Technical advances in the existing modalities may also have an impact on how imaging is used in this setting. For SPECT MPI, high speed and high efficiency cameras are now available that allow high quality imaging with lower radionuclide doses, reducing radiation exposure, and with protocols that are completed more quickly. In an observational study of over 1,400 ED patients who underwent CCTA or stress testing (with ECG or imaging with a high efficiency SPECT camera), Duvall and co-workers reported that similar proportions of patients were directly discharged from the ED with either testing strategy, but the CCTA group had longer length of stay, higher mean effective radiation dose exposure, and more follow-up testing130. The higher efficiency SPECT cameras also enable the possibility of “stress-only” imaging, which when combined with attenuation correction techniques for selected patients significantly reduces the time and dose for radionuclide imaging.131
The technology to perform CCTA also continues to evolve. In an initial study, Achenbach and colleagues reported on using a new CT acquisition scan mode to achieve high quality CCTA imaging with acquisition times of approximately 4 minutes and radiation exposures < 1mSv.132 Min and colleagues have published a series of reports, including a multicenter trial, assessing the ability of the noninvasive CT images to evaluate fractional flow reserve associated with a stenosis133. Once mature, this technology may attenuate the apparent increase in downstream catheterizations that have been observed in some CCTA studies, by enabling interrogation of the physiologic significance of a stenosis seen on CCTA.
Thus, the technologies involved in imaging patients in this setting continue to evolve, as do the potential ways to better select patients for optimally efficient use of imaging. The evaluation of imaging techniques for ED patients with suspected ACS in clinical trials has been leading the way in setting an example of how to rigorously assess technology for clinical use, resulting in a high level evidence base that can assist clinicians in selecting the right test for the right patient.
Dr. Alpert: Dr. Udelson and colleagues have presented an exhaustive and outstanding review of a very common and important clinical problem. It should be considered essential reading for physicians and for physicians-in-training in emergency medicine, in cardiovascular medicine and internal medicine. The authors are to be congratulated for this most important contribution.
Figure 17:
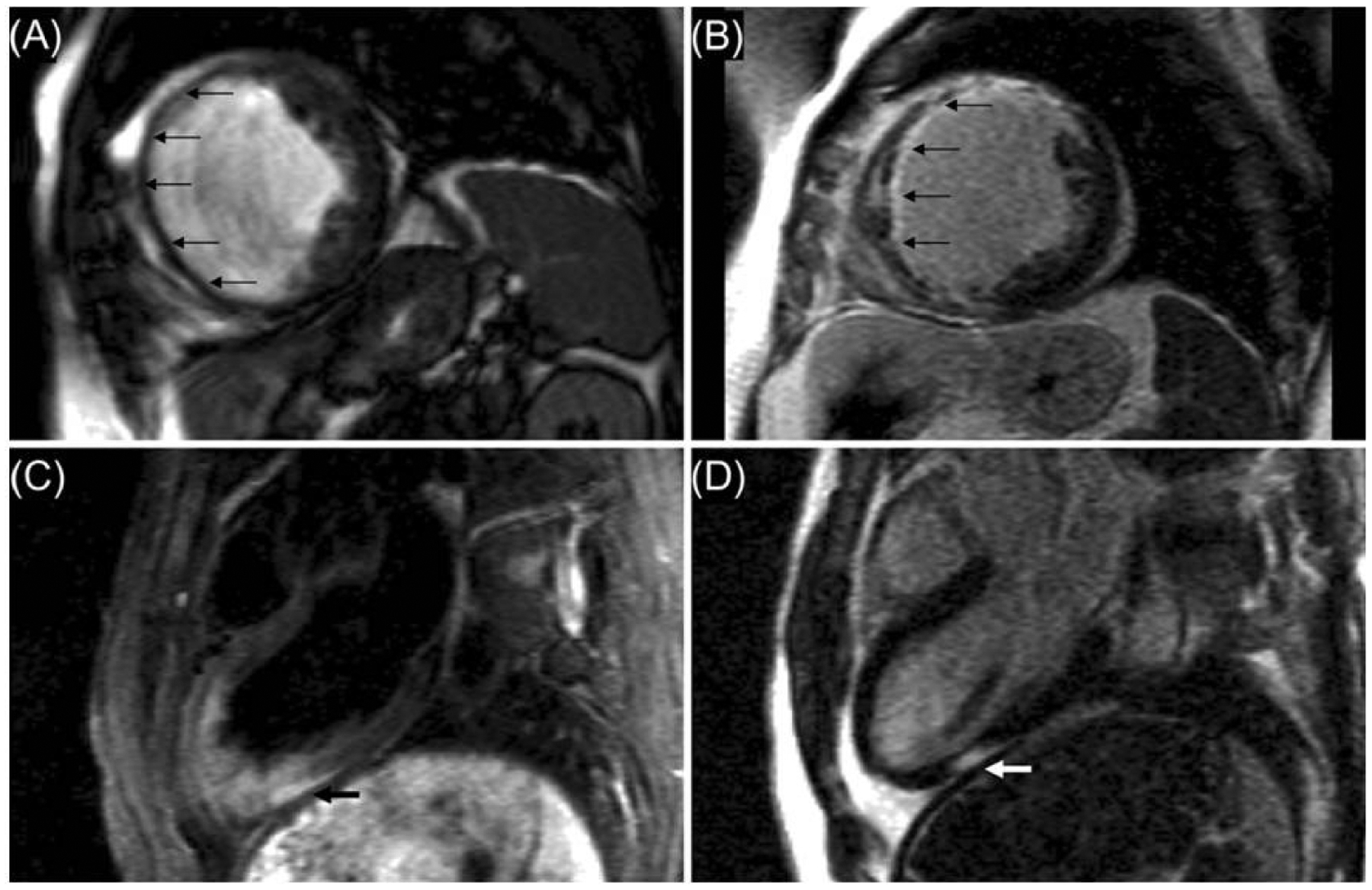
CMR imaging for detecting etiologies of chest pain in ED patients distinct from CAD as cause of clinical ACS. Panels A and B demonstrate the typical appearance of infarction related to CAD, with wall thinning of the anterior wall, septum and inferoseptum in Panel A (arrows), and near transmural gadolinium enhancement seen in Panel B (arrows). In contrast, the images shown in Panels C and D represent regional myocarditis, seen in a patient with a troponin-positive ACS clinical syndrome, but no severe stenosis seen on subsequent coronary angiography. Panel C is a T2-weighted long-axis view, and an area of focally increased signal is seen in the inferolateral wall suggesting inflammation (black arrow). In Panel D, gadolinium-enhanced imaging shows in the corresponding region patchy, mostly epicardial late enhancement, indicating fibrosis (white arrow). This pattern is most suggestive of acute myocarditis as an etiology of the clinical syndrome. Reproduced from reference 125.
Support relevant to this work
Dave: none
Ferencik: support from the American Heart Association (13FTF16450001)
Hoffmann: National Heart, Lung, and Blood Institute (U01HL092040; U01HL092022; 5T32 HL076136 and 5K24HL113128)
