Abstract
Eight 4-carboalkoxyvalerolactones (CRVLs), varying in the composition of their alkyl (R) side chains, were synthesized from malic acid and subjected to ring-opening transesterification polymerization (ROTEP) using diphenyl phosphate [DPP, (PhO)2PO2H] as a catalyst. Each CRVL produced a semicrystalline poly(4-carboalkoxyvalerolactone) (PCRVL), and the nature of the R group impacted the thermal transitions of these polyesters. Bulk polymerizations at 70 °C allowed for preparation of high molar mass samples that contained small amounts of branching, as evidenced by 1H NMR spectroscopy, MALDI spectrometry, size-exclusion chromatography, and eliminative degradation. Tensile testing of these lightly branched, high molar mass samples revealed that these polyesters are tough (tensile toughness values up to 88 ± 33 MJ•m−3) and have Young’s moduli (E) up to 186 ± 13 MPa. The acid- and base-catalyzed hydrolytic degradation of the PCRVLs was quantitatively monitored using total organic carbon analysis, and effect of the alkyl chain length on PCRVL hydrolysis rate was determined. Finally, the methyl ester variant of these malic acid-derived thermoplastics is known to be chemically recyclable.
Graphical Abstract

INTRODUCTION
With the growing demand to replace petroleum-derived plastics with biobased alternatives, polyesters such as poly(lactic acid) (PLA) and poly(3-hydroxyalkanoates) [P(3-HAs)]1 are leading replacements. However, broad applicability of these polymers has been limited due to their relatively narrow range of properties.2 Research has focused on modifying the mechanical properties of these polymers through, for example, the incorporation of branching3,4,5 or the use of comonomers to prepare statistical6 or block copolymers.7,8,9 Alternatively, exploration of new biobased monomers and polymers with tunable and complementary properties to those currently available is also an important contemporary research endeavor.10,11,12
Side chain substituents play an important role in dictating the thermal and mechanical properties of polymers.13,14,15 The differences can be either dramatic [e.g., the methyl group in poly(propylene) vs. the carboxyl group in poly(acrylic acid)] or subtle in nature. That is, even slight modifications in backbone substituents can lead to useful changes in polymer properties.16,17 These subtle changes are exemplified by poly[alkyl (meth)acrylates], where glass transition temperature (Tg) and entanglement molar mass (Me) can be controlled by modifying the alkyl chain on the monomer used for polymerization.18
We previously demonstrated19 that poly(4-carbomethoxyvalerolactone) [PCMeVL, Figure 1a] from CMeVL has thermal properties distinct from alkyl-substituted valerolactones.20 Notably, CMeVL is the first racemic substituted valerolactone monomer to afford a semicrystalline polyester. This polymer, PCMeVL, is also chemically recyclable by two independent routes: (i) reverse-ring-opening transesterification polymerization (reverse-ROTEP) back to CMeVL and (ii) base-induced eliminative degradation to a methacrylate analogue. The latter chemical recycling pathway is enabled by the presence of the carboalkoxy group present in every repeat unit. Additionally, this side chain ester allowed for a post-polymerization isomerization from linear PCMeVL to an isomeric, hyperbranched polyester using a highly active zinc catalyst.21
Figure 1.

(a) Previous work: polymerization of CMeVL to its linear polyester PCMeVL and (post-polymerization) isomerization to hyperbranched PCMeVL. (b) This work: polymerization of eight CRVLs that vary in the alkyl group within each carboalkoxy side chain to linear PCRVLs.
Here we report the synthesis and thermal and mechanical properties of poly(4-carboalkoxyvalerolactones) (PCRVLs, Figure 1b) using [DPP, (PhO)2PO2H] as the catalyst for ROTEP of the CRVL monomers. The PCRVLs differ in the identity of the alkyl side chain (i.e., R group) of the carboalkoxy units. This subtle modification leads to polyesters with complementary and tunable properties to those of PCMeVL as well as other sustainable polymers such as P(3-HAs) and PLA. These PCRVLs should also be chemically recyclable in two ways and may be capable of a post-polymerization isomerization to a hyperbranched polyester. Furthermore, we explore the hydrolytic degradation of PCRVLs and demonstrate that the alkyl sidechain can modify the rate of degradation.
RESULTS AND DISCUSSION
Monomer syntheses.
CRVL monomers containing an ethyl (CEtVL), isopropyl (CiPrVL), or n-butyl (CnBuVL) carboalkoxy group were synthesized following our previous procedure for CMeVL (Figure 2).19 Malic acid (1) was heated in sulfuric acid (65 °C for 16 to 24 h) to form coumalic acid (2-H).22,23 Ethanol, isopropanol, or n-butanol was added directly to this reaction mixture (65 °C for 4 to 18 h) to form alkyl coumalates 2-R in 37–59% yield following distillation and a single recrystallization. Hydrogenation of 2-R using Pd/C gave CEtVL, CiPrVL, and CnBuVL. The 2-methylglutaric acid monoalkyl ester was always observed as a byproduct; these likely arise from hydrogenolysis of an allylic C–O bond in an intermediate 2,6-dihydrocoumalate or a hydropalladation/elimination event followed by further reduction of the cleaved lactone. In each case, the acid byproduct was easily removed from the CRVL by extraction into aqueous base. The final yield of each purified lactone monomer was between 56 and 67% with no apparent trend across R groups.
