SUMMARY
BACKGROUND:
Performance characteristics of novel rapid drug susceptibility tests (DST) for Mycobacterium tuberculosis may change when moving from research to implementation in actual public health practice. We describe the performance characteristics of a direct, rapid DST when implemented in Lima, Peru.
METHODS:
A district laboratory validated conventional proportions and nitrate reductase methods. We collected data on samples submitted for DST from January 2005 to June 2007 and calculated frequency of testing and results, and median time to test results.
RESULTS:
A total of 4102 DSTs were performed by conventional DST and 895 by nitrate reductase. Results were obtained from 72.8% of samples by conventional DST and from 70.2% of those processed by Griess; respectively 26.4% and 31.5% were multidrug-resistant tuberculosis. The median time from sample collection to test result was 31 days for Griess vs. 99 days for conventional DST.
CONCLUSIONS:
Preliminary experience with the Griess method demonstrates favorable performance under program conditions.
Keywords: tuberculosis, multidrug-resistant, laboratories, DST, resource-poor settings
RÉSUMÉ
CONTEXTE:
Les caractéristiques de performance des tests novateurs rapides de détermination de la sensibilité aux médicaments antituberculeux (DST) peuvent varier lorsqu’on passe du stade de recherche à leur mise en œuvre dans une pratique de santé publique sur le terrain. Nous décrivons les caractéristiques de performance d’un DST direct et rapide mis en œuvre à Lima, Pérou.
MÉTHODES:
La méthode conventionnelle des proportions et le test de réduction des nitrates (Griess) ont été validés dans un laboratoire de district. Nous avons colligé les données sur les échantillons soumis pour DST entre janvier 2005 et juin 2007 et calculé la fréquence des tests et des résultats et la durée médiane avant l’obtention des résultats.
RÉSULTATS:
On a réalisé 4102 DST par la méthode conventionnelle et 895 par le test de réduction des nitrates. Les résultats ont été obtenus sur 72,8% des échantillons par DST conventionnelle et sur 70,2% de ceux-ci traités par la méthode de Griess ; une tuberculose multirésistante a été décelée respectivement dans 26,4% et 31,5% des cas. La durée médiane séparant la collecte des échantillons et le résultat du test a été de 31 jours pour la méthode de Griess contre 99 jours pour la DST conventionnelle.
CONCLUSIONS:
Une expérience préliminaire avec la méthode de Griess démontre des performances valables dans des conditions de programme.
RESUMEN
MARCADEREFERENCIA:
Las características de la perfomance de una nueva prueba rápida de susceptibilidad a drogas (DST) para M. tuberculosis pueden cambiar cuando se pasa de investigación a la implementación en la práctica de salud pública actual. Describimos las características de la perfomance de una DST rápida, directa cuando se implementó en Lima, Perú.
MÉTODOS:
Un laboratorio distrital validó métodos de proporciones convencional y nitrato reductasa. Recolectamos información sobre muestras enviadas para DST de enero de 2005 a junio de 2007 y calculamos la frecuencia de la prueba y resultados, y el tiempo medio para los resultados.
RESULTADOS:
En todo, 4102 DST fueron realizadas por DST convencional, 895 por nitrato reductasa. Los resultados fueron obtenidos de 72,8% de muestras por DST convencional y de 70,2% de aquellas procesadas por Griess ; respectivamente 26,4% y 31,5% fueron tuberculosis multirresistante. El tiempo medio de recolección de muestras para resultados de la prueba fue 31 días para Griess versus 99 días para DST convencional.
CONCLUSIONES:
Experiencia preliminar con el método Griess demuestra perfomance favorable bajo condiciones de programa.
MULTIDRUG-RESISTANT tuberculosis (MDR-TB, defined as resistance to both isoniazid [INH] and rifampicin [RMP]) has emerged as a major threat to tuberculosis (TB) control. The urgent need for timely diagnosis of drug-resistant strains has stimulated efforts to develop rapid drug susceptibility test (DST) methods suitable for implementation in low- and middle-income settings. Ideal performance characteristics of such tests include high sensitivity and specificity, speed, low cost, technical ease and low biosafety risk. While many promising novel methods have emerged, the performance characteristics of any DST method may change when moving from the research and development stage to implementation on a population level in actual public health practice.1 Factors on many levels influence this process. At the center are biosafe laboratory capacity, adequately trained and motivated personnel, equipment and supplies, information systems, quality control systems, and TB epidemiology. These are in turn shaped by government policy, economics, public and private health care systems, post-secondary and medical education, and financial resources. In this context, test uptake, utilization and financing will control the potential impact on health. Previous studies of new diagnostic tests have not taken these factors into account.
In Peru, the National Tuberculosis Control Program (NTP) and the National Reference Laboratory (NRL) at the Instituto Nacional de Salud developed a strategy to expand laboratory infrastructure for MDR-TB control that was published formally as government policy in 2006.2 This strategy included: 1) systematic algorithms to identify and refer patients at increased risk of MDR-TB; 2) increased use of culture and DST for patients with risk of MDR-TB; 3) improvements in central and district laboratory infrastructure and biosafety; 4) decentralization of DST to first-line drugs using conventional indirect methods to district reference laboratories; 5) rapid screening for INH and RMP resistance among patients at increased risk.
