Abstract
Background and aims
The ovule is a synapomorphy of all seed plants (gymnosperms and angiosperms); however, there are some striking differences in ovules among the major seed plant lineages, such as the number of integuments or the orientation of the ovule. The genetics involved in ovule development have been well studied in the model species Arabidopsis thaliana, which has two integuments and anatropous orientation. This study is approached from what is known in arabidopsis, focusing on the expression patterns of homologues of four genes known to be key for the proper development of the integuments in arabidopsis: AINTEGUMENTA (ANT), BELL1, (BEL1), KANADIs (KANs) and UNICORN (UCN).
Methods
We used histology to describe the morphoanatomical development from ovules to seeds in Gnetum gnemon. We carried out spatiotemporal expression analyses in G. gnemon, a gymnosperm, which has a unique ovule morphology with an integument covering the nucellus, two additional envelopes where the outermost becomes fleshy as the seed matures, and an orthotropous orientation.
Key Results
Our anatomical and developmental descriptions provide a framework for expression analyses in the ovule of G. gnemon. Our expression results show that although ANT, KAN and UCN homologues are expressed in the inner integument, their spatiotemporal patterns differ from those found in angiosperms. Furthermore, all homologues studied here are expressed in the nucellus, revealing major differences in seed plants. Finally, no expression of the studied homologues was detected in the outer envelopes.
Conclusions
Altogether, these analyses provide significant comparative data that allows us to better understand the functional evolution of these gene lineages, providing a compelling framework for evolutionary and developmental studies of seeds. Our findings suggest that these genes were most likely recruited from the sporangium development network and became restricted to the integuments of angiosperm ovules.
Keywords: AINTEGUMENTA, BELL1, Gnetales, integument, KANADI, seed evolution, neosynangial hypothesis, UNICORN
Introduction
The seed is a synapomorphy of all extant seed plants (gymnosperms and angiosperms), and develops from an ovule or integumented megasporangium. As sporangia are conserved throughout vascular plants, the origin of the integument is a defining step in seed evolution (Brenner and Stevenson, 2006). There are some key differences in the number of integuments present in seed plants as well as accessory structures that cover seeds in gymnosperms. Due to extra structures covering the nucellus, the ovules of Gnetales (Ephedra, Gnetum, Welwitschia) are strikingly different from those of all other extant gymnosperms. In the genera Ephedra and Welwitschia two envelopes cover the nucellus, and in the genus Gnetum three envelopes cover it (Martens, 1971; Takaso, 1985; Takaso and Bouman, 1986; Endress, 1996; Rydin et al., 2010). Each of the three envelopes in Gnetum has been variously interpreted as integuments, a cupule or a megasporophyll based on comparisons with pteridosperms (extinct seed-bearing fern-like plants), other gymnosperms or angiosperms (Martens, 1971; Takaso and Bouman, 1986). In all three genera of Gnetales, it is generally agreed that the inner envelope is homologous to an integument that elongates and forms the micropyle; the homology of the additional envelopes is still debated but it is not debated that these form a seed coat unique to Gnetales (Takaso and Bouman, 1986; Ickert-Bond, 2003; Hollander and Vander Wall, 2009; Hollander et al., 2010; Ickert-Bond and Rydin, 2011). Furthermore, the relationships among seed plants (Ginkgoales, Gnetales, Cycadales, Coniferales and angiosperms) are still debated. With morphological data, Gnetales and angiosperms are sister clades, which is known as the ‘anthophyte’ hypothesis (Crane, 1985; Doyle and Donoghue, 1986, 1992; Loconte and Stevenson, 1990). This is due to extensive morphological convergences between Gnetales and angiosperms, which include the presence of an additional integumentary envelope (Crepet et al., 1991; Crane et al., 1995). On the other hand, molecular data have recovered different topologies according to the genes and analyses used, but recent analyses indicate that Gnetales are nested within Coniferales (Ruhfel et al., 2014; Wickett et al., 2014; Forest et al., 2018).
Focusing specifically on the differences between Gnetales and angiosperms, it is worth noting that while in Gnetales the initiation sequence of the envelopes is acropetal (i.e. the outer envelope develops first and then the inner envelope), in most angiosperms it is basipetal, i.e. the integument (inner envelope) develops first (Takaso, 1985; Endress, 1996; Rydin et al., 2010). On the other hand, regarding the development of the envelopes in Gnetales, the outer envelope emerges in a horseshoe shape, starting on two sides of the ovule and later becoming uniformly distributed around the entire ovule, which is known as bilobed development (Takaso, 1984, 1985). Finally, in Gnetales the integument is vascularized with four bundles (Takaso, 1985; Rydin et al., 2010) while in most angiosperms the integuments are not vascularized (Takaso, 1985; Endress, 2010, 2011; Rydin et al., 2010).
A number of functional studies, mainly focused on the model species Arabidopsis thaliana (arabidopsis; reviewed in Colombo et al., 2008), have revealed that the initiation, identity and development of the integuments involve a complex molecular circuitry that includes several transcription factors (Elliott et al., 1996; Baker et al., 1997; Skinner et al., 2004). For the development of the ovule, in arabidopsis WUSCHEL (WUS) is a key gene; it is expressed in the nucellus and generates a downstream signal for integument development (Gross-Hardt et al., 2002). In gymnosperms, the WUS homologue GgWUS/WOX5 is expressed in the nucellus and the pollen cones of Gnetum gnemon (Nardmann et al., 2009). Initially in arabidopsis, the MADS-box genes SEEDSTICK (STK), SHATTERPROOF1 (SHP1) and SHP2 act redundantly to determine the initiation of integument primordia (Favaro et al., 2003; Pinyopich et al., 2003; Brambilla et al., 2007; Losa et al., 2010), but the expression patterns of the homologue, GGM3, in Gnetum are different: it is expressed in the nucellus, integument and the pollen cones (Becker et al., 2003).
Later, in the development of the ovule, the initiation of the integument also seems to be determined by AINTEGUMENTA (ANT), the main role of which is cell proliferation, affecting the initiation and proper growth of plant organs. In ovules of ant mutants there are no integuments as integument primordia are not formed and megasporogenesis is blocked (Elliott et al., 1996; Klucher et al., 1996; Baker et al., 1997; Gasser et al., 1998). BELL1 (BEL1) is similar to ANT: bel1 mutants do not develop integuments; however, unlike ant, bel1 shows significant growth in the chalazal region, where an amorphous structure develops instead of integuments (Robinson-Beers et al., 1992; Modrusan et al., 1994; Ray et al., 1994; Reiser et al., 1995; Brambilla et al., 2007; Bencivenga et al., 2012).
