Abstract
This study aims to describe the sociodemographic determinants associated with exposure to Zika Virus (ZIKV) in pregnant women during the 2015–2016 epidemic in Salvador, Brazil.
Methods
We recruited women who gave birth between October 2015 and January 2016 to a cross-sectional study at a referral maternity hospital in Salvador, Brazil. We collected information on their demographic, socioeconomic, and clinical characteristics, and evaluated their ZIKV exposure using a plaque reduction neutralization test. Logistic regression was then used to assess the relationship between these social determinants and ZIKV exposure status.
Results
We included 469 pregnant women, of whom 61% had a positive ZIKV result. Multivariate analysis found that lower education (adjusted Prevalence Rate [aPR] 1.21; 95%CI 1.04–1.35) and food insecurity (aPR 1.17; 95%CI 1.01–1.30) were positively associated with ZIKV exposure. Additionally, age was negatively associated with the infection risk (aPR 0.99; 95%CI 0.97–0.998).
Conclusion
Eve after controlling for age, differences in key social determinants, as education and food security, were associated with the risk of ZIKV infection among pregnant women in Brazil. Our findings elucidate risk factors that can be targeted by future interventions to reduce the impact of ZIKV infection in this vulnerable population.
Author summary
The Zika virus (ZIKV) epidemic in Brazil has intensified global concern about congenital defects associated with intrauterine exposure. Social determinants are factors that reinforce and contribute to the transmission and spread of ZIKV as well as other arboviruses like Dengue. We performed a cross-sectional study to describe the prevalence of ZIKV and the contribution of social determinants to transmission among pregnant women during the 2015–2016 ZIKV epidemic in Salvador, Brazil. We found that 61% of pregnant women were ZIKV seropositive. We also found that lower education level, food insecurity and lower maternal age were associated with higher ZIKV infection risk. These findings contribute to understanding the role of social determinants in ZIKV transmission, providing key social factors that can be combined with pre-existing tactics (vector control and environmental improvement) to create policies and interventions which reduce social inequalities and risk of infection in vulnerable populations like pregnant women.
Introduction
Zika virus (ZIKV) has become an emerging global public health problem [1–3] with 87 countries reporting ZIKV outbreaks in 2019 [4]. The ZIKV epidemic which occurred in Brazil between 2015 and 2016 is currently the largest of these on record. The magnitude of ZIKV presence in Brazil may be related to the extensive habitat and abundance of the Aedes aegypti mosquito, which is its main vector [5,6], as well as to the vulnerability of the local population due to their lack of prior immunity [7]. Around 80% of people infected with ZIKV are asymptomatic while the rest present symptoms such as headache, fever, arthralgia and rash that can last between a few days and a week [8]. The paradigm shifts, however, in the case of vertical transmission, and studies during the Brazilian epidemic brought to light significant maternal-child health issues associated with ZIKV infection. [9,10]. Intrauterine exposure to ZIKV can result in Congenital Zika Syndrome (CZS), which includes a range of physical and neurological impairments, such as microcephaly [9–11].
The spread of the ZIKV epidemic across Brazil was not uniform. Although the disease was registered in most Brazilian states, the epicenter of this disease was in the northeastern region, where the highest rates of congenital abnormalities were registered [12,13]. In Brazil between 2015 to 2017 were registered 2,751 cases of CZS among them 69.5% were reported in the northeast region [14]. One possible explanation for this disease prevalence heterogeneity within the country may be the role of social and environmental factors which vary across regions. [15,16]. Social inequalities such as food insecurity, unemployment and low schooling have been linked to problems with infrastructure and sanitation which may allow the spread and proliferation of these diseases [17]. This has been previously shown in the case of dengue virus (DENV) infection, another flavivirus, closely related to ZIKV, for which prior studies suggest that these variables help to explain the impact and distribution of the disease [18]. In fact, these social determinants are known to correlate with increased susceptibility to numerous diseases, many of which have been classified as neglected, meaning that they primarily affect people living in poverty around the world [19]. As a result, it is not surprising that in Brazil significant disparities have been observed in the demographic characteristics of mothers of children born with CZS, 80% of whom are young, identify as black or brown, have a low income, and live in less well-developed regions of the country [20].