Udelson: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures relevant to this work
Dave: None
Ferencik: None
Hoffmann: Siemens and Heart Flow: Unrestricted Research grants to Massachusetts General Hospital
Udelson: None
References
- 1.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief. 2010;1–8. [PubMed] [Google Scholar]
- 2.Pope JH, Auferdeide TP, Ruthazer R, Woolard RH, Fledman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;57:e215–367 [DOI] [PubMed] [Google Scholar]
- 4.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- 5.Sanchis J, Bodí V, Núñez J, Bosch X, Loma-Osorio P, Mainar L, et al. Limitations of clinical history for evaluation of patients with acute chest pain, non-diagnostic electrocardiogram, and normal troponin. Am J Cardiol. 2008;101:613–7. [DOI] [PubMed] [Google Scholar]
- 6.Turnipseed SD, Trythall WS, Diercks DB, Laurin EG, Kirk JD, Smith DS, et al. Frequency of acute coronary syndrome in patients with normal electrocardiogram performed during presence or absence of chest pain. Acad Emerg Med. 2009;16:495–9. [DOI] [PubMed] [Google Scholar]
- 7.Singer AJ, Brogan GX, Valentine SM, McCuskey C, Khan S, Hollander JE. Effect of duration from symptom onset on the negative predictive value of a normal ECG for exclusion of acute myocardial infarction. Ann Emerg Med. 1997;29:575–9. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. [DOI] [PubMed] [Google Scholar]
- 9.Kontos MC, Shah R, Fritz LM, Anderson FP, Tatum JL, Ornato JP, et al. Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J Am Coll Cardiol. 2004;43:958–65. [DOI] [PubMed] [Google Scholar]
- 10.Newby LK, Christenson RH, Ohman EM, Armstrong PW, Thompson TD, Lee KL, et al. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. Circulation. 1998;98:1853–1859. [DOI] [PubMed] [Google Scholar]
- 11.Farkouh ME, Aneja A, Reeder GS, Smars PA, Bansilal S, Lennon RJ, et al. Clinical risk stratification in the emergency department predicts long-term cardiovascular outcomes in a population-based cohort presenting with acute chest pain: primary results of the Olmsted county chest pain study. Medicine. 2009;88:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–8. [DOI] [PubMed] [Google Scholar]
- 13.Antman E, Cohen M, Bernink PJLM, Mccabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina / non – ST elevation MI. J Am Med Assoc. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 14.Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang W-C, Lee KL, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation?: results from an international trial of 9461 patients. Circulation. 2000;101:2557–2567. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–53. [DOI] [PubMed] [Google Scholar]
- 16.De Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26:865–72. [DOI] [PubMed] [Google Scholar]
- 17.Selker HP, Beshansky JR, Griffith JL, Aufderheide TP, Ballin DS, Bernard SA, et al. Use of the Acute Cardiac Ischemia Time-Insensitive Predictive Instrument (ACI-TIPI) to assist with triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia. Ann Int Med. 1998;129:845–855. [DOI] [PubMed] [Google Scholar]
- 18.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–9. [DOI] [PubMed] [Google Scholar]
- 19.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. [DOI] [PubMed] [Google Scholar]
- 20.Than M, Cullen L, Reid CM, Lim SH, Aldous S, Ardagh MW, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377:1077–84. [DOI] [PubMed] [Google Scholar]
- 21.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59:23C–30C. [DOI] [PubMed] [Google Scholar]
- 22.Wackers FJ, Lie KI, Liem KL, Sokole EB, Samson G, van der Schoot JB, et al. Thallium-201 scintigraphy in unstable angina pectoris. Circulation. 1978;57:738–42. [DOI] [PubMed] [Google Scholar]