Once the integuments are initiated, the planar development of the integument is controlled by another set of genes, members of the large transcription factor families KANADI (KAN) and Class III HD-ZIP (C3HDZ), which are known to determine the polarity of the lateral organs, by regulating a common set of direct target genes, many of which are linked to auxin signalling (Kerstetter et al., 2001; Izhaki and Bowman, 2007; Reinhart et al., 2013; Huang et al., 2014). In arabidopsis, there are four KANADI (KAN) paralogues, KAN1 to 4 (McAbee et al., 2006), among which KAN1 and KAN2 also play a role in the development of the outer integument, determining its abaxial polarity (Kerstetter et al., 2001; Bowman et al., 2002; Eshed et al., 2001, 2004; McAbee et al., 2006). ABERRANT TESTA SHAPE (ATS) also known as KANADI 4, determines the abaxial polarity of the inner integument, resulting in a mutant phenotype that shows the two integuments fused (Leon-Kloosterziel et al., 1994; McAbee et al., 2006; Kelley et al., 2012). UNICORN (UCN) is another gene that plays a role in the planar development of the integuments and is expressed in the outermost cell layers of the outer integument. The ucn mutants exhibit multicellular protrusions, also described as extra-integuments that develop from the outer integument (Schneitz et al., 1997; Enugutti et al., 2012, 2013).
Studies on the molecular genetics of ovule development have mainly focused on arabidopsis and a few other angiosperms (Dong et al., 2000; Yamada et al., 2003; Brown et al., 2010; Dash and Malladi, 2012; Lora et al., 2015; Skinner et al., 2016; Arnault et al., 2018). Expression studies of genes involved in the polarity of the integument in the early diverging angiosperm Amborella trichopoda suggest that this genetic network is conserved across angiosperms (Arnault et al., 2018). Although these studies in gymnosperms are still rare, in Gnetum parvifolium and Pinus thunbergii the expression of ANT homologues, GpANTL1 and PtANTL1, respectively, has been studied in the young strobili, where they were found to be expressed in the nucellus and primordia of all envelopes (Yamada et al., 2008). The study of these genes in gymnosperms is of great importance in improving our understanding of the evolution and development of the ovule. Spatiotemporal expression analyses constitute a solid tool to assess their putative function, given the lack of functional genetic tools for these taxa.
Our study focused on G. gnemon, which has a key phylogenetic position and apparent morphological similarities to angiosperms, and is fundamental for assessing the evolution of these genes in seed plants. Determining if their role in the development of the integument is conserved outside angiosperms allows us to better understand their impact on the morphological evolution of the ovules and to elucidate whether the ovule genetic network is conserved in seed plants. Moreover, an insight into the genes acting in integument development makes it possible to clarify the nature of the envelopes in G. gnemon from a molecular perspective. We have performed detailed spatiotemporal expression analyses for six homologues of the four gene families ANT, BEL1, KAN and UCN, which have been previously identified with phylogenetic analyses and named GneANT, Melbel1, GnmoKAN1 and 2, and GnmoUCN and GnmoUCN2 (Becker et al., 2002; Kim et al., 2006; Zumajo-Cardona and Ambrose, 2020).
These expression analyses made it possible to establish that the genes initially described as being involved in the development of the integument in angiosperms are not conserved in all seed plants. In turn, these genes appear to be involved in the development of the megasporangium in G. gnemon, suggesting that the ancestral function of the genes may be the development of sporangia. Furthermore, according to the expression patterns reported here in early-divergent seed plants, our results provide evidence that supports the interpretation of integuments as sterilized sporangia. Given the complexity of the ovule genetic network, it is difficult for us to extrapolate the results obtained here to other gymnosperms, and studies in representatives of Ginkgoales, Cycadales and Coniferales are still required.
MATERIALS AND METHODS
Anatomy of the strobili and ovules of Gnetum gnemon
Strobili at different developmental stages were collected from the Nolen Greenhouses at the New York Botanical Garden (Voucher 2153/2002*C; NYBG) and immediately fixed in formaldehyde–acetic acid–ethanol (FAA; 3.7 % formaldehyde, 5 % glacial acetic acid, 50 % ethanol) for 3 h. The fixed material was manually dehydrated through an alcohol–Histo-Clear II (National Diagnostics, Atlanta, GA) series and embedded in Paraplast X-TRA (Fisher Healthcare, Houston, TX, USA). The samples were sectioned at 8–12 μm with an AO Spencer 820 (GMI, MN, USA) rotary microtome. Sections were stained with Johansen’s safranin, to identify lignification, cuticle and accumulation of tannins, and 0.5 % Astra Blue and mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA). Sections were viewed with a Zeiss Axioplan compound microscope equipped with a Nikon DXM1200C digital camera with ACT-1 software.
Expression analyses by in situ hybridization
Gnetum gnemon strobili were collected as described in the preceding section for the anatomical study. After a 3-h fixation, samples were dehydrated in an ethanol series and then transferred to fresh Paraplast X-TRA and stored at 4 °C until use. Samples were sectioned on a Microm HM3555 rotary microtome at 10 μm. Sequences were initially identified through a BLAST search using arabidopsis sequences as query (BEL1 = At5g41410; KAN1 = At5g16560; KAN2 = At1g32240; KAN3 = At4g17695; KAN4 = At5g42630; UCN = At1g51170). The homology of these sequences has been established with previous phylogenetic analyses by several authors (BELL1, named Melbel1, initially characterized by Becker et al., 2002; ANT by Yamada et al., 2008; Melbel1, KANs and UCNs by Zumajo-Cardona and Ambrose, 2020). Maximum likelihood analyses with selected sequences were performed using the RaxML-BlackBox available through the Cipres Portal (Stamatakis et al., 2008; Miller et al., 2012), to corroborate the homology of the Gnetum sequences used here (Supplementary Data Figs S1 and S2). DNA templates for RNA probe synthesis were obtained by PCR amplification of 300- to 500-bp fragments. To ensure specificity, the probe templates were designed to amplify the flanks of the domains: the Melbel1 probe amplifies the 5′ sequence flanking the homeodomain; GneKAN1 amplifies the 3′ region flanking the GARP domain; the GneKAN2 sequence is incomplete but the probe was designed towards the 3′ end as well; GneUCN1 and 2 probes amplify a region at the 3′ end with no functional motifs (Supplementary Data Table S1, Fig. S3). Fragments were cleaned using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA). Digoxigenin-labelled RNA probes were prepared using T7 RNA polymerase (Roche, Switzerland), murine RNAse inhibitor (New England Biolabs, Ipswich, MA, USA) and Digoxigenin d-UTP RNA Labeling Mix (Roche, Switzerland) according to each manufacturer’s protocol. RNA in situ hybridization was performed according to Ambrose et al. (2000) and Ferrandiz et al. (1999), optimized to hybridize overnight at 55 °C. In situ hybridized sections were subsequently dehydrated and permanently mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA). Sections were viewed and photographed with a Zeiss Axioplan compound microscope equipped with a Nikon DXM1200C digital camera.