Figure 2.

Synthesis of CRVLs from malic acid.
tert-Butyl coumalate (2-tBu) was much more difficult to synthesize. Common methods to convert carboxylic acids to tert-butyl esters include utilizing isobutylene with a Brønsted acid or reaction of t-BuOH with an acid chloride. However, in our hands, neither of these strategies gave better than about 15% yield of 2-tBu.24 Instead,25 exposure of 2-H to di-tert-butyl dicarbonate [(Boc)2O] with DMAP and t-BuOH26 afforded a 10–35% yield of 2-tBu (and 67% of CtBuVL following hydrogenation).
To expand the set of CRVLs, we also prepared 4-carbobenzoxy, 4-carbo(2-ethylhexoxy), and 4-carboundecoxyvalerolactones [CBnVL, C2EtHexVL, and CC11VL]. The two-step synthesis starting from malic acid and sulfuric acid previously described (Figure 2) was not suitable for this series, likely because these less polar alcohols were not miscible with the H2SO4 reaction mixture. Also, addition of these alcohols to coumalic acid chloride (S1) often led to esterification of the pyrone ester moiety. Deprotonation of 2-H with Na2CO3 in DMF and displacement of alkyl bromides emerged as effective for making 2-Rs that contained longer alkyl chains. Hydrogenation of these 2-Rs gave similar conversion to CRVLs and 2-methylglutaric acid monoalkyl esters. However, this product/byproduct pair was more difficult to separate for these greasier analogs by simple partitioning into a basic aqueous solution.
These challenges led us to develop an alternative synthesis to obtain CRVLs with longer alkyl chains (Figure 3). 2-Methyleneglutaric acid (3, prepared in two steps from methyl acrylate)27 was treated with HBr•AcOH to give bromodiacid 4. Exposure of 4 to Hünig’s base (diisopropylethylamine, DIPEA) in DCM caused cyclization to lactone 5, which was then alkylated to CRVLs using Na2CO3 and alkyl bromides. This two-step sequence afforded CBnVL, C2EtHexVL, and CC11VL in 42–70% yield, in these instances following chromatographic purification on silica gel.
Figure 3.

Two-step synthesis of CBnVL, C2EtHexVL, and CC11VL from 3 (derived in two steps from methyl acrylate).
Characterization of low molar mass PCRVLs.
Each of the new CRVLs just described was polymerized using a 50:1:0.5 ratio of CRVL:BnOH:DPP (neat, ambient temperature, 20–24 h, Figure 4). Each PCRVL was purified by precipitation and characterized by 1H and 13C NMR spectroscopy, size-exclusion chromatography (SEC), thermogravimetric analysis (TGA) [see Supporting Information (SI)], and differential scanning calorimetry (DSC). The measured molar masses [1H NMR spectroscopy (and SEC, see SI)] for these samples matched well with those targeted, and the SEC traces exhibited unimodal distributions with low dispersities (Đ), suggesting that the polymerization proceeded without significant intermolecular transesterification with the polymer main chain or side chain esters. TGA analysis also showed degradation temperatures (according to onset of significant mass loss) typical of polyesters.28
