Abstract
Background and Aims
Abiotic and biotic stresses related to climate change have been associated with increased crown defoliation, decreased growth and a higher risk of mortality in many forest tree species, but the impact of stresses on tree reproduction and forest regeneration remains understudied. At the dry, warm margin of species distributions, flowering, pollination and seed maturation are expected to be affected by drought, late frost and other stresses, eventually resulting in reproduction failure. Moreover, inter-individual variation in reproductive performance versus other performance traits (growth, survival) could have important consequences for population dynamics. This study investigated the relationships among individual crown defoliation, growth and reproduction in a drought-prone population of European beech, Fagus sylvatica.
Methods
We used a spatially explicit mating model and marker-based parentage analyses to estimate effective female and male fecundities of 432 reproductive trees, which were also monitored for basal area increment and crown defoliation over 9 years.
Key Results
Female and male fecundities varied markedly between individuals, more than did growth. Both female fecundity and growth decreased with increasing crown defoliation and competition, and increased with size. Moreover, the negative effect of defoliation on female fecundity was size-dependent, with a slower decline in female fecundity with increasing defoliation for the large individuals. Finally, a trade-off between growth and female fecundity was observed in response to defoliation: some large trees maintained significant female fecundity at the expense of reduced growth in response to defoliation, while some other defoliated trees maintained high growth at the expense of reduced female fecundity.
Conclusions
Our results suggest that, while decreasing their growth, some large defoliated trees still contribute to reproduction through seed production and pollination. This non-coordinated decline of growth and fecundity at individual level in response to stress may compromise the evolution of stress-resistance traits at population level, and increase forest tree vulnerability.
Keywords: Defoliation, drought, female and male fecundity, growth, trade-off, marginal population, Mediterranean forest, mixed effect mating model, microsatellite, parentage analyses, Fagus sylvatica
INTRODUCTION
Climate and land-use changes can have major and complex effects on forest vegetation dynamics (McDowell et al., 2020). Indeed, increasing episodic forest disturbances, such as windthrow or wildfire, together with land-use changes and changes in chronic drivers of forest dynamics (e.g. rising temperature, vapour pressure deficit and CO2) lead to both compounding and antagonistic impacts that alter demographic processes of tree growth, mortality and recruitment. Many studies have focused on how global change can lead to massive forest decline (Briceño-Elizondo et al., 2006; Camarero et al., 2015) and tree mortality (Allen et al., 2010; Adams et al., 2017). However, the long-term response of forests is at least as dependent on recruitment, as the number and composition of recruits determines the demography (Beckage et al., 2005) and the adaptive potential (Hampe and Petit, 2005) of tree populations. Hence, we need more studies investigating the impacts of stresses related to climate or global change on tree reproduction and forest regeneration.
The warm and dry margins of tree species distributions are expected, and already observed, to suffer massive forest decline, driven by climate change and its consequences (Jump et al., 2009; Anderegg et al., 2019). Most importantly, prolonged droughts and high temperatures have been extensively associated with decreasing tree growth and forest productivity (Zhao and Running, 2010; Zimmermann et al., 2015), increasing crown defoliation and leaf fall (Dobbertin, 2005; Galiano et al., 2011) and higher risk of tree mortality (Allen et al., 2010; Adams et al., 2017; Anderegg et al., 2019). There is also an increasing concern that the advance in spring phenology currently observed in many species exposes them to a higher risk of late frost, with damaging effects on crown development (Charrier et al., 2015; Bigler and Bugmann, 2018). Finally, the few existing studies monitoring tree reproduction and forest regeneration over time (i.e. the diachronic approach; see Bontemps et al., 2013 for a definition) suggest that, at the rear edge of tree species’ distributions, reproduction abilities are lowered by climatic stresses, in particular drought (Cecich and Sullivan, 1999; Fernández-Martínez et al., 2012). However, such observational, diachronic studies are limited by the rarity of long-term reproduction-monitoring data sets (Clark et al., 1999). Moreover, masting (i.e. the synchronized, intermittent production of large amounts of seeds) is common in forest trees (Pearse et al., 2016), which makes the interpretation of reproduction time series challenging (Caignard et al., 2017). Hence, we need complementary approaches to generalize whether climate stresses systematically decrease seed production and forest regeneration at the rear edge of tree species distribution.
Knowledge of species physiology predicts diverse, antagonistic effects of climate stress on reproductive performance. On the one hand, abiotic stresses such as droughts or late frosts are indeed expected to directly reduce plant sexual reproduction through altered reproductive phenology (i.e. the timing of flowering and fruiting), a higher risk of pollen abortion or pollination failure, a shorter seed maturation cycle and/or a higher risk of seed abortion (Hedhly et al., 2009; Zinn et al., 2010; Bykova et al., 2012). Moreover, indirect negative effects are also expected: plant sexual reproduction can be strongly dependent on complex relationships between climate, plants and seed predators (Bogdziewicz et al., 2020; Clark et al., 2021). Also, by decreasing photosynthetic activity, leaf fall may reduce the amount of stored resources to invest in growth and reproduction in the next year (Obeso, 1988). On the other hand, stresses have also been hypothesized to shift patterns of resource allocation and act like a cue stimulating higher reproductive effort (Lee, 1988; Bréda et al., 2006; Pulido et al., 2014; Wiley et al., 2017; Lauder et al., 2019).
In their conifer-centred review, Lauder et al. (2019) proposed that trees under stress may exhibit either fight behaviour (i.e. increased allocation to survival at the expense of reproduction) or flight behaviour (i.e. increased allocation to reproduction at the expense of growth and survival). They hypothesize that flight behaviours increase as drought stress escalates the likelihood of mortality in a given location. The clearest experimental evidence of flight behaviour can be found in the literature on (fruit) tree orchards. Among the cultural practices allowing early and abundant flowering, water stress is used to enhance flower initiation in conifers, while hot, dry summers are reported to induce abundant seed crops in both conifers and broadleaved species (Meilan, 1997). Another practice relies on circumferential girdles (the removal of a swath of the bark, down to the phloem, around the entire stem), which are associated with reduced vegetative growth and increased fruiting (Bonnet-Masimbert and Webber, 2012). Finally, pruning (the reduction of crown leaf area) is also recommended to favour reproductive development while reducing vegetative growth in fruit trees (Karimi et al., 2017).
This study investigates the effects of climatic stresses on sexual reproduction and growth using an observational synchronic approach, which consists in comparing the reproductive and growth performances of declining versus non-declining trees within a single population. As in Camarero et al. (2015), we consider crown defoliation as an indicator of stress, and analyse the relationships between crown defoliation, growth and fecundity at the inter-individual scale to first test whether defoliation leads to a decline in fecundity and growth. Moreover, by investigating the correlation between growth and fecundity in response to defoliation, we also test two alternative hypotheses. Hypothesis 1 (H1) is that crown defoliation is associated with a proportional decrease in growth and reproduction, so that the relationship between reproduction and growth does not change with increasing crown defoliation. Alternatively, hypothesis 2 (H2) holds that if defoliation or stresses act like a cue stimulating reproductive performances at the expense of reduced growth, then the relationship between reproduction and growth should change with increasing crown defoliation.
A main originality of our study is the use of a spatially explicit mating model and marker-based parentage analyses to estimate individual tree fecundity (e.g. Oddou-Muratorio et al., 2018). Male and female basic fecundities (i.e. the numbers of pollen grains and seeds produced) have traditionally been estimated before dispersal through the resource allocated to male (i.e. biomass/number of pollen grains or staminate flowers) and female (i.e. biomass/number of ovules, seeds, ovuliferous flowers or fruits) functions. The development of marker-based approaches for parentage reconstruction has then allowed realized reproductive successes to be estimated from genotypes of seedlings and their potential parents, and used as a proxy of fitness (Conner et al., 1996; Elle and Meagher, 2000). The next step was to combine genotypes with spatial locations of sampled individuals, through spatially explicit mating models, to disentangle the effect of fecundity from that of spatial design on reproductive success (Oddou-Muratorio et al., 2005; Burczyk et al., 2006; Goto et al., 2006; Oddou-Muratorio and Klein, 2008; Moran and Clark, 2011). This approach avoids the spatial bias typically generated by sampling seedlings non-uniformly with respect to the positions of their parents or by the confounding effects of heterogeneous spatial distribution of mates. With this aim, dispersal is explicitly modelled using pollen and seed dispersal kernels. We used a previously developed Bayesian framework to estimate individual male and female individual effective fecundities (mixed-effect mating model, MEMM; Klein et al., 2008; Oddou-Muratorio et al., 2018). In this mating model for monoecious species, each adult individual is considered in turn as the potential father and mother of each sampled seedling. The MEMM hence estimates the effective amount of pollen achieving successful fertilization, and of seeds achieving successful germination.
