Abstract
Background:
Americans increasingly use cannabis, including those with psychiatric disorders. Yet little is known about cannabis use among individuals with obsessive-compulsive disorder (OCD). Thus, we conducted the first survey of cannabis users with OCD.
Methods:
Adults with OCD (i.e., prior professional diagnosis and/or score above the cutoff on a validated scale) who reported using cannabis were recruited from internet sources to complete a survey querying demographic information, medical/psychiatric history, cannabis use patterns, and perceived cannabis effects.
Results:
Of 1096 survey completers, 601 met inclusion criteria. Inhalation/cannabis flower were the most common method/formulation participants endorsed; most identified using high-potency cannabis products; 42% met criteria for cannabis use disorder. Nearly 90% self-reported using cannabis medicinally, 33.8% had a physician’s recommendation, and 29% used specifically to manage OCD symptoms. Most participants reported cannabis improved obsessions/compulsions; those with increased obsession severity perceived less benefit. Finally, most participants were not receiving evidence-based OCD treatment, and the odds of receiving treatment decreased with increased cannabis use.
Conclusions:
In this survey, participants with OCD reported both subjective benefits and harms from cannabis use. Future research should clarify the risks and benefits of cannabis use to those with OCD and develop treatment models to better support this population.
Keywords: Obsessive Compulsive Disorder, Cannabis, Cannabinoids, Treatment, Cannabis Use Disorder
1. Introduction
Obsessive-compulsive disorder (OCD) is a disabling illness affecting approximately 2-3% of the population (Stein et al., 2019). Individuals with OCD experience intrusive thoughts (obsessions) and repetitive behaviors (compulsions), which lead to functional impairment. Emerging preclinical findings indicate that abnormal functioning in the brain’s endocannabinoid system (ECS) may contribute to OCD symptoms (Kayser et al., 2019). The ECS is a regulatory neurotransmitter system that is involved in various homeostatic functions throughout the central nervous system and is targeted by cannabis and related substances (cannabinoids; Lu & MacKie, 2016). Both rodent models and human neuroimaging studies link ECS activity to changes in neurocognitive functions involved in OCD pathology; specifically, excessive threat response (Apergis-Schoute et al., 2017; Pietrzak et al., 2014), impaired fear learning (Jenniches et al., 2016; McLaughlin et al., 2015), and excessive deployment of habitual behavioral strategies (Gremel et al., 2016; Voon et al., 2015). In preclinical studies, ECS modulators attenuated threat responses (Mayo et al., 2019), facilitated fear extinction (Hammoud et al., 2019), and promoted goal-directed over habitual behavior action selection (Gianessi et al., 2021). Cannabis and other ECS modulators might therefore affect obsessions and/or compulsions.
However, the directionality of these potential effects is unclear: For example, agents that facilitate activity at the cannabinoid 1 receptor have been found to both promote and protect against habitual behavior development in different studies (Gianessi et al., 2021; Gremel et al., 2016), and thus might increase or decrease compulsions. Due to a lack of research in patients, it remains unclear whether cannabinoids are beneficial or harmful to individuals with OCD.
While research on cannabis and cannabinoids in OCD is emerging slowly, popular interest in their recreational and therapeutic uses is exploding. Between 2008 and 2018, the number of American adults reporting near-daily cannabis use grew by 4.8 million (Lipari & Park-Lee, 2019). Cannabis products are increasingly marketed as treatments for psychiatric conditions like OCD and anxiety (Richter & Levy, 2014; Subritzky et al., 2016), and as of May 2021, 37 states and the District of Columbia (DC) have legalized medicinal cannabis, while 17 states and DC permit recreational cannabis use. Physician-recommended cannabis is increasingly accessible to Americans, but medicinal cannabis use (i.e., using to treat one or more medical conditions) also occurs regardless of state policies or physician supervision (Azcarate et al., 2020). The exact prevalence of medicinal or recreational cannabis use among individuals with OCD is unknown. However, in 2019, a specialty clinical program reported that nearly 30% of adults seeking intensive OCD treatment endorsed current or past cannabis use (Storch & Kay, 2019).
As trends towards greater access and legalization continue, there is a growing public health need to understand why individuals with OCD use cannabis and the risks and benefits of cannabinoids to this population. To explore this issue, the present study surveyed a large sample of adults with both OCD (based on scores on a validated diagnostic scale or self-reported professional diagnosis) and cannabis/cannabinoid use.
1.1. Prior studies involving cannabis use and OCD
1.1.1. Observational studies of OCD symptoms in cannabis users
Small observational studies suggest that cannabis can have mixed effects on OCD symptoms. For instance, in 132 adolescents receiving residential treatment for cannabis use, greater pre- and post-treatment OCD symptom severity predicted more frequent post-treatment cannabis use, possibly reflecting participants’ self-medication with cannabis (Albertella & Norberg, 2012). Similarly, another study including 44 users of both cannabis and ecstasy found that greater OCD symptom severity at baseline and 18-month follow-up was associated with more frequent cannabis use (Daumann et al., 2004). These studies are limited by the fact that participants were not primarily diagnosed with or treated for OCD.
Another study used a mobile app to track acute changes after smoking cannabis in 87 medicinal cannabis users who self-identified as having OCD symptoms (Mauzay et al., 2021). Participants reported short-term improvement in OCD symptoms and anxiety in more than 90% of the tracked cannabis use sessions, although pre-session symptom severity remained static over the course of repeated sessions. Study limitations included a modest sample size and lack of validated assessments of OCD symptoms and diagnosis.
1.1.2. Clinical studies of cannabinoids in patients with OCD
Directly studying cannabis in human trials is challenging because of its Schedule I classification by the US Drug Enforcement Administration (DEA). Trials of single synthetic cannabinoids or isolated cannabis constituents have been more feasible to conduct given that many lack Schedule I restrictions: Agents tested include nabilone, a synthetic analogue of Δ-9-tetrahydrocannabinol (THC, the primary psychoactive component in cannabis) which is FDA-approved for treating chemotherapy-associated nausea and vomiting (Fraser, 2009; Kayser, Raskin, et al., 2020), and Epidiolex, an isolate of cannabidiol (CBD, another cannabis constituent with different mechanistic properties than THC), which is FDA-approved for two rare forms of pediatric epilepsy (Gaston, Bebin, Cutter, Liu, & Szaflarski, 2017; Hurd et al., 2019).
Three case reports document symptomatic improvement in patients with OCD who received dronabinol, an oral form of THC (J. J. Cooper & Grant, 2017; Schindler, Anghelescu, Regen, & Jockers-Scherubl, 2008). In another case report, a patient’s symptoms resolved over 20 months of medicinal cannabis treatment (Szejko et al., 2020). There have been only two small cannabinoid trials in individuals with OCD. The first, a randomized, placebo-controlled, within-subjects human laboratory study in 12 adults, found that smoked cannabis containing primarily THC or CBD did not differ from placebo in its acute effects on OCD symptoms (Kayser, Haney, et al., 2020). In a second small pilot trial, 11 patients received nabilone over four weeks. Results suggested that nabilone had little effect on OCD symptoms on its own, but may have augmented the effects of exposure-based psychotherapy when both were delivered simultaneously (Kayser, Raskin, et al., 2020).
However, all of these studies were limited by small sample sizes, and the nabilone study lacked a placebo control. Moreover, these single synthetic and isolated cannabinoids are chemically and functionally distinct from cannabis flower, which contains >140 constituents with varied psychoactive properties (Russo & Marcu, 2017) and is by far the most common cannabinoid that recreational and medicinal cannabis users report using (Sexton et al., 2016a).
