Abstract
Recent evidence indicates that the corneal back surface astigmatism (CBSA) contributes to the refractive state of the eye in cataract surgery, especially with the implantation of toric intraocular lenses. But this has been met with some scepticism. A review of key studies performed over the past three decades shows that the mean CBSA power ranges from 0.18(±0.16)D to 1.04(±0.20)D. The clinical assessment of CBSA is problematic. There is poor agreement between the current automated systems for assessment of CBSA and it is assumed that these systems directly measure the CBSA. But CBSA cannot be measured directly in vivo. A historical review of methods used to quantify the curvature of the posterior corneal surface reveals that CBSA estimated by current systems is based on values for corneal front surface astigmatism, corneal refractive index, central corneal thickness, corneal thickness at peripheral locations and the exact distance between the corneal apex and each one of these peripheral locations. Doubts and errors in these values, coupled with the precise details of the algorithm incorporated to estimate CBSA, are the likely sources of the lack of agreement between current systems. These systematic errors cloud the assessment of CBSA. Mean CBSA may be low, but it varies from case to case. There is a clear need for a realistic, practical procedure for clinicians to independently calibrate systems for estimating CBSA. This would help to reduce uncertainty and the discrepancies between instruments designed to measure the same parameter.
Keywords: cornea, back surface, astigmatism, radius, thickness, refractive index
Introduction
The classical texts identified the corneal back surface as being a relatively inert intra-ocular optical boundary that had a negligible impact on the overall optical performance of the eye.1,2 Nevertheless, since 2000 there has been a resurgence of interest in the curvature, and in particular the astigmatism, of the corneal back surface. A quick check on “PubMed” leads to more than 200 hits after typing in “corneal posterior surface power.” Changes in corneal back surface astigmatism are expected after most forms of keratoplasty,3–5 in cases of keratoconus,3,6–8 Fuchs’ endothelial corneal dystrophy,8,9 and during refractive therapies including orthokeratology.10 In addition, corneal back surface astigmatism is claimed to impact on the performance of the eye in relation to refractive surgery11,12 and planning for cataract surgery especially in astigmatic eyes.13–25 Some investigators reported that corneal back surface astigmatism remains stable after refractive surgery, and this would have a marginal impact on later routine phacoemulsification.26–32 This is certainly not the case in patients that underwent more invasive procedures, such as radial keratotomy, where profound changes in corneal back surface astigmatism can occur.33–35 Thus, it is reasonable to expect corneal back surface astigmatism to impact on refraction, and IOL power selection when such extraordinary cases request cataract surgery.
Current Approaches for the Assessment of Back Corneal Astigmatism
Total corneal astigmatism is modified by routine phacoemulsification and this alteration appears to be driven by the change in corneal back surface astigmatism on an individual case-by-case basis.36 But, to what extent does corneal back surface astigmatism vary within a typical sample drawn from a normal population? Table 1 lists the mean corneal back surface astigmatic power values reported in some of the key publications on this topic. The earliest publication reported a mean (±s.d.) corneal back surface astigmatic power value of 0.41(±0.03)D.37 The lowest, highest and typical mean values are 0.18(±0.03)D,38 1.04(±0.20)D,39 and 0.26 (±0.15)D,40 respectively. Most reported mean values were small, verging on the insignificant, but the range of values can be >0.75D.22,41–43 The difference between the lowest and highest means is about 6.5 times greater when compared with the typical average ±s.d value. Corneal back surface astigmatism cannot be measured directly. It is an estimate based on other measurements and assumptions. The lack of cohesion between these studies could be due to genuine differences between population samples or systematic differences in the way values for the corneal back surface astigmatism are derived.
Table 1.
Key Previous Estimates of Corneal Back Surface Astigmatism
| Authors | Year | Instrument/Method | Values (D) |
|---|---|---|---|
| Dunne et al37 | 1992 | Purkinje Images | 0.23 (±0.02, n=40, F). |
| 0.37 (±0.03, n=40, M) | |||
| Oshika et al38 | 1998 | Scanning Slit Video | 0.18 (±0.16, n=50) |
| Liu et al39 | 1999 | Orbscan | 0.37 (±0.18, n=94) over 3.0mm |
| 0.52 (±0.25, n=94) over 5.0mm | |||
| 1.04 (±0.20, n=94) over 7.0mm | |||
| Dubbleman et al63 | 2006 | Scheimpflug imaging | 0.31 (n=114) |
| Kamiya et al64 | 2015 | Pentacam HR | 0.44 (±0.12, n=53) |
| Klijn et al22 | 2016 | Pentacam HR | 0.31 (±0.17,0.01 to 0.82, n=77) |
| Rydström et al40 | 2016 | Pentacam HR | 0.26 (±0.15, n=402) |
| Choi et al65 | 2018 | Scheimpflug imaging | 0.39 (±0.18, n=33) |
| Cui et al42 | 2019 | Cassini | 0.35 (0.04–0.89, n=117) |
| Pentacam HR | 0.29 (0.00–0.80, n=117) | ||
| Li et al43 | 2019 | Sirius | 0.31 (±0.17, 0.05–1.09, n=121) |
| Wylęgała et al48 | 2020 | Galilei G6 | 0.44 (n=94) over 4.0mm |
| Casia 2 | 0.52 (n=94) over 3.2mm | ||
| Revo NX | 0.05 (n=94) over 3.0mm |
Notes: All mean values for astigmatism have been converted into plus cylinder format where necessary; Values in parentheses are ±sd and ranges according to the corresponding publication (reference number is in superscript); Details of instruments, or methods, used by each group of investigators are noted as cited in the relevant publication.