Our study is focused on a major European tree species (the European beech, Fagus sylvatica), considered to be sensitive to summer drought. Beech is a monoecious, wind-dispersed species, and shows a masting behaviour triggered both by weather and plant resource status (Vacchiano et al., 2017). Hacket-Pain et al. (2017) showed that summer droughts combined with masting years were associated with reduced growth, while growth was not reduced in mast years without summer drought, nor when summer droughts occurred during non-mast years. There is overall little evidence of a direct effect of drought on beech seed production, except in Bréda et al. (2006), which reported increased seed production associated with leaf fall in high drought years, even though this relationship between crown defoliation and fruit production may not be directly causal. Our study site is a drought-prone, rear-edge natural population of F. sylvatica in southern France (Supplementary Data Fig. S1), where crown defoliation and mortality have been surveyed since 2003 (Petit-Cailleux et al., 2020). We estimated effective female and male fecundities cumulated from 2002 to 2012. Growth over the same period was assessed through inventory data, completed by ring-width measurements. Finally, this study is based on the well-accepted hypothesis that recurrent defoliation is related to physiological stresses associated with climate change, and symptomatic of declining health in beech (Bréda et al., 2006; Peñuelas and Boada, 2003). Supporting this hypothesis, a companion study on 4327 trees individually surveyed in the same population showed that crown defoliation increases the risk of mortality (Petit-Cailleux et al., 2020). Moreover, simulations with a process-based physiological model indicated that the mortality rate in this population is driven by a combination of drought-related processes such as loss of hydraulic conductance or carbon reserve depletion, and late frost damages (Petit-Cailleux et al., 2020).
MATERIALS AND METHODS
Study site
Located on the foothills of Eastern Pyrenees in southern France, the Massane Forest National Nature Reserve (42°28′41″N, 3°1′26″E) was created in 1973. It covers 336 ha on the highest part of the Massane valley, from 600 to 1127 m a.s.l., and is only around 5 km from the Mediterranean Sea. With a mean annual temperature of 11.95 °C and 1164.9 mm of mean annual precipitation (monitored on site since 1976 and 1960 respectively; Supplementary Data Fig. S1A), the site is under a meso-Mediterranean climate influence (sensuQuézel and Médail, 2003). This site is one of the European beech locations most prone to water stress (Supplementary Data Fig. S1B).
More than half of the reserve consists of an old-growth forest, where no logging operation has been performed since at least 1886. The canopy is dominated by European beech (Fagus sylvatica) in mixture with downy oak (Quercus pubescens), maples (Acer opalus, A. campestris, A. monspessulanum) and holly (Ilex aquifolium). A 10 ha fenced plot has excluded cow grazing since 1956. All trees from this protected plot have been monitored since 2002.
Adult seed–tree inventory and phenotyping
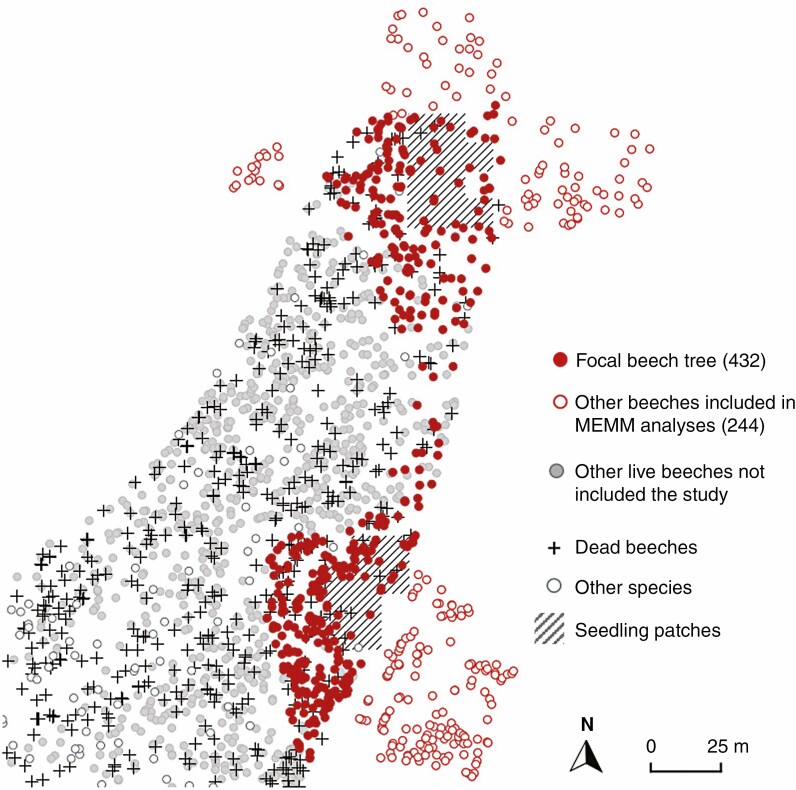
This study was conducted on two circular-shaped plots (as classically used in parentage analyses) covering 0.17 ha in total, where all the 683 live adult beeches were mapped and material was collected for genetic analyses in 2012 (red circles in Fig. 1). Although beech reproduction is mostly sexual, vegetative reproduction may occasionally occur, with the production of stump shoots resulting in multiple stems (i.e. several ramets for a single genet). In obvious cases of vegetative reproduction (i.e. root-connected stems), we sampled only the largest ramet of each genet for genetic analyses.
Fig. 1.
Study site and sampling design. Red filled circles represent the 432 beech trees for which individual fecundity, growth and defoliation were assessed. Hatched squares represent the seedling patches used to estimate fecundity through parentage analyses and MEMMs. Open red circles represent the 244 beech trees outside of the protected area and included in the fecundity analyses (but not phenotyped for growth and defoliation). Grey circles and crosses represent other beeches within the protected area not included in the fecundity analyses either because they were far from sampled seedlings (circles) or because they were dead in 2012 (+). Open circles represent other species within the protected area.
Only 439 among the 683 collected beeches were included within the protected plot and monitored since 2002 (filled circles in Fig. 1). The monitoring consisted first in measuring tree size as the diameter at breast height (DBH) in 2002 and 2012, which allowed us to derive the basal area (BA = π × DBH2/4). Individual radial growth was measured by the basal area increment (herein BAI) between 2002 and 2012, as estimated by BAI = π(DBH22012 − DBH22002)/4.
The presence of major dead branches or foliage loss was recorded each year between 2004 and 2012 as a qualitative measure (1 = presence; 0 = absence). Note that only branch mortality of the current year is accounted for. This simple estimate of defoliation can be applied to a large number of individuals (e.g. 4327 trees in Petit-Cailleux et al., 2020). Here, we used the sum of these nine annual defoliation (DEF) scores as an integrative, qualitative ordered measure. A high DEF score means that a tree either suffers recurrent defoliation or/and is not able to recover from previous defoliation.
The conspecific local density (herein ) was estimated as the number of beech neighbours found within a radius of dmax (see below) around each mother-tree. We also used the Martin-Ek index (Martin and Ek, 1984) to quantify the intensity of competition on a focal individual i. This index (herein ) accounts simultaneously for the diameter and the distance of each beech competitor j from the competed individual i:
| (1) |
where DBHi and DBHj are the DBH (in centimetres) of the competed individual i and of competitor j (any adult tree of any species with DBHj > DBHi), is the total number of competitors in a given radius dmax (in metres) around each individual i, and dij is the distance between individuals i and j. We computed a total of 20 variables and 20 variables, by considering dmax values between 1 and 20 m with a 1-m step. The variables were strongly and positively correlated with each other, and so were the variables, but variables were not correlated with variables (Supplementary Data Fig. S2).
