SUMMARY
Objectives:
Diabetes is a risk factor for active tuberculosis (TB). Data are limited regarding the association between diabetes and TB drug resistance and treatment outcomes. We examined characteristics of TB patients with and without diabetes in a Peruvian cohort at high risk for drug-resistant TB. Among TB patients with diabetes (TB–DM), we studied the association between diabetes clinical/management characteristics and TB drug resistance and treatment outcomes.
Methods:
During 2005–2008, adults with suspected TB with respiratory symptoms in Lima, Peru, who received rapid drug susceptibility testing (DST), were prospectively enrolled and followed during treatment. Bivariate and Kaplan–Meier analyses were used to examine the relationships of diabetes characteristics with drug-resistant TB and TB outcomes.
Results:
Of 1671 adult TB patients enrolled, 186 (11.1%) had diabetes. TB–DM patients were significantly more likely than TB patients without diabetes to be older, have had no previous TB treatment, and to have a body mass index (BMI) ≥18.5 kg/m2 (p < 0.05). In patients without and with previous TB treatment, the prevalence of multidrug-resistant TB was 23% and 26%, respectively, among patients without diabetes, and 12% and 28%, respectively, among TB–DM patients. Among 149 TB–DM patients with DST results, 104 (69.8%) had drug-susceptible TB and 45 (30.2%) had drug-resistant TB, of whom 29 had multidrug-resistant TB. There was no association between diabetes characteristics and drug-resistant TB.Of 136 TB–DM patients with outcome information, 107 (78.7%) had a favorable TB outcome; active diabetes management was associated with a favorable outcome.
Conclusions:
Diabetes was common in a cohort of TB patients at high risk for drug-resistant TB. Despite prevalent multidrug-resistant TB among TB–DM patients, the majority had a favorable TB treatment outcome.
Keywords: Tuberculosis, Multi-drug resistance, Diabetes, Peru
1. Introduction
Globally, there are an estimated 8.8 million new tuberculosis (TB) cases each year, and approximately 340 million people are living with diabetes.1,2 Diabetes is a known risk factor for the development of active TB,3–5 and an estimated 15% of patients with TB in countries with a high TB burden have diabetes.6 The association between diabetes and TB is an area of growing interest due to the persistently high prevalence of both diseases internationally and the expected increase in diabetes incidence and deaths over the coming decades.2,7,8
Due to global increases in multidrug-resistant TB (MDR-TB),9 the importance of understanding the relationship between diabetes and MDR-TB is growing.10 For example, studies from India, the Philippines, Spain, and Turkey have shown that diabetes is prevalent among patients with MDR-TB, with 10–23% of MDR-TB patients having concomitant diabetes.11–14 Other studies have reported an independent association between diabetes and MDR-TB,15,16 but data are limited regarding factors associated with drug resistance among TB patients with diabetes (TB–DM). Furthermore, among TB–DM patients, there remains a paucity of information about the relationship between diabetes control and clinical care characteristics and TB outcomes.4,17
The greatest TB and MDR-TB burdens occur in low- and middle-income countries, where the largest increases in diabetes prevalence and incidence are expected during the next 20 years.4,8 In 2009, the incidence of TB in Peru was 113 per 100 000 persons, which is the highest in South America.18 The national prevalence of MDR-TB among patients never treated and previously treated for TB (one of the strongest known risk factors for MDR-TB) is also high, at 5.0% and 23.6%, respectively.9 Similarly, there is a growing burden of diabetes in Peru. The prevalence of diabetes in urban areas was recently reported to be 7.0%;14 based on limited data, the national prevalence is estimated to be 5.1–6.0% among adults19 and is expected to increase to 7.3% by 2030.7 Given the high burden of TB and diabetes in Peru, the complexities in managing patients with these co-morbidities, and the challenges that exist in linking TB and diabetes services, it is important to better understand the relationship between diabetes and drug-resistant TB to help inform efforts to optimize patient care. Therefore, in this study we aimed to (1) describe the characteristics of TB patients screened for MDR-TB in Peru with and without diabetes; (2) describe the diabetes clinical characteristics and medical care associated with drug resistance among patients with TB and diabetes; and (3) determine diabetes care characteristics associated with favorable TB treatment outcomes.
2. Methods
2.1. Setting and program description
In 2005, the Peruvian National Tuberculosis Program (PNTP) and the National Reference Laboratory (NRL) initiated a pilot program in two districts in Lima to strengthen its TB laboratory network, implement rapid diagnostic strategies for drug-resistant TB, and decentralize drug susceptibility testing (DST) to district laboratories.20 Patients with risk factors for MDR-TB, as defined by the PNTP, had rapid screening for drug-resistant TB using DST by the direct nitrate reductase assay (Griess method). Diabetes was one of the indications for rapid DST per the PNTP criteria.21 Additional details about this programmatic strategy of rapid screening for MDR-TB have been described elsewhere.20,22,23
2.2. Data collection
Patients who had suspected or confirmed TB were prospectively enrolled in two (of four) health districts of Lima from January 2005 through May 2008. Health care workers from 54 health centers in Lima Ciudad and Lima Este identified patients with risk factors for MDR-TB, and then patient sputum samples were sent to the district laboratory for DST. Observational data were collected on all individuals referred for DST using standardized data collection forms. For TB patients, HIV status was recorded as a part of the routine PNTP guidelines. Chest radiographs performed <1 year before the enrollment date or <1 month after the enrollment date were reviewed. Socio-demographic, clinical, bacteriologic, and risk factor data were also collected at baseline. Patients were then followed throughout TB treatment.
Patients with diabetes were identified from their history recorded in the medical records. From each of these patients, information about date of diabetes diagnosis, result, and type of last glucose test were collected. To supplement these data, additional diabetes-related information from before and during TB treatment were abstracted from existing records at health clinics and hospitals in Lima where patients with diabetes received medical care. The following information was collected: type and date of diabetes diagnosis, type of medications used, diabetes-related care and complications prior to and during enrollment, and laboratory results during the year prior to and during study enrollment.
2.3. Definitions
Definitions of MDR-TB and diabetes as defined by the World Health Organization (WHO) were used for this project.24,25 MDR-TB was defined as infection with Mycobacterium tuberculosis with in vitro resistance to at least isoniazid and rifampin. Drug-resistant TB was defined as M. tuberculosis with in vitro resistance to any first-line anti-TB drug. Patients identified as having diabetes in the medical records had their diabetes confirmed by any one of the following criteria: fasting plasma glucose ≥7.0 mmol/l (126mg/dl), 2-h plasma glucose/random blood glucose ≥11.1 mmol/l (200 mg/dl), glycosylated hemoglobin (HbA1c) ≥7.0%, or treatment with insulin or oral hypoglycemic agents. Any values for HbA1c or urine glucose during the previous year before enrollment start date were also recorded. Diabetes control was defined by documentation of controlled diabetes status by a nurse or physician in the patient’s medical records prior to TB treatment. Diabetes care during TB treatment was defined using a combination of the following measures: recorded endocrinologist visit, diabetes control documented in the medical record, recorded use of diabetes medications, or three or more measures of blood glucose. The level of diabetes care during treatment was defined as frequent care (three or four of the measures mentioned above), some care (one or two measures), and none (0 measures). A favorable TB treatment outcome was defined as cured or completed, and a poor treatment outcome was defined as default, failed, or died, in accordance with WHO guidelines.26 If final outcome information was unavailable, converted sputum culture during treatment was also considered favorable.