It has been projected that more than half of the world’s population will live in tropical regions by 2030 [21]. Therefore it is urgently necessary to elucidate the social and environmental determinants associated with ZIKV infection, in order to improve future strategies to control ZIKV and other emerging arboviruses in these tropical regions. In particular, we should focus on identifying these risk factors in vulnerable populations, especially pregnant women, in order to construct improved prenatal care recommendations for them in the face of future emerging diseases. Furthermore, understanding the social determinants of ZIKV infection will also facilitate the targeting of other effective disease-prevention measures, like vector control, to these vulnerable and high-risk groups. In this study, we assess the sociodemographic, economic, and social factors associated with ZIKV infection, describe the prevalence and the most common clinical characteristics of this infection in women who gave birth at a referral hospital in the city of Salvador, Brazil.
Methods
Ethics statement
The project was approved by the Human Research Ethics Committee of The General Hospital Roberto Santos, through the Certificate of Presentation for Ethical Appreciation (CAAE) number 5344216.1.1001.5028 and Yale University (1603017343). All women who were eligible and participated in the study, read and signed the Free and Informed Consent Form (ICF) before the collection of clinical data and biological samples.
Study site and Socioeconomic Survey
We performed a cross-sectional study at Roberto Santos General Hospital (Hospital Geral Roberto Santos—HGRS), a referral hospital in Salvador, Brazil. Salvador (population, 2.9 million in 2020) is located in the state of Bahia in northeastern Brazil [22]. HGRS is the largest tertiary referral health center in the state, with 640 beds and an average of 1,300 hospitalizations per month. HGRS has an obstetric center that is responsible for 140 births per month, and which receives patients with high-risk pregnancies from throughout the nearby region.
In this study we recruited pregnant women who resided in Salvador, Brazil and who gave birth at the HGRS obstetric center, between October 1, 2015 and January 31, 2016. Women were enrolled during the postpartum period or during the second year of their child’s life. A structured questionnaire was used by a trained team (including physicians and nurses) to survey participants about their demographic, socioeconomic and clinical history (including symptoms related to ZIKV during pregnancy). We measured food insecurity during pregnancy using a two-item screening method developed by Hager E.R et al [23]. Participants were asked whether the following statements were true or false: 1) Within the pregnancy we worried whether our food would run out before we got money to buy more, and 2) Within the pregnancy the food we bought just did not last and we did not have money to get more. Hager et al. reported that an affirmative response to at least one of these two items showed 97% sensitivity and 83% specificity and was therefore highly effective at identifying food insecurity. We categorized participants as “at risk of food insecurity” if they answered “yes” to either of the screening questions. Additionally, participants categorized as at risk of food insecurity by the screening, completed an short 12-item questionnaire from the Brazilian Food Insecurity Scale (Escala Brasileira de Insegurança Alimentar – EBIA)[24]. All information about the pregnancy period was collected retrospectively during postpartum or their child’s second year of life. The subject’s clinical history and prenatal Brazilian monitoring program card were used to reduce memory bias.
ZIKV exposure
ZIKV exposure was determined by Plaque Reduction Neutralization Test (PRNT) [19]. PRNT was performed on serum samples collected at birth and during mother-child follow ups between 2 to 3 years after birth. The antibody titer for PRNT was defined based on a 50% reduction in inhibition of virus inoculum (PRNT50). Serum samples were considered positive when the antibody titers were ≥ 20 for ZIKV. PRNT50 assays were also performed for the four dengue serotypes (DENV 1, DENV 2, DENV 3 and DENV 4) in a subgroup of samples collected during the delivery period, in order to assess the possibility of cross reaction between the two flaviviruses. For mothers from whom blood samples were collected more than once, PRNT results were compared in order to validate the results of the assay.
Data analysis
Data collection was performed using electronic questionnaires, and responses were stored using the REDCap (Research Electronic Data Capture) data collection and data management system and saved on the Gonçalo Moniz Institute (IGM) server. Statistical analyses were performed using RStudio software (RStudio Version 1.2.5033).
We summarized the data using descriptive statistics. Categorical data were compared using Fisher’s exact test. Quantitative data were compared using the Mann-Whitney test. Agreement analysis between PRNT results at birth and during the follow up (2 to 3 years after the birth) were analyzed using Cohen’s Kappa coefficient of agreement and 95% Confidence Interval (95% CI). Additionally, Spearman’s correlation coefficients were calculated to evaluate the correlation between both PRNT results. Bivariate binomial logistic regressions were performed to assess the risk factors and the symptoms associated with infection, and those with a significance level <0.20 were included in the multivariate models. We used Prevalence Ratio (PR) to measure the associations, with a 95% CI and a p-value <0.05 was considered statistically significant.