1.1.3. Surveys of OCD symptoms in cannabis users
Given these challenges, direct patient surveys have become an important tool to better understand how cannabis interacts with psychiatric symptoms and disorders. Four prior anonymous surveys have explored the relationship between OCD symptoms and cannabis use. The first, a cross-sectional survey of an unselected birth cohort of 930 American 18-year-olds, found a higher rate of cannabis dependence in those who met OCD criteria (n=7 out of 37) than those who did not (n=45 out of 893; i.e., 19% versus 5%; Douglass, Moffitt, Dar, McGEE, & Silva, 1995). Three others (all in cannabis-using young adults, n=159, 430, and 177, respectively) found that self-reported OCD symptom severity (as measured by the Obsessive-Compulsive Inventory, Revised [OCI-R]) predicted cannabis misuse (Bakhshaie et al., 2020; Buckner et al., 2007; Spradlin et al., 2017). Two of these found that the relationship between OCD symptom severity and cannabis use was mediated by coping motivations (i.e., cannabis use to relieve stress and negative affect; Bakhshaie et al., 2020; Spradlin et al., 2017); one also identified an association between higher scores on the obsessing symptom subdomain and increased cannabis-related problems (e.g., failure to complete work/school obligations due to cannabis use; Spradlin et al., 2017). However, because few participants in these surveys met OCD criteria (n=37, 47, 57, and 50, respectively), the generalizability of their findings is unclear.
1.2. Rationale for the present study
To begin exploring patterns and effects of cannabis use in OCD, we conducted the first internet survey of adults with OCD (based on either self-report of diagnosis by a mental health professional or score on a validated symptom severity scale) and experience using cannabis products (≥1 reported lifetime use). Our goals were to characterize a population of cannabis users with OCD, examine their patterns of cannabis use (i.e., methods and formulations; medicinal vs. recreational use), and assess their self-reported perceptions of cannabis effects on OCD symptoms. Based on the extant literature, we made three hypotheses: First, prior research indicates that cannabis effects may vary based on demographics. Older age (Sexton, Cuttler, & Mischley, 2019), male gender (Z. D. Cooper & Craft, 2018b), and lower scores on the obsessing OCI-R subdomain (Spradlin et al., 2017) have all been associated with higher perceived benefit from cannabis. Thus, we expected that participants who were older, male, and reported less severe obsessions would have better subjective response to cannabis (i.e., higher YBOC-CS scores). Second, considering the two previous surveys linking OCD symptom severity to cannabis misuse (Bakhshaie et al., 2020; Spradlin et al., 2017), we also hypothesized that more severe OCD symptoms would be associated with more frequent cannabis use and higher risk for cannabis use disorder (CUD). Finally, because some patients may use cannabis as a replacement for psychiatric medication (Corroon et al., 2017) and cannabis use may also limit motivation to seek or access to evidence-based OCD treatment (Storch & Kay, 2019), we hypothesized that more frequent cannabis use would reduce the likelihood that participants were currently receiving evidence-based treatment for OCD. For simplicity, throughout this report we use the term “cannabis” to refer to both cannabis plant material and cannabis-related substances (e.g., cannabis concentrates with varied THC/CBD concentrations); we defined these terms explicitly in all survey content.
2. Methods
2.1. Recruitment
The survey was developed by the authors and conducted under the auspices of the (Removed to preserve anonymity). With Institutional Review Board approval (Protocol #7745), it was posted online on SurveyMonkey.com between 1/7/2019 and 12/23/2019. Participants self-referred from advertisements on a variety of internet sites including forums on major portals (e.g., Reddit, Yahoo), social media pages (e.g., Facebook, Twitter, Instagram), consumer organizations (e.g., the International OCD Foundation, Anxiety and Depression Association of America, MQ: Transforming Mental Health), the nOCD mobile app, and (our website, details removed to preserve anonymity). Previous participants in research at our clinic who expressed interest in future studies and met eligibility criteria were also invited to participate.
We recruited adults (age≥18) who self-identified as having OCD symptoms and at least one lifetime use of cannabis, cannabis products (e.g., edibles), or cannabinoid isolates (e.g., THC capsules, CBD oil). Advertisements linked to a page informing participants that the survey would ask questions about their OCD symptoms and experiences using cannabinoids, that no identifying information would be collected, and that they would be offered entry into a raffle for a $100 gift card upon survey completion. Participants electronically provided informed consent before answering any survey questions.
2.2. Survey Content
2.2.1. Overview
Participants were queried about their background and demographics (e.g., age, gender, race, ethnicity, occupation). We also asked about their history of OCD symptoms (i.e., illness onset, receipt of professional diagnosis), prior OCD treatments (including selective serotonin reuptake inhibitors [SSRIs] or cognitive behavioral therapy [CBT] consisting of exposure and response prevention [EX/RP], the recommended first-line treatments for OCD; Koran et al., 2007) and history of other psychiatric and medical comorbidities and treatment. When querying about past psychotherapy treatment, we asked patients who had received CBT to specify whether this included exposure (i.e., EX/RP), as in prior surveys (Patel et al., 2017). Using the validated questionnaires listed below, we assessed OCD symptom severity and dimensions, patterns of cannabis use, CUD symptoms, and self-report of change in OCD symptoms following cannabis use.
In addition to these scales, the survey asked participants to describe qualitatively how cannabis affected their obsessions, compulsions, and anxiety symptoms, and to provide any relevant information the questionnaires did not otherwise capture. On average, participants spent 16 minutes completing the survey.
2.2.2. Validated measures
a). Obsessive-Compulsive Inventory, Revised (OCI-R; Huppert et al., 2007):
The OCI-R is an 18-item self-report questionnaire evaluating OCD symptom severity, which has been validated against the clinician-administered Yale-Brown Obsessive-Compulsive Scale (YBOCS). The OCI-R is widely-used to assess for OCD symptoms in nonclinical samples (Goodman et al., 1989). The scale measures symptom severity overall and in six specific symptom clusters (checking, hoarding, neutralizing, obsessing, ordering, and washing). Higher scores suggest greater symptom severity (overall and for individual symptom subdomains), with scores of 15-19, 20-34, and ≥35 indicating mild, moderate, and severe symptoms, respectively. Prior research found a cutoff of 21 was optimal for distinguishing individuals with OCD from non-anxious controls (Foa et al., 2002), although higher cutoffs have also been proposed (Abramovitch et al., 2020). Consistent with our prior survey work (Patel et al., 2017), we used a cutoff of 21 to define clinically significant OCD given our goal of recruiting individuals with a range of symptom severities. As a conservative measure, we also conducted a sensitivity analysis using a higher cutoff as described below.
b). Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU; Cuttler & Spradlin, 2017):
The DFAQ-CU is a 33-item scale evaluating patterns of cannabis use, forms of cannabis used (e.g., cannabis flower, concentrates, edibles), and methods used to ingest cannabis (e.g., cigarettes, pipes, vaping). The DFAQ-CU includes frequency and quantity subscales; higher scores indicate more frequent and greater quantity of cannabis used. The measure also queries participants about their knowledge of the THC and CBD content of the products they typically use.
c). Cannabis Use Disorder Identification Test, Revised (CUDIT-R; Adamson et al., 2010):
This 8-item scale asks about symptoms of cannabis use disorder (CUD) over the past 6 months (e.g., failure to meet responsibilities). Higher scores suggest greater CUD symptomatology; scores ≥8 indicate problematic cannabis use, and scores ≥12 suggest probable CUD.
d). Yale-Brown Obsessive-Compulsive Challenge Scale (YBOC-CS):
The YBOC-CS is a 10-item Likert scale asking about current OCD symptoms that was previously used in pharmacological challenge studies of ketamine (Rodriguez et al., 2011, 2013) and our human laboratory study of cannabis (Kayser, Haney, et al., 2020). In this survey, participants were asked to indicate how cannabis typically affects their OCD symptoms with regard to each of the ten items queried by the YBOC-CS (e.g., time spent, degree of control over, and overall severity of obsessions and compulsions).
