Abstract
Most NIR-IIb fluorophores are nanoparticle-based probes with long retention ( ≈ 1 month or longer) in the body. Here, we applied a novel cross-linked coating to functionalize core/shell lead sulfide/cadmium sulfide quantum dots (PbS/CdS QDs) emitting at ≈ 1600 nm. The coating was comprised of an amphiphilic polymer followed by three crosslinked amphiphilic polymeric layers (P3 coating), imparting high biocompatibility and > 90% excretion of QDs within 2 weeks of intravenous administration. The P3-QDs were conjugated to an engineered anti-CD8 diabody (Cys-diabody) for in vivo molecular imaging of CD8 + cytotoxic T lymphocytes (CTLs) in response to anti-PD-L1 therapy. Two-plex molecular imaging in combination with down-conversion Er nanoparticles (ErNPs) was performed for real-time in vivo monitoring of PD-L1 positive tumor cells and CTLs with cellular resolution by non-invasive NIR-IIb light sheet microscopy. Imaging of angiogenesis in the tumor microenvironment and of lymph nodes deep in the body with a signal-to-background ratio of up to ≈ 170 was also achieved using P3-QDs.
Keywords: excretion, imaging agents, nanomedicine, nanoparticles, second near-infrared window
Introduction
Inorganic nanoparticles have been widely used as contrast-enhancing imaging probes,[1a,b] drug delivery systems,[1c–e] and therapeutic agents[1f,g] in preclinical studies to exploit their unique chemical and physical properties. Despite considerable successes in achieving advanced functionalities of nanoparticles in small animal models, few inorganic nanoparticles have been approved for clinical use owing to potential toxicity concerns and long retention in the body,[2] since most nanoparticles cannot be rapidly excreted through the renal route due to large sizes relative to the kidney cutoff. It has been shown that nanoparticles can retain in the reticuloendothelial system (RES) over the course of a few months to years.[3] It is imperative to increase the biocompatibility and eliminate the long-term retention of nanoparticle-based imaging and therapeutic agents, overcoming a major roadblock to clinical translation of nanomedicine.[4]
Fluorescence imaging has been a powerful tool for researchers and clinicians to visualize structures and dynamics of biological systems down to single-cell and single-molecule resolution)[5] Conventional fluorescence imaging focuses on detecting in a narrow wavelength range of the electromagnetic spectrum (visible window: 400–700 nm or NIR-I window: 700–900 nm), often suffering from high autofluorescence signals from endogenous biological molecules and feature smearing and signal loss due to light scattering by tissues.[6] As a result, only superficial structures can be resolved with a conventional imaging contrast.[7] In recent years, fluorescence imaging in the second near-infrared window (1000–1700 nm, NIR-II) has attracted increasing attention due to suppressed tissue scattering and diminished autofluorescence, enabling deep tissue one-photon optical imaging with high clarity.[8] In particular, fluorescence imaging in the long end of the NIR-II window (1500–1700 nm, NIR-IIb window) can afford further improved tissue penetration up to sub-centimeter level due to near-zero autofluorescence and suppressed light scattering.[9] Several probes have been explored as candidates for in vivo NIR-IIb fluorescence imaging, including carbon nanotubes,[9a,b] rare-earth based down-shifting nanoparticles,[9c–f] semiconducting quantum dots[9g,h] and J-aggregates of organic molecules.[9i] These NIR-IIb contrast agents have been deployed for through scalp/skull imaging of the brain vasculatures,[9a,h] ultrafast hemodynamic tracking of the blood flow,[9d,f,h,j] deep-tissue tumor detection with a high signal-to-background ratio,[9c] and multi-plex molecular mapping of target proteins at the cellular level.[10] Nevertheless, most of these NIR-IIb probes are based on nanoparticles, and none have been reported to be renally excretable thus far. It is important to investigate the functionality as well as long-term fate of these novel materials to alleviate long-term safety concerns.
Recently, we devised cross-linked coating layers for rare-earth Er based down-shifting NIR-IIb luminescent nanoparticles (ErNPs).[9d] The coating was comprised of an amphiphilic hydrolyzed poly(maleic anhydride-alt-1-octadecene) (PMH) layer followed by a layer of 8Arm-PEG-NH2, a layer of PAA, and a layer of mixed mPEG- NH2 and 8Arm-PEG-NH2 at a mass ratio of 5:1 (branched PEG-linear PAA-branched PEG, denote P3 coating). The P3 coating enabled ≈90 % biliary excretion of the water-dispersible ErNPs within 2 weeks after intravenous injection, which was much faster than the excretion of rare-earth nanoparticles without cross-linked surface coating (> 90% in 44 days).[9c,d] In this work, we applied this novel cross-linked coating to several nanoparticle imaging probes, including core/shell lead sulfide/cadmium sulfide quantum dots (PbS/CdS QDs)[9f] and well-known superparamagnetic iron oxide nanoparticles (SPIONs).[11] We found that more than 90% of the P3-coated quantum dots (P3-QDs) were cleared from the body through the biliary pathway within 2 weeks of intravenous administration, without any obvious in vivo toxicity observed. The rapid excretion and low toxicity of P3-QDs alleviated potential side-effect risks for animals, and allowed long-term study of preclinical animal models. The bright and long-wavelength emission of the biocompatible quantum dots under 808/1319 nm excitation enabled noninvasive imaging of the CD8 + cytotoxic T lymphocytes (CTLs) in the tumor microenvironment in response to cancer immunotherapy using programmed cell death ligand-1 (PD-L1) blockade antibody.[12] In combination with P3-ErNPs,[9d] two-plex molecular imaging was achieved in the NIR-IIb window to monitor the interaction between PD-L1 positive tumor cells and CD8 positive T cells in real time at the cellular level by NIR-LSM. In addition, P3-QDs facilitated high-resolution molecular imaging of CD105 on tumor vasculatures in the NIR-IIb window using quantum dots conjugated to TRC105 chimeric antibody.[13] Non-targeted P3-QD was used to image lymph nodes non-invasively with an unprecedented signal-to-background ratio of 170, well exceeding the FDA-approved fluorescent dye indocyanine green (ICG) for imaging lymphatic nodes and vasculatures. Finally, we demonstrated that the P3 coating method could afford rapid excretion of SPIONs as well, suggesting a general strategy to produce rapid-excretable and biocompatible nanoparticles for in vivo imaging and therapy.