Figure 4.
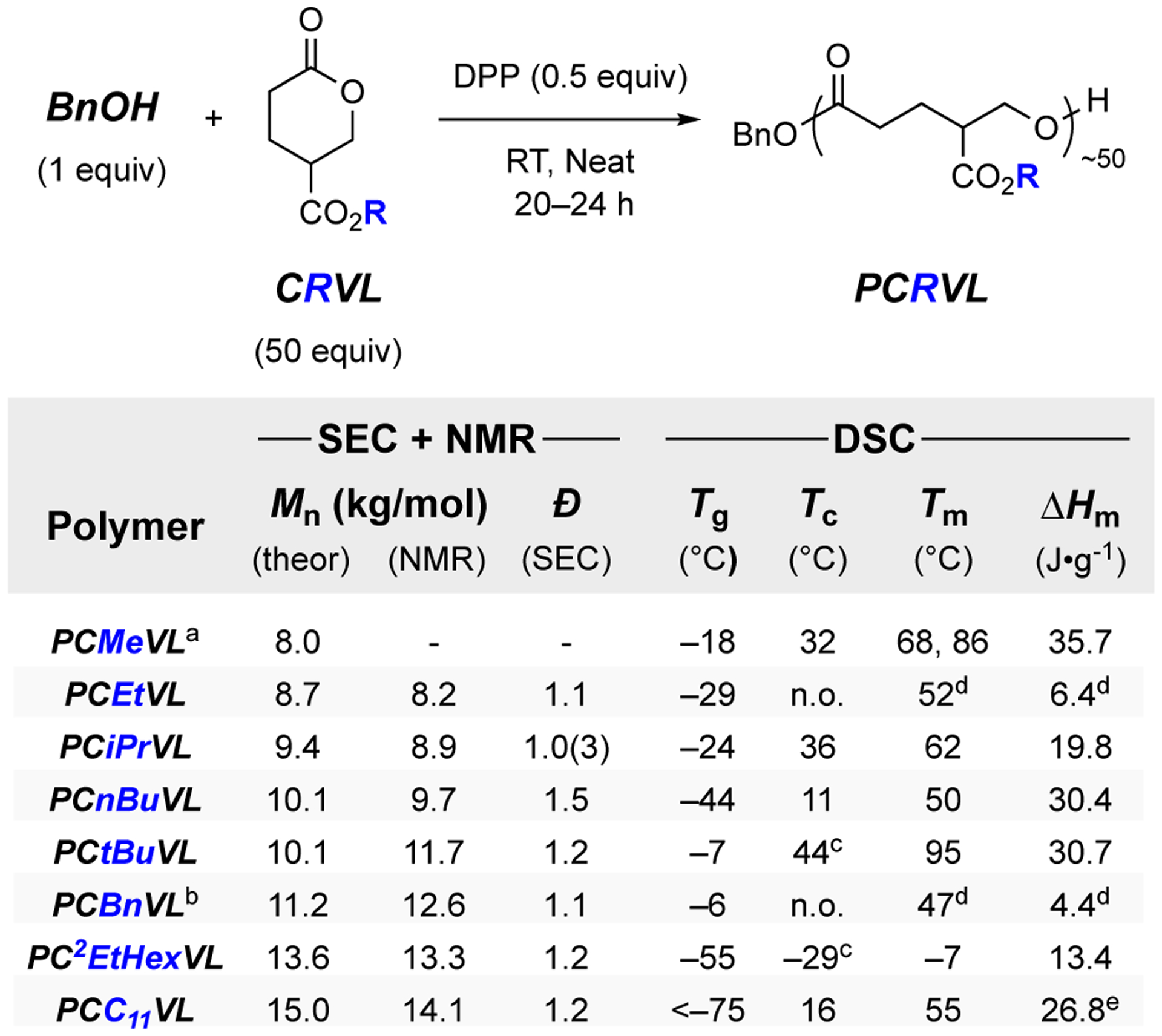
Polymerization of CRVLs to PCRVLs using DPP and benzyl alcohol (BnOH) neat at room temperature. Each PCRVL had ca. 50 repeat units per polymer chain. DSC results were taken on the third heating cycle, unless specified otherwise; each DSC sample was cooled from 150 °C to −60 °C at a rate of 5 °C•min−1 and subsequently heated to 150 °C (also at 5 °C•min−1) for analysis. No Tc was observed for PCEtVL or PCBnVL (n.o. = not observed).
aData from our previous report.19 bContains an acetylated end group. cTc observed during cooling prior to heating cycle. dNo Tm (or ΔHm) was observed after the first heating cycle; these values are from the first heating cycle. eA very subtle melting endotherm was observed for PC11VL beginning at ca. −50 °C until the major endotherm at 55 °C. The ΔHm over this entire range (−50 to 75 °C) is 73.3 J•g−1 (see Figure S17g).
Notably, each PCRVL was semicrystalline and the set of thermal characteristics [Tg, crystallization temperature (Tc), melting temperature (Tm), and enthalpy of melting (ΔHm)] of each was influenced by the nature of the alkyl group in the side chain ester (Figure 4). In the linear alkyl series, polymers with alkyls larger than methyl in the side chain esters had lower Tg values (e.g., −44 °C for PCnBuVL vs. −18 °C for PCMeVL). Overall, the lowest Tm was observed for PC2EtHexVL (−7 °C), and the highest for PCtBuVL (95 °C). The undecyl-containing PCC11VL showed an intermediate Tm to those of PCMeVL and PCnBuVL, a phenomenon that suggests crystallization of both the polymer backbone and side chain alkyl moieties of PCC11VL.29 Finally, the ΔHm values and the crystallization rates in PCRVLs varied greatly from sample to sample. In particular, PCEtVL and PCBnVL were the slowest to crystallize and no melting endotherms were observed for either of these samples after the initial DSC heating cycle.
Last, we repeated the polymerizations for PCtBuVL, PC2EtHexVL, and PCC11VL and monitored their equilibrium monomer conversion. Each reached 97–99% conversion, indicating that the alkyl substituents play a minor role in the free energy of polymerization.20
High molar mass PCRVLs: Polymerization and molar mass distribution.