- 23.Wackers FJ, Lie KI, Liem KL, Sokole EB, Samson G, van der Schoot J, et al. Potential value of thallium-201 scintigraphy as a means of selecting patients for the coronary care unit. Br Heart J. 1979;41:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbons RJ, Verani MS, Behrenbeck T, Pellikka PA, O’Connor MK, Mahmarian JJ, et al. Feasibility of tomographic 99mTc-hexakis-2-methoxy-2-methylpropyl- isonitrile imaging for the assessment of myocardial area at risk and the effect of treatment in acute myocardial infarction. Circulation. 1989;80:1277–1286. [DOI] [PubMed] [Google Scholar]
- 25.Christian TF, Clements IP, Gibbons RJ. Noninvasive identification of myocardium at risk in patients with acute myocardial infarction and nondiagnostic electrocardiograms with technetium-99m-Sestamibi. Circulation. 1991;83:1615–20. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons RJ, Miller TD, Christian TF. Infarct size measured by single photon emission computed tomographic imaging with 99mTc-sestamibi?: a measure of the efficacy of therapy in acute myocardial infarction. Circulation. 2000;101:101–108. [DOI] [PubMed] [Google Scholar]
- 27.Bilodeau L, Théroux P, Grégoire J, Gagnon D, Arsenault A. Technetium-99m sestamibi tomography in patients with spontaneous chest pain: correlations with clinical, electrocardiographic and angiographic findings. J Am Coll Cardiol. 1991;18:1684–91. [DOI] [PubMed] [Google Scholar]
- 28.Varetto T, Cantalupi D, Altieri A, Orlandi C. Emergency room technetium-99m Sestamibi imaging to rule out acute myocardial ischemia in patients with nondiagnostic electrocardiograms. J Am Coll Cardiol. 1993;22:1804–8. [DOI] [PubMed] [Google Scholar]
- 29.Hilton TC, Thompson RC, Williams HJ, Saylors R, Fulmer H, Stowers SA. Technetium-99m sestamibi myocardial perfusion imaging in the emergency room evaluation of chest pain. J Am Coll Cardiol. 1994;23:1016–22. [DOI] [PubMed] [Google Scholar]
- 30.Tatum JL, Jesse RL, Kontos MC, Nicholson CS, Schmidt KL, Roberts CS, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med. 1997;29:116–25. [DOI] [PubMed] [Google Scholar]
- 31.Kontos MC, Jesse RL, Schmidt KL, Ornato JP, Tatum JL. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997;30:976–82. [DOI] [PubMed] [Google Scholar]
- 32.Heller GV, Stowers SA, Hendel RC, Herman SD, Daher E, Ahlberg AW, et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011–1017. [DOI] [PubMed] [Google Scholar]
- 33.Duca MD, Giri S, Wu AH, Morris RS, Cyr GM, Ahlberg A, et al. Comparison of acute rest myocardial perfusion imaging and serum markers of myocardial injury in patients with chest pain syndromes. J Nucl Cardiol. 1999;6:570–6. [DOI] [PubMed] [Google Scholar]
- 34.Kosnik JW, Zalenski RJ, Shamsa F, Harris R, Mittner J, Kozlowski J, et al. Resting sestamibi imaging for the prognosis of low-risk chest pain. Acad Emerg Med. 1999;6:998–1004. [DOI] [PubMed] [Google Scholar]
- 35.Kontos MC, Jesse RL, Anderson FP, Schmidt KL, Ornato JP, Tatum JL. Comparison of myocardial perfusion imaging and cardiac troponin I in patients admitted to the emergency department with chest pain. Circulation. 1999;99:2073–2078. [DOI] [PubMed] [Google Scholar]
- 36.Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Heller GV, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia. J Am Med Assoc. 2002;288:2693–2700. [DOI] [PubMed] [Google Scholar]
- 36a.Schaeffer MW, Brennan TD, Hughes JA et al. Resting radionuclide myocardial perfusion imaging in a chest pain center including an overnight delayed image acquisition protocol. J Nucl Med Technol. 2007;35:242–5 [DOI] [PubMed] [Google Scholar]