RESULTS
Anatomy of the ovules and seeds of G. gnemon
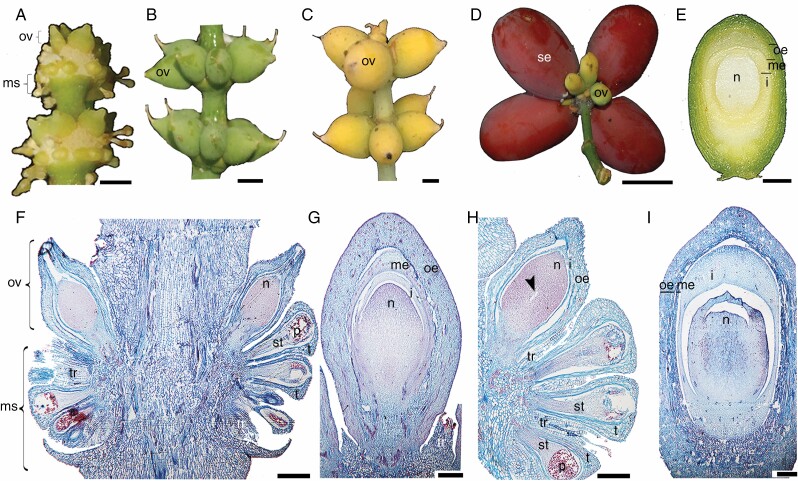
Ovules and microsporangia form on specialized structures called strobili. Strobili develop in the axil of a leaf; each strobilus consists of a long axis bearing numerous pairs of decussate bracts. Some strobili are bisexual; both ovules and microsporangia develop in the axil of the same bract, all around the axis (Chamberlain, 1935), thus forming at each node of the strobilus an upper ring of ovules and several rings of basipetal microsporangia (Fig. 1A). However, in G. gnemon some strobili are only ovulate (Fig. 1A–D; Takaso and Bouman, 1986). The ovules are characterized by an integument and two additional envelopes, resulting in three protective layers covering the megagametophyte. As the ovule matures the outermost layer changes colour from green and coriaceous (Fig. 1B) to yellow, indicating that it is ready to be pollinated (Fig. 1C). After pollination, it turns into a red fleshy seed (Fig. 1D). The layers covering the megagametophyte, going from the inside to the outside, are the integument, the middle envelope and the outer envelope (Endress, 1996; Rydin et al., 2006; Fig. 1E). The microsporangia are unilocular (Fig. 1F). The ovules that develop on a staminate strobilus (forming a bisexual strobilus) have one envelope, and hence these ovules have an integument covered by an additional envelope (Fig. 1F), whereas the ovules that develop on an ovulate strobilus have three layers covering the megagametophyte, as shown by the well-developed ovule illustrated in Fig. 1G (Beccari, 1877; Strasburger, 1879; Takaso and Bouman, 1986). A well-developed ovule has the envelopes and the integument completely developed and overtopping the nucellus. At the following stage, the megaspore mother cell is formed in the centre of the nucellus (Fig. 1H). After pollination, the nucellus begins to degenerate and the integument closes over the nucellus. The three envelopes form the seed coat: the outer envelope starts to become fleshy, leaving apparent spaces between the cells forming the ‘fleshy outer’ region of the seed; the middle envelope is sclerenchymatic, forming the ‘stony layer’ and the inner envelope (integument) compresses, forming a ‘papery layer’ (Takaso and Bouman, 1986; Fig. 1I). All layers surrounding the megagametophyte are completely separated from each other, including the integument (the inner layer), which is also separated from the megagametophyte (Fig. 1F–I). The ovules born on bisexual strobili are usually abortive; if the ovule is young it does not affect the development of microsporangia on the same node (Fig. 1F). However, if the ovule has reached an advanced stage of development, nearby microsporangia will abort while those that are further from the mature ovule will develop properly (Fig. 1H; Chamberlain, 1935).
Fig. 1.
Morphoanatomical development of the ovules in G. gnemon. (A) Strobili with a ring of ovules and rings of microsporangia developing basipetally at each node. (B, C) Ovules at different stages of development, developing on ovulate strobili. (D) Red fleshy seed. (E) Inside of the seed showing three different layers protecting the nucellus. (F) longitudinal section throughout strobili, sterile ovule with one envelope surrounding the integument and the oldest microsporangium next to the ovule. (G) Ovule with the nucellus covered by the integument, middle envelope and outer envelope. (H) Strobilus with functional ovule, where the gametophyte has reached the free nuclear stage; the functional microsporangium is at the bottom. (I) Mature ovule after fertilization. The middle envelope is fusing with the outer envelope and the nucellus has started to degenerate. i, integument; me, middle envelope; ms, microsporangia; n, nucellus; oe, outer envelope; ov, ovule; p, pollen; se, seed; st, sporogenous tissue; t, tapetum; tr, trichome. Scale bars: (A–C, E) = 0.5 cm, (D) = 1 cm, (F–H) = 50 μm, (I) = 25 μm.
Expression of AINTEGUMENTA homologue
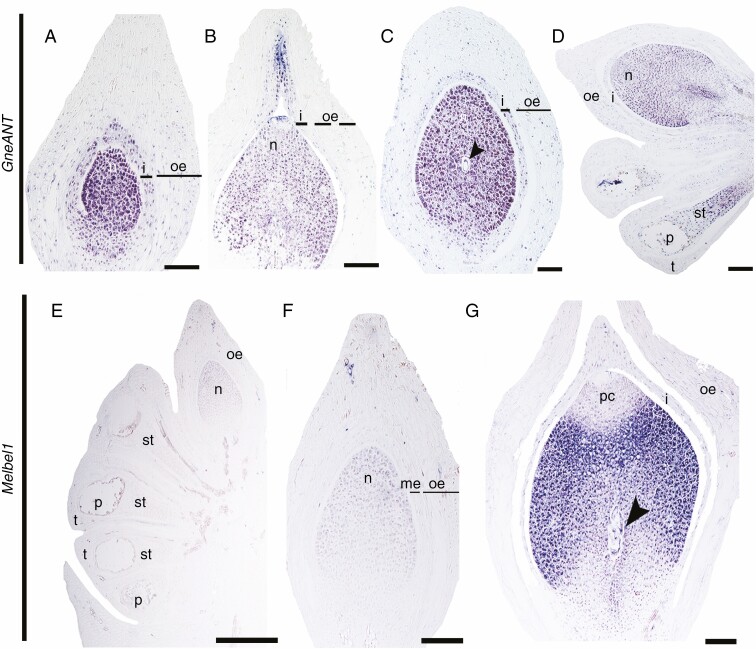
Expression analyses have been previously performed during very early stages of ovule development in Gnetum parviflorum (GpANTL1), where expression was detected in the abaxial side of all envelope primordia, integument primordia and the nucellar tip (Yamada et al., 2008). Here we investigated the expression of one ANT homologue in Gnetum that has been previously identified with phylogenetic analyses (Yamada et al., 2008; Supplementary Data Fig. S2). Due to the availability of plant material, our expression analyses focused on strobili later in development. During early stages of ovule development GneANT is expressed in the nucellus and the entire integument; there is no expression in the outer envelope (Fig. 2A). As the micropyle develops and the ovule is ready to be fertilized, expression in the integument is restricted to the apical region that forms the micropyle (Fig. 2B). GneANT expression is maintained in the nucellus and the integument throughout ovule development, including the stage when the megaspore mother cell is formed (Fig. 2C) and after meiosis (Fig. 2D). GneANT expression is also detected in the sporogenous tissue of the microsporangia and the pollen grains (Fig. 2D).
Fig. 2.