2.3. Sample
Participants were included in final analyses if they met all three of the following criteria: a) met OCD criteria, based on self-reported professional diagnosis or an OCI-R score≥21, b) reported they had used cannabis at least once, and c) completed all required (i.e., non-qualitative) survey items.
2.4. Data Analysis
Statistical analyses were performed using SPSS25 (SPSS Statistics for Windows, Version 25.0, IBM Corp. Armonk, NY). Little’s Missing Completely at Random (MCAR) test was non-significant (p=.273), suggesting data were unlikely to be missing at random, and the overall amount of missing data was low (<5%, with individual models missing between 4.7 and 10.1% of observations). Thus, pairwise deletion was used to account for missing data (Tabachnick, B. G. & Fidell, 2019). We computed descriptive statistics for demographic characteristics, psychiatric/medical history and comorbidity, OCD symptom severity, CUD symptoms, cannabis use patterns, and self-reported response to cannabis (i.e., YBOC-CS). We used multivariate linear regression to test the hypothesis that demographic variables and/or OCD symptom dimensions would predict participants’ subjective response to cannabis. We also computed bivariate correlations (for continuous variables) and chi-square statistics (for categorical variables) between OCD- and cannabis-related outcomes to test the hypothesis that OCD symptoms would be associated with increased cannabis use and CUD symptoms. Finally, we used multiple logistic regression to test the hypothesis that cannabis use would predict whether participants were currently receiving evidence-based treatment for OCD. Considering recent literature proposing an OCI-R cutoff of 27 to establish OCD diagnosis, we conducted a sensitivity analysis by re-computing the above statistics in the subset of participants with OCI-R scores ≥27. As this did not change the overall outcomes of any of our analyses, all results hereafter reflect the entire group of participants who met inclusion criteria. All statistical tests were two-tailed and conducted using α=0.05; given the exploratory nature of this study, no corrections were made for multiple comparisons.
3. Results
3.1. Demographics
Of the 1476 individuals who provided consent, 1096 started the survey (response rate: 74.2%). Of those 1096, 601 participants met eligibility criteria (i.e., likely OCD diagnosis, at least one lifetime cannabis use episode, and survey completion). Demographic characteristics of participants are presented in Table 1; there were no significant differences in demographic characteristics between the 1096 who started the survey and the 601 who met eligibility criteria and were included in subsequent analyses.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICSa
| Variable | Participants,b n=601 |
|---|---|
| Age | 28.6±8.6, 18-72 |
| Gender | |
| Female | 283 (47.1) |
| Male | 288 (47.9) |
| Transgender/Other | 30 (5.0) |
| Single | 329 (54.7) |
| Education | |
| <High School | 9 (1.6) |
| High School Graduate | 82 (13.6) |
| Attended College (≥1 year) | 408 (67.6) |
| Attended Grad School (≥1 year) | 103 (17.2) |
| Employment | |
| Working | 363 (60.4) |
| Student | 119 (19.8) |
| Homemaker, Retired | 29 (4.9) |
| Disabled | 34 (5.7) |
| Unemployed | 56 (9.3) |
| Hispanic | 36 (6.4) |
| Race | |
| White | 498 (82.9) |
| Black | 13 (2.2) |
| Asian | 18 (3.0) |
| Other | 72 (11.9) |
Values shown as means (±SD), range; for frequencies, as n (%)
Includes participants who met OCD criteria (OCI-R≥21 or prior professional diagnosis), endorsed lifetime cannabis use, and completed required survey sections
The 601 eligible participants included residents of 47/50 US states as well as DC, Puerto Rico, and the US Virgin Islands. Cannabis’ legal status has recently changed in several of these states. Based on state policies at the time this survey was administered, 44.0% of responses were from states where cannabis was fully-legal (i.e., both recreational and medicinal use had been legalized), 41.3% from states where only medicinal use was legal, and 14.7% from states where cannabis use was fully-illegal. The five most-represented states included those where cannabis was fully-legal (California, Michigan), only medicinal use was legal (New York, Florida), and cannabis was fully-illegal (Texas).
3.2. OCD diagnosis and symptom severity
The 601 participants were 28.6 years old on average (SD 8.6, range 18-72), with a female:male ratio of approximately 1:1; most participants were white and non-Hispanic. Mean OCI-R score was 33.4 (SD 13.1, median 33.0. Mean age of OCD symptom onset was 9.9 years (SD 5.1, median 9.0). Of the 601 participants, 522 (86.9%) were eligible based on OCI-R scores ≥21, while 79 (13.1%) scored <21 but were eligible based on a previous OCD diagnosis from a mental health professional. In total, 238 of the 601 eligible participants (39.6%) reported a prior OCD diagnosis, which they received on average at age 19.0 years (SD 6.6, median 18.0).
3.3. Psychiatric and Medical Comorbidities
Table 2 depicts rates of self-reported comorbid conditions. Most participants reported at least one medical or psychiatric diagnosis aside from OCD. The most commonly-endorsed psychiatric comorbidities were generalized anxiety disorder, major depressive disorder, and posttraumatic stress disorder (PTSD); chronic pain, migraine, and asthma were the most common medical comorbidities.
TABLE 2.
CLINICAL CHARACTERISTICS (N=601)a
| Variable | Value, n (%) | |
|---|---|---|
| Mean OCI-R Score | 33.4±13.9, 1-72 | |
| Age of OCD Symptom Onset | 9.9±5.1, 1-30 | |
| Age of first cannabis use | 17.3±4.2, 6-46 | |
| Psychiatric Comorbidity - any | 481 (80.0) | |
| Generalized anxiety disorder | 334 (55.6) | |
| Major depressive disorder | 225 (37.4) | |
| Post-traumatic stress disorder | 166 (27.6) | |
| Social anxiety disorder | 151 (24.1) | |
| Personality disorder | 110 (18.3) | |
| Eating disorder | 100 (16.6) | |
| Panic disorder | 96 (16.0) | |
| Substance use disorder | 72 (12.0) | |
| Bipolar affective disorder | 59 (9.8) | |
| Autism spectrum disorder | 41 (6.8) | |
| Primary psychotic illness | 18 (3.0) | |
| Mean # of Psychiatric Comorbidities | 2.43 (SD: 2.03) | |
| Medical Comorbidity - any | 361 (60.1) | |
| Chronic pain | 131 (21.1) | |
| Migraine | 110 (18.3) | |
| Asthma | 94 (15.6) | |
| Thyroid disease | 38 (6.3) | |
| Mean # of Medical Comorbidities | 1.09 (SD: 1.26) | |
| Treatment History (N=601) | Current | Lifetime |
| Any Treatment | 226 (37.6) | 450 (74.9) |
| Medications | 188 (31.3) | 315 (52.4) |
| SRIs (SSRIs or clomipramine) | 155 (25.8) | 236 (39.3) |
| Psychotherapy | 100 (16.6) | 334 (55.5) |
| Residential | 4 (0.7) | 68 (11.3) |
| Transcranial Magnetic Stimulation | 2 (0.3) | 10 (1.8) |
| Alternative (mindfulness meditation, yoga, herbal supplements) | 162 (27.0) | 360 (59.9) |
| Lifetime Psychotherapy Treatment (N=334) | Value, n (%) | |
| Exposure and response prevention | 99 (16.5) | |
| Cognitive therapy (without exposure) | 257 (42.8) | |
| Acceptance and commitment therapy | 67 (11.1) | |
| Psychodynamic psychotherapy | 35 (5.8) | |
Includes only analyzed participants
3.4. Treatment Utilization
As shown in Table 2, fewer than half of participants reported currently receiving treatment for OCD, and the most frequently endorsed current treatment was “alternative treatments” (e.g., mindfulness meditation, yoga, herbal supplements). More than half (n=315, 53.8%) reported lifetime medication use, including 236 participants (39.3%) who received serotonin reuptake inhibitors (SRIs, including SSRIs and clomipramine). The most frequently-endorsed lifetime psychotherapy treatment was CBT (but without exposure) in 257 participants (42.8%); only 99 participants (15.5%) endorsed lifetime EX/RP. About a quarter (n=160 participants, 26.6%) endorsed at least one psychiatric hospitalization.
