Abstract
The molecular and cellular basis for cataract development in mice lacking dystrophin, a scaffolding protein that links the cytoskeleton to the extracellular matrix, is poorly understood. In this study, we characterized lenses derived from the dystrophin-deficient mdx3cv mouse model. Expression of Dp71, a predominant isoform of dystrophin in the lens, was induced during lens fiber cell differentiation. Dp71 was found to co-distribute with dystroglycan, connexin-50 and 46, aquaporin-0, and NrCAM as a large cluster at the center of long arms of the hexagonal fibers. Although mdx3cv mouse lenses exhibited dramatically reduced levels of Dp71, only older lenses revealed punctate nuclear opacities compared to littermate wild type (WT) lenses. The levels of dystroglycan, syntrophin, and dystrobrevin which comprise the dystrophin-associated protein complex (DAPC), and NrCAM, connexin-50, and aquaporin-0, were significantly lower in the lens membrane fraction of adult mdx3cv mice compared to WT mice. Additionally, decreases were observed in myosin light chain phosphorylation and lens stiffness together with a significant elevation in the levels of utrophin, a functional homolog of dystrophin in mdx3cv mouse lenses compared to WT lenses. The levels of perlecan and laminin (ligands of α-dystroglycan) remained normal in dystrophin-deficient lens fibers. Taken together, although mdx3cv mouse lenses exhibit only minor defects in lens clarity possibly due to a compensatory increase in utrophin, the noted disruptions of DAPC, stability, and organization of membrane integral proteins of fibers, and stiffness of mdx3cv lenses reveal the importance of dystrophin and DAPC in maintaining lens clarity and function.
Keywords: Cataract, dystrophin, connexins, myosin, utrophin, extracellular matrix
I. Introduction
The ocular lens is an avascular and transparent organ that plays a crucial role in vision by focusing incident light on to the retina. A monolayer of cuboidal epithelial cells covers the anterior surface of the lens, with long, differentiated fiber cells occupying the rest of the organ [1]. The lens is enclosed in a thick collagenous capsule comprising various extracellular matrix components and therefore does not undergo cell shedding, with cells being retained throughout life once they are formed. Epithelial cells at the equator or bow region of the lens continuously exit from the cell cycle to elongate and differentiate into ribbon-like long fiber cells [1]. As lens epithelial cells differentiate into fiber cells, they elongate, migrate, and develop adhesive interactions anteriorly with epithelial cells through apical to apical interactions and posteriorly with the extracellular matrix of the capsule. Newly differentiated fibers organize themselves over older fibers much like the layers of an onion, with older fibers progressively losing all cellular organelles, and undergoing tight compaction, radial organization, and achieving a characteristic hexagonal geometry [1, 2]. As fiber cells differentiate, they express lens abundant and specific proteins including the cystallins, cytoskeletal, and transport proteins [1]. Protein turnover is minimal in the lens since terminally differentiated lens fibers do not possess cellular organelles [3].
Transparency and accommodation are crucial determinants of the image focusing ability of the ocular lens and depend upon precise hexagonal geometry, radial packing, membrane organization, and deformability of lens fiber cells [1, 2, 4]. While ample evidence supports a direct and vital role for the membrane skeleton (the spectrin/actin network) in maintaining lens fiber cell shape, packing, membrane organization, and mechanical properties [2, 5, 6], we have a minimal understanding of the identity and role of scaffolding or adaptor proteins that regulate the anchoring interactions of cytoskeletal proteins with transmembrane proteins to form and maintain distinct functional complexes or domains in the lens fibers [6].
The structural and functional integrity of differentiated fibers critically depend on membrane transport mechanisms including the connexin-based gap junctions, aquaporin-0-based water channels, and ion channels for microcirculation of ions, solutes, and water. Disruptions in transport mechanisms have been demonstrated to impair lens growth, integrity, and transparency [7–9]. Given the importance of membrane domain organization and stability of various transport, channel, and adhesion proteins for maintenance of activity of these proteins in the lens, we need to identify the molecular mechanisms regulating the tethering of membrane proteins to the cytoskeleton and signaling proteins in the lens fibers [6].
In recent studies, we demonstrated the importance of ankyrins and periaxin in linking the spectrin-actin cytoskeleton to lens fiber membrane proteins, and that deficiency of ankyrin-B and periaxin disrupts membrane organization and mechanical properties of the lens [6]. Deficiency of ankyrin-B and periaxin was found to be associated with disruption of the dystrophin-dystroglycan complex (DGC) in lens fibers and alteration in lens mechanical stability suggesting the functional significance of ankyrins, periaxin, and the DGC complex in maintaining lens fiber membrane organization, cell adhesion, signaling, and mechanical integrity [6]. Interestingly, connexin-50 deficiency in the lens has also been reported to disrupt the association of dystroglycan with ball and socket interdigitations in fiber cells [10]. Although the importance of dystrophin in lens function was evident from the reports of incidence of cataract in patients with a mutation in dystrophin [11], and in experimental models lacking dystrophin [12, 13], overall, we have a very limited understanding of the role of dystrophin and the dystrophin-associated protein complex (DAPC) in lens membrane organization and mechanical stability.
Dystrophin, a spectrin related protein that connects the cytoskeleton with the extracellular matrix (ECM) through the plasma membrane, is expressed in a variety of tissues, most notably in skeletal, cardiac muscles, and neurons [14, 15]. Dystrophin is an X chromosome-linked protein with a molecular mass of 427 kDa [14]. Full-length dystrophin has four functional domains comprising an N-terminal actin-binding domain, the central rod domain consisting of spectrin repeats, a cysteine-rich region which binds to the intracellular region of the transmembrane β-dystroglycan, and the C-terminal domain that binds to syntrophins [16]. Besides the full-length form of dystrophin, several amino-terminally truncated isoforms of this protein are generated via the use of tissue-specific internal promoters [16, 17]. Dp71, one of the shortest isoforms of dystrophin, is expressed in many tissues including the ocular lens [13]. The DAPC is a large multi-protein complex that plays a critical role in mechanical stability and membrane organization of channel proteins and signaling proteins by mediating interactions between the cytoskeleton, membrane, and ECM [18, 19].
Although the role of the DAPC in heart and skeletal muscle tissue is well understood in maintaining mechanical stability during contraction and relaxation of muscle tissue [16, 19], its function in lens architecture and function remains far from understood [13]. Since maintenance of mechanical stability of lens fibers is necessary to support lens deformability and resilience during visual accommodation [5, 20, 21], we believe that the DAPC may be required for lens architecture, membrane organization, signaling, mechanical stability, and function.
Therefore, the goal of this study was to characterize the DAPC in lens tissue and to determine how the deficiency of dystrophin may affect the stability of DAPC, membrane proteins, adhesion proteins, and the mechanical properties and transparency of the lens. Towards this, we investigated the stability of DAPC, organization of lens cytoskeletal and membrane-associated proteins, the status of extracellular ligands of dystroglycan, myosin II activity, and lens stiffness in dystrophin-deficient mdx3cv mice. Our findings reveal that dystrophin and the DAPC are required for the organizational integrity of channel and cell adhesion proteins in lens fiber cell membranes, myosin II activity, and for the tensile properties of the ocular lens.
2. Methods
2.1. Mice
C57BL/6J (wild-type) and B6.Cg-Dmdmdx−3Cv/J (mdx3cv) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Heterozygous mice were generated in-house by crossing wild type C57BL/6J mice with mdx3cv mice. Homozygous mutant mdx3cv and littermate wild type (WT) mice were genotyped by tail DNA via PCR analysis based on primer competition [22]. Mouse studies were performed in compliance with the National Institutes of Health guide for the care and use of laboratory animals with approval from the Duke University Medical Center IACUC (Institutional Animal Care and Use Committee). Both male and female mice were used in the described studies.
2.2. RT-PCR and qRT-PCR
To determine the relative expression level of different isoforms of dystrophin (splice variants) in mouse lens, total RNA was extracted from pooled lenses (4 lenses/sample) of neonatal (P2) and P21 mice using RNeasy Micro kit (Cat. No. 74004; Qiagen, Inc., Valencia, CA, USA) and reverse transcribed using the Advantage RT-for-PCR Kit (Cat. No. 639506; Takara Bio USA, Inc., Mountain View, CA, USA), as we described previously [23]. Reverse-transcribed single-stranded DNA, different isoform-specific forward and reverse oligonucleotide PCR primers of dystrophin (Table S1) and Titanium® Taq PCR Kit (Cat. No. 639210; Takara Bio USA, Inc.) were used to amplify the dystrophin specific DNA. Amplified DNA products were separated by agarose gel electrophoresis and visualized using Gel Red Nucleic Acid Stain (Cat. No. 41002; Biotium, Hayward, CA, USA) and viewed with a Bio-Rad ChemiDoc™ Touch Imaging System; Hercules, CA, USA. The DNA products were sequenced to confirm identity.
To determine relative levels of Dp71 expression in WT and mdx3cv lenses, qRT-PCR reactions were carried out using the iQ SYBR Green Kit (Cat. No. 64277547; Bio-Rad), Dp71 specific oligonucleotide primer sets (Table S1) and CFX 96™ Real-Time PCR detection system (Bio-Rad). Fold differences in expression of Dp71 in WT and mdx3cv mouse lenses were calculated by the comparative threshold method and normalized to the expression of housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as described by the manufacturer.