- 37.Better N, Karthikeyan G, Vitola J, Fatima A, Peix A, Novak MD, et al. Performance of rest myocardial perfusion imaging in the management of acute chest pain in the emergency room in developing nations (PREMIER trial). J Nuc Card. 2012;19:1146–53. [DOI] [PubMed] [Google Scholar]
- 38.Stowers SA, Eisenstein EL, Wackers FJ, Berman DS, Blackshear JL, Jones AD Jr, et al. An economic analysis of an aggressive diagnostic strategy with single photon emission computed tomography myocardial perfusion imaging and early exercise stress testing in emergency department patients who present with chest pain but nondiagnostic electrocardiograms. Ann Emerg Med. 2000;35:17–25. [DOI] [PubMed] [Google Scholar]
- 39.Kontos M, Haney A, Ornato J, Jesse R, Tatum J. Value of simultaneous functional assessment in association with acute rest perfusion imaging for predicting short- and long-term outcomes in emergency department patients with chest pain. J Nucl Cardiol. 2008;15:774–782. [DOI] [PubMed] [Google Scholar]
- 40.Fram DB, Azar RR, Ahlberg AW, Gillam LD, Mitchel JF, Kiernan FJ, et al. Duration of abnormal SPECT myocardial perfusion imaging following resolution of acute ischemia: an angioplasty model. J Am Coll Cardiol. 2003;41:452–459. [DOI] [PubMed] [Google Scholar]
- 41.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. Circulation. 2009;119:e561–87. [DOI] [PubMed] [Google Scholar]
- 42.Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram MY, Goodbody AE, et al. Metabolic imaging with beta-methyl-p-[(123)I]-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation. 200;112:2169–74. [DOI] [PubMed] [Google Scholar]
- 43.Kontos MC, Dilsizian V, Weiland F, DePuey G, Mahmarian JJ, Iskandrian AE, et al. Iodofiltic acid I 123 (BMIPP) fatty acid imaging improves initial diagnosis in emergency department patients with suspected acute coronary syndromes: a multicenter trial. J Am Coll Cardiol. 2010;56:290–9. [DOI] [PubMed] [Google Scholar]
- 44.Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction a prospective study using two-dimensional echocardiography. Circulation. 1991;84:I85–92. [PubMed] [Google Scholar]
- 45.Lewis WR. Echocardiography in the evaluation of patients in chest pain units. Cardiol Clin. 2005;23:531–9 [DOI] [PubMed] [Google Scholar]
- 46.Kontos MC, Arrowood JA, Paulsen WH, Nixon JV. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med. 1998;31:550–7. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki H, Charuzi Y, Beeder C, Sugiki Y, Lew AS. Utility of echocardiography for the early assessment of patients with nondiagnostic chest pain. Am Heart J. 1986;112:494–7. [DOI] [PubMed] [Google Scholar]
- 48.Peels CH, Visser CA, Kupper AJF, Visser FC, Roos JP. Usefulness of Two-Dimensional Echocardiography for Immediate Detection of Myocardial Ischemia in the Emergency Room. Am J Cardiol. 1990;65:687–691. [DOI] [PubMed] [Google Scholar]
- 49.Weston P, Alexander JH, Patel MR, Maynard C, Crawford L, Wagner GS. Hand-held echocardiographic examination of patients with symptoms of acute coronary syndromes in the emergency department: the 30-day outcome associated with normal left ventricular wall motion. Am Heart J. 2004;148:1096–101. [DOI] [PubMed] [Google Scholar]
- 50.Atar S, Feldman A, Darawshe A, Siegel RJ, Rosenfeld T. Utility and diagnostic accuracy of hand-carried ultrasound for emergency room evaluation of chest pain. Am J Cardiol. 2004;94:408–9. [DOI] [PubMed] [Google Scholar]
- 51.Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225–30. [DOI] [PubMed] [Google Scholar]
- 52.Anantharam B, Chahal N, Chelliah R, Ramzy I, Gani F, Senior R. Safety of contrast in stress echocardiography in stable patients and in patients with suspected acute coronary syndrome but negative 12-hour troponin. Am J Cardiol. 2009;104:14–8. [DOI] [PubMed] [Google Scholar]