For use in uniaxial tensile testing, higher molar mass samples (ca. 100 kg•mol−1) of PCMeVL, PCEtVL, PCiPrVL, and PCnBuVL were prepared (Figure 5a). Initial attempts to polymerize CMeVL to PCMeVL in bulk to this high of a molar mass required 5 days to reach 90% conversion at room temperature (RT, 0.4 mol% of DPP). Therefore 70 °C was used for the polymerization temperature for each of these CRVLs, which resulted in ca. 90% monomer conversion within 9 to 24 h. The SEC chromatograms of the higher molar mass PCMeVL, PCEtVL, PCiPrVL, and PCnBuVL showed bimodal distributions wherein the larger peak had a slightly lower than targeted molar mass (e.g., for PCiPrVL see Figure 5b). The shorter retention volume signal had ca. twice the Mw of that of the major component. This is in contrast to the lower molar mass samples, bulk polymerized at room temperature, which were unimodal. Monitoring the SEC behavior of PCiPrVL (CiPrVL:BnOH = ca. 300:1) over the course of the polymerization revealed that this bimodal distribution was present even at low monomer conversion. The polymer distribution further broadened over time, and the bimodality persisted until ca. 9 h, at which point the two signals had converged (Figure 5c). These observations are consistent with slow chain transfer reactions occurring during the polymerization, a previously observed phenomenon30,32 that has been mathematically modeled.30,31
Figure 5.
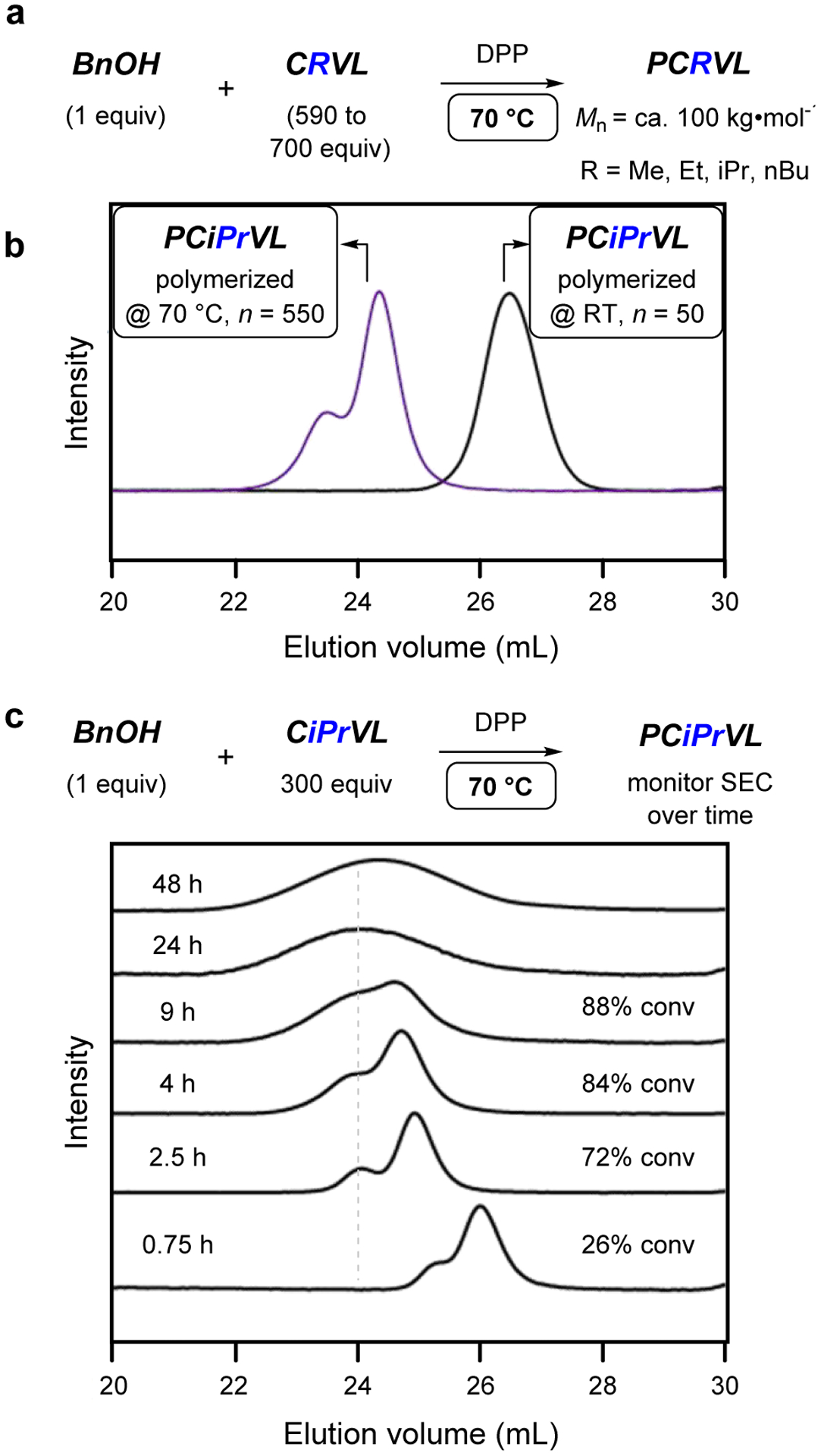
(a) Polymerization of CRVLs (R = Me, Et, iPr, nBu) to PCRVLs at 70 °C with targeted molar masses of ca. 100 kg•mol−1. (b) Comparative SEC behavior of PCiPrVL with ca. 550 (polymerized at 70 °C) vs. ca. 50 (polymerized at RT) repeat units per chain.