Abbreviations: D, units of astigmatic power (dioptres); F, females; M, males.
The current crop of automated systems designed to provide values of corneal back surface astigmatism estimate the radius of curvature at multiple locations over the corneal back surface. Full descriptions of the more widely available systems can be found in a range of review articles and numerous websites.44–48 These radii are converted into power values, using the standard lens surface formula, assuming the refractive indices of the cornea and aqueous humour are 1.376 and 1.336, respectively. When the orthogonal radii of the corneal back surface are 5.8mm and 6.8mm the corresponding respective powers are −6.90D and −5.88D. Ray tracing through classical model eyes shows the impact on the refractive error is about 1.00D when the radius at this surface changes by 1mm.2 There is a near 1:1 relationship between a change in corneal back surface astigmatism and change in overall refraction. The algorithm built in each device utilises these data to produce dioptral maps of the surfaces, identifies the orthogonal axes where the averaged dioptral values are highest and lowest in a small series of concentric zones covering the central cornea. The estimation of corneal back surface astigmatism is wholly reliant upon the limitations of the exact method employed to determine the radii over the central region of the corneal back surface.
It is reasonable to assume that there should be close agreement between different instruments designed to measure the same parameter. However, this is not the case regarding the corneal back surface astigmatism. There is a distinct lack of affinity between the corneal back surface astigmatism values obtained by one instrument when compared with another.16,42,48–51 For example, Nasser et al50 reported the limit of agreement between the Sirius and Pentacam devices for the estimation of corneal back surface astigmatism ranged from −0.56D to +0.31D. Wylęgała et al48 compared the performance of Casia, Galilei G6 and Revo NX for the estimation of corneal back surface astigmatism in a series of 94 eyes. The mean values for the corneal back surface astigmatism according to these systems were 0.52D, 0.44D and 0.05D, respectively. Savini et al51 compared the Sirius and Pentacam devices for the estimation of total corneal astigmatism and found the differences between the two systems amounted to ≥0.50D in over 20% of cases and the axes differed by >10° in over 40% of cases.
The diversity and range of these values lead to the conclusion that results from different instruments are not readily interchangeable. This impacts on our confidence in these estimates and questions the value and clinical relevance of corneal back surface astigmatism. But why are the current advanced technologies yielding such differences? To seek possible answers and gain a better understanding of the relative importance of corneal back surface astigmatism, it is necessary to consider how corneal back surface astigmatism has been estimated in the past, the rationale underpinning some of these procedures and the repeatability and validity of these various techniques.
Estimating Corneal Back Surface Radii, Hence the Astigmatism
During the early stages of 20th century, Tscherning1 declared the radius of the corneal back surface was 6.22mm while Gullstrand2 declared this radius was 6.80mm. A review of the pertinent literature shows that there was a paucity of interest in the radius and shape of the corneal back surface for about six decades. However, there are two publications where the quoted values are quite disparate. In 1973, Lowe and Clarke52 reported that the mean radius of this surface along the vertical meridian was typically 6.46mm, and seventeen years later Camellin53 estimated the mean radius was 5.84mm. This difference of +0.62mm changes the overall power of the eye by approximately −0.75D.54 This translates to a difference in astigmatism if the difference was orthogonal. Under normal circumstances it is hardly likely for the radius of curvature along one meridian at the corneal back to change by more than 0.50mm. Thus, little attention was paid to investigating the astigmatism and overall shape of the corneal back surface.
Tscherning1 measured the corneal back surface curvature by injecting the anterior chamber of cadaver eyes with liquid gelatin until it took up the shape of the corneal surface. The cornea was removed, after the gelatin had hardened, to reveal a convex cast of the corneal back surface. The radius of the cast mirrors the curvature of the corneal back surface. Moving on from this totally invasive method, he developed a non-invasive procedure for calculating corneal back surface astigmatism using an ophthalmophakometer (sic). The result obtained using this procedure is dependent upon five variables. Therefore, the validity of the final calculation of corneal back surface astigmatism is totally reliant upon the error in each one of the five variables.
