Abstract
Background:
Few Western studies have evaluated the long-term oncologic outcomes of minimally invasive surgery (MIS) approaches to gastrectomy for gastric cancer. Here, we sought to compare the outcomes of minimally invasive vs. open gastrectomies, and of laparoscopic vs. robotic gastrectomies, at a high-volume cancer center in the United States.
Methods:
We analyzed data on all patients undergoing curative gastrectomy for gastric adenocarcinoma from January 2007 to June 2017. Postoperative complications and disease-specific survival (DSS) was compared between surgical approaches.
Results:
Among 845 patients, median follow-up was 38.5 months. Stage-stratified 5-year DSS did not significantly differ between open surgery (n = 534) and MIS (n = 311). MIS resulted in significantly fewer complications, which was confirmed by adjusted comparison (OR 0.70 [0.49–1.00], p = 0.049). DSS did not differ between the two groups after adjustment (HR 0.83 [0.55–1.25], p = 0.362). Robotic operations (n = 190) had fewer conversions to open procedure (p = 0.010), shorter operative time (212 vs. 240 min, p < 0.001), more dissected nodes (27 vs. 22, p < 0.001), fewer complications of Clavien-Dindo grade ≥ III (5.8% vs. 13.2%, p = 0.023), and shorter postoperative stay (5 vs. 6 days, p = 0.045) compared with laparoscopic operations (n = 121). DSS did not differ between the laparoscopic and robotic groups.
Conclusion:
We demonstrate the long-term survival and oncologic equivalency of MIS gastrectomy with the open approach in a Western cohort, supporting the use of MIS at centers with adequate experience in appropriately selected patients.
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death worldwide, with over one million new diagnoses and nearly 800,000 deaths in 20181. Surgical resection is the only potential curative treatment, and survival can be improved by the addition of perioperative chemotherapy in advanced cases2–5.
The adoption of minimally invasive surgery (MIS) for gastric cancer has rapidly increased worldwide. Its operative and oncological safety has been demonstrated mainly in Asian countries, where more early-stage cancers are more frequent than in the United States, and patients generally have lower BMI and fewer comorbidities, leading to earlier adoption of this approach6–11. Although two randomized prospective trials10,12 and a large-scale multicenter historical cohort study13 of MIS approaches for advanced gastric cancer have demonstrated operative safety and oncologic equivalency in Asian populations, trials have not yet evaluated patients with gastric cancer in other settings, especially in the Western part of the world where neoadjuvant chemotherapy is frequent, and long-term oncological outcomes have not been reported.
Since the introduction of robotic gastrectomy for gastric cancer surgery in 200314, its operative feasibility has been compared to open and conventional laparoscopic approaches in Korea and Japan15–18. As for MIS generally, there are few reports of outcomes after robotic gastrectomy from Western countries19–25, and its operative feasibility and oncological equivalency for advanced gastric cancer remains uncertain.
We previously reported the feasibility and short- and long-term safety of laparoscopic gastrectomy at our center26,27.The aim of this study was to compare the operative and long-term oncologic outcomes of all laparoscopic and robotic gastrectomies with the open approach. Our second aim was to compare feasibility, clinicopathologic metrics, complications, and stage-specific oncologic outcomes of laparoscopic versus robotic gastrectomy in gastric cancer patients treated at a single high-volume cancer center in the United States.
Methods
Patient characteristics and clinicopathological data
Patients were identified from the surgical gastric cancer database at our center following Institutional Review Board approval. Inclusion criteria were histologically confirmed primary gastric adenocarcinoma and R0 resection. Exclusion criteria were gastroesophageal junction cancer and incomplete clinicopathological data. Demographics, clinicopathological characteristics, treatment information, and length of follow-up were determined from the database and review from medical records. Tumor stage, lymph node status, and TNM stage were classified according to the 8th edition of the AJCC staging system28. Clinical TNM stage was determined by CT scan of the chest, abdomen, and pelvis with or without endoscopic ultrasound. Diagnostic laparoscopy was used to stage locoregional cancer and rule out occult metastatic disease for patients with tumors classified as clinical T3 or higher or clinical N-positive disease. Patients with clinical T3 or higher and/or node-positive disease were offered neoadjuvant treatment after 2006, following publication of the MAGIC study demonstrated its survival benefit3. Pathological chemotherapy response was assessed by an experienced gastric cancer pathologist on a scale from 0 to 100%.
Surgical approach was selected based on discussion with patients. Patients who were eligible for MIS approaches were offered this approach or referred to a surgeon who offers this approach. Occasionally, patients deemed eligible for MIS gastrectomy declined this approach, and their preference was always honored. Relative contraindications to MIS approaches included limited physical status, serious co-morbidities considered to present high risk, multiple prior operations, or BMI > 40 kg/m2. Patients whose procedures converted to the open approach during surgery were classified as having MIS surgery according to the intent-to-treat concept.
Length of stay was defined as the number of days from the date of operation to the date of discharge. Tumor size was the maximum tumor dimension on final pathology of the resected specimen. Postoperative complications within 30 days of surgery were recorded and classified according to the Clavien-Dindo system29.
Follow-up data
Follow-up after curative resection consisted of outpatient visits every 3–6 months for the first 2 years and every 6–12 months for 3–5 years, at which blood tests (complete blood count, chemistry panel, and in some cases, carcinoembryonic antigen and carbohydrate antigen 19–9) and CT scan with or without endoscopy were performed. Survival was measured from the date of surgery, disease-specific survival (DSS) and overall survival (OS) were measured to the date of death or the last follow-up, whichever occurred first. Death from recurrent gastric cancer was considered as an event in DSS. Patients who died postoperatively were included in the survival analysis.
Statistical analysis
Patient demographics, clinicopathological characteristics, early postoperative outcomes, and survival were compared between patients undergoing MIS and open gastrectomy, and between those MIS patients undergoing laparoscopic versus robotic gastrectomy. Categorical variables were compared using the chi-square test, Fisher’s exact test, or logistic regression analysis. Continuous variables using the Mann-Whitney U test. Survival was compared using Kaplan-Meier methods with log-rank test and Cox regression analysis. Univariable analyses were performed for all potential confounding variables and effect modifiers for survival. All variables with a p-value of < 0.1 in the univariable analysis were included as independent variables in the multivariable analysis. Data were expressed as median (interquartile ranges [IQR]), odds ratio (OR), or hazard ratio (HR) with 95% confidence interval (95% CI), unless otherwise stated. Statistical calculations were conducted using SPSS® software version 25 (IBM, Armonk, New York, USA). P values of < 0.05 (two-tailed) were considered to be statistically significant.