2.3. Tissue Fixation and Sectioning
Embryonic, neonatal, and postnatal mouse eyes were fixed for cryostat or paraffin sectioning. For frozen tissue sectioning, embryonic heads (E12.5, E16.5, and P1) were fixed in 4% paraformaldehyde for 24 h at 4 °C, transferred into 5%, and 30% sucrose in PBS (phosphate-buffered saline) on successive days at 4 °C. Tissues were embedded in optimal cutting temperature (OCT) media (Tissue-Tek, Torrance, CA, USA), and cut into 10-μm-thick sections (sagittal plane) using a Microm™ HM550 Cryostat (GMI, Ramsey, MN, USA).
For paraffin sections, tissue specimens (P1, and P30 mice) were fixed in 3.7% buffered formalin for 48 h at room temperature, embedded in paraffin, and 5-μm-thick sections (both sagittal and equatorial plane) were generated and used as we described earlier [6].
2.4. Immunofluorescence and Imaging
Sagittal and equatorial plane sections derived from cryofixed and paraffin-embedded mouse eyes were used for immunofluorescence analyses. The paraffin tissue sections were deparaffinized and rehydrated using xylene and absolute ethyl alcohol, respectively, as we described earlier [6]. Specimens were then placed in preheated antigen retrieval solution (0.1 M sodium citrate buffer, pH 6.0) and heated for 20 min at 100 °C in a water bath. After rinsing, the tissue sections were blocked for 10 min in a humidified chamber using medical background Sniper reducing solution (Cat. No. BS966H; Biocare Medical, Concord, CA).
Air-dried tissue cryosections were treated with Image-iT FX signal enhancer (Invitrogen, Eugene, OR, USA) and blocked using blocking buffer (5% globulin-free BSA and 5% filtered goat serum in 0.3% Triton X-100 containing PBS) for 30 min each. Primary antibodies (Table S2) were added in the blocking buffer and incubated with tissue sections overnight at 4 °C in a humidified chamber.
For paraffin sections, primary antibodies were added (see Supplementary Table S2 for the details) in Tris-buffered saline containing 1% bovine serum albumin either individually or in combination with other primary antibodies (for double labeling) and incubated with tissue sections overnight at 4 °C in a humidified chamber. The slides were washed and incubated with either Alexa fluor 488 or 568 conjugated secondary antibodies (Invitrogen; at 1:500 dilution) or both (for double labeling) under dark for 2 h at room temperature. Slides were then washed and mounted using Vectashield® Antifade Mounting Medium (Cat. No: H-1000; Vector Laboratories, Burlingame, CA), with images being captured using a Nikon Eclipse 90i confocal laser scanning microscope. For all immunofluorescence analyses described in this study, a minimum of three independent specimens (biological replicates) was analyzed, with three serial sections from each specimen. Lens fibers from the cortical region (100 μm from the lens epithelium using equatorial sections) were imaged to evaluate the distribution profile of the proteins of interest.
Mouse lenses were dissected immediately from freshly enucleated eyeballs in phenol-free DMEM medium (Cat No: 11054–020, Gibco, Gaithersburg, MD) and imaged using Zeiss Stemi 2000-C Trinocular Stereo Microscope with Axiocam ERc 5s.
2.5. Extraction of ECM proteins from lens fibers
Freshly enucleated lenses from one-month-old mouse eyes were micro-dissected using a dissecting microscope to remove the capsule/epithelium. The fiber mass was then used for ECM extraction by sequentially separating the cytosolic, nuclear, membrane, cytoskeletal and ECM enriched fractions as described by Naba et. al. [24] (a stepwise protocol is described schematically in Supplementary Fig. S1). Briefly, about 100 mg of lens fiber tissue was homogenized at 4 °C using a glass homogenizer and 0.1 M HEPES buffer (pH 7.9) containing 5 mM MgCl2, 100 mM KCl, 2 mM EDTA, 0.5 M sucrose, 5% glycerol, 1 mM sodium orthovanadate and protease inhibitors. From this initial tissue homogenate, the cytosolic, nuclear, membrane, cytoskeletal and ECM enriched fractions were sequentially extracted using the respective buffers and detergents as described by Naba et. al [24]. The ECM-enriched pellet obtained in the last step was resuspended in 8 M urea buffer containing 100 mM ammonium bicarbonate and incubated at 37 °C with continuous agitation at 1,400 rpm for 2 h and subsequently sonicated gently. This ECM-enriched fraction was mixed with 4x Laemmli buffer (10% SDS, 20% glycerol, 1 M Tris-HCl (pH 6.8), 0.5% Bromophenol blue, 5% β-mercaptoethanol and 100 mM dithiothreitol) and separated on a gradient (4–20%) SDS (sodium dodecyl sulphate) -polyacrylamide gel. The gel was stained with Gelcode Blue (Cat. No. 24590; Thermo Fisher Scientific, Waltham, MA.), destained with deionized water and the protein bands were excised from the gel and subjected to in-gel tryptic digestion as we described earlier [25].
2.6. Mass spectrometry
Peptide mixtures obtained from the in-gel-trypsin-digest procedure referenced above were analyzed using a nanoAcquity UPLC system coupled to a Synapt G2 HDMS mass spectrometer (Waters Corp, Milford, MA). Peptides were separated on a 75 μm × 100 mm column with 1.7 μm C18 BEH particles (Waters) using a 90-min gradient of 6 to 32% acetonitrile with 0.1% formic acid at a flow rate of 0.3 μl/min and a column temperature of 45 °C. Data-dependent analysis (DDA) was conducted for each peptide digest using a 0.8 s MS scan followed by MS/MS acquisition on the top three ions with a charge greater than one. MS/MS scans for each ion used an isolation window of ~3 Da, a maximum of 3s per precursor, and dynamic exclusion for 120 s within 1.2 Da. DDA data were converted to searchable files using Protein Lynx Global Server 2.5 (Waters Corporation) and searched against the Uniprot human database (2018) using Mascot server 2.5 with the following parameters: maximum one missed cleavage site, carbamidomethylation at Cys residues as fixed modification and Met oxidation, Asn and Gln deamidation as variable modifications. Precursor ion mass tolerance was set to 20 ppm, and fragment mass tolerance to 0.2 Da. Mascot data were imported into Scaffold 4.4 (Proteome Software Inc) to merge all the data for a sample represented by multiple gel bands, identify a false discovery rate for protein identification, group proteins and perform spectral counting based protein quantification. Acceptance criteria for protein identification required identification of at least two peptides for each protein with a confidence interval percentage, CI% over 99%, corresponding to a false discovery rate of <1%.
2.7. Lens stiffness analysis
Changes in the compressive stiffness of WT and mdx3cv mouse lenses were analyzed using an RSA III micro-strain analyzer (TA Instruments, New Castle, DE) equipped with parallel plate tools as we described earlier [6]. The samples were strained at a constant rate of 0.05 mm/s for 35 s until sample rupture occurred. Data were acquired and plotted in real-time using TA Orchestrator software. Applied stress was calculated by dividing measured changes in applied force by the area. Slope values before lens rupture were calculated from the linear range of slope[6].
2.8. Immunoblotting
To analyze the distribution profile of different lens proteins in membrane rich and cytosolic fractions, whole lenses (P30, P120) from both WT and mdx3cv mice were dissected free of other tissue. Lens tissue (4–5 lenses/pooled sample) was homogenized using a Dounce glass homogenizer and cold (4 °C) hypotonic buffer containing 10 mM Tris buffer, pH 7.4, 0.2 mM MgCl2, 5 mM N-ethylmaleimide, 2 mM sodium orthovanadate, 10 mM sodium fluoride, 60 μM phenylmethylsulfonyl fluoride, 0.4 mM iodoacetamide, along with complete Mini, EDTA-free protease inhibitor cocktail tablet and PhosSTOP phosphatase inhibitor cocktail tablet, 1 per 10 ml buffer, respectively (Roche, Manheim, Germany). Lysates were centrifuged at 800 x g for 20 min and the supernatants were further centrifuged at 100,000 x g for 1 h at 4 °C using a Beckman tabletop ultracentrifuge. The pellets were suspended in a sample buffer containing 8 M urea, 20 mM Tris, 23 mM glycine, 0.5 M DTT, and saturated sucrose, further sonicated and used as the membrane-enriched fraction, while the supernatant was used as a cytosolic fraction. To determine the different isoforms of dystrophin in P2 and P21, and utrophin in P30 and 4-mo-old mouse lenses, lens lysates were prepared in the hypotonic buffer, centrifuged at 800 x g for 20 min, and supernatants were analyzed. Lens capsule/epithelium samples isolated from P30 mice were also homogenized as described above to generate 800xg supernatants for analysis of dystrophin.