Decentralization of first-line DST to district laboratories has eliminated the bottle-neck of first-line DST at the central level and enabled the NRL to serve the country as a real reference laboratory: performing species identification and second-line DST on referred isolates, providing supervision, training, and quality assurance for district laboratories, and performing broth culture and DST for smear-negative and paucibacillary patients with human immunodeficiency virus (HIV) infection, children and health care workers (HCWs).
As part of this strategy, the NRL worked with the Massachusetts State Laboratory Institute (MSLI) to validate a novel rapid method—the direct Griess method—to identify resistance to INH and RMP at modest cost.3 This colorimetric method uses a nitrate reductase reaction to indicate growth of Mycobacterium tuberculosis on a modified Löwenstein-Jensen (LJ) medium 1–3 weeks before colonies become visible. When compared to other novel rapid DST methods, the nitrate reductase method has performed favorably in terms of sensitivity, specificity, simplicity and cost for rapid DST in lower-income settings.4–7 The validation of this method in the Peruvian NRL yielded a sensitivity and specificity of respectively 99.1% and 100% for INH and 93.5% and 100% for RMP. The average time from sputum processing to DST result was 28 days. These results are similar to other validation reports of the Griess method,4,5,7–16 and are even comparable to the performance of broth-based systems at a small fraction of the time and cost.17,18
After successful validation by the NRL, the Peruvian NTP and NRL proceeded with the implementation of the Griess method in two district reference laboratories as the next step toward nationwide implementation. In the present study, we describe the performance characteristics of the Griess method in programmatic use in the first of these two districts.
METHODS
Study setting and TB treatment program
The decentralization of DST began in 2005. The Lima Ciudad reference laboratory served 45 health establishments (24 health centers, 9 health posts and 12 hospitals) with a population of 1 577 090 in an area of approximately 100 km2. In June 2006, Peruvian health districts were reorganized. The number of health establishments under the jurisdiction of this laboratory tripled and the total population increased to 3 785 688 inhabitants. The reference laboratory of Lima Ciudad is staffed by four biologists, one laboratory technician and one support person. In addition to Griess, the laboratory performs approximately 10 000 mycobacterial cultures and 800 conventional DSTs per year (in 2005) and supervises quality control of smear microscopy and culture performed by the laboratories of the Ministry of Health in the district.
Physicians at local health centers, health posts and hospitals evaluate patients and request DST according to NTP norms. Microscopy is performed at the local health center or hospital. The sample is transported to the district laboratory by a courier that visits daily or twice weekly, depending on demand. Upon receipt at the district laboratory, laboratory personnel determine if the request meets requisites for Griess:
at least one indication for rapid DST per NTP norms
no prior documentation of MDR-TB and/or treatment with second-line drugs (otherwise sent directly to NRL for complete first- and second-line DST)
smear-positive (at least 10 acid-fast bacilli per 100 fields)
minimum volume of 3 ml
ideally, age of specimen no more than 4 h at room temperature and no more than 72 h under refrigeration prior to Griess processing.
All samples are registered in a web-based electronic information system.19 A trained biologist is exclusively dedicated to processing Griess samples on a daily basis. Culture and DST results are entered into the information system, verified by the laboratory director and transmitted electronically and in paper form to the health establishment. Any isolate with resistance to INH and/or RMP is sent to the NRL for DST against first- and second-line drugs by the proportion method on Middlebrook agar plates. Physicians treat patients based on Griess results until the results of full DST to first- and second-line drugs are available. The Griess method thus serves as a rapid, inexpensive screening test for smear-positive sputum specimens from high-risk patients.
Laboratory methods
The Griess method has been described in detail else-where.3 Smear-positive sputum specimens are digested and decontaminated with 2% NaOH/N-acetyl cysteine (NALC). The sample is then centrifuged at 3000 × g in a refrigerated centrifuge. Aliquots of 0.2 ml of sputum sediment are distributed into four tubes: INH at 0.2 mg/l, RMP at 40 mg/l, and two drug-free control tubes. The tubes are incubated at 37°C and read at 28 days by introducing 0.5 ml of freshly-made Griess reagent containing one part 50% concentrated hydrochloric acid mixed with two parts 0.2% sulfanilamide and two parts 0.1% n-1-naphthylethylenediamine dihydrochloride. If the control tube turns purple, the same amount of reagent is introduced into the drug-containing tubes and the color intensity is compared to the control tube. If the sample is resistant to INH and/or RMP, the remaining drug-free control tube is sent to the NRL for the full panel of first- and second-line drugs. Biologists performing DST at the NRL are not blinded to the DST results, but typically do not review them at the time of sample processing and reading. If Griess fails to yield a DST result (e.g., no growth or contaminated), further assessment depends on whether the drug-free control becomes culture-positive. If so, this sample is submitted for indirect conventional DST against first-line drugs at the district laboratory. If negative, the provider may submit an additional sample if the patient remains smear-positive.