2.4. Data management and analyses
Data were double-entered into an Epi-Info database (Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA). All analyses were performed using SAS 9.3 (Cary, NC, USA). We limited our analysis to all adult (≥15 years) TB patients who were enrolled into the study during January 2005-May 2008. Patients aged less than 15 years were excluded from the analysis because both childhood diabetes (primarily type 1) and TB differ clinically from adult disease. TB–DM patients were excluded from analyses of the association of diabetes characteristics (prior to TB treatment) with drug resistance pattern if TB drug susceptibility data were not available (n = 35).
The Chi-square test or Fisher’s exact test was used to calculate p-values for categorical variables. The Student’s t-test and analysis of variance (ANOVA) were used to compare differences in normally distributed continuous variables, and the Wilcoxon-Mann-Whitney rank sums test was used when continuous variables were not normally distributed. The associations between patient characteristics and study outcomes were examined in bivariate analyses. A two-sided p-value of less than 0.05 was considered statistically significant throughout the analyses. Product limit survival estimates were created using Kaplan–Meier curves, and the log-rank test was used to evaluate statistical difference in survival time. Cox proportional hazards models were used to estimate the rate of culture conversion using survival time. Time to TB culture conversion for survival estimation was calculated as the number of days between the date a sputum sample was sent for DST and the first date a negative sputum sample was collected that was followed by at least one additional consecutive negative sputum sample ≥30 days apart.
2.5. Ethical approval
The institutional review boards at Brigham and Women’s Hospital and the Peruvian National Institute of Health reviewed and approved the study. This activity was approved by the US CDC as program evaluation and not as human subject research.
3. Results
3.1. Characteristics of TB patients screened for MDR-TB in Peru
A total of 1671 adult patients with culture-positive TB were screened for MDR-TB and enrolled in the prospective cohort (Figure 1). The majority of patients were male (66%) and the median age was 31 years (range 15–83 years). Baseline drug susceptibility testing results were available for 1190 patients; 394 (33.1%) of these had MDR-TB. One-hundred and eighty-six patients (11.1%) had diabetes.
Figure 1.
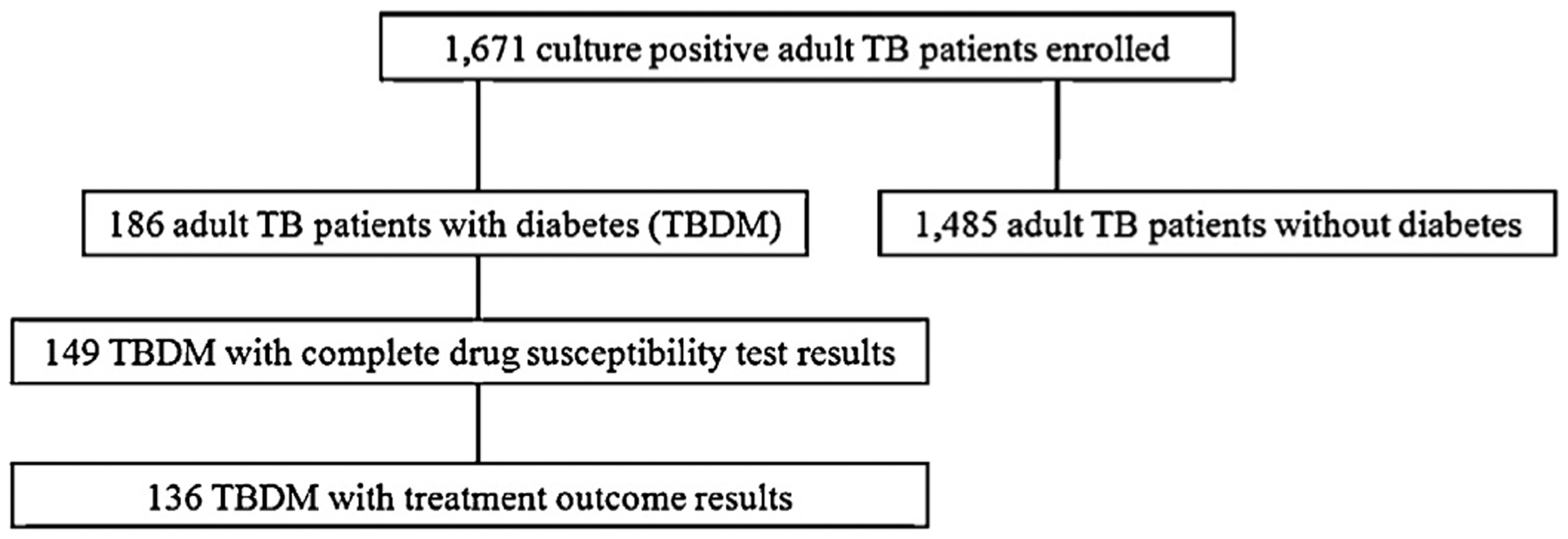
Tuberculosis (TB) patients at risk for multidrug-resistant TB enrolled, screened for diabetes mellitus (DM), and followed up forTB treatment outcomes in Peru.
TB–DM patients were significantly more likely than those without diabetes to be older (median age in years 54 (range 31–80) vs. 39 (range 15–83)), married or partnered (65.6% vs. 35.1%), and have less than a primary education (25.8% vs. 11.5%) (Table 1). They were also less likely to have had previous TB treatment (23.1% vs. 54.2%). TB–DM patients had a significantly lower proportion of bilateral lung cavitations (3.2% vs. 5.1%) compared to TB patients without diabetes (p < 0.05). More TB–DM patients than TB patients without diabetes had positive cultures at baseline (p < 0.05). TB–DM patients were also significantly less likely to have HIV infection (0.5% and 13.3%) and to be undernourished (BMI < 18.5 kg/m2) (4.8% vs. 22.2%). Overall, TB–DM patients were significantly less likely than those without diabetes to have MDR-TB (15.6% vs. 24.6%, p < 0.01). Because of the strong influence of previous TB treatment on drug resistance status, we examined the relationship between diabetes and drug resistance stratified by treatment history (Figure 2), and among those with previous TB treatment, 27.9% of TB–DM patients and 26.1% without diabetes had MDR-TB at baseline.
Table 1.
