Abstract
An uncontrolled CD4+ T cell response is a critical hallmark of autoimmune diseases. IL-10, which can be produced by both effector and regulatory CD4+ T cells, plays an essential role in the inhibition of autoimmunity. MicroRNAs (miRNAs) are key molecules involved in regulating immune responses. However, how miR-10a regulates CD4+ T cell function in the pathogenesis of intestinal immune responses is not fully understood. Here, we show that the mice with deficient miR-10a in CD4+ T cells were more resistant to intestinal inflammation upon inflammatory insult. miR-10a-deficient CD4+ CD45Rbhi T cells were less colitogenic in Rag−/− mice, in which CD4+ T cell production of IL-10 was increased. miR-10a-deficient CD4+ T cells expressed a higher expression of IL-10 in vitro. Blocking the IL-10/IL-10R pathway in vivo aggravated colitis induced by miR-10a-deficient CD4+ CD45Rbhi T cells. Mechanically, miR-10a suppressed CD4+ T cell production of IL-10 through targeting Prdm1, which encodes Blimp1. We further show that that CD4+ T cells lacking Blimp1 produced lower levels of IL-10 and induced more severe colitis in Rag−/− mice. These data thus establish the role of miR-10a in the inhibition of IL-10 production in CD4+ T cells to regulate intestinal homeostasis.
Introduction
As key mediators in regulating immune responses, CD4+ T cells are involved in the pathogenesis of autoimmune diseases (1, 2). There are two major functional subtypes of CD4+ T cells, regulatory T cells (Treg) and effector T cells, mainly T helper (Th)1, Th2, and Th17 cells. While aberrant effector CD4+ T cell responses lead to immunopathology, Treg cells suppress excessive immune responses to maintain immune homeostasis, which is primarily attributed to TGF-β and IL-10 production (3, 4). Interestingly, effector CD4+ T cells can also produce IL-10, which forms a negative loop restricting effector T cell responses (5), thereby suppressing autoimmune diseases (6, 7). CD4+ T cell-specific IL-10 conditional knockout mice, despite having an intact IL-10 gene in other cell types, develop spontaneous colitis closely resembling the phenotype in complete IL-10 deficient mice, indicating a crucial role for T cell production of IL-10 in inhibiting colitis development (8). Several transcription factors have been shown to be associated with IL-10 production in T cells, including Gata3, c-Maf, Irf1, Irf4, and Blimp-1 (9–12). However, how CD4+ T cell production of IL-10 is negatively regulated remains incompletely defined.
MicroRNAs (miRNAs) are small non-coding RNA molecules, which affect a wide range of genes through post-transcriptional regulation. Thirty to eighty percent of human genes are predicted to be regulated by miRNAs (13), indicating an indispensable role of miRNAs in human health and disease (14–16). miR-10a has been reported to regulate several functions in a variety of cells (17–19), including CD4+ T cells (20–22). Retinoic acid and TGF-β induce miR-10a in CD4+ T cells (21), and miR-10a is highly expressed in Treg cells (20, 21). miR-10a restricts the conversion of Treg cells into follicular helper T cells by targeting Bcl6 and Nocr2, indicating that miR-10a promotes Treg stability (21). However, it has also been reported that miR-10 compromises the suppressive activity of Treg cells (22). Moreover, miR-10a inhibits Th17 cell differentiation in the presence of retinoic acid (21). We previously showed that miR-10a regulates IL-12/IL-23p40 production in dendritic cells, which affects T cell differentiation (23, 24). However, the effect of miR-10a on effector CD4+ T cells, including IL-10 production, is still not completely understood. More importantly, it is still unclear whether miR-10a in CD4+ T cells regulates intestinal homeostasis and the pathogenesis of colitis.
In this study, we showed, by using two animal models of colitis, that miR-10a-deficient CD4+ T cells are less pathogenic in the induction of colitis, which is at least partially mediated by the upregulation of IL-10 production. Moreover, miR-10a-deficient CD4+ T cells produce higher levels of IL-10. Mechanically, miR-10a directly suppresses Prdm1, which encodes Blimp1. Higher expression of Prdm1 contributes to higher levels of IL-10 production in miR-10a-deficient CD4+ T cells. Additionally, Blimp1-deficient CD4+ T cells produce lower levels of IL-10 and induce more severe colitis in Rag−/− mice.
Material and Methods
Mice
B6. Cg-Tg(Cd4-cre)1Cwi/BfluJ (Cd4cre) mice, B6. 129S7-Rag1 tm1Mom /J (Rag−/−) mice, and B6.129-Prdm1 tm1clme/J (Prdm1fl/fl) mice were purchased from The Jackson Laboratory. Cd4cremiR-10fl/fl and Cd4cremiR-10fl/+ mice were generated by crossing Cd4cre mice with miR-10afl/fl mice, and Cd4crePrdm1fl/fl and Cd4crePrdm1fl/+ mice were generated by crossing Cd4cre mice with Prdm1fl/fl mice. miR-10afl/fl mice were generated at InGenious Targeting Laboratory, Inc, NY, US. miR10a targeting region was cloned from a mouse BAC clone RP23–65E5 (chr11, 96103070~96331948), and miR10a targeting vector, which contained the LoxP-Frt- Neomycin-LoxP-Frt cassette, was obtained through recombination of the miR10a targeting region into the backbone vector (Promega). miR10a targeting vector was linearized by Not I enzyme and then electroporated in C57BL/6 embryonic stem cells. ES cells were selected with the criteria of the presence of distal LoxP and Neomycin cassette. Genotyping of miR10afl/fl mice was performed using PCR. All the mice used in this study are cohoused, sex- and gender-matched littermates. All mice were maintained on a 12 hour-light/dark cycle in the specific pathogen free animal facilities of UTMB. All the mouse experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. All the mice used are littermates and cohoused.
DSS-induced Colitis Model
Cd4cremiR-10fl/+ or Cd4cremiR-10fl/fl littermates were administered 1.65% DSS (w/v) in drinking water for 7 days and changed to regular drinking water for additional 3 days.
CD4+ CD45Rbhi T Cell Transfer Model
Splenic CD4+ CD45Rbhi cells (1 × 105/ mouse) from different mouse strains were intravenously (i. v.) transferred to Rag−/− mice. Mice were sacrificed based on weight change (around 85% original weight).
For blocking the IL-10/IL-10R pathway in vivo, Rag−/− recipients were administered anti-IL-10R antibody (25 mg/kg, PRID: AB_1107694, Bio X cell) intraperitoneally (i.p.) every other day, and other groups of mice were treated with anti-IgG antibody as control.
RAW 264.7 cell line
Mouse RAW 264.7 cells (male) were cultured in compete DMEM media containing penicillin-streptomycin and 10% FBS. For transfection, cells were cultured in Opti-MEM™ I Reduced Serum Medium. Cells were cultured in a humidified incubator with 5% CO2 at 370°C.
Primary Mouse CD4+ T Cell Cultures
Plates were coated with 5 μg/ml anti-mouse CD3 (PRID: AB_1107634; Bio X cell) for 4 hours at 37°C. Splenic CD4+ T cells were then seeded in the coated plates at a concentration of 2 × 105/ml in the presence of 2 μg/ml anti-mouse CD28 (PRID: AB_1107624, Bio X cell) in complete RPMI 1640 media containing penicillin-streptomycin, L-glutamine, sodium pyruvate, β-mercaptoethanol, and 10% FBS. Half of the media was replaced on day 3. Cells were harvested on day 2 for gene analysis, on day 3 for protein analysis measured by ELISA, and on day 5 for protein analysis measured by flow cytometry. Cells were cultured in a humidified incubator with 5% CO2 at 37°C.
Mouse CD4+ T cell isolation, activation, and differentiation
After blood cell lysis, splenic cells were incubated with anti-mouse CD4 magnetic particles (250 μl/ mouse) for 30 min at 4°C. CD4+ T cells were then purified through the Cell Separation Magnet. CD4+ T cells were activated with anti-mouse CD3 (5 μg/ml) anti-mouse CD28 (2 μg/ml) under neutral (without additional cytokines and antibodies), Th1 (10 ng/ml recombinant mouse IL-12, Biolegend), Th17 (2 ng/ml recombinant human TGF-β, Biolegend; 50 ng/ml recombinant mouse IL-6, Biolegend; 10 μg/ml anti-mouse IFN-γ, Bio X cell; and 5 μg/ml anti-mouse IL-4, Bio X cell), or Treg (5 ng/ml recombinant human TGF-β, Biolegend; and 10 μg/ml anti-mouse IFN-γ, Bio X cell) conditions.
Mouse CD8+ T cell isolation
After blood cell lysis, splenic cells were incubated with anti-mouse CD8 magnetic particles (250 μl/spleen) for 30 min at 4℃. CD8+ T cells were then purified through the Cell Separation Magnet.