Lowe and Clark52 captured slit-lamp images of the corneal profile and matched the shapes of the corneal surfaces with contours of known curvature. The physical limitations of the procedure meant that only an estimate for the corneal back surface radius along the vertical meridian could be made in each case. During that time, the resolution of profile photography for estimating the dimensions of the cornea was heavily criticised for being unreliable.55
Royston et al56,57 modified Tscherning’s non-invasive procedure by capturing the 1st and 2nd Purkinje images of fixed targets positioned along 3 meridians, measuring the corneal front surface radii along the same 3 meridians and the central corneal thickness. The sizes of the Purkinje images together with the other measurements were used to calculate the corneal back surface radii along these meridians using a simple formula. In a series of 15 eyes, the RMS difference between the profile photography and Purkinje image methods for corneal back surface radius was 0.097mm (range 0 to 0.20mm). This is equivalent to a difference in corneal back surface power of about 0.10D.57 The test–retest reliability of the Purkinje image technique, based on estimates of corneal back surface astigmatism obtained from 5 eyes, equates to less than 0.03D for power and 3° for the axis. A subsequent paper by the same authors reported mean corneal back surface astigmatism values equivalent to 0.23D (±0.02D, n=40) in females and 0.37D (±0.03D, n=40) in males.37 The corresponding axis values were 88.6° (±4.8) and 91.7° (±4.5). Lam and Douthwaite58 used a similar procedure based on Purkinje images and reported that the typical difference between vertical and horizontal corneal back surface radii values was about 0.25mm. This equates to a corneal back surface astigmatism of about 0.30D. To facilitate these procedures, the 1st and 2nd Purkinje images must remain separate and free of any overlap. This limitation means that corneal back surface assessments are made at distances about 3mm, or more, away from the apex of the cornea. Hence, the estimate of corneal back surface astigmatism is for a region of the central cornea no less than 6mm in diameter. It has been shown that the back surface of the cornea flattens gradually from the apex.54,59 The radius at the apex of the back surface of the cornea is likely to be smaller compared with the figures obtained by these other methods. The actual astigmatism at the apex of the back surface of the cornea could exceed 0.30D in some cases.
Camellin53 devised a geometric method for calculating the radius of the corneal back surface from measurements of front corneal surface radius and topographic corneal thickness. A further development of this technique was used to estimate not only the radius of the corneal back surface but also its' asphericity.54,59
Fundamental Basis for the Estimation of Corneal Back Surface Radius and Astigmatism
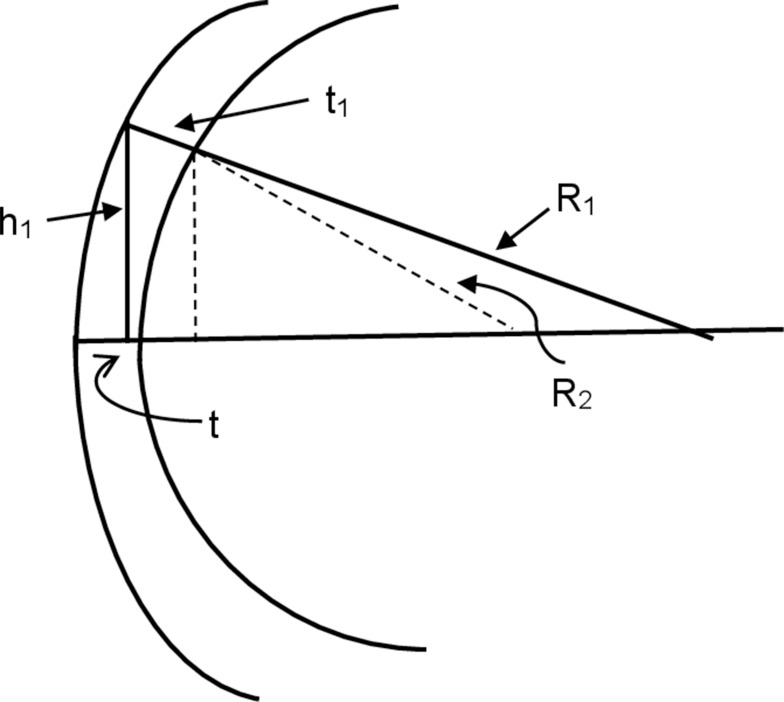
The precise details of the algorithms incorporated into any of the current generation of clinical instruments designed to determine corneal back surface astigmatism are, to some extent, trade secrets. However, Figure 1 shows how corneal back surface radii and astigmatism can be derived. Figure 1 is a diagrammatic representation of a corneal section where the front and back surfaces are centred about a common geometric axis. It can be shown that the radius of the back surface (R2) can be estimated using the following:
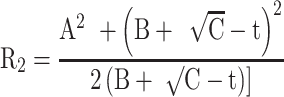 |
Eq.1 |
Figure 1.
Diagram of a corneal section for the calculation of back surface radius. h1= distance from the apex of the cornea and a peripheral location on corneal surface, t1= thickness of the cornea at this peripheral location on corneal surface, t = thickness at the apex of the cornea, R1 = corneal front surface radius, R2 = corneal back surface radius.
Where, h1= distance from the apex of the cornea and a peripheral location on corneal surface, t1= thickness of the cornea at this peripheral location on corneal surface (ie, the length of the perpendicular stretching from this peripheral location on the corneal front surface to the corneal back surface), t = thickness at the apex of the cornea, R1 = corneal front surface radius, A = h1 – (h1·t1/R1), B= R1- √(R12 – h12) and C= t12 + (h1·t1/R1)2.
The actual methods built-in the automated instruments for deriving the corneal back surface astigmatism are likely variations of the schema shown in Figure 1. The estimate of corneal back surface radius, hence the astigmatism, is dependent upon four measured variables, t, t1, h1 and R1. The estimates of t1 and t also depend on the refractive index of the cornea.60
The numerical effect of errors in the measurement of, or changes in, the four variables in the estimation of R2 can be quantified as follows:61
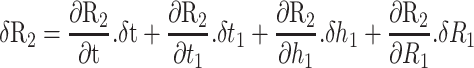 |
Eq.2 |
The partial differential equations demonstrating the theoretical effects on the estimation of R2 are shown in Table 2.