Characteristics of adult tuberculosis patients with and without diabetes screened for drug-resistant tuberculosis in Lima, Peru, 2005–2008 (N = 1671)
| Characteristics | TB with DM, n (%) (n = 186) | TB without DM, n (%) (n = 1485) | Total, n (%) (N = 1671) |
|---|---|---|---|
| Demographics | |||
| Sex | |||
| Male | 114 (61.3) | 989 (66.6) | 1103 (66.0) |
| Female | 72 (38.7) | 496 (38.7) | 568 (34.0) |
| Age, yearsa | |||
| 15–24 | 0 | 506 (34.1) | 506 (30.3) |
| 25–44 | 33 (17.7) | 714 (30.1) | 747 (44.7) |
| 45–64 | 125 (67.2) | 218 (14.7) | 343 (20.5) |
| >64 | 28 (15.1) | 47 (3.2) | 75 (4.5) |
| Marital statusa | |||
| Married/partner | 122 (65.6) | 521 (35.1) | 643 (38.5) |
| Divorced/separated/widow | 37 (19.9) | 172 (11.6) | 209 (12.5) |
| Single | 27 (14.5) | 791 (53.3) | 818 (49.0) |
| Employment | |||
| Employed/otherb | 123 (66.1) | 885 (59.6) | 1008 (60.3) |
| Unemployed | 63 (33.9) | 596 (40.1) | 659 (39.4) |
| Educationa | |||
| None/incomplete primary | 48 (25.8) | 170 (11.5) | 218 (13.1) |
| Some secondary | 52 (28.0) | 482 (32.5) | 534 (32.0) |
| Graduated secondary or more | 85 (45.7) | 824 (55.5) | 909 (54.4) |
| TB characteristics | |||
| Baseline AFB smear result | |||
| Positive | 171 (91.9) | 1303 (87.7) | 1474 (88.2) |
| Negative | 14 (7.5) | 161 (10.8) | 175 (10.5) |
| Missing | 1 (0.5) | 21 (1.4) | 22 (1.3) |
| Baseline culture resulta | |||
| Positive | 138 (74.2) | 1059 (71.3) | 1197 (71.6) |
| Negative | 24 (12.9) | 308 (20.7) | 332 (19.9) |
| Missing | 24 (12.9) | 118 (8.0) | 142 (8.5) |
| Previous TB treatmenta | |||
| Yes | 43 (23.1) | 805 (54.2) | 848 (50.7) |
| No | 143 (76.9) | 680 (45.8) | 823 (49.3) |
| Baseline drug susceptibilitya | |||
| Pan-susceptible | 105 (56.5) | 537 (36.2) | 642 (38.4) |
| Drug-resistant | 17 (9.1) | 137 (9.2) | 154 (9.2) |
| MDR | 29 (15.6) | 365 (24.6) | 394 (23.6) |
| Missing | 35 (18.8) | 446 (30.0) | 481 (28.8) |
| Extrapulmonary | |||
| Yes | 3 (1.6) | 34 (2.3) | 37 (2.2) |
| No | 183 (98.4) | 1450 (97.6) | 1633 (97.7) |
| Duration of symptoms, weeks | |||
| Mean (SD) | 12.1 (11.6) | 15.3 (21.9) | 15.0 (21.0) |
| Median (IQR) | 8.7 (11.6) | 8.9 (15.9) | 8.9 (14.9) |
| TB symptomsc | |||
| Weight loss | 158 (85.0) | 1205 (81.1) | 1363 (81.6) |
| Dyspnea | 25 (13.4) | 273 (18.4) | 298 (17.8) |
| Blood in sputum | 6 (3.2) | 93 (6.3) | 99 (5.9) |
| Bilateral cavitationa | |||
| Yes | 6 (3.2) | 75 (5.1) | 81 (4.9) |
| No | 177 (95.2) | 1357 (91.4) | 1534 (91.8) |
| Missing | 3 (1.6) | 53 (3.6) | 56 (3.4) |
| Risk factorsc | |||
| HIV-positivea | 1 (0.5) | 197 (13.3) | 198 (11.9) |
| Any household TB contacta | 10 (5.4) | 347 (23.4) | 357 (21.4) |
| Household MDR contacta | 7 (3.8) | 199 (13.4) | 206 (12.3) |
| Current tobacco | 9 (4.8) | 57 (3.8) | 66 (4.0) |
| Current alcohol | 10 (5.4) | 125 (8.4) | 135 (8.1) |
| Low BMI (<18.5 kg/m2)a | 9 (4.8) | 326 (22.2) | 335 (20.3) |
AFB, acid-fast bacillus; BMI, body mass index; DM, diabetes mellitus; IQR, interquartile range; MDR, multidrug-resistant; SD, standard deviation; TB, tuberculosis. Some cells may not sum to 100% due to missing data. For any variable with more than 1% missing, percent missing is indicated.
Mantel-Haenszel p-value <0.05.
‘Other’ includes student, housewife, and retired.
Not mutually exclusive categories, should not add up to 100%, column percentages are listed.
Figure 2.
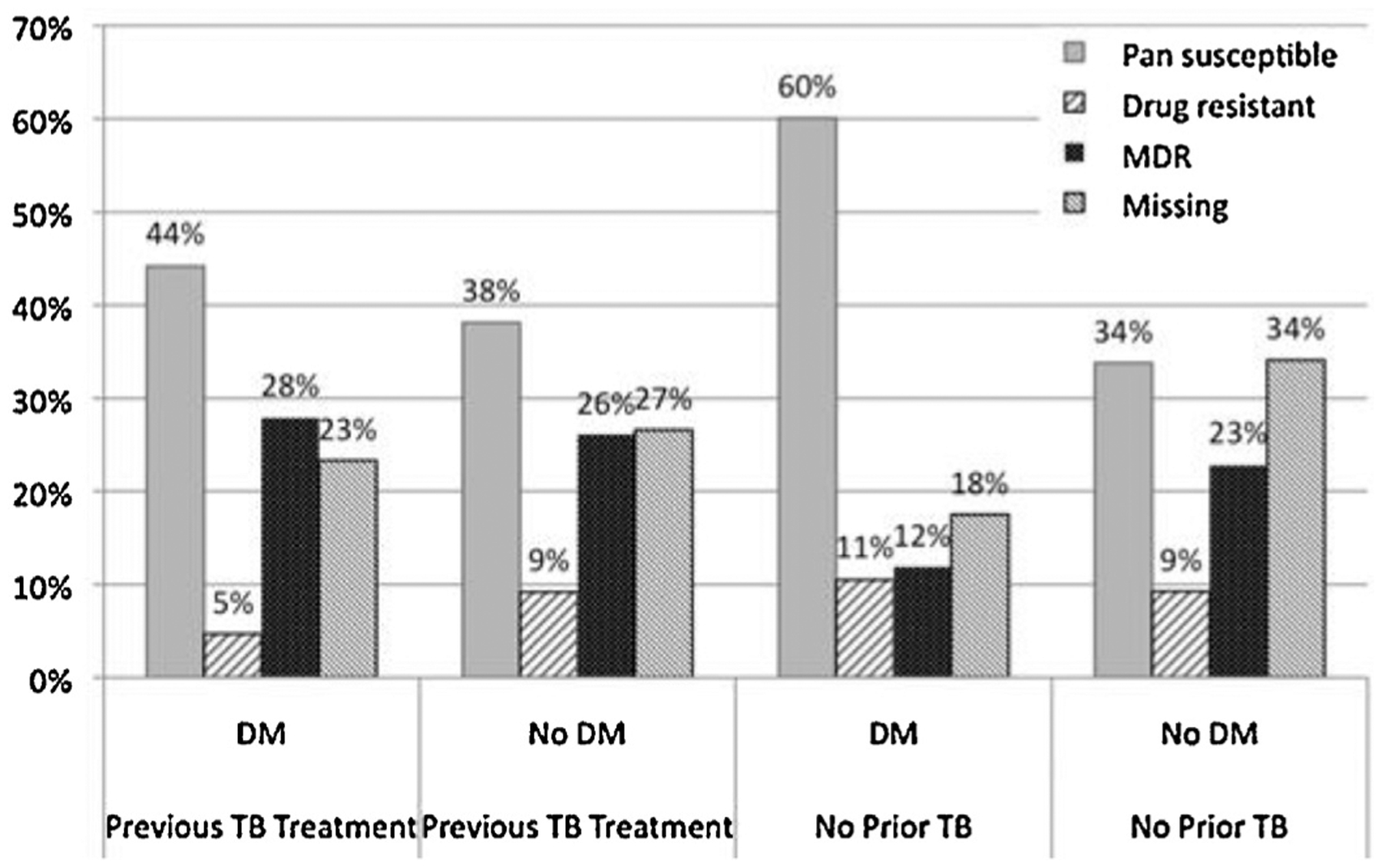
Anti-tuberculosis (TB) drug susceptibility results for 1671 Peruvian TB patients with and without diabetes (DM) stratified by previous TB treatment history (drug-resistant refers to any resistance to first-line drugs, but is not multidrug-resistant).