Expression analyses at different stages of ovule development using in situ hybridization. (A–D) Expression patterns of GneANT. (E–G) Expression of Melbel1. Arrowhead points to the megaspore; i, integument; me, middle envelope; n, nucellus; oe, outer envelope; ov, ovule; p, pollen; st, sporogenous tissue; t, tapetum. Scale bars: (A–C, G) = 25 μm, (D, F) = 50 μm, (E) = 100 μm.
Expression of BELL1 homologue
A homologue of BELL1 in G. gnemon, named Melbel1, has been identified by phylogenetic analyses (Becker et al., 2002). Thus, to better understand the putative function of BEL1 homologues in ovule development of G. gnemon, we performed spatiotemporal expression analyses at different stages of development. In young sterile developing ovules, Melbel1 is detected at low levels in the nucellus and pollen grains (Fig. 2E). In the next stage, as the nucellus continues growing, these expression patterns are maintained (Fig. 2F). As the ovule matures, Melbel1 is still expressed in the nucellus and in the megaspores (after meiosis). Strikingly, no expression is detected in the apical region of the nucellus, the pollen chamber or the integument or the outer envelope (Fig. 2G).
Expression of KANADI homologues
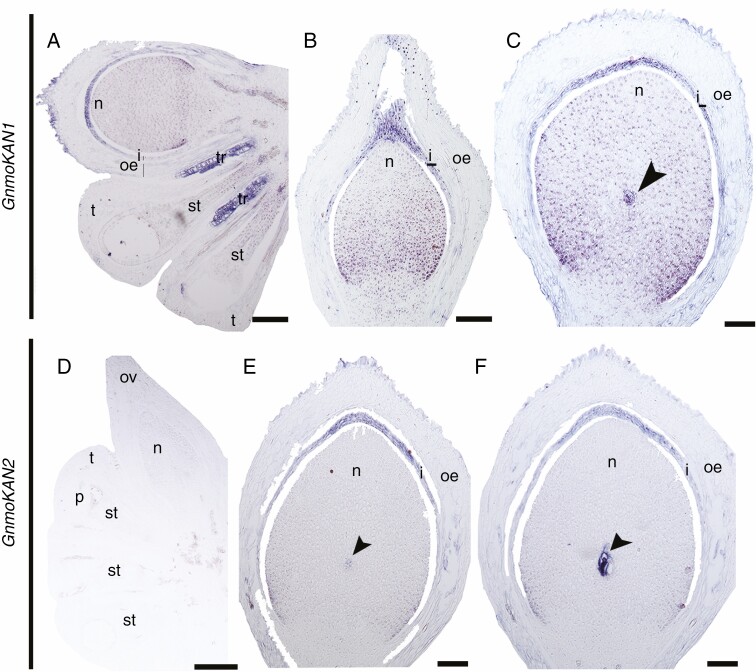
Two KAN homologues have been identified for G. gnemon, named GnmoKAN1 and GnmoKAN2 (Zumajo-Cardona and Ambrose, 2020). The position of these two sequences within gymnosperms is not yet clear, but all gymnosperm homologues are sister clades to the angiosperm-specific clades KAN1 and KAN2/3, which are also involved in integument polarity in arabidopsis (Kerstetter et al., 2001; Bowman et al., 2002; Eshed et al., 2004; McAbee et al., 2006; Zumajo-Cardona and Ambrose, 2020). With the spatiotemporal expression analyses we detected that the two G. gnemon homologues show different expression patterns, suggesting some degree of sub-functionalization (Fig. 3). GnmoKAN1 is expressed in the nucellus and in the apical portion of the integument during early stages of ovule development (Fig. 3A), and its expression patterns are maintained throughout ovule development, when the micropyle is formed (Fig. 3B), and as the ovule matures, when it is also found to be expressed in the megaspore mother cell (Fig. 3C). However, GnmoKAN2 is expressed at low levels in the nucellus of a young developing ovule (Fig. 3D). At the next stage, when the megaspore begins to develop, GnmoKAN2 is specifically expressed in the megaspore mother cell and towards the apical region of the integument (Fig. 3E). These expression patterns are maintained as the megaspore undergoes meiosis (Fig. 3F).
Fig. 3.
Expression analyses of the two KAN homologues in G. gnemon at different stages of ovule development. (A–C) Expression patterns of GneKAN1. (D–F) Expression patterns of GneKAN2. Arrowhead points to the megaspore; i, integument; me, middle envelope; n, nucellus; oe, outer envelope; ov, ovule; p, pollen; st, sporogenous tissue; t, tapetum. Scale bars: (A) = 100 μm, (B, F) = 25 μm, (C–E) = 50 μm.
Expression of UNICORN homologues
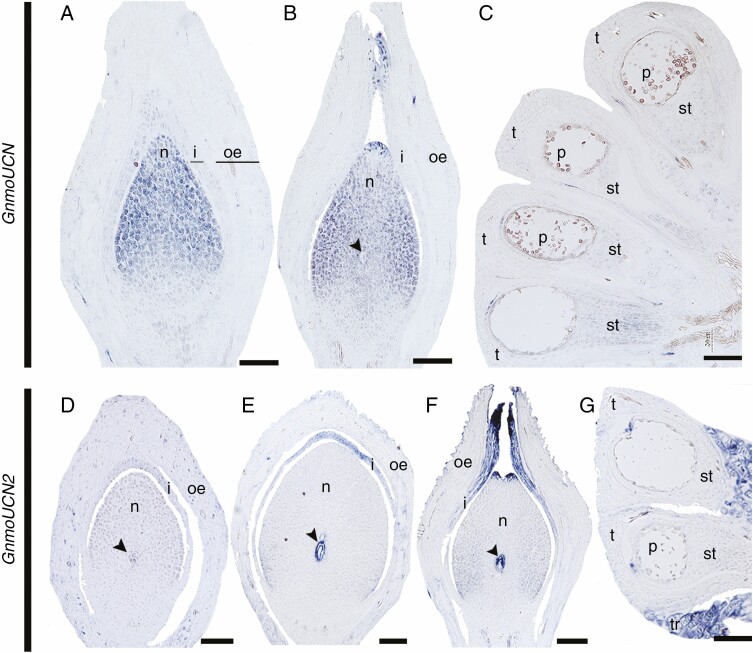
Two UCN homologues have been identified in G. gnemon, named GnmoUCN and GnmoUCN2, both belonging to the same clade within gymnosperms (Zumajo-Cardona and Ambrose, 2020). However, the two paralogues show different expression patterns (Fig. 4). GnmoUCN is expressed in the nucellus in the young developing ovule (Fig. 4A). The expression in the nucellus is maintained as the ovule develops and forms the megaspore, where it is also expressed. In addition, GnmoUCN expression is also found in the apical region of the integument forming the micropyle (Fig. 4B). Low levels of GnmoUCN expression are detected in the sporogenous tissue of the microsporangia (Fig. 4C). On the other hand, GnmoUCN2 is expressed specifically in the megaspore mother cell (Fig. 4D) and this expression is maintained as the megaspore undergoes meiosis. At this stage, GnmoUCN2 is also expressed in the apical region of the integument (Fig. 4E). These expression patterns are maintained when the micropyle begins to close; moreover, GnmoUCN2 expression is also detected in the pollen chamber, in the apical region of the nucellus, as it begins to degenerate (Fig. 4F). No expression is detected in the microsporangium (Fig. 4G).