Table 2.
Partial Differential Equations for the Relative Error, 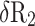 , for in the Calculation of Corneal Back Surface Radius
, for in the Calculation of Corneal Back Surface Radius
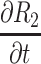 |
[2(t - B - √C)V - 2U]/V2 |
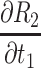 |
[4(K1t1) + 2t1 −2h1K1 + {2K2(t1+K1t1)/√[t12+(K1t1)2]}]/V – U{2t1(1+K1)/ √[t12+(K1t1)2]}/ V2 |
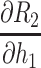 |
h1(2+2K52+4K5)+[2K8(K6+K7)]/V – U(K6 + 2K7)/V2 |
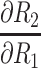 |
2{V[K11 - K12 + ([B-t+ √C]x[1- K9 –K10])] – U[1 - K9 -(h12t12/R13√C)]}/V2 |
Notes: Where, with reference to Figure 1, h1= distance from the apex of the cornea and a peripheral location on corneal surface, t1= the length of the perpendicular stretching from this peripheral location on the corneal front surface to the peripheral corneal surface (ie, the thickness of the cornea at this peripheral location); t = thickness at the apex of the cornea; R1 = corneal front surface radius, and A = h1 - (h1t1/R1); B= R1 - √(R12 – h12); C= t12 + (h1t1/R1)2; U= A2 + (B + √C – t)2; V= 2(B + √C - t); K1= h1/R1; K2= B-t; K3=K1+1; K4= h1t1; K5= t1/R1; K6= h1/√(R12 - h12); K7 = h1K52/√(t12 + [h1K5]2); K8 = B + √(t12 + [h1K5]2; K9 = R1/√(R12 - h12); K10 = h12t12/R13√C]; K11= h12t1/R12; K12= h12t12/R13.
For typical values of R1 (7.7mm), h1 (2.0mm), t1 (0.52mm), and t (0.50mm) reduces Equation 2 to:
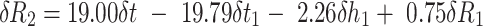 |
Eq.3 |
When errors in the measurement of δR1, δh1, δt1 and δt = +0.01mm, then δR2 = −0.02mm. This is an indication of the accuracy in the determination, that is the difference between the true and estimated value, of R2. The value of δR2 is negative and indicative of an overestimate of corneal back radius.
In normal eyes, the average change in the astigmatic power of the ocular surface during short intervals of blink suppression is about 0.48D over the central pupillary region of the cornea.62 Such a change in ocular surface power would occur when the orthogonal difference in ocular surface radii is about 0.1mm. Therefore, it is reasonable to adopt +0.1mm as a more realistic clinical estimate for δR1. For δR1=+0.1mm, keeping δR1, δh1, δt1 and δt = +0.01mm, then δR2 = +0.04mm. The value of δR2 is now positive and indicative of an underestimate of corneal back radius. This value for δR2 is equivalent to an error in corneal back surface power of about −0.04D. As this is the result of a change in ocular surface astigmatism, then 0.04D is a reasonable estimate for the absolute limit of accuracy in the practical determination of corneal back surface astigmatic power.
Nasser et al50 found the limits of agreement between the Sirius and Pentacam for the estimation of corneal front surface astigmatism ranged from −0.1mm to 0.12mm. The extent of this range, 0.22mm, equates to a range in the limit of agreement for corneal front surface astigmatism of over 1.25D. This being the range where most of the differences in the estimation of corneal front surface astigmatism lie. Adopting these as values, −0.1mm and 0.12mm, for δR1 in Equation 3 results in hypothetical values for δR2 ranging from −0.10mm to +0.06mm when δh1, δt1 and δt = +0.01mm. The actual limits of agreement for the corneal back surface astigmatism (in mm) reported by Nasser et al50 ranged from −0.54mm to +0.33mm. This range, 0.87mm, is 5.4 times greater than the hypothetical range of δR2 values of −0.10mm to +0.06mm. In addition, inter-device differences in the estimation of mean corneal thickness can be up to 0.030mm.48 Thus, other factors, other values for the uncertainties or differences in h1, t1 or t besides +0.01mm could account for the range −0.54mm to +0.33mm. Equation 3 predicts uncertainties in δt1 and δt are not expected to have much impact on δR2 when the uncertainties in these parameters are in the same direction, either positive or negative, as such directional uncertainties will cancel each other out. However, by how much does δR2 vary should δt1=+0.01mm along one meridian and −0.01mm along another? According to Equation 3, for δR1 = +0.1mm, δh1 and δt = +0.01mm, δR2 = −0.11mm when δt1=+0.01mm and +0.29mm when δt1=−0.01mm. This positive-to-negative shift in δt1 alters δR2 by +0.40mm. This change in δR2 increases to +0.59mm when the δt1 values are adjusted to −0.015mm and +0.015mm. Such uncertainty in δR2 is equivalent to an uncertainty in corneal back surface astigmatism, and overall refraction, of just under 0.75D. Clearly, there is a profound impact on δR2 when the regional uncertainties in δt1 consist of positive and negative values. Inter-device differences for the estimation of corneal back surface astigmatism are amplified when the direction (positive or negative) of uncertainties in the pachymetric estimations is inconsistent. This partially, but not fully, accounts for the reported limits of agreement in the estimation of corneal back surface astigmatism ranging from −0.54mm to +0.33mm.50
Turning to δh1, by how much should this potential source of uncertainty fluctuate to account for these wide limits of agreement? According to Equation 3, when δR1 = +0.1mm and δt1 and δt = +0.01mm, the value of δR2 shifts from −0.54mm to +0.34mm by changing δh1 from +0.27mm to −0.12mm. The impact of this hypothetical shift in δh1 on the estimation of corneal back surface astigmatism is just below 1D. Therefore, it is possible for small systematic, meridionally sensitive, differences in the algorithms used by these devices to contribute towards the variations in corneal back surface astigmatism estimates between instruments. Random errors, occurring during the clinical comparison of devices designed to quantify corneal back surface astigmatism, would also contribute to the variance in the data. But such random errors would mask any real differences in the estimation of corneal back surface astigmatism and prevent statistically significant differences from being detected. When the differences between the acquired results are either clinically or statistically significant different, then the reasons are most likely due to systematic differences in the way different devices operate to quantify the same parameter.