Results
Rapidly Excretable P3-QDsfor NIR-IIb Imaging
PbS/CdS core/shell quantum dots were synthesized in organic phase using a procedure described previously (see Supporting Information).[9g] To transfer the oleate-capped quantum dots from nonpolar organic solution to aqueous solution for applications in biological environment, we modified surface of the quantum dots by cross-linked hydrophilic polymers[9d] (see Supporting Information for detailed reactions). The inner-most layer was made of hydrolyzed poly(maleic anhydride-alt-1-octadecene) (PMH, molecular weight: 30000–50000). The hydrophobic side chains of PMH interacted with alkyl chains on the surface of PbS/CdS quantum dots via van der Waals forces, while the carboxyl groups provided sites for cross-linking reactions. A layer of branched 8Arm-PEG-NH2 (molecular weight: 40 000), a layer of linear PAA (molecular weight: 1800), and a layer of mixed mPEG-NH2 (molecular weight: 5000) and 8Arm-PEG-NH2 (molecular weight: 40000) at a mass ratio of 5:1 were subsequently coated on the surface through EDC chemistry (Figure 1 a). Each additional polymeric layer cross-linked with the underlying layer, preventing the polymers from detachment. Two PEG layers in the coating design was also novel, imparting high hydrophilicity to the particles.
Figure 1.
Surface modification of PbS/CdS quantum dots. a) Schematic illustration of PbS/CdS quantum dots with hydrophilic cross-linked polymeric layers (P3 coating). b) Absorption spectrum of P3-QDs in PBS buffer. c) Emission spectrum of P3-QDs in PBS buffer, excited by an 808-nm laser. d) Dynamic light scattering analysis of P3-QDs in PBS buffer, P3-QDs had a number averaged hydrodynamic diameter of 26.5 nm.
The resulting P3-QDs showed an emission peak at ≈1650 nm in aqueous solution with an absolute quantum yield of 1.5 % under the excitation of an 808-nm laser (Figure 1 b,c). The dip of the emission spectrum around 1450 nm was attributed to the overtone absorption of the water molecules (Figure S1).[14] Dynamic light scattering analysis of the P3-QDs showed a hydrodynamic diameter of 26.5 nm at a physiological pH (Figure 1 d). The quantum dots with P3 crosslinked polymer coating exhibited high stability in PBS buffer at room temperature and the physiological temperature of 37 °C over a course of 4 weeks monitored, exhibiting negligible decay in photoluminescence and little aggregation upon centrifugation at 14000 rpm. In contrast, quantum dots with regular non-crosslinked surface coating as done in ref. [9g] showed obvious fluorescence decay and agglomeration through the same treatment steps (Figure S2).
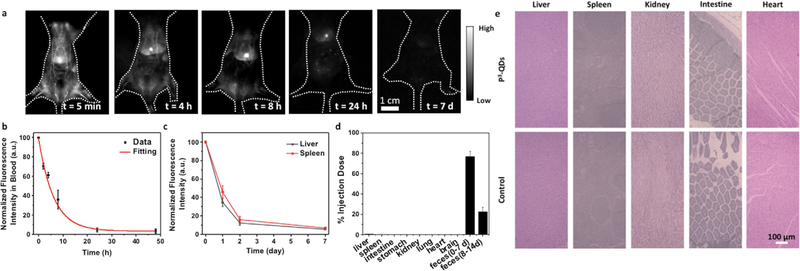
To investigate pharmacokinetics and biodistribution, P3-QDs were injected intravenously into balb/c mice (n = 5), and wide-field NIR-IIb images were recorded at different time points post injection (p.i.) (Figure 2 a). By measuring the fluorescence intensity in femoral artery,[15] we derived a blood circulation half-time of 4.75 hours (Figure 2 b). Fluorescence intensity in major organs, such as liver and spleen, increased within ≈ 4 hours p.i., followed by a gradual decline over the course of 2 weeks (Figure 2 a,c, Figure S3). Ex vivo NIR-IIb imaging of the major organs at 2 weeks post injection (p.i). showed minimal fluorescence signal, suggesting excretion of the particles (Figure S4). We observed NIR-IIb fluorescence signal in the feces of the mice, indicating structural intactness of the nanoparticles after clearance (Figure S4c). To analyze the biodistribution of P3-QDs quantitatively, the mice injected with P3-QDs were euthanized 14 days p.i., and the amount of lead in major organs and feces were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES). We observed that ≈ 95 % of the imaging agent was excreted from the body within 2 weeks (Figure 2 d), which was much faster than excretion of a previous QD coated by an amphiphilic polymer followed by a layer of mixed mPEG-NH2 and 8Arm-PEG-NH2 (mass ratio = 3:1). The latter case had fewer layers of coating and a much lower degree of crosslinking, with ≈ 76 % the quantum dots excreted in 4 weeks.[9g] None of the mice injected with P3-QDs showed any obvious health problems monitored over weeks to months. Histological studies found similar results as un-treated control, suggesting no obvious damage to major organs (Figure 2 e). Clearly the P3 coating was superior in affording rapid clearance and biocompatibility to nanoparticles.
Figure 2.
Rapid-excretable lead sulfide-cadmium sulfide core–shell quantum dots with P3 coating. a) Wide-field NIR-IIb (1500–1700 nm) fluorescence images of mice (n = 5) showed decay of fluorescence signal over time. The mice were excited by an 808 nm laser at a power density of 70 mWcm−2. b) NIR-IIb fluorescence signal in femoral artery of the mice (n = 5) at different time points post injection. The P3-QDs showed a blood circulation half time of 4.75 hours. c) Fluorescence signal of liver and spleen over the course of 2 weeks. d) Biodistribution of P3-QDs in major organs and feces at 14 days p.i. measured by ICP analysis of lead. Feces from day 0 to day 7, and day 8 to day 14 were collected and analyzed separately. e) Histological sections of hematoxylin and eosin (H&E)-stained major organs from healthy mice and mice injected with P3-QDs (2 weeks post injection). No difference can be discerned between the two, due to > 90% excretion of the QDs from the mouse and no apparent toxic effects.