Results
During the period of the study, from October 1st 2015 to January 31st 2016, HGRS provided delivery service to 685 pregnant women from Salvador, Brazil. We enrolled 469 (68,5%) of these women in our study, of whom 157 (33.5%) were enrolled when they gave birth and 312 (66.5%) were enrolled two years later at the time of their child’s developmental follow up appointment (Fig 1).
Fig 1. Study flowchart.
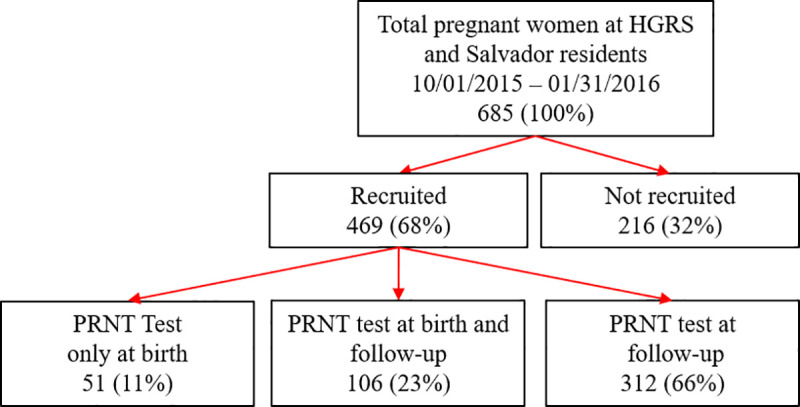
Among the 157 women with a ZIKV PRNT result at delivery, 106 (22.6%) women also had a ZIKV PRNT result during the follow up (2 to 3 years after delivery). This subsample was used to evaluate the seroconcordance and confirm the correlation between the ZIKV PRNT results at delivery and at follow up. We found a 93% concordance (74 positives and 25 negatives) between both different sample collection time points (Kappa = 0.83; 95% CI 0.77–0.98), as well as a significant positive correlation β = 0.75 (p <0.01) between quantitative PRNT values in the two periods (Fig 2). The overall evaluation revealed ZIKV neutralizing antibodies (nAb) in 287 samples, indicating a seroprevalence of 61.2%, as shown in Table 1 alongside the demographic, socioeconomic, and clinical characteristics of the interviewed women.
Fig 2. Correlation between ZIKV PRNT50 titer at birth and ZIKV PRNT50 titer at follow up between 106 women with samples at birth and samples at follow-up.
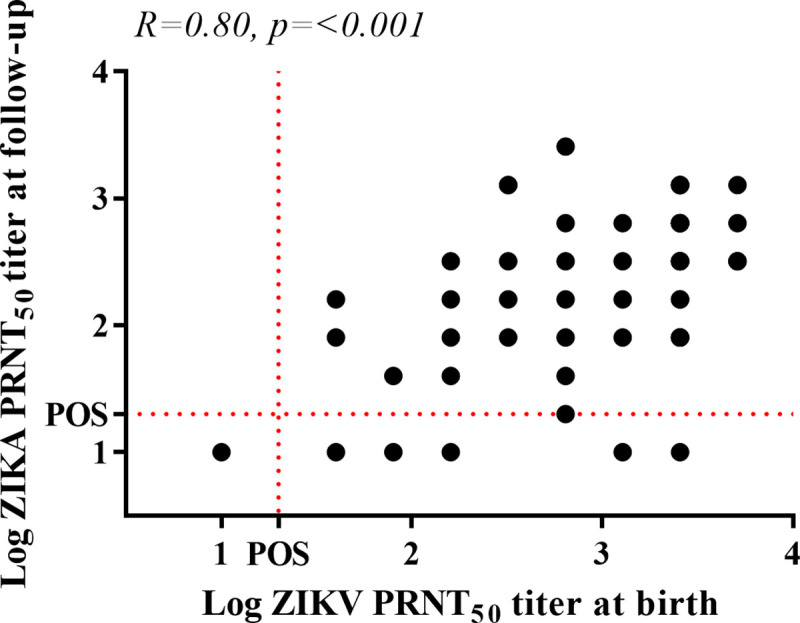
Table 1. Demographic and clinical characteristics of pregnant women in the study.