Fig. 4.
Expression analyses of the two UCN homologues in G. gnemon at different stages of ovule development. (A–C) Expression patterns of GneUCN. (D–G) Expression patterns of GneUCN2. Arrowhead points to the megaspore; i, integument; me, middle envelope; n, nucellus; oe, outer envelope; ov, ovule; p, pollen; st, sporogenous tissue; t, tapetum. Scale bars: (A–D, G) = 50 μm, (E–F) = 25 μm.
Discussion
Within the ovule, the integument is one of the most important structures, with roles spanning from the protection of the female gametophyte to reproduction. In angiosperms, cross-talk between the integuments with the megagametophyte and with the fertilization products ensures the proper development of the seed (Figueiredo and Köhler, 2016). When the integuments become the seed coat, the cross-signalling with the endosperm ensures its coordinated growth, and in addition the seed coat ensures successful seed dispersal, protects the embryo from stress and influences dormancy, germination and seed longevity (Figueiredo and Köhler, 2014; Neuman and Hay, 2020).
The ovule is a salient synapomorphy of all seed plants, but there are major morphological differences across seed plants. Regarding Gnetum, its ovules have a unique morphology not found in any other plant. With an orthotropous orientation, its ovules have an integument, which is separated from the nucellus, and two additional envelopes that cover the ovule; the seed is fleshy, thus ensuring seed dispersal by animals (Fig. 1D; Takaso and Bouman, 1986). In contrast, the ovules of arabidopsis exhibit the typical ovule morphology of angiosperms, with two integuments completely fused to the nucellus and with anatropous orientation due to the asymmetrical growth of the outer integument (Schneitz et al., 1995). In gymnosperms, the integument plays key roles in fertilization, as the ovule directly receives the pollen. In Gnetales, the integuments elongate over the nucellus, leaving a small opening, the micropyle, producing a pollination drop on which the pollen lands; subsequently the pollination drop is withdrawn to the pollen chamber, on which the pollen germinates; at this stage the micropyle closes (Takaso and Bouman, 1986; Tomlinson et al., 1991; reviewed in Rudall, 2021).
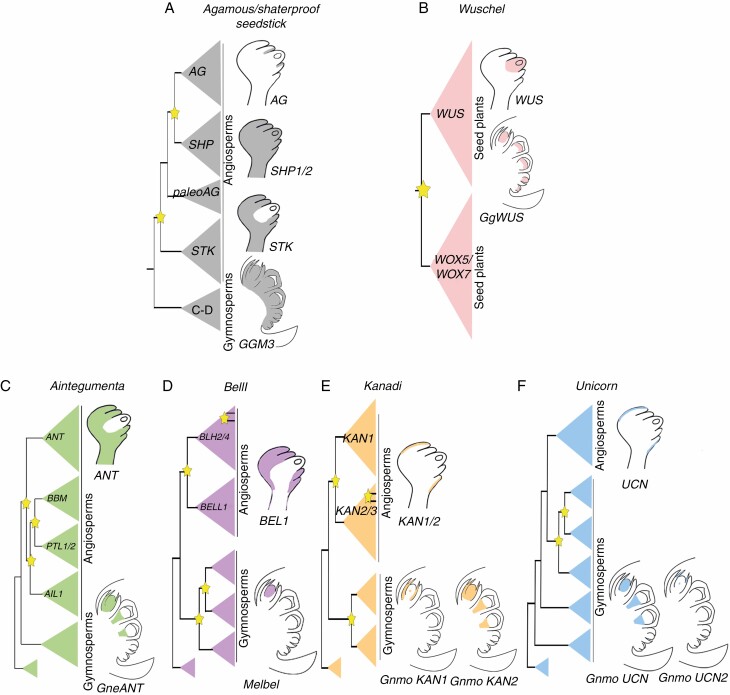
Understanding the functional evolution of the genetic network of ovule development and its impact on the morphological evolution of land plants remains difficult because it has been mainly studied in arabidopsis (Colombo et al., 2008), including ANT, BEL1, KANs and UCN genes, involved in integument development. Each of these genes belongs to a different family of transcription factors, each with a unique evolutionary history and having, in turn, multiple independent duplication events across seed plants and with homologues within land plants (Fig. 5; Kim et al., 2006; Zumajo-Cardona and Ambrose, 2020).
Fig. 5.
Schematic representation showing the expression of the different genes known to be involved in ovule development in A. thaliana and what is known so far in Gnetum spp. Information on seedless plants (ferns, lycophytes or mosses) was included when available for reproductive structures . MADS-box gene data (A) are based on Ray et al. (1994), Becker et al. (2002), Losa et al. (2010) and Pabón-Mora et al., 2014. WUSCHEL data (B) are based on Gross-Hardt et al. (2002) and Nardmann et al. (2009). Yellow stars pointing to duplication events in each gene lineage.
AINTEGUMENTA, from sporangia development in early embryophytes to integument identity in seed plants
ANT is a plant-specific gene, a member of the large transcription factor family APETALA2/ETHYLENE-RESPONSIVE ELEMENT-BINDING FACTOR (AP2/ERF; Nole-Wilson and Krizek, 2006; Kim et al., 2006). The ANT homologue in the fern Ceratopteris richardii, CerANT, is expressed in the sperm, in the archegonial neck canal just before fertilization (gametophyte structure) and in the fertilized egg, the zygote, but not in the egg cell before fertilization; expression is also detected in the fiddlehead (sporophyte; Bui et al., 2017). Overexpression of CerANT promotes apogamy, where the sporophyte develops from the gametophyte but without fertilization of the gametes (Bui et al., 2017). Expression analyses in young developing ovules in the gymnosperms P. thunbergii (PtANTL1) and G. parvifolium (GpANTL1) show expression in the nucellus and integument (Yamada et al., 2008). Similarly, we found that the expression in the nucellus and in the integument is maintained throughout ovule development in G. gnemon. These results suggest that the expression of ANT homologues is conserved in P. thunbergii and Gnetum spp. and that it is maintained throughout ovule development (Yamada et al., 2008; Fig. 2A–D). In addition, we detected expression in the pollen grains (i.e. microspores), which suggests that ANT homologues were retained in gymnosperms as key factors in the development of the megaspores and microspores (i.e. gametophyte development), similar to what is found in ferns (Fig. 2A–D).
In angiosperms, in species such as Petunia × hybrida, Antirrhinum majus (Manchado-Rojo et al., 2014) and Malus domestica (Dash and Malladi, 2012), ANT homologues have been identified and functionally characterized, showing that these genes act in the development of both integuments, or the only integument in Petunia, as well as in the control of leaf size (Dash and Malladi, 2012; Manchado-Rojo et al., 2014). Likewise, in arabidopsis the ant mutants do not develop integuments, megasporogenesis is blocked, the number of floral organs decreases, and the size of the leaves is smaller (Elliott et al., 1996; Klucher et al., 1996; Baker et al., 1997; Gasser et al., 1998). Studies in arabidopsis showed that pleiotropic roles of ANT in plant development are the result of its control over cell proliferation during organogenesis, affecting initiation, growth and intrinsic organ size, including ovules and floral organs as well as shoots and leaves (Elliott et al., 1996; Mizukami and Fischer, 2000), which seems to be the conserved function across core eudicots.