Specimens without indications for direct, rapid DST are decontaminated with 4% NaOH for 15 min and inoculated onto Ogawa medium. For positive cultures, the district laboratory performs DST for first-line drugs (INH, RMP, streptomycin [SM], and ethambutol [EMB]) on LJ media by the proportions method and/or sends the isolate to the NRL for first- and second-line DST. The NRL uses the proportions method on MB7H10 agar plates.20 Smear-negative and paucibacillary sputum samples from high-risk patients, including HCWs, HIV-positive patients and children, are sent to the NRL for direct culture and indirect DST using BACTEC 460 TB (Becton Dickinson, Franklin Lakes, NJ, USA).21
The NRL has standard operating procedures and quality control protocols for all methods. The director of the TB laboratory at the NRL and directors of the district laboratories are responsible for monitoring laboratory performance.
Data collection
All data were collected from the web-based electronic information system, which contains basic socio-demographic and clinical data on patients, as well as all laboratory results. Data were extracted by creating an SQL query of all samples consecutively received by the laboratory from January 2005 to June 2007, for which conventional and/or Griess DST were requested and/or performed.
Analysis
We present the frequency of testing, culture and DST results, and time to test results among samples processed in the Lima Ciudad district laboratory using conventional and Griess methods. If a sample was first processed by Griess and then by indirect conventional culture, we considered the time to test result only for Griess. We assessed factors associated with the time (days) from specimen registration to result by plotting Kaplan-Meier estimates and included confounding variables in a multiple Cox proportional-hazards model. Observation time was defined as the number of days from the date of DST request to the date of result (e.g., DST result, contamination or no growth). We censored observations on the date of analysis (30 August 2007). To account for missing data, we generated a second Cox proportional-hazards model that included dummy variables for missing data regarding whether the patient was in treatment at the time of sample collection and smear microscopy status. In this model, we also imputed method-specific medians of time from sample collection to inoculation.
RESULTS
Description of implementation process
Implementing Griess had several prerequisites. First, the NRL validated and implemented conventional DST against first-line drugs. Next, as the supervisory Supranational Reference Laboratory for Peru, the MSLI trained NRL personnel in the direct Griess method. The NRL then successfully validated the method against MSLI by parallel testing of isolates (Table 1). In turn, the NRL trained district laboratory personnel in the Griess method, first at the NRL and then in the district laboratory. Laboratories were also renovated and expanded to provide adequate infrastructure to expand DST capacity under biosafe conditions. The Lima Ciudad reference laboratory conducted its validation procedure for conventional DST (by the indirect proportions method on LJ medium) and purchased equipment (refrigerated centrifuge, incubators, autoclave, biosafety cabinet) and supplies. A Lima Ciudad biologist then conducted a validation study of the Griess method by processing 50 consecutive clinical isolates using the Griess method and submitting all cultures to the NRL for DST using the agar plate proportions method (Table 2). These results met validation requirements, and Griess has since been made publicly available. In April-May 2007, the district laboratory performed acceptable quality assurance under NRL supervision (Table 3).
Table 1.
Validation of Griess method, NRL vs. supranational reference laboratory, from Solis et al.3
| Drugs | Conventional method | Griess method | ||||
|---|---|---|---|---|---|---|
| Resistant n | Susceptible n | Sensitivity % (95%CI) | Specifi city % (95%CI) | Accuracy % (95%CI) | ||
| INH | Resistant (n = 114) Susceptible (n = 78) |
113 0 |
1 78 |
99.1 (95.2–100) | 100 (95.4–100) | 99.5 (97.1–100) |
| RMP | Resistant (n = 108) Susceptible (n = 84) |
101 0 |
7 84 |
93.5 (87.0–97.3) | 100 (95.7–100) | 96.4 (92.6–98.5) |
NRL = National Reference Laboratory; CI = confidence interval; INH = isoniazid; RMP = rifampicin.
Table 2.
Validation of Griess method, district laboratory vs. NRL
| Drugs | Conventional method | Griess method | ||||
|---|---|---|---|---|---|---|
| Resistant n | Susceptible n | Sensitivity % (95%CI) | Specifi city % (95%CI) | Accuracy % (95%CI) | ||
| INH | Resistant (n = 30) Susceptible (n = 20) |
30 1 |
0 19 |
100 (88.4–100) | 95.0 (75.1–99.9) | 98.0 (89.4–100) |
| RMP | Resistant (n = 30) Susceptible (n = 20) |
29 0 |
1 20 |
96.7 (82.8–99.9) | 100 (83.2–100) | 98.0 (89.4–100) |
NRL = National Reference Laboratory; CI = confidence interval; INH = isoniazid; RMP = rifampicin.
Table 3.