Offspring sampling and genotyping
To estimate adult fecundity, we sampled 365 seedlings located amidst the 683 genotyped adult beeches in 2012 (shaded quadrats in Fig. 1). Seedlings were sampled exhaustively within a selected number of quadrats at the centre of each circular plot: 165 seedlings germinated in spring 2012 (masting in 2011), and 200 seedlings germinated from spring 2011 back to spring 2001 (age was estimated using annual bud scars). Qualitative surveys indicated that masting occurred in years 2002, 2004, 2006 and 2009. In this study the two seedlings cohorts were mixed to estimate cumulative reproduction from 2001 to 2012.
The genotypes of the 683 live adult beeches and 365 seedlings were scored at a combination of 18 microsatellite loci (Supplementary Data Table S1). DNA extraction, PCR amplifications and genotype scoring with a MegaBACE 1000 sequencer were performed using the conditions described by Oddou-Muratorio et al. (2018). The total number of alleles observed in each cohort was >95 (Supplementary Data Table S1). Adult genotypes revealed seven pairs of clones among the adult beeches. We checked that these clones were always spatially clustered, and kept only one ramet for each genet in the following analyses (i.e. 676 adult beeches).
Inference of male and female relative fecundities: MEMM analyses
Male and female fecundities (F♂ and F♀ respectively) (FM and FF respectively) of each adult tree were jointly estimated with the pollen and seed dispersal kernels in a Bayesian framework (MEMM approach; Klein et al., 2008; Oddou-Muratorio et al., 2018). Briefly, MEMM considers that each sampled seedling originates either (1) from a mother tree located outside the study site (implying seed immigration) or (2) from a mother tree located within the study site. The latter case includes three possible origins of the fertilizing pollen: (1) pollen immigration; (2) selfing; or (3) pollination by a male tree located within the study site. The approach bypasses parentage assignation and focuses instead on the fractional contribution of all adults, either as female or as male parent, to each seedling (see Appendix A1 for details). For instance, the probability π Sij of each sampled female tree j contributing to the seedling pool at the spatial location of seedling i is modelled as:
| (2) |
where F♀j and F♀l are the female fecundities of mother j and l, respectively; dij and dil are the distances between seedling i and mother j and l, respectively; and θs is the seed dispersal kernel. Both the seed and pollen dispersal kernels (θs and θp) are modelled using a power-exponential function. All the parameters of the model are estimated in a Bayesian framework (Appendix A1). Note that F♀ (and F♂) estimates are relative, with the average F♀ value (or FM value) over the entire parent population fixed to 1.
For the estimation, we accounted for typing errors at microsatellite loci, with two possible types of mistyping: in the first type, the allele read differs only by one motif repeat from the true allele with a probability Perr1, while in the second type the allele read can be any allele observed at this locus with a probability Perr2. We considered a mixture of the two error types, with Perr1 = 0.01 and Perr2 = 0.01. We performed ten Markov chain Monte Carlo (MCMC) runs of 10 000 steps, each with an additional 500 first MCMC steps as burn-in, checked that the different chains converged to the same value visually, and then combined the ten chains. Individual female (F♀) and male (F♂) fecundities were summarized by their median value across the 100 000 iterations.
Adult subsampling for dendrochronological analysis
We selected 90 trees within the protected plot, for which we sampled cores to measure ring widths. These 90 trees were chosen to represent contrast in terms of defoliation and female fecundity (Supplementary Data Fig. S3). Cores were extracted in February 2016 at 1.30 m above ground. After sanding, cores were scanned at high resolution (1200 dpi). Boundary rings were read using CooRecorder v 9.0. Ring widths were transcribed, individual series were checked for missing rings and dating errors and mean chronologies were calculated using Cdendro 9.0 (CDendro 9.0 and CooRecorder 9.0; Cybis Elektronik & Data, Sweden). Using the sum of ring widths’ increments between 2002 and 2012 (Σrw), the growth of the 90 individuals between 2002 and 2012 was estimated as: BAIwood = π((DBH2002/2 + Σ rw)2 − DBH22002/4).
Statistical analyses of the ecological drivers of growth and fecundity
Our objective herein was to test whether defoliation significantly affected individual growth and female/male fecundity cumulated across the 2002–12 period. For each response variable independently (i.e. growth as measured by BAI and fecundity as estimated with MEMM), we considered the following initial linear model:
| (3) |
where all the predictors are quantitative variables (Supplementary Data Table S2). Besides the target defoliation factor (DEF), this model includes one size-related factor (DBH2002) and two competition-related factors ( and ). Size and competition are considered here as ‘nuisance’ parameters, likely to blur the signal between defoliation, growth and reproduction. A quadratic effect of DBH2002 was also included, as growth and sometimes fecundity are known to be proportional to basal area. Density and the competition index can both be relevant in capturing the competition effect on growth or fecundity; moreover, their influence may vary with the distance up to which competitors are accounted for. Therefore, we first selected the best and the best terms for each response variable independently using the model described by eqn (3) without interaction terms, and retaining the radius distance dmax leading to the highest coefficient of determination (R2). Then, we included interaction terms, i.e. the three last terms in eqn (3), to investigate specific effects of defoliation depending on individual size or on the level of competition.
The model was fitted on 432 focal adult beech trees within the protected plot (Fig. 1) for which BAI was estimated from inventory data. All response variables were log-transformed to approach Gaussian distribution and to account for the higher variance associated with higher fecundity or higher growth. We visually inspected the relationship between each predictor and each response variable (Supplementary Data Fig. S4). For each response variable, we selected the most parsimonious model based on the Akaike information criterion (AIC) using the functions ‘lm’ and ‘step’ in R 3.3 (R Core Team, 2018). The residuals were visually inspected through a plot of residuals versus predicted. Interaction effects were visualized with the package ‘jtools’ (Long, 2020).
Collinearity resulting from correlations between predictor variables is expected to affect the statistical significance of correlated variables by increasing type-II errors (Schielzeth, 2010). To evaluate this risk, we computed variance inflation factors (VIFs) associated with each term retained in the best model with the R package ‘car’ (Fox and Weisberg, 2019).
Statistical analyses of the joint defoliation effects on female fecundity and growth
Our objective here was to focus on the two variables (growth and female fecundity) responding to defoliation (see the Results section) and to investigate how the relationship between these two variables varied with defoliation. We first compared the effects of defoliation on female fecundity versus growth after centring and normalizing fecundity and growth, and by using the best models fitted with eqn (3) to estimate the effect of defoliation on these transformed variables.
Then, we investigated the individual correlation between raw relative female fecundity and growth for non-defoliated trees (DEF = 0) versus defoliated trees (DEF > 0). Note that a part of these correlations may be due to variation in size and/or competition. Moreover, they do not account for the quantitative nature of DEF. To overcome these limitations, we further investigated the trade-off between growth and female fecundity using the following linear model:
| (4) |
where BAI and the interaction between BAI and DEF are added to the model described by eqn (3) above. A quadratic effect of DBH2002 was also included.
RESULTS
Patterns of covariation of defoliation, tree size and competition
Recurrent crown defoliation was overall limited in the 432 individuals, with 95 trees with a non-null DEF-value (mean = 0.37; Supplementary Data Table S2). Defoliation increased with tree size; the significant interaction between DBH2002 and competition (mediated by competition in a radius of 19 m) or density (mediated by density in a radius of 20 m) reflected a stronger effect of size on defoliation as competition increased (Supplementary Data Fig. S5).