Protein concentration was determined in both 800 x g supernatant and cytosolic fractions using the Pierce™ BCA Protein Assay Kit (Cat. No. 23225; Thermo Fisher Scientific), and in membrane-enriched fractions using the Pierce™ 660nm Protein Assay Reagent (Cat. No. 22660; Thermo Fisher Scientific). Equal amounts of protein from both WT and mdx3cv mice membrane fractions and 800 x g supernatants (30 μg) were thoroughly mixed with freshly prepared 4x SDS/PAGE Laemmli buffer containing 40 mM DTT and incubated for 5 min at 100 °C. These protein samples were separated on 8–10% acrylamide gels or 4–20% Mini-Protean® TGX Stain-Free™ gradient SDS-polyacrylamide gels (Bio-Rad) and electrophoretically transferred to nitrocellulose membrane (Bio-Rad). Nitrocellulose membranes were probed with different primary antibodies (Table S2 for details) and subsequently with appropriate secondary antibody as we described earlier [6]. GAPDH and Na+ K+ ATPase were immunoblotted as loading controls, where required for cytosolic and membrane-enriched fractions, respectively. Blots were developed by chemiluminescence using Chemidoc™ Touch (Bio-Rad), and bands were quantified using ImageJ Software.
2.9. Myosin Light-Chain Phosphorylation
Myosin light-chain (MLC) phosphorylation status in mdx3cv and WT mouse lenses (4-mo-old) was determined as we described earlier [9]. Briefly, freshly enucleated lenses (2 lenses/sample) were initially treated with 10% ice-cold trichloracetic acid and 0.5 M DTT for 5 min. Lens tissue was then homogenized with urea buffer (8 M urea, 20 mM Tris, 23 mM glycine, 0.5 M DTT saturated in sucrose) containing protease and phosphatase inhibitor cocktails and centrifuged at 800 x g. Protein concentration in the supernatant was determined using a Pierce™ 660nm Protein Assay Reagent (Cat. No. 22660; Thermo Fisher Scientific), according to manufacturer’s protocol. Lysates (200 μg protein) were subjected to urea/glycerol-polyacrylamide gel electrophoresis and immunoblot analysis using di-phospho-MLC (Thr18/Ser19) and MLC specific antibodies (Table S2 for details).
2.10. Statistical analysis
Student’s t-test was performed to determine the significance of differences (P<0.05) between the WT and mdx3cv lens specimens. Values are presented as mean±SEM of 4–10 biological replicates.
3. Results
3.1. Expression of Dp71, the predominant isoform of lens dystrophin is induced during fiber cell differentiation.
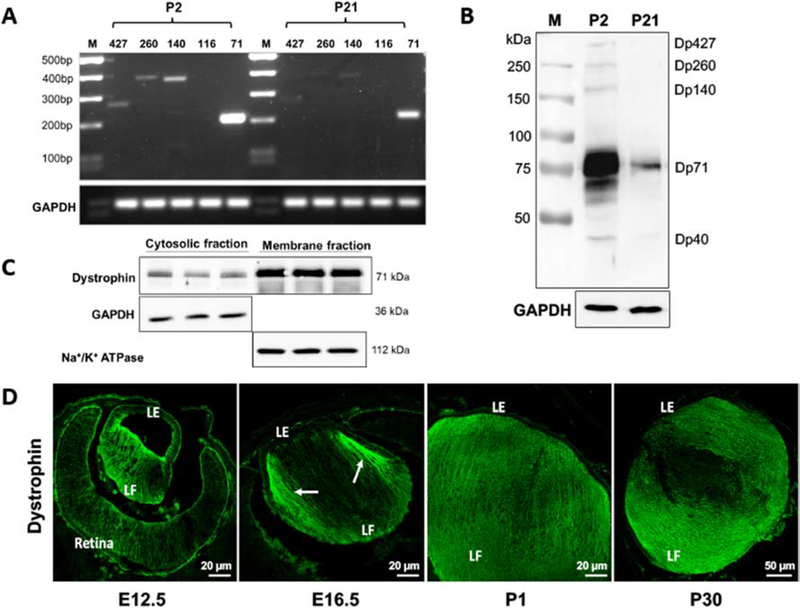
The expression profile of different isoforms of dystrophin in the lens was initially evaluated using RT-PCR analysis of RNA derived from P2 and P21 WT mouse lenses. This analysis revealed the expression of Dp427, Dp260, Dp140, and Dp71, with Dp71 being the most abundantly expressed isoform in both P2 and P21 lenses. The expression of all dystrophin isoforms was found to be downregulated in P21 lenses relative to the levels noted in P2 lenses (Fig. 1A). Subsequently, immunoblotting analysis of P2 and P21WT lens homogenates (800 x g supernatant, 30 μg protein) using a polyclonal dystrophin antibody confirmed Dp427, Dp260, Dp140, Dp71, and Dp40 isoforms of dystrophin in P2 lenses, and the presence of only Dp71 in P21 lenses, indicating that Dp71 is the predominant dystrophin isoform in lens tissue (Fig. 1B). Since equal amounts of protein were used from P2 and P21 lenses to evaluate the profile of dystrophin proteins, we conclude that the reduced amounts of dystrophin isoforms detected in P21 lenses including Dp71, could be accounted for partly by a ‘dilutional effect’ owing to the robust increase in expression of fiber abundant proteins including crystallins, aquaporin-0 and connexins in P21 lens relative to P2 lenses. Additionally, dystrophin appears to be predominantly a membrane-associated protein in the lens based on immunoblot analysis of cytosolic and membrane-enriched fractions obtained from P30 lens homogenates (Fig. 1C).
Figure 1: The expression and distribution profile of dystrophin in the mouse lens.
A) RT-PCR analysis of P2 and P21 mouse lenses reveals the expression of different isoforms of dystrophin, with Dp71 being the predominant species. B) Immunoblotting-based detection of dystrophin isoforms including Dp427, Dp260, Dp140, Dp71, and Dp40 in P2 and P21 mouse lenses. Compared to P2 lenses, only Dp71 expression is detected in P21 lenses. C) Immunoblotting analysis of distribution between the cytosolic and membrane-enriched fractions of P30 lenses reveals that dystrophin localizes predominantly to the membrane fraction. Data is shown for three biological replicates. GAPDH and Na+/K+ ATPase were immunoblotted as loading controls for the cytosolic and membrane-enriched fractions, respectively. D) Immunofluorescence-based analysis of distribution profile reveals the induction of dystrophin expression during fiber cell differentiation, with a robust upregulation in the differentiating secondary fibers (arrows) relative to the primary fibers. In embryonic, neonatal, and post-natal lenses, dystrophin is distributed robustly to lens fibers relative to the epithelium. Dp: Dystrophin; LE: Lens epithelium; LF: Lens fibers; M: Markers. Scale bar indicates image magnification.
Regarding the expression pattern of dystrophin in the developing lens, immunofluorescence-based analysis of E12.5 and E16.5 eyes revealed a robust increase in the expression of this protein in differentiating primary and secondary lens fibers. Interestingly, the expression appears to be more intense in the differentiating secondary lens fibers relative to primary fibers (Fig. 1D; arrows), which is evident in E16.5 specimens. Relatively, based on immunofluorescence analysis, dystrophin appears to be distributing intensely to the lens fibers in both P1 and P30 lenses compared to the epithelium (Fig. 1D). Immunoblotting analysis of lens capsule/epithelium derived from P30 mice confirms the presence of Dp71 in the epithelial fraction (Fig. S2).
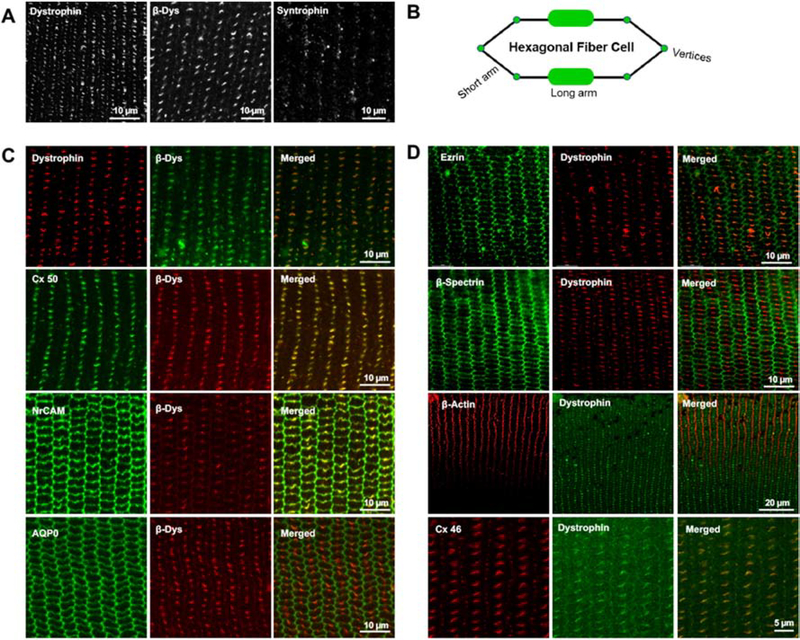
3.2. Dystrophin and β-dystroglycan exhibit a clustered distribution pattern at the center of the long arms of hexagonal lens fibers.