Enzyme-linked immunosorbent assay (ELISA)
After centrifuging at 350 × g for 8 minutes, cell or colonic tissue culture supernatants were collected and stored at −80°C. Cytokine levels in supernatants were measured using commercial ELISA MAX™ Deluxe Sets (TGF-β, IL-6, and IL-10, Biolegend). According to the manufacturer’s instruction, capture antibodies were coated in 96-well plates overnight at 4°C. After blocking with PBS containing 1% BSA, supernatants were added in duplicate for 2 hours, followed by incubation of detection antibodies for another 1 hour at room temperature. Finally, streptavidin conjugated with horseradish peroxidase was incubated for 1 hour, and the substrate was then incubated for 30 minutes at room temperature.
Quantitative real-time PCR (qRT-PCR)
After washing with PBS, cells were lysed in Trizol. Total RNA was then extracted and purified by chloroform and isopropanol. Complementary DNA was synthesized using the Quanta bio qScripttm cDNA Synthesis Kit, and qRT-PCR was then performed using iTaq Universal SYBR@ Green Supermix. Primers: Il10 (forword): AGCCGGGAAGACAATAACTG; Il10 (reverse): GGAGTCGGTTAGCAGTATGTTG; Prdm1 (forward): CTCAACACTCTCATGTAAGAGGC; Prdm1 (reverse): AGCATGACCTGACATTGACACC. For measuring the miR-10a expression, RNA was reverse transcribed using TaqMan@ MicroRNA Reverse Transcription Kit. qRT-PCR was then performed using SsoAdvanced™ Universal Probe Supermix. Aliquots of PCR products were visualized by electrophoresis on 2 % agarose gels. miR-10a primers for reverse transcription (Part number #4427975) and PCR (Part number #4440887) were predesigned and purchased from ThermoFisher.
Isolation of Intestinal Lamina Propria Cells
Colons were cut into 1-cm pieces, put into 50-ml centrifuge tubes, washed with cold PBS, and then shaken at 300 RPM in PBS containing 0.5 mM EDTA, 10 mM HEPES, and 2% FBS for 40 minutes at 37°C. After washing with cold PBS, tissues were then transferred into gentleMACS™ tubes containing RPMI 1640 media containing 0.5 mg/ml Collagenase IV, 2 μg/ml DNAse, and 2% FBS, and incubated under the program “37_m_LPDK_1” on gentleMACS™ Dissociator. The cell suspension was then filtered through a 100-μm cell strainer, and lamina propria cells were purified by density gradient centrifuge using 40%/ 70% Percoll.
For purifying the intestinal lamina propria CD4+ T cells, we incubated the intestinal lamina propria cells with anti-mouse CD4 magnetic particles (150 μl/spleen) for 30 minutes at 4°C. CD4+ T cells were then purified through the Cell Separation Magnet.
Flow cytometry
Cells were stimulated with 50 ng/ml Phorbol-12-myristate 13-acetate (Sigma Aldrich) and 750 ng/ml ionomycin (ThermoFisher) for 2 hours, and then treated with 0.7 μl/ml Brefeldin A (eBiosicence) for another 3 hours. After blocking with anti-mouse CD16/32 (Biolegend), cells were stained for dead cells using Live/dead Fixable Near-IR Dead Cell Stain kit (ThermoFisher) and for surface markers with BV421 anti-mouse CD4 (Biolegend). Cells were then fixed and permeabilized using the Foxp3/Transcription Factor Fixation/Permeabilization set (ThermoFisher), followed by intracellular staining with PE anti-mouse IL-10 (Biolegend), FITC anti-mouse IFN-γ (Biolegend), PE/cyanine7 anti-mouse IL-17 (Biolegend), and APC anti-mouse Foxp3 antibodies (ThermoFisher). Events were collected on a BD LSR II/Fortessa™ and analyzed by FlowJo.
CD4+ CD45Rbhi T Cell Sorting
Mouse splenic CD4+ T cells were isolated using anti-mouse magnetic particles, washed with cold PBS containing 2% FBS, and stained with APC anti-mouse CD4 antibody (Biolegend) and PE anti-mouse CD45Rb antibody (Biolegend) for 30 minutes at 4°C. Cells were then sorted by BD FACSAria™ III and washed once with RPIM 1640 media before transfer.
Western blot
Total protein was extracted from CD4+ T cells using RIPA lysis buffer containing protease and phosphatase inhibitor cocktail and phenylmethylsulfonyl fluoride. 25 μg cellular protein/sample was loaded into TGX gels for electrophoresis, followed by transferring the protein into polyvinylidene difluoride membranes. After blocking with 0.1% Tween Casein Blocking Buffer, membranes were incubated with the primary antibody (1:500, anti-Blimp1 (6D3), Santa Cruz) overnight at 4°C. After washing, the membranes were incubated with the secondary antibody (1:2000) for 1 hour at room temperature. Blots were detected using the ChemiDoc™ Imaging System. After stripping with the stripping buffer, the membranes were re-blocked and incubated with another primary antibody (1:2000, anti-βactin (D6A8), Cell Signaling Technology), followed by incubation with secondary antibody (1:2000). The blots were also detected using the ChemiDoc™ Imaging System.
Plasmid Transfection and Dual Luciferase Assay
RAW 264.7 cells (2 × 105/ml) were plated overnight and changed to Opti-MEM™ Medium. Psi-CHECK-2 plasmid containing 3’UTR of WT-prdm1 or Mut-prdm1 was transfected according to the manufacturer’s instruction. Plasmid construction: WT-prdm1 (forward): TCGAGTCGACAATCAGGGTGCCGC; WT-prdm1 (reverse): GGCCGCGGCACCCTGATTGTCGAC; Mut-prdm1 (forward): TCGAGTCGACAATTAAGATGCCGC; Mut-prdm1 (reverse): GGCCGCGGCATCTTAATTGTCGAC.
Nucleofection
CD4+ T cells were activated with anti-mouse CD3 (5 μg/ml) anti-mouse CD28 (2 μg/ml) for 24 hours. Cells were collected and resuspended in P3 Primary Cell 4D-NucleofectorTM X Solution containing 100 nM Prdm1 siRNA (ON-TARGETplustm Prdm1 siRNA, Dharmacon) or Control siRNA (ON-TARGETplustm Control siRNA, Dharmacon) and transferred to Nucleocuvette™ Vessels (3 × 105 cells/vessel). Vessels were placed into the retainer of the 4D-Nucelfector™ X Unit, and cells were nucleofected under the program of DN-100. Cells were then cultured for another 2 days for analysis of il10 expression.
Material Availability
This study did not generate new unique reagents, and miR-10afl/fl mice generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Statistics
In this study, all data were analyzed using GraphPad Prism 8. The differences between groups were considered statistically significant when p < 0.05 by two-way Student’s t test or Mann–Whitney U test or one-way ANOVA test as indicated in the figure legends. All data are presented as mean ± SD.
Results
miR-10a in CD4+ T cells does not affect intestinal homeostasis under steady conditions.
First, we determined if miR-10a is efficiently knocked out in CD4+ T cells from CD4+ T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice. We found that miR-10a was undetectable in CD4+ T cells from Cd4cremiR-10fl/fl mice (Supplementary Figure 1A). Additionally, although CD8+ T cells expressed a relatively lower level of miR-10a compared with CD4+ T cells, the expression of miR-10a was similar in CD8+ T cells from wild type (WT) Cd4cremiR-10afl/+ mice and CD4+ T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice (Supplementary Figure 1B). To investigate whether miR-10a expression in CD4+ T cells regulates intestinal homeostasis under steady conditions, we sacrificed 10-week age-matched WT Cd4cremiR-10afl/+ mice and CD4 T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice and found there was no intestinal inflammation in both WT and CD4 T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice (Figure 1A). Additionally, CD4+ T cell phenotypes in intestinal lamina propria were determined. As shown in Figure 1B–C, there were no differences in intestinal IFN-γ+ Th1, IL-17+ Th17, Foxp3+ Treg, and IL-10+ CD4+ T cell. Segmented filamentous bacteria, which induces Th17 in the intestine (25), is low in our animal facility, which contributes to the low expression of intestinal Th17 in mice. Taken together, these data suggest that miR-10a in CD4+ T cells does not affect intestinal homeostasis under steady conditions.
Figure 1. Deficiency of miR-10a in CD4+ T cells does not affect intestinal CD4+ T cell differentiation in vivo under steady conditions.