Results
Demographics and clinicopathological characteristics
Of the 1,405 patients with gastric cancer who underwent surgical treatment with curative intent at our center between January 2007 and June 2017, 845 met inclusion criteria for this study (Figure 1). Patients were excluded if their cancer occurred in the gastroesophageal junction cancer (Siewert type I and II, and Siewert type III which required transthoracic approach) (n = 460), if they underwent endoscopic resection (n = 3), wedge resection (n = 30), gastrectomy for remnant gastric cancer (n = 26), or non-curative resection (n = 37), or if clinicopathological data were incomplete (n = 4).
Figure 1. Patient selection.
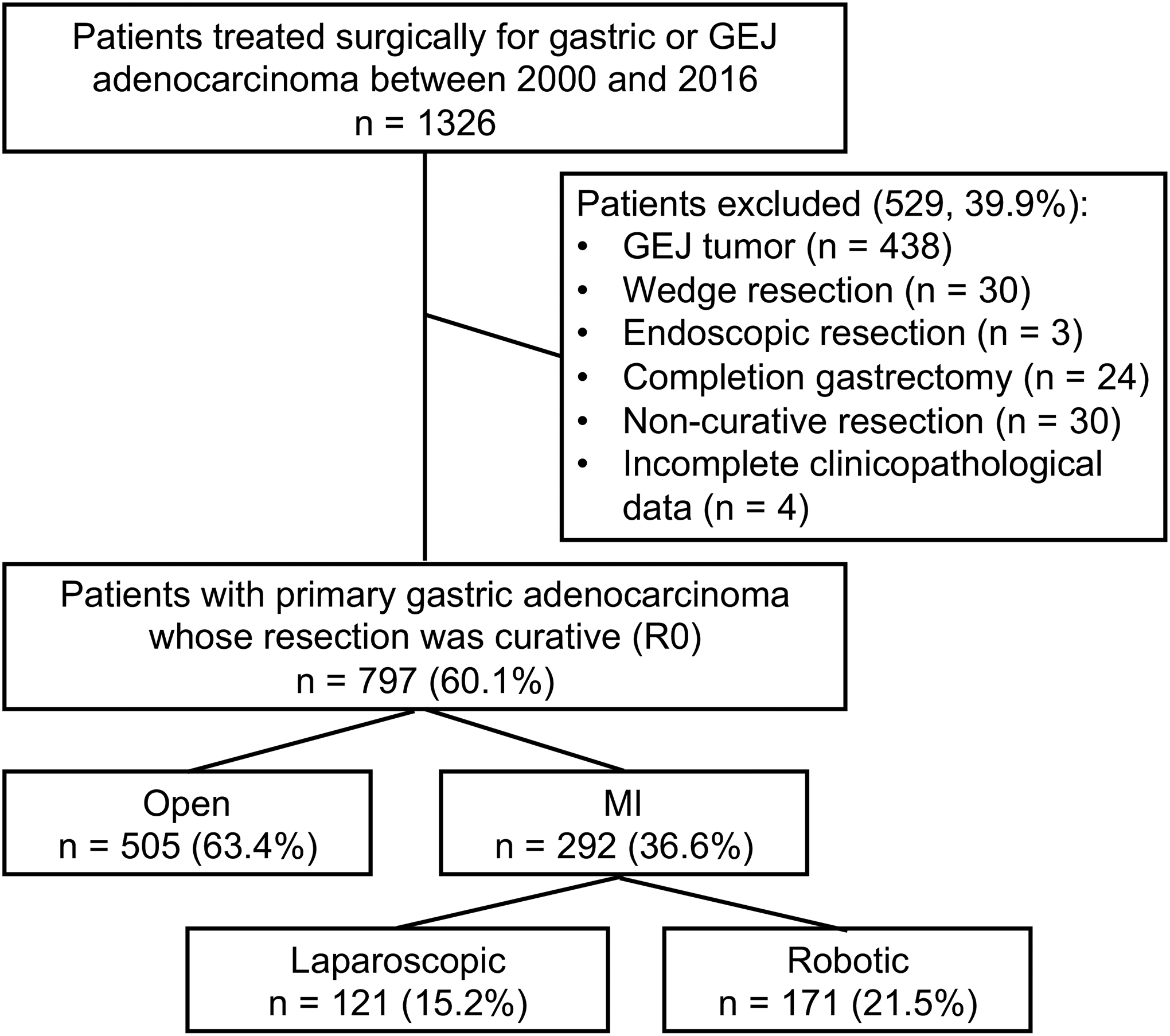
GEJ, gastroesophageal junction; MIS, minimally invasive surgery.
Of the 845 patients who underwent R0 gastrectomy for gastric cancer, open gastrectomy was performed in 534 (63.2%) and MIS gastrectomy in 311 (36.8%) patients. MIS patients were younger (63 vs. 66 years, p < 0.001) and underwent gastrectomy more recently. Fewer MIS patients had an American Society of Anesthesiologists (ASA) physical status of IV (1.0% vs. 7.3%, p < 0.001), whereas comorbidity did not differ between the two groups (56.3% vs. 55.1%, p = 0.732). Fewer MIS patients had received preoperative neoadjuvant chemotherapy (36.7% vs. 43.6%, p = 0.047) compared with the open group (Table 1). In addition, MIS patients had smaller tumors (2.2 vs. 3.0 cm, p < 0.001) that were more frequently located in the lower third of the stomach (p < 0.001), and thus underwent distal gastrectomy at higher rates (69.1% vs. 55.8%, p < 0.001) than open gastrectomy patients. D2 or more extensive lymphadenectomy was performed at a similar rate regardless of surgical approach (p = 0.768). Among patients undergoing MIS gastrectomy, operative time was significantly longer (222 vs. 163 min, p < 0.001), but more lymph nodes (25 vs. 23, p = 0.040) were retrieved. Patients undergoing MIS gastrectomy had earlier stage tumors: fewer MIS patients had a pathologic tumor of T2 or higher (41.5% vs. 63.3%, p < 0.001), pathologic positive nodes (35.4% vs. 45.9%, p = 0.025), and were classified as pathologic stage II or higher (39.9% vs. 58.8%, p < 0.001).