Quality assurance of Griess method at district laboratory vs. NRL
| Drugs | Conventional method | Griess method | |||||
|---|---|---|---|---|---|---|---|
| Resistant n | Susceptible n | Sensitivity % (95%CI) | Specifi city % (95%CI) | Accuracy % (95%CI) | |||
| INH | Resistant (n = 8) Susceptible (n = 12) |
8 2 |
0 10 |
100 (63.0–100) | 83.3 (51.6–97.9) | 90.0 (68.3–98.8) | |
| RMP | Resistant (n = 5) Susceptible (n = 15) |
5 0 |
0 15 |
100 (47.8–100) | 100 (78.2–100) | 100 (83.2–100) | |
NRL = National Reference Laboratory; CI = confidence interval; INH = isoniazid; RMP = rifampicin.
Laboratory performance
Lima Ciudad implemented conventional DST in January 2005 and Griess in December 2005. From January 2005 to June 2007, Lima Ciudad performed a total of 4997 DSTs: 4102 by the conventional proportions method, and 895 by Griess (Figure 1). Prior to expansion of the district in June 2006, the Lima Ciudad laboratory averaged 36 Griess DSTs and 91 conventional DSTs per month. After the district expanded, the workload increased to an average of 52 Griess and 202 conventional DSTs per month.
Figure 1.
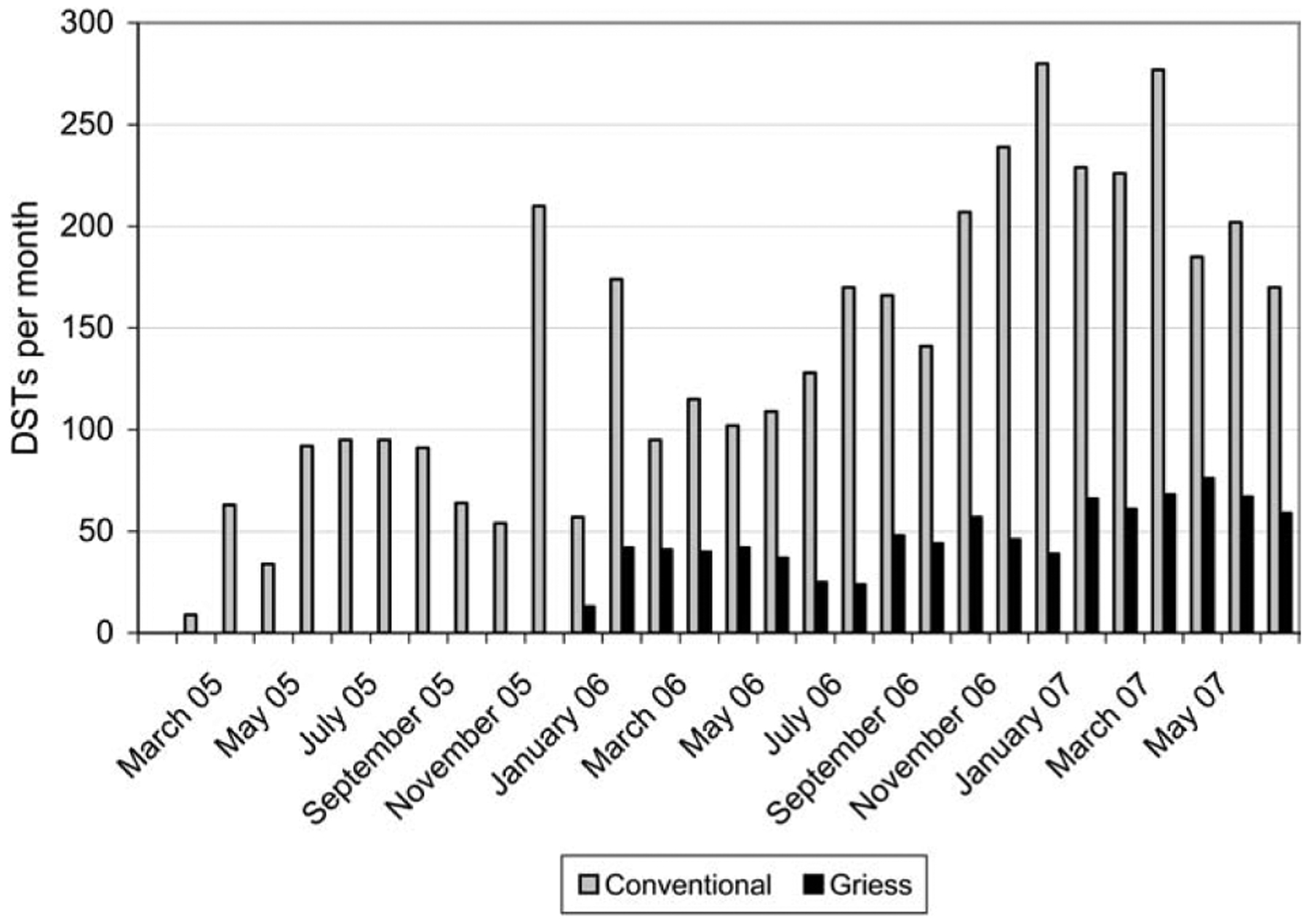
Monthly DST by method, February 2005–June 2007. DST = drug susceptibility test.