Furthermore, it appears that expression patterns of ANT are not conserved in seed plants; in arabidopsis expression is restricted to the integuments, but in Gnetum it is found not only in the integuments but also in the nucellus (Figs 2 and 5C). It is worth noting that ANT is expressed in the micropyle region, where the integument has active cell division (Fig. 2C), and that it is not expressed in either of the outer envelopes, indicating that these structures use genes other than ANT for cell proliferation.
Altogether, the ancestral function of ANT seems to be in gametophytic development within the sporangia, as seen in ferns and in gymnosperms (Bui et al., 2017; Yamada et al., 2008; Fig. 2A–D). Apparently, during integument evolution in seed plants, ANT homologues have been recruited for its development (Yamada et al., 2008). It is important to note that ANT in arabidopsis is a gene with pleiotropic roles, being involved not only in integument initiation but also in primordia development of all plant organs except roots (Elliott et al., 1996).
Changes in the expression patterns of BELL1 suggest major changes in its interactions among land plants
BEL1 belongs to the Three Amino acid Loop Extension (TALE) homeobox family of transcription factors that are conserved among eukaryotes (Reiser et al., 1995; Bürglin, 1997; Bellaoui et al., 2001). Functional studies in the moss Physcomitrella patens show that PpBELL1 is a master regulator of the gametophyte–sporophyte transition; loss of function of PpBELL1 generates bigger egg cells unable to form embryos, suggesting that BELL1 was the key to facilitating the diversification of land plants (embryophytes; Horst et al., 2016). Expression in the gymnosperm G. gnemon, where the homologue Melbel1 is expressed in the nucellus and the megaspore after meiosis, suggests that the function in the proper formation of the egg cell may be conserved (Fig. 2E–G). In angiosperms the function in ovule development seems to be conserved. In the monocot Hordeum, two BEL1 homologues have been identified, JUBEL1 and 2 showing expression in the meristematic tissues and primordia of the ovule (Müller et al., 2001). In other angiosperms, such as Malus domestica, analyses of the BELL1 homologue, known as MDH1, suggest that its function in the ovule is conserved to that in arabidopsis, where BEL1 acts in the proper development of the integuments (Dong et al., 2000). In arabidopsis, the bel1 mutant causes significant growth in the chalazal region, producing an asymmetrical fleshy structure at the base of the nucellus, but no true integument is formed. Thus, the function of BEL1 in the development of the integument seems to be due to the interaction with the carpel identity dimer AGAMOUS-SEPATALLATA3 (AG/SEP3) and to the repression of WUSCHEL (WUS) towards the nucellus (Brambilla et al., 2007). Another interaction, which may be related to the function of BEL1 in integument formation shown by expression analyses, is the repression of SPOROCYTELESS (SPL), a master regulator of nucellus-forming pathways upregulating PIN-FORMED 1 (PIN1) and WUS (Sieber et al., 2004; Bencivenga et al., 2012; Yamada et al., 2019).
We infer that the function of BEL1 homologues in development of the egg cell may be conserved in bryophytes and gymnosperms (Fig. 2E–G; Horst et al., 2016). Although our results do not cover embryo development, the expression patterns of Melbel1 found in the nucellus and the megaspore mother cell suggest that it could be directly or indirectly involved in embryo development in G. gnemon as well. However, this function does not seem to be conserved in angiosperms, where BELL1 homologues play key roles in formation of the ovule primordia and integument, suggesting major differences across seed plants. Overall, this leads us to suggest that major changes in the functional evolution of the BELL1 gene lineage occurred following a duplication event that took place before the diversification of angiosperms (Fig. 5D).
Integument polarity genes KANADI and UNICORN show different expression patterns in seed plants
The KAN genes, a subset of the large GARP family of transcription factors, are present in all eukaryotes and are characterized by the plant-specific GARP DNA-binding domain (Riechmann et al., 2000; Hosoda et al., 2002; Zhang et al., 2009). Expression studies in the lycophyte Selaginella moellendorffii show that there are three KAN-specific homologues differentially expressed throughout sporangium development, participating in its initiation (SmKAN1, 2) through sporocyte formation (SmKAN3; Zumajo-Cardona et al., 2019). In the fern Equisetum hyemale the expression of KAN homologues was assessed in vegetative tissue, where it was found in leaf primordia and the abaxial side of each leaf (Zumajo-Cardona et al., 2019).
The expression patterns we found in the gymnosperm G. gnemon, where the two paralogues are expressed in the nucellus (megasporangium) and in the megaspore, are similar to those in S. moellendorffii (Fig. 3). Our results show different expression patterns between the two G. gnemon paralogues, suggesting a partial sub-functionalization event. While GnmoKAN1 is expressed in the nucellus, integument and megaspore, GnmoKAN2 is expressed in the megaspore and the apical region of the integument (Fig. 3). Expression or functional studies in the reproductive structures of ferns are still required to better hypothesize whether this function is conserved in lycophytes, ferns and gymnosperms.
In angiosperms, the KAN genes are known for their role in establishing leaf polarity, specifically the abaxial side of the leaf, similar to that found in ferns (Zumajo-Cardona et al., 2019). This function has been shown to be conserved in monocot homologues: Milkweed pod1 in Zea mays (Candela et al., 2008) and SHALLOT-LIKE1 in Oryza sativa (Zhang et al., 2009). In arabidopsis they are also responsible for specifying the abaxial identity of integuments. KAN1 and 2 are responsible for the planar identity of the outer integument, while ATS (also known as KAN4) is responsible for the planar identity of the inner integument (Leon-Kloosterziel et al., 1994; Kerstetter et al., 2001; Bowman et al., 2002; Eshed et al., 2004; McAbee et al., 2006; Kelley et al., 2012).
While KAN1/2 expression is detected in the outer integument of arabidopsis, we only detected expression of GmnoKAN1 and GmnoKAN2 in the single integument of G. gnemon and not in any of the outer envelopes. Therefore, the development of these structures does not require KAN function. As for integument polarity, no observations have been reported in other angiosperms, and no polar expression was detected here in the integument of Gnetum (Fig. 3). Hence, three scenarios are possible: (1) the function in integument polarity is not conserved across seed plants; (2) there are major changes in the regulatory network involved in ovule development in Gnetum; or (3) polar expression patterns of KAN are only present during early stages of development. Although the anatomy of the integument and the envelopes is homogeneous, it is important to emphasize that expression and functional studies are still needed, focusing on the development of the ovule in different species of seed plants. Previous studies in lycophytes together with our findings allow us to hypothesize that the ancestral function of the KAN genes is in the development of the sporangium and that it is conserved among lycophytes and gymnosperms.