Table 1. Demographic and clinicopathological information and surgical outcomes among patients undergoing open vs. MIS gastrectomy.
Data are presented as n (%) if categorical and median (IQR) if continuous.
| Open (534) | MIS (311) | p | |
|---|---|---|---|
| Sex | 0.328 | ||
| Male | 295 (55.2) | 161 (51.8) | |
| Female | 239 (44.8) | 150 (48.2) | |
| Age, years | 66 (56–76) | 63 (50–71) | < 0.001 |
| BMI, kg/m2 | 26.7 (23.4–29.8) | 26.2 (23.2–30.0) | 0.261 |
| ASA status | < 0.001 | ||
| I | 12 (2.2) | 15 (4.8) | |
| II | 146 (27.3) | 102 (32.8) | |
| III | 337 (63.1) | 191 (61.4) | |
| IV | 39 (7.3) | 3 (1.0) | |
| Comorbidity | |||
| Hypertension | 203 (38.0) | 105 (33.8) | 0.215 |
| Hypercholesteremia/lipidemia | 160 (30.0) | 82 (26.4) | 0.265 |
| Coronary artery disease | 70 (13.1) | 27 (8.7) | 0.052 |
| Other heart disease | 48 (9.0) | 25 (8.0) | 0.635 |
| Diabetes | 76 (14.2) | 48 (15.4) | 0.634 |
| Respiratory disease | 57 (10.7) | 38 (12.2) | 0.493 |
| Chronic kidney disease | 48 (9.0) | 14 (4.5) | 0.016 |
| Liver disease | 13 (2.4) | 8 (2.6) | 0.901 |
| DVT/PE | 23 (4.3) | 4 (1.3) | 0.016 |
| Any of above | 294 (55.1) | 175 (56.3) | 0.732 |
| Tumor location | < 0.001 | ||
| Upper third | 127 (23.8) | 33 (10.6) | |
| Middle third | 167 (31.3) | 110 (35.4) | |
| Lower third | 213 (39.9) | 146 (46.9) | |
| Whole stomach or multiple | 27 (5.1) | 22 (7.1) | |
| Year of surgery | < 0.001 | ||
| 2007–2010 | 216 (40.4) | 62 (19.9) | |
| 2011–2014 | 189 (35.4) | 144 (46.3) | |
| 2015–2017 | 129 (24.2) | 105 (33.8) | |
| Type of gastrectomy | < 0.001 | ||
| Distal | 298 (55.8) | 215 (69.1) | |
| Total | 227 (42.5) | 96 (30.9) | |
| Proximal | 9 (1.7) | 0 (0) | |
| Lymph node dissection | 0.768 | ||
| D1 | 32 (6.0) | 17 (5.5) | |
| D1+ | 22 (4.1) | 16 (5.1) | |
| D2 | 479 (89.7) | 278 (89.4) | |
| D3 | 1 (0.2) | 0 (0) | |
| Operative time, min | 163 (128–203) | 222 (187–256) | < 0.001 |
| Dissected nodes | 23 (17–32) | 25 (18–34) | 0.040 |
| Tumor size, cm | 3 (1.6–5.5) | 2.2 (1.3–4.0) | < 0.001 |
| Unknown | 7 (1.3) | 8 (2.6) | |
| Neoadjuvant chemotherapy | 233 (43.6) | 114 (36.7) | 0.047 |
| Neoadjuvant radiotherapy | 5 (0.9) | 1 (0.3) | 0.423 |
| Adjuvant chemotherapy | 165 (30.9) | 91 (29.3) | 0.617 |
| Adjuvant radiotherapy | 26 (4.9) | 16 (5.1) | 0.859 |
| Pathological T factor | < 0.001 | ||
| 1 | 172 (32.2) | 172 (55.3) | |
| 2 | 62 (11.6) | 32 (10.3) | |
| 3 | 140 (26.2) | 56 (18.0) | |
| 4 | 136 (25.5) | 41 (13.2) | |
| CR | 24 (4.5) | 10 (3.2) | |
| Pathological N status | 0.025 | ||
| 0 | 289 (54.1) | 201 (64.6) | |
| 1 | 101 (18.9) | 41 (13.2) | |
| 2 | 74 (13.9) | 36 (11.6) | |
| 3 | 70 (13.1) | 33 (10.6) | |
| Pathological stage | < 0.001 | ||
| I | 196 (36.7) | 177 (56.9) | |
| II | 160 (30.0) | 64 (20.6) | |
| III | 154 (28.8) | 60 (19.3) | |
| ypT0 N any | 24 (4.5) | 10 (3.2) | |
| Lymphovascular invasion | 260 (48.7) | 102 (32.8) | < 0.001 |
| Lauren classification | 0.436 | ||
| Intestinal | 251 (47.0) | 132 (42.4) | |
| Diffuse | 167 (31.3) | 107 (34.4) | |
| Mixed | 109 (20.4) | 70 (22.5) | |
| Unknown | 7 (1.3) | 2 (0.6) | |
| Postoperative stay, days | 7 (6–9) | 6 (5–7) | <0.001 |
| Complications | |||
| Any | 174 (32.6) | 64 (20.6) | < 0.001 |
| Grade I/II | 107 (20.0) | 37 (11.9) | 0.002 |
| Grade III or greater | 67 (12.5) | 27 (8.7) | 0.085 |
| Mortality | 6 (1.1) | 2 (0.6) | 0.717 |
| Any complications | |||
| In pStage I (n = 373) | 55 (28.1) | 34 (19.2) | 0.045 |
| In pStage II/III (n = 438) | 115 (36.6) | 26 (21.0) | 0.002 |
| In neoadjuvant chemotherapy (+) (n = 347) | 79 (33.9) | 24 (21.1) | 0.014 |
BMI, body mass index; ASA, American Society of Anesthesiologists; CR, complete response; DVT, deep vein thrombosis; PE, pulmonary embolism
Postoperative events and complications
The overall incidence of all complications within 30 postoperative days was lower among patients undergoing MIS gastrectomy compared with open gastrectomy in the unadjusted cohort (20.6% vs. 32.6%, p < 0.001), although there was no difference in the rate of major complications (Clavien-Dindo ≥ III). The lower rate of complications persisted when comparing patients with the same stage of gastric cancer (i.e. pStage I, 19.2 vs. 28.1%, p = 0.045, pStage II/III 21.0 vs. 36.6%, p = 0.002) and among patients receiving neoadjuvant chemotherapy (21.1% vs. 33.9%, p = 0.014) (Table 1). After adjustment with uni- and multivariable analysis, the MIS patients had significantly fewer overall complications compared with the open patients (OR 0.70 [95%CI 0.49–1.00], p = 0.049) (Supplemental table 1). Thirty-day mortality did not significantly differ. Postoperative stay was shorter after MIS gastrectomy (6 vs. 7 days, p < 0.001).