P3-QDs-anti-CD8 and P3-ErNPs-anti-PD-L1 for Molecular Imaging of Immunotherapy
Next, we explored P3-QDs for NIR-IIb molecular imaging. Despite the successful application of checkpoint blockade therapy in a wide range of tumor types,[16] many patients fail to respond to the therapy,[17a] often associated with limited infiltration of CD8 + cytotoxic T lymphocytes (CTLs).[17b,c] To investigate PD-L1 + tumor cells and CD8 + CTLs simultaneously in vivo, we performed two-plex NIR-IIb molecular imaging using P3-QDs-anti-CD8 and ErNPs-anti-PD-L1 by detecting emission at ≈1600 nm for both types of probes. The drastically different fluorescence lifetimes of P3-QDs and ErNPs were utilized to differentiate the two probes and their respective molecular targets for two-plex molecular imaging in the NIR-IIb window (see Supporting Information).[9d]
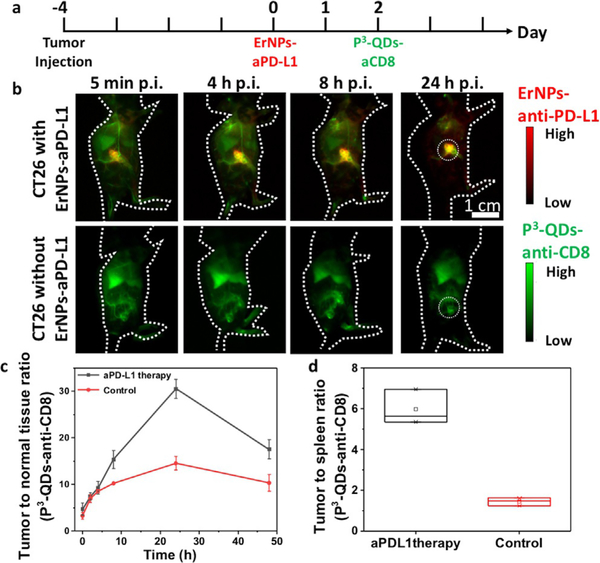
P3-ErNPs-anti-PD-L1 were first administered intravenously into the tail vein of balb/c mice (n = 3) with xenograft CT26 tumors known to respond to PD-L1 blockade immunotherapy.[18] The P3-ErNPs-anti-PD-L1 complex was used as a theranostic agent for both molecular imaging of PD-L1 and for immunotherapy as the PD-L1 antibodies conjugated to P3-ErNP retained therapeutic effect.[9d] The CT26 tumor-to-normal-tissue ratio (T/NT) revealed by imaging the tumor targeting ErNPs-anti-PD-L1 probes increased steadily over time and peaked at ≈ 36.7 at 48 hours p.i. (Figure S5). To map out immune responding CD8 + CTLs in vivo, an engineered anti-CD8 antibody fragment from rat-anti-mouse CD8a YTS169.4.2.1 hybridoma (cys-diabody, cDb)[19a] was conjugated to P3-QDs through EDC chemistry (see Supporting Information). The engineered cDb was shown to be nondepleting in vivo due to the lack of Fc domain,[19a b] making it suitable for imaging CD8 + CTLs without affecting immunotherapy. P3-QDs-anti-CD8 were injected through the tail vein of the same balb/c mice (n = 3) 48 hours after the administration of ErNPs-anti-PD-L1 (see Figure 3 a for the schedule of the experiment). A high tumor-to-normal-tissue ratio (T/NT) of 30.5 and tumor-to-spleen ratio (T/Sp) of 5.98 (Figure 3 b,c,d, Figure S6) were observed in the P3-QDs-anti-CD8 channel in the tumor, which was high due to immune responses to the injected P3-ErNPs-anti-PD-L1 48 h prior to the CD8 imaging. As a control, balb/c mice (n = 3) bearing CT26 tumors without ErNPs-anti-PDL1 treatment and the associated immune response were imaged by injected P3-QDs-anti-CD8, showing a much lower T/NT of 14.6 and T/Sp of 1.44 in the P3-QDs-anti-CD8 channel (Figure 3 b,c,d, Figure S6). The increased T/NT and T/Sp suggested trafficking of T cells from splenic organs and infiltration of activated CD8 + CTLs into CT26 tumor upon anti-PD-L1 therapy.[16b,c]
Figure 3.
P3-QDs-anti-CD8 diabody conjugate for in vivo molecular Imaging of CD8+ T cells. a) Treatment scheme. Balb/c mice were inoculated subcutaneously with CT26 tumors on the right side of the back. P3-ErNPs-anti-PD-L1 were injected intravenously when the tumor size reached 4 mm (typically 3–4 days after inoculation), and P3-QDs-anti-CD8 were injected 2 days after the injection of ErNPs-anti-PD-Ll. b) Wide-field NIR-IIb (1500–1700 nm) fluorescence images of mice with CT26 tumors injected with P3-QDs-anti-CD8, with and without ErNPs-anti-PD-L1 therapy (n = 3 for each group) at different time points after the injection of P3-QDs-anti-CD8. P3-QDs-anti-CD8 were excited by an 808-nm continuous-wave laser, and ErNPs-anti-PD-L1 were excited by a 975-nm pulse laser (duration = 14 ms). Fluorescence emission in the NIR-IIb window were collected for both channels. c) Tumor-to-normal-tissue ratio of P3-QDs-anti-CD8 channel over the course of 48 hours after the injection of P3-QDs-anti-CD8. d) Tumor-to-spleen ratio of the P3-QDs-anti-CD8 channel 24 hours after the administration of P3-QDs-anti-CD8.
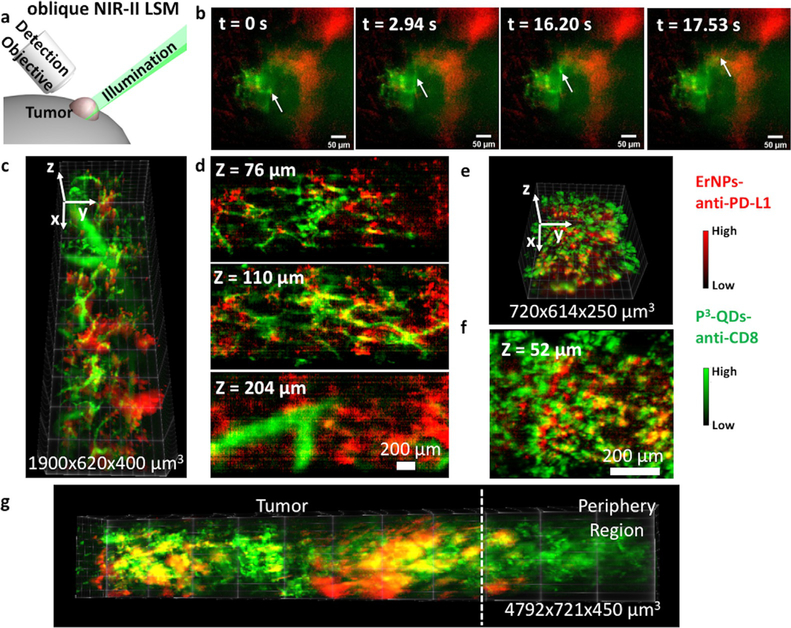
NIR-II light-sheet microscope (NIR-II-LSM) enabled noninvasive, high-resolution, and three-dimensional imaging of live mammals, allowing dynamic monitoring of CD8 + CTLs at the cellular level.[10] We performed 45° oblique LSM for two-plex imaging of ErNPs-anti-PD-L1 and P3-QDs-anti-CD8 (Figure 4 a) using a 1,319-nm laser excitation for QDs for maximum tissue penetration and propagation of the light sheet[9g,10] while detecting at ≈ 1600 nm to minimize scattering of the emitted light. Dynamic imaging (9 frames per second) of ErNPs-anti-PD-L1 and P3-QDs-anti-CD8 at a fixed illumination plane was taken at 2 hours after the administration of P3-QDs-anti-CD8 (50 hours after the administration of ErNPs-anti-PD-L1). The signal of the ErNPs-anti-PD-L1 channel (red color) remained unchanged during video recording, corresponding to labeled PD-L1 + tumor cells by ErNPs-anti-PD-L1 out-of-circulation and extravasated from the blood vessels. The blood vessels were bright in the P3-QDs-anti-CD8 channel (green color) due to the injected conjugates were still in circulation at 2 h p.i. Interestingly, we observed some bright spots in the P3-QDs-anti-CD8 channel moving in vessels over time, suggesting individual CD8 + CTLs labeled by P3-QDs-anti-CD8 migrating in the tumor microenvironment in response to immunotherapy (Figure 4 b and Video S1).