| Characteristic | No. responses | Total (N = 469)a | Zika positive (N = 287)a | Zika negative (N = 182)a | p-valueb |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 468 | 26 (20–32) | 25 (20–31) | 27 (21–33) | 0.017 |
| Ethnicity | 414 | 0.95 | |||
| White | 16 (3.9) | 10 (3.9) | 6 (3.9) | ||
| Black | 212 (51) | 134 (52) | 78 (50) | ||
| Brown | 182 (44) | 113 (44) | 69 (45) | ||
| Others | 4 (1.0) | 2 (0.8) | 2 (1.3) | ||
| Education | 417 | 0.016 | |||
| Less than 9 years (No high school) | 292 (70) | 169 (66) | 123 (77) | ||
| More than 9 years (Some high school) | 125 (30) | 88 (34) | 37 (23) | ||
| Number of residents in the house | 398 | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.59 |
| Worked before pregnancy | 414 | 194 (47) | 119 (47) | 75 (47) | >0.99 |
| Worked with a formal contract | 325 | 96 (30) | 53 (27) | 43 (33) | 0.33 |
| Dissatisfied with family income | 409 | 233 (57) | 146 (59) | 87 (54) | 0.41 |
| Income | 263 | >0.99 | |||
| below minimum wage | 27 (10) | 16 (10) | 11 (10) | ||
| above minimum wage | 236 (90) | 142 (90) | 94 (90) | ||
| Receives government financial assistance | 102 | 61 (60) | 44 (63) | 17 (53) | 0.39 |
| Food insecurity screening | |||||
| At risk of food insecurity | 409 | 247 (60) | 158 (63) | 89 (56) | 0.12 |
| Diagnoses and exposures | |||||
| Poor self-perceived health | 412 | 21 (5.1) | 16 (6.3) | 5 (3.1) | 0.17 |
| Pre-existing diseases | 417 | 92 (22) | 66 (25) | 26 (16) | 0.038 |
| Sexually transmitted disease | 411 | 27 (6.6) | 18 (7.0) | 9 (5.8) | 0.69 |
| TORCHSc | 469 | 10 (2.1) | 6 (2.1) | 4 (2.2) | >0.99 |
| Symptoms during pregnancy | |||||
| Rash | 469 | 51 (11) | 49 (17) | 2 (1.1) | <0.001 |
| Fever | 469 | 45 (9.6) | 41 (14) | 4 (2.2) | <0.001 |
| Cough | 469 | 15 (3.2) | 12 (4.2) | 3 (1.6) | 0.18 |
| Headache | 469 | 48 (10) | 44 (15) | 4 (2.2) | <0.001 |
| Arthralgia | 469 | 49 (10) | 46 (16) | 3 (1.6) | <0.001 |
| Muscle ache | 469 | 46 (9.8) | 43 (15) | 3 (1.6) | <0.001 |
| Conjunctivitis | 469 | 9 (1.9) | 9 (3.1) | 0 (0) | 0.014 |
| Alcohol use during the pregnancy | 389 | 103 (26) | 71 (29) | 32 (22) | 0.12 |
| Smoking during the pregnancy | 389 | 24 (6.2) | 17 (7.0) | 7 (4.8) | 0.51 |
aMedian (IQR); n (%)
bWelch Two Sample t-test; Fisher’s exact test
cTORCH, Toxoplasmosis, Rubella, Cytomegalovirus, Herpes infections and Others
Bold numbers indicate statistically significant differences (p <0.05) between ZIKV positive and ZIKV negative women
Most of the pregnant women were young adults with a median age of 25 years (IQR 20–31), 212 (51.2%) self-identified as black, and 125 (30.0%) had less than 9 years of schooling (ie. had not attended high school). We found that pregnant women with a positive ZIKV PRNT result were more likely to be younger [ZIKV (+) 25 years old vs ZIKV (-) 27 years old; p = 0.017] and less educated [ZIKV (+) 34.2% vs ZIKV (-) 22.5%; p = 0.016]. Among 247 (52.6%) pregnant women meeting the standard for food insecurity based on our screening, 158 (54.9%) had a positive ZIKV PRNT result and 90 (47.1%) had a negative ZIKV result, with a p-value of 0.127 (Table 1). Additionally, within the subsample of participants who completed the full food insecurity, a higher proportion of answers associated with food insecurity was shown in participants with a positive ZIKV PRNT, however this difference was not statistically significant (S1 Table). Our multivariate analysis showed that age in years (aPR 0.99; CI 0.97–0.998), schooling (aPR 1.21; CI 1.04–1 .35) and food insecurity (aPR 1.17; CI 1.01–1 .30) were all associated with increased risk of a positive ZIKV PRNT result (Table 2).