The cellular distribution pattern of dystrophin and its canonical membrane binding partner β-dystroglycan and their colocalization with different transmembrane and cytoskeletal proteins were evaluated by immunofluorescence analysis with confocal imaging using equatorial sections (paraffin-embedded) derived from P30 mouse lenses. Images were captured from the lens cortical region (100 μm from the lens epithelium). Dystrophin exhibits an intense and clustered distribution pattern localizing discretely at the center of the long arms of fiber cells with relatively minor distribution evident at the vertices of hexagonal fiber cells (Fig. 2A). Similarly, β-dystroglycan and syntrophin also reveal a clustered distribution pattern at the center of the long arms of hexagonal fiber cells (Fig. 2A). Fig. 2B shows a schematic representation of dystrophin and dystroglycan distribution in hexagonal lens fiber cell. As expected, dystrophin exhibits colocalization with β-dystroglycan (Fig. 2C). Connexin-50 (Cx50) and connexin-46 (Cx46) exhibit a very similar cellular distribution pattern and colocalization with dystrophin and β-dystroglycan in lens fibers (Figs. 2C and 2D). In contrast, β-actin, β-spectrin, and ezrin immunofluorescence distribute intensely to the short arms and vertices of lens fibers, with much less evidence for colocalization with either dystrophin or β-dystroglycan (Fig. 2D). In contrast, the aquaporin-0 (AQP0) and NrCAM signal was found to distribute uniformly throughout the hexagonal fiber cell plasma membrane with evidence for colocalization with β-dystroglycan at the center of the long arms of the fibers, a finding that was more obvious for NrCAM (Fig. 2C).
Figure 2: Distribution and colocalization patterns of dystrophin and β-dystroglycan in mouse lens fibers.
A) Immunofluorescence analysis of lens sections (equatorial plane) derived from P30 mice reveals a discrete distribution profile for dystrophin, β-dystroglycan, and syntrophin with the proteins exhibiting a large plaque-like organization pattern at the center of the long arms and a relatively reduced distribution at the vertices of the hexagonal fiber cells. Images are taken from the outer cortex region (100 μm from the epithelium) of the lens. B) This panel shows a schematic representation of dystrophin and β-dystroglycan distribution in hexagonal lens fiber cells. C & D) Colocalization of dystrophin or β-dystroglycan with Cx50 & Cx46, AQP0, NrCAM, ezrin, β-actin, and β-spectrin in the lens fibers of P30 mouse. While Cx50 & Cx46 show a strong colocalization with dystrophin or β-dystroglycan, colocalization with NrCAM, and AQP0 is evident only at the center of the long arms of the fibers. No evidence was found for colocalization of β-actin, β-spectrin, and ezrin with dystrophin or β-dystroglycan. Scale bar; 10 μm for all images except for β-actin; 20 μm. β-Dys: β-Dystroglycan.
3.3. Dystrophin deficient mdx3cv mice exhibit late-onset punctate nuclear opacities
To understand the role of dystrophin and the DAPC in maintaining lens growth, architecture, and transparency, we characterized lenses derived from dystrophin-deficient mdx3cv mice. Mdx3cv is a well-characterized mouse model carrying an ENU (N-ethyl-N-nitrosourea) induced mutation in the dystrophin gene, which results in a >90% deficiency of all dystrophin isoforms in various tissues [26]. Mdx3cv lenses from one-month-old mice were confirmed to be significantly deficient in Dp71 expression by ~ 90% based on RT-PCR (Fig. 3A) and qRT-PCR (Fig. 3B) analyses compared to littermate WT mouse lenses. Similarly, immunoblot analysis of 800 x g supernatants of mdx3cv mouse lenses (P1) detected almost no Dp71 and much-reduced levels of other isoforms of dystrophin, expect the higher molecular weight (>260 kDa) isoform, while WT lenses exhibit a prominent immunopositive band corresponding to Dp71 (Fig. 3C). Consistent with the immunoblot-based results, immunofluorescence analysis of dystrophin distribution revealed a dramatic reduction in P1 mdx3cv mouse lens sections (sagittal) compared to WT lenses (Fig. 3D).
Figure 3: Phenotype of mdx3cv mouse lenses.
A & B) RT-PCR and qRT-PCR based analyses, respectively, show a significant decrease (~90%) in Dp71 expression in one-month-old mdx3cv mouse lenses compared to littermate WT lenses. In panel A, data were shown for 3 to 4 biological replicates. Minus RT (-RT) indicates control reactions carried out without reverse transcriptase. C) Immunoblotting analysis of 800 x g supernatants confirms a dramatic decrease (almost undetectable) in Dp71 in P1 mdx3cv mouse lenses compared to WT lenses. Data from two representative biological replicates are shown. D) Similarly, immunofluorescence analysis of the P1 lens cryosections reveals a dramatic reduction in dystrophin staining in the mdx3cv mice compared to the WT mouse. E) Lenses derived from mdx3cv mice (one- and 6-month-old) show only a marginal but significant decrease in wet weight compared to littermate WT lenses. F) 7-month-old mdx3cv mice showed small and punctate opacities at the center of the lens compared to littermate WT lenses (identified with yellow arrows). GAPDH was immunoblotted as the loading control. Data in all the panels represent mean±SEM of 4 biological replicates. *P < 0.05, **P < 0.01, and ***P < 0.001.
Mdx3cv mice were found to be relatively slow breeders compared to WT controls, but litter size was comparable across the two groups of mice. While mdx3cv mice showed a marginal but significant decrease in body weight (by ~15 %) relative to WT controls at one month of age, no differences were evident between the two groups of animals at six months of age (data not shown). Similar to body weight, the lenses derived from one and six-month-old mdx3cv mice showed only a marginal but significant decrease in wet weight by ~14% and 6%, respectively compared to WT lenses (Fig. 3E). Interestingly, although mature lenses from the mdx3cv mice (1–4 months old) maintain transparency, lenses derived from older mdx3cv mice (older than 6 months, both males and females; based on 8 lenses derived from 4 mice) consistently revealed punctate nuclear opacities compared to the respective WT lenses (Fig. 3F, indicated with arrows).
3.4. Disruption of the dystrophin-associated protein complex (DAPC) in mdx3cv mouse lens
Dystrophin binds to various proteins including dystrobrevin, syntrophin, sarcoglycans, sarcospan, syncoilin, and nNOS in addition to dystroglycan and exists as a large multiprotein complex called the DAPC [16, 19]. This complex interacts with various membrane proteins, cytoskeletal proteins, and signaling proteins, and disruptions in the stability of DAPC have been shown to lead to different forms of muscle dystrophies [16, 18, 19]. Based on RNA-seq analysis (GSE143909), we identified the expression of various genes of the DAPC proteins in one-month-old mouse lenses (Table 1; based on two biological replicates), indicating the existence of DAPC in the lens. Having confirmed the expression of various DAPC components in the lens, we undertook an evaluation of DAPC stability in mdx3cv lenses. To this end, we isolated the membrane-enriched fraction of lenses derived from one month- and 4-month-old mdx3cv and littermate WT mice to determine the levels of various DAPC components by immunoblot analysis. This analysis revealed a significant decrease in the levels of β-dystroglycan, dystrobrevin, and syntrophin along with Dp71 in both one and 4-month-old mdx3cv lenses compared to WT lenses. Figs. 4A–D show data from one-month-old lenses, with very similar data being observed in 4-month-old lenses (Fig. S3). Consistent with the immunoblotting data, immunofluorescence analysis showed a significant decrease of β-dystroglycan staining in mdx3cv lenses (one-month-old, equatorial sections) compared to WT lenses (Fig. 4C and D), collectively indicating a disruption or compromise in the DAPC integrity and potentially the DAPC regulated activities in the lens under deficiency of dystrophin.
Table 1:
Expression of the DAPC genes in mouse lens based on RNA-seq analysis.
| Gene | Relative Units* |
|---|---|
| Dystrophin | 716 |
| Dystrogylcan | 5700 |
| Dystrobrevin binding protein 1 | 5000 |
| Dystrobrevin-α | 3000 |
| Dystrobrevin-β | 300 |
| Dystrophin binding protein 1 | 40 |
| Sarcoglycan-α | 35 |
| Sarcoglycan-β | 570 |
| Sarcoglycan-γ | 20 |
| Sarcoglycan-δ | 60 |
| Sarcoglycan-ε | 560 |
| Sarcospan | 620 |
| Syntrophin acidic 1 | 9000 |
| Syntrophin basic 1 | 1900 |
| Syntrophin basic 2 | 1100 |
| Utrophin | 1500 |
| Drp2 (Dystrophin related protein 2) | 121 |
| nNOS 1 | 170 |
| Laminin α1 | 2400 |
| Laminin α2 | 3700 |
| Laminin α3 | 650 |
| Laminin α5 | 3900 |
| Laminin β1 | 4500 |
| Laminin β2 | 800 |
| Laminin β3 | 30 |
| Laminin γ1 | 4100 |
| Laminin γ2 | 90 |
| Laminin γ3 | 22 |
| Agrin | 650 |
| Perlecan | 37250 |
| Fukutin (FKTN) | 1920 |
| Fukutin related protein (FKRP) | 1292 |
| Protein-o-mannosyl transferase 1 (POMT1) | 705 |
| POMT2 | 411 |
| POMGNT1 | 1767 |
| DPM2 | 506 |
| DPM3 | 340 |
Values are the mean of two biological replicates of one-month-old lenses.
Figure 4: Disruption of the DAPC stability in dystrophin-deficient mouse lenses.