Of the 4377 samples submitted for conventional DST, 275 (6.3%) were rejected for clinical reasons (e.g., no indication for DST, duplicates, etc). Of the remaining 4102 isolates, a total of 1116 (27.2%) specimens could not yield conventional DST results because they were culture-negative (21.7%), paucibacillary (4.8%) or contaminated (0.7%) (Figure 2). If we excluded the 1756 smear-negative samples, 2034 (86.7%) DST results were obtained from the remaining 2346 samples that were processed. Among 895 samples processed by Griess, 202 (22.6%) were culture-negative and 65 (7.3%) were contaminated. As shown in Table 4, among samples successfully processed by conventional DST, 26.4% were MDR-TB and 55.9% were susceptible to all drugs tested. A small proportion of individuals tested by conventional DST were found to have monoresistance (7.1%) or polyresistance (6.1%), which would not have been identified by Griess.
Figure 2.
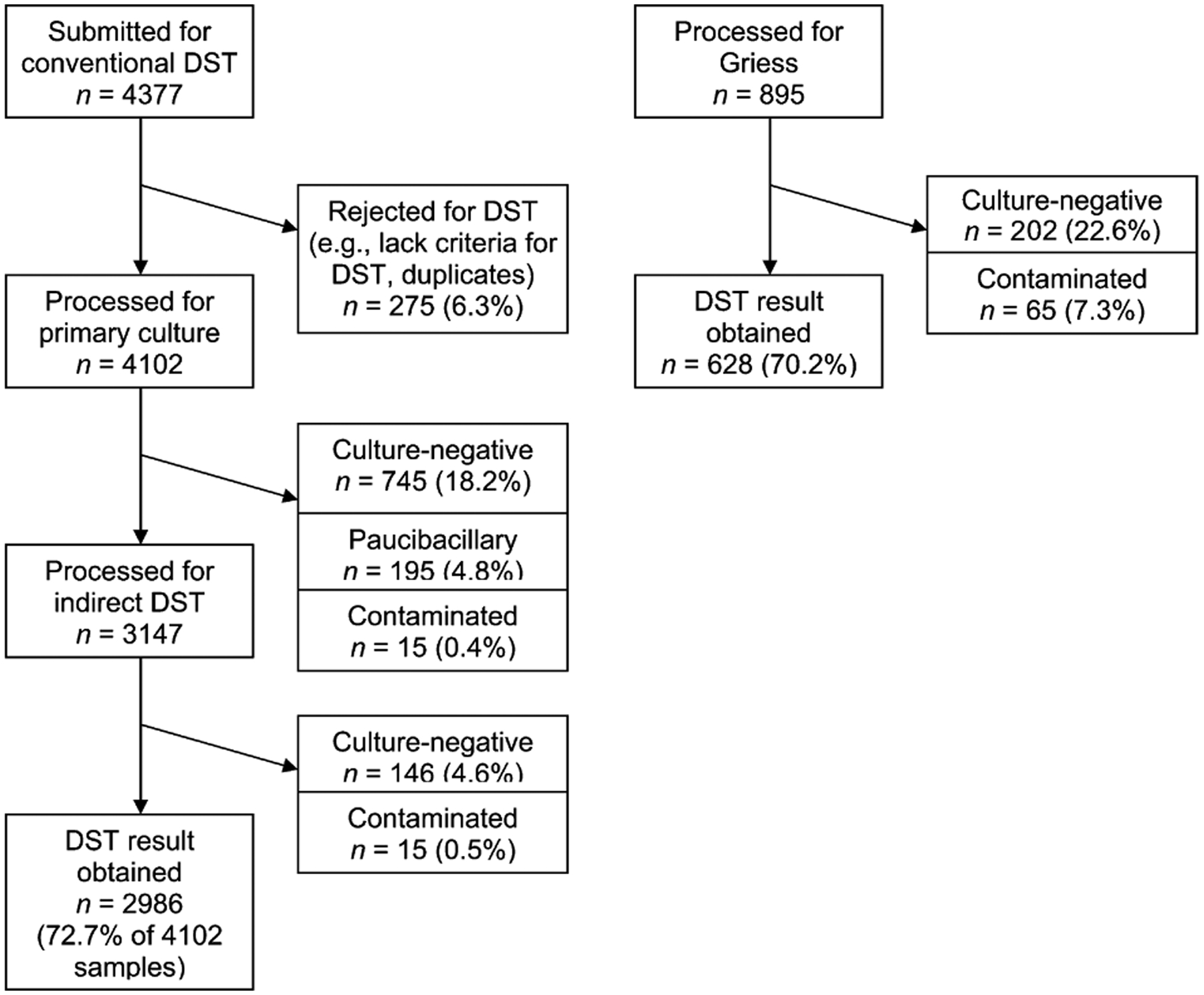
Culture results of Griess and conventional DST in district laboratory. DST = drug susceptibility test.