UNICORN (UCN) encodes a functional AGC VIII kinase and acts in determining the planar identity of the outer integument (Schneitz et al., 1997; Enugutti et al., 2012, 2013). UCN also appears to be implicated in the planar growth of other plant organs, such as petals (Enugutti et al., 2012). UCN suppresses ectopic growth of integuments through two independent processes: by attenuating the protein kinase 3-PHOSPHOINOSITIDE-DEPENDENT PROTEIN KINASE 1 (PDK1) in the cytoplasm, which is involved in the stress response, and on the other hand by promoting growth (Flynn et al., 2000) and repressing ABERRANT TESTA SHAPE (ATS) in the nucleus (Scholz et al., 2019). There are two copies in G. gnemon: GnmoUCN and GnmoUCN2; although our results may not be conclusive for determining whether these homologues are also involved in integument polarity, it seems that this function is not conserved in G. gnemon because (1) GnmoUCN is expressed only in the nucellus (Fig. 4A-C), (2) GnmoUCN2 is expressed in the megaspore and in the apical region of the integument that forms the micropyle (Fig. 4D–G), and (3) we found no differences in the expression of UCN homologues in G. gnemon between the adaxial and abaxial sides of the integument (Fig. 4).
In arabidopsis UCN is known to interact with ATS in planar development, an interaction that seems to be maintained with other KAN genes (Enugutti et al., 2012; Enugutti and Shneitz, 2013). Interestingly, GnmoKAN1 and 2 and GnmoUCN2 are expressed in the distal region of the integument, which will form the micropyle; to assess if the interaction is maintained in Gnetum and their role in proximo-distal development of the integument, further studies are required.
Phylogenetic analyses show a complex evolutionary history for the KAN and UCN lineages (Zumajo-Cardona and Ambrose, 2020). Indeed, the lack of functional characterization outside arabidopsis does not allow us to predict the evolutionary history of this lineage. However, their function in integument polarity does not seem to be conserved between arabidopsis and G. gnemon. In gymnosperms, the KAN homologues predate a duplication event which resulted in KAN1 and KAN2/3 clades in angiosperms (Fig. 5E). This suggests that the KAN function in integument polarity may have occurred as a neo-functionalization event in angiosperms. Regarding the UCN lineage, it has undergone five gymnosperm-specific duplication events (Fig. 5F). While the angiosperm inner integument is considered to be homologous to the single gymnosperm integument (Crane, 1985; Doyle and Donoghue, 1986; Gasser and Skinner, 2019), interestingly, KAN and UCN homologues that are expressed in the arabidopsis outer integument are expressed in the single gymnosperm integument.
Evolution of the ovule development genes and what it may imply in the morphological evolution of ovules
The genes that we studied here are known to be involved in proper development of the integument (Baker et al., 1997; Gasser and Skinner, 2019). However, for the integuments to begin to develop, the identity of the ovule must have been established. To gain a better understanding of the putative molecular evolution of the ovule genetic network, our discussion here focuses on what is known about these genes across seed plants. The ovule identity protein complex is formed by three MADS-box proteins: SEEDSTICK (STK), SEPALLATA (SEP) and SHATERPROOF (SHP), which stabilize the BEL1-SEP3-AGAMOUS (AG) complex, to regulate the identity of the integument (Colombo et al., 2008). In addition, STK, SHP1 and SHP2 specify the fate of the integument cells, and later on ANT promotes the initiation and growth of these cells (Losa et al., 2010). The expression patterns of AG, SHP and STK in arabidopsis are found in the placenta and expression is maintained in the ovule, where their expression diverges, becoming specific to different regions (Fig. 5A; Ray et al., 1994; Losa et al., 2010). Expression studies carried out on the homologue in G. gnemon, GGM3, revealed that it is expressed in the entire ovule and in the pollen cones (Fig. 5A; Becker et al., 2002). These expression patterns as well as the evolution of the gene lineage suggest that these genes play pleiotropic roles in Gnetum and that, after multiple angiosperm-specific duplication events, these genes have become restricted to the ovule (Fig. 5A). Furthermore, the expression of AG, SHP and STK in the ovule further suggests a sub-functionalization event (Becker et al., 2002; Kramer et al., 2004; Pabón-Mora et al., 2014).
In arabidopsis, WUSCHEL is well known for its function in meristem identity (Laux et al., 1996; Mayer et al., 1998; Schoof et al., 2000). But in the ovule WUS is required for the proper establishment of the chalaza, the distal region of the ovule from which the integuments develop, and to induce the formation of the integuments. In fact, wus mutants do not develop integuments (Gross-Hardt et al., 2002; Sieber et al., 2004). Moreover, the expression of WUS is restricted to the nucellus, activating a downstream signal that derives from the nucellus and induces organ initiation in the adjacent chalaza cells (Fig. 5B), revealing that WUS activity is not in the cells where it is expressed, but forms a short-range signalling module repeatedly during plant development (Gross-Hardt et al., 2002; Colombo et al., 2008). Expression studies in the Gnetum homologue, known as GgWUS, exhibit expression in the nucellus, similar to that in arabidopsis ovules, suggesting that the expression and putative function of WUS in the ovule is conserved across seed plants (Fig. 5B; Nardmann et al., 2009). The WUS clade appears to be seed-plant-specific as the result of multiple duplication events specific to this lineage, in the clade T3WOX where it belongs (Gehring et al., 1990; Nardmann et al., 2009; Wu et al., 2019). In the fern Ceratopteris richardii, T3WOX homologues are expressed in the young tissues of the plant and in the root apical meristem (Nardmann and Werr, 2012; Youngstrom et al., 2019). Due to the four major duplication events of this gene lineage and the differences in the expression patterns, the evolution of T3WOX genes is complex, with functional changes in the major plant lineages.
Class III HD-Zip genes in arabidopsis, including the paralogues CORONA (CNA), PHABULOSA (PHB) and PHAVOLUTA (PHV), have also been reported to be involved in the proper establishment of the planar polarity of the integuments, where they are expressed adaxially (Sieber et al., 2004; Kelley and Gasser, 2009; Kelley et al., 2009). In addition, functional studies revealed that BEL1, CNA, PHB and PHV genes restrict WUS expression to the nucellus, probably independently (Yamada et al., 2016). Homologues of Class III HD-Zip genes have been identified across land plants, vascular plants seem to have undergone independent duplication events and lycophyte homologues are preduplication genes (Vasco et al., 2016). So far, no expression analyses have been performed in gymnosperms. But expression patterns in ferns show these genes on the adaxial side of the leaf. In addition, Class III HD-Zip homologues are expressed in the sporangia of the lycophyte Selaginella moellendorffii and the fern Psilotum nudum, suggesting that sporangia development may be the ancestral function of this gene lineage (Vasco et al., 2016).
Our results allow us to conclude that the ancestral function of integument genes is most likely in sporangium development and that these genes were subsequently recruited for integument development in angiosperms (Fig. 5C–F). The ANT, KAN and UCN homologues were also found to be expressed in the integument of G. gnemon. However, in arabidopsis this expression seems to be restricted to the integuments. The evolutionary history of these genes has been shown to be complex and most of the gymnosperm homologues are pre-duplication genes (i.e. BEL1, KAN and UCN; Fig. 5). The major differences observed in the expression patterns, suggest that their specific functions in integument development may be the result of sub- or neo-functionalization events in angiosperms. However, expression studies are still required, at early stages of ovule development in Gnetum as well as in other gymnosperm species, to better hypothesize on the functional evolution of these genes.