A & B) Evaluation of DAPC protein levels in membrane-enriched fractions derived from mdx3cv lenses (one-month-old) by immunoblot analysis and densitometric quantification revealed significant decreases in the levels of β-dystroglycan, dystrobrevin, syntrophin along with dystrophin compared to the corresponding WT control samples. C & D) Immunofluorescence based analysis of the distribution of β-dystroglycan in mdx3cv mouse lens fibers (equatorial paraffin sections) reveals not only a much smaller size in its clustered distribution pattern but also significantly reduced staining intensity compared to the respective WT lens fibers. Na+/K+ ATPase was immunoblotted as the loading control. Data in panels B & D represent mean±SEM of 4 biological replicates. *P < 0.05; ***P < 0.001. Scale bar indicates image magnification.
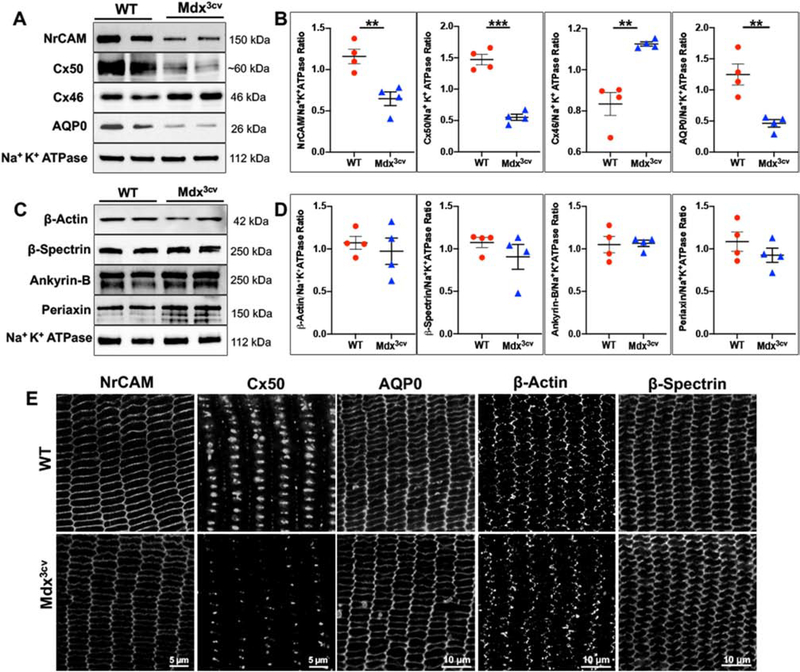
3.5. Disruption of the membrane organization of NrCAM, Cx50, and AQP0 in dystrophin-deficient lenses
To determine how dystrophin deficiency and disruption of the DAPC impact membrane organization and stability of some predominant channel proteins and cell adhesion proteins in lens fibers, we used immunoblotting analysis to determine the levels of Cx50, Cx46, NrCAM, and AQP0 in membrane-enriched fractions derived from one month and 4-month-old mdx3cv and littermate WT lenses. This analysis revealed a significant decrease in the levels of Cx50, NrCAM, and AQP0 in mdx3cv lenses compared to WT lenses from both one- and 4-month-old mice. Interestingly, in contrast to Cx50, a slight but significant increase was noted in the levels of Cx46 in mdx3cv lenses relative to WT lenses. Figures 5A–E show representative data from onemonth-old lenses. Fig. S2 shows data from 4-month-old lenses.
Figure 5: Dystrophin deficiency-induced changes in membrane organization and stability of channel, cell adhesive, scaffolding, and cytoskeletal proteins in lens fibers.
A-D) Immunoblot analysis with subsequent quantification was performed to evaluate changes in the levels of Cx50, Cx46, AQP0, NrCAM, ankyrin-B, periaxin, β-actin, and β-spectrin in membrane-enriched fractions of mdx3cv and WT lenses (one-month-old). These analyses revealed a significant decrease in protein levels of NrCAM, Cx50, AQP0, and an increase in protein levels of Cx46 in mdx3cv lenses compared to WT lenses. In contrast, no differences were detected in the levels of β-actin, β-spectrin, ankyrin-B, and periaxin between mdx3cv and WT lenses. E) Immunofluorescence –based analysis revealed a dramatic decrease in Cx50 plaque size and staining, a notable decrease in immunostaining for AQP0 and NrCAM along the long arms. In contrast, there were no noticeable differences in the distribution pattern of β-actin and β-spectrin between the mdx3cv and WT lens fibers. Na+/K+ ATPase was immunoblotted as the loading control. Data represent mean±SEM of 4 biological replicates. **P < 0.01; **P < 0.001. Scale bar: Image magnification.
Using the same membrane-enriched fractions described above, we also evaluated levels of the predominant membrane skeletal proteins β-actin and β-spectrin, and membrane scaffolding proteins including ankyrin-B and periaxin by immunoblotting analysis. No significant differences were noted in the levels of the aforementioned proteins between mdx3cv and WT lens membrane fractions from one- or four-month-old animals (Figs. 5C, D).
Immunofluorescence analysis supported the results derived from immunoblotting analyses, revealing a dramatic reduction in Cx50 staining in equatorial sections from one-month-old mdx3cv lenses relative to WT lenses. Although the staining of AQP0 and NrCAM was not as dramatically reduced as that observed for Cx50, there seems to be a reduction in immunostaining for both AQP0 and NrCAM at the long arms of hexagonal fibers from mdx3cv lenses compared to WT lenses (Fig. 5E). In contrast, immunostaining for β-actin and β-spectrin was found to be very similar in equatorial sections from one-month-old mdx3cv and WT lenses, as shown in Fig. 5E.
3.6. Dystrophin deficiency decreases lens stiffness
Having observed disruptions in the stability and membrane organization of the DAPC, Cx50, AQP0, and NrCAM in dystrophin-deficient lenses, we asked whether dystrophin deficiency affects tensile properties of the lens. For this, we evaluated changes in lens stiffness by performing stress/strain analysis using a microstrain analyzer. Lenses from both two and four-month-old mdx3cv mice exhibited a moderate but significant decrease (by ~20% and 16%, respectively) in Young’s modulus (stiffness) compared to littermate WT lenses (Figs. 6A and B). As expected, the stiffness of 4-month-old lenses was higher that of 2-month-old lenses in both wild type and mutant mice, with the age-dependent difference in stiffness being significant only for mutant lenses.
Figure 6: Decreased lens stiffness in dystrophin-deficient mouse lenses.
A & B) Lenses derived from 2 and 4-month-old mdx3cv and respective WT mice were evaluated for changes in stiffness using a microstrain analyzer. The analysis revealed a moderate but significant decrease in Young’s modulus (stiffness) in dystrophin-deficient mdx3cv lenses compared to WT lenses. As expected, the stiffness of four-month-old lenses (both wild type and mutant) was higher than two-month-old lenses, with the difference in age-dependent increase of stiffness being significant (P<0.05) only in the mutant groups of lenses. Values are mean±SEM of 8 biological replicates. *P < 0.05.
3.7. Dystrophin deficiency affects myosin II activity in lens
Since the DAPC plays a critical role not only in maintaining mechanical stability and membrane organization but also in the activities of ion transporters and signaling proteins [16], we evaluated whether dystrophin deficiency influences the contractile and relaxation properties of the lens. Immunoblotting analysis of myosin light chain phosphorylation was utilized to explore this possibility, since MLC is a key regulator of myosin II activity. Myosin II plays a crucial role in regulating contractile activity of various non-muscle tissues via calcium-dependent and independent mechanisms/pathways [27]. The levels of phospho-MLC were noted to be significantly decreased in dystrophin-deficient mdx3cv lenses (from 4-month-old mice; one-month-old lenses were not analyzed) as compared to WT lenses. Levels of total MLC protein remained unchanged in mdx3cv lenses compared to WT lenses as shown in Figs. 7A–C. These results together with the changes observed in tensile properties of mdx3cv lenses show the importance of dystrophin and the DAPC in maintaining the contractile and mechanical properties of the ocular lens.
Figure 7: Dystrophin deficiency impairs myosin light chain phosphorylation (pMLC) in the lens.
A) To determine whether dystrophin deficiency impacts myosin II activity in the lens, we determined the status of MLC phosphorylation, a key regulator of myosin II activity, by immunoblotting analysis. The levels of pMLC were significantly decreased in mdx3cv lenses compared to WT lenses and total MLC levels remain unchanged in mdx3cv lenses compared to WT lenses. B & C) Quantitative changes in the levels of pMLC and MLC in mdx3cv mouse lenses in comparison with WT lenses. Values are mean±SEM of 4 biological replicates. **P<0.01.
3.8. Compensatory upregulation of utrophin expression in dystrophin-deficient lenses
Utrophin (product of UTRN), an autosomal homolog of dystrophin is expressed in various tissues, and shares both structural and sequence homology with dystrophin including actin and dystroglycan binding characteristics [28, 29]. Utrophin has been shown to exhibit a compensatory upregulation of expression under dystrophin deficiency in different tissues [30–32]. Since we had noted the expression of utrophin in our mouse lens RNA-seq data (Table 1), we analyzed the levels of utrophin in the 800 x g supernatants of mdx3cv and WT lenses by immunoblotting analysis. One-month-old and 4-month-old WT mouse lenses showed a prominent immunopositive protein band with a molecular mass of much higher than 250 kDa marker corresponding to the high molecular mass full-length utrophin protein (Fig. 8A and 8C). In mdx3cv lenses, there was a significant increase in the levels of high molecular mass (>250 kDa) utrophin in both one month and 4-month-old lenses relative to the respective WT lenses (Figs. 8A–D), indicating a compensatory increase in utrophin levels in the lens under deficiency of dystrophin as has been reported in other tissues[30–32].