Inter-individual variation in relative fecundity and growth
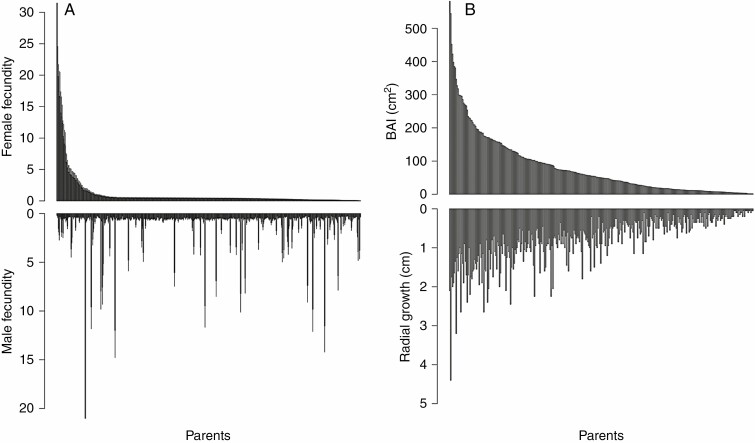
The distributions of relative female and male individual fecundities estimated by MEMM were strongly L-shaped (Fig. 2A). Female fecundities varied from 0.03 to 32.44 (median = 0.42, mean = 1, s.d. = 2.78), while male fecundities varied from 0.17 to 21.16 (median = 0.48, mean = 1, s.d. = 1.86). By comparison, the distributions of growth values were less L-shaped than those of fecundity (Fig. 2B). In the data set of 432 adult trees, where cumulated growth from 2002 to 2012 was estimated through inventory data, DBH radial growth varied from 0 to 4.4 cm (median = 0.45, mean = 0.60, s.d. = 0.62), while BAI varied from 0 to 581.22 cm2 (median = 23.98, mean = 61.58, s.d. = 86.87).
Fig. 2.
Distribution of individual (A) relative female (top) and male (bottom) fecundities estimated with MEMM, and (B) absolute growth estimated by BAI (top) or radial growth (bottom) for the 432 adult trees. Parents on the x-axis are ranked in decreasing order of female fecundity (A) or BAI (B).
In the subset of 90 cored trees, where cumulated growth from 2002 to 2012 was estimated through ring-width data, radial growth varied from 0.17 to 2.70 cm (median = 0.97, mean = 1.03, s.d. = 0.57), while BAI varied from 7.8 to 805.89 cm2 (median = 126.30, mean = 180.07, s.d. = 172.7). Moreover, for these 90 cored trees, the correlation between inventory-based and ring-width-based radial growth was 0.84 (P < 0.001), while the correlation between inventory-based and ring-width-based BAI was 0.68 (P < 0.001). The lower correlation for BAI values was due to the largest trees, for which inventory data generally underestimated growth (Supplementary Data Fig. S6).
Ecological drivers of fecundities and growth
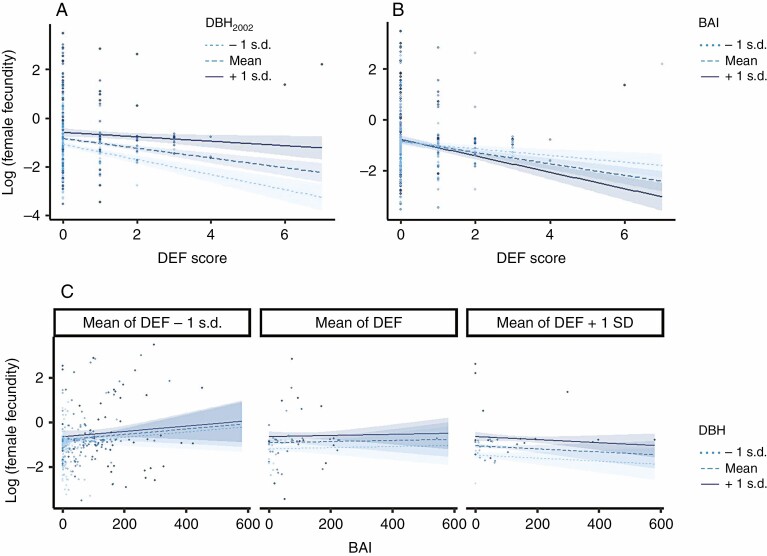
Defoliation, size and competition overall explained a significant part of the variation in growth (61 %) and female fecundity (12 %), while competition alone was found to marginally explain a small part of the variation in male fecundity (<1 %). In the whole data set of 432 individuals, the most parsimonious model showed that female fecundity significantly decreased with defoliation and competition (mediated by competition in a radius of 10 m), while it increased with DBH2002 and density (mediated by density in a radius of 10 m; Table 1A). For instance, the 10 % of trees with the largest DBH were associated with 36 % of the total female fecundity. The 10 % of trees with the lowest competition index were associated with 27 % of the total female fecundity. Moreover, the interaction between DEF and DBH2002 was significant, reflecting a weaker negative effect of defoliation on female fecundity as tree size increased (Fig. 3A). By contrast, male fecundity was only marginally (and negatively) affected by competition (mediated by density in a radius of 5 m; Table 1B). Finally, growth (as measured by BAI) significantly decreased with defoliation and competition (mediated by competition in a radius of 7 m), and increased with DBH2002 and density (mediated by density in a radius of 10 m; Table 1C). We did not find significant interactions between defoliation and size on growth. For all fitted models, VIFs (Table 1) were all <10, ruling out any serious multicollinearity issue. Diagnostic plots confirmed the quality of the fitted models (Supplementary Data Fig. S7).
Table 1.
Analysis of variance table for (A) female fecundity (F♀), (B) male fecundity (F♂) and (C) BAI in response to ecological determinants included in eqn (3). Results of the most parsimonious model are shown: its adjusted R2, the type-III sum of squares (SSQ) and degrees of freedom associated with each term. For each predictor, we give the estimate of its effect, the standard error and associated t and P-values. VIFs were computed with R package ‘CAR’. All the response variables were log-transformed. Results are based on the whole data set of 432 individuals for F♀ and F♂, and on the 341 individuals with non-null BAI for BAI
| Predictor | R 2 | SSQ | d.f. | Estimate | s.e. | T | P-value | VIF |
|---|---|---|---|---|---|---|---|---|
| (A) Log(F♀) | 0.12 | <0.001 | ||||||
| DEF | 9.33 | 1 | −0.349 | 0.111 | −3.148 | 0.002 | 4.13 | |
| DBH2002 | 11.97 | 1 | 0.013 | 0.004 | 3.564 | <0.001 | 2.19 | |
| Compet10 | 7.9 | 1 | −0.033 | 0.011 | −2.896 | 0.004 | 1.43 | |
| Dens10 | 7.46 | 1 | 0.010 | 0.003 | 2.815 | 0.005 | 1.35 | |
| DEF:DBH2002 | 7.28 | 1 | 0.006 | 0.002 | 2.780 | 0.006 | 4.61 | |
| Residuals | 401.29 | 426 | ||||||
| (B) Log(F♂) | 0.004 | 0.097 | ||||||
| Dens5 | 1.695 | 1 | −0.01 | 0.01 | −1.662 | 0.10 | – | |
| Residuals | 263.854 | 430 | ||||||
| (C) log(BAI) | 0.61 | <0.001 | ||||||
| DEF | 4.89 | 1 | −0.153 | 0.058 | −2.646 | 0.00853 | 1.05 | |
| DBH2002 | 139.39 | 2 | 15.494 | 1.167 | 13.275 | <0.001 | 1.21 | |
| (DBH2002)2 | −5.539 | 0.886 | −6.251 | <0.001 | ||||
| Compet7 | 20.92 | 1 | −0.090 | 0.016 | −5.473 | <0.001 | 1.27 | |
| Dens14 | 4.78 | 1 | 0.005 | 0.002 | 2.617 | 0.00927 | 1.16 | |
| Residuals | 234.02 | 335 |
Italic type correspond to the statistics (R², P) associated with the model itself.
Compet7, Compet10, Compet14, competition index within 7, 10 and 14 m, respectively; Dens5, Dens10, Dens14, number of neighbours within 5, 10 and 14m, respectively.
Fig. 3.