Figure 4.
In vivo two-plex LSM imaging of CT26 tumors. a) Scheme of in vivo NIR-II oblique LSM showing positions of the illumination light, the detection objective, and the tumor. b) Video-rate LSM imaging of a CT26 tumor at a fixed illumination plane 2 hours after the injection of P3-QDs-anti-CD8. The arrow showed the motion of CD8 + T cells in tumor microenvironment. Green color: P3-QDs-anti-CD8 channel, red color: ErNPs-anti-PD-L1 channel. Reconstructed 3D LSM image of a CT26 tumor obtained c) 6 hours or e) 24 hours after the injection of P3-QDs-anti-CD8. d,f) Corresponding two-plex xy images at different z positions. g) Reconstructed 3D LSM image of a CT26 tumor obtained 24 hours after the injection of P3-QDs-anti-CD8 showing tumor and the periphery region.
3D images were reconstructed from the oblique LSM scan at 6 h and 24 h p.i. of P3-QDs-anti-CD8 (Figure 4 c–f). At 6 h p.i., the quantum dots were still in circulation, and the signal mainly revealed the tumor vasculatures (Figure 4 c,d). Over time, most P3-QDs-anti-CD8 were cleared from the blood vessels at 24 h p.i. (Figure 4 e,f), which allowed distribution analysis of CD8 + CTLs. By evaluating the fluorescence signal in various regions in both channels, we observed that PD-L1 positive tumor cells mainly distributed in the core region of the CT26 tumor, while significant amount of CD8 + CTLs were in the periphery of the tumor microenvironment (Figure 4 g). The spatial distribution is consistent with our previous observation,[9g] and suggested that infiltration of CD8 + CTLs started from the tumor periphery.[20]
Molecular Imaging of Angiogenesis in Tumors with P3-QDs-TRC105
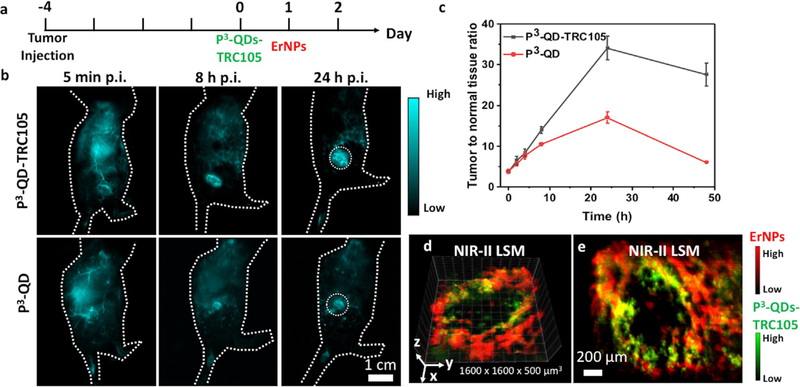
Next, we employed P3-QD as a probe for molecular imaging of angiogenesis in tumor models. TRC105 is a monoclonal antibody to human CD105[13a] highly expressed on proliferating human endothelial cells,[13a,b,c] and cross-reacts with murine CD105.[13d] P3-QDs were conjugated to TRC105 through EDC chemistry. Compared with free quantum dots, the conjugate showed a larger hydrodynamic size of 34.1 nm in aqueous solution, indicating successful conjugation (Figure S7). Balb/c mice were inoculated with 4T1 murine breast tumors on the flank, and injected with either free P3-QDs or P3-QDs-TRC105 conjugate from the tail vein (Figure 5 a, n = 3 for each group). Since 4T1 tumors expressed a high level of CD105 on tumor neovasculature,[21] we observed higher T/NT (≈ 34 vs. 17) at 24 h p.i. (Figure 5 b,c) in the case of active P3-QDs-TRC105 targeting of angiogenesis than free quantum dots accumulated in the tumor region through the passive enhanced permeability and retention (EPR) effect.[22]
Figure 5.
P3-QDs-TRC105 conjugate for in vivo molecular imaging of angiogenesis. a) Experiment scheme. Balb/c mice were injected subcutaneously with 4T1 tumors on the right side of the back. P3-QDs-TRC105 were injected intravenously when the tumor size reached 4 mm (typically 3–4 days after inoculation), and non-targeted ErNPs were injected 24 hours after the administration of P3-QDs-TRC105. b) Wide-field NIR-IIb fluorescence images of mice with 4T1 tumors injected with P3-QDs-TRC105 or non-targeted P3-QDs (n = 3 each group). The mice were excited with an 808-nm laser at a power density of 70 mWcm−2. c) Tumor-to-normal-tissue ratio over the course of 48 hours p.i. d) Reconstructed 3D LSM image (5x objective) of P3-QDs-TRC105 (green) and non-targeted ErNPs (red). The image was obtained 24 hours after the injection of P3-QDs-TRC105 (30 minutes after the injection of ErNPs). e) Maximum intensity projection of the 3D image shown in (d). The maximum intensity value in the x–y plane within a thickness of 100 μm along the z direction was displayed.