(c) Overlays of normalized SEC distributions taken over the course of the polymerization for CiPrVL to PCiPrVL at 70 °C.
1H NMR and MALDI analyses further supported the hypothesis that chain transfer reactions were occurring. For example, the 1H NMR spectrum of PCnBuVL indicated fewer terminal hydroxymethyl groups compared to benzyloxymethyl initiator end group resonances (POLY-CH2OH to PhCH2O- ratio = 1.3 to 2.0). Additionally, MALDI analysis of a low molar mass sample of PCnBuVL [number-average molar mass (Mn) = ca. 2.5 kg•mol−1 polymerized at 70 °C for 24 h to mimic the conditions used for the synthesis of the high Mn samples] contained signals for five unique series of molar mass distributions (Figure 6a). The two most-intense distributions correspond to sodiated or potassiated linear PCnBuVL (BnOH + #•CnBuVL + Na+ or BnOH + #•CnBuVL + K+), and the next most intense corresponds to backbone ester cleavage (pyrolytic to oligomeric alkenes and carboxylic acids), which occurs during the MALDI excitation.21 The remaining two, smallest series of peaks are indicative of intramolecular cyclization to form small amounts of cyclic chains. The first of these corresponds to cyclization of the terminal hydroxyl group in PCnBuVL with a side chain ester (blue, loss of nBuOH); the second supports cyclization into a backbone ester (green, loss of the mass of n monomer units and BnOH) (Figure 6b). These analyses suggest that the higher temperature for polymerization leads to small amounts of transesterification reactions of the side chain and backbone esters. The conclusion here is that these PCRVLs contain small amounts of cyclic and branched chains.
Figure 6.

(a) A portion of the MALDI spectrum of PCnBuVL (Mn = 2.5 kg•mol−1) polymerized with BnOH and DPP at 70 °C (see Figure S1 for the full spectrum). (b) Two modes of cyclization give rise to small amounts of cyclic polyesters in PCnBuVL, as evidenced by masses corresponding to the loss of nBuOH (blue) or the loss of (n monomer units plus) BnOH (green).
Other studies using PCMeVL support the interpretation that branching or cyclization had only occurred to a small extent. When PCMeVL was subjected to DBU to effect eliminative degradation of the polymer backbone, very minor 1H NMR resonances (<1%) associated with degradative subunits characteristic of branched PCMeVL were detected.21 Additionally, a polymerization of CMeVL with DPP at 85 °C for 4 days (polymerization at this temperature reaches equilibrium monomer conversion within hours) showed the ca. 5% growth of a new methyl ester singlet (3.67 ppm), which is again associated with branching through side chain transesterfication.21
High molar mass PCRVLs: Thermal and mechanical properties.
The thermal behavior as assessed by DSC of each high molar mass PCRVL was noticeably different from their analogous lower molar mass samples. The DSC traces of three PCiPrVL samples (ca. 50, 100, and 550 repeat units per chain) showed similar ΔHm values on their first heating cycle. However, a reduction in the ΔHm was observed on the following heating cycles and this was more pronounced as the molar mass of the PCiPrVL sample increased (Figure 7a). This suggests that the crystallization rate of the larger polymers is suppressed.33
Figure 7.

(a) DSC thermograms of PCiPrVL with varying Mn taken on the third heating cycle (5 °C•min−1) that show a reduction in ΔHm as the Mn of each sample increases. This suggests that the crystallization rate of each sample is reduced with higher Mn. (b) DSC annealing study of PCiPrVL. One sample was heated to 150 °C (10 °C•min−1), cooled to and held at 22 °C for the annealing time, reheated to 150 °C (10 °C•min−1), and annealed again (for longer time). The top trace (23 h) is of the bulk sample of the fully annealed PCiPrVL prior to mechanical testing.
We also studied the crystallization of each PCRVL using DSC heat-and-hold annealing experiments for a range of temperatures (Figures S4, S6, S8, and S9) and hold times (Figures 7b, S5, S7, and S10). An example of the effect of hold time is shown in Figure 7b. PCiPrVL was heated to 150 °C, cooled (10 °C•min−1) to and held at 22 °C (see SI for annealing temperature scan)34 for the indicated time, and reheated to 150 °C (10 °C•min−1). This cycle was repeated for each of the subsequent, longer hold times. These DSC traces (Figure 7b) show that little crystallization occurs within the first hour of annealing (ΔHm = 3.9 J•g−1 after 1 h) and that PCiPrVL crystallizes the most from 1 to 4 hours (ΔHm = 16.0 J•g−1 after 4 h). There is little subsequent change in the overall ΔHm (final ΔHm = 20.0 J•g−1). However, with longer annealing times the lower Tm slowly shifts to higher temperatures, reaching its final value at 47 °C.