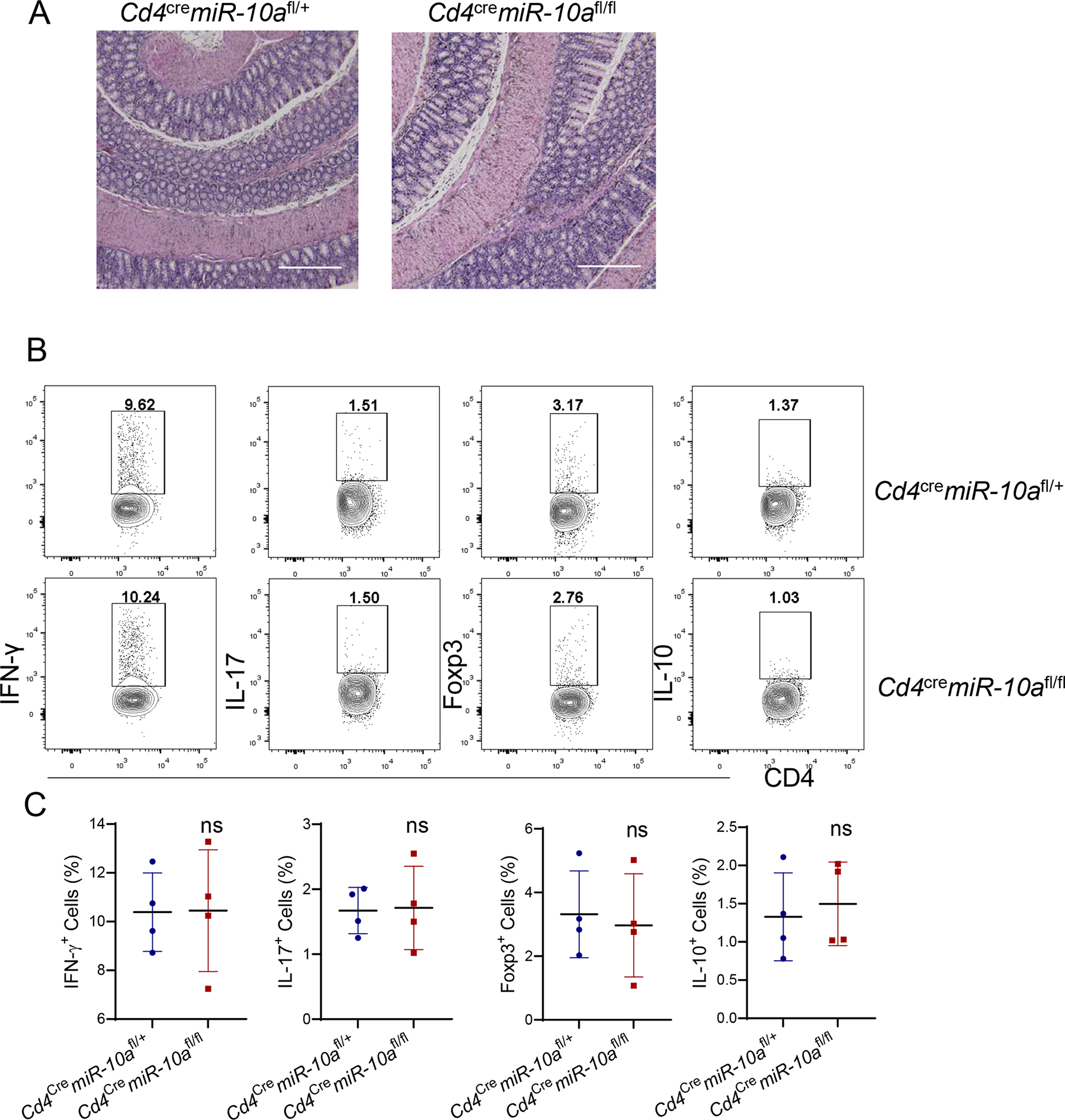
10-week age-matched wild type (WT) Cd4cremiR-10afl/+ and CD4 T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice were sacrificed. (A) Representative H&E staining of colon was shown. Scale bars, 300 μm. (B-C) Flow cytometry profile (B) and Quantification (C) of IFN-γ +, IL-17+, Foxp3+, and IL-10+ CD4+ T cells in intestinal lamina propria were shown. All data are presented as mean ± SD. One representative of two independent experiments. Unpaired Student’s t test; ns, not significant.
miR-10a-deficient CD4+ T cells are less pathogenic in the induction of colitis.
To investigate whether miR-10a expression in CD4+ T cells regulates intestinal inflammation, the DSS-induced colitis model, an acute chemical-induced colitis model (26), was conducted in wild type (WT) Cd4cremiR-10afl/+ mice and CD4 T cell-specific miR-10a deficient Cd4cremiR-10afl/fl mice. Mice were administered DSS in their drinking water for 7 days, followed by regular drinking water for additional 3 days. Weight changes were monitored every day. We found that WT Cd4cremiR-10afl/fl mice showed less weight loss from day 8 compared to miR-10a deficient Cd4cremiR-10afl/+ mice (Supplementary Figure 2A). Consistent with the weight loss, miR-10a deficient Cd4cremiR-10afl/fl mice developed less severe colitis, evidenced by lower pathological scores (Supplementary Figures 2B–C) and less production of pro-inflammatory cytokines, including TNF-α and IL-6, in colonic tissues (Supplementary Figures 2D–E), indicating that CD4+ T cell expression of miR-10a regulates the pathogenesis of colitis.
To further determine the role of CD4+ T cell expression of miR-10a in the induction of colitis, the CD4+ CD45Rbhi T cell transfer model, a T cell-mediated chronic colitis model (27), was used. CD4+ CD45Rbhi T cells from WT Cd4cremiR-10afl/+ mice or miR-10a deficient Cd4cremiR-10afl/fl mice were transferred to Rag−/− mice and mouse weight changes were monitored once a week. The Rag−/− recipients of miR-10-deficient CD4+ CD45Rbhi T cells showed less weight loss than the mice that received WT CD4+ CD45Rbhi T cells (Figure 2A). miR-10-deficient CD4+ CD45Rbhi T cells induced less severe colitis than WT CD4+ CD45Rbhi T cells (Figure 2B–C). Moreover, lower levels of TNF-α and IL-6 were found in colonic tissues of Rag−/− mice reconstituted with miR-10-deficient CD4+ CD45Rbhi T cells compared with Rag−/− mice that received WT CD4+ CD45Rbhi T cells (Figure 2D–E). Furthermore, the percentages of intestinal IFN-γ+ CD4+ T cells were lower, but IL-10+ CD4+ T cells were higher in Rag−/− mice that received miR-10-deficient CD4+ CD45Rbhi T cells compared with those in Rag−/− mice reconstituted with WT CD4+ CD45Rbhi T cells. There were no differences in IL-17+ CD4+ T cells and Foxp3+ CD4+ T cells (Figure 2F–G). Collectively, these data indicate that miR-10-deficient CD4+ T cells produce more IL-10 and are less colitogenic.
Figure 2. miR-10a-deficient CD4+ T cells induce less severe colitis.