NIR-II LSM was used to image the 3D vasculature structures inside the tumor at a high resolution. Non-targeted P3-ErNPs were administered 24 hours after the injection of P3-QDs-TRC105, and two-plex LSM scan was performed immediately after the injection of ErNPs. All blood vessels were imaged by circulating P3-ErNPs (red color), while only vessels that overexpressed CD105 were labeled by P3-QDs-TRC105 (green color) injected 24 h earlier and mostly out of circulation. The overlap of the two color channels (yellow color in the Figure) mapped out the parts of vasculatures overexpressing CD105 within the tumor (Figure 5 d,e, Figure S8) labeled by the P3-QDs-TRC105 in vivo. There was negligible overlap of the two color channels within a 4T1 tumor when non-targeted P3-QDs were administered (Figure S9), suggesting that free P3-QDs did not bind to tumor vasculatures non-specifically. Such deep tissue NIR-IIb molecular imaging of tumor vasculatures at single-vessel level was not possible with one-photon NIR-I probes previously.[21b]
Lymph Node Mapping with P3-QDs
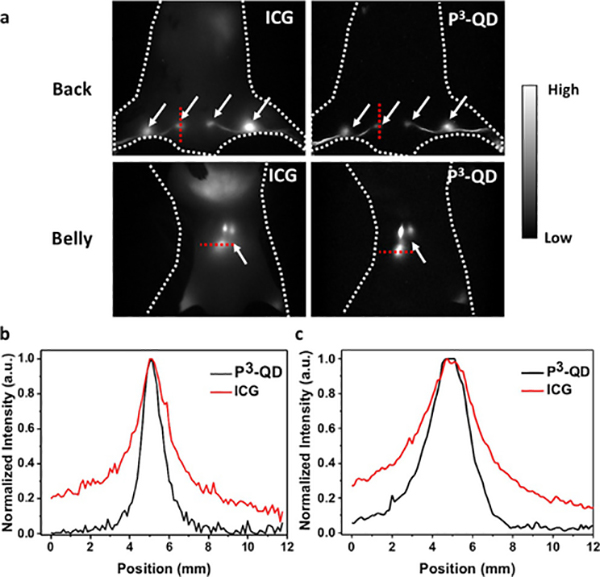
The P3-QD was employed for mapping lymph nodes and lymphatic vessels. For comparison, the clinical gold standard NIR dye ICG was also used. Non-targeted P3-QDs and ICG were co-injected into the footpad or the base of the tail of a mouse simultaneously, followed by gentle tissue massaging. The footpad of the mouse mainly drained to the sacral, popliteal and lumbar lymph nodes (Figure 6 a), while the base of the tail mainly drained to the inguinal and auxiliary nodes (Figure S10).[23] Imaging of ICG was done by exploiting the emission tail extending to the > 1000 nm NIR-II window[24] to reduce light scattering, and a lymph node-to-background ratio of 7.98 was observed for the shallow lymph nodes (sacral and popliteal nodes), and 6.14 for the deep ones (lumbar nodes) (Figure 6 b,c). In contrast, NIR-IIb imaging using P3-QDs afforded an unprecedented ratio of 170.1 and 49.1 for shallow and deep lymph nodes, respectively (Figure 6 b,c). NIR-IIb imaging was clearly superior to ICG based NIR-II tail emission imaging.
Figure 6.
Lymph node imaging with P3-QDs and ICG. a) Wide-field fluorescence images of a balb/c mouse co-injected with ICG (1100–1200 nm) and P3-QDs (1500–1700 nm) from the foot pads. The mice were excited with an 808-nm laser at a power density of 70 mWcm−2. White arrows indicate the positions of the lymph nodes (sacral, popliteal and lumbar lymph nodes are shown here). Fluorescence intensity cross-sectional profile of b) sacral and c) lumbar lymph nodes in NIR-II (1000−1200 nm)/NIR-IIb. Locations of the line profiles are shown in (a).
Comparison of the full width of half maximum (FWHM) of the lymph nodes at different depth clearly showed reduced broadening of the features when using P3-QDs as the NIR-IIb imaging probe. The two shallow popliteal lymph nodes showed a FWHM of 1.09 and 2.23 mm in the 1000–1200 nm and NIR-IIb window, respectively. The two deep lumbar lymph nodes showed a FWHM of 2.46 and 3.62 mm in the 1000–1200 nm and NIR-IIb window, respectively (Figure 6 b,c). In both optical windows, fluorescence signal could be visualized from the intestine, and the fluorescence signal of the injection site decayed over time (Figure S11), suggesting that both P3-QD and ICG agents could be excreted by the hepatocytes into the small intestine via the bile, and then cleared from the body.[25]
Rapidly Excreted Magnetic Nanoparticles with P3 Coating
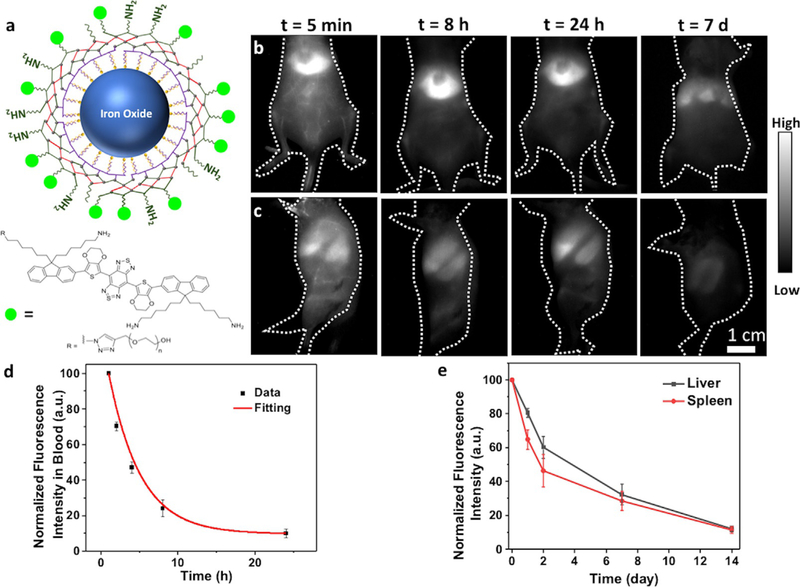
Lastly, since superparamagnetic iron oxide nanoparticles (SPIONs) have been widely used as magnetic resonance imaging contrast agents, drug delivery vehicles and cancer hyperthermia materials,[11] we demonstrate the generality of our cross-linked surface functionalization for P3 coating of SPIONs. It has been demonstrated that SPIONs coated with non-crosslinked PEGs could remain in liver and spleen for up to 70 days.[26c] Oleic acid-coated SPIONs were synthesized according to a previously described method (see Supporting Information for details),[26] and transferred to aqueous phase through the interaction with PMH. Subsequently, layer by layer 8-Arm-PEG-NH2, PAA and mixed 8-Arm-PEG and mPEG-NH2 were conjugated to the surface by EDC chemistry. Finally, a NIR-II small-molecule dye IR-FEPC[27] emitting in 1000–1200 nm was conjugated to the amine groups of the 8-Arm-PEG-NH2 using EDC chemistry. The emission of IR-FEP is stable under physiological conditions,[27b] which allowed tracing SPIONs in vivo for a long period of time (Figure 7 a).
Figure 7.
Excretion of iron oxide nanoparticles with P3 coating. a) Schematic illustration of iron oxide nanoparticles with P3 coating and conjugated to IR-FEPC. Wide-field NIR-II (1000–1200 nm) fluorescence images of mice showed decay of the fluorescence signal over time in b) liver and c) spleen. The mice were excited by an 808 nm laser at a power density of 70 mWcm2. d) NIR-II fluorescence signal in femoral artery of the mice at different time points post injection. The iron oxide nanoparticles showed a blood circulation half time of 2.86 hours. e) Fluorescence signal decay of liver and spleen over the course of 2 weeks.