Table 2. Multivariable analysis of sociodemographic factors associated with a positive ZIKV PRNT50 result between 377 women in Brazil.
| Characteristics | PR | 95% CI | p-value | aPR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.99 | 0.98–1a | 0.017 | 0.99 | 0.97–1b | 0.023 |
| Education | 1.19 | 1.04–1.32 | 0.016 | 1.21 | 1.04–1.35 | 0.020 |
| Food insecurity risk at screening | 1.13 | 0.96–1.34 | 0.120 | 1.17 | 1.01–1.30 | 0.038 |
Prevalence Ratio (PR), Adjusted prevalence ratio (aPR)
a Upper limit of CI = 0.997
b Upper limit of CI = 0.998
We also evaluated the clinical symptoms associated with ZIKV exposure. Unsurprisingly, we found that pregnant women who tested positive for ZIKV reported a higher frequency of symptoms during pregnancy, such as rash [ZIKV (+) 17.1% vs ZIKV (-) 1.1%; p <0.01], fever [ZIKV (+) 14.3% vs ZIKV (-) 2.2%; p <0.01), headache [ZIKV (+) 15.3% vs ZIKV (-) 2.2%; p <0.01), arthralgia [ZIKV (+) 16.0% vs ZIKV (-) 1.6%; p <0.01), muscle pain [ZIKV (+) 15.0% vs ZIKV (-) 1.6%; p <0.01) and conjunctivitis [ZIKV (+) 3.1% vs 0%; p = 0.01) in relation to mothers with negative results. In the multivariate analysis, only rash (aPR 1.52; 1.26–1.62) showed an association with ZIKV infection after adjusting for the presence of arthralgia symptoms (Table 3).
Table 3. Multivariable analysis of symptoms during pregnancy in 469 women associated with a positive ZIKV PRNT50 result.
| Characteristics | PR | 95% CI | p-value | aPR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Rash | 1.68 | 1.53–1.87 | <0.001 | 1.52 | 1.26–1.62 | 0.008 |
| Arthralgia | 1.64 | 1.47–1.83 | <0.001 | 3.16 | 0.95–1.36 | 0.101 |
Prevalence Ratio (PR), Adjusted prevalence ratio (aPR)
Confidence interval (CI)
Discussion
During the 2015–2016 Brazilian ZIKV epidemic, the northeast region of the country bore the brunt of the disease burden, however for the role of social and environmental factors in geographical disparities in infection rates remains to be identified [10,25]. This regional phenomenon could also be observed in our study, in a referral obstetric center at HGRS, in Salvador, Northeastern of Brazil. We found that among 469 pregnant women recruited to our study, 61% had been infected with ZIKV. We also found that low education and food insecurity were positively associated with ZIKV exposure, while age in years was negatively associated with ZIKV antibodies.
The large proportion (61%) of pregnant women in our study with PRNT-confirmed ZIKV infections is consistent with the prevalence found in another study in Salvador, which tracked a community cohort in a low income neighborhood, and found a rate of infection of 73% [12] as well as another ZIKV serosurvey which included pregnant women that found a seroprevalence of 63.3% [10]. Emphasizing the contrast between northeastern and southern Brazil, cross sectional studies on other regions of Brazil found significantly lower seroprevalences [26].
We identified significant relationships between maternal ZIKV exposure and two socioeconomic variables: maternal schooling and household food insecurity. Those two determinants of health play a foundational role, not just in determining the risk of ZIKV infection, but in a wide range of public health challenges [27–30]. One possible explanation for the association between low education and higher vulnerability to infection is that education is associated with better knowledge and safety practices regarding infectious diseases [27,28]. This is consistent with several studies analyzing the role of education level in the knowledge, attitudes and practices related to ZIKV and DENV transmission [27–29]. In Belo Horizonte, Brazil, during a seven-year study, seven dengue epidemic waves were recorded, with regions possessing a higher prevalence of adults with low levels of education being the most heavily impacted [27]. Moreover, other studies in two cities of Brazil have found associations between low economic conditions and low educational level was associated with less use preventive practice against Zika and higher ZIKV prevalence [10,31]. But this phenomena is not restricted to regional areas, also it was register in all Brazil territory, where the most affected region was the northeast that it is a lower developed region with lower income rates and lower education levels [32].
Like education level, food insecurity is a social determinant of health which is associated with poverty and low income [33,34]. Moreover, food insecurity and subsequent malnutrition can directly promote human susceptibility to infection diseases, while infectious diseases themselves can sometimes inhibit the body’s digestive processes and exacerbate malnutrition in turn [30]. For example, there is evidence that vitamin A and D supplementation can reduce the risk of DENV infection, while in the context of ZIKV the risk of CZS-associated microcephaly is increased among individuals who fail to consume enough protein in their diets [35,36].