Disease-specific survival (DSS) and overall survival (OS)
Median follow-up was 38.5 (IQR 20.8–59.7) months, and slightly but not significantly longer for patients undergoing MIS gastrectomy (42.9 vs. 37.0 months; p = 0.066). The median DSS and OS was - and -months for the entire cohort; - and -months for the open patients, - and -months for the MIS patients. The 1-, 3-, and 5-year DSS was 96.8%, 84.3%, and 76.9% for the entire cohort; 96.2%, 80.6%, and 73.8% for the open patients and 97.6%, 90.4%, and 81.6% for the MIS patients, respectively (Figure 2a). The 1-, 3-, 5-year OS was 93.6%, 77.1%, and 65.2% for the entire cohort; 92.2%, 71.8%, and 60.2% for the open patients and 96.0%, 86.4%, and 73.6% for the MIS patients, respectively. Figure 2b–d shows that after stratification for pathological stage, DSS did not differ between the two groups. Specifically, even after adjustment with tumor location, year of surgery, type of surgery, tumor size, Lauren type, presence of lymphovascular invasion, neoadjuvant and adjuvant chemotherapy, pTN status, and postoperative complications, DSS did not differ between the two groups (HR 0.83 [95%CI 0.55–1.25], p = 0.362) (Table 2).
Figure 2. Comparison of disease-specific survival between patients undergoing open vs. MIS gastrectomy.
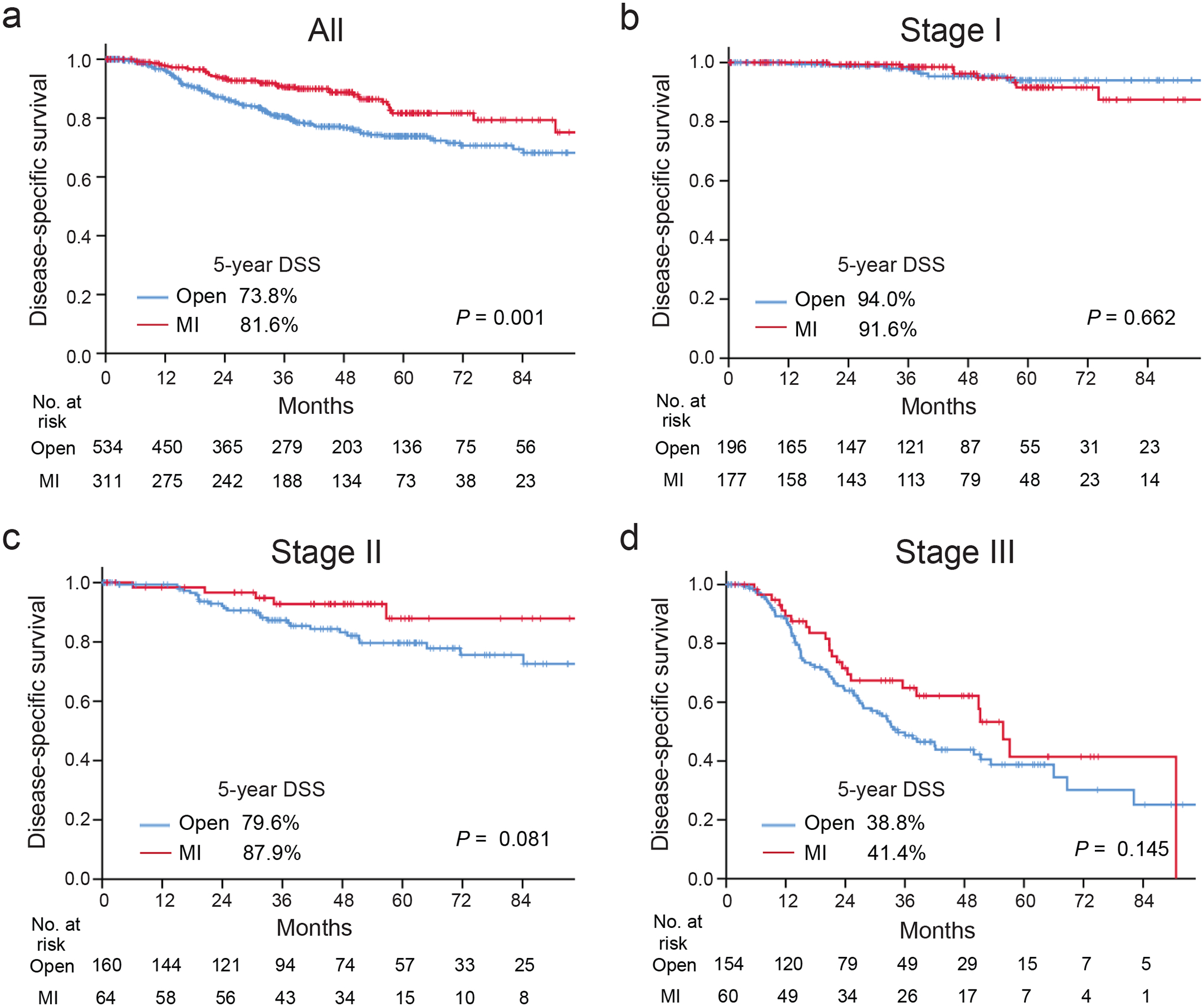
(a) Entire cohort, (b-d), those with pathological stage I (b), II (c), and III (d).
Table 2.