Figure 8: Compensatory upregulation of utrophin in dystrophin-deficient lenses.
Since mdx3cv mouse lenses showed only small punctate nuclear opacities, we used immunoblotting analysis to determine the levels of utrophin, a functional homolog of dystrophin, in lenses (800 x g supernatant) from one and 4-month-old mdx3cv and respective WT mice. A & B and C & D show significantly increased levels of utrophin in both one- and 4-month-old dystrophin-deficient mdx3cv lenses relative to WT lenses, respectively. The band above 250kDa was used for quantification. GAPDH was immunoblotted for loading control. Values are mean±SEM of 4 biological replicates. **P < 0.01.
3.9. Identification of α-dystroglycan ECM ligands in lens fibers
The dystrophin-dystroglycan complex links the intracellular actin cytoskeleton to the ECM through transmembrane dystroglycan [33]. Dystroglycan, which is encoded by a single gene undergoes posttranslational cleavage resulting in two subunits; α-dystroglycan and β-dystroglycan. The C-terminal and N-terminal regions of β-dystroglycan, a single-pass transmembrane protein, bind to dystrophin and α-dystroglycan, respectively [34]. The cell surface-localized α-dystroglycan, which is also glycosylated enzymatically in turn, binds to ECM ligands including, laminin, perlecan, and agrin [34–36]. The mouse lens expresses many of the genes involved in dystroglycan glycosylation including POMT1, POMT2, POMGNT, FKTN, and DPM (Table 1). Moreover, mutations in some of these genes, and impairment of dystroglycan binding to ECM proteins have been shown to lead to cataract, indicating the importance of dystrophin/dystroglycan complex in maintaining lens function [37, 38]. To identify α-dystroglycan binding ECM ligands in lens fibers, we isolated the ECM enriched fraction from the one-month-old mouse lens fiber mass and performed proteomics analysis as described in the Methods section. Table 2 lists the different ECM proteins identified in the ECM-enriched fraction of the mouse lens fiber mass. Importantly, laminins and perlecan (Hspg2), which are the known ECM ligands of α-dystroglycan are readily detectable in the lens fiber mass. Additionally, several isoforms of collagen, and nidogen-1 and 2 were also detected. These data were based on 4 biological replicates.
Table 2:
Extracellular Matrix proteins identified in the mouse lens fiber mass based on proteomics analysis.
| Protein Name | Accession No. | Alternate ID | MW (kDa) | Peptide# (unique) | % Coverage |
|---|---|---|---|---|---|
| Basement membrane-specific heparan sulfate proteoglycan core protein (Perlecan) | PGBM | Hspg2 | 398 | 42 | 15 |
| Laminin subunit gamma 1 | LAMC1 | Lamc1 | 177 | 16 | 13 |
| Laminin subunit beta 1 | LAMB1 | Lamb1 | 197 | 17 | 13 |
| Laminin subunit alpha 2 | LAMA2 | Lama2 | 344 | 5 | 2 |
| Nidogen-1 | NID1 | Nid1 | 136 | 27 | 27 |
| Nidogen-2 | NID2 | Nid2 | 154 | 6 | 6 |
| Collagen alpha 2(IV) chain | CO4A2 | Col4a2 | 167 | 10 | 9 |
| Collagen alpha 1(IV) chain | CO4A1 | Col4a1 | 161 | 8 | 7 |
| Collagen alpha 2(I) chain | CO1A2 | Col1a2 | 129 | 2 | 1 |
| Collagen alpha 1(XII) chain | COCA1 | Col12a1 | 340 | 2 | 1 |
| Collagen alpha 4(VI) chain | CO6A4 | Col6a4 | 251 | 2 | 1 |
| Collagen alpha 1(XXIV) chain | COOA1 | Col24a1 | 175 | 2 | 1 |
| Collagen alpha 1(XVIII) chain | COIA1 | Col18a1 | 182 | 2 | 2 |
| Extracellular matrix protein FRAS1 | FRAS1 | Fras1 | 442 | 2 | 0.1 |
In addition to mass spectrometry-based identification, we confirmed the presence of perlecan, laminin, and endostatin producing collagen (Col 18a1) in lens fibers by immunoblot analysis, with these proteins being easily detected in the membrane-enriched fraction of mouse lens fibers (Fig. 9A).
Figure 9: Detection and distribution of α-dystroglycan ECM ligands in lens fibers.
A) Immunoblotting based detection of perlecan, laminin, and endostatin producing collagen XVIII (Col 18a) in WT mouse lens fiber insoluble fractions. B & C) Immunoblot and densitometry-based evaluation for changes in the levels of perlecan and laminin in the membrane fraction of lens fibers derived from one-month-old mdx3cv and WT lenses showed no differences between the two groups. Values in panels B and C represent mean±SEM of 3–4 biological replicates.
Immunoblotting analysis of membrane lysates derived from lens fiber mass samples of one-month-old mdx3cv and littermate WT mice (30 μg) revealed that dystrophin deficiency does not affect perlecan and laminin levels in the lens (Figs. 9B and 9C).
4. Discussion
In this study, we focused on addressing the functional significance of the DAPC in lens fiber cell cytoarchitecture, membrane organization, and mechanical stability since the absence of dystrophin has been reported to lead to cataract development. Using the dystrophin-deficient mdx3cv mouse model, we investigated how the deficiency of dystrophin influences the stability and organization of membrane proteins, DAPC, membrane skeleton, cell shape, myosin II activity, and the stiffness of lens. Interestingly, although mdx3cv mouse lenses were >90% deficient in dystrophin, only older mice showed small punctate nuclear opacities and upregulation of utrophin, a functional homolog of dystrophin. Despite compensatory elevated levels of utrophin, membrane organization, and stability of the DAPC, gap junctional, water channel, and cell junctional proteins were disrupted in mdx3cv lenses indicating the importance of dystrophin and the DAPC in maintaining membrane organization and the stability of several transmembrane proteins. We also obtained evidence for the role of dystrophin and DAPC in maintaining myosin II activity and tensile properties of the lens, and the presence of laminin and perlecan which are known ligands of α-dystroglycan in lens fibers. Collectively, these findings indicate that the increased levels of utrophin likely provide a partial compensatory response under dystrophin deficiency, and potentially slow down development of mature cataracts in mdx3cv mice despite the noted disruptions in the stability of DAPC and membrane organization of Cx50 and NrCAM in lens fibers.
The ribbon-like long differentiated fibers that constitute the bulk of the lens, maintain a close lateral adhesion and compaction [1, 4]. The plasma membrane, transmembrane proteins, membrane-associated cytoskeletal and scaffolding proteins are the major components of lens fibers [1, 2, 6–8]. Although differentiated fibers lose all cellular organelles [3], they maintain active transport mechanisms through gap junctions, water channels, and ion channels [7–9, 39], and cell-cell adhesive interactions are required for lens architecture and function [40–42]. Importantly, lens shape undergoes frequent changes during visual accommodation [21, 43]. Maintenance of the properties of deformability and mechanical resilience are integral for the ability of the lens to accommodate without undergoing mechanical damage [5, 20, 21, 44]. Therefore, the molecular mechanisms regulating the tethering of cytoskeletal proteins to membrane proteins, and binding interactions of membrane proteins with the extracellular matrix are expected to play a crucial role in the organization of membrane proteins and mechanical stability of the lens plasma membrane. The DAPC is a well-recognized macromolecular complex that links the intracellular cytoskeleton to the ECM through the plasma membrane [16, 19]. Perturbations in the integrity of this multi-protein complex have been shown to lead to various types of muscle dystrophies [16, 18, 45], and cardiac and neurological abnormalities [15, 19, 46, 47]. Based on these well-established characteristics of the DAPC, we reasoned that this multiprotein complex likely plays a crucial role in maintaining lens integrity and function.
Although the cataract phenotype has been reported in humans and mouse models under deficiency of various dystrophin isoforms, there is no clear or conclusive understanding regarding whether the occurrence of cataract phenotype is isoform-specific or results from the involvement of multiple isoforms [11–13]. In this study, we characterized lenses derived from the mdx3cv mouse and unlike previously used models including the mdx mouse (which has a mutation in the dystrophin gene but is not deficient in Dp71) and Dp71 knockout mice which are not deficient in full-length dystrophin, mdx3cv mice are reported to be deficient in all isoforms of dystrophin [11, 26, 48].