The DSC traces of the final, annealed, bulk samples of PCMeVL, PCEtVL, PCiPrVL, and PCnBuVL are shown in Figure 8. These show slightly higher Tgs and slightly lower Tms than their analogous lower molar mass samples (see Figure 4). Overall, PCMeVL has the highest Tm values (49 and 78 °C) and the largest ΔHm (35.4 J•g−1). PCnBuVL has the lowest Tm (45 °C), and PCEtVL (surprisingly) has the smallest ΔHm (13.0 J•g−1) in this series. The ΔHms trend from largest to lowest for PCMeVL, PCnBuVL, PCiPrVL, and PCEtVL.
Figure 8.

DSC thermograms of PCRVLs prior to mechanical testing. Each sample was annealed, cooled to −60 °C, and heated to 150 °C at a heating rate of 10 °C•min−1. Due to the broadening of the melting exotherm of PCnBuVL while cooling to −60 °C, another DSC run was performed and cooled only to 20 °C prior to heating to 150 °C. PCMeVL was annealed at 35 °C for 24 h followed by room temperature for 4 days. PCEtVL, PCiPrVL, and PCnBuVL were annealed at room temperature for 5 days.
We next analyzed the high molar mass PCRVLs using uniaxial tensile testing (Figure 9). The alkyl group on each carboalkoxy significantly impacted the mechanical properties. In this series, PCMeVL has the most rigid, strongest, and toughest tensile features in terms of elastic modulus (E = 186 ± 13 MPa), tensile strength (σB = 34.5 ± 9.1 MPa), yield stress (σy = 11.4 ± 0.3 MPa), strain at break (εB = 480 ± 100%), and tensile toughness (88 ± 33 MJ•m−3). The E, σB, σy, and toughness of these samples decreases in the series PCMeVL to PCiPrVL to PCEtVL and to PCnBuVL. In a contrasting fashion the strain at break (εB) decreases from PCEtVL ≈ PCiPrVL ≈ PCMeVL > PCnBuVL.
Figure 9.

Stress-strain curves and tabulated results for four PCRVLs. Each sample was uniaxially extended at 50 mm•min−1 until sample failure. Plotted curves are representative samples. Values labeled with subscript “Y” correspond to the stress or strain at yield and values labeled with subscript “B” correspond to stress or strain at “break” (sample failure).
The tensile properties of PCMeVL are comparable to many plastics used today (low-density polyethylene: E = ca. 250 MPa and εB = ca. 400–500%; high-density polyethylene: σB = ca. 32 MPa).1,35 Additionally, PCMeVL behaves similarly to poly(3-hydroxypropionate)36 and is much tougher than PLA (ca. 2 MJ•m−3)2—two of today’s leading biobased polymers.
Hydrolytic degradation.
Given the varied thermal and physical properties of PCMeVL, PCEtVL, PCiPrVL, and PCnBuVL, we sought to investigate the differences in their hydrolytic degradability. Each polymer was immersed in 0.1 M NaOH, HCl, or phosphate-buffered saline (PBS, pH 7.4) and the degradation was monitored using total organic carbon (TOC) analysis of the degradation supernatant at various timepoints. Room temperature degradation experiments for the PCRVLs indicated only minimal hydrolytic degradation (≤ 3 %C after 13 days) in both acidic and basic media. We therefore repeated the degradation experiments at elevated temperature (80 °C, above the Tm of all four polymers). Under these conditions, a similar degradation trend was observed for each polymer: hydrolysis under basic conditions was most rapid, hydrolysis under acidic conditions was slower, and hydrolysis in the PBS buffer conditions was much slower (Figure S23). Each polymer was fully degraded under acidic and basic conditions by the 13-day time point; however, the steric bulk of the alkyl group was seen to affect the degradation rate (Figure 10). As the number of carbon atoms in the alkyl side chain increased, the rate of degradation in both basic and acidic conditions decreased. We speculate that the increased hydrophobicity of the polymers having larger alkyl groups reduces the susceptibility of PCRVLs to hydrolysis. That is, under both basic and acidic degradation conditions, the trend for the hydrolysis rates across all four polymers was PCMeVL > PCEtVL > PCiPrVL > PCnBuVL.
Figure 10.

Hydrolytic degradation studies of PCRVLs at 80 °C in (a) 0.1 M NaOH or (b) 0.1 M HCl. The %C data were obtained by comparing the total organic carbon content of the aqueous solution to the amount of carbon present in the initial mass of polymer; each point is an average of triplicate experiments and the error bars represent standard deviations from the mean.
We also investigated the hydrolysis products formed during the high-temperature degradation studies. To determine the products formed during the hydrolysis of the PCRVLs, we sampled the degradation media at the final time point (i.e., 22-day) using 1H NMR spectroscopy (D2O as solvent). In each case, we observed two primary hydrolysis products: 2-(hydroxymethyl)pentanedioic acid (6, or the dicarboxylate 7 under basic conditions) and the alcohol freed by hydrolysis of the alkoxy moiety from the pendant carboalkoxy group (Figure 11, see NMR spectra on Figures S24 and S25). NMR analysis of the degradation of PCiPrVL in aq NaOH at an earlier time point (3 days) showed, again, the formation of ca. equimolar amounts of 7 and isopropanol, suggesting that side chain and polymer backbone hydrolyses were occurring at similar rates.