Comparison of DSS between the open and MIS group with adjustment by univariable and multivariable COX regression analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| MIS approach | 0.55 | 0.38–0.79 | 0.001 | 0.83 | 0.55–1.25 | 0.362 |
| Age > 60 years | 1.22 | 0.87–1.72 | 0.245 | |||
| Female sex | 0.82 | 0.59–1.14 | 0.245 | |||
| ASA status III or IV (vs. I or II) | 1.30 | 0.90–1.86 | 0.160 | |||
| Any comorbidity | 0.81 | 0.59–1.12 | 0.205 | |||
| Tumor location | ||||||
| Upper third | 1 | 1 | ||||
| Middle third | 0.72 | 0.46–1.12 | 0.149 | 1.06 | 0.64–1.77 | 0.810 |
| Lower third | 0.70 | 0.46–1.06 | 0.088 | 1.29 | 0.71–2.33 | 0.408 |
| Whole or multiple | 0.63 | 0.27–1.51 | 0.303 | 2.81 | 1.10–7.22 | 0.031 |
| Year of surgery | ||||||
| 2007–2010 | 1 | 1 | ||||
| 2011–2014 | 0.84 | 0.59–1.20 | 0.340 | 1.01 | 0.69–1.47 | 0.977 |
| 2015–2017 | 0.52 | 0.31–0.89 | 0.017 | 0.39 | 0.22–0.69 | 0.001 |
| Total or proximal gastrectomy (vs. Distal gastrectomy) | 1.81 | 1.31–2.50 | < 0.001 | 1.72 | 1.05–2.83 | 0.031 |
| Operative time > 180 min | 0.92 | 0.67–1.28 | 0.631 | |||
| > 20 dissected nodes | 0.93 | 0.67–1.30 | 0.688 | |||
| Tumor > 3 cm | 3.40 | 2.39–4.85 | < 0.001 | 1.26 | 0.84–1.90 | 0.266 |
| Lauren classification | ||||||
| Intestinal | 1 | 1 | ||||
| Diffuse | 1.93 | 1.32–2.83 | 0.001 | 1.11 | 0.72–1.73 | 0.634 |
| Mixed | 1.94 | 1.28–2.97 | 0.003 | 1.35 | 0.86–2.11 | 0.194 |
| Lymphovascular invasion | 4.95 | 3.37–7.26 | < 0.001 | 1.38 | 0.83–2.29 | 0.213 |
| Neoadjuvant chemotherapy | 2.46 | 1.77–3.43 | < 0.001 | 1.88 | 1.30–2.71 | 0.001 |
| Adjuvant chemotherapy | 1.69 | 1.22––2.34 | 0.001 | 0.84 | 0.59–1.20 | 0.335 |
| pT | ||||||
| 1 | 1 | 1 | ||||
| 2 | 2.08 | 0.88–4.91 | 0.094 | 0.91 | 0.34–2.41 | 0.846 |
| 3 | 4.62 | 2.54–8.42 | < 0.001 | 1.91 | 0.93–3.93 | 0.077 |
| 4 | 16.47 | 9.50–28.53 | < 0.001 | 5.20 | 2.52–10.72 | < 0.001 |
| CR | 1.31 | 0.30–5.72 | 0.722 | 1.15 | 0.25–5.32 | 0.861 |
| pN | ||||||
| 0 | 1 | 1 | ||||
| 1 | 3.38 | 2.03–5.63 | < 0.001 | 2.54 | 1.41–4.57 | 0.002 |
| 2 | 5.84 | 3.56–9.58 | < 0.001 | 3.26 | 1.78–5.95 | < 0.001 |
| 3 | 13.85 | 8.86–21.67 | < 0.001 | 5.14 | 2.85–9.26 | < 0.001 |
| Any complications | 1.43 | 1.02–2.01 | 0.038 | 0.88 | 0.59–1.29 | 0.503 |
| Grade III or greater complication | 1.04 | 0.61–1.77 | 0.896 |
ASA, American Society of Anesthesiologists; CR, complete response
Laparoscopic versus robotic approach
Among patients who underwent MIS gastrectomy, we compared the operative and oncological outcomes between those treated by laparoscopic (n = 121) and robotic (n = 190) approaches. There was no difference in patient characteristics, type of gastrectomy, or pathological tumor or lymph node status (Table 3). In the robotic group, operative time was shorter (212 vs. 240 min, p < 0.001), there were fewer conversions to open procedures (25.8% vs. 39.7%, p = 0.010), and more nodes were dissected (27 vs. 22, p < 0.001). Although there was no difference in overall complications (18.9% vs. 23.1%, p = 0.373), there were significantly fewer postoperative complications of grade III or greater in the robotic group (5.8% vs. 13.2%, p = 0.023) (Table 3). Overall and stage-specific DSS did not differ between the two approaches (p = 0.873) (Figure 3).
Table 3. Demographic and clinicopathological information and surgical outcomes among patients undergoing laparoscopic vs. robotic gastrectomy.
Data are presented as n (%) if categorical and median (IQR) if continuous.