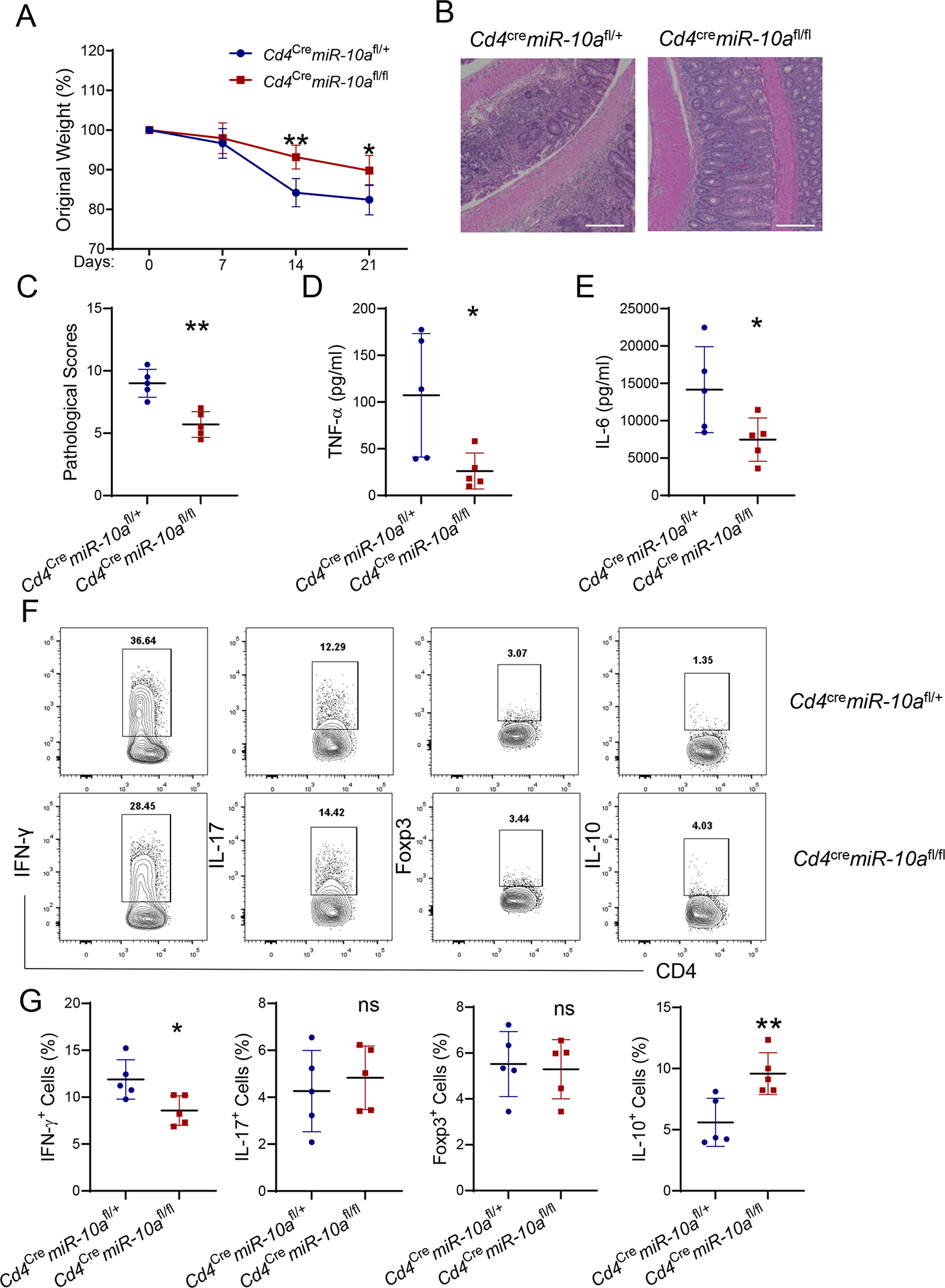
CD4+ CD45Rbhi T cells (1 × 105/ mouse) of Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice were transferred to Rag−/− mice (n = 5/group). Mice were sacrificed 3 weeks post cell transfer. (A) Weight changes of the recipient mice were monitored. (B) Representative H&E staining of colon was shown. Scale bars, 300 μm. (C) Pathological scores were assessed. (D-E) TNF-α (D) and IL-6 (E) production was measured in colonic tissues. (F-Q) Flow cytometry profile (F) and quantification (G) of IFN-γ+, IL-17+, Foxp3+, and IL-10+ CD4+ T cells in intestinal lamina propria from the recipient mice were shown. All data are presented as mean ± SD. One representative of three independent experiments. (A, and D-G) unpaired Student’s t test; (C) Mann–Whitney U test; *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
miR-10a-deficient CD4+ T cells produce higher levels of IL-10.
Next, we investigated whether miR-10a regulates CD4+ T cell responses in vitro. Splenic CD4+ T cells were isolated from WT Cd4cremiR-10afl/+ mice or miR-10a deficient Cd4cremiR-10afl/fl mice and activated with anti-CD3 mAb and anti-CD28 mAb under different polarization conditions, including neutral conditions without exogenous cytokines, Th1 (with IL-12), Th17 (with TGFβ and IL-6), and Treg (with TGFβ) conditions. Although miR-10a-deficient CD4+ T cells expressed slightly higher levels of IFN-γ than WT CD4+ T cells under neutral conditions (Supplementary Figure 3A–B), there was no difference in IFN-γ expression between miR-10a-deficient and WT CD4+ T cells under Th1 conditions (Supplementary Figure 3C–D). miR-10a-deficient CD4+ T cells produced a similar level of IL-17 under both neutral and Th17 conditions (Supplementary Figure 3A–B and E–F). miR-10a deficiency did not affect Treg polarization under both neutral and Treg conditions (Supplementary Figure 3A–B and G–H). Interestingly, IL-10+ cells were increased in miR-10a-deficient CD4+ T cells compared with WT CD4+ T cells under all conditions (Figure 3A–B). Consistently, IL-10 secretion was also increased in miR-10a-deficient CD4+ T cells measured by ELISA (Figure 3C). Furthermore, miR-10a-deficient CD4+ T cells expressed a higher mRNA level of il10 under all conditions (Figure 3D). Taken together, these data indicate that miR-10a negatively regulates IL-10 production in CD4+ T cells.
Figure 3. miR-10a-deficient CD4+ T cells produce a higher level of IL-10.
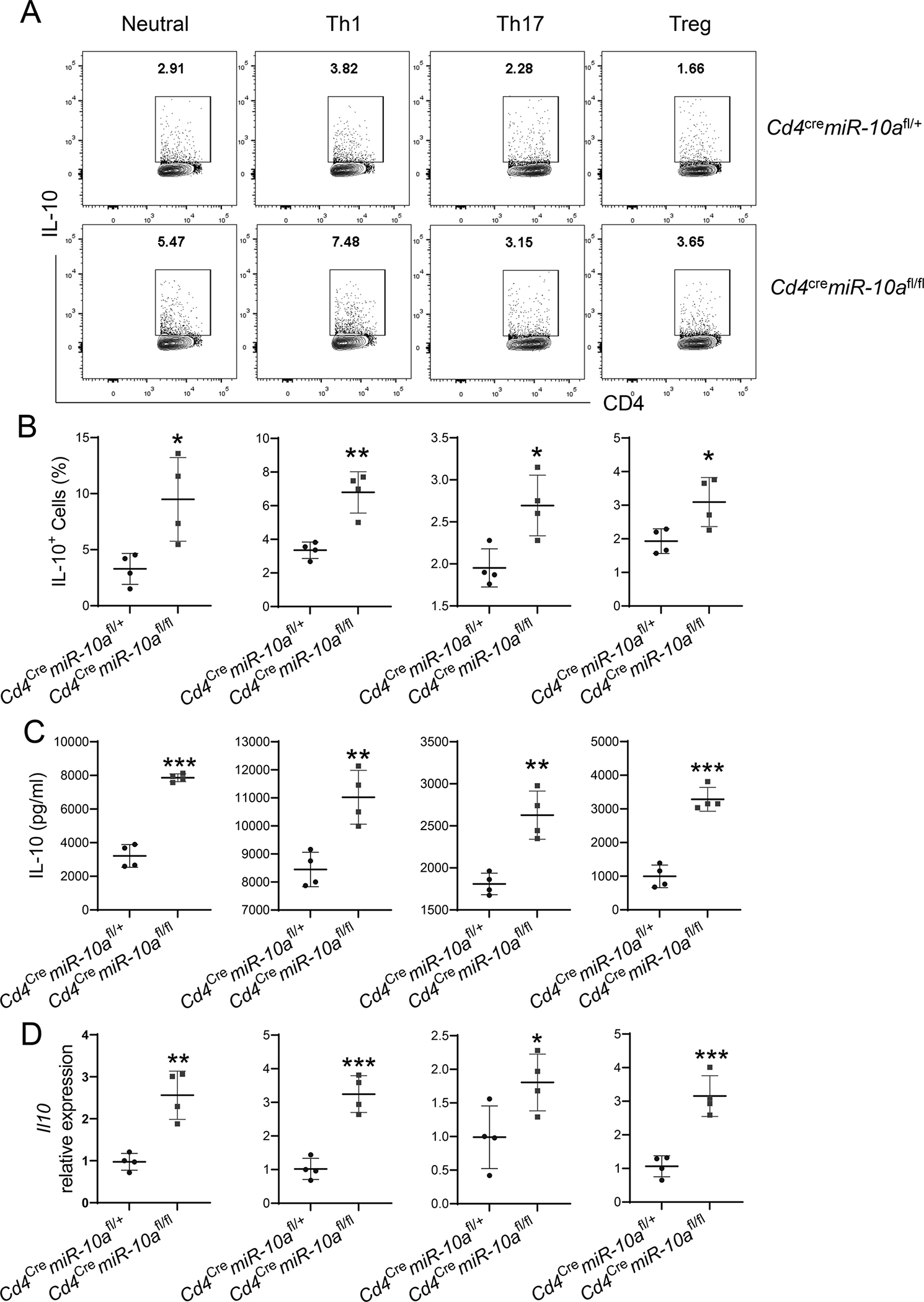
Splenic CD4+ T cells (n = 4/group) were isolated from Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice and activated with anti-CD3 and anti-CD28 mAb under neutral, Th1, Th17, and Treg conditions. (A-B) Flow cytometry profile (A) and quantification (B) of IL-10-producing CD4+ T cells were shown 5 days post activation. (C) IL-10 production in culture supernatants was measured by ELISA 3 days post activation. (D) l10 mRNA expression in CD4+ T cells was measured by qRT-PCR 2 days post activation. All data are presented as mean ± SD. One representative of three independent experiments. (A-D) unpaired Student’s t test; *p<0.05, **p<0.01, ***p<0.001.
Blockade of the IL-10/IL-10R pathway aggravates colitis induced by miR-10a-deficient CD4+ CD45Rbhi T cells.