3.5. Cannabis Use Patterns
As shown in Table 3, of the 601 participants, 539 reported using cannabis at least one day over the past month (mean: 20.1 days, SD 11.8, range 0-31), and 344 (57.3%) reported using cannabis at least daily. On average, participants reported that onset of their OCD symptoms preceded their first cannabis use episode (ages 9.9±5.4 vs. 17.3±4.2, respectively).
TABLE 3.
PATTERNS OF CANNABIS USE (N=601)
| Variable | Valuea | |
|---|---|---|
| Mean cannabis use days (past month) | 20.1±11.8, 0-31 | |
| Mean CUDIT-R Score | 11.5±6.1, 0-29 | |
| CUDIT-R Item Scores (scale of 0-4) | ||
| 1. Frequency of use (past month) | 3.2±1.2, 0-4 | |
| 2. Hours intoxicated per day | 1.7±1.2, 0-4 | |
| 3. Unable to stop using | 0.7±1.3, 0-4 | |
| 4. Failure to meet expectations | 0.5±0.8, 0-4 | |
| 5. Time spent getting, using or recovering | 0.9±1.3, 0-4 | |
| 6. Memory or concentration problems | 1.2±1.4, 0-4 | |
| 7. Use in dangerous situations (e.g., driving) | 0.8±1.3, 0-4 | |
| 8. Desire to reduce or stop use | 2.3±1.7, 0-4 | |
| Primary method used | ||
| Vaporizing | 183 (30.4) | |
| Joints/Blunts | 105 (17.5) | |
| Pipes | 113 (18.8) | |
| Bongs (water pipes) | 104 (17.3) | |
| Edibles | 41 (6.8) | |
| Primary formulation used | ||
| Cannabis flower | 388 (64.6) | |
| Concentrates | 149 (24.8) | |
| Edible forms | 34 (5.7) | |
| Typical THC content | All cannabis flower usersb, n=472 | All concentrate usersb, n=379 |
| “I don’t know” | 171 (36.2) | 97 (25.6) |
| High THC (>10%) | 279 (59.2) | 264 (69.7) |
| Low THC (<10%) | 22 (4.6) | 18 (4.7) |
| Typical CBD content | ||
| “I don’t know” | 212 (45.7) | 136 (37.4) |
| High CBD (>10%) | 96 (20.7) | 90 (24.7) |
| Low CBD (<10%) | 156 (33.6) | 138 (37.9) |
Values shown as means (±SD), range; for frequencies, as n (%)
Subset of participants who reported using this formulation at least 25% of the time, shown as n (%)
Table 3 also depicts the methods and formulations of cannabis that participants endorsed using. Participants were queried about both their primary (i.e., most commonly-used) methods/formulations and any others they used at least 25% of the time. Of the 601 participants, the most common primary method was vaporizing (endorsed by around one-third), followed by joints/blunts (endorsed by around one-fifth). Cannabis flower was the most commonly primary formulation (endorsed by nearly 65%), followed by cannabis concentrates (endorsed by about 25%).
For formulations used at least 25% of the time, participants were asked about their knowledge of the typical THC and CBD content of products they used. Responses were grouped into high (i.e., >10%), low (i.e., <10%), or unknown THC/CBD concentrations. Of the 42.1% of participants who used edibles (n=251), nearly 75% reported being unaware of their typical THC/CBD contents; thus we focused on cannabis flower (n=472) and cannabis concentrate users (n=379). The most common THC concentration reported by both cannabis flower and cannabis concentrate users was high-THC (by 59.2% and 69.7%, respectively), followed by “I don’t know” (by 36.2% and 25.6%, respectively). Around a third of concentrate users (n=126 [33.2%]) reported using products with extremely high THC contents (>80%). Regarding CBD concentrations, cannabis flower users most commonly reported “I don’t know” (45.7%) followed by low-CBD (33.6%); reports from concentrate users were evenly divided between “I don’t know” and low-CBD (37.4 vs. 37.9%, respectively).
3.6. Medicinal Cannabis Use
Of the 601 participants, 203 (33.8%) reported having a physician’s recommendation to use cannabis; indications included OCD, anxiety, PTSD, depression, insomnia and pain. However, only 66 (11.0%) reported using cannabis exclusively medicinally: 447 (74.3%) reported both medicinal and recreational use, and 88 (14.6%) reported only recreational use. Of the 601 participants, 175 (29.1%) reported they used cannabis specifically to manage OCD symptoms.
3.7. Subjective Cannabis Effects
On the YBOC-CS, most participants reported that cannabis typically improved their obsessions n=457 [68.3%]), but to varying degrees (“a little better”, n=225 [37.4%]; “a lot better”, n=188 [31.3%]). Similarly, most reported that cannabis improved their compulsions (n=393 [65.4%]; “a little better”, n=220 [36.6%]; “a lot better”, n=173 [28.8%]). A subset reported that cannabis worsened obsessions (n=104, 17.3%) or compulsions (n=81, 13.8%). Fifty (8.3%) and 127 (21.1%) reported that cannabis had no effect on overall obsessions and compulsions, respectively.
Correlation analysis revealed significant positive associations between subjective cannabis effects (i.e., YBOC-CS scores, with higher scores indicating greater perceived benefit) and all OCI-R subdomains except obsessing (all correlations > 0.10, all P’s < .005). Multivariate linear regression revealed two significant predictors of YBOC-CS scores: Total medical comorbidities, an increase in which was associated with greater perceived benefit, and the obsessing symptom subdomain, for which higher scores were associated with less perceived benefit (Table 4; see Supplemental Materials for detailed results of bivariate correlations and regression analyses).
TABLE 4.
Multivariate Linear Regression Model for Prediction of Subjective Cannabis Effectsa
| Variableb | Effect on Slope |
||
|---|---|---|---|
| β | t | p | |
| Age | 0.049 | 1.014 | 0.311 |
| Gender | 0.014 | 0.310 | 0.757 |
| Age of onset | −0.080 | −1.728 | 0.085 |
| Total Medication Trials | 0.023 | 0.470 | 0.638 |
| Total Medical Comorbidities* | 0.128 | 2.564 | 0.011 |
| Total Psychiatric Comorbidities | 0.009 | 0.181 | 0.856 |
| Checking | 0.035 | 0.651 | 0.515 |
| Hoarding | 0.021 | 0.385 | 0.700 |
| Neutralizing | 0.079 | 1.506 | 0.133 |
| Ordering | 0.043 | 0.736 | 0.462 |
| Washing | 0.076 | 1.461 | 0.145 |
| Obsessing* | −0.126 | −2.472 | 0.014 |
p < .05
based on YBOC-CS scores; higher score=greater perceived benefit
Variables of a priori interest (age, gender, OCI-R subscales) and those correlated with YBOC-CS at p<.1 were entered simultaneously into the model
Qualitative reports described a range of cannabis effects on OCD symptoms, though most focused on its subjective benefits. 17 participants (2.8%) described integrating cannabis into psychotherapy treatment (for example, using before EX/RP sessions). Figure 1 includes selected examples representing the range of responses.
Figure 1. Sample responses to open-ended questions about cannabis effectsa.