| Laparoscopic (121) | Robotic (190) | p | |
|---|---|---|---|
| Sex | 0.882 | ||
| Male | 62 (51.2) | 99 (52.1) | |
| Female | 59 (48.8) | 91 (47.9) | |
| Age, years | 64 (54–72) | 61 (47–69) | 0.085 |
| BMI, kg/m2 | 26.8 (23.4–31.2) | 25.7 (23.2–29.2) | 0.073 |
| Any comorbidity | 56 (46.3) | 119 (62.6) | 0.005 |
| Year of surgery | < 0.001 | ||
| 2007–2010 | 62 (51.2) | 0 (0) | |
| 2011–2014 | 52 (43.0) | 92 (48.4) | |
| 2014–2017 | 7 (5.8) | 98 (51.6) | |
| Type of gastrectomy | 0.554 | ||
| Distal | 86 (71.1) | 129 (67.9) | |
| Total | 35 (28.9) | 61 (32.1) | |
| Proximal | 0 (0.0) | 0 (0.0) | |
| Lymph node dissection | 0.387 | ||
| D1 | 4 (3.3) | 13 (6.8) | |
| D1+ | 7 (5.8) | 9 (4.7) | |
| D2 | 110 (90.9) | 168 (88.4) | |
| Operative time, min | 240 (202–286) | 212 (180–246) | < 0.001 |
| Converted to open | 48 (39.7) | 49 (25.8) | 0.010 |
| Dissected nodes | 22 (15–31) | 27 (20–38) | < 0.001 |
| Tumor size, cm | 2.5 (1.5–4.0) | 2.2 (1.3–3.8) | 0.477 |
| Unknown | 8 (7.1) | 0 (0.0) | |
| Neoadjuvant chemotherapy | 38 (31.4) | 76 (40.0) | 0.125 |
| Neoadjuvant radiotherapy | 0 (0.0) | 1 (0.5) | 0.611 |
| Adjuvant chemotherapy | 41 (33.9) | 50 (26.3) | 0.153 |
| Adjuvant radiotherapy | 9 (7.4) | 7 (3.7) | 0.144 |
| Pathological T factor | 0.580 | ||
| 1 | 64 (52.9) | 108 (56.8) | |
| 2 | 16 (13.2) | 16 (8.4) | |
| 3 | 20 (16.5) | 36 (18.9) | |
| 4 | 18 (14.9) | 23 (12.1) | |
| CR | 3 (2.5) | 7 (3.7) | |
| Pathological N status | 0.824 | ||
| 0 | 78 (64.5) | 123 (64.7) | |
| 1 | 18 (14.9) | 23 (12.1) | |
| 2 | 12 (9.9) | 24 (12.6) | |
| 3 | 13 (10.7) | 20 (10.5) | |
| Pathological stage | 0.930 | ||
| I | 70 (57.9) | 107 (56.3) | |
| II | 24 (19.8) | 40 (21.1) | |
| III | 24 (19.8) | 36 (18.9) | |
| ypT0 Nany | 3 (2.5) | 7 (3.7) | |
| Lymphovascular invasion | 41 (33.9) | 61 (32.1) | 0.745 |
| Lauren classification | 0.277 | ||
| Intestinal | 46 (38.0) | 86 (45.3) | |
| Diffuse | 49 (40.5) | 58 (30.5) | |
| Mixed | 24 (19.8) | 46 (24.2) | |
| Unknown | 2 (1.7) | 0 (0) | |
| Postoperative stay, days | 6 (5–7) | 5 (4–7) | 0.045 |
| Mortality | 1 (0.8) | 1 (0.5) | 0.628 |
| Complication | |||
| Any | 28 (23.1) | 36 (18.9) | 0.373 |
| Grade III or greater | 16 (13.2) | 11 (5.8) | 0.023 |
BMI, body mass index; ASA, American Society of Anesthesiologists; CR, complete response
Figure 3. Comparison of disease-specific survival between patients undergoing gastrectomy by a laparoscopic vs. robotic approach.
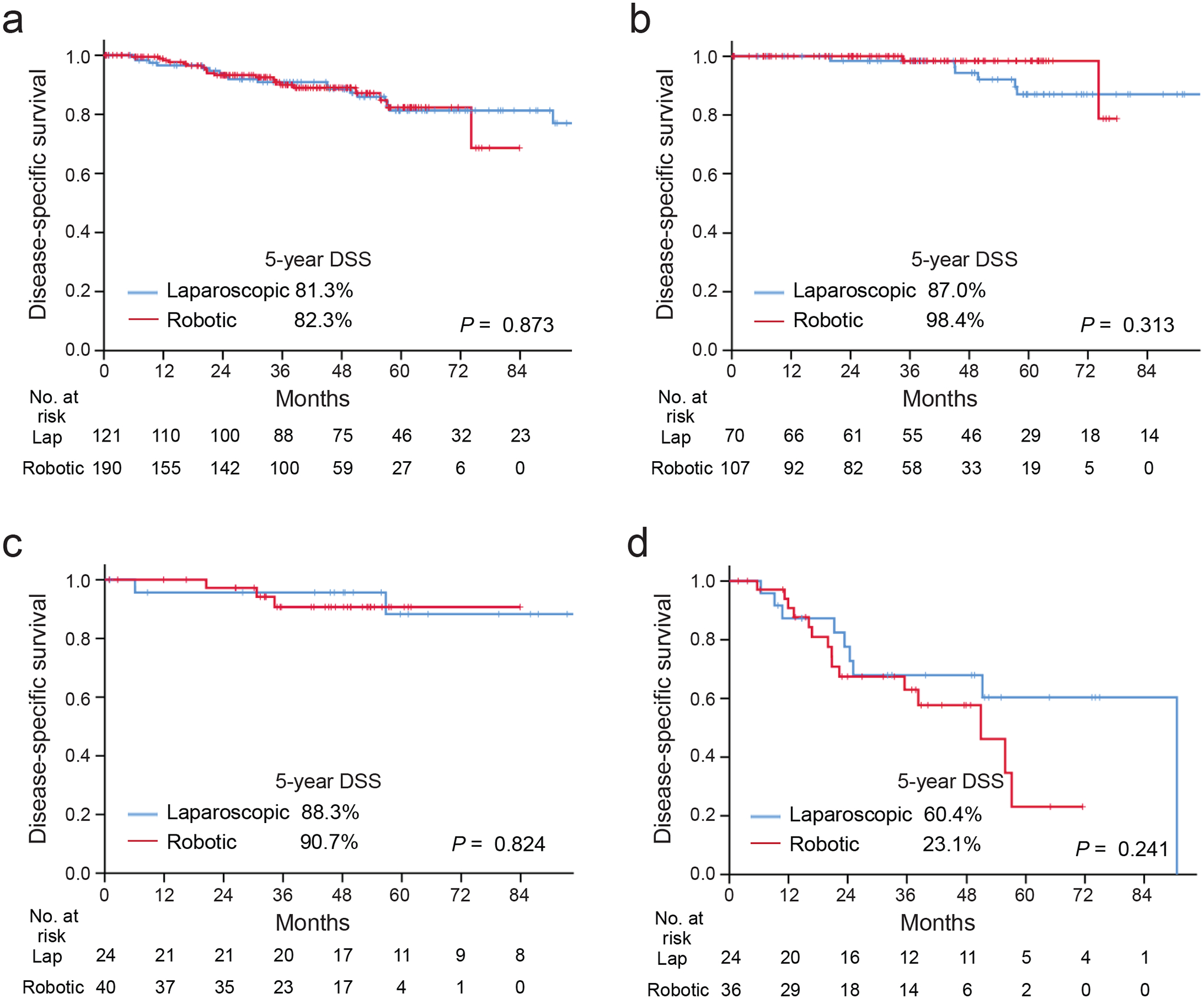
(a) All 292 patients undergoing MIS gastrectomy, (b-d) those with pathological stage I (b), II (c), or III (d).