Table 4.
DST results of Griess and conventional DST in district laboratory
| Method | Samples N | Pan-susceptible* n (%) | Resistant only to INH* n (%) | Resistant only to RMP* n (%) | Other monoresistance* n (%) | Polyresistance* n (%) | MDR n (%) |
|---|---|---|---|---|---|---|---|
| Conventional DST | 2986† | 1667 (55.9) | 88 (3.0) | 49 (1.6) | 212 (7.1) | 182 (6.1) | 787 (26.4) |
| Griess DST | 628 | 298 (47.5) | 83 (13.2) | 49 (7.8) | — | — | 198 (31.5) |
Among all drugs tested (HRES for conventional, HR only for Griess).
Result not available for 1 sample processed by conventional DST.
DST = drug susceptibility test; INH, H = isoniazid; RMP, R = rifampicin; MDR = multidrug resistance; E = ethambutol; S = streptomycin.
As shown in Table 5, smear-positive samples were associated with shorter time to DST result, while TB treatment at the time of sample collection and longer delays in sputum processing time were inversely associated with time to DST results. The Griess method was associated with shorter time to result (adjusted hazard ratio [aHR] 1.55, 95% confidence interval [CI] 1.38–1.74); this association did not change when accounting for missing data (aHR 1.51, 95%CI 1.36–1.68).
Table 5.
Factors associated with time to DST result
| Factor | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) |
|---|---|---|
| Positive AFB | 2.56 (2.36–2.77) | 2.96 (2.63–3.34) |
| Receiving treatment at time of sample collection | 0.69 (0.63–0.76) | 0.65 (0.58–0.72) |
| Days from sputum collection to inoculation | 0.95 (0.94–0.96) | 0.98 (0.97–0.99) |
| Griess method | 2.04 (1.87–2.22) | 1.55 (1.38–1.74) |
DST = drug susceptibility test; CI = confidence interval; AFB = acid-fast bacilli.
The median number of days from specimen collection to inoculation of Griess samples and from inoculation to Griess results was 1 day, with an interquartile range (IQR) of respectively 1–2 days and 29 days (IQR 26–31) (Figure 3). For indirect conventional DST, the median time from collection to inoculation of DST was 49 days (IQR 42–57) and the time from DST inoculation to result was 49 days (IQR 47–53).
Figure 3.
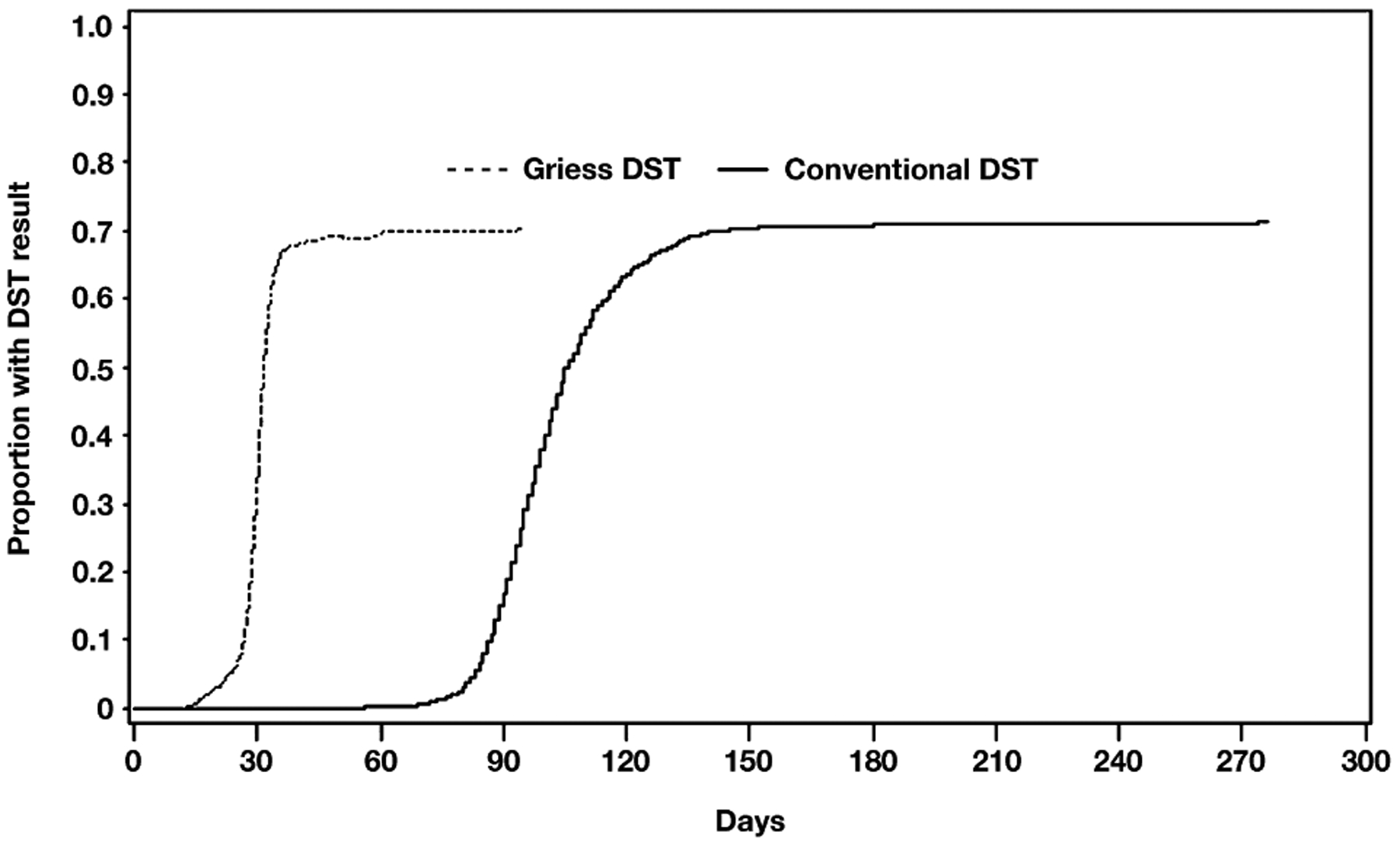
Time to DST result, by method. DST = drug susceptibility test.