We found that young pregnant women were more likely to have a positive ZIKV PRNT test result. Similar results were found in a study of ZIKV in Puerto Rico, where the incidence rate decreased in older women and those most affected were pregnant women between 20–29 years old [37]. The difference that we found was small, however, with less than a 2-year difference in median age between positive and negative participants. The relatively low contribution of age could be due to the limited age range allowed for by our exlusive enrollment of pregnant women, although previous studies have also failed to identify large differences in ZIKV risk between age groups [10].
We found that ZIKV positive participants reported fever (14%), rash (17%) and other nonspecific symptoms at a higher rate during pregnancy than ZIKV negative participants. This is similar to previous studies which reported that around 80% of ZIKV infected individuals were asymptomatic and that among those who reported symptoms, the principal complaints were rash, fever, arthralgia, myalgia, fatigue, headache, and conjunctivitis [1,38]. Furthermore, in the multivariate analysis, we found that women who reported rash during their pregnancy were 68% more likely to have a positive ZIKV PRNT result. Again, this is consistent with prior literature. For example, among symptomatic cases during a ZIKV outbreak on Yap Island, rash was the most reported complaint (90% of cases), alongside arthritis and arthralgia (65% of cases) [1].
Our study has some important limitations. As a cross-sectional study, it is difficult to establish causality, and further prospective studies will be required in the future. Additionally, we collected data about the pregnancy retrospectively, which creates a risk of memory bias. We took careful steps to reduce this memory bias, and interviews were performed by trained health professionals who used the subject’s medical record and prenatal Brazilian monitoring program card to reduce bias. Another study limitation was that we did not collect blood samples for all participants at the same time. That said, we found a high concordance (93%) of PRNT results between the samples from the delivery period and those performed at follow up (2 to 3 years later). This finding is important in order to assess the exposure to ZIKV after the outbreak, a finding which may be a valuable tool for epidemiological surveillance actions and which may help to provide guidance for women in childbearing age about ongoing risk of exposure to the virus. Finally, PRNT results for the four DENV serotypes were available only for 23% of the recruited mothers. Still, when we compared DENV infection rates, between our ZIKV positive and negative subjects, we did not find any statistically significant differences (S2 Table), suggesting that environmental exposure to disease-carrying arthropod vectors may not have varied substantially between groups.
Social determinants of health are factors associated with an increased risk of contracting a wide range of diseases [39]. ZIKV is a worldwide public health issue, however, as in the case of dengue and other mosquito-borne infections, the risk of ZIKV infection can be linked to several primary sociodemographic determinants. We identified three key factors: low education, food insecurity, and younger age which are associated with the risk of ZIKV infection in a vulnerable population located in an undeveloped region. We believe that these findings may facilitate prediction of areas and individuals who may be at a particularly elevated risk of infection. Furthermore, we hope that these findings can be combined with pre-existing knowledge about environmental factors such as mosquito prevalence, which are also major risk factors for infection with arthropod-borne diseases like ZIKV and DENV to create public health campaigns that focus on reducing social inequalities in addition to enacting vector control and other basic environmental interventions.