3.2. Characteristics of TB–DM patients by drug resistance pattern
Of the 186 adult TB–DM patients in the study, 149 (80.1%) had complete baseline TB drug susceptibility data. Of these, 104(69.8%) had drug-susceptible TB and 45 (30.2%) had drug-resistant TB, of whom 29 had MDR-TB (Table 2). There were no significant differences in the demographic or TB characteristics (data not shown) or diabetes characteristics (Table 2) comparing TB–DM patients with and without drug-resistant TB. However, a significantly greater proportion of patients with any drug-resistant TB had a household contact with TB (13.3% vs. 3.9%), including MDR-TB (13.3% vs. 1.0%), and were more likely to have been previously treated for TB (31.1% vs. 17.3%) (p < 0.05 for all comparisons).
Table 2.
Diabetes characteristics prior to tuberculosis treatment and tuberculosis risk factors by drug resistance pattern among tuberculosis patients with diabetes in Lima, Peru, 2005–2008 (N = 149)
| Characteristics | Susceptible TB, n (%) (n = 104) | Any DR-TB,a n (%) (n =45) | Total, n (%) (N = 149) |
|---|---|---|---|
| DM-related factors prior to TB treatment | |||
| Type of DM | |||
| Type 2 | 86 (82.7) | 38 (84.4) | 124 (83.2) |
| Type 1 | 2 (1.9) | 1 (2.2) | 2 (2.0) |
| Unclassified | 16 (15.4) | 6 (13.3) | 22 (14.8) |
| Duration of DM, years | |||
| Mean (SD) | 8.1 (8.9) | 6.8 (8.2) | 7.7 (8.7) |
| Median (IQR) | 5.0 | 4.0 | 5.0 |
| FBGb | |||
| Mean (SD) | 205.9 (100.7) | 184.9 (55.6) | 198.5 (87.4) |
| Median (IQR) | 197.5 (124.5) | 170.2 (76.5) | 178.0 (105.0) |
| Tertiles of mean FBG | |||
| <153 | 10 (9.6) | 5 (11.1) | 15 (10.1) |
| 153–252 | 16 (15.4) | 10 (22.2) | 26 (17.5) |
| >252 | 7 (6.7) | 3 (6.7) | 10 (6.7) |
| No measures | 71 (68.3) | 27 (60.0) | 98 (65.8) |
| Prior DM medicationc | |||
| Insulin or oral agent | 55 (52.9) | 24 (53.3) | 79 (53.0) |
| None/unknown | 49 (47.1) | 21 (46.7) | 70 (47.0) |
| Any DM complicationsd | |||
| Yes | 28 (26.9) | 10 (22.2) | 38 (25.5) |
| No | 76 (73.1) | 35 (77.8) | 111 (74.5) |
| Ever DM hospitalization | |||
| Yes | 7 (6.7) | 5 (11.1) | 12 (8.1) |
| No | 97 (93.3) | 40 (88.9) | 137 (91.9) |
| Risk factorse | |||
| HIV-positive | 0 | 0 | 0 |
| Any household TB contactf | 4 (3.9) | 6 (13.3) | 10 (6.7) |
| Household MDR contactf | 1 (1.0) | 6 (13.3) | 7 (4.7) |
| Current tobacco | 5 (4.8) | 2 (4.4) | 7 (4.7) |
| Current alcohol | 4 (3.9) | 2 (4.4) | 6 (4.0) |
| Low BMI (<18.5 kg/m2) | 6 (5.8) | 3 (6.8) | 9 (6.1) |
| Previous TB treatmentf | 18 (17.3) | 14 (31.1) | 32 (21.5) |
| >1 MDR risk factorg | 11 (10.6) | 9 (20.0) | 20 (13.4) |
BMI, body mass index; DM, diabetes mellitus; DR, drug-resistant; FBG, fasting blood glucose; IQR, interquartile range; MDR, multidrug-resistant; SD, standard deviation; TB, tuberculosis.
Includes 16 DR and 29 MDR patients.
Fasting blood glucose (FBG): mean ofFBG measures taken before TB treatment; 18/45 resistant patients and 33/104 susceptible had measurements available.
Prior DM medication: insulin, oral hypoglycemic agents, or both taken prior to study enrollment.
Complications: include poor glycemic control, eye disease, renal failure, cardiovascular disease, peripheral neuropathy, or hospitalization due to DM before study enrollment.
Not mutually exclusive categories, should not add up to 100%, column percentages are listed.
Mantel-Haenszel p-value <0.05.
Greater than one MDR-TB risk factor identified by the Peruvian National TB Control Program.
3.3. Clinical and diabetes care characteristics by TB treatment outcomes among TB–DM patients
Complete TB treatment outcome data were available for 136 (73.1%) TB–DM patients (Figure 1). Of these, 107 (78.7%) had a favorable outcome (cured, completed treatment, or converted cultures) and 29 (21.3%) had a poor outcome (failed, default, or death) (Table 3). Diabetes care during TB treatment differed across treatment outcome groups: among patients with favorable TB treatment outcomes, 67.3% received diabetes management with diet or medicine, compared to 44.8% of patients with a poor TB treatment outcome (p < 0.05). More TB–DM patients with a favorable TB treatment outcome received an oral hypoglycemic (44.9%) or both an oral hypoglycemic and insulin (21.5%), but fewer received insulin alone (9.4%) compared to those with poor TB treatment outcomes (27.6%, 10.3%, and 20.7%, respectively); however, overall more TB–DM patients with a favorable treatment outcome received any diabetes medications during TB treatment (75.5%) compared to those with a poor TB treatment outcome (58.6%) (p = 0.07). While 89.9% of TB–DM with drug-susceptible disease had favorable outcomes, only 48.6% of those with drug-resistant TB had favorable outcomes (p < 0.05).