Conclusion
The actual astigmatism at the back surface of the cornea cannot yet be measured directly. This astigmatism is an estimate based on several assumptions including the topographic changes in the thickness and front surface radii of the cornea. The hypothetical analysis stemming from Equation 1 is based on several assumptions, such as both corneal surfaces are centred on a common axis, the length of the distances separating the surfaces is perpendicular to the front surface, and it is two dimensional. The analysis does not readily cater for differences in the axes of astigmatism between the corneal surfaces. Furthermore, the dimensions of R1, t, t1 and h1 must be known. None of these factors is measured directly except for h1. R1 is an indirect estimate based on the calibration procedures adopted by the manufacturer. Non-contact pachymeters measure the width of the corneal section at various locations and the final computation of t and t1 rests solely upon the algorithm employed by the manufacturer to interpret the dimensions of the corneal section. The computed t and t1 values are wavelength dependent and influenced by the refractive index value adopted by the manufacturer. It comes as no surprise that different instruments will provide different estimates of corneal back surface astigmatism when the machines estimate topographic variations in corneal thickness by different rules and algorithms. In addition, the automated systems designed to measure corneal back astigmatism locate the apex of the corneal front surface, while the subject fixates centrally as part of the operational procedure. It is assumed that the location of the 1st Purkinje image is coincident with the apex of the corneal front surface and the visual axis passes through the centre of the pupil. Thus, the true values of both angles kappa and alpha also carry the potential to impact on the estimation of corneal back surface astigmatism. The estimate of corneal back surface astigmatism is an educated guess. It would be a well-educated guess if there was closer, more clinically acceptable, agreement between the results of one instrument and another. It is incumbent on the industry to reach a compromise so that different technologies yield largely similar results. It would be useful if a universally acceptable and realistic model, a procedure, was made available allowing clinics to independently calibrate their system for estimating corneal back surface astigmatism. Such actions would help reduce uncertainty and the discrepancies between instruments designed to measure the same parameter.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tscherning MME. The optic system of the eye. In: Tscherning MME, Weiland C, editors. Physiological Optics- Dioptrics of the Eye, Functions of the Retina, Ocular Movements and Binocular Vision. Philadelphia: Keystone; 1904:27. [Google Scholar]
- 2.Gullstrand A, Southall JPC, editor. Helmholtz’s Treatise on Physiological Optics. Volumes I and II Appendix 1911. 1st ed. New York: Dover; 1962:325–351 [Google Scholar]
- 3.Savini G, Barboni P, Carbonelli M, Hoffer KJ. Repeatability of automatic measurements by a new Scheimpflug camera combined with Placido topography. J Cataract Refract Surg. 2011;37(10):1809–1816. doi: 10.1016/j.jcrs.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 4.Ham L, Dapena I, Moutsouris K, et al. Refractive change and stability after Descemet membrane endothelial keratoplasty. Effect of corneal dehydration-induced hyperopic shift on intraocular lens power calculation. J Cataract Refract Surg. 2011;37(8):1455–1464. doi: 10.1016/j.jcrs.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 5.Langenbucher A, Szentmáry N, Spira C, Seitz B, Eppig T. Corneal power after Descemet stripping automated endothelial keratoplasty (DSAEK) - Modeling and concept for calculation of intraocular lenses. Z Med Phys. 2016;26(2):120–126. doi: 10.1016/j.zemedi.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Mostafa EM. Comparison between corneal elevation maps using different reference surfaces with Scheimpflug–Placido topographer. Int Ophthalmol. 2017;37(3):553–558. doi: 10.1007/s10792-016-0291-7 [DOI] [PubMed] [Google Scholar]
- 7.de Luis Eguileor B, Arriola-Villalobos P, Pijoan Zubizarreta JI, et al. Multicentre study: reliability and repeatability of Scheimpflug system measurement in keratoconus. Br J Ophthalmol. 2021;105(1):22–26. doi: 10.1136/bjophthalmol-2019-314954 [DOI] [PubMed] [Google Scholar]
- 8.Ramos IC, Belin MW, Valbon BF, et al. Keratoconus associated with Corneal Guttata. Int J Kerat Ect Cor Dis. 2012;1(3):173–178. [Google Scholar]