- 53.Lepper W, Belcik T, Wei K, Lindner JR, Sklenar J, Kaul S. Myocardial contrast echocardiography. Circulation. 2004;109:3132–5. [DOI] [PubMed] [Google Scholar]
- 54.Kontos MC, Hinchman D, Cunningham M, Miller JJ, Cherif J, Nixon J. Comparison of contrast echocardiography with single-photon emission computed tomographic myocardial perfusion imaging in the evaluation of patients with possible acute coronary syndromes in the emergency department. Am J Cardiol. 2003;91:1099–1102. [DOI] [PubMed] [Google Scholar]
- 55.Kaul S, Senior R, Firschke C, Wang X, Lindner J, Villaneuva FS, et al. Incremental value of cardiac imaging in patients presenting to the emergency department with chest pain and without ST-segment elevation: a multicenter study. Am Heart J. 2004;148:129–36. [DOI] [PubMed] [Google Scholar]
- 56.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–66.21349406 [Google Scholar]
- 57.Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40:251–6. [DOI] [PubMed] [Google Scholar]
- 59.Zalenski RJ, McCarren M, Roberts R, Rydman RJ, Jovanovic B, Das K, et al. An evaluation of a chest pain diagnostic protocol to exclude acute cardiac ischemia in the emergency department. Arch Intern Med. 1997;157:1085–1091. [PubMed] [Google Scholar]
- 60.Polanczyk CA, Johnson PA, Hartley LH, Walls RM, Shaykevich S, Lee TH. Clinical correlates and prognostic significance of early negative exercise tolerance test in patients with acute chest pain seen in the hospital emergency department. Am J Cardiol. 1998;81:288–92. [DOI] [PubMed] [Google Scholar]
- 61.Gibler WB, Runyon JP, Levy RC, Sayre MR, Kacich R, Hattemer CR. A rapid diagnostic and treatment center for patients with chest pain in the emergency department. Ann Emerg Med. 1995;25:1–8. [DOI] [PubMed] [Google Scholar]
- 62.Fesmire FM, Hughes AD, Fody EP, Jackson AP, Fesmire CE, Gilbert MA,et al. The Erlanger chest pain evaluation protocol: A one-year experience with serial 12-lead ECG monitoring, two-hour delta serum marker measurements, and selective nuclear stress testing to identify and exclude acute coronary syndromes. Ann Emerg Med. 2002;40:584–594. [DOI] [PubMed] [Google Scholar]
- 63.Lim SH, Anantharaman V, Sundram F, Chan ES-Y, Ang ES, Yo SL, et al. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol. 2013;20:1002–12. [DOI] [PubMed] [Google Scholar]
- 64.Bholasingh R, Cornel JH, Kamp O, van Straalen JP, Sanders GT, Tijssen JG, et al. Prognostic value of predischarge dobutamine stress echocardiography in chest pain patients with a negative cardiac troponin T. J Am Coll Cardiol. 2003;41:596–602. [DOI] [PubMed] [Google Scholar]
- 65.Trippi JA, Lee KS, Kopp G, Nelson DR, Yee KG, Cordell WH. Dobutamine stress tele-echocardiography for evaluation of emergency department patients with chest pain. J Am Coll Cardiol. 1997;30:627–32. [DOI] [PubMed] [Google Scholar]
- 66.Nucifora G, Badano LP, Sarraf-Zadegan N, Karavidas A, Trocino G, Scaffidi G, et al. Comparison of early dobutamine stress echocardiography and exercise electrocardiographic testing for management of patients presenting to the emergency department with chest pain. Am J Cardiol. 2007;100:1068–73. [DOI] [PubMed] [Google Scholar]
- 67.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography. Circulation. 2008;117:1478–97. [DOI] [PubMed] [Google Scholar]
- 68.Diver DJ, Bier JD, Ferreira PE, Sharaf BL, McCabe C, Thompson B, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol. 1994;74:531–537. [DOI] [PubMed] [Google Scholar]
- 69.Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, et al. Clinical and Therapeutic Profile of Patients Presenting With Acute Coronary Syndromes Who Do Not Have Significant Coronary Artery Disease. Circulation. 2000;102:1101–1106. [DOI] [PubMed] [Google Scholar]
- 70.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. [DOI] [PubMed] [Google Scholar]