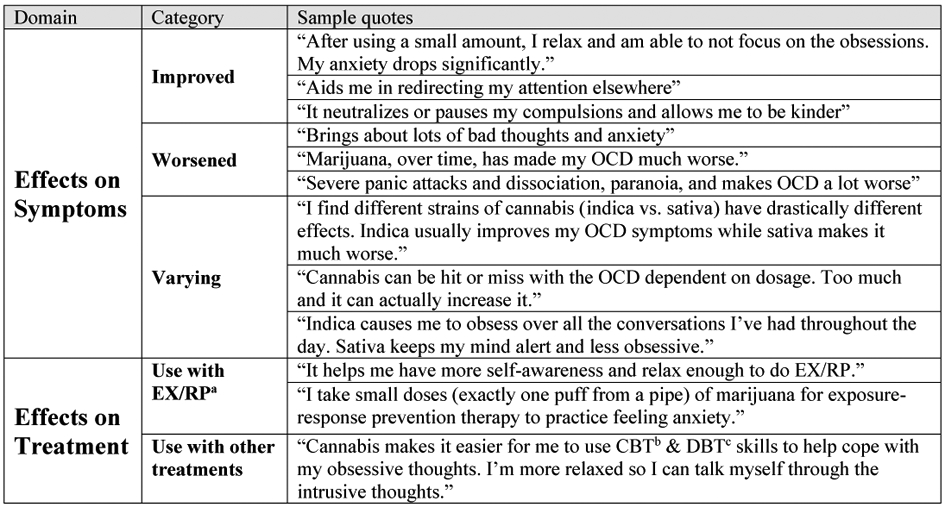
aEX/RP, exposure and response prevention; b CBT, cognitive behavioral therapy; cDBT, dialectical behavior therapy
3.8. Problematic Cannabis Use
Of the 601 participants, 253 (42.1%) met CUD criteria (CUDIT-R≥12), and 394 (65.6%) for problematic cannabis use (CUDIT-R≥8). Participants meeting CUD criteria were less likely to be in treatment (n=78, 31%; χ2=7.4, df=1, p=.007) and less likely to have ever received psychiatric medications (n=133, 53%; χ2=11.9, df=1, p<.001) or CBT (n=33, 13%, χ2=4.8, df=1, p=.028). They were more likely to be male (n=140, 55%; χ2=17.5, df=2, p<.001) and to endorse comorbid substance use disorder (n=41, 16.2%; χ2=4.0, df=1, p=.006) and migraine (n=35, 14%; χ2=6.54, df=1, p=.011). There were no correlations between total OCD symptoms and cannabis use frequency or CUD symptoms as assessed by total CUDIT-R scores. Given that frequent cannabis use may not be problematic per se, we also computed correlations without the CUDIT-R frequency items (i.e., only items 3-8). This did not change our findings; thus, subsequent analyses included the CUDIT-R total score. There were significant negative correlations between several individual OCD symptom subdomains and individual CUDIT-R item scores. The exception to this was with hoarding symptoms, which were positively correlated with the CUDIT-R frequency items; details in Supplemental Materials).
We used multiple logistic regression to explore whether demographic, OCD-related, and cannabis-related predictors affected participants’ current treatment status (i.e., in OCD treatment vs. not; Table 5). The odds of being in treatment for OCD decreased with more frequent cannabis use (p=0.003); in contrast, earlier age of OCD symptom onset (p<0.001) and higher scores on the obsessing OCI-R subdomain (p<0.001) increased the likelihood of current OCD treatment.
TABLE 5.
Multiple Logistic Regression Model for Prediction of Treatment Statusa
| Variableb | B | SE | OR | 95% CI for OR |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | −0.02 | 0.01 | 0.98 | 0.96 | 1.01 |
| Age of onset** | −0.09 | 0.02 | 0.92 | 0.88 | 0.95 |
| Total Medical Comorbidities | 0.02 | 0.09 | 1.02 | 0.86 | 1.22 |
| Total Psychiatric Comorbidities** | 0.16 | 0.06 | 1.17 | 1.05 | 1.31 |
| Checking | −0.06 | 0.04 | 0.95 | 0.95 | 1.02 |
| Hoarding | 0.01 | 0.04 | 1.01 | 0.94 | 1.09 |
| Neutralizing | 0.04 | 0.03 | 1.04 | 0.98 | 1.11 |
| Ordering | −0.01 | 0.05 | 0.99 | 0.90 | 1.09 |
| Washing | −0.01 | 0.03 | 0.99 | 0.93 | 1.06 |
| Obsessing** | 0.19 | 0.04 | 1.21 | 1.13 | 1.31 |
| Cannabis use frequency** | −0.06 | 0.02 | 0.94 | 0.90 | 0.98 |
| %Medicinal (vs. recreational) use | −0.00 | 0.00 | 1.00 | 0.99 | 1.00 |
p < .05
p < .005
Treatment status coded as a binary outcome (0=not currently in treatment, 1=currently in treatment)
Variables with p<.1 on independent t-tests were forwarded stepwise into the logistic regression model
4. Discussion
To our knowledge, this is the first study to assess patterns of cannabis use in a large sample of individuals with a diagnosis of OCD (based on a prior diagnosis by a healthcare profession or well-established cutoff on the OCI-R, a validated self-report form). There were five major findings. First, individuals with OCD who use cannabis were willing to provide very detailed information regarding their patterns of cannabis use in an online survey. In this sample, participants most commonly used inhalation methods (i.e., smoking or vaporization) to ingest cannabis and most often used cannabis flower over other formulations. Most participants reported either using products with high-THC/low-CBD concentrations or being unaware of the THC/CBD content of the products they used. Second, nearly 90% reported using cannabis for medicinal purposes, despite the fact that only about a third had a physician’s recommendation to do so. Further, 29% said they used cannabis specifically to manage OCD symptoms. Third, most participants reported that cannabis improved their OCD symptoms, although greater severity of obsessions was associated with less perceived benefit. Fourth, our sample experienced high rates of cannabis-associated problems: 42% met criteria for CUD, and nearly 70% for problematic cannabis use. Finally, most participants were not currently receiving evidence-based OCD treatment, and the likelihood of this treatment decreased as cannabis use frequency increased.
Americans increasingly use cannabis for both recreational and medicinal purposes (Lin et al., 2016). Like all psychotropic agents, cannabis has the potential to cause beneficial or harmful effects; yet unlike most medical interventions, medicinal cannabis use often occurs without physician oversight or regard to state policies (Azcarate et al., 2020). Cannabis users increasingly report medicinal use to alleviate psychiatric symptoms (including OCD symptoms; Mauzay et al., 2021) and some describe using cannabis as a substitute for psychotropic medications (Corroon et al., 2017). Yet, a 2019 meta-analyses including 83 studies found insufficient evidence to support treating any psychiatric condition with cannabinoids (Black et al., 2019). Meanwhile, both acute and chronic cannabis use has been linked to adverse psychiatric consequences (De Aquino et al., 2018; Hindley et al., 2020). In this context, there is a pressing public health need to understand how cannabis interacts with psychiatric illnesses like OCD. The present survey was designed with this need in mind.
In this first exploration of the methods and formulations by which individuals with OCD use cannabis, participants most commonly reported using inhalation methods to ingest cannabis flower, patterns of use that reflect findings from other studies of cannabis users (Azcarate et al., 2020; Sexton et al., 2016b; Spindle et al., 2019). Notably, some participants reported using high-potency cannabis products (i.e., containing high THC and low CBD); these included nearly a third of concentrate users who reported using products containing >80% THC. These data are consistent with findings from large epidemiological studies showing increasing use of high-THC cannabis products in the US (ElSohly et al., 2016; Hasin, 2018a), which is potentially concerning given that these products are associated with greater risk for adverse psychiatric consequences (Hindley et al., 2020). Because of our design (e.g., recruiting participants from online venues), it is unclear to what extent this reflects the broader population of adults with OCD who use cannabis. Nonetheless, our findings indicate that clinicians should ask about cannabis use in their patients with OCD and consider counseling cannabis users about potential risks associated with high-potency cannabis products.