Interaction plots for (A) DEF and DBH2002 effects on female fecundity, (B) DEF and BAI effects on female fecundity, and (C) BAI, DBH2002 and DEF effects on female fecundity. Regression lines are plotted for three values of each moderator variable, corresponding to ±1 s.d. from the mean. Confidence interval at 80 % are shown around each regression line. Points are the observations.
To compare the effects of defoliation on fecundity and growth, we centred and normalized female fecundity and BAI, and ran the best models for each response variable. The average decline in response to a 1-unit increase in DEF was −0.06 for female fecundity (s.e. = 0.10; measured in the standard unit of the trait) versus −0.10 for BAI (s.e. = 0.04).
Joint defoliation effects on female fecundity and growth
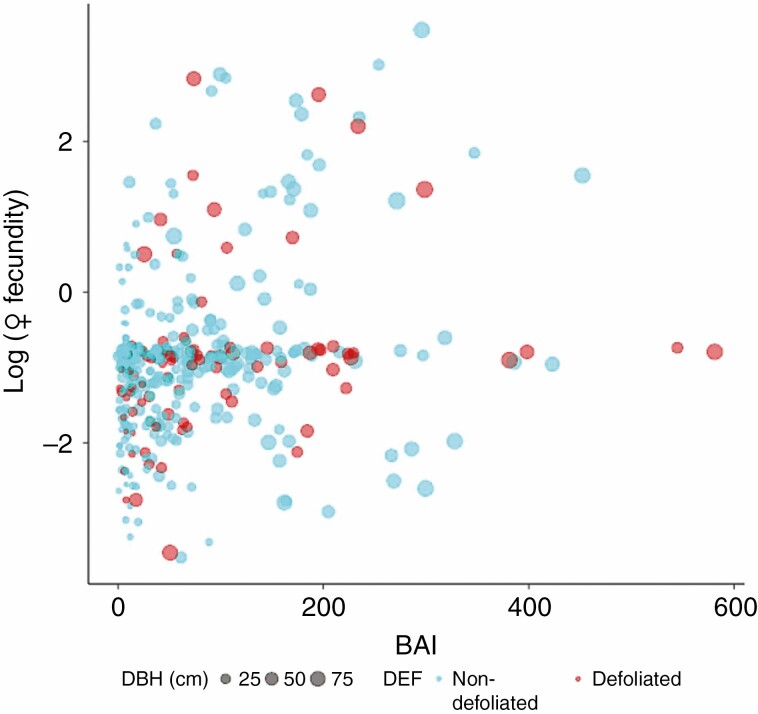
The raw female fecundities and BAIs were significantly and positively correlated in the 337 non-defoliated trees (ρ = 0.31, P < 0.001), but not in the 95 defoliated trees (ρ = 0.13, P = 0.2; Fig. 4).
Fig. 4.
Correlation between growth, measured by BAI and female fecundity plotted on a log scale. The size of the dots is proportional to tree diameter (DBH2002). This is a scatter plot of raw data, not of model predictions.
The linear model for female fecundity including BAI as a predictor [eqn (4)] allowed us to disentangle the effects of defoliation, size and competition on the relationship between female fecundity and growth. In addition to the previous effects, a significant interaction between BAI and defoliation was detected (Table 2): female fecundity overall decreased with increasing defoliation, but this decrease was faster and stronger for trees with a higher BAI (Fig. 3B). The complex interaction between BAI, DEF and DBH2002 on female fecundity resulted in a defoliation-dependent trade-off between growth (as estimated by BAI) and female fecundity. The female fecundity of the non-defoliated trees (Fig. 3C, leftmost panel) increased with BAI (no trade-off), whereas the female fecundity of the most defoliated trees (Fig. 3C, rightmost panel) decreased with increasing BAI (trade-off). Moreover, the female fecundity of small trees (Supplementary Data Fig. S8, left panel) always decreased in response to increasing defoliation, whatever their BAI, whereas the female fecundity of large trees (Supplementary Data Fig. S8, right panel) did not change in response to increasing DEF. This last result, combined with the observed trade-off between female fecundity and growth in response to defoliation (Fig. 3C, right panel), suggests that some large trees maintained high female fecundity under stressful conditions at the expense of reduced growth. Diagnostic plots confirmed the quality of the fitted models (Supplementary Data Fig. S9).
Table 2.
Analysis of variance table for female fecundity in response to ecological determinants included in eqn (4). Results are based on the whole data set of 432 individuals. See Table 1 legend for further explanation
| Predictor | R 2 | SSQ | d.f. | Estimate | s.e. | t | P-value | VIF |
|---|---|---|---|---|---|---|---|---|
| 0.13 | <0.001 | |||||||
| BAI | 0.56 | 1 | 0.001 | 0.001 | 0.773 | 0.440 | 2.468 | |
| DEF | 11.04 | 1 | −0.384 | 0.112 | −3.433 | 0.001 | 4.231 | |
| DBH | 6.24 | 1 | 0.011 | 0.004 | 2.582 | 0.010 | 3.171 | |
| Compet10 | 7.49 | 1 | −0.032 | 0.011 | −2.828 | 0.005 | 1.444 | |
| Dens10 | 6.85 | 1 | 0.009 | 0.003 | 2.704 | 0.007 | 1.354 | |
| BAI:DEF | 4.09 | 1 | −0.001 | 0.001 | −2.091 | 0.037 | 2.857 | |
| DEF:DBH | 11.39 | 1 | 0.009 | 0.003 | 3.488 | 0.001 | 6.750 | |
| Residuals | 397.03 | 424 |
Italic type correspond to the statistics (R², P) associated with the model itself.
DISCUSSION
The results of our study supported the hypothesis of an overall negative effect of climatic stresses on reproduction and growth in the study beech population. Moreover, the declines in growth and fecundity in response to stress varied between individuals: some defoliated trees maintained significant female fecundity at the expense of reduced growth, while others maintained high growth at the expense of reduced female fecundity. Hypothesis H1 (a coordinated decrease in growth and reproduction in response to stress) was thus rejected. We discuss below the ecological and physiological processes underlying these patterns, and their long-term consequences for the adaptive response of the beech to water stress, before summarizing the methodological insights of our study.
Climate stress reduced and generated a trade-off between female fecundity and growth
This study is among the rare ones to bring observational evidence that increasing crown defoliation decreases individual female fecundity. This negative effect of climate stress on tree reproductive performances is consistent with the few diachronic studies based on time-series data sets, which reported a reduction over time of seed production in European Mediterranean ecosystems, attributed to increasing drought duration and severity (Cecich and Sullivan, 1999; Pérez-Ramos et al., 2010). These findings are also supported by the few results available so far from experiments manipulating stresses in situ. For instance, by manipulating temperature during pollen dispersal and germination, Flores-Rentería et al. (2018) demonstrated negative impacts of high temperatures on the pollen viability of Pinus edulis. Bykova et al. (2018) showed that water deficit increases pollen abortion in Quercus ilex. Also in Q. ilex, Pérez-Ramos et al. (2010) showed that reduced water availability increased the rate of acorn abortion, while Sánchez-Humanes and Espelta (2011) showed that increased drought reduces acorn production. However, the mechanisms mediating climate effects on reproduction may differ strongly between species, and even between Quercus and Fagus genera (Journé et al., 2021a), and we still lack studies testing how different climate variables affect the stages of the reproductive cycle of the beech.
Our results also indicated a negative effect of climate stress on tree growth, which has been reported by many previous studies (Zhao and Running, 2010; Zimmermann et al., 2015). There is still some debate on the physiological processes causing radial growth variations in response to drought stress (Hayat et al., 2017; Mund et al., 2020). High temperatures and low precipitation undoubtedly directly affect photosynthesis and hydraulic conductance, inducing source limitations to tree growth and reproduction. However, at the same time the large number of physiological processes involved in the response to drought (including meristem and cell growth rates) and the time-sequencing of physiological responses throughout successive annual cycles (with carry-over effects) result in a complex system of sink and source limitations driven by drought conditions (Mund et al., 2020). In particular, growth is under direct environmental control, such as through water limitation, is not determined only by the amount of available carbon, and stops before photosynthesis with increasing drought (Eckes-Shephard et al., 2021).