To analyze pharmacokinetics and excretion of the as-prepared P3-coated SPIONs (P3-SPIONs), wide-field whole-body NIR-II (1000–1200 nm) fluorescence imaging of injected mice was performed at different time points following intravenous administration of the nanoparticles. The nanoparticles mainly accumulated in the liver and spleen of the mouse (Figure 7 b,c). Fluorescence intensity in the blood circulation was imaged over 24 hours, and the blood circulation half time of P3-SPIONs was determined to be 2.86 hours (Figure 7 d). Fluorescence signal in liver and spleen decayed over time, with < 15 % of the original signal remaining after 2 weeks due to excretion (Figure 7 e). ICP-OES analysis of iron in major organs and feces showed that 92% of the nanoparticles was excreted from the body (Figure S12). The results with SPIONs suggested that the P3 surface modification is a versatile method of imparting aqueous solubility and biocompatibility to nanoparticles.
Discussion
In the past decades nanoparticles have been widely explored for biomedical applications owing to their unique physiochemical properties, such as large surface area, tunable surfaces for functionalization and distinctive optical or magnetic properties compared to their bulk materials.[25] Despite the technology advancements achieved in small animal models, very few nanoparticles have been approved for clinical use due to safety concerns. Most nanoparticles have a hydrodynamic size larger than the 5.5-nm cutoff of the renal filtration,[28] which prevents clearance of the materials through the kidney. It has been reported that the prolonged accumulation of nanomaterials may cause significant changes of organ indices and serum biochemical parameters.[29] To alleviate the long-term toxicity side effect, surface of the nanomaterials has to be tailored to allow fast clearance from the body. We recently developed the P3 coating method for functionalization of ErNPs.[9d] The hydrophilic polymer layers formed a highly cross-linked network to stabilize the nanoparticles in aqueous environment, and prevented them from aggregation, allowing rapid biliary excretion of the nanomaterials. In this work, we generalized the surface coating strategy to other nanomaterials, including PbS/CdS quantum dots and iron oxide nanoparticles. The stable surface coating lead to > 90 % clearance of the nanoparticles from the body within two weeks in both cases, which together with the earlier P3-ErNP result suggest that the P3 coating can be used as a versatile method for nanoparticle functionalization for high biocompatibility and low retention inside the body. This could be a key progress toward clinical translation of nanomaterials to utilize their unique physical and chemical properties.
Our long-term stability data (Figure S2) showed the P3 cross-linked surface coating prevented the polymers from detaching from the nanoparticles in vivo, maintained a highly hydrophilic surface by the covalently linked inner and outer PEG layers, and avoided strong binding and interactions with proteins and tissues in vivo. These could be a key to prevent long term trapping of nanoparticles by cells and organisms and enable rapid clearance from the body. In general, nanomaterials can be removed by hepatocytes and phagocytic cells in the liver after endocytosis and enzymatic break-down.[25] The biliary excretion pathway is highly influenced by the surface functionalization of the nanomaterials. It has been shown that nanoparticles captured in RES can persist for a long time and may induce chronic tissue inflammation, generate reactive oxygen species (ROS), and cause dissolution and release of toxic elements if not properly functionalized.[30] The P3-coated nanoparticles exhibited no toxicity in vivo and no harmful effects over the course of weeks to months, allowing possibilities for clinical translation.
The P3 cross-linked nanoparticles could give nanomedicine a boost in terms of clinical translation. For surface tumors such as melanoma and head and neck cancer, non-invasive NIR-IIb fluorescence molecular imaging of PD-L1 using rapid-excretable nanoparticles could complement or replace ex vivo biopsy-based methods currently being used in clinics to assess response to immunotherapy. More importantly, noninvasive NIR-II molecular imaging of CD8 + CTLs in vivo and tracking the T cells over time could provide a direct, longitudinal assessment of a patient’s response to immunotherapy. Compared to other imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), NIR-IIb fluorescence imaging provides the benefits of high spatial resolution and real-time temporal feedback. Further, multi-plex fluorescence imaging can be achieved by applying fluorophores with different properties (emission wavelength and/or lifetime), which allows in vivo mapping of two or more molecular targets simultaneously. As shown in this work, multi-plex in vivo fluorescence imaging facilitates monitoring the interaction between immune cells and tumor cells in the tumor microenvironment, which can be used as an indicator of the likelihood of response to immunotherapy.[16a]
Besides diagnostic imaging and assessment of immunotherapy, NIR-IIb Imaging may also facilitate imaging guided surgery. In vivo fluorescence imaging can complement other imaging modalities which cannot provide real-time feedback for intraoperative applications. We have demonstrated that by employing specific targeting ligands such as antibodies to PD-L1 or CD105, P3-functionalized NIR-IIb fluorescent nanoparticles could afford a ≈10 times higher T/NT (≈40) compared to FDA-approved fluorescent dyes like ICG.[24b] This will allow identification of resection margins with a significantly improved precision. NIR-IIb fluorophores also enables mapping of sentinel lymph nodes with a much higher signal-to-background ratio compared to ICG and methylene blue. It can help the observation and assessment of lymphatic tumor metastasis after cancer therapy, which has been shown to play an important role in increasing the survival rates.[31] P3 coating also opens up the possibility of clinical translation for other nanoparticles. For example, P3-SPIONs have been widely investigated as a contrast agent for magnetic resonance imaging.[11]
In addition to medical imaging, nanoparticles can also be utilized as therapeutic agents or drug delivery systems as demonstrated in small animal models.[1c–g] For instance, P3-SPOINs can be used for both MRI imaging and as an agent for hyperthermia-based cancer therapy due to their ability to generate heat in a magnetic field.[11]
Conclusion
In summary, we developed cross-linked coating layers for functionalization of nanoparticles. The P3 cross-linked surface coating enabled rapid clearance of the nanoparticles from the body through RES uptake and subsequent biliary excretion. The cross-linked surface coating could help to reduce the side effects caused by long-term retention of a wide range of nanomaterials, establishing a foundation for nanomedicine based theranostics for clinical translation for human use.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (DP1-NS-105737). We thank Charles P. Theuer (TRACON Pharmaceuticals Inc.) for providing the TRC105 antibody. We thank Stanford Animal Histology Services (AHS) for preparing H&E stained tissues for histology analysis.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.202008083.
Contributor Information
Zhuoran Ma, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
Feifei Wang, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
Yeteng Zhong, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
Felix Salazar, Department of Molecular Imaging and Therapy, Beckman Research, Institute of the City of Hope, Duarte, CA (USA).
Jiachen Li, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
Mingxi Zhang, State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan (China).
Fuqiang Ren, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
Anna M. Wu, Department of Molecular Imaging and Therapy, Beckman Research, Institute of the City of Hope, Duarte, CA (USA)
Hongjie Dai, Department of Chemistry and Bio-X, Stanford University Stanford, CA 94305 (USA).