Patients who received neoadjuvant chemotherapy
Among the 347 patients who received neoadjuvant chemotherapy, generally representing patients with locally advanced gastric cancers, we compared operative and oncological outcomes between the open (n = 233) and MIS (n = 114) groups (Table 4). Patients who underwent open gastrectomy were slightly older (63 vs. 62 years, p = 0.014), had higher BMI (26.8 vs. 26.0 kg/m2, p = 0.050), and were more likely to have tumors located in the upper third of the stomach (p = 0.002). There was no significant difference in pathological stage (p = 0.179). In the MIS group, there were fewer postoperative complications (as described above), and postoperative stay was shorter (6 vs. 7 days, p < 0.001) (Table 4). DSS was similar (p = 0.662).
Table 4. Demographic and clinicopathological information and surgical outcomes among patients who received neoadjuvant chemotherapy.
Data are presented as n (%) if categorical and median (IQR) if continuous.
| Open (233) | MIS (114) | p | |
|---|---|---|---|
| Sex | 0.809 | ||
| Male | 134 (57.5) | 64 (56.1) | |
| Female | 99 (42.5) | 50 (43.9) | |
| Age, years | 63 (55–70) | 62 (49–69) | 0.014 |
| BMI, kg/m2 | 26.8 (23.5–29.8) | 26.0 (23.1–29.1) | 0.050 |
| Tumor location | 0.002 | ||
| Upper third | 78 (33.5) | 16 (14.0) | |
| Middle third | 70 (30.0) | 45 (39.5) | |
| Lower third | 82 (35.2) | 50 (43.9) | |
| Whole stomach/multiple | 3 (1.3) | 3 (2.6) | |
| Neoadjuvant radiotherapy | 5 (2.1) | 1 (0.9) | 0.668 |
| Adjuvant chemotherapy | 109 (46.8) | 50 (43.9) | 0.608 |
| Adjuvant radiotherapy | 7 (3.0) | 6 (5.3) | 0.368 |
| Year of surgery | < 0.001 | ||
| 2007–2010 | 81 (34.8) | 18 (15.8) | |
| 2011–2014 | 86 (36.9) | 63 (55.3) | |
| 2015–2017 | 66 (28.3) | 33 (28.9) | |
| Type of gastrectomy | 0.032 | ||
| Distal | 114 (48.9) | 72 (63.2) | |
| Total | 117 (50.2) | 42 (36.8) | |
| Proximal | 2 (0.9) | 0 (0.0) | |
| Pathological T factor | 0.010 | ||
| ypT1 | 20 (8.6) | 25 (21.9) | |
| ypT2 | 31 (13.3) | 14 (12.3) | |
| ypT3 | 87 (37.3) | 41 (36.0) | |
| ypT4 | 71 (30.5) | 24 (21.1) | |
| ypT0 (CR) | 24 (10.3) | 10 (8.6) | |
| Pathological N status | 0.241 | ||
| ypN0 | 104 (44.6) | 56 (49.1) | |
| ypN1 | 52 (22.3) | 15 (13.2) | |
| ypN2 | 41 (17.6) | 22 (19.3) | |
| ypN3 | 36 (15.5) | 21 (18.4) | |
| Pathological Stage | 0.179 | ||
| I | 35 (15.0) | 27 (23.7) | |
| II | 91 (39.1) | 35 (30.7) | |
| III | 83 (35.6) | 42 (36.8) | |
| ypT0 Nany | 24 (10.3) | 10 (8.8) | |
| Pathological response rate, % | 30 [10–80] | 50 [20–80] | 0.204 |
| Mortality | 2 (0.9) | 0 (0.0) | 0.450 |
| Complications | |||
| Any | 79 (33.9) | 24 (21.1) | 0.014 |
| Grade III or greater | 28 (12.0) | 6 (5.3) | 0.047 |
| Postoperative stay, days | 7 (5–9) | 6 (5–7) | <0.001 |
BMI, body mass index; ASA, American Society of Anesthesiologists; CR, complete response
Discussion
Our single-institution experience demonstrates the operative safety of minimally invasive gastrectomy among US patients with early and advanced gastric cancer, including significantly fewer complications and long-term oncologic equivalency.
After stratification by pathologic stage and adjustment with clinicopathological and operative variables, DSS did not differ between patients undergoing MIS vs. open surgery. These results demonstrate that the MIS approach is oncologically equivalent to the open approach in a US gastric cancer population. This study is unique in that it includes patients with locally advanced gastric cancer, some of whom were treated with neoadjuvant chemotherapy (41.1%), which is not routinely used in similar populations of Asian gastric cancer patients, in which the majority of studies comparing MIS and open approaches have been performed8,13,30,31.
Our findings of equivalent DSS contrast with those of a prior retrospective study comparing the outcomes of open and MIS gastrectomy for gastric cancer among US patients using a national clinical database24. That study found that open gastrectomy was an independent predictor of worse overall survival (OS), with 5-year OS of 47.7% compared with 51.9% after MIS gastrectomy (p < 0.001)24. Our study is focusing on DSS, an endpoint unavailable from administrative databases, and that more specifically reflects the effects of the disease and its treatment. Further, our study was conducted in a single academic institution and includes more patients who received neoadjuvant chemotherapy (41.1% vs. 19.1%).
Consistent with past reports, we found that MIS gastrectomy was associated with fewer complications compared with open surgery10,32, and this difference was also observed among patients who received neoadjuvant chemotherapy. Though serious morbidity such as anastomotic leak and operative mortality were similar between the two approaches, the difference in minor complications nonetheless has important implications for patient recovery and reduced length of stay.
Our finding of shorter operative time for robotic gastrectomy contrasts with the majority of studies comparing robotic and laparoscopic gastrectomy15–17,21. This may reflect our institutional experience; as we started performing robotic gastrectomy in 2012 after achieving our learning curve with over 100 laparoscopic gastric procedures. Since then, the robotic approach has been applied for most cases that were eligible for an MIS approach.
Our findings support the oncological safety of robotic gastrectomy, consistent with other recent studies of the long-term outcomes of robotic gastrectomy18,22,24,25,33, though most were conducted in Asia, where relatively more patients have early-stage gastric cancer. For example, a propensity-score matching analysis of long-term outcomes between patients undergoing laparoscopic vs. robotic gastrectomy found no difference in 5-year OS (94.2% vs. 93.2%; p = 0.527) or recurrence-free survival (92.6% vs. 90.7%; p = 0.229)18. This large study included a high proportion of patients with pathological stage I disease (83%) and none who received neoadjuvant chemotherapy18. Studies from Western countries have been smaller, but similarly reported that survival is similar regardless of surgical approach22,34.