Table 3.
Clinical and diabetes care by tuberculosis treatment outcome among tuberculosis patients with diabetes in Lima, Peru, 2005–2008 (N = 136)
| Characteristic | Favorable: Cure, complete,a n (%) (n = 107; 78.7%) | Poor: Failed, default, died, n (%) (n = 29; 21.3%) | Total, n (%) (N = 136; 100%) |
|---|---|---|---|
| Before TB treatment | |||
| Type of DM | |||
| Type 2 | 90 (84.1) | 25 (86.2) | 115 (84.6) |
| Type 1 | 3 (2.8) | 0 | 3 (2.2) |
| Unclassified | 14 (13.1) | 4 (13.8) | 18 (13.2) |
| DM duration, years | |||
| Mean (SD) | 8.2 (8.7) | 8.9 (10.1) | 8.1 (8.7) |
| Median (IQR) | 5.5 (8.0) | 5.0 (8.0) | 5.5 (7.0) |
| Mean FBG | |||
| Mean (SD) | 188.9 (75.8) | 243.1 (127.5) | 198.5 (87.4) |
| Median (IQR) | 178.0 (94.7) | 230.0 (149.7) | 178.0 (105.0) |
| Tertiles of mean FBGb | |||
| <153 | 11 (10.3) | 3 (10.3) | 15 (10.7) |
| 153–252 | 21 (19.6) | 2 (6.9) | 26 (17.5) |
| >252 | 5 (4.7) | 5 (17.2) | 10 (6.7) |
| No measures | 70 (65.4) | 19 (65.5) | 98 (65.8) |
| Prior DM medication | |||
| Insulin or oral agent | 59 (55.1) | 15 (51.7) | 74 (54.4) |
| None/unknown | 48 (44.9) | 14 (48.3) | 62 (45.6) |
| Any DM complications | |||
| Yes | 17 (15.9) | 3 (10.3) | 20 (14.7) |
| No | 90 (84.1) | 26 (89.7) | 116 (85.3) |
| Ever DM hospitalization | |||
| Yes | 11 (10.3) | 0 | 11 (8.1) |
| No | 96 (89.7) | 29 (100.0) | 125 (91.9) |
| During TB treatment | |||
| Mean FBG | |||
| Mean (SD) | 175.9 (52.0) | 157.4 (76.2) | 170.3 (58.4) |
| Median (IQR) | 169.7 (73.0) | 161.0 (120.6) | 162.3 (89.0) |
| Tertiles of mean FBGb | |||
| <136 | 11 (10.3) | 5 (17.2) | 20 (13.4) |
| 136–214 | 25 (23.4) | 3 (10.3) | 28 (18.8) |
| >214 | 10 (9.4) | 3 (10.3) | 15 (10.1) |
| No measures | 61 (57.0) | 18 (62.1) | 86 (57.7) |
| Glucose control | |||
| Below median | 20 (18.7) | 5 (17.2) | 25 (18.4) |
| Above median | 26 (24.3) | 6 (20.7) | 32 (23.5) |
| No documentation | 61 (57.0) | 18 (62.1) | 79 (58.1) |
| Record mentioned control | |||
| Controlled | 31 (29.0) | 9 (31.0) | 40 (29.4) |
| Not controlled/no mention | 76 (71.0) | 20 (69.0) | 96 (70.6) |
| Managementc | |||
| Diet or medicine | 72 (67.3) | 13 (44.8) | 85 (62.5) |
| None/missing | 35 (32.7) | 16 (55.2) | 51 (37.5) |
| DM medications | |||
| Any | 81 (75.7) | 17 (58.6) | 98 (72.1) |
| None | 26 (24.3) | 12 (41.4) | 38 (27.9) |
| DM medicationsc | |||
| Insulin | 10 (9.4) | 6 (20.7) | 16 (11.8) |
| Oral hypoglycemic | 48 (44.9) | 8 (27.6) | 56 (41.2) |
| Both | 23 (21.5) | 3 (10.3) | 26 (19.1) |
| None | 26 (24.3) | 12 (41.4) | 38 (27.9) |
| Endocrinologist visit | |||
| Yes | 36 (33.6) | 6 (20.7) | 42 (30.9) |
| No/missing | 71 (66.4) | 23 (79.3) | 94 (69.1) |
| Any complicationsd | |||
| Yes | 22 (20.6) | 3 (10.3) | 25 (18.4) |
| No | 85 (79.4) | 26 (89.7) | 111 (81.6) |
| DM care during TB treatmente | |||
| Frequent care | 35 (32.7) | 5 (17.2) | 40 (29.4) |
| Some care | 48 (44.9) | 12 (41.4) | 60 (44.1) |
| None | 24 (22.4) | 12 (41.4) | 36 (26.5) |
| Other measures | |||
| Drug susceptibilityc | |||
| DR | 18 (16.8) | 19 (65.5) | 37 (27.2) |
| Susceptible | 89 (83.2) | 10 (34.5) | 99 (72.8) |
| BMI, kg/m2 | |||
| <18.5 | 6 (5.6) | 2 (6.9) | 8 (5.9) |
| 18.5–24.99 | 58 (54.2) | 15 (51.7) | 73 (53.7) |
| 25–29.99 | 34 (31.8) | 9 (31.0) | 43 (31.6) |
| ≥30 | 9 (8.4) | 2 (6.9) | 11 (8.1) |
| Age | |||
| Mean (SD) | 54.7 (9.8) | 50.8 (10.8) | 53.9 (10.1) |
| Median (IQR) | 55.0 (13.0) | 49.0 (14.0) | 54.0 (14.0) |
BMI, body mass index; DM, diabetes mellitus; DR, drug-resistant; FBG, fasting blood glucose; IQR, interquartile range; SD, standard deviation; TB, tuberculosis.
Or if no outcome, culture conversion result.
Mean of patient fasting blood glucose measures.
Mantel-Haenszel general association p-value <0.05.
Any complications from glycemic control, eye disease, renal insufficiency, cardiovascular disease, peripheral neuropathy, or hospitalization due to DM.
DM care during TB treatment: combines during-treatment measures of endocrinologist visit, mentioned DM control, recorded DM medications, or ≥3 measures of blood glucose. ‘Frequent care’ indicates three or four of the during-treatment measures, ‘Some care’ indicates one or two, and ‘None’ indicates 0.