Another major finding of this study was the negative correlation between growth and reproduction for defoliated trees, contrasting with the positive correlation for non-defoliated trees. Several studies have tested the existence of a negative correlation between growth and reproduction at the individual level, as a signature of the possible trade-off between these functions. The key assumption underlying this trade-off is that reproduction is costly and competes with growth for resources (Obeso, 1988; Koenig and Knops, 1998; Thomas, 2011). By contrast, the absence of correlation is usually interpreted as independence between these functions in terms of the resource pool (Obeso, 1988; Knops et al., 2007; Pulido et al., 2014). A trade-off between growth and reproduction has already been found for beech (Hacket-Pain, 2017, 2018; Lebourgeois et al., 2018). Hacket-Pain et al. (2017) found that masting years (i.e. years with high seed production) are negatively correlated with growth and that this trade-off is more pronounced during drought years due to resource scarcity.
These different results hence support the general idea that the correlation between reproduction and growth depends on the level of resources available (van Noordwijk and de Jong, 1986; Obeso, 1988), a trade-off being present only under limiting resources, i.e. crown defoliation in our case. Moreover, the detailed analysis of the interactions between defoliation, size and growth on female fecundity showed that those defoliated trees maintaining high female fecundity were the largest ones, suggesting that crown defoliation could shift the allocation of carbon to reproduction above a given tree size (Genet et al., 2010; Thomas, 2011). Besides the literature on forest seed orchards and fruit trees orchards, one of the rare studies supporting this hypothesis is that of Wiley et al. (2017), who experimentally defoliated black oak, a tree species that matures its acorns over 2 years. Recovery following defoliation was shown to involve substantial allocation shifts, with carbohydrate storage and already initiated reproduction cycles (i.e. maturation of 2-year acorns) being favoured relative to growth and new reproductive cycles (i.e. flowering and production of new 1-year acorns).
Long-term consequences for adaptive response of beech to stress
This study showed that defoliated beech trees reduce their fecundity and growth in varying proportions depending on the individual. Some trees maintained significant female fecundity at the expense of reduced growth (i.e. flight behaviour as defined by Lauder et al., 2019), while other trees showed the reverse, i.e. fight behaviour, where growth is maintained at the expense of reduced female fecundity. Moreover, as male fecundity was insensitive to crown defoliation, the defoliated trees that were subject to less intra-specific competition also contributed to reproduction through male function. Hence, we can reject hypothesis H1, that the relationship between reproduction and growth does not change with increasing crown defoliation. Note, however, that the alternative hypothesis, H2 (defoliation or stresses act like a cue stimulating reproductive performances at the expense of reduced growth), was only supported for some individuals.
This response to stress could have major consequences for the short-term evolutionary dynamics of the local population. Indeed, assuming that at least some of the traits underlying vulnerability to stresses are under genetic control, we showed here that the most vulnerable individuals (those that are the most impacted by stress) still contribute to regeneration, which could lead the population to evolve traits compromising its adaptation to stress. By contrast, if the defoliated individuals showed a coordinated decrease in their growth and reproduction (H1), their potentially non-adapted genotypes could be purged more efficiently. Still, the fact that the study population is composed of a diversity of individuals that promote either growth or reproduction in response to stress could also give it an adaptive advantage in the long term, depending on the climate change scenario that will actually take place. For example, if climatic conditions become so difficult that all trees eventually decline, and that even the most ‘fighting’ individuals can neither grow nor survive, then the flight strategy may be the only viable one (e.g. by migrating to higher altitudes and establishing new populations).
Demonstrating that these fight and flight behaviours actually occur as adaptive strategies and deriving demo-genetic scenarios for the adaptive response of the population to stress would, however, require further investigations. The coexistence within the same population of several adaptive strategies for the allocation to reproduction versus growth in response to stress may be the rule rather than the exception (Bontemps et al., 2017), and could be the result of ongoing, antagonistic selection within genetically diverse tree populations (Hampe and Petit, 2005). However, demonstrating the existence of these strategies would first require disentangling how genetic factors versus microenvironment variation (stress exposure) and ontogeny each contribute to the intra-individual variation in allocation to reproduction versus growth. Then, projections of possible evolutionary changes in response to drought stress would need to be quantitatively investigated (Hamanishi and Campbell, 2011).
Methodological insights
Our synchronic approach, comparing reproductive performances among trees with different levels of defoliation, thus usefully complements other observational, diachronic studies based on the analysis of reproduction and climate time series. However, it relies on several hypotheses, which need to be carefully evaluated. First, we assumed that crown defoliation is an indicator of higher intrinsic sensitivity to drought stresses, and/or a higher impact of drought stresses due to lower availability of resources in a heterogeneous environment. The companion study of Petit-Cailleux et al. (2020) supports this hypothesis. Still, our qualitative measure of defoliation suffered some limitations. Being the sum over 9 years of annual binary scores (0, no defoliation; 1, major dead branch or foliage loss), our DEF variable was likely to be informative about the recurrence of defoliation, but less informative about its intensity (in terms of percentage of the crown affected). Although out of the scope of this study, a better quantification of the intensity of defoliation would allow a more thorough investigation of the effects of defoliation on growth and reproduction.
Second, we used a spatially explicit mating model (MEMM) and marker-based parentage analyses to estimate individual fecundity, which had both advantages and drawbacks. Unlike traditional resource-based estimates of seed/pollen production, MEMM estimates of fecundity have the main advantage of integrating several processes of the regeneration loop. Indeed, being evaluated after mating, dispersal, germination and possibly early mortality preceding the sampling stage, MEMM estimates are considered as better proxies of fitness than seed/pollen biomass or numbers (Oddou-Muratorio et al., 2018). Note that MEMM estimates account specifically for individual effects (either maternal or genetic) that affect survival independently on location; for instance, two trees producing the same number of seeds (same basic fecundity) but with different intrinsic quality of germination or survival, are expected to have different MEMM estimates of fecundity. By contrast, MEMM estimates are expected to be insensitive to spatial processes such as density-dependent mortality affecting seedling survival depending on their location (Klein et al., 2013). Early mortality is massive in trees, and has been shown to reshuffle the reproductive success ranking (but not the MEMM fecundity ranking) of adult oak trees, where gravity-dispersed seedlings suffer higher density-dependent mortality than seedlings dispersed over a long distance (Gerzabek et al., 2017). Finally, a main drawback of the MEMM is that it estimates effective amounts of pollen or seed produced by each individual relative to other plants; these relative fecundities hence convey no information on the absolute contribution of defoliated or non-defoliated individuals to regeneration, and thus on the demographic impact of defoliation. Still, it should be noted that recruitment and seed production are abundant in the study site, despite its marginality and the presence of declining trees. These overall good reproduction abilities ensure that our relative measures of fecundity are not biased by overall very low recruitment.
In conclusion, this study brings new insights on the reproductive and adaptive response to stress of European beech, by investigating the inter-individual variation in the impact of stress-induced defoliation on fecundity and wood growth within a natural population at the warm, dry margin of the species’ distribution. Elucidating the causal processes underlying these relationships deserves further investigation (Supplementary Data Fig. S10). In particular, accounting for the non-observed level of resource allocated to reproduction and growth would require either an estimation of this resource (e.g. Mund et al., 2020) or specific methodological approaches [e.g. path analyses (Shipley, 2016) or other Bayesian tools introducing the level of resource as a latent variable (Journé et al., 2021b)]. Our study overall stresses the need to investigate simultaneously the impact of climate change on reproduction, growth and survival, and how the inter-individual variations in these responses may affect the adaptation or maladaptation of forest tree populations in the face of climate change.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: genetic data for the adult and offspring population. Table S2: size, competition and defoliation variables measured in adult trees. Figure S1: climate characteristics of the study site. Figure S2: patterns of covariation among competition index and density. Figure S3: sampling design for the 90 cored individuals. Figure S4: preliminary check of the quality of linear models. Figure S5: effect of size and competition on defoliation. Figure S6: relationship between growth estimated from ring width and growth estimated from inventory data. Figure S7: diagnostic plot for the linear regression model described by eqn (3). Figure S8: interaction plots for DEF, BAI and DBH2002 effects on female fecundity. Figure S9: diagnostic plot for the linear regression model described by eqn (4). Figure S10: conceptual framework for the impacts of abiotic and biotic stresses on individual plant performance.