Supporting information
(XLS)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank to the mothers and children who participated in this study and the support of health workers at HGRS, in Salvador, Brazil. We would also like to thank the team members from the Collective Health institute at the Oswaldo Cruz Foundation and the team from the School of Public Health at Yale University.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by grants from the National Institutes of Health (NIAID R01 AI052473 (AK), R25 U01AI088752 (AK), FIC R01 TW009504 (AK), R25 TW009338 (AK), F31 AI114245 (AK), R01 AI121207 (AK) (www.niaid.nih.gov/), Wellcome Trust (102330/Z/13/Z (FC); 218987/Z/19/Z (FC) (www.wellcome.ac.uk/), Bahia State Research Support Foundation - FAPESB ZIKA-FAPESB T.O. n° PET0021/2016 (MGR) (www.fapesb.ba.gov.br), Coordination for the Improvement of Higher Education – CAPES, from Brazil-Finance Code 001 (scholarship to JPAT), (www.capes.gov.br/), the São Paulo Research Foundation – FAPESP (LCSF) (www.fapesp.br), under Project 2016/20045-7 and the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 734584 (DBA) (www.ec.europa.eu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Duffy MR, Chen T-H, Thane Hancock W, Powers AM, Kool JL, Lanciotti RS, et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine. 2009. pp. 2536–2543. doi: 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 2.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19. Available: https://www.ncbi.nlm.nih.gov/pubmed/24721538 [PubMed] [Google Scholar]
- 3.Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. The Lancet. 2017. pp. 2099–2109. doi: 10.1016/S0140-6736(17)31450-2 [DOI] [PubMed] [Google Scholar]
- 4.Countries and territories with current or previous Zika virus transmission. [cited 28 Oct 2020]. Available: https://www.who.int/emergencies/diseases/zika/countries-with-zika-and-vectors-table.pdf
- 5.Brito C. Zika Virus: A New Chapter in the History of Medicine. Acta Med Port. 2015;28: 679–680. doi: 10.20344/amp.7341 [DOI] [PubMed] [Google Scholar]
- 6.Musso D, Ko AI, Baud D. Zika Virus Infection—After the Pandemic. New England Journal of Medicine. 2019. pp. 1444–1457. doi: 10.1056/NEJMra1808246 [DOI] [PubMed] [Google Scholar]
- 7.Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, et al. Rapid Spread of Zika Virus in The Americas—Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis. 2016;44: 11–15. doi: 10.1016/j.ijid.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Moghadas SM, Shoukat A, Espindola AL, Pereira RS, Abdirizak F, Laskowski M, et al. Asymptomatic Transmission and the Dynamics of Zika Infection. Sci Rep. 2017;7: 5829. doi: 10.1038/s41598-017-05013-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47: 6–7. doi: 10.1002/uog.15831 [DOI] [PubMed] [Google Scholar]
- 10.Netto EM, Moreira-Soto A, Pedroso C, Höser C, Funk S, Kucharski AJ, et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. MBio. 2017;8. doi: 10.1128/mBio.01390-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar Ticona JP, Nery N Jr, Ladines-Lim JB, Gambrah C, Sacramento G, de Paula Freitas B, et al. Developmental outcomes in children exposed to Zika virus in utero from a Brazilian urban slum cohort study. PLoS Negl Trop Dis. 2021;15: e0009162. doi: 10.1371/journal.pntd.0009162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Barraquer I, Costa F, Nascimento EJM, Nery N, Castanha PMS, Sacramento GA, et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science. 2019. pp. 607–610. doi: 10.1126/science.aav6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady OJ, Osgood-Zimmerman A, Kassebaum NJ, Ray SE, de Araújo VEM, da Nóbrega AA, et al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: An observational analysis of over 4 million births. PLOS Medicine. 2019. p. e1002755. doi: 10.1371/journal.pmed.1002755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro MC, Han QC, Carvalho LR, Victora CG, França GVA. Implications of Zika virus and congenital Zika syndrome for the number of live births in Brazil. Proc Natl Acad Sci U S A. 2018;115: 6177–6182. doi: 10.1073/pnas.1718476115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali S, Gugliemini O, Harber S, Harrison A, Houle L, Ivory J, et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl Trop Dis. 2017;11: e0005135. doi: 10.1371/journal.pntd.0005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler D. Brazil asks whether Zika acts alone to cause birth defects. Nature. 2016;535: 475–476. doi: 10.1038/nature.2016.20309 [DOI] [PubMed] [Google Scholar]
- 17.Oliveira KK de F, de França Oliveira KK, Caprara A. Face social do controle do Aedes: em um bairro periférico de Fortaleza, Brasil, as mulheres tomam a palavra. Ciência & Saúde Coletiva. 2019. pp. 2983–2992. doi: 10.1590/1413-81232018248.21522017 [DOI] [PubMed] [Google Scholar]