- 9.Wacker K, McLaren JW, Patel SV. Directional posterior corneal profile changes in fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2015;56(10):5904–5911. doi: 10.1167/iovs.15-17311 [DOI] [PubMed] [Google Scholar]
- 10.Queirós A, Villa-Collar C, Gutiérrez ÁR, et al. Anterior and posterior corneal elevation after orthokeratology and standard and customized LASIK surgery. Eye Contact Lens. 2011;37(6):354–358. doi: 10.1097/ICL.0b013e318232e32d [DOI] [PubMed] [Google Scholar]
- 11.Cheng AC, Ho T, Lau S, Lam DS. Evaluation of the apparent change in posterior corneal power in eyes with LASIK using Orbscan II with magnification compensation. J Refract Surg. 2009;25(2):221–228. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Lopes BT, Li H, et al. Unintended changes in ocular biometric parameters during a 6-month follow-up period after FS-LASIK and SMILE. Eye Vis (Lond). 2021;8(1):9. PMID: 33741072; PMCID: PMC7977186. doi: 10.1186/s40662-021-00232-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng LS, Tsai CY, Tsai RJ, Liou SW, Ho JD. Estimation accuracy of surgically induced astigmatism on the cornea when neglecting the posterior corneal surface measurement. Acta Ophthalmol. 2011;89(5):417–422. doi: 10.1111/j.1755-3768.2009.01732.x [DOI] [PubMed] [Google Scholar]
- 14.Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080–2087. doi: 10.1016/j.jcrs.2012.08.036 [DOI] [PubMed] [Google Scholar]
- 15.Miyata K, Otani S, Honbou N, Minami K. Use of Scheimpflug corneal anterior-posterior imaging in ray-tracing intraocular lens power calculation. Acta Ophthalmol. 2013;91(7):546–549. doi: 10.1111/aos.12139 [DOI] [PubMed] [Google Scholar]
- 16.Tejedor J, Guirao A. Agreement between refractive and corneal astigmatism in pseudophakic eyes. Cornea. 2013;32(6):783–790. doi: 10.1097/ICO.0b013e31826dd44b [DOI] [PubMed] [Google Scholar]
- 17.Eom Y, Kang SY, Kim HM, Song JS. The effect of posterior corneal flat meridian and astigmatism amount on the total corneal astigmatism estimated from anterior corneal measurements. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1769–1777. doi: 10.1007/s00417-014-2737-9 [DOI] [PubMed] [Google Scholar]
- 18.Nemeth G, Berta A, Szalai E, Hassan Z, Modis L Jr. Analysis of surgically induced astigmatism on the posterior surface of the cornea. J Refract Surg. 2014;30(9):604–608. doi: 10.3928/1081597X-20140723-01 [DOI] [PubMed] [Google Scholar]
- 19.Savini G, Versaci F, Vestri G, Ducoli P, Næser K. Influence of posterior corneal astigmatism on total corneal astigmatism in eyes with moderate to high astigmatism. J Cataract Refract Surg. 2014;40(10):1645–1653. doi: 10.1016/j.jcrs.2014.01.046 [DOI] [PubMed] [Google Scholar]
- 20.Savini G, Næser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(2):827–835. doi: 10.1167/iovs.14-15903 [DOI] [PubMed] [Google Scholar]
- 21.Preussner PR, Hoffmann P, Wahl J. Impact of posterior corneal surface on toric intraocular lens (IOL) calculation. Curr Eye Res. 2015;40(8):809–814. doi: 10.3109/02713683.2014.959708 [DOI] [PubMed] [Google Scholar]
- 22.Klijn S, van der Sommen CM, Sicam VA, Reus NJ. Value of posterior keratometry in the assessment of surgically induced astigmatic change in cataract surgery. Acta Ophthalmol. 2016;94(5):494–498. [DOI] [PubMed] [Google Scholar]
- 23.Park DY, Lim DH, Hwang S, Hyun J, Chung TY. Comparison of astigmatism prediction error taken with the Pentacam measurements, Baylor nomogram, and Barrett formula for toric intraocular lens implantation. BMC Ophthalmol. 2017;17(1):156. PMID: 28836956; PMCID: PMC5571625. doi: 10.1186/s12886-017-0550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandeira F, Morral M, Elies D, et al. Transitional conic toric intraocular lens for the management of corneal astigmatism in cataract surgery. Clin Ophthalmol. 2018;12:1071–1079. PMID: 29922036. doi: 10.2147/OPTH.S148599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Ye Z, Chen W, Li J, Yan X, Li Z. Management of cataract in patients with irregular astigmatism with regular central component by phacoemulsification combined with toric intraocular lens implantation. J Ophthalmol. 2020:3520856. PMID: 32411427. doi: 10.1155/2020/3520856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitz B, Torres F, Langenbucher A, Behrens A, Suárez E. Posterior corneal curvature changes after myopic laser in situ keratomileusis. Ophthalmology. 2001;108(4):666–672. doi: 10.1016/S0161-6420(00)00581-9 [DOI] [PubMed] [Google Scholar]