There are several limitations to this study. First, its retrospective nature makes our results susceptible to biases inherent to all retrospective studies. Furthermore, we focus on the comparison of postoperative complications and DSS between the open and MIS patients in this study and any other results are unadjusted, which requires a careful interpretation of other outcomes in this study. Second, we started performing robotic gastrectomies in 2012 and therefore the robotic gastrectomy group includes more recent patients compared with open gastrectomy. These more recent cases were subject to evidence-based changes in the optimal regimen of perioperative treatment, which could have influenced survival outcomes. Third, patients whose surgery was intraoperatively converted to the open approach were classified as having MIS surgery (39.7% in laparoscopic and 25.8% in robotic group) according to the intent-to-treat concept, and converted cases might also have influenced the operative outcomes. This study included a considerable number of patients receiving neoadjuvant chemotherapy, In addition, many patients undergoing the robotic approach were more recent as the robotic approach has replaced the conventional laparoscopic approach in recent years. Future studies comparing robotic approaches with laparoscopic or open approaches as well as studies focusing on advanced gastric cancer patients receiving neoadjuvant chemotherapy will continue to clarify and define appropriate indications and selection for the MIS approach.
Conclusion
We demonstrate that MIS gastrectomy is feasible and leads to equivalent long-term oncologic outcomes and fewer complications compared with the open approach for selected patients at a high-volume gastric cancer center in the West, both overall and when stratified by stage.
Supplementary Material
Synopsis.
This retrospective study found that minimally invasive gastrectomy including laparoscopic and robotic gastrectomy had superior short-term outcomes compared with the open approach. Five-year DSS did not differ between minimally invasive and open gastrectomy.
Acknowledgement
We would like to acknowledge Jessica Moore, MS, staff editor at MSK, for editing this manuscript.
Disclosures:
YYJ has received research funding provided to the institution for other studies from Rgenix, Boehringer Ingelheim, Bayer, Genentech/Roche, Bristol-Myers Squibb, Eli Lilly, and Merck and served on advisory boards for Rgenix, Merck Serono, Bristol-Myers Squibb, Eli Lilly, Pfizer, Bayer, Imugene, Merck, Daiichi-Sankyo, and AstraZeneca. GYK has received research funding provided to the institution for other studies from AROG Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Merck, Pieris Pharmaceuticals, and Zymeworks, and consulting, speaking, or travel fees from Bristol Myers Squibb, Merck, and Pieris. MAS is a consultant for Boston Scientific. MN is a consultant for Lumendi, Boston Scientific, and Olympus. No other authors have financial relationships to disclose.
Funding:
This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. [DOI] [PubMed] [Google Scholar]
- 6.Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13(4):238–244. [DOI] [PubMed] [Google Scholar]
- 7.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251(3):417–420. [DOI] [PubMed] [Google Scholar]
- 8.Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142–151. [DOI] [PubMed] [Google Scholar]
- 9.Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699–708. [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Hyung WJ, Yang HK, et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270(6):983–991. [DOI] [PubMed] [Google Scholar]
- 11.Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39(11):2734–2741. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321(20):1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinoshita T, Uyama I, Terashima M, et al. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage II/III Gastric Cancer: A Multicenter Cohort Study in Japan (LOC-A Study). Ann Surg. 2019;269(5):887–894. [DOI] [PubMed] [Google Scholar]
- 14.Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83(6):1429–1444. [DOI] [PubMed] [Google Scholar]
- 15.Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146(9):1086–1092. [DOI] [PubMed] [Google Scholar]
- 16.Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29(3):673–685. [DOI] [PubMed] [Google Scholar]
- 17.Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263(1):103–109. [DOI] [PubMed] [Google Scholar]
- 18.Obama K, Kim YM, Kang DR, et al. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21(2):285–295. [DOI] [PubMed] [Google Scholar]
- 19.Caruso R, Vicente E, Quijano Y, et al. Robotic assisted gastrectomy compared with open resection: a case-matched study. Updates Surg. 2019;71(2):367–373. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese R, Maggioni D, Sansonna F, et al. Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol. 2009;35(3):281–288. [DOI] [PubMed] [Google Scholar]
- 21.Cianchi F, Indennitate G, Trallori G, et al. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg. 2016;16(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Procopiuc L, Tudor S, Manuc M, Diculescu M, Vasilescu C. Open vs robotic radical gastrectomy for locally advanced gastric cancer. Int J Med Robot. 2016;12(3):502–508. [DOI] [PubMed] [Google Scholar]
- 23.Glenn JA, Turaga KK, Gamblin TC, Hohmann SF, Johnston FM. Minimally invasive gastrectomy for cancer: current utilization in US academic medical centers. Surg Endosc. 2015;29(12):3768–3775. [DOI] [PubMed] [Google Scholar]
- 24.Hendriksen BS, Brooks AJ, Hollenbeak CS, Taylor MD, Reed MF, Soybel DI. The Impact of Minimally Invasive Gastrectomy on Survival in the USA. J Gastrointest Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 25.Coratti A, Fernandes E, Lombardi A, et al. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41(8):1106–1113. [DOI] [PubMed] [Google Scholar]
- 26.Kelly KJ, Selby L, Chou JF, et al. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann Surg Oncol. 2015;22(11):3590–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Yoon C, Xu B, et al. Long-Term Survival after Minimally Invasive Versus Open Gastrectomy for Gastric Adenocarcinoma: A Propensity Score-Matched Analysis of Patients in the United States and China. Ann Surg Oncol. 2020;27(3):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 29.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. [DOI] [PubMed] [Google Scholar]
- 30.Honda M, Hiki N, Kinoshita T, et al. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage I Gastric Cancer: The LOC-1 Study. Ann Surg. 2016;264(2):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HH, Han SU, Kim MC, et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019;5(4):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263(1):28–35. [DOI] [PubMed] [Google Scholar]
- 33.Nakauchi M, Suda K, Susumu S, et al. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc. 2016;30(12):5444–5452. [DOI] [PubMed] [Google Scholar]
- 34.Solaini L, Bazzocchi F, Pellegrini S, et al. Robotic vs open gastrectomy for gastric cancer: A propensity score-matched analysis on short- and long-term outcomes. Int J Med Robot. 2019;15(5):e2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