- 71.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. [DOI] [PubMed] [Google Scholar]
- 72.Meijboom WB, Meijs MFL, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CAG, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. [DOI] [PubMed] [Google Scholar]
- 73.Chow BJW, Freeman MR, Bowen JM, Levin L, Hopkins RB, Provost Y, et al. Ontario multidetector computed tomographic coronary angiography study: field evaluation of diagnostic accuracy. Arch Intern Med. 2011;171:1021–1029. [DOI] [PubMed] [Google Scholar]
- 74.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152:167–177. [DOI] [PubMed] [Google Scholar]
- 75.Schuetz GM, Schlattmann P, Dewey M. Use of 3×2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. Br Med J. 2012;345:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, Macneill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. [DOI] [PubMed] [Google Scholar]
- 77.Leber AW, Becker A, Knez A, Ziegler von F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–677. [DOI] [PubMed] [Google Scholar]
- 78.Petranovic M, Soni A, Bezzera H, Loureiro R, Sarwar A, Raffel C, et al. Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: evaluation of nonculprit coronary lesions. J Cardiovasc Comput Tomogr. 2009;3:24–31. [DOI] [PubMed] [Google Scholar]
- 79.Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging. 2011;4:537–548. [DOI] [PubMed] [Google Scholar]
- 80.Ferencik M, Nieman K, Achenbach S. Noncalcified and calcified coronary plaque detection by contrast-enhanced multi-detector computed tomography: a study of interobserver agreement. J Am Coll Cardiol. 2006;47:207–209. [DOI] [PubMed] [Google Scholar]
- 81.Lehman SJ, Schlett CL, Bamberg F, Lee H, Donnelly P, Shturman L, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging. 2009;2:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun J, Zhang Z, Lu B, Yu W, Yang Y, Zhou Y, Wang, et al. Identification and quantification of coronary atherosclerotic plaques: a comparison of 64-MDCT and intravascular ultrasound. AJR Am J Roentgenol. 2008;190:748–754. [DOI] [PubMed] [Google Scholar]
- 83.Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr. 2011;5:35–43. [DOI] [PubMed] [Google Scholar]
- 84.Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762–1768. [DOI] [PubMed] [Google Scholar]
- 85.Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863–871. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) Trial. J Am Coll Cardiol. 2011;58:1414–1422. [DOI] [PubMed] [Google Scholar]
- 87.Chang S-A, Choi SI, Choi E-K, Kim H-K, Jung J-W, Chun EJ, et al. Usefulness of 64-slice multidetector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J. 2008;156:375–383. [DOI] [PubMed] [Google Scholar]
- 88.Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251–2260. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marwan M, Pflederer T, Schepis T, Seltmann M, Klinghammer L, Muschiol G, et al. Accuracy of dual-source CT to identify significant coronary artery disease in patients with uncontrolled hypertension presenting with chest pain: comparison with coronary angiography. Int J Cardiovasc Imaging. 2011; [DOI] [PubMed] [Google Scholar]
- 91.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295–304. [DOI] [PubMed] [Google Scholar]
- 92.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
- 93.Hansen M, Ginns J, Seneviratne S, Slaughter R, Premaranthe M, Samardhi H, et al. The value of dual-source 64-slice CT coronary angiography in the assessment of patients presenting to an acute chest pain service. Heart Lung Circ. 2010;19:213–218. [DOI] [PubMed] [Google Scholar]
- 94.Nasis A, Meredith IT, Nerlekar N, Cameron JD, Antonis PR, Mottram PM, et al. Acute chest pain investigation: utility of cardiac CT angiography in guiding troponin measurement. Radiology. 2011;260:381–389. [DOI] [PubMed] [Google Scholar]