ACKNOWLEDGEMENTS
We are grateful to our colleagues Tonya Lander, Francois Lefèvre and Etienne K. Klein, as well as to PCI Ecology editor Georges Kunstler, Annals of Botany handling editor Alex Fajardo and five anonymous reviewers for discussions and comments on previous versions of this manuscript. We also thank Nicolas Mariotte (INRA URFM, Avignon) for wood core sampling, Jean Thevenet (INRA UEFM, Avignon) for sample management, and Marianne Correard (INRA UEFM, Avignon) for Fig. 2. The MEMM software for estimating female and male fecundities is available at https://informatique-mia.inra.fr/biosp/memm. The data set analysed is available online under the zenodo platform (DOI: 10.5281/zenodo.3516305). More detailed data (genotypes) and R scripts for statistical analyses are available from the corresponding author. A previous version of this manuscript (available at bioRxiv, 10.1101/474874) has been peer-reviewed and recommended by Peer Community In Ecology (https://doi.org/10.24072/pci.ecology.100033). Author contributions: S.O.M. designed the study with E.M., analysed the data and wrote the manuscript. J.A.M., J.G., E.M. and C.H. mapped and measured the trees and sampled the seedlings. H.D. and C.P. measured and analysed ring-width data. M.L. analysed genetic data with S.O.M. All the authors contributed to improvement of the manuscript.
FUNDING
This work was partly supported by the European Union ERA-NET BiodivERsA program (TIPTREE project, grant number BiodivERsA2-2012–15) and by the French Agence Nationale de la Recherche (MeCC project, grant number ANR-13-ADAP-0006).
DATA AVAILABILITY
The data set analysed is available online under the zenodo platform (https://doi.org/10.5281/zenodo.3516305).
LITERATURE CITED
- Adams HD, Zeppel MJB, Anderegg WRL, et al. 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature Ecology & Evolution 1: 1285–1291. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Anderegg WRL, Anderegg LDL, Kerr KL, Trugman AT. 2019. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Global Change Biology 25: 3793–3802. [DOI] [PubMed] [Google Scholar]
- Beckage B, Lavine M, Clark JS. 2005. Survival of tree seedlings across space and time: estimates from long-term count data. Journal of Ecology 93: 1177–1184. [Google Scholar]
- Bigler C, Bugmann H. 2018. Climate-induced shifts in leaf unfolding and frost risk of European trees and shrubs. Scientific Reports 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdziewicz M, Kelly D, Thomas PA, Lageard JGA, Hacket-Pain A. 2020. Climate warming disrupts mast seeding and its fitness benefits in European beech. Nature Plants 6: 88–94. [DOI] [PubMed] [Google Scholar]
- Bonnet-Masimbert M, Webber JE. 2012. From flower induction to seed production in forest tree orchards. Tree Physiology 15: 419–426. [DOI] [PubMed] [Google Scholar]
- Bontemps A, Klein EK, Oddou-Muratorio S. 2013. Shift of spatial patterns during early recruitment in Fagus sylvatica: evidence from seed dispersal estimates based on genotypic data. Forest Ecology and Management 305: 67–76. [Google Scholar]
- Bontemps A, Davi H, Lefèvre F, Rozenberg P, Oddou-Muratorio S. 2017. How do functional traits syndromes covary with growth and reproductive performance in a water-stressed population of Fagus sylvatica? Oikos 126: 1472–1483. [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. 2006. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science 63: 625–644. [Google Scholar]
- Briceño-Elizondo E, Garcia-Gonzalo J, Peltola H, Matala J, Kellomäki S. 2006. Sensitivity of growth of Scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. Forest Ecology and Management 232: 152–167. [Google Scholar]
- Burczyk J, Adams WT, Birkes DS, Chybicki IJ. 2006. Using genetic markers to directly estimate gene flow and reproductive success parameters in plants on the basis of naturally regenerated seedlings. Genetics 173: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykova O, Chuine I, Morin X, Higgins SI. 2012. Temperature dependence of the reproduction niche and its relevance for plant species distributions. Journal of Biogeography 39: 2191–2200. [Google Scholar]
- Bykova O, Limousin J-M, Ourcival J-M, Chuine I. 2018. Water deficit disrupts male gametophyte development in Quercus ilex. Plant Biology 20: 450–455. [DOI] [PubMed] [Google Scholar]
- Caignard T, Kremer A, Firmat C, Nicolas M, Venner S, Delzon S. 2017. Increasing spring temperatures favor oak seed production in temperate areas. Scientific Reports 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. 2015. To die or not to die: early warnings of tree dieback in response to a severe drought. Journal of Ecology 103: 44–57. [Google Scholar]
- Cecich RA, Sullivan NH. 1999. Influence of weather at time of pollination on acorn production of Quercus alba and Quercus velutina. Canadian Journal of Forest Research 29: 1817–1823. [Google Scholar]
- Charrier G, Ngao J, Saudreau M, Améglio T. 2015. Effects of environmental factors and management practices on microclimate, winter physiology, and frost resistance in trees. Frontiers in Plant Science 6: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JS, Beckage B, Camill P, et al. 1999. Interpreting recruitment limitation in forests. American Journal of Botany 86: 1–16. [PubMed] [Google Scholar]
- Clark JS, Andrus R, Aubry-Kientz M, et al. 2021. Continent-wide tree fecundity driven by indirect climate effects. Nature Communications 12: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JK, Rush S, Jennetten P. 1996. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). I. Selection through lifetime female fitness. Evolution 50: 1127–1136. [DOI] [PubMed] [Google Scholar]
- Dobbertin M. 2005. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. European Journal of Forest Research 124: 319–333. [Google Scholar]
- Eckes-Shephard AH, Tiavlovsky E, Chen Y, Fonti P, Friend AD. 2021. Direct response of tree growth to soil water and its implications for terrestrial carbon cycle modelling. Global Change Biology 27: 121–135. [DOI] [PubMed] [Google Scholar]
- Elle E, Meagher TR. 2000. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist 156: 622–636. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez M, Belmonte J, Maria Espelta J. 2012. Masting in oaks: disentangling the effect of flowering phenology, airborne pollen load and drought. Acta Oecologica 43: 51–59. [Google Scholar]
- Flores-Rentería L, Whipple AV, Benally GJ, Patterson A, Canyon B, Gehring CA. 2018. Higher temperature at lower elevation sites fails to promote acclimation or adaptation to heat stress during pollen germination. Frontiers in Plant Science 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2019. An R companion to applied regression, 3rd edn. Thousand Oaks, CA: Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- Galiano L, Martínez-Vilalta J, Lloret F. 2011. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytologist 190: 750–759. [DOI] [PubMed] [Google Scholar]
- Genet H, Bréda N, Dufrêne E. 2010. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiology 30: 177–192. [DOI] [PubMed] [Google Scholar]
- Gerzabek G, Oddou-Muratorio S, Hampe A. 2017. Temporal change and determinants of maternal reproductive success in an expanding oak forest stand. Journal of Ecology 105: 39–48. [Google Scholar]
- Goto S, Shimatani K, Yoshimaru H, Takahashi Y. 2006. Fat-tailed gene flow in the dioecious canopy tree species Fraxinus mandshurica var. japonica revealed by microsatellites. Molecular Ecology 15: 2985–2996. [DOI] [PubMed] [Google Scholar]
- Hacket-Pain AJ, Lageard JGA, Thomas PA. 2017. Drought and reproductive effort interact to control growth of a temperate broadleaved tree species (Fagus sylvatica). Tree Physiology 37: 744–754. [DOI] [PubMed] [Google Scholar]