- 27.Seitz B, Langenbucher A, Torres F, Behrens A, Suárez E. Changes of posterior corneal astigmatism and tilt after myopic laser in situ keratomileusis. Cornea. 2002;21(5):441–446. doi: 10.1097/00003226-200207000-00001 [DOI] [PubMed] [Google Scholar]
- 28.Du CX, Shen Y, Huang ZM, Xin SH. Characteristics and correlative factors of posterior corneal surface changes after laser in situ keratomileusis. Zhonghua Yan Ke Za Zhi. 2005;41(6):488–491. [PubMed] [Google Scholar]
- 29.Twa MD, Roberts C, Mahmoud AM, Chang JS Jr. Response of the posterior corneal surface to laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2005;31(1):61–71. doi: 10.1016/j.jcrs.2004.09.032 [DOI] [PubMed] [Google Scholar]
- 30.Ciolino JB, Khachikian SS, Cortese MJ, Belin MW. Long-term stability of the posterior cornea after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33(8):1366–1367. doi: 10.1016/j.jcrs.2007.04.016 [DOI] [PubMed] [Google Scholar]
- 31.Nishimura R, Negishi K, Saiki M, et al. No forward shifting of posterior corneal surface in eyes undergoing LASIK. Ophthalmology. 2007;114(6):1104–1110. doi: 10.1016/j.ophtha.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 32.Khairat YM, Mohamed YH, Moftah IA, Fouad NN. Evaluation of corneal changes after myopic LASIK using the Pentacam®. Clin Ophthalmol. 2013;7:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camellin M, Savini G, Hoffer KJ, Carbonelli M, Barboni P. Scheimpflug camera measurement of anterior and posterior corneal curvature in eyes with previous radial keratotomy. J Refract Surg. 2012;28(4):275–279. doi: 10.3928/1081597X-20120221-03 [DOI] [PubMed] [Google Scholar]
- 34.Meduri A, Urso M, Signorino GA, Rechichi M, Mazzotta C, Kaufman S. Cataract surgery on post radial keratotomy patients. Int J Ophthalmol. 2017;10(7):1168–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsyrenzhapova EK, Rozanova OI, Seliverstova NN. Analysis of optical properties of posterior surface of cornea in patients after anterior radial keratotomy. Acta Biomed Sci. 2019;4(4):24–29. doi: 10.29413/ABS.2019-4.4.3 [DOI] [Google Scholar]
- 36.Tutchenko L, Patel S, Voytsekhivskyy O, Skovron M, Horak O. The influence of routine uncomplicated phacoemulsification on the orthogonality of the cornea. Indian J Ophthalmol. 2021;69(5):1073–1079. doi: 10.4103/ijo.IJO_1168_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunne MC, Royston JM, Barnes DA. Normal variations of the posterior corneal surface. Acta Ophthalmol (Copenh). 1992;70(2):255–261. doi: 10.1111/j.1755-3768.1992.tb04133.x [DOI] [PubMed] [Google Scholar]
- 38.Oshika T, Tomidokoro A, Tsuji H. Regular and irregular refractive powers of the front and back surfaces of the cornea. Exp Eye Res. 1998;67(4):443–447. doi: 10.1006/exer.1998.0558 [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Huang AJ, Pflugfelder SC. Evaluation of corneal thickness and topography in normal eyes using the Orbscan corneal topography system. Br J Ophthalmol. 1999;83(7):774–778. doi: 10.1136/bjo.83.7.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rydström E, Westin O, Koskela T, Behndig A. Posterior corneal astigmatism in refractive lens exchange surgery. Acta Ophthalmol. 2016;94(3):295–300. doi: 10.1111/aos.12965 [DOI] [PubMed] [Google Scholar]
- 41.Miyake T, Shimizu K, Kamiya K. Distribution of posterior corneal astigmatism according to axis orientation of anterior corneal astigmatism. PLoS One. 2015;10(1):e0117194. PMID: 25625283; PMCID: PMC4307987. doi: 10.1371/journal.pone.0117194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui XH, Yoo YS, An Y, Joo CK. Comparison of keratometric measurements between color light-emitting diode topography and Scheimpflug camera. BMC Ophthalmol. 2019;19(1):98. PMID: 31027491; PMCID: PMC6486681. doi: 10.1186/s12886-019-1106-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Zhang J, Yin X, Li J, Cao Y, Lu P. Distribution and related factors of corneal regularity and posterior corneal astigmatism in cataract patients. Clin Ophthalmol. 2019;13:1341–1352. doi: 10.2147/OPTH.S212946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gosavi VV, Hardten DR. Developments in diagnostic tools for corneal ectasia. Expert Rev Ophthalmol. 2010;5(4):475–481. doi: 10.1586/eop.10.40 [DOI] [Google Scholar]
- 45.Ramamurthy S, Reddy JC, Jhanji V. Topography and tomography in the diagnosis of corneal ectasia. Expert Rev Ophthalmol. 2015;10(3):215–228. doi: 10.1586/17469899.2015.1044979 [DOI] [Google Scholar]
- 46.Oliveira CM, Ribeiro C, Franco S. Corneal imaging with slit-scanning and Scheimpflug imaging techniques. Clin Exp Optomol. 2011;94(1):33–42. doi: 10.1111/j.1444-0938.2010.00509.x [DOI] [PubMed] [Google Scholar]