Consistent with a previous report [13], in this study, we found that the expression of Dp71, which is a predominant dystrophin isoform in mouse lens, is induced during fiber cell differentiation, with the protein distributing robustly to fiber cells relative to the epithelium in mature lenses. Although, we detected the expression of several isoforms of dystrophin including the full length, and the 260 kDa, 140 kDa, and 40 kDa forms in P2 lenses, these isoforms were barely detectable in P21 lenses by immunoblotting analysis indicating maturation-dependent differential regulation of expression of dystrophin isoforms in the lens. Moreover, on a relative basis, there seems to be a robust upregulation of dystrophin expression in differentiating secondary fibers compared to the primary fibers. However, the deficiency of dystrophin in the mdx3cv mouse lens did not result in overt changes in lens differentiation based on the expression and distribution pattern of the differentiation-specific proteins. Only older mdx3cv mice (>6 months) showed small punctate opacities in the nucleus. Interestingly, the mdx3cv mice revealed an upregulated expression of utrophin, a homolog of dystrophin [49]. Therefore, we speculate that a compensatory increase in utrophin along with low levels of dystrophin expression observed in mdx3cv mice appears to prevent the development of mature cataracts in this mouse model. As observed for the lens tissue in this study, an increase in the levels of utrophin has also been reported under deficiency of dystrophin in other tissues[30–32]. In contrast to the mdx3cv mouse model, it is not known whether or not utrophin levels are altered in the Dp71 null mouse, which has been shown to develop progressive cataracts starting from two-months of age [13]. In addition to the complete absence of Dp71 in the Dp71 null mice, the specifics of the genetic background of the reported lens phenotype in Dp71 null mice are not known [13]. Unlike Dp71 null lenses, the adult mdx3cv lens did not exhibit overt changes in fiber cell organization, shape, or organization of actin and spectrin cytoskeleton, a finding that may be related to the minor lens phenotype noted in the mdx3cv lens as compared to the prominent phenotype reported in adult Dp71 null lenses [13].
One of the consistent findings in the mdx3cv lenses was the disruption of the DAPC and perturbation of membrane organization and stability of Cx50, NrCAM, and to a lesser extent of AQP0, as evidenced by the altered distribution profiles and decreased levels of these proteins. Significantly, these changes were associated with disruptions in the membrane organization and the integrity of DAPC in lens fibers. Membrane distribution of dystrophin and dystroglycan, which are key components of the DAPC, and protein levels of β-dystroglycan, syntrophin, and dystrobrevin, considered other major components of the DAPC, were significantly decreased in lens fibers deficient in dystrophin, revealing disruption of structural and functional integrity of the DAPC in mdx3cv lenses. The DAPC plays a key role in membrane organization of various channel proteins and transport proteins by linking the cytoskeleton to plasma membrane proteins [16, 19]. Further, this complex also regulates the scaffolding of various signaling proteins with the plasma membrane, influencing the activity of these proteins [16].
For example, Cx50 colocalizes with β-dystroglycan and distributes as a large cluster at the center of the large arm of the fiber cell [6, 10]. In mdx3cv lenses, the membrane organization of both Cx50 and β-dystroglycan is dramatically disrupted. Although we did not obtain direct evidence for an interaction between Cx50 and DAPC as underlying their observed colocalization in lens fibers, these proteins may interact via syntrophin. Syntrophins contain a PDZ domain [50], and Cx50 interacts with PDZ domain proteins including ZO-1 [51]. Membrane distribution of Cx46 on the other hand which may not bind to PDZ domain proteins was not disrupted, with the levels of this protein being slightly increased in dystrophin-deficient lens fibers. Similar to Cx50, NrCAM has also been shown to interact with certain PDZ domain proteins in neurons [52]. Therefore, it is possible that NrCAM in lens fibers interacts with and binds to the PDZ domain of syntrophin.
We had previously reported that deficiency of periaxin, which is a PDZ domain protein similar to syntrophin, results in disruption of membrane organization of dystroglycan, a component of DAPC, and decreases in the protein levels of dystrophin and NrCAM in lens fibers [6]. In contrast, the deficiency of dystrophin in mdx3cv lenses appears to have no effects on either membrane organization or protein levels of periaxin, indicating that the involvement of periaxin lies upstream of DAPC in membrane scaffolding interactions of lens fibers. Aquaporin-4 has been reported to interact with the DAPC, with dystrophin deficiency disrupting the membrane organization and activity of aquaporin-4 in skeletal muscle and brain [53, 54]. While only subtle changes were evident in the distribution profile of AQP0 within hexagonal lens fibers of mdx3cv lenses, AQP0 protein levels were significantly decreased relative to WT lenses, indicating a plausible involvement of the DAPC in maintaining water channel stability in the lens. This latter conclusion was consistent with the results reported in the Dp71 null mouse lenses [13]. Although we did not find any obvious changes in the lens epithelial phenotype in mdx3cv mice compared to WT lenses, it will be important to determine the effects of Dp71 deficiency on the lens epithelium in future studies, since we have confirmed the presence of this protein in the epithelium.
Mdx3cv lenses revealed decreased lens stiffness very similar to ankyrin-B and periaxin deficient lenses [6]. Interestingly, in the mdx3cv mouse lens, and in the ankyrin-B and periaxin deficient lenses [6], decreased lens stiffness was associated with disruption of the DAPC and decreased myosin light chain phosphorylation, a key regulator of myosin II contractile activity [27]. Regulation of myosin light chain phosphorylation is mediated via both, calcium dependent and independent mechanisms [27]. It is plausible that disruption of the DAPC under deficiency of dystrophin alters the activity of various ion channels (including the gap junctions, L-type channels, and piezo channels), resulting in changes in the intracellular calcium levels and thereby in myosin light chain phosphorylation and myosin II activity, collectively affecting the tensile properties of the lens.
5. Conclusions
This study reports a detailed characterization of the dystrophin-deficient mdx3cv mouse lens revealing that dystrophin deficiency induces only late-onset mild punctate nuclear opacities, and a compensatory upregulation of utrophin, a functional homolog of dystrophin. However, the disruptions in DAPC integrity, impaired myosin II activity, tensile properties, and membrane organization and stability of Cx50, NrCAM, and AQP0 noted in dystrophin-deficient mouse lenses uncovers an important role for dystrophin and DAPC in maintaining fiber cell organization and stability of membrane integral channel and cell adhesion proteins, and ultimately mechanical properties of the ocular lens.
Supplementary Material
Highlights.
Aged dystrophin deficient mdx3cv mouse develops punctate nuclear lens opacities.
Dystrophin deficiency disrupts the integrity of DAPC in the lens.
Dystrophin deficiency impairs the stability of lens fiber membrane integral proteins.
Dystrophin deficient lens exhibits a compensatory increase in utrophin expression.
Mdx3cv mouse exhibits a decrease in lens stiffness.
Acknowledgments
We thank Vann Bennett, Ph.D., Joseph Horwitz, Ph.D., Harold P. Erickson, Ph.D., and Takako Sasaki, Ph.D., for providing anti- ankyrin-B, anti-AQP0, anti-laminin, and anti-Collagen XVIII antibodies, respectively. We also thank Rupalatha Maddala, Ph.D., for her help in immunofluorescence analysis, and Mark Walters, Ph.D., for his help with the analysis of lens stiffness.
Grants
This work was supported by the National Institutes of Health (R01-EY 025096).
Abbreviations:
- AQP0
Aquaporin-0
- Cx
Connexin
- DAPC
Dystrophin associated-protein complex
- DGC
Dystrophin-dystroglycan complex
- DDA
Data Dependent Acquisition
- DTT
Dithiothreitol
- ECM
Extracellular matrix
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- MLC
Myosin light chain
- MS/MS
Tandem Mass Spectrometry
- PAGE
Polyacrylamide gel electrophoresis
- PCR
Polymerase chain reaction
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- WT
Wild type
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Bassnett S, Shi Y, Vrensen GF, Biological glass: structural determinants of eye lens transparency, Philos Trans R Soc Lond B Biol Sci 366(1568) (2011) 1250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cheng C, Nowak RB, Fowler VM, The lens actin filament cytoskeleton: Diverse structures for complex functions, Exp Eye Res 156 (2017) 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bassnett S, On the mechanism of organelle degradation in the vertebrate lens, Exp Eye Res 88(2) (2009) 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kuszak JR, Peterson KL, Brown HG, Electron microscopic observations of the crystalline lens, Microsc Res Tech 33(6) (1996) 441–79. [DOI] [PubMed] [Google Scholar]