Figure 11.

Products observed from the hydrolysis of PCRVLs.
CONCLUSIONS
CRVLs that vary in the nature of their alkyl side chains were synthesized from malic acid. The ROTEP of each CRVL using DPP as a catalyst resulted in thermoplastic, semicrystalline PCRVLs. These showed unimodal distributions when polymerized at room temperature (for lower molar mass samples) and bimodal distributions when polymerized at 70 °C (for higher molar mass samples). 1H NMR spectroscopy, MALDI spectrometry, size-exclusion chromatography, and eliminative degradation indicated that this bimodality is likely caused by chain transfer reactions. The nature of the alkyl group in carboalkoxy substituents significantly impacted the Tg, Tm, and ΔHm as well as the mechanical properties of these (chemically recyclable19) polyesters. In particular, PCMeVL, PCEtVL, and PCiPrVL were tough (up to 88 ± 33 MJ•m−3) and had Young’s moduli up to 186 ± 13 MPa. These PCRVL polymers behaved similarly to some commercial polymers used today. Furthermore, the rates of base- and acid-catalyzed hydrolytic degradation of the PCRVLs were found to be dependent on the alkyl group; larger alkyl chains imparted slower degradation character.
EXPERIMENTAL SECTION
All experimental information and data are gathered in the Supporting Information document, which has the following outline:
General experimental protocols.
Preparation and characterization of small molecules
Preparation and characterization of polymers
Polymer testing
Polymer characterization data
SI references
Copies of 1H and 13C NMR spectra
Supplementary Material
ACKNOWLEDGMENTS
This work was conducted with financial support from (i) an NSF-funded Center for Chemical Innovation, the NSF Center for Sustainable Polymers (CHE-1901635), and (ii) the University of Minnesota Doctoral Dissertation Fellowship program. Some NMR data were collected on instruments that were purchased through the NIH Shared Instrumentation Grant Program (S10OD011952).
Footnotes
Publisher's Disclaimer: This document is confidential and is proprietary to the American Chemical Society and its authors. Do not copy or disclose without written permission. If you have received this item in error, notify the sender and delete all copies.
Supporting Information.
“This material is available free of charge via the Internet at http://pubs.acs.org.”
For content information, see the outline in “Experimental Section” (immediately above).
The authors have no competing financial interests to declare.
REFERENCES
- 1.Andreeßen B; Taylor N; Steinbüchel. Poly(3-hydroxypropionate): A promising alternative to fossil fuel-based materials. Appl. Environ. Microbiol 2014, 80 6574–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagarajan V; Mohanty AK; Misra M Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustainable Chem. Eng 2016, 4, 2899–2916. [Google Scholar]
- 3.Corneillie S; Smet M PLA architectures: The role of branching. Polym. Chem 2015, 6, 850–867. [Google Scholar]
- 4.Gu L; Xu Y; Fahnhorst GW; Macosko CW Star vs long chain branching of poly(lactic acid) with multifunctional aziridine. J. Rheol 2017, 61, 785–796. [Google Scholar]
- 5.Haugan IN; Maher MJ; Chang AB; Lin T-P; Grubbs RH; Hillmyer MA; Bates FS – Consequences of grafting density on the linear viscoelastic behavior of graft polymers. ACS Macro Lett 2018, 7, 525–530. [DOI] [PubMed] [Google Scholar]
- 6.Qian H; Wohl AR; Crow JT; Macosko CW; Hoye TR A strategy for control of “random” copolymerization of lactide and glycolide: Application to synthesis of PEG-b-PLGA block polymers having narrow dispersity. Macromolecules, 2011, 44, 7132–7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aluthge DC; Xu C; Othman N; Noroozi N; Hatzikiriakos SG; Mehrkhodavandi P PLA-PHB-PLA triblock copolymers: Synthesis by sequential addition and investigation of mechanical and rheological properties. Macromolecules 2013, 46, 3965–3974. [Google Scholar]
- 8.Schneiderman DK; Hillmyer MA Scalable production of mechanically tunable block polymers from sugar. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 8357–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts A; Kurokawa N; Hillmyer MA Strong, resilient, and sustainable aliphatic polyester thermoplastic elastomers. Biomacromolecules 2017, 18, 1845–1854. [DOI] [PubMed] [Google Scholar]
- 10.Belgacem MN, Gandini A, Eds.; Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, 2008. [Google Scholar]
- 11.Gandini A; Lacerda TM From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci 2015, 48, 1–39. [Google Scholar]
- 12.Wang Z; Ganewatta MS; Tang C Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci 2020, 101, 1–14. [Google Scholar]