DISCUSSION
Within 20 years after RMP was introduced, outbreaks of MDR-TB heralded a global pandemic that highlighted the need for rapid culture and DST. To treat MDR-TB, fluoroquinolones were combined with older TB drugs; 15 years later, extensively drug-resistant TB (XDR-TB) makes us ask, ‘Are we learning from history’s lessons or repeating them?’22 In this study, we describe the successful implementation of a rapid, simple, inexpensive DST in a district laboratory under the super vision of the NRL. To our knowledge, this is one of the first published evaluations of programmatic implementation of rapid DST in a low- or middle-income setting. Even when a method performs favorably under research conditions, programmatic implementation with subsequent quality assurance are needed to evaluate the performance of the method in actual public health practice.23 In the second year after full implementation of conventional and rapid DST, we report several lessons from this experience.
First, maintaining acceptable performance of both DST methods required close supervision and trouble-shooting by the NRL. A biologist trained in the methods visited the district laboratories frequently to observe the processing technique. When unexpected results were encountered, we performed a series of operational assessments to identify any contributing factors. Our laboratory information system24 proved instrumental for these operational assessments by generating almost immediate reports. As test methods move into district laboratories closer to the primary point of care, variability in their performance is to be expected.22 Through quality assurance, continued training, implementation of standard operating procedures and diligent monitoring, we were able to maintain excellent performance of the methods under program conditions.
Second, the Griess method has performed well under programmatic conditions as a rapid test to screen for MDR-TB at the district level. Reagents and equipment were easy to procure and distribute. Training and validation of the method in both national and district laboratories was feasible. The cost of processing a sample using Griess is US$4.80 (not including labor or capital costs) compared with US$10.00 for conventional DST. The method proved simple and robust; performance did not deteriorate over time. Both validation and subsequent quality assurance in the district laboratory demonstrated high sensitivity for detecting resistance to INH and RMP. Although the specificity of detecting INH resistance was 83% in the first round of quality assurance, we felt this was acceptable for a screening test in which all drug-resistant isolates are sent for first- and second-line DST at the NRL. The proportion of samples yielding DST results was lower among smear-positive samples processed by Griess compared with conventional indirect DST, given higher rates of contamination (7.3% vs. 0.8%) and insufficient growth (22.6% vs. 12.5%). However, because samples were processed for Griess vs. conventional DST based on different clinical criteria, it is difficult for us to determine how much these performance differences are related to the processing methods. In such cases, the district laboratory uses one of the Griess control tubes to perform indirect conventional DST, if the isolate grows. Most importantly, the direct method significantly shortened the time to test result, with a median of 31 days (IQR 28–33) from sample collection to result for Griess, compared with 99 days (IQR 91–109) for conventional DST.
CONCLUSION
Rapid DST to identify MDR-TB was successfully implemented in the one of the most populous health districts in Peru. Preliminary experience with the Griess method demonstrates favorable performance under program conditions. Ongoing efforts to streamline multiple facets of program and laboratory management were integral to the DST implementation process.
Acknowledgements
The authors would like to thank A Sloutsky, M Stowell and P Jenson for their support of the activities described in this manuscript. This study was funded by the Bill & Melinda Gates Foundation, the National Institute of Allergy and Infectious Diseases, Bethesda, MD, the Heiser Foundation, Wantagh, NY, and the Infectious Diseases Society of America, Arlington, VA, USA.