We next investigated whether the less colitogenic property of miR-10a-deficient T cells is attributed to their higher levels of IL-10 production. We transferred CD4+ CD45Rbhi T cells from WT Cd4cremiR-10afl/+ mice or miR-10a deficient Cd4cremiR-10afl/fl mice to Rag−/− mice. One group of mice with miR-10a-deficient CD4+ CD45Rbhi T cells were treated with anti-IL-10R antibody intraperitoneally (i.p.) every other day, and the other groups of mice with miR-10a-deficient CD4+ CD45Rbhi T cells or WT CD4+ CD45Rbhi T cells were treated with anti-IgG antibody to serve as controls. Treatment of anti-IL-10R antibody aggravated colitis in Rag−/− mice that received miR-10a-deficient CD4+ CD45Rbhi T cells, which was characterized by more weight loss (Figure 4A), increased pathological scores (Figures 4B–C), and higher levels of TNF-α and IL-6 production in colonic tissues (Figures 4D–E). In contrast, there were no statistical differences of weight loss (Figure 4A), pathological scores (Figures 4B–C), and pro-inflammatory cytokines (Figures 4D–E) between Rag−/− mice that received WT CD4+ CD45Rbhi T cells and Rag−/− mice reconstituted with miR-10a-deficient CD4+ CD45Rbhi T cells after administration of the anti-IL-10R antibody. Anti-IL-10R antibody treatment increased the percentage of IFN-γ+ CD4+ T cells in the intestine of Rag−/− mice that received miR-10a-deficient CD4+ CD45Rbhi cells to the levels similar to the Rag−/− mice reconstituted with WT CD4+ CD45Rbhi T cells (Figure 4F–G). Additionally, the percentages of IL-17+ CD4+ T cells and Foxp3+ T cells were similar among all three groups of mice (Figure 4F–G). Furthermore, Rag−/− mice that received miR-10a-deficient CD4+ CD45Rbhi T cells, with or without administration of the anti-IL-10R antibody, showed higher levels of IL-10 compared to Rag−/− mice reconstituted with WT CD4+ CD45Rbhi T cells (Figure 4F–G). Taken together, these data indicate that increased IL-10 production in miR-10a-deficient CD4+ T cells inhibits their capacity to induce colitis.
Figure 4. Blocking IL-10/IL-10R pathway in vivo aggravates colitis induced by miR-10a-deficient CD4+ CD45Rbhi cells.
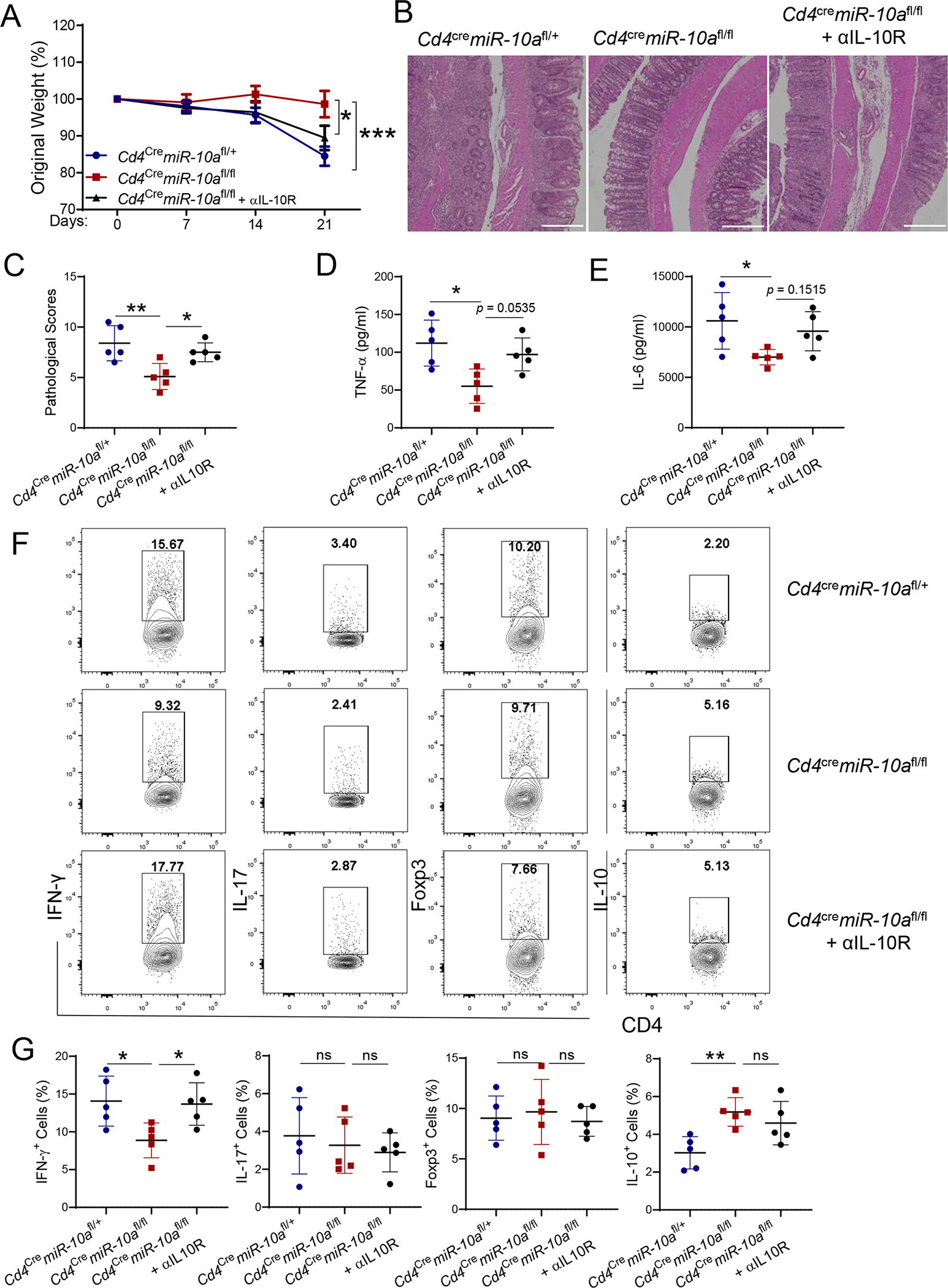
CD4+ CD45Rbhi T cells (1 × 105/ mouse) of Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice were transferred to Rag−/− mice (n = 5/group). One group of mice transferred with miR-10a-deficient CD4+ CD45Rbhi T cells were administered anti-IL-10R antibody (25 mg/kg) i.p. every other day, and the other two groups of mice were treated with anti-IgG antibody as control. (A) Weight changes of the recipient mice were monitored. (B) Representative H&E staining of colon was shown. Scale bars, 300 μm. (C) Pathological Scores were assessed. (D-E) TNF-α (D) and IL-6 (E) secretion in colonic tissues was measured. (F-G) Flow cytometry profile (F) and quantification (G) of IFN-γ+, IL-17+, Foxp3+, and IL-10+ CD4+ T cells in the intestinal lamina propria were shown. All data are presented as mean ± SD. One representative of three independent experiments. (A, and D-G) One-way ANOVA followed by Tukey’s multiple comparisons; (C) Mann–Whitney U test; *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
miR-10a suppresses CD4+ T cell production of IL-10 through targeting prdm1.
Next, we investigated how miR-10a inhibits IL-10 expression in CD4+ T cells. The targets of miR-10a were predicted using ComiR, which runs TargetScan, miRanda, and PITA publicly available scripts, to compute the binding sites (28, 29). IL-10 is not the direct target gene of miR-10a. Among transcription factors which have been shown to promote IL-10 production in CD4+ T cells, prdm1, which encodes Blimp1, but not IRF1, IRF4, c-Maf, and AhR (6, 12, 30), is one of the predicted target genes of miR-10a (Figure 5A). To determine whether miR-10a directly suppresses prdm1, a dual-luciferase assay was conducted. As shown in Figure 5B, miR-10a precursor and miR-10a inhibitor inhibited and enhanced, respectively, luciferase expression in psiCHECK-2 vector fused with the predicted seed sequence in the 3’-UTR of Prdm1 (Figure 5B). Meanwhile, miR-10a precursor and miR-10a inhibitor did not affect luciferase activity in both vector alone and mutant vectors (Figure 5B), suggesting that Prdm1 is the direct target of miR-10a. Consistently, Prdm1 mRNA expression and Blimp1 protein levels were increased in miR-10a-deficient CD4+ T cells compared with that in WT CD4+ T cells from Cd4cre miR-10afl/+ mice under neutral, Th1, Th17, and Treg culture conditions (Figure 5C–D). Additionally, we measured the Prdm1 expression in CD4+ T cells re-isolated from the intestine of Rag−/− mice receiving WT or miR-10a-deficient CD4+ CD45Rbhi T cells. Prdm1 expression was higher in intestinal CD4+ T cells of Rag−/− mice reconstituted with miR-10a-deficient T cells (Figure 5E), which corroborates that the higher level of IL-10 in miR-10-deficient CD4+ T cells is due to the increased expression of Prdm1. Next, we investigated whether prdm1 contributes to higher levels of IL-10 production in miR-10a-deficient CD4+ T cells. Splenic CD4+ T cells from WT Cd4cremiR-10afl/+ mice and miR-10a deficient Cd4cremiR-10afl/fl mice were transfected with Prdm1 siRNA or control siRNA. The efficiency of nucleofection was determined (Supplementary Figure 4A). As shown in Figure 5F, Prdm1 siRNA suppressed il10 expression in miR-10a-deficient CD4+ T cells compared with the control siRNA. Although Prdm1 siRNA transfected WT CD4+ T cells expressed a lower level of il10 compared with T cells transfected with control siRNA, it did not reach statistical significance (Figure 5F). Additionally, silencing Prdm1 did not affect the expression of IFN-γ, IL-17, and Foxp3 (Supplementary Figure 4B–D). Collectively, these data indicate that miR-10a inhibits CD4+ T cell expression of IL-10 through suppressing prdm1.
Figure 5. miR-10a inhibits CD4+ T cell production of IL-10 through suppressing Prdm1.
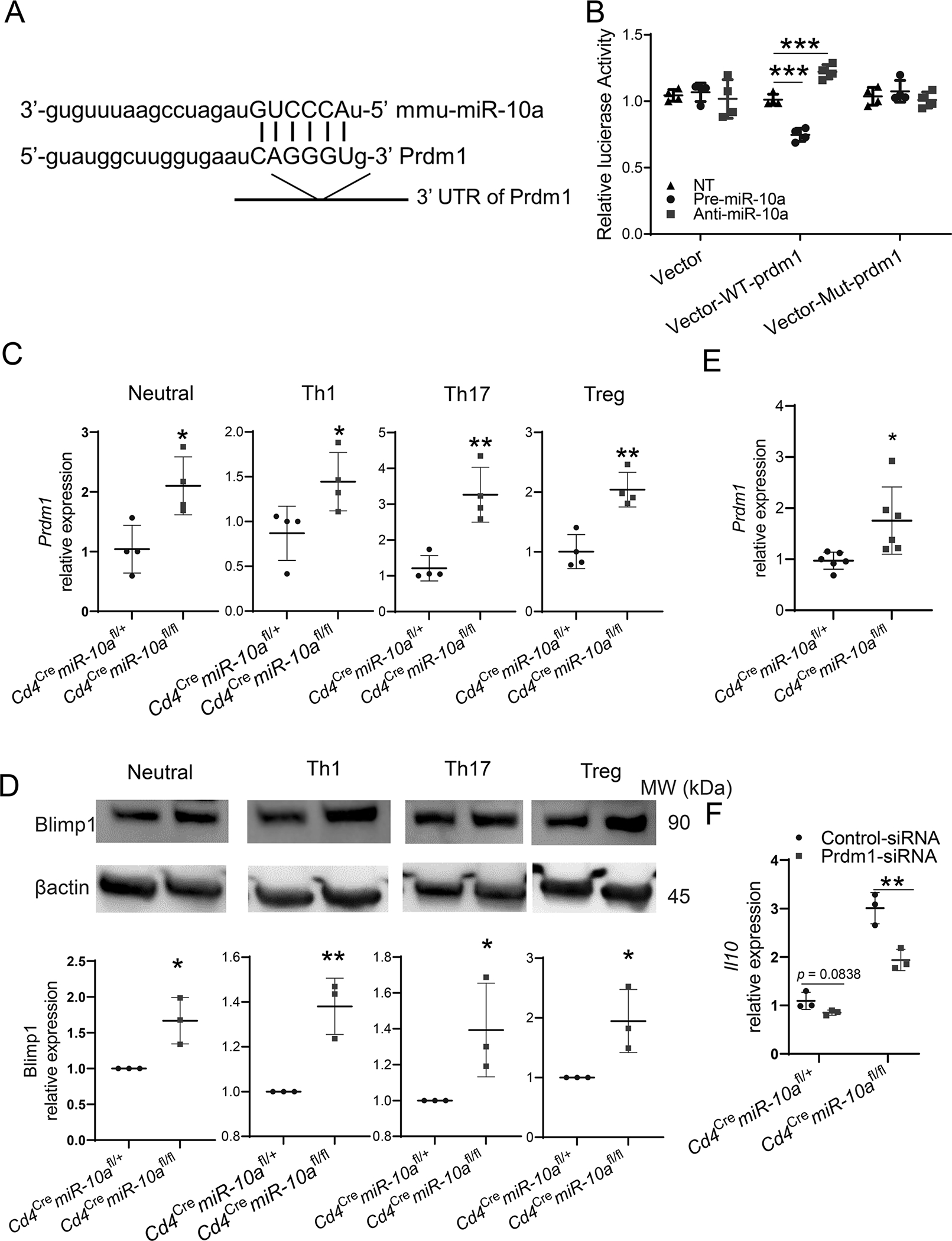
(A) Mus musculus miR-10a/Prdm1 alignment was shown. (B) Luciferase activity of Prdm1 WT and mutant (Mut) 3’ UTR constructs (n = 3/group) was measured. (C) Prdm1 expression in CD4+ T cells (n = 4/group) from Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice was measured 2 days post activation by using qRT-PCR. (D) Blimp1 protein in CD4+ T cells (n = 3/group) from Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice was measured 3 days post activation by using Western blot. (E) CD4+ CD45Rbhi T cells (1 × 105/ mouse) of Cd4cremiR-10afl/+ and Cd4cremiR-10afl/fl mice were transferred to Rag−/− mice (n = 6/group). Mice were sacrificed 3 weeks post cell transfer. Prdm1 expression was measured by using qRT-PCR in CD4+ T cells re-isolated from the intestine of Rag−/− mice receiving WT or miR-10a-deficient T cells. (F) Il10 expression in CD4+ T cells (n = 4/group) from Cd4cremiR-10afl/+ mice and Cd4cremiR-10afl/fl mice was measured 2 days post nucleofection of Prdm1 siRNA or control siRNA. All data are presented as mean ± SD. One representative of two or three independent experiments. (B-F) unpaired Student’s t test; *p<0.05, **p<0.01.
Blimp1-deficient CD4+ CD45Rbhi T cells induce more severe colitis in Rag−/− mice.
To investigate whether miR-10a’s target gene Blimp1 is critical in regulating CD4+ T cell induction of colitis, we isolated CD4+ CD45Rbhi T cells from WT Cd4crePrdm1fl/+ mice or Blimp1-deficient Cd4crePrdm1fl/fl mice and transferred those cells to Rag−/− mice. Weights were monitored every week, and mice were sacrificed 4 weeks post cell transfer. Blimp1-deficient CD4+ CD45Rbhi T cells induced more weight loss (Figure 6A) and more severe colitis (Figure 6B–C) compared with WT CD4+ CD45Rbhi T cells in Rag−/− mice. Furthermore, intestinal TNF, but not IL-6, production was higher in Rag−/− mice that received Blimp1-deficient CD4+ T cells than those that received WT T cells (Figure 6D–E). Intestinal LP IFN-γ+ CD4+ T cells and IL-17+ CD4+ T cells were increased, and Foxp3+ CD4+ T cells were decreased in Rag−/− mice that received Blimp1-deficient CD4+ T cells compared with those that received control T cells (Figure 6F–G). Importantly, there were fewer IL-10+ CD4+ T cells in the intestinal LP from Rag−/− mice reconstituted with Blimp1-deficient CD4+ T cells compared with those reconstituted with WT CD4+ T cells (Figure 6F–G). Put together, these data indicate that the expression of Blimp1, the target gene of miR-10a, in CD4+ T cells contributes to intestinal homeostasis through induction of T cell IL-10 production.
Figure 6. Blimp1-deficient CD4+ T cells induce more severe colitis.
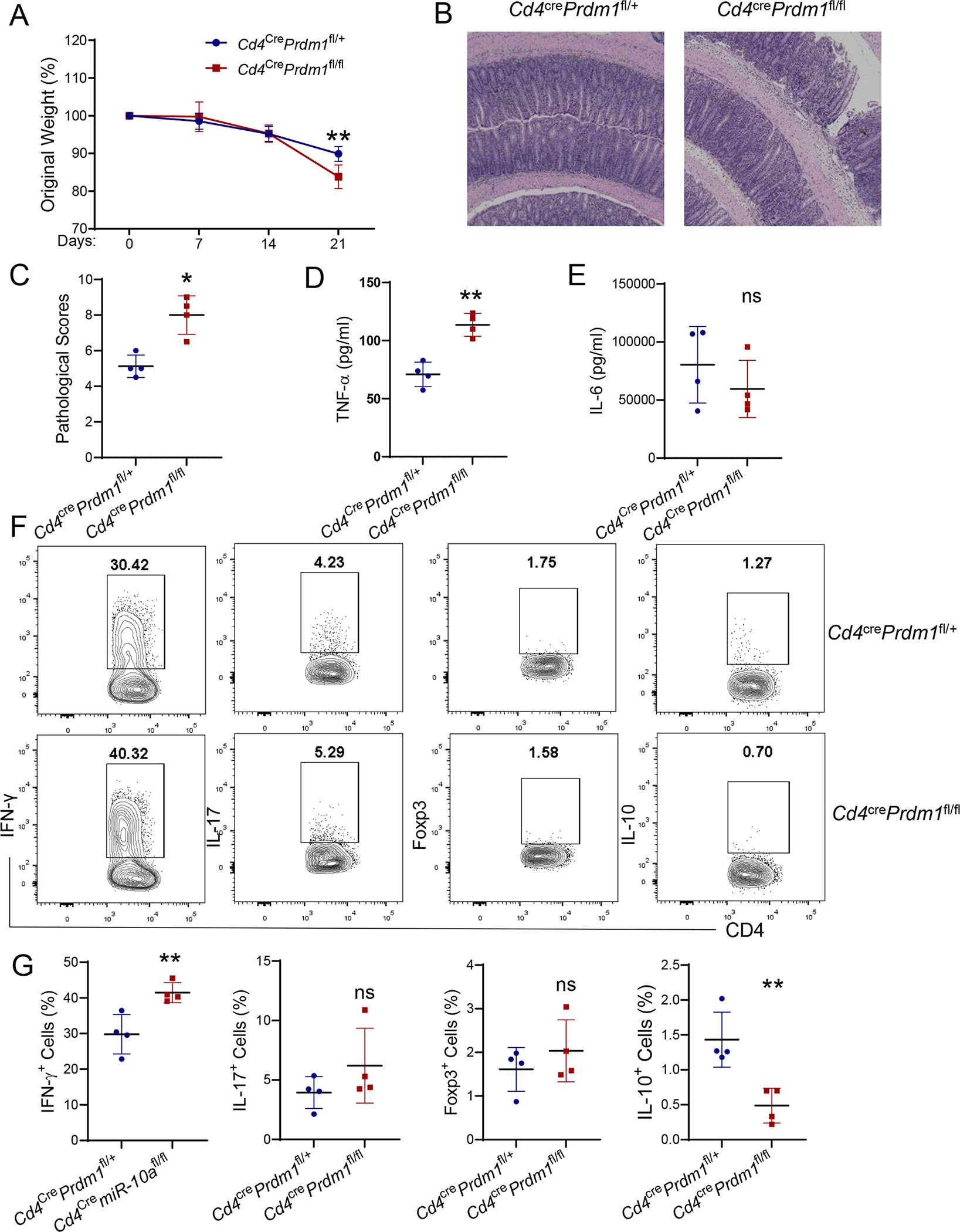
CD4+ CD45Rbhi T cells (1 × 105/ mouse) from Cd4crePrdm1fl/+ and Cd4crePrdm1fl/fl mice were transferred to Rag−/− mice (n = 4/group). Mice were sacrificed 3 weeks post cell transfer. (A) Weight changes of the recipient mice were monitored. (B) Representative H&E staining of colon was shown. Scale bars, 300 μm. (C) Pathological scores were assessed. (D-E) TNF-α (D) and IL-6 (E) secretion in colonic tissues was shown. (F-G) Flow cytometry profile (F) and quantification (G) of IFN-γ+, IL-17+, Foxp3+, and IL-10+ CD4+ T cells in intestinal lamina propria from the recipient mice were shown. All data are presented as mean ± SD and are representative of three independent experiments. (A, and D-G) unpaired Student’s t test; (C) Mann–Whitney U test; *p<0.05, **p<0.01; ns, not significant.
Discussion
Emerging evidence indicates the importance of miRNAs in orchestrating immune responses (31, 32). As key mediators in regulating various autoimmune inflammation, the functions of CD4+ T cells were greatly affected by miRNAs (33, 34). In addition to pro-inflammatory properties, CD4+ T cells also possess anti-inflammatory activities, including the production of IL-10 (35). Although CD4+ T cell production of IL-10 has been well recognized as a critical immunoregulator for suppressing excessive immune responses, how it is regulated is still not completely understood. Our current study demonstrated that miR-10a suppresses IL-10 production through targeting prdm1, which provides novel insights into how miRNAs affect CD4+ T cell production of IL-10 to regulate intestinal inflammation.
Several miRNAs have been reported to be dysregulated in inflammatory bowel disease (IBD) (36, 37). For example, miR-16 and miR-31 are increased in intestinal biopsies from patients with Crohn’s disease (CD) (38, 39), whereas miR-26a and miR-29a are reduced in the intestine from both patients with CD and ulcerative colitis (UC) (40). In previous studies, we found that miR-10a inhibits IL-12/IL-23p40 production in DCs, which affects Th1 and Th17 cell function in IBD (23, 24), indicating a potential role of miR-10a in regulating the pathogenesis of IBD. Furthermore, miRNAs affect the severity of colitis by regulating various functions of CD4+ T cells (15, 16, 41). However, whether miR-10a in CD4+ T cells affects intestinal inflammation remains unclear. We found that miR-10a deficient CD4cre miR-10afl/fl mice develop less severe colitis upon inflammatory insult. Moreover, miR-10a-deficient CD4+ CD45Rbhi T cells induce less severe colitis, revealing a critical role of CD4+ T cell expression of miR-10a in the pathogenesis of IBD.
IL-10 is a broad anti-inflammatory cytokine, which contributes to the maintenance of intestinal homeostasis (42). Although several miRNAs have been identified to regulate IL-10 in different cells (43), it is still poorly understood how miRNAs regulate IL-10 in CD4+ T cells, which are one of the major IL-10-producing cells. In addition to Treg, effector T cells also produce IL-10 to restrict their pro-inflammatory activities, which is an important self-regulatory function of CD4+ T cells (5). We found that both miR-10-deficient effector and regulatory CD4+ cells produce elevated IL-10 in vitro. Furthermore, intestinal LP miR-10a-deficient CD4+ T cells produce higher IL-10 in vivo under inflammatory conditions, which at least partially contributes to the less severe colitis induced by miR-10a-deficient CD4+ T cells. However, there is no difference in IL-10 between miR-10a-deficient CD4+ T cells and WT CD4+ T cells under steady conditions, which might be due to the low expression of IL-10 in intestinal CD4+ T cells under steady conditions. Although miR-10a has been reported to regulate Treg stability (21), our data showed that it does not affect Treg differentiation in vitro and in vivo. In addition, miR-10a deficiency in CD4+ T cells does not affect the differentiation of naïve T cells to Th1 and Th17 in vitro, indicating that miR-10a specifically affects IL-10 production in CD4+ T cells.
Several transcription factors, including Blimp1, have been reported to induce IL-10 production in CD4+ T cells (12, 30, 44, 45). Blimp1 is critical in regulating T cell homeostasis and function, including proliferation, apoptosis, differentiation, and cytokine production (46, 47). Interestingly, IL-10 is downregulated in Blimp1-deficient CD4+ T cells (8, 47). Furthermore, Blimp1 has been shown to enhance CD4+ T cell production of IL-10 through direct binding the transcriptional start site in the il10 locus (12). In this study, we found that il10 is not the direct target gene of miR-10a. Interestingly, miR-10a directly suppresses Prdm1, which encodes Blimp1. Prdm1 expression is increased in miR-10a-deficient CD4+ T cells, and downregulation of Prdm1 compromises IL-10 production in miR-10a-deficient CD4+ T cells. However, inhibition of Prdm1 does not affect the differentiation from naïve T cells to Th1, Th17, and Treg cells, indicating Blimp1 is specifically required for the increased production of IL-10 in CD4+ T cells lacking miR-10a. Consistent with previous finding that Blimp1-decificent naïve T cells induced more severe colitis (46), Blimp1-deficient CD4+ CD45Rbhi T cells induce more severe colitis with lower levels of IL-10 production in intestinal CD4+ T cells, indicating the importance of the Blimp1/IL-10 pathway in the anti-inflammatory property of miR-10-deficient CD4+ T cells.
In summary, our study reveals a novel role of CD4+ T cell expression of miR-10a in regulating intestinal inflammation, which is at least partially mediated by the upregulation of IL-10. Mechanically, miR-10a directly suppresses Prdm1 to inhibit IL-10 production in CD4+ T cells. Therefore, this study provides a potential therapeutic target for treating IBD.
Supplementary Material
Key points:
MicroRNA-10a negatively regulates CD4+ T cell IL-10 production through Prdm1.
MicroRNA-10a-deficient CD4+ T cells induce less severe intestinal inflammation.
Acknowledgment
We appreciate Dr. Sherry Haller of The University of Texas Medical Branch for proofreading the manuscript.
Grant Support:
This work was supported by the National Institutes of Health grants DK105585, DK112436, DK125011, AI150210, and DK124132, the University of Texas System STARs award (YC), and James W. McLaughlin Fellowship Fund, UTMB (WY).
Abbreviations:
- miRNAs
microRNAs
- Treg
regulatory T cell
- Th
T helper
- ELISA
Enzyme-linked immunosorbent assay
- qRT-PCR
Quantitative real-time PCR
- WT
wild-type
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Geremia A, Biancheri P, Allan P, Corazza GR, and Di Sabatino A. 2014. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity reviews 13: 3–10. [DOI] [PubMed] [Google Scholar]
- 2.Wu W, Chen F, Liu Z, and Cong Y. 2016. Microbiota-specific Th17 Cells: Yin and Yang in Regulation of Inflammatory Bowel Disease. Inflammatory bowel diseases 22: 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, and Rudensky AY. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, Wang S, and Chen W. 2017. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 46: 660–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankovic D, Kugler DG, and Sher A. 2010. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal immunology 3: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, and Cong Y. 2018. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nature communications 9: 3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M, He C, Chen L, Yang W, Wu W, Chen F, Cao AT, Yao S, Dann SM, Dhar TGM, Salter-Cid L, Zhao Q, Liu Z, and Cong Y. 2019. RORgammat Represses IL-10 Production in Th17 Cells To Maintain Their Pathogenicity in Inducing Intestinal Inflammation. Journal of immunology 202: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, and Müller W. 2004. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. The Journal of experimental medicine 200: 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutz S, and Ouyang W. 2016. Regulation of Interleukin-10 Expression. Advances in experimental medicine and biology 941: 89–116. [DOI] [PubMed] [Google Scholar]
- 10.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, and Kallies A. 2011. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology 12: 304–311. [DOI] [PubMed] [Google Scholar]
- 11.Karwacz K, Miraldi ER, Pokrovskii M, Madi A, Yosef N, Wortman I, Chen X, Watters A, Carriero N, Awasthi A, Regev A, Bonneau R, Littman D, and Kuchroo VK. 2017. Critical role of IRF1 and BATF in forming chromatin landscape during type 1 regulatory cell differentiation. Nature immunology 18: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kuhl AA, Heimesaat MM, Esser C, Im SH, Radbruch A, Rutz S, and Scheffold A. 2014. Role of Blimp-1 in programing Th effector cells into IL-10 producers. The Journal of experimental medicine 211: 1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, and Clark AG. 2012. Impact of microRNA regulation on variation in human gene expression. Genome research 22: 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, and Satsangi J. 2015. MicroRNAs: new players in IBD. Gut 64: 504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge Y, Sun M, Wu W, Ma C, Zhang C, He C, Li J, Cong Y, Zhang D, and Liu Z. 2019. MicroRNA-125a suppresses intestinal mucosal inflammation through targeting ETS-1 in patients with inflammatory bowel diseases. Journal of autoimmunity 101: 109–120. [DOI] [PubMed] [Google Scholar]
- 16.He C, Shi Y, Wu R, Sun M, Fang L, Wu W, Liu C, Tang M, Li Z, Wang P, Cong Y, and Liu Z. 2016. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-alpha in IBD. Gut 65: 1938–1950. [DOI] [PubMed] [Google Scholar]
- 17.Long MJ, Wu FX, Li P, Liu M, Li X, and Tang H. 2012. MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer letters 324: 186–196. [DOI] [PubMed] [Google Scholar]
- 18.Ohuchida K, Mizumoto K, Lin C, Yamaguchi H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M, and Tanaka M. 2012. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Annals of surgical oncology 19: 2394–2402. [DOI] [PubMed] [Google Scholar]
- 19.Hu R, Pan W, Fedulov AV, Jester W, Jones MR, Weiss ST, Panettieri RA Jr., Tantisira K, and Lu Q. 2014. MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 28: 2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, Lund AH, and Bluestone JA. 2012. MicroRNA 10a marks regulatory T cells. PloS one 7: e36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, and O’Shea JJ. 2012. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nature immunology 13: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelada S, Sethupathy P, Okoye IS, Kistasis E, Czieso S, White SD, Chou D, Martens C, Ricklefs SM, Virtaneva K, Sturdevant DE, Porcella SF, Belkaid Y, Wynn TA, and Wilson MS. 2013. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS pathogens 9: e1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W, He C, Liu C, Cao AT, Xue X, Evans-Marin HL, Sun M, Fang L, Yao S, Pinchuk IV, Powell DW, Liu Z, and Cong Y. 2015. miR-10a inhibits dendritic cell activation and Th1/Th17 cell immune responses in IBD. Gut 64: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 24.Xue X, Feng T, Yao S, Wolf KJ, Liu CG, Liu X, Elson CO, and Cong Y. 2011. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. Journal of immunology 187: 5879–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, and Littman DR. 2009. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittem CG, Williams AD, and Williams CS. 2010. Murine Colitis modeling using Dextran Sulfate Sodium (DSS). Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, and Grisham MB. 2009. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. American journal of physiology. Gastrointestinal and liver physiology 296: G135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronnello C, Hartmaier R, Arora A, Huleihel L, Pandit KV, Bais AS, Butterworth M, Kaminski N, Stormo GD, Oesterreich S, and Benos PV. 2012. Novel modeling of combinatorial miRNA targeting identifies SNP with potential role in bone density. PLoS computational biology 8: e1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronnello C, and Benos PV. 2013. ComiR: Combinatorial microRNA target prediction tool. Nucleic acids research 41: W159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, Wei CY, Zhou WJ, Zhu XY, Shao J, Li DJ, and Li MQ. 2017. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORgammat/Blimp-1 signal to promote the progression of endometriosis. Cell death & disease 8: e2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Runtsch MC, Round JL, and O’Connell RM. 2014. MicroRNAs and the regulation of intestinal homeostasis. Frontiers in genetics 5: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, and Chen J. 2019. microRNAs as therapeutic targets in intestinal diseases. ExRNA 1: 23. [Google Scholar]
- 33.Liu J, Wu CP, Lu BF, and Jiang JT. 2013. Mechanism of T cell regulation by microRNAs. Cancer biology & medicine 10: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saki N, Abroun S, Soleimani M, Hajizamani S, Shahjahani M, Kast RE, and Mortazavi Y. 2015. Involvement of MicroRNA in T-Cell Differentiation and Malignancy. International journal of hematology-oncology and stem cell research 9: 33–49. [PMC free article] [PubMed] [Google Scholar]
- 35.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, and Wraith DC. 2013. Regulation of adaptive immunity; the role of interleukin-10. Frontiers in immunology 4: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman CG, and Pekow J. 2015. The emerging role of miRNAs in inflammatory bowel disease: a review. Therapeutic advances in gastroenterology 8: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer JS 2016. MicroRNAs: how many in inflammatory bowel disease? Current opinion in gastroenterology 32: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, and Kwon JH. 2010. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflammatory bowel diseases 16: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Welker NC, Zhao Z, Li Y, Zhang J, Reuss SA, Zhang X, Lee H, Liu Y, and Bronner MP. 2014. Novel specific microRNA biomarkers in idiopathic inflammatory bowel disease unrelated to disease activity. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 27: 602–608. [DOI] [PubMed] [Google Scholar]
- 40.Fasseu M, Treton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, Aparicio T, Daniel F, Soule JC, Moreau R, Bouhnik Y, Laburthe M, Groyer A, and Ogier-Denis E. 2010. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PloS one 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Dai S, Qiu C, Wang T, Zhou Y, Xue C, Yao J, and Xu Y. 2020. MicroRNA-219a-5p suppresses intestinal inflammation through inhibiting Th1/Th17-mediated immune responses in inflammatory bowel disease. Mucosal immunology 13: 303–312. [DOI] [PubMed] [Google Scholar]
- 42.Kole A, and Maloy KJ. 2014. Control of intestinal inflammation by interleukin-10. Current topics in microbiology and immunology 380: 19–38. [DOI] [PubMed] [Google Scholar]
- 43.Saraiva M, and O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nature reviews. Immunology 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 44.Wang YH, Tsai DY, Ko YA, Yang TT, Lin IY, Hung KH, and Lin KI. 2019. Blimp-1 Contributes to the Development and Function of Regulatory B Cells. Frontiers in immunology 10: 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montes de Oca M, Kumar R, de Labastida Rivera F, Amante FH, Sheel M, Faleiro RJ, Bunn PT, Best SE, Beattie L, Ng SS, Edwards CL, Muller W, Cretney E, Nutt SL, Smyth MJ, Haque A, Hill GR, Sundar S, Kallies A, and Engwerda CR. 2016. Blimp-1-Dependent IL-10 Production by Tr1 Cells Regulates TNF-Mediated Tissue Pathology. PLoS pathogens 12: e1005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, and Calame K. 2006. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nature immunology 7: 457–465. [DOI] [PubMed] [Google Scholar]
- 47.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, and Nutt SL. 2006. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nature immunology 7: 466–474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