- Hacket-Pain AJ, Ascoli D, Vacchiano G, et al. 2018. Climatically controlled reproduction drives interannual growth variability in a temperate tree species. Ecology Letters 21: 1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanishi ET, Campbell MM. 2011. Genome-wide responses to drought in forest trees. Forestry 84: 273–283. [Google Scholar]
- Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8: 461–467. [DOI] [PubMed] [Google Scholar]
- Hayat A, Hacket-Pain AJ, Pretzsch H, Rademacher TT, Friend AD. 2017. Modeling tree growth taking into account carbon source and sink limitations. Frontiers in Plant Science 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. 2009. Global warming and sexual plant reproduction. Trends in Plant Science 14: 30–36. [DOI] [PubMed] [Google Scholar]
- Journé V, Caignard T, Hacket-Pain A. 2021a. Leaf phenology correlates with fruit production in European beech (Fagus sylvatica) and in temperate oaks (Quercus robur and Quercus petraea). European Journal of Forest Research. doi: 10.1007/s10342-021-01363-2. [DOI] [Google Scholar]
- Journé V, Papaïx J, Walker E, et al. 2021. b. A hierarchical Bayesian model to investigate trade-offs between growth and reproduction in a long-lived plant. Ecosphere. doi: 10.1101/2021.01.26.428205. [DOI] [Google Scholar]
- Jump AS, Mátyás C, Peñuelas J. 2009. The altitude-for-latitude disparity in the range retractions of woody species. Trends in Ecology & Evolution 24: 694–701. [DOI] [PubMed] [Google Scholar]
- Karimi F, Igata M, Baba T, et al. 2017. Summer pruning differentiates vegetative buds to flower buds in the rabbiteye blueberry (Vaccinium virgatum Ait.). Horticulture Journal 86: 300–304. [Google Scholar]
- Klein EK, Desassis N, Oddou-Muratorio S. 2008. Pollen flow in the wildservice tree, Sorbus torminalis (L.) Crantz. IV. Whole interindividual variance of male fecundity estimated jointly with the dispersal kernel. Molecular Ecology 17: 3323–3336. [DOI] [PubMed] [Google Scholar]
- Klein EK, Bontemps A, Oddou-Muratorio S. 2013. Seed dispersal kernels estimated from genotypes of established seedlings: does density-dependent mortality matter? Methods in Ecology and Evolution 4: 1059–1069. [Google Scholar]
- Knops JM, Koenig WD, Carmen WJ. 2007. Negative correlation does not imply a tradeoff between growth and reproduction in California oaks. Proceedings of the National Academy of Sciences of the USA 104: 16982–16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig WD, Knops JMH. 1998. Scale of mast-seeding and tree-ring growth. Nature 396: 225–226. [Google Scholar]
- Lauder JD, Moran EV, Hart SC. 2019. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiology 39: 1071–1085. [DOI] [PubMed] [Google Scholar]
- Lebourgeois F, Delpierre N, Dufrêne E, et al. 2018. Assessing the roles of temperature, carbon inputs and airborne pollen as drivers of fructification in European temperate deciduous forests. European Journal of Forest Research 137: 349–365. [Google Scholar]
- Lee TD. 1988. Patterns of fruit and seed production. In: Lovett-Doust J, Lovett-Doust L, eds. Plant reproductive ecology: patterns and strategies. New York: Oxford University Press, 179–202. [Google Scholar]
- Long JA. 2020. jtools: Analysis and Presentation of Social Scientific Data. R package version 2.1.0.https://cran.r-project.org/package=jtools. [Google Scholar]
- Martin GL, Ek AR. 1984. A comparison of competition measures and growth models for predicting plantation red pine diameter and height growth. Forest Science 30: 731–743. [Google Scholar]
- McDowell NG, Allen CD, Anderson-Teixeira K, et al. 2020. Pervasive shifts in forest dynamics in a changing world. Science 368: eaaz9463. [DOI] [PubMed] [Google Scholar]
- Meilan R. 1997. Floral induction in woody angiosperms. New Forests 14: 179–202. [Google Scholar]
- Moran EV, Clark JS. 2011. Estimating seed and pollen movement in a monoecious plant: a hierarchical Bayesian approach integrating genetic and ecological data. Molecular Ecology 20: 1248–1262. [DOI] [PubMed] [Google Scholar]
- Mund M, Herbst M, Knohl A, et al. 2020. It is not just a ‘trade-off’: indications for sink- and source-limitation to vegetative and regenerative growth in an old-growth beech forest. New Phytologist 226: 111–125. [DOI] [PubMed] [Google Scholar]
- van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. American Naturalist 128: 137–142. [Google Scholar]
- Obeso JR. 1988. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Oddou-Muratorio S, Klein EK. 2008. Comparing direct vs. indirect estimates of gene flow within a population of a scattered tree species. Molecular Ecology 17: 2743–2754. [DOI] [PubMed] [Google Scholar]
- Oddou-Muratorio S, Klein EK, Austerlitz F. 2005. Pollen flow in the wildservice tree, Sorbus torminalis (L.) Crantz. II. Pollen dispersal and heterogeneity in mating success inferred from parent-offspring analysis. Molecular Ecology 14: 4441–4452. [DOI] [PubMed] [Google Scholar]
- Oddou-Muratorio S, Gauzere J, Bontemps A, Rey JF, Klein EK. 2018. Tree, sex and size: ecological determinants of male vs. female fecundity in three Fagus sylvatica stands. Molecular Ecology 27: 3131–3145. [DOI] [PubMed] [Google Scholar]
- Pearse IS, Koenig WD, Kelly D. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytologist 212: 546–562. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Boada M. 2003. A global change-induced biome shift in the Montseny mountains (NE Spain). Global Change Biology 9: 131–140. [Google Scholar]
- Pérez-Ramos IM, Ourcival JM, Limousin JM, Rambal S. 2010. Mast seeding under increasing drought: results from a long-term data set and from a rainfall exclusion experiment. Ecology 91: 3057–3068. [DOI] [PubMed] [Google Scholar]
- Petit-Cailleux C, Davi H, Lefevre F, et al. 2020. Comparing statistical and mechanistic models to identify the drivers of mortality within a rear-edge beech population. bioRxiv 645747 ver. 7 peer-reviewed and recommended by PCI Ecology. doi: 10.24072/pci.ecology.100070 [DOI]
- Pulido F, Moreno G, Garcia E, Obrador JJ, Bonal R, Diaz M. 2014. Resource manipulation reveals flexible allocation rules to growth and reproduction in a Mediterranean evergreen oak. Journal of Plant Ecology 7: 77–85. [Google Scholar]
- Quézel P, Médail F. 2003. Écologie et biogéographie des forêts du bassin méditerranéen. Paris: Elsevier. [Google Scholar]
- R Core Team . 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/. [Google Scholar]
- Sánchez-Humanes B, Espelta JM. 2011. Increased drought reduces acorn production in Quercus ilex coppices: thinning mitigates this effect but only in the short term. Forestry 84: 73–82. [Google Scholar]
- Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution 1: 103–113. [Google Scholar]
- Shipley B. 2016. Cause and correlation in biology: a user’s guide to path analysis, structural equations and causal inference with R. Cambridge: Cambridge University Press. [Google Scholar]
- Thomas PA. 2011. Age-related changes in tree growth and functional biology: the role of reproduction. In: Meinzer F, Lachenbruch B, Dawson T, eds. Tree physiology: size- and age-related changes in tree structure and function, Vol. 4. Dordrecht, Netherlands: Springer, 33–64. [Google Scholar]
- Vacchiano G, Hacket-Pain A, Turco M, et al. 2017. Spatial patterns and broad-scale weather cues of beech mast seeding in Europe. New Phytologist 215: 595–608. [DOI] [PubMed] [Google Scholar]
- Wiley E, Casper BB, Helliker BR. 2017. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. Journal of Ecology 105: 412–424. [Google Scholar]
- Zhao M, Running SW. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329: 940–943. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Hauck M, Dulamsuren C, Leuschner C. 2015. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European mixed forests. Ecosystems 18: 560–572. [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set analysed is available online under the zenodo platform (https://doi.org/10.5281/zenodo.3516305).