As in other studies of cannabis users (Lin et al., 2016; Turna et al., 2019; Wall et al., 2019), most participants endorsed a mix of medicinal and recreational use. One-third of participants had a physician’s recommendation to use cannabis for indications including anxiety and OCD; 29% of participants (including those with and without a physician’s recommendation) used cannabis specifically to treat OCD symptoms. These findings are notable given that OCD is not currently an indication for medicinal cannabis in any state (“State Medical Marijuana Laws,” 12/21/2020), and around 15% of participants resided in states where cannabis was fully-illegal. Discretionary cannabis recommendations (i.e., for indications beyond those specifically approved by state law) are currently permitted in at least nine states and may explain some of our findings. However, recent surveys of medicinal cannabis users suggest that the majority of such use may occur without a physician’s recommendation or knowledge (Azcarate et al., 2020; Turna et al., 2020). Thus, it is reasonable to propose that our results also reflect self-medication of OCD symptoms with cannabis, either outside of a medical context or possibly in individuals with a physician’s recommendation for a condition other than OCD.
As noted in the Introduction, in an observational study using a mobile app of 87 medicinal cannabis users with self-reported OCD, participants generally reported acute improvements in OCD symptoms following cannabis use (Mauzay et al., 2021). Consistent with these findings, more than 60% of participants in the present online survey indicated that cannabis typically improved their obsessions and compulsions (as measured by the YBOC-CS). Although prior research suggests that cannabis effects may vary with gender (Z. D. Cooper & Craft, 2018a) and age (Gorey et al., 2019), we did not find that these variables predicted YBOC-CS scores. However, as hypothesized, we did find that greater severity of obsessions (assessed via the OCI-R) predicted worse perception of cannabis effects. Moreover, increased severity of obsessing correlated with less frequent cannabis use. One explanation for these findings could be that cannabis adversely affects obsessions. In this case, patients with prominent obsessions may perceive fewer benefits and thus use less often. Obsessional individuals might also be more likely to seek evidence-based care, perhaps due to the nature of obsessions themselves, or if self-medication of these symptoms with cannabis is ineffective; indeed, we found that participants with increased obsessions were more likely to be in treatment. As others have noted (Spradlin et al., 2017), a third possibility is that obsessional individuals ruminate about adverse cannabis effects and overreport these problems. Regardless of the explanation, our results provide further evidence supporting a link between obsessing and adverse cannabis effects from (and less frequent use of) cannabis.
Counter to our initial hypothesis, we found no correlation between OCD symptom severity (measured by the OCI-R) and problematic cannabis effects (measured by the CUDIT-R). This contrasts with prior survey findings (Spradlin et al., 2017), which could possibly be due to differences in sample: All participants included in our analysis met criteria for OCD, compared to only 13% of participants in the prior study. Nonetheless, our participants experienced substantial cannabis-related impairment in functioning, with nearly half meeting criteria for CUD, and close to 70% for problematic cannabis use (based on their CUDIT-R score). Alarmingly, the CUD rate we observed was over twice the rate for all lifetime cannabis users (19.5%) reported by the 2012-2013 National Epidemiologic Survey on Alcohol and Related Disorders (Hasin et al., 2016). Though not an epidemiological study and prone to recruitment bias, this survey raises a possibility that future studies should examine: That compared to the general population, those with OCD may be more susceptible to developing cannabis-associated problems. Our data suggest that clinicians treating patients with OCD should monitor for and provide additional counseling about the potential risk for CUD and other harms related to cannabis use (particularly when obsessive symptoms are prominent).
Confirming our hypothesis, more frequent cannabis use decreased participants’ likelihood of currently receiving treatment for OCD in this sample. Furthermore, although nearly 75% of participants reported a lifetime history of psychiatric treatment, a minority had ever received first-line OCD treatments, including SRIs in only 39.3% and EX/RP in only 15.9%. Why participants were not receiving treatment is unclear. One interpretation is that some individuals with OCD attempt to manage their symptoms with cannabis in lieu of pursuing evidence-based treatments, which aligns with our finding that most participants perceived cannabis as benefitting their OCD symptoms. Alternatively, cannabis use may interfere with receipt of evidence-based treatment, for example when cannabis is an exclusion criterion for enrolling in treatment (as is the case in some specialty programs; Storch & Kay, 2019) or if CUD symptoms limit patients’ ability to seek out or engage in care (e.g., inability to follow through with therapy appointments). Given the risks associated with untreated OCD and cannabis-related problems, our findings suggest that providers of OCD treatment may need to re-evaluate “zero-tolerance” policies around cannabis use. Instead, harm reduction strategies or CUD treatments could be integrated with existing approaches. Indeed, effective treatment models for individuals with OCD and comorbid substance use already exist (Fals-Stewart & Schafer, 1992); expanding these could improve access to care for individuals with OCD and cannabis misuse.
Unexpectedly, around 3% of participants indicated using cannabis during psychotherapy, including before exposure exercises. Though beyond the scope of this survey, understanding the prevalence and motivations for this use in patients with OCD has important treatment implications. For example, if patients use cannabis to cope with exposure-related anxiety, this may become an avoidance behavior that undermines the mechanisms associated with this treatment. Yet there is evidence that THC can facilitate at least one of these mechanisms (i.e., extinction learning, Raber et al., 2019). Thus, cannabinoids delivered concurrently with exposure-based treatment might actually augment its effects in some contexts (as our preliminary study of nabilone in patients with OCD supports (Kayser, Raskin, et al., 2020). Future research should examine how cannabis use impacts the mechanisms and clinical efficacy of exposure treatment.
Strengths of this study include a large sample size of adults meeting OCD criteria (n=601) and use of well-validated measures of OCD symptoms (the OCI-R) and cannabis use (the DFAQ-CU and CUDIT-R). Nonetheless, interpretation of our findings is limited due to our use of a cross-sectional survey design relying on self-reports, without independent confirmation of OCD diagnosis, treatment history, or cannabis use. Moreover, because we recruited participants from online forums, our findings (e.g., that most participants perceived cannabis as beneficial towards their OCD symptoms) could reflect selection bias of individuals who would complete such a study. Perhaps related to this recruitment strategy, participants were predominantly white, non-Hispanic, and highly educated, which may impact the sample’s generalizability. Finally, expectancy bias (to which subjective cannabis effects are notoriously susceptible; see Chait et al., 1988; Fillmore, Mulvihill, & Vogel-Sprott, 1994; Kirk, Doty, & De Wit, 1998) may have affected our data.
5. Future Directions
These survey results suggest that cannabis use may be associated with a range of positive and negative subjective effects in individuals with OCD, may be used instead of evidence-based OCD treatments, and may lead to problematic cannabis use and/or CUD. As cannabis use among the general population grows increasingly common (both in the US and worldwide; Hasin, 2018b), use by individuals with OCD is almost certain to expand as well. Thus, improving our understanding of cannabis’ potential risks and benefits to those with OCD is crucial. Future research is needed to determine if our survey results are replicable in a larger, more nationally-representative sample. Placebo-controlled trials will be critical in order to objectively test the acute and chronic effects of different cannabis preparations in patients with OCD while accounting for expectancy effects. Similarly, controlled studies are needed to dissect the effects of different cannabis constituents (e.g., THC/CBD) on OCD symptoms. Investigators must also clarify why cannabis users are not accessing OCD treatment, how cannabis use interacts with evidence-based OCD care, and what explains the link between obsessing symptoms and adverse cannabis effects. Finally, it is time to develop effective treatment models for patients with both OCD and problematic cannabis use.
Supplementary Material
Highlights.
In an internet survey, we examined patterns of cannabis use in adults with OCD (n=601)
Participants reported experiencing both subjective benefits and harms from cannabis use
Participants with more severe obsessions perceived fewer benefits from cannabis and used less often
More frequent cannabis use reduced participants’ odds of receiving evidence-based OCD treatment
We discuss implications for treating cannabis users with OCD and topics for future research
Acknowledgments
The authors would like to acknowledge the staff at the Columbia University/New York State Psychiatric Institute Anxiety Disorders Clinic, in particular Dr. John Markowitz, who contributed feedback and revisions to an earlier draft of this manuscript.