Data for the time until TB culture conversion were available for 149 (80.0%) of the 186 TB–DM patients. The mean time to culture conversion was 152 days and the median was 171 days (range 1300 days). Among TB–DM patients, those with documented diabetes control during TB treatment converted TB cultures from positive to negative more quickly (p < 0.01) than patients without any record of diabetes control (Figure 3, A–C). After stratification by TB treatment history, among TB–DM patients with no history of previous TB treatment, those with documented diabetes control converted sputum cultures more quickly (p = 0.01) (Figure 3, B and C). After controlling for TB treatment history and drug susceptibility, the hazard rate of culture conversion among those with documented diabetes control was 2.2 (95% confidence interval 1.1, 4.1) times the rate of patients with no documented control (Table 4). Similarly, Figure 4A shows that TB–DM patients who received frequent diabetes care during TB treatment converted cultures to negative more quickly than TB–DM patients with no diabetes care (p < 0.05), and this significant difference remained even after stratifying by treatment history (Figure 4, B and C). After controlling for TB treatment history and drug susceptibility, the hazard rate of culture conversion among those with frequent diabetes care during TB treatment was 1.8 (95% confidence interval 0.9, 3.7) times the rate of patients with some care during TB treatment (Table 4).
Figure 3.
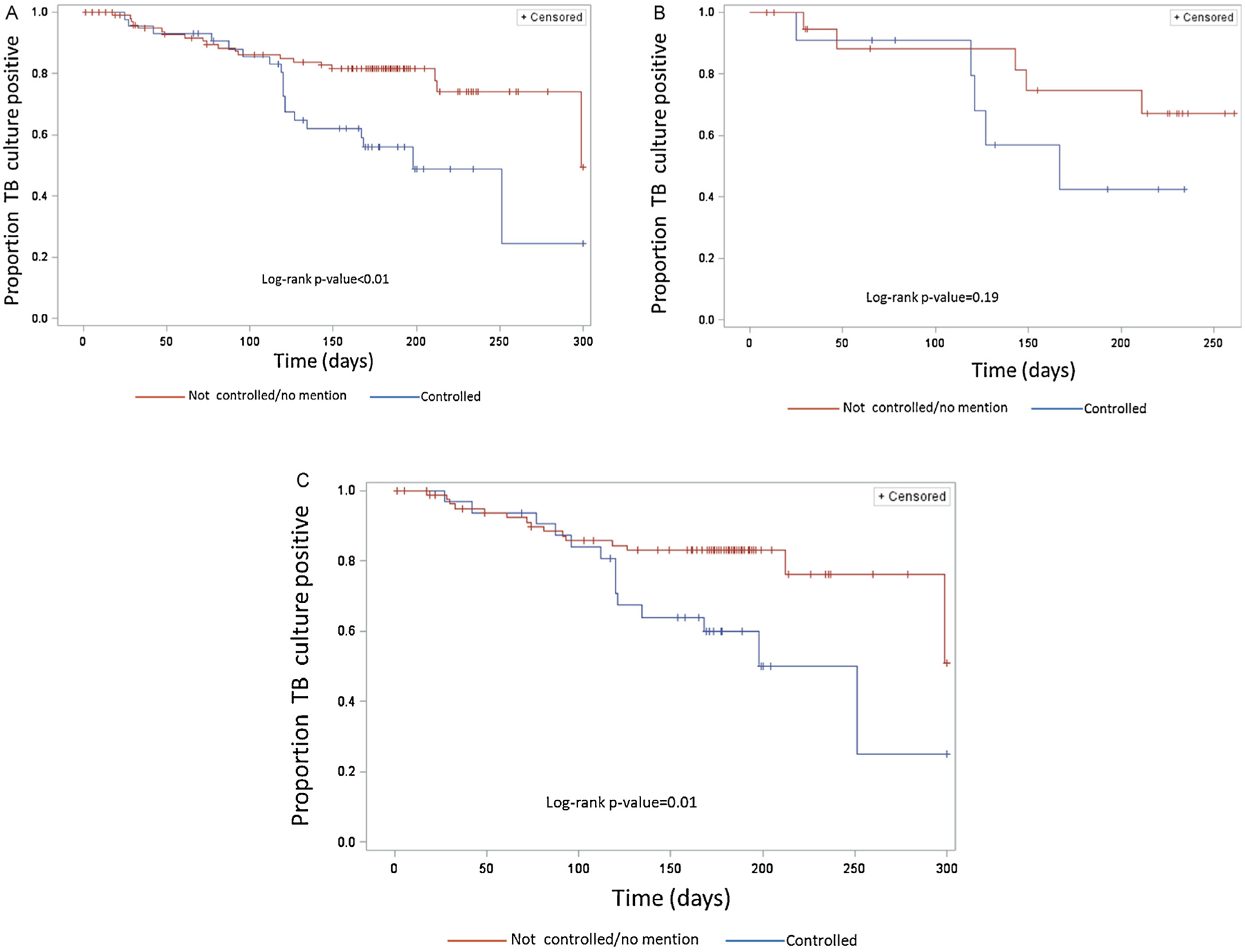
(A) Kaplan–Meier curves for time until tuberculosis (TB) culture conversion from positive to negative by diabetes control status among TB patients with diabetes mellitus (n = 149); control status determined by medical record indication. (B) Kaplan–Meier curves for time until TB culture conversion from positive to negative by diabetes control status among TB patients with diabetes mellitus who have been previously treated for TB (n = 32). (C) Kaplan–Meier curves for time until TB culture conversion from positive to negative by diabetes control status among patients with diabetes mellitus who have not been previously treated for TB (n = 117).
Table 4.
Hazard ratio of time to culture conversion among tuberculosis patients with diabetes mellitus, Peru 2005–2008 (N = 149)
| Hazard ratio (95% CI) | Adjusted hazard ratioa (95% CI) | |
|---|---|---|
| Record mentioned control | ||
| Controlled | 2.5 (1.3, 4.6) | 2.2 (1.1, 4.1) |
| Not controlled/no mention | 1 | 1 |
| DM care during TB treatmentb | ||
| Frequent care | 2.1 (1.2, 4.2) | 1.8 (0.9, 3.7) |
| Some care | 1 | 1 |
| None | 0.7 (0.2, 2.0) | 0.8 (0.3, 2.3) |
CI, confidence interval; DM, diabetes mellitus; TB, tuberculosis.
Adjusted for previous TB treatment and any drug resistance.
DM care duringTB treatment: combines during-treatment measures ofendocrinologistvisit, mentioned DM control, recorded DM medications, or ≥3 measures ofblood glucose. ‘Frequent care’ indicates three or four of the during-treatment measures, ‘Some care’ indicates one or two, and ‘None’ indicates 0.
Figure 4.