- 95.Chow BJW, Joseph P, Yam Y, Kass M, Chen L, Beanlands RS, et al. Usefulness of computed tomographic coronary angiography in patients with acute chest pain with and without high-risk features. Am J Cardiol. 2010;106:463–469. [DOI] [PubMed] [Google Scholar]
- 96.Ferencik M, Schlett CL, Bamberg F, Truong QA, Nichols JH, Pena AJ, et al. Comparison of traditional cardiovascular risk models and coronary atherosclerotic plaque as detected by computed tomography for prediction of acute coronary syndrome in patients with acute chest pain. Acad Emerg Med. 2012;19:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takakuwa KM, Keith SW, Estepa AT, Shofer FS. A meta-analysis of 64-section coronary CT angiography findings for predicting 30-day major adverse cardiac events in patients presenting with symptoms suggestive of acute coronary syndrome. Acad Radiol. 2011;18:1522–1528. [DOI] [PubMed] [Google Scholar]
- 98.Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, et al. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging. 2010;3:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bezerra HG, Loureiro R, Irlbeck T, Bamberg F, Schlett CL, Rogers I, et al. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr. 2011;5:382–391. [DOI] [PubMed] [Google Scholar]
- 100.Dirksen MS, Jukema JW, Bax JJ, Lamb HJ, Boersma E, Tuinenburg JC, et al. Cardiac multidetector-row computed tomography in patients with unstable angina. Am J Cardiol. 2005;95:457–461. [DOI] [PubMed] [Google Scholar]
- 101.Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, et al. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012;98:1510–1517. [DOI] [PubMed] [Google Scholar]
- 102.Branch KR, Busey J, Mitsumori LM, Strote J, Caldwell JH, Busch JH, et al. Diagnostic performance of resting CT myocardial perfusion in patients with possible acute coronary syndrome. AJR Am J Roentgenol. 2013;200:W450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwasaki K, Matsumoto T. Myocardial perfusion defect in patients with coronary artery disease demonstrated by 64-multidetector computed tomography at rest. Clin Cardiol. 2011;34:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferencik M, Schlett CL, Ghoshhajra BB, Kriegel MF, Joshi SB, Maurovich-Horvat P, et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol. 2012;110:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pflederer T, Marwan M, Schepis T, Ropers D, Seltmann M, Muschiol G, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis. 2010;211:437–444. [DOI] [PubMed] [Google Scholar]
- 106.Kim SY, Kim K-S, Seung MJ, Chung JW, Kim JH, Mun SH, et al. The culprit lesion score on multi-detector computed tomography can detect vulnerable coronary artery plaque. Int J Cardiovasc Imaging. 2010;26:245–252. [DOI] [PubMed] [Google Scholar]
- 107.Koo B-K, Erglis A, Doh J-H, Daniels DV, Jegere S, Kim H-S, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 108.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo B-K, van Mieghem C, et al. Diagnostic Accuracy of Fractional Flow Reserve From Anatomic CT Angiography. J Am Med Assoc. 2012;:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;61:880–892. [DOI] [PubMed] [Google Scholar]
- 111.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society of Cardiovascular Computed Tomography, American College of Radiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, North American Society for Cardiovascular Imaging, Society for Cardiovascular Angiography and Interventions, Society for Cardiovascular Magnetic Resonance. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–55. [DOI] [PubMed] [Google Scholar]
- 112.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. [DOI] [PubMed] [Google Scholar]