Funding/Support
This work was supported by a National Institute of Mental Health (NIMH) T32 Training Grant in Mood, Anxiety and Related Disorders and an NIMH Loan Repayment Award (Grant Nos. T32MH15144 and L30MH120715, both to RK). Given her role as an Editorial Board Member, BS had no involvement in the peer-review of this article and had no access to information regarding its peer-review. The authors otherwise have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitch A, Abramowitz JS, Riemann BC, & McKay D (2020). Severity benchmarks and contemporary clinical norms for the Obsessive-Compulsive Inventory-Revised (OCI-R). Journal of Obsessive-Compulsive and Related Disorders, 27, 100557. 10.1016/j.jocrd.2020.100557 [DOI] [Google Scholar]
- Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, & Sellman JD (2010). An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R)☆. Drug and Alcohol Dependence, 110(1–2), 137–143. 10.1016/j.drugalcdep.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Albertella L, & Norberg MM (2012). Mental Health Symptoms and their Relationship to Cannabis Use in Adolescents Attending Residential Treatment. Journal of Psychoactive Drugs, 44(5), 381–389. 10.1080/02791072.2012.736808 [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Gillan CM, Fineberg NA, Fernandez-Egea E, Sahakian BJ, & Robbins TW (2017). Neural basis of impaired safety signaling in Obsessive Compulsive Disorder. Proceedings of the National Academy of Sciences, 114(12), 3216–3221. 10.1073/pnas.1609194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcarate PM, Zhang AJ, Keyhani S, Steigerwald S, Ishida JH, & Cohen BE (2020). Medical Reasons for Marijuana Use, Forms of Use, and Patient Perception of Physician Attitudes Among the US Population. Journal of General Internal Medicine, 35(7). 10.1007/s11606-020-05800-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshaie J, Storch EA, Tran N, & Zvolensky MJ (2020). Obsessive-Compulsive Symptoms and Cannabis Misuse: The Explanatory Role of Cannabis Use Motives. Journal of Dual Diagnosis, 1–11. 10.1080/15504263.2020.1786616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, Farrell M, & Degenhardt L (2019). Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. The Lancet Psychiatry, 6(12), 995–1010. 10.1016/S2215-0366(19)30401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, & Schmidt NB (2007). Marijuana use motives and social anxiety among marijuana-using young adults. Addictive Behaviors, 32(10), 2238–2252. 10.1016/j.addbeh.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, & Schuster CR (1988). Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology, 94(2), 206–212. 10.1007/bf00176846 [DOI] [PubMed] [Google Scholar]
- Cooper JJ, & Grant J (2017). Refractory OCD Due to Thalamic Infarct With Response to Dronabinol. The Journal of Neuropsychiatry and Clinical Neurosciences, 29(1), 77–78. 10.1176/appi.neuropsych.16030053 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, & Craft RM (2018a). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology, 43(1), 34–51. 10.1038/npp.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, & Craft RM (2018b). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. In Neuropsychopharmacology (Vol. 43, Issue 1, pp. 34–51). Nature Publishing Group. 10.1038/npp.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, Mischley L, & Sexton M (2017). Cannabis as a substitute for prescription drugs – a cross-sectional study. Journal of Pain Research, Volume 10, 989–998. 10.2147/JPR.S134330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, & Spradlin A (2017). Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU). PLoS ONE, 12(5), e0178194. 10.1371/JOURNAL.PONE.0178194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Hensen G, Thimm B, Rezk M, Till B, & Gouzoulis-Mayfrank E (2004). Self-reported psychopathological symptoms in recreational ecstasy (MDMA) are mainly associated with regular cannabis use: Further evidence from a combined cross-sectional/longitudinal investigation. Psychopharmacology, 173(3–4), 398–404. 10.1007/s00213-003-1719-0 [DOI] [PubMed] [Google Scholar]
- De Aquino JP, Sherif M, Radhakrishnan R, Cahill JD, Ranganathan M, & D’Souza DC (2018). The Psychiatric Consequences of Cannabinoids. Clinical Therapeutics, 40(9), 1448–1456. 10.1016/j.clinthera.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Douglass HM, Moffitt TE, Dar R, McGEE R, & Silva P (1995). Obsessive-Compulsive Disorder in a Birth Cohort of 18-Year-Olds: Prevalence and Predictors. Journal of the American Academy of Child and Adolescent Psychiatry, 34(11), 1424–1431. 10.1097/00004583-199511000-00008 [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, & Church JC (2016). Changes in cannabis potency over the last 2 decades (1995-2014): Analysis of current data in the United States. Biological Psychiatry, 79(7), 613–619. 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, & Schafer J (1992). The treatment of substance abusers diagnosed with obsessive-compulsive disorder: An outcome study. Journal of Substance Abuse Treatment, 9(4), 365–370. 10.1016/0740-5472(92)90032-J [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, & Vogel-Sprott M (1994). The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology, 115(3), 383–388. 10.1007/BF02245081 [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, & Salkovskis PM (2002). The obsessive-compulsive inventory: Development and validation of a short version. Psychological Assessment, 14(4), 485–496. 10.1037/1040-3590.14.4.485 [DOI] [PubMed] [Google Scholar]
- Fraser GA (2009). The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neuroscience and Therapeutics, 15(1), 84–88. 10.1111/j.1755-5949.2008.00071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston TE, Bebin EM, Cutter GR, Liu Y, & Szaflarski JP (2017). Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia, 58(9), 1586–1592. 10.1111/epi.13852 [DOI] [PubMed] [Google Scholar]
- Gianessi CA, Groman SM, & Taylor JR (2021). The effects of fatty acid amide hydrolase inhibition and monacylglycerol lipase inhibition on habit formation in mice. European Journal of Neuroscience, ejn.15129. 10.1111/ejn.15129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, & Charney DS (1989). The Yale-Brown Obsessive Compulsive Scale: II. Validity. Archives of General Psychiatry, 46(11), 1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- Gorey C, Kuhns L, Smaragdi E, Kroon E, & Cousijn J (2019). Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. In European Archives of Psychiatry and Clinical Neuroscience (Vol. 269, Issue 1, pp. 37–58). Dr. Dietrich Steinkopff Verlag GmbH and Co. KG. 10.1007/s00406-019-00981-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, & Costa RM (2016). Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron, 90(6), 1312–1324. 10.1016/j.neuron.2016.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud MZ, Peters C, Hatfield JRB, Gorka SM, Phan L, Milad MR, & Rabinak CA (2019). Influence of Δ9-tetrahydrocannabinol on long-term neural correlates of threat extinction memory retention in humans. Neuropsychopharmacology. 10.1038/s41386-019-0416-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018a). US Epidemiology of Cannabis Use and Associated Problems. In Neuropsychopharmacology (Vol. 43, Issue 1, pp. 195–212). Nature Publishing Group. 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018b). US Epidemiology of Cannabis Use and Associated Problems. In Neuropsychopharmacology (Vol. 43, Issue 1, pp. 195–212). Nature Publishing Group. 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, Zhang H, & Grant BF (2016). Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. In American Journal of Psychiatry (Vol. 173, Issue 6, pp. 588–599). American Psychiatric Association. 10.1176/appi.ajp.2015.15070907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley G, Beck K, Borgan F, Ginestet CE, McCutcheon R, Kleinloog D, Ganesh S, Radhakrishnan R, D’Souza DC, & Howes OD (2020). Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. The Lancet Psychiatry, 7(4), 344–353. 10.1016/S2215-0366(20)30074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert JD, Walther MR, Hajcak G, Yadin E, Foa EB, Simpson HB, & Liebowitz MR (2007). The OCI-R: Validation of the subscales in a clinical sample. Journal of Anxiety Disorders, 21(3), 394–406. 10.1016/j.janxdis.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, & Salsitz E (2019). Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. American Journal of Psychiatry, 176(11), 911–922. 10.1176/appi.ajp.2019.18101191 [DOI] [PubMed] [Google Scholar]
- Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, & Zimmer A (2016). Anxiety, Stress, and Fear Response in Mice with Reduced Endocannabinoid Levels. Biological Psychiatry, 79(10), 858–868. 10.1016/j.biopsych.2015.03.033 [DOI] [PubMed] [Google Scholar]
- Kayser RR, Haney M, Raskin M, Arout C, & Simpson HB (2020). Acute Effects of Cannabinoids on Symptoms of Obsessive-Compulsive Disorder: A Human Laboratory Study. Depression and Anxiety, da.23032. 10.1002/da.23032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser RR, Raskin M, Snorrason I, Hezel DM, Haney M, & Simpson HB (2020). Cannabinoid Augmentation of Exposure-Based Psychotherapy for Obsessive-Compulsive Disorder. Journal of Clinical Psychopharmacology, 40(2), 207–210. 10.1097/JCP.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser RR, Snorrason I, Haney M, Lee FS, & Simpson HB (2019). The Endocannabinoid System: A New Treatment Target for Obsessive Compulsive Disorder? Cannabis and Cannabinoid Research, 4(2). 10.1089/can.2018.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, Doty P, & De Wit H (1998). Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacology, Biochemistry, and Behavior, 59(2), 287–293. 10.1016/s0091-3057(97)00414-0 [DOI] [PubMed] [Google Scholar]
- Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB, & American Psychiatric Association. (2007). Practice guideline for the treatment of patients with obsessive-compulsive disorder. The American Journal of Psychiatry, 164(7 Suppl), 5–53. http://www.ncbi.nlm.nih.gov/pubmed/17849776 [PubMed] [Google Scholar]
- Lin LA, Ilgen MA, Jannausch M, & Bohnert KM (2016). Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addictive Behaviors, 61, 99–103. 10.1016/j.addbeh.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari RN, & Park-Lee E (2019). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. https://www.samhsa.gov/data/ [Google Scholar]
- Lu HC, & MacKie K (2016). An introduction to the endogenous cannabinoid system. Biological Psychiatry, 79(7), 516–525. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzay D, LaFrance EM, & Cuttler C (2021). Acute Effects of Cannabis on Symptoms of Obsessive-Compulsive Disorder. Journal of Affective Disorders, 279, 158–163. 10.1016/j.jad.2020.09.124 [DOI] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, & Heilig M (2019). Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase (FAAH): a randomized, controlled experimental medicine trial. Biological Psychiatry. 10.1016/j.biopsych.2019.07.034 [DOI] [PubMed] [Google Scholar]
- McLaughlin NCR, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, Fadok R, Spofford C, Rasmussen SA, Milad MR, & Greenberg BD (2015). Extinction retention and fear renewal in a lifetime obsessive-compulsive disorder sample. Behavioural Brain Research. 10.1016/j.bbr.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Galfavy H, Kimeldorf MB, Dixon LB, & Simpson HB (2017). Patient preferences and acceptability of evidence-based and novel treatments for obsessive-compulsive disorder. Psychiatric Services, 68(3), 250–257. 10.1176/appi.ps.201600092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Huang Y, Corsi-Travali S, Zheng MQ, Lin SF, Henry S, Potenza MN, Piomelli D, Carson RE, & Neumeister A (2014). Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology, 39(11), 2519–2528. 10.1038/npp.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Raber J, Arzy S, Bertolus JB, Depue B, Haas HE, Kangas M, Kensinger E, Lowry CA, Marusak HA, Minnier J, Mouly A-M, Muehlberger A, Norrholm SD, Peltonen K, Pinna G, Rabinak C, Shiban Y, Soreq H, van der Kooij MA, … Boutros SW (2019). Current understanding of fear learning and memory in humans and animal models and the value of a linguistic approach for analyzing fear learning and memory in humans. Neuroscience & Biobehavioral Reviews. 10.1016/j.neubiorev.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Richter KP, & Levy S (2014). Big Marijuana — Lessons from Big Tobacco. New England Journal of Medicine, 371(5), 399–401. 10.1056/nejmp1406074 [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Flood P, & Simpson HB (2011). Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. The Journal of Clinical Psychiatry, 72(4), 567–569. 10.4088/JCP.10l06653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, & Simpson HB (2013). Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(12), 2475–2483. 10.1038/npp.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, & Marcu J (2017). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Advances in Pharmacology, 80, 67–134. 10.1016/BS.APHA.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Schindler F, Anghelescu I, Regen M, & Jockers-Scherubl M (2008). Improvement in Refractory Obsessive Compulsive Disorder with Dronabinol. American Journal of Psychiatry, 165(4), 536–537. 10.1176/appi.ajp.2007.07060946 [DOI] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, Finnell JS, & Mischley LK (2016a). A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis and Cannabinoid Research, 1(1), 131–138. 10.1089/can.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, Finnell JS, & Mischley LK (2016b). A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis and Cannabinoid Research, 1(1), 131–138. 10.1089/can.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, & Mischley LK (2019). A Survey of Cannabis Acute Effects and Withdrawal Symptoms: Differential Responses Across User Types and Age. In Journal of Alternative and Complementary Medicine (Vol. 25, Issue 3, pp. 326–335). Mary Ann Liebert Inc. 10.1089/acm.2018.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Bonn-Miller MO, & Vandrey R (2019). Changing landscape of cannabis: novel products, formulations, and methods of administration. In Current Opinion in Psychology (Vol. 30, pp. 98–102). Elsevier B.V. 10.1016/j.copsyc.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradlin A, Mauzay D, & Cuttler C (2017). Symptoms of obsessive-compulsive disorder predict cannabis misuse. Addictive Behaviors, 72(January), 159–164. 10.1016/j.addbeh.2017.03.023 [DOI] [PubMed] [Google Scholar]
- State Medical Marijuana Laws. (n.d.). Retrieved July 12, 2020, from https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, van den Heuvel OA, & Simpson HB (2019). Obsessive–compulsive disorder. Nature Reviews Disease Primers, 5(1), 52. 10.1038/s41572-019-0102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, & Kay BC (2019). Commentary on Spradlin et al.: Is marijuana use common in OCD? In Addictive Behaviors. 10.1016/j.addbeh.2017.07.028 [DOI] [PubMed] [Google Scholar]
- Subritzky T, Lenton S, & Pettigrew S (2016). Legal cannabis industry adopting strategies of the tobacco industry. Drug and Alcohol Review, 35(5), 511–513. 10.1111/dar.12459 [DOI] [PubMed] [Google Scholar]
- Szejko N, Fremer C, & Müller-Vahl KR (2020). Cannabis Improves Obsessive-Compulsive Disorder—Case Report and Review of the Literature. Frontiers in Psychiatry, 11, 1. 10.3389/fpsyt.2020.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG & Fidell L (2019). Using Multivariate Statistics (Seventh Edition). Pearson. https://lccn.loc.gov/2017040173 [Google Scholar]
- Turna J, Balodis I, Munn C, Van Ameringen M, Busse J, & MacKillop J (2020). Overlapping patterns of recreational and medical cannabis use in a large community sample of cannabis users. Comprehensive Psychiatry, 102, 152188. 10.1016/j.comppsych.2020.152188 [DOI] [PubMed] [Google Scholar]
- Turna J, Simpson W, Patterson B, Lucas P, & Van Ameringen M (2019). Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. Journal of Psychiatric Research, 111, 134–139. 10.1016/j.jpsychires.2019.01.024 [DOI] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, & Bullmore ET (2015). Disorders of compulsivity: A common bias towards learning habits. Molecular Psychiatry, 20(3), 345–352. 10.1038/mp.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MM, Liu J, Hasin DS, Blanco C, & Olfson M (2019). Use of marijuana exclusively for medical purposes. Drug and Alcohol Dependence, 195, 13–15. 10.1016/j.drugalcdep.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