- 13.Bicerano J In Prediction of Polymer Properties, 3rd ed.; Marcel Dekker: New York, 2002. [Google Scholar]
- 14.Boyle BM; Heinz O; Miyake GM; Ding Y Impact of the pendant group on the chain conformation and bulk properties of norbornene imide-based polymers. Macromolecules 2019, 52, 3426–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball-Jones NR; Fahnhorst GW; Hoye TR; Poly(isoprenecarboxylates) from glucose via anhydromevalonolactone. ACS Macro Lett 2016, 5, 1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark HF Internal polyolefins and a few highly substituted polyvinyls. In Advances in Polyolefins: The World’s Most Widely Used Polymers; Seymour RB, Cheng TC, Eds.; Springer: New York, 1987; pp 15–22, DOI: 10.1007/978-1-4757-9095-5. [DOI] [Google Scholar]
- 17.Aime JP; Ramakrishnan S; Chance RR; Kim MW The effect of substituent groups on polymer conformation in good solvent: Polyoctene and polydecene. J. Phys. France 1990, 51, 963–975. [Google Scholar]
- 18.Penzel E Polyacrylates. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Vol. 28, pp 515–536. [Google Scholar]
- 19.Fahnhorst GW; Hoye TR A carbomethoxylated polyvalerolactone from malic acid: Synthesis and divergent chemical recycling. ACS Macro Lett 2018, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneiderman DK; Hillmyer MA Aliphatic polyester block polymer design. Macromolecules 2016, 49, 2419–2428. [Google Scholar]
- 21.Fahnhorst GW; Stasiw DE; Tolman WB; Hoye TR Isomerization of linear to hyperbranched polymers: Two isomeric lactones converge via metastable isostructural polyesters to a highly branched analogue. ACS Macro Lett 2018, 7, 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Pechmann H On the cleavage of α-oxyacids. Liebigs Ann. Chem 1891, 264, 261–309. [Google Scholar]
- 23.Wiley RH; Smith NR Coumalic acid. Org. Synth 1951, 31, 23–2 [Google Scholar]
- 24.Phillips AJ; Nasveschuk CG; Henderson JA; Liang Y; Chen C-L; Duplessis M; He M; Larzarski K Amine-linked C3-glutarimide degronimers for target protein degradation. World patent WO2017197051(A1) November 16, 2017. [Google Scholar]
- 25.Yamashita T; Nishikawa H; Kawamota T Scale-up synthesis of a deuterium-labeled cis-cyclobutane-1,3-dicarboxylic acid derivative using continuous photo flow chemistry. Tetrahedron 2019, 75, 617–623. [Google Scholar]
- 26.Takeda K; Akiyama A; Nakamura H; Takizawa S-I; Mizuno Y; Takayanagi H; Harigaya Y Dicarbonates: Convenient 4-dimethylaminopyridine catalyzed esterification reagents. Synthesis 1994, 10, 1063–1066. [Google Scholar]
- 27.Tello-Aburto R; Lucero AN; Rogelj S A Catalytic approach to the MH-031 lactone: Application to the synthesis of geralcin analogs. Tetrahedron Lett 2014, 55, 6266–6268. [Google Scholar]
- 28.See, for example: Kopinke F-D; Remmler M; Mackenzie K; Möder M; Wachsen O Thermal decomposition of biodegradable polyester—II. Poly(lactic acid). Polym. Degrad. Stabil 1996, 53, 329–342. [Google Scholar]
- 29.Jordan EF; Feldeise DW; Wrigley AN Side-chain crystallinity.1. Heats of fusion and melting transitions on selected homopolymers having long side chains. J. Polym. Sci., Part A-1: Polym. Chem 1971, 9, 1835–1851. [Google Scholar]
- 30.Baran J; Duda A; Kowalski A; Szymanski R; Penczek S Intermolecular chain transfer to polymer with chain scission: General treatment and determination of kp/ktr in l,l-lactide polymerization. Macromol. Rapid Commun 1997, 18, 325–333. [Google Scholar]
- 31.Penczek S; Duda A; Szymanski R Intra- and intermolecular chain transfer to macromolecules with chain scission. The case of cyclic esters. Macromol. Symp 1998, 132, 441–449. [Google Scholar]
- 32.Zhang D; Hillmyer MA; Tolman WB Catalytic polymerization of a cyclic ester derived from a “cool” natural precursor. Biomacromolecules 2005, 6, 2091–2095. [DOI] [PubMed] [Google Scholar]
- 33.Hiemenz PC; Lodge TP Crystalline polymers: Kinetics of nucleation and growth. In Polymer Chemistry; CRC Press: Boca Raton, Florida, 2007; pp 536–545. [Google Scholar]
- 34. The appropriate annealing temperature for these PCRVLs was determined by DSC heat and hold experiments at four to five temperatures near each sample’s Tc (see SI). The temperature that provided the largest ΔHm was used to anneal each sample.
- 35.Callister WD; Rethwisch DG Characteristics, applications, and processing of polymers. In Materials Science and Engineering: An Introduction, 8 ed.; John Wiley & Sons, Inc.: Hoboken, 2010; p 572. [Google Scholar]
- 36.Meng D-C; Shi Z-Y; Wu L-P; Zhou Q; Wu Q; Chen J-C Chen G-Q Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab. Eng 2012, 14, 317–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