References
- [1] a).Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R, Nano Lett 2008, 8, 4593–4596; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Marangoni VS, Neumann O, Henderson L, Kaffes CC, Zhang H, Zhang R, Bishnoi S, Ayala-Orozco C, Zucolotto V, Bankson JA, Nordlander P, Halas NJ, Proc. Natl. Acad. Sci. USA 2017, 114, 6960–6965; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H, Nano Res 2008,1, 203–212; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang J, Yuan Z, Wang Y, Chen W, Luo G, Cheng S, Zhuo R, Zhang X, J. Am. Chem. Soc 2013, 135, 5068–5073; [DOI] [PubMed] [Google Scholar]; e) Ali HR, Ali MRK, Wu Y, Selim SA, Abdelaal HFM, Nasr EA, El-Sayed MA, Bioconjugate Chem 2016,27, 2486–2492; [DOI] [PubMed] [Google Scholar]; f) Wang M, Song J, Zhou F, Hoover AR, Murray C, Zhou B, Wang L, Qu J, Chen WR, Adv. Sci 2019, 6,1802157; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C, ACS Nano 2016, 10, 2436–2446. [DOI] [PubMed] [Google Scholar]
- [2] a).Fadeel B, Garcia-Bennett AE, Adv. Drug Delivery Rev 2010, 62, 362–374; [DOI] [PubMed] [Google Scholar]; b) Anselmo AC, Mitragotri S, Bioeng. Transl. Med 2019, 4, e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3] a).Liu Z, Davis C, Cai W, He L, Chen X, Dai H, Proc. Natl Acad. Sci. USA 2008,105,1410–1415; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H, Nat. Nanotechnol 2007, 2, 47–52. [DOI] [PubMed] [Google Scholar]
- [4] a).Liu C, Hou Y, Gao M, Adv. Mater 2014, 26, 6922–6932; [DOI] [PubMed] [Google Scholar]; b) Tsoi KM, Dai Q, Alman BA, Chan WCW, Acc. Chem. Res 2013, 46, 662–671; [DOI] [PubMed] [Google Scholar]; c) Francis AP, Devasena T, Toxicol. Ind. Health 2018, 34, 200–210. [DOI] [PubMed] [Google Scholar]
- [5] a).sahl SJ, Hell SW, Jakobs S, Nat. Rev. Mol. Cell Biol 2017, 18, 685–701; [DOI] [PubMed] [Google Scholar]; b) Sevick-Muraca EM, Caskey CT, Austin CP, Hoxie JA, Annu. Rev. Med 2012, 63, 217–231. [DOI] [PubMed] [Google Scholar]
- [6] a).Hong G, Diao S, Antaris AL, Dai H, Chem. Rev 2015,115, 10816–10906; [DOI] [PubMed] [Google Scholar]; b) Hong G, Antaris AL, Dai H, Nat. Biomed. Eng 2017,1, 0010. [Google Scholar]
- [7].Starosolski Z, Bhavane R, Ghaghada KB, Vasudevan SA, Kaay A, Annapragada A, PLoS One 2017,12, e0187563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8] a).Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H, Nat. Nanotechnol 2009, 4, 773–780; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Y, Hong G, Zhang Y, Chen G, Li F, Dai H, Wang Q, ACS Nano 2012, 6, 3695–3702; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hong G, Zou Y, Antaris AL, Diao S, Wu D, Cheng K, Zhang X, Chen C, Liu B, He Y, Wu JZ, Yuan J, Zhang B, Tao Z, Fukunaga C, Dai H, Nat. Commun 2014, 5, 4206; [DOI] [PubMed] [Google Scholar]; d) Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, Zhang X, Yaghi OK, Alamparambil ZR, Hong X, Cheng Z, Dai H, Nat. Mater 2016, 15, 235–242; [DOI] [PubMed] [Google Scholar]; e) Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Kuo CJ, Dai H, Nat. Photonics 2014, 8, 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Diao S, Blackburn JL, Hong G, Antaris AL, Chang J, Wu JZ, Zhang B, Cheng K, Kuo CJ, Dai H, Angew. Chem. Int. Ed 2015,54,14758–14762; Angew. Chem. 2015,127,14971–14975; [DOI] [PubMed] [Google Scholar]; b) Diao S, Hong G, Antaris AL, Blackburn JL, Cheng K, Cheng Z, Dai H, Nano Res 2015, 8, 3027–3034; [Google Scholar]; c) Zhong Y, Ma Z, Zhu S, Yue J, Zhang M, Antaris A, Yuan J, Cui R, Wan H, Zhou Y, Wang W, Huang NF, Luo J, Hu Z, Dai H, Nat. Commun 2017,8,737; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhong Y, Ma Z, Wang F, Wang X, Yang Y, Liu Y, Zhao X, Li J, Du H, Zhang M, Cui Q, Zhu S, Sun Q, Wan H, Tian Y, Liu Q, Wang W, Garcia KC, Dai H, Nat. Biotechnol 2019,37,1322–1331; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fan Y, Wang P, Lu Y, Wang R, Zhou L, Zheng X, Li X, Piper JA, Zhang F, Nat. Nanotechnol 2018, 13, 941–946; [DOI] [PubMed] [Google Scholar]; f) Naczynski DJ, Tan MC, Zevon M, Wall B, Kohl J, Kulesa A, Chen S, Roth CM, Riman RE, Moghe PV, Nat. Commun 2013, 4, 2199; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhang M, Yue J, Cui R, Ma Z, Wan H, Wang F, Zhu S, Zhou Y, Kuang Y, Zhong Y, Pang D-W, Dai H, Proc. Natl. Acad. Sci. USA 2018,115, 6590–6595; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Bruns OT, Bischof TS, Harris DK, Franke D, Shi Y, Riedemann L, Bartelt A, Jaworski FB, Carr JA, Rowlands CJ, Wilson MWB, Chen O, Wei H, Hwang GW, Montana DM, Coropceanu I, Achorn OB, Kloepper J, Heeren J, So PTC, Fukumura D, Jensen KF, Jain RK, Bawendi MG, Nat. Biomed. Eng 2017, 1, 0056; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Sun C, Li B, Zhao M, Wang S, Lei Z, Lu L, Zhang H, Feng L, Dou C, Yin D, Xu H, Cheng Y, Zhang F, J. Am. Chem. Soc 2019,141,19221–19225; [DOI] [PubMed] [Google Scholar]; j) Ma Z, Zhang M, Yue J, Alcazar C, Zhong Y, Doyle TC, Dai H, Huang NF, Adv. Funct. Mater 2018, 28, 1803417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang F, Wan H, Ma Z, Zhong Y, Sun Q, Tian Y, Qu L, Du H, Zhang M, Li L, Ma H, Luo J, Liang Y, Li WJ, Hong G, Liu L, Dai H, Nat. Methods 2019,16, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mahmoudi M, Sant S, Wang B, Laurent S, Sen T, Adv. Drug Delivery Rev 2011, 63, 24–46. [DOI] [PubMed] [Google Scholar]