- 47.Piñero DP. Technologies for anatomical and geometric characterization of the corneal structure and anterior segment: a review. Semin Ophthalmol. 2015;30(3):161–170. doi: 10.3109/08820538.2013.835844 [DOI] [PubMed] [Google Scholar]
- 48.Wylęgała A, Mazur R, Bolek B, Wylęgała E. Reproducibility, and repeatability of corneal topography measured by Revo NX, Galilei G6 and Casia 2 in normal eyes. PLoS One. 2020;15(4):e0230589. PMID: 32240192; PMCID: PMC7117679. doi: 10.1371/journal.pone.0230589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savini G, Carbonelli M, Sbreglia A, Barboni P, Deluigi G, Hoffer KJ. Comparison of anterior segment measurements by 3 Scheimpflug tomographers and 1 Placido corneal topographer. J Cataract Refract Surg. 2011;37(9):1679–1685. doi: 10.1016/j.jcrs.2011.03.055 [DOI] [PubMed] [Google Scholar]
- 50.Nasser CK, Singer R, Barkana Y, Zadok D, Avni I, Goldich Y. Repeatability of the Sirius imaging system and agreement with the Pentacam HR. J Refract Surg. 2012;28(7):493–497. doi: 10.3928/1081597X-20120619-01 [DOI] [PubMed] [Google Scholar]
- 51.Savini G, Næser K, Schiano-Lomoriello D, Ducoli P. Total corneal astigmatism measurements: agreement between 2 rotating Scheimpflug cameras. Cornea. 2017;36(4):463–469. doi: 10.1097/ICO.0000000000001117 [DOI] [PubMed] [Google Scholar]
- 52.Lowe RF, Clark BA. Posterior corneal curvature. Correlations in normal eyes and in eyes involved with primary angle-closure glaucoma. Br J Ophthalmol. 1973;57(7):464–470. doi: 10.1136/bjo.57.7.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camellin M. Proposed formula for the dioptric power evaluation of the posterior corneal surface. Refract Corneal Surg. 1990;6(4):261–264. doi: 10.3928/1081-597X-19900701-07 [DOI] [PubMed] [Google Scholar]
- 54.Patel S, Marshall J, Fitzke FW. Shape and radius of posterior corneal surface. Refract Corneal Surg. 1993;9(3):173–181. doi: 10.3928/1081-597X-19930501-05 [DOI] [PubMed] [Google Scholar]
- 55.Smith TW. Corneal topography. Doc Ophthalmol. 1977;43(2):249–276. doi: 10.1007/BF01569199 [DOI] [PubMed] [Google Scholar]
- 56.Royston JM, Dunne MC, Barnes DA. Measurement of posterior corneal surface toricity. Optom Vis Sci. 1990;67(10):757–763. doi: 10.1097/00006324-199010000-00002 [DOI] [PubMed] [Google Scholar]
- 57.Royston JM, Dunne MC, Barnes DA. Measurement of the posterior corneal radius using slit lamp and Purkinje image techniques. Ophthalmic Physiol Opt. 1990;10(4):385–388. doi: 10.1111/j.1475-1313.1990.tb00886.x [DOI] [PubMed] [Google Scholar]
- 58.Lam AK, Douthwaite WA. A pilot study on the measurement of central posterior corneal radius in Hong Kong Chinese using Purkinje image technique. Ophthalmic Physiol Opt. 1997;17(1):68–74. doi: 10.1111/j.1475-1313.1997.tb00526.x [DOI] [PubMed] [Google Scholar]
- 59.Lam AK, Douthwaite WA. Measurement of posterior corneal asphericity on Hong Kong Chinese: a pilot study. Ophthalmic Physiol Opt. 1997;17(4):348–356. doi: 10.1111/j.1475-1313.1997.tb00066.x [DOI] [PubMed] [Google Scholar]
- 60.Patel S. Some theoretical factors governing the accuracy of corneal thickness measurement. Ophthalmic Physiol Opt. 1981;1(3):193–203. doi: 10.1111/j.1475-1313.1981.tb00391.x [DOI] [PubMed] [Google Scholar]
- 61.Thomas GB. Calculus and Analytic Geometry Parts I & II. 1st ed. London: Addison-Wesley; 1972:824. [Google Scholar]
- 62.Mrukwa Kominek E, Sarnat-Kucharczyk M, Patel S. The impact of exposure on the magnitude of astigmatism formed within the precorneal tear film over the central optical zone of the cornea in ocular surface disease. Cont Lens Anterior Eye. 2020;43(3):261–267. doi: 10.1016/j.clae.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 63.Dubbelman M, Sicam VA, Van der Heijde GL. The shape of the anterior and posterior surface of the aging human cornea. Vision Res. 2006;46(6–7):993–1001. doi: 10.1016/j.visres.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 64.Kamiya K, Shimizu K, Yamagishi M, Igarashi A, Kobashi H. Anterior and posterior corneal astigmatism after refractive lenticule extraction for myopic astigmatism. J Ophthalmol PMID: 26097749; PMCID: PMC4443750. 2015;2015:915853. doi: 10.1155/2015/915853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi Y, Eom Y, Song JS, Kim HM. Comparison of anterior, posterior, and total corneal astigmatism measured using a single Scheimpflug camera in healthy and keratoconus Eyes. Korean J Ophthalmol. 2018;32(3):163–171. doi: 10.3341/kjo.2017.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]