- 18.McMichael AJ. Environmental and social influences on emerging infectious diseases: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2004;359: 1049–1058. doi: 10.1098/rstb.2004.1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotez PJ, Murray KO, Buekens P. The Gulf Coast: a new American underbelly of tropical diseases and poverty. PLoS Negl Trop Dis. 2014;8: e2760. doi: 10.1371/journal.pntd.0002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diniz D, Gumieri S, Bevilacqua BG, Cook RJ, Dickens BM. Zika virus infection in Brazil and human rights obligations. Int J Gynaecol Obstet. 2017;136: 105–110. doi: 10.1002/ijgo.12018 [DOI] [PubMed] [Google Scholar]
- 21.Messina JP, Brady OJ, Pigott DM, Golding N, Kraemer MUG, Scott TW, et al. The many projected futures of dengue. Nat Rev Microbiol. 2015;13: 230–239. doi: 10.1038/nrmicro3430 [DOI] [PubMed] [Google Scholar]
- 22.IBGE. Estimativas da população residente para os municípios e para as unidades da federação com data de referência em 1° de julho de 2019. IBGE. [cited 8 Feb 2021]. Available: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=2101662
- 23.Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126: e26–32. doi: 10.1542/peds.2009-3146 [DOI] [PubMed] [Google Scholar]
- 24.Aliaga MA, Ribeiro MS, Santos SMC dos, Trad LAB. Participatory food and nutrition security assessment in a community of Salvador, Brazil. Ciênc saúde coletiva. 2020;25: 2595–2604. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Musa SS, Fu H, He D, Qin J. Simple framework for real-time forecast in a data-limited situation: the Zika virus (ZIKV) outbreaks in Brazil from 2015 to 2016 as an example. Parasit Vectors. 2019;12: 1–13. doi: 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocelin HJS, Catão RC, Freitas PSS, Prado TN, Bertolde AI, Castro MC, et al. Analysis of the spatial distribution of cases of Zika virus infection and congenital Zika virus syndrome in a state in the southeastern region of Brazil: Sociodemographic factors and implications for public health. Int J Gynaecol Obstet. 2020;148 Suppl 2: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Mattos Almeida MC, Caiaffa WT, Assunção RM, Proietti FA. Spatial vulnerability to dengue in a Brazilian urban area during a 7-year surveillance. J Urban Health. 2007;84: 334–345. doi: 10.1007/s11524-006-9154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Quijano FA, Martínez-Vega RA, Rodriguez-Morales AJ, Rojas-Calero RA, Luna-González ML, Díaz-Quijano RG. Association between the level of education and knowledge, attitudes and practices regarding dengue in the Caribbean region of Colombia. BMC Public Health. 2018;18: 1–10. doi: 10.1186/s12889-018-5055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maharajan MK, Rajiah K, Belotindos J-AS, Basa MS. Social Determinants Predicting the Knowledge, Attitudes, and Practices of Women Toward Zika Virus Infection. Front Public Health. 2020;8: 170. doi: 10.3389/fpubh.2020.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farhadi S, Ovchinnikov RS. The relationship between nutrition and infectious diseases: A review. Biomedical and Biotechnology Research Journal (BBRJ). 2018;2: 168. [Google Scholar]
- 31.Melo VAD, Silva JRS, Corte RL. Personal protective measures of pregnant women against Zika virus infection. Rev Saude Publica. 2019;53: 72. doi: 10.11606/s1518-8787.2019053001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IBGE. Pesquisa Nacional por Amostra de Domicílios Contínua. [cited 29 May 2021]. Available: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101736_informativo.pdf
- 33.Compton MT. Food Insecurity as a Social Determinant of Mental Health. Psychiatr Ann. 2014;44: 46–51. [Google Scholar]
- 34.Ivers LC, Cullen KA. Food insecurity: special considerations for women. Am J Clin Nutr. 2011;94: 1740S–1744S. doi: 10.3945/ajcn.111.012617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weger-Lucarelli J, Auerswald H, Vignuzzi M, Dussart P, Karlsson EA. Taking a bite out of nutrition and arbovirus infection. PLoS Negl Trop Dis. 2018;12: e0006247. doi: 10.1371/journal.pntd.0006247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbeito-Andrés J, Pezzuto P, Higa LM, Dias AA, Vasconcelos JM, Santos TMP, et al. Congenital Zika syndrome is associated with maternal protein malnutrition. Sci Adv. 2020;6: eaaw6284. doi: 10.1126/sciadv.aaw6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozier M, Adams L, Febo MF, Torres-Aponte J, Bello-Pagan M, Ryff KR, et al. Incidence of Zika Virus Disease by Age and Sex—Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep. 2016;65: 1219–1223. doi: 10.15585/mmwr.mm6544a4 [DOI] [PubMed] [Google Scholar]
- 38.Anna R. Plourde EMB. A Literature Review of Zika Virus. Emerg Infect Dis. 2016;22: 1185. doi: 10.3201/eid2207.151990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuti M, Cunha GM, Paploski IAD, Kasper AM, Silva MMO, Tavares AS, et al. Spatial Distribution of Dengue in a Brazilian Urban Slum Setting: Role of Socioeconomic Gradient in Disease Risk. PLOS Neglected Tropical Diseases. 2015. p. e0003937. doi: 10.1371/journal.pntd.0003937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