- 113.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537 [DOI] [PubMed] [Google Scholar]
- 114.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, et al. Cardiac magnetic resonance with t2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844 [DOI] [PubMed] [Google Scholar]
- 115.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, et al. Delayed enhancement and t2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416 [DOI] [PubMed] [Google Scholar]
- 116.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333 [DOI] [PubMed] [Google Scholar]
- 117.Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the ce-marc trial. Circulation. 2013 [DOI] [PubMed] [Google Scholar]
- 118.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (ce-marc): A prospective trial. Lancet. 2012;379:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heitner JF, Klem I, Rasheed D, Chandra A, Kim HW, Van Assche LM, et al. Stress cardiac MR imaging compared with stress echocardiography in the early evaluation of patients who present to the emergency department with intermediate-risk chest pain. Radiology. 2013:130557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432 [DOI] [PubMed] [Google Scholar]
- 121.Miller CD, Hwang W, Hoekstra JW, Case D, Lefebvre C, Blumstein H, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010;56:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, et al. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller CD, Case LD, Little WC, Mahler SA, Burke GL, Harper EN, et al. Stress CMR reduces revascularization, hospital readmission, and recurrent cardiac testing in intermediate-risk patients with acute chest pain. JACC Cardiovasc Imaging. 2013;6:785–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miller CD, Hoekstra JW, Lefebvre C, Blumstein H, Hamilton CA, Harper EN, et al. Provider-directed imaging stress testing reduces health care expenditures in lower-risk chest pain patients presenting to the emergency department. Circ Cardiovasc Imaging. 2012;5:111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249 [DOI] [PubMed] [Google Scholar]
- 126.Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, et al. Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging. 2007;25:957–964 [DOI] [PubMed] [Google Scholar]
- 127.Hendel RC, Patel MR, Kramer CM, Poon M et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiacmagnetic resonance imaging: a report of the American College of Cardiology Foundation/American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006;48:1475–97 [DOI] [PubMed] [Google Scholar]
- 128.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 200925;301:831–41 [DOI] [PubMed] [Google Scholar]
- 129.Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable High Sensitivity Cardiac Troponin T Level in the Emergency Department and Risk of Myocardial Infarction. J Am Coll Cardiol. 2014: Epub before print. doi: 10.1016/j.jacc.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 130.Duvall WL, Savino JA, Levine EJ, Sanz J, Baber U, Lin JT, et al. A comparison of coronary CTA and stress testing using high-efficiency SPECT MPI for the evaluation of chest pain in the emergency department. J Nucl Cardiol 2014;21:305–18 [DOI] [PubMed] [Google Scholar]
- 131.Duvall WL, Wijetunga MN, Klein TM, Hingorani R, Bewley B, Khan SM, et al. Stress-only Tc-99m myocardial perfusion imaging in an emergency department chest pain unit. J Emerg Med. 2012;42:642–50 [DOI] [PubMed] [Google Scholar]
- 132.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–6 [DOI] [PubMed] [Google Scholar]
- 133.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK,et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–45 [DOI] [PMC free article] [PubMed] [Google Scholar]