Furthermore, the unique morphology of Gnetum ovules allows us to address hypotheses on the possible origin of the ovule. The three envelopes initiate in acropetal order: the outer develops first from the lateral sides of the ovule primordia, then the middle, and then the integument shortly after, as a smooth outgrowth encircling the nucellus (Takaso and Bouman, 1986; Herr, 1995). The integument grows rapidly, surpassing the two envelopes forming the micropyle, and the exposed part forms multiple apical lobes (Fig. 1; Takaso and Bouman, 1986). These lobes seem to be similar to the integumentary lobes of Palaeozoic ovules (Herr, 1995). Interestingly, none of the genes studied here is expressed in the middle or outer envelopes of Gnetum ovules; most of them are restricted or strongly expressed in the apical region of the integument (i.e. GneANT, GnmoKAN1/ 2, GnmoUCN and GnmoUCN2; Figs 2–4).
Concerning the mechanisms that led to the evolution of ovules as a major synapomorphy of seed plants, three hypotheses are still debated and remain valid. (1) The integuments covering the megasporangia appeared as a new structure; this is known as the ‘de novo hypothesis’ (Meeuse, 1966). (2) The integuments are the result of the fusion of vegetative structures, telomes, around the sporangium – the ‘telome hypothesis’ (De Haan, 1920; Walton, 1953); this hypothesis is supported by the fusion of integumentary lobes in the Palaeozoic ovules. (3) The integuments are the result of sterilization of sporangia around the only sporangium that remains functional – the ‘synangial hypothesis’ (Benson, 1904). The synangial hypothesis was accepted (Boesewinkel and Bouman, 1967; Takhtajan, 1981) and later modified (Kenrick and Crane, 1997) by following the vascular traces of the Palaeozoic ovules, thus also providing evidence for the ‘neo-synangial hypothesis’. Palaeontological and morphological evidence so far seems equivocal for these three hypotheses. However, our studies in the integument genes in G. gnemon synthesized with previous studies in seedless plants (bryophytes, lycophytes and ferns) suggest two possible scenarios for ovule evolution. The first is that these genes were co-opted from the sporangium development network and became specific for integument development in angiosperms, which, is likely to have occurred, since plants are modular organisms (Fig. 5). In the second scenario the integuments are the result of fusion of integumentary lobes that once were fertile. The neo-synangial hypothesis is supported by fossil evidence and by the expression patterns of these genes in the micro-/megasporangium and in the apical region of the integument (Yamada et al., 2019). Moreover, anatomical development of ovules in Cycadales and the fossil record of Genomosperma kidstonii seem to support the synangial hypothesis (Rothwell and Scheckler, 1988; Sánchez-Tinoco and Engelman, 2004, 2005). The possibility that both scenarios have occurred is therefore plausible.
The envelopes of Gnetum do not seem to have genetic similarities with the integument of angiosperms, supporting the hypothesis that there is one integument in Gnetum and that the nature of the two additional envelops remains enigmatic. Differential expression analyses in dissected tissues from ovules are still required, which could reveal new candidate genes involved in the development of the different structures of seeds in Gnetum. Crucially, given the unique morphology of Gnetum ovules and the complex evolutionary history of these gene lineages, added to the uncertainty over monophyletic or polyphyletic origin of the ovule, it is difficult to extrapolate the results obtained here to other gymnosperms.
Finally, among seed plants the phylogenetic position of Gnetales has always been ambiguous. According to morphological data, Gnetales appears to be sister to angiosperms (Crane, 1985; Doyle and Donoghue 1986, 1992; Loconte and Stevenson, 1990), but the molecular data do not support this hypothesis. Gnetales, instead, seems to be sister to different groups of conifers, Pinaceae and non-Pinaceae (Ruhfel et al., 2014; Wickett et al., 2014; Forest et al., 2018). Our data highlight the genetic differences among ovules of Gnetales and angiosperms, but to gain a better understanding of the evolution of this group of plants, revealing the similarities through developmental genetic studies in conifers is of great importance.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: maximum likelihood analyses using selected sequences across land plants showing the phylogenetic position of G. gnemon homologues for BELL1, KANADI and UNICORN. Figure S2: maximum likelihood analyses using selected sequences of AINTEGUMENTA across seed plants. Figure S3: primer sequence and location for each sequence of interest used for the expression studies. Table S1: list of primer sequences used to make the probes.
ACKNOWLEDGEMENTS
The authors thank Dr Dennis Stevenson for helpful discussions and Marc Hachadourian, Director of the NYBG’s Nolen Greenhouses, for access to the Gnetum gnemon collections and plant care. Special thanks to the three anonymous reviewers whose comments helped improve this manuscript. C.Z.-C. designed and performed the experiments. C.Z.-C. and B.A.A. wrote the manuscript. B.A.A obtained the funding. All authors read and approved the final manuscript. The authors declare that there is no conflict of interest.
Funding
This research was funded by the Eppley Foundation for Research to B.A.A.
LITERATURE CITED
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ. 2000. Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Molecular Cell 5: 569–579. [DOI] [PubMed] [Google Scholar]
- Arnault G, Vialette ACM, Andres-Robin A, Fogliani B, Gâteblé G, Scutt CP. 2018. Evidence for the extensive conservation of mechanisms of ovule integument development since the most recent common ancestor of living angiosperms. Frontiers in Plant Science 9: 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Robinson-Beers K, Villanueva JM, Gaiser JC, Gasser CS. 1997. Interactions among genes regulating ovule development in Arabidopsis thaliana. Genetics 145: 1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari O. 1877. Della organogenia dei flori feminei del Gnetum gnemon L. Un Nouvo Giorno in Italia 9: 91–100. [Google Scholar]
- Becker A, Bey M, Bürglin TR, Saedler H, Theissen G. 2002. Ancestry and diversity of BEL1-like homeobox genes revealed by gymnosperm (Gnetum gnemon) homologs. Development Genes and Evolution 212: 452–457. [DOI] [PubMed] [Google Scholar]
- Becker A, Saedler H, Theissen G. 2003. Distinct MADS-box gene expression patterns in the reproductive cones of the gymnosperm Gnetum gnemon. Development Genes and Evolution 213: 567–572. [DOI] [PubMed] [Google Scholar]
- Bellaoui M, Pidkowich MS, Samach A, et al. 2001. The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell 13: 2455–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencivenga S, Simonini S, Benková E, Colombo L. 2012. The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis. Plant Cell 24: 2886–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. 1904. The origin of flowering plants. New Phytologist 3: 49–51. [Google Scholar]
- Boesewinkel FD, Bouman F. 1967. Integument initiation in Juglans and Pterocarya. Acta Botanica Neerlandica 16: 86–101. [Google Scholar]
- Bowman JL, Eshed Y, Baum SF. 2002. Establishment of polarity in angiosperm lateral organs. Trends in Genetics 18: 134–141. [DOI] [PubMed] [Google Scholar]
- Brambilla V, Battaglia R, Colombo M, et al. 2007. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell 19: 2544–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner ED, Stevenson