References
- 1.Small PM, Perkins MD. More rigour needed in trials of new diagnostic agents for tuberculosis. Lancet 2000; 356: 1048–1049. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Yagui M, Asencios L, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis 2008. May [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solis LA, Shin SS, Han LL, Llanos F, Stowell M, Sloutsky A. Validation of a rapid method for detection of M. tuberculosis resistance to isoniazid and rifampin in Lima, Peru. Int J Tuberc Lung Dis 2005; 9: 760–764. [PMC free article] [PubMed] [Google Scholar]
- 4.Mengatto L, Chiani Y, Imaz MS. Evaluation of rapid alternative methods for drug susceptibility testing in clinical isolates of Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz 2006; 101: 535–542. [DOI] [PubMed] [Google Scholar]
- 5.Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 2005; 55: 500–505. [DOI] [PubMed] [Google Scholar]
- 6.Palomino JC. Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. Eur Respir J 2005; 26: 339–350. [DOI] [PubMed] [Google Scholar]
- 7.Palomino JC, Martin A, Portaels F. Rapid drug resistance detection in Mycobacterium tuberculosis: a review of colouri-metric methods. Clin Microbiol Infect 2007; 13: 754–762. [DOI] [PubMed] [Google Scholar]
- 8.Coban AY, Birinci A, Ekinci B, Durupinar B. Drug susceptibility testing of Mycobacterium tuberculosis with nitrate reductase assay. Int J Antimicrob Agents 2004; 24: 304–306. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Khan IA, Verma V, Qazi GN. Microplate nitrate reductase assay versus Alamar Blue assay for MIC determination of Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2005; 9: 939–941. [PubMed] [Google Scholar]
- 10.Lemus D, Montoro E, Echemendia M, Martin A, Portaels F, Palomino JC. Nitrate reductase assay for detection of drug resistance in Mycobacterium tuberculosis: simple and inexpensive method for low-resource laboratories. J Med Microbiol 2006; 55: 861–863. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Montoro E, Lemus D, et al. Multicenter evaluation of the nitrate reductase assay for drug resistance detection of Mycobacterium tuberculosis. J Microbiol Methods 2005; 63: 145–150. [DOI] [PubMed] [Google Scholar]
- 12.Musa HR, Ambroggi M, Souto A, Angeby KA. Drug susceptibility testing of Mycobacterium tuberculosis by a nitrate reductase assay applied directly on microscopy-positive sputum samples. J Clin Microbiol 2005; 43: 3159–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poojary A, Nataraj G, Kanade S, Mehta P, Baveja S. Rapid antibiotic susceptibility testing of Mycobacterium tuberculosis: its utility in resource poor settings. Indian J Med Microbiol 2006; 24: 268–272. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Sharma S, Sharma SK, Meharwal SK, Jindal SK, Sharma M. Drug susceptibility of Mycobacterium tuberculosis to primary antitubercular drugs by nitrate reductase assay. Indian J Med Res 2004; 120: 468–471. [PubMed] [Google Scholar]
- 15.Angeby KA, Klintz L, Hoffner SE. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J Clin Microbiol 2002; 40: 553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Affolabi D, Odoun M, Martin A, Palomino JC, Anagonou S, Portaels F. Evaluation of direct detection of Mycobacterium tuberculosis rifampin resistance by a nitrate reductase assay applied to sputum samples in Cotonou, Benin. J Clin Microbiol 2007; 45: 2123–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrigo M, Aragon LM, Alcaide F, et al. Multicenter laboratory evaluation of the MB/BacT Mycobacterium detection system and the BACTEC MGIT 960 system in comparison with the BACTEC 460TB system for susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2007; 45: 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortoli E, Benedetti M, Fontanelli A, Simonetti MT. Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major anti-tuberculous drugs: comparison with the radiometric BACTEC 460TB method and the agar plate method of proportion. J Clin Microbiol 2002; 40: 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaya JA, Shin SS, Yagui MJ, et al. A web-based laboratory information system to improve quality of care of tuberculosis patients in Peru: functional requirements, implementation and usage statistics. BMC Med Inform Decis Mak 2007; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent P, Kubica G. Public health mycobacteriology: a guide for the level III laboratory. Atlanta, GA, USA: Centers for Disease Control and Prevention, 1985. [Google Scholar]
- 21.Heifets L Drug susceptibility testing. Clin Lab Med 1996; 16: 641–656. [PubMed] [Google Scholar]
- 22.Dukes Hamilton C, Sterling TR, Blumberg HM, et al. Extensively drug-resistant tuberculosis: are we learning from history or repeating it? Clin Infect Dis 2007; 45: 338–342. [DOI] [PubMed] [Google Scholar]
- 23.Quezada CM, Kamanzi E, Mukamutara J, et al. Implementation validation performed in Rwanda to determine whether the INNO-LiPA Rif.TB line probe assay can be used for detection of multidrug-resistant Mycobacterium tuberculosis in low-resource countries. J Clin Microbiol 2007; 45: 3111–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaya J, Shin S, Yagui M, Yale G, Suarez C, Asencios L. Implementing and evaluating a laboratory information system to optimize the treatment of tuberculosis patients in Peru. Int J Tuberc Lung Dis 2006; 10 (Suppl 1): S58–S59. [Google Scholar]