- [12].Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im S, Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Investigators IT, N. Engl. J. Med 2018, 379, 2108–2121. [DOI] [PubMed] [Google Scholar]
- [13] a).Brossa A, Buono L, Bussolati B, Oncotarget 2018, 9, 22680; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fonsatti E, Maio M, J. Transl. Med 2004, 2, 18; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Paauwe M, ten Dijke P, Hawinkels LJ, Expert Opin. Ther. Targets 2013, 17, 421–435; [DOI] [PubMed] [Google Scholar]; d) Chen F, Nayak TR, Goel S, Valdovinos HF, Hong H, Theuer CP, Barnhart TE, Cai W, Mol. Pharm 2014,11, 4007–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Curcio JA, Petty CC, J. Opt. Soc. Am 1951, 41, 302–304. [Google Scholar]
- [15] a).Zhang X-D, Wang H, Antaris AL, Li L, Diao S, Ma R, Nguyen A, Hong G, Ma Z, Wang J, Zhu S, Castellano JM, Wyss-Coray T, Liang Y, Luo J, Dai H, Adv. Mater 2016, 28, 6872–6879; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang W, Ma Z, Zhu S, Wan H, Yue J, Ma H, Ma R, Yang Q, Wang Z, Li Q, Qian Y, Yue C, Wang Y, Fan L, Zhong Y, Zhou Y, Gao H, Ruan J, Hu Z, Liang Y, Dai H, Adv. Mater 2018, 30, 1800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16] a).Zou W, Wolchok JD, Chen L, Sci. Transl. Med 2016, 8, 328rv4; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Iams WT, Porter J, Horn L, Nat. Rev. Clin. Oncol 2020, 17, 300–312; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Seidel JA, Otsuka A, Kabashima K, Front. Immunol 2018, 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17] a).Hegde PS, Chen DS, Immunity 2020, 52, 17–35; [DOI] [PubMed] [Google Scholar]; b) Herbst RS, Soria J, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS, Nature 2014, 515, 563–567; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Leclerc M, Voilin E, Gros G, Corgnac S, de Montpreville V, Validire P, Bismuth G, Mami-Chouaib F, Nat. Commun 2019, 10, 3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mosely SIS, Prime JE, Sainson RCA, Koopmann JO, Wang DYQ, Greenawalt DM, Ahdesmaki MJ, Leyland R, Mullins S, Pacelli L, Marcus D, Anderton J, Watkins A, Ulrichsen JC, Brohawn P, Higgs BW, McCourt M, Jones H, Harper JA, Morrow M, Valge-Archer V, Stewart R, Dovedi SJ, Wilkinson RW, Cancer Immunol Res 2017, 5, 29–41. [DOI] [PubMed] [Google Scholar]
- [19] a).Tavare R, Escuin-Ordinas H, Mok S, McCracken MN, Zettlitz KA, Salazar FB, Witte ON, Ribas A, Wu AM, Cancer Res 2016, 76, 73–82; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu AM, Methods 2014, 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20] a).Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, J. Clin. Oncol 2009, 27, 5944–5951; [DOI] [PubMed] [Google Scholar]; b) Li X, Gruosso T, Zuo D, Omeroglu A, Meterissian S, Guiot MC, Salazar A, Park M, Levine H, Proc. Natl. Acad. Sci. USA 2019,116, 3678–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21] a).Fonsatti E, Nicolay HJM, Altomonte M, Covre A, Maio M, Cardiovasc. Res 2010,86,12–19; [DOI] [PubMed] [Google Scholar]; b) Yang Y,Zhang Y, Hong H, Liu G, Leigh BR, Cai W, Eur J Nucl. Med. Mol. Imaging 2011, 38, 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Greish K, Cancer Nanotechnology, Springer, Heidelberg, 2008, pp. 25–37. [Google Scholar]
- [23].Harrell MI, Iritani BM, Ruddell A, J. Immunol. Methods 2008, 332, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24] a).Carr JA, Franke D, Caram JR, Perkinson CF, Saif M, Askoxylakis V, Datta M, Fukumura D, Jain RK, Bawendi MG, Bruns OT, Proc. Natl. Acad. Sci. USA 2018, 115, 4465–4470; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, Su S, Sun X, Shi X, Li C, Zhou T, Zhang Y, Chi C, He P, Xia X, Chen Y, Gambhir SS, Cheng Z, Tian J, Nat. Biomed. Eng 2020,4,259–271. [DOI] [PubMed] [Google Scholar]
- [25].Bourquin J, Milosevic A, Hauser D, Lehner R, Blank F, Petri-Fink A, Rothen-Rutishauser B, Adv. Mater 2018, 30, 1704307. [DOI] [PubMed] [Google Scholar]
- [26] a).Park J, An K, Huang Y, Park J-G, Noh H-J, Kim J-Y, Park J-H, Hwang N-M, Hyeon T, Nat. Mater 2004, 3, 891–895; [DOI] [PubMed] [Google Scholar]; b) Bloemen M, Brullot W, Luong TT, Geukens N, Gils A, Verbiest T, J. Nanopart. Res 2012,14, 1100; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Keselman P, Yu EY, Zhou XY, Goodwill PW, Chandrasekharan P, Ferguson RM, Khandhar AP, Kemp SJ, Krishnan KM, Zheng B, Conolly SM, Phys. Med. Biol 2017, 62, 3440–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27] a).Zhu S, Herraiz S, Yue J, Zhang M, Wan H, Yang Q, Ma Z, Wang Y, He J, Antaris AL, Zhong Y, Diao S, Feng Y, Zhou Y, Yu K, Hong G, Liang Y, Hsueh AJ, Dai H, Adv. Mater 2018, 30, 1705799; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wan H, Yue J, Zhu S, Uno T, Zhang X, Yang Q, Yu K, Hong G, Wang J, Li L, Ma Z, Gao H, Zhong Y, Su J, Antaris AL, Xia Y, Luo J, Liang Y, Dai H, Nat. Commun 2018, 9, 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV, Nat. Biotechnol 2007,25,1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang S-T, Wang X, Jia G, Gu Y, Wang T, Nie H, Ge C, Wang H, Liu Y, Toxicol. Lett 2008,181, 182–189. [DOI] [PubMed] [Google Scholar]
- [30].Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M, Nat. Mater 2009, 8, 543–557. [DOI] [PubMed] [Google Scholar]
- [31].Wu Y-L, Huang Z-F, Wang S-Y, Yang X-N, Ou W, Lung Cancer 2002, 36, 1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.