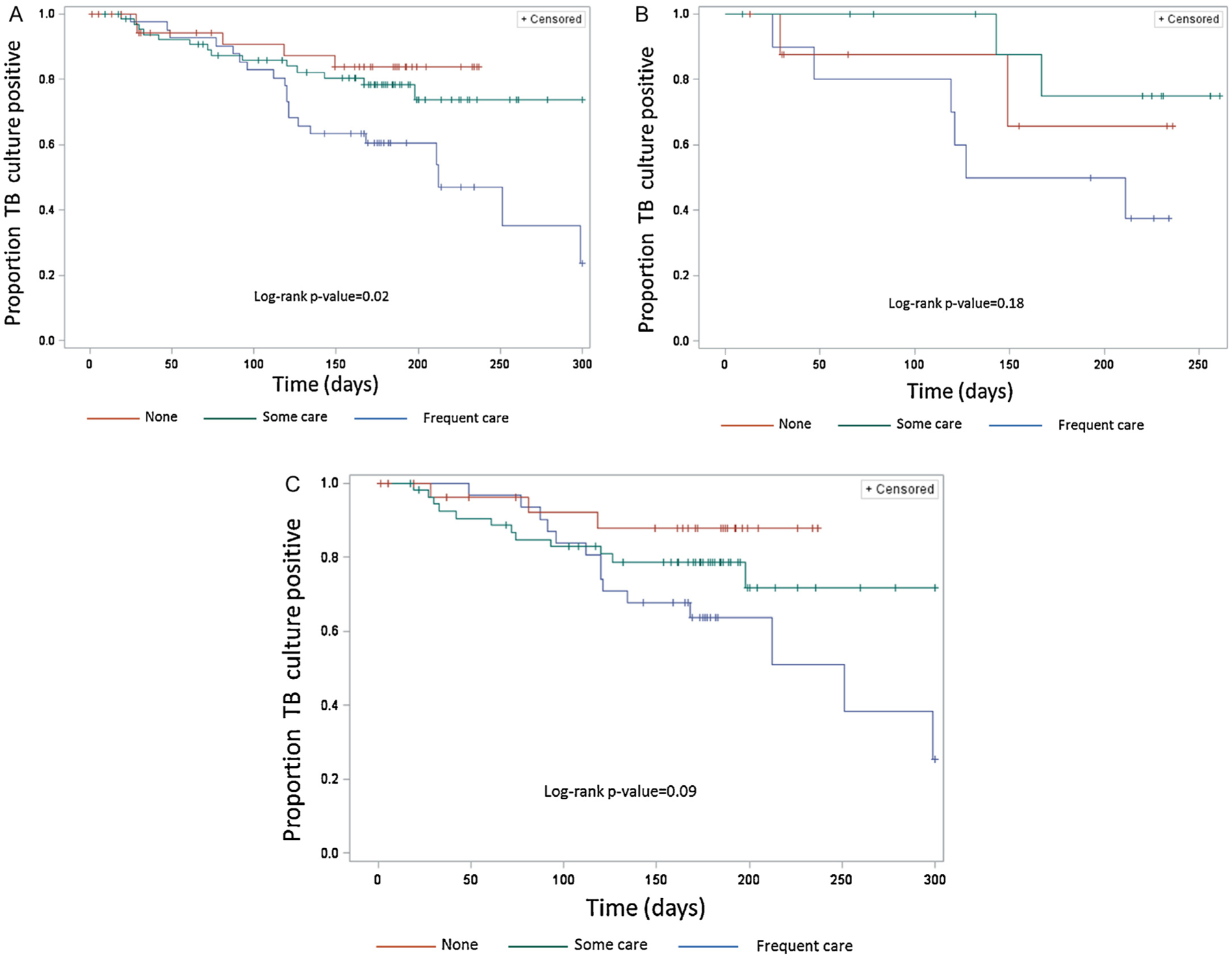
(A) Kaplan–Meier curves for time until tuberculosis (TB) culture conversion from positive to negative by diabetes care during TB treatment among TB patients with diabetes mellitus (n = 149). Frequent diabetes care during TB treatment was defined as at least three of the four following measures: at least one endocrinologist visit, medical record mentioned diabetes is controlled, recorded oral hypoglycemic agent or insulin use, or ≥3 documented blood glucose exams. Some care during TB treatment was defined as one or two of the aforementioned measures and none was defined as 0 of these measures. (B) Kaplan–Meier curves for time until TB culture conversion from positive to negative by diabetes care during TB treatment among TB patients with diabetes mellitus who have been previously treated for TB (n = 32). (C) Kaplan–Meier curves for time until TB culture conversion from positive to negative by diabetes care during TB treatment among patients with diabetes mellitus who have not been previously treated for TB (n = 117).
4. Discussion
In this study of a Peruvian cohort of TB patients at increased risk of MDR-TB, we evaluated the role of diabetes in TB clinical presentation, drug resistance patterns, and treatment outcomes. Among TB patients enrolled in this cohort, 11.1% had diabetes. Furthermore, 11.9% of never previously treated and 27.9% of previously treated TB–DM patients had MDR-TB. Among TB–DM patients we found that drug-resistant TB was associated with having a household TB contact and previous TB treatment, and a favorable TB treatment outcome was associated with diabetes management, drug-susceptible TB, and receiving diabetes medications during TB treatment.
Consistent with previous studies, we found TB–DM patients were more likely to be married or partnered, older, and less educated than TB patients without diabetes.27–31 It is important for health providers to better understand who may be at increased likelihood of having a comorbid illness to better identify high-risk patients and ensure they receive the necessary care for both diseases. Patient-centered strategies to treatment, such as those promoted in the International Standards for TB Care, should be applied in the management of TB–DM patients.32
TB–DM patients were more likely to be culture-positive at the time of diagnosis but less likely to have had previous TB treatment, have MDR-TB, or have bilateral lung cavitation than TB patients without diabetes. Although diabetes is known to modify clinical features and radiological manifestations of pulmonary TB patients, previous studies have reported inconsistent results.4,28,33 Previous studies have reported a lower proportion of previous TB treatment,34 MDR-TB,33 and cavitation34,35 among TB–DM patients compared to TB patients without diabetes, while other studies have found the opposite.36,37 Because our cohort consisted of TB patients with at least one risk factor for MDR-TB, our finding that 11.9% of never previously treated TB–DM patients had MDR-TB and 22.8% of never previously treated non-diabetes patients had MDR-TB may not suggest a lack of association between MDR-TB and diabetes, but rather may reflect a high prevalence of even stronger risk factors than diabetes in our cohort.
Overall, almost 79% of TB–DM cases had a favorable outcome, a result consistent with recent studies of TB–DM patient treatment outcomes.34,38 We found favorable TB outcomes were associated with receiving diabetes care, specifically the use of a provider-indicated diet or medicine to manage diabetes, including the use of an oral hypoglycemic or both an oral hypoglycemic and insulin. Additionally, we showed that diabetes control and frequent diabetes care during TB treatment resulted in a significantly shorter time to TB culture conversion. These results suggest that regardless of the complex clinical care required for both diseases, TB treatment effectiveness can be improved among TB patients with well-managed diabetes.
Our findings suggest optimal diabetes control should be part of TB–DM patient management in Peru. Furthermore, TB–DM patients in Peru are at risk of MDR-TB, and screening for drug resistance among TB–DM is the important first step to effectively diagnose MDR-TB in this group. With increasing access to rapid diagnostics, universal screening for drug resistance is feasible for the national TB program.
Given the high prevalence of diabetes in this cohort of TB patients, our findings also support the importance of conversely screening TB patients for diabetes. The detection of diabetes in TB patients and linking these persons to care (management with diet and/or medicine) may improve TB treatment outcomes. Additionally, active TB screening among persons with diabetes, particularly those with uncontrolled diabetes, may be worthwhile for TB control in Peru, especially since diabetes patients visit their health care providers more frequently compared to other groups at risk of TB. Further studies are needed to assess the feasibility and cost-effectiveness of such interventions.
There were several limitations in this study. First, patients were selected for this study because of specific risk factors for drug-resistant TB. Therefore, the observations reported in Table 1 cannot be applied to the general TB population in Peru. Without a comparison group representing the experience of general TB patients without diabetes, our results only highlight differences in patients with diabetes compared to other TB patients already determined to have an increased MDR-TB risk. Previous TB treatment was the strongest, most common risk factor for drug resistance. The prevalence of TB drug resistance among patients with diabetes was greater than its prevalence among TB patients in general, but the magnitude of the increased risk was less than the magnitude of increased risk associated with previous TB treatment. Second, misclassification of study patients by diabetes status and diabetes clinical features is possible due to measurement error. However, we do not have reason to believe any misclassification would be disproportionately distributed by factors related to drug susceptibility or treatment outcome, consequently if bias exists it is likely toward the null effect. Third, although this study represents one of the largest reported TB–DM cohorts, missing drug susceptibility and treatment outcome data precluded multivariable analyses due to sample size.
Currently few published studies have examined diabetes clinical and care characteristics associated with drug-resistant TB, TB treatment outcome, or time to culture conversion. A strength of this study is that it examined a large cohort of TB–DM patients with detailed information on diabetes clinical history both before and during TB treatment, including glucose levels, duration of diabetes disease, type of diabetes management (including type of medication used and access to endocrinologists), and reported diabetes complications or hospitalizations. Our findings highlight the importance of linking TB and diabetes diagnostic and treatment services in Peru.
Acknowledgements
This work was supported by the US Centers for Disease Control and Prevention (US CDC), NIAID K23 AI054591-01A2, the Bill & Melinda Gates Foundation, Heiser Foundation, and the Infectious Diseases Society of America. Some of the authors of this publication are employed by the US CDC.
Footnotes
Conflict of interest:
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: Disclaimer:
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2011. Geneva: WHO; 2011. [Google Scholar]
- 2.World Health Organization. Diabetes. Fact sheet No. 312. Geneva: WHO; 2011. [Google Scholar]
- 3.Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Di; 2012;54:818–25. [DOI] [PubMed] [Google Scholar]
- 4.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009;9:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, Raviglione MC. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet 2010;375(9728):1814–29. [DOI] [PubMed] [Google Scholar]
- 7.International Diabetes Federation. International Diabetes Federation diabetes atlas, 4th ed., Brussels: IDF; 2009. [Google Scholar]
- 8.Magee MJ, Blumberg HM, Narayan KM. Commentary: Co-occurrence of tuberculosis and diabetes: new paradigm of epidemiological transition. Int J Epidemiol 2011;40:428–31. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: WHO; 2010. [Google Scholar]
- 10.Chang JT, Dou HY, Yen CL, Wu YH, Huang RM, Lin HJ, et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug-resistance. J Formos Med Assoc 2011;110:372–81. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Gothi D, Joshi J. Multidrug resistant tuberculosis: role of previous treatment with second line therapy on treatment outcome. Lung India 2007;24:54–7. [Google Scholar]
- 12.Aragon J, Litonjua A, Tupasi T, Quelapio I. Prevalence of type 2 diabetes among multi-drug resistant tuberculosis (MDR-TB) patients seen in Makati Medical Center under the directly observed therapy plus (DOTS PLUS) program. Philippine Journal of Internal Medicine 2003;41:7–10. [Google Scholar]
- 13.Tanrikulu AC, Hosoglu S, Ozekinci T, Abakay A, Gurkan F. Risk factors for drug resistant tuberculosis in southeast Turkey. Trop Doct 2008;38:91–3. [DOI] [PubMed] [Google Scholar]
- 14.Garcia F, Solis J, CalderonJ, Luque E, Zacarias E. Prevalence of diabetes mellitus and related risk factors in an urban population. Revista de la Sociedad Peruana de Medicina Interna 2007;20:90–4. [Google Scholar]
- 15.Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997. Chest 2001;120:1514–9. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Hoch SP, Whitney E, McCormick JB, Crespo G, Smith B, Rahbar MH, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis 2008;40:888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SW, Shin JW, Kim JY, Park IW, Choi BW, Choi JC, et al. The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 2012;7:1305–10. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global tuberculosis control: WHO report 2010. Geneva: WHO; 2010. [Google Scholar]
- 19.Barcelo A Diabetes in the Americas. Epidemiol Bull 2001;22:1–3. [PubMed] [Google Scholar]
- 20.Shin SS, Yagui M, Ascencios L, Yale G, Suarez C, Quispe N, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis 2008;14:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministerio de Salud. Norma tecnica de salud para el control de la tuberculosis. Lima, Peru: Ministerio de Salud; 2006. [Google Scholar]
- 22.Shin SS, Yagui M, Ascencios L, Yale G, Suarez C, Quispe N, et al. Targeted drug-resistance testing strategy for multidrug-resistant tuberculosis detection, Lima, Peru, 2005–2008. Emerg Infect Dis 2011;17:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin SS, Asencios L, Yagui M, Yale G, Suarez C, Bayona J, et al. Impact of rapid drug susceptibility testing for tuberculosis: program experience in Lima, Peru. Int J Tuberc Lung Dis 2012;16:1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: a report of a WHIO/IDF consultation. Geneva: WHO; 2006. [Google Scholar]
- 25.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: WHO; 2006. [PubMed] [Google Scholar]
- 26.World Health Organization. Treatment of tuberculosis: guidelines for national programmes, 4th ed., Geneva: WHO; 2009. [Google Scholar]
- 27.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009;80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 2010;15:1289–99. [DOI] [PubMed] [Google Scholar]
- 29.Nissapatorn V, Kuppusamy I, Jamaiah I, Fong MY, Rohela M, Anuar AK. Tuberculosis in diabetic patients: a clinical perspective. Southeast Asian J Trop Med Public Health 2005;36(Suppl 4):213–20. [PubMed] [Google Scholar]
- 30.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004;27:1584–90. [DOI] [PubMed] [Google Scholar]
- 31.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, McCormick JB. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect 2007;135:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuberculosis Coalition for Technical Assistance. International Standards for Tuberculosis Care (ISTC). The Hague: Tuberculosis Coalition for Technical Assistance; 2006. [Google Scholar]
- 33.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis 2006;10:74–9. [PubMed] [Google Scholar]
- 34.Wang CS, Yang CJ, Chen HC, Chuang SH, Chong IW, Hwang JJ, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect 2009;137:203–10. [DOI] [PubMed] [Google Scholar]
- 35.Bacakoglu F, Basoglu OK, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration 2001;68:595–600. [DOI] [PubMed] [Google Scholar]
- 36.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 2007;45: 428–35. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Salazar-Lezama MA, Vargas MH. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis 2001;5: 455–61. [PubMed] [Google Scholar]
- 38.Chiang CY, Lee JJ, Yu MC, Enarson DA, Lin TP, Luh KT. Tuberculosis outcomes in Taipei: factors associated with treatment interruption for 2 months and death. Int J Tuberc Lung Dis 2009;13:105–11. [PubMed] [Google Scholar]