- [5].Cheng C, Nowak RB, Amadeo MB, Biswas SK, Lo WK, Fowler VM, Tropomyosin 3.5 protects the F-actin networks required for tissue biomechanical properties, J Cell Sci 131(23) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maddala R, Walters M, Brophy PJ, Bennett V, Rao PV, Ankyrin-B directs membrane tethering of periaxin and is required for maintenance of lens fiber cell hexagonal shape and mechanics, Am J Physiol Cell Physiol 310(2) (2016) C115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schey KL, Petrova RS, Gletten RB, Donaldson PJ, The Role of Aquaporins in Ocular Lens Homeostasis, Int J Mol Sci 18(12) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mathias RT, White TW, Gong X, Lens gap junctions in growth, differentiation, and homeostasis, Physiol Rev 90(1) (2010) 179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maddala R, Nagendran T, de Ridder GG, Schey KL, Rao PV, L-type calcium channels play a critical role in maintaining lens transparency by regulating phosphorylation of aquaporin-0 and myosin light chain and expression of connexins, PLoS One 8(5) (2013) e64676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang E, Geng A, Maniar AM, Mui BW, Gong X, Connexin 50 Regulates Surface Ball-and-Socket Structures and Fiber Cell Organization, Invest Ophthalmol Vis Sci 57(7) (2016) 3039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mirabella M, Galluzzi G, Manfredi G, Bertini E, Ricci E, De Leo R, Tonali P, Servidei S, Giant dystrophin deletion associated with congenital cataract and mild muscular dystrophy, Neurology 51(2) (1998) 592–5. [DOI] [PubMed] [Google Scholar]
- [12].Kurihara T, Kishi M, Saito N, Komoto M, Hidaka T, Kinoshita M, Electrical myotonia and cataract in X-linked muscular dystrophy (mdx) mouse, J Neurol Sci 99(1) (1990) 83–92. [DOI] [PubMed] [Google Scholar]
- [13].Fort PE, Darche M, Sahel JA, Rendon A, Tadayoni R, Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization, Mol Vis 20 (2014) 1480–90. [PMC free article] [PubMed] [Google Scholar]
- [14].Hoffman EP, Brown RH Jr., Kunkel LM, Dystrophin: the protein product of the Duchenne muscular dystrophy locus, Cell 51(6) (1987) 919–28. [DOI] [PubMed] [Google Scholar]
- [15].Sunada Y, Campbell KP, Dystrophin-glycoprotein complex: molecular organization and critical roles in skeletal muscle, Curr Opin Neurol 8(5) (1995) 379–84. [PubMed] [Google Scholar]
- [16].Constantin B, Dystrophin complex functions as a scaffold for signalling proteins, Biochim Biophys Acta 1838(2) (2014) 635–42. [DOI] [PubMed] [Google Scholar]
- [17].Muntoni F, Torelli S, Ferlini A, Dystrophin and mutations: one gene, several proteins, multiple phenotypes, Lancet Neurol 2(12) (2003) 731–40. [DOI] [PubMed] [Google Scholar]
- [18].Ehmsen J, Poon E, Davies K, The dystrophin-associated protein complex, J Cell Sci 115(Pt 14) (2002) 2801–3. [DOI] [PubMed] [Google Scholar]
- [19].Batchelor CL, Winder SJ, Sparks, signals and shock absorbers: how dystrophin loss causes muscular dystrophy, Trends Cell Biol 16(4) (2006) 198–205. [DOI] [PubMed] [Google Scholar]
- [20].Cheng C, Parreno J, Nowak RB, Biswas SK, Wang K, Hoshino M, Uesugi K, Yagi N, Moncaster JA, Lo WK, Pierscionek B, Fowler VM, Age-related changes in eye lens biomechanics, morphology, refractive index and transparency, Aging (Albany NY) 11(24) (2019) 12497–12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang K, Pierscionek BK, Biomechanics of the human lens and accommodative system: Functional relevance to physiological states, Prog Retin Eye Res 71 (2019) 114–131. [DOI] [PubMed] [Google Scholar]
- [22].Shin JH, Hakim CH, Zhang K, Duan D, Genotyping mdx, mdx3cv, and mdx4cv mice by primer competition polymerase chain reaction, Muscle Nerve 43(2) (2011) 283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maddala R, Nagendran T, Lang RA, Morozov A, Rao PV, Rap1 GTPase is required for mouse lens epithelial maintenance and morphogenesis, Dev Biol 406(1) (2015) 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Naba A, Clauser KR, Hynes RO, Enrichment of Extracellular Matrix Proteins from Tissues and Digestion into Peptides for Mass Spectrometry Analysis, J Vis Exp (101) (2015) e53057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho LTY, Skiba N, Ullmer C, Rao PV, Lysophosphatidic Acid Induces ECM Production via Activation of the Mechanosensitive YAP/TAZ Transcriptional Pathway in Trabecular Meshwork Cells, Invest Ophthalmol Vis Sci 59(5) (2018) 1969–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cox GA, Phelps SF, Chapman VM, Chamberlain JS, New mdx mutation disrupts expression of muscle and nonmuscle isoforms of dystrophin, Nat Genet 4(1) (1993) 87–93. [DOI] [PubMed] [Google Scholar]
- [27].Somlyo AP, Somlyo AV, Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II, J Physiol 522 Pt 2 (2000) 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tinsley JM, Blake DJ, Zuellig RA, Davies KE, Increasing complexity of the dystrophin-associated protein complex, Proc Natl Acad Sci U S A 91(18) (1994) 8307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blake DJ, Tinsley JM, Davies KE, Utrophin: a structural and functional comparison to dystrophin, Brain Pathol 6(1) (1996) 37–47. [DOI] [PubMed] [Google Scholar]
- [30].Perkins KJ, Davies KE, Alternative utrophin mRNAs contribute to phenotypic differences between dystrophin-deficient mice and Duchenne muscular dystrophy, FEBS Lett 592(11) (2018) 1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weir AP, Burton EA, Harrod G, Davies KE, A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle, J Biol Chem 277(47) (2002) 45285–90. [DOI] [PubMed] [Google Scholar]
- [32].Pons F, Nicholson LV, Robert A, Voit T, Leger JJ, Dystrophin and dystrophin-related protein (utrophin) distribution in normal and dystrophin-deficient skeletal muscles, Neuromuscul Disord 3(5–6) (1993) 507–14. [DOI] [PubMed] [Google Scholar]
- [33].Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP, Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix, Nature 355(6362) (1992) 696–702. [DOI] [PubMed] [Google Scholar]
- [34].Barresi R, Campbell KP, Dystroglycan: from biosynthesis to pathogenesis of human disease, J Cell Sci 119(Pt 2) (2006) 199–207. [DOI] [PubMed] [Google Scholar]
- [35].Colombelli C, Palmisano M, Eshed-Eisenbach Y, Zambroni D, Pavoni E, Ferri C, Saccucci S, Nicole S, Soininen R, McKee KK, Yurchenco PD, Peles E, Wrabetz L, Feltri ML, Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin, J Cell Biol 208(3) (2015) 313–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Endo T, Glycobiology of alpha-dystroglycan and muscular dystrophy, J Biochem 157(1) (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [37].van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, Huynen MA, Verrips A, Walsh CA, Barth PG, Brunner HG, van Bokhoven H, POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome, J Med Genet 42(12) (2005) 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Topaloglu H, Yetuk M, Talim B, Akcoren Z, Caglar M, Merosin-positive congenital muscular dystrophy with mental retardation and cataracts: a new entity in two families, Eur J Paediatr Neurol 1(4) (1997) 127–31. [DOI] [PubMed] [Google Scholar]
- [39].Delamere NA, Mandal A, Shahidullah M, The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium, J Ocul Pharmacol Ther 32(8) (2016) 504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Logan CM, Rajakaruna S, Bowen C, Radice GL, Robinson ML, Menko AS, N-cadherin regulates signaling mechanisms required for lens fiber cell elongation and lens morphogenesis, Dev Biol 428(1) (2017) 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maddala R, Rao PV, Switching of alpha-Catenin From Epithelial to Neuronal Type During Lens Epithelial Cell Differentiation, Invest Ophthalmol Vis Sci 58(9) (2017) 3445–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].More MI, Kirsch FP, Rathjen FG, Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation, J Cell Biol 154(1) (2001) 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weeber HA, van der Heijde RG, On the relationship between lens stiffness and accommodative amplitude, Exp Eye Res 85(5) (2007) 602–7. [DOI] [PubMed] [Google Scholar]
- [44].Fudge DS, McCuaig JV, Van Stralen S, Hess JF, Wang H, Mathias RT, FitzGerald PG, Intermediate filaments regulate tissue size and stiffness in the murine lens, Invest Ophthalmol Vis Sci 52(6) (2011) 3860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Martin PT, Dystroglycan glycosylation and its role in matrix binding in skeletal muscle, Glycobiology 13(8) (2003) 55R–66R. [DOI] [PubMed] [Google Scholar]
- [46].Finsterer J, Cripe L, Treatment of dystrophin cardiomyopathies, Nat Rev Cardiol 11(3) (2014) 168–79. [DOI] [PubMed] [Google Scholar]
- [47].Waite A, Brown SC, Blake DJ, The dystrophin-glycoprotein complex in brain development and disease, Trends Neurosci 35(8) (2012) 487–96. [DOI] [PubMed] [Google Scholar]
- [48].Sarig R, Mezger-Lallemand V, Gitelman I, Davis C, Fuchs O, Yaffe D, Nudel U, Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: differential activity of the Dp71 promoter during development, Hum Mol Genet 8(1) (1999) 1–10. [DOI] [PubMed] [Google Scholar]
- [49].Nguyen TM, Le TT, Blake DJ, Davies KE, Morris GE, Utrophin, the autosomal homologue of dystrophin, is widely-expressed and membrane-associated in cultured cell lines, FEBS Lett 313(1) (1992) 19–22. [DOI] [PubMed] [Google Scholar]
- [50].Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS, Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains, Cell 84(5) (1996) 757–67. [DOI] [PubMed] [Google Scholar]
- [51].Chai Z, Goodenough DA, Paul DL, Cx50 requires an intact PDZ-binding motif and ZO-1 for the formation of functional intercellular channels, Mol Biol Cell 22(23) (2011) 4503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dirks P, Thomas U, Montag D, The cytoplasmic domain of NrCAM binds to PDZ domains of synapse-associated proteins SAP90/PSD95 and SAP97, Eur J Neurosci 24(1) (2006) 25–31. [DOI] [PubMed] [Google Scholar]
- [53].Crosbie RH, Dovico SA, Flanagan JD, Chamberlain JS, Ownby CL, Campbell KP, Characterization of aquaporin-4 in muscle and muscular dystrophy, FASEB J 16(9) (2002) 943–9. [DOI] [PubMed] [Google Scholar]
- [54].Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME, Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein, Proc Natl Acad Sci U S A 98(24) (2001) 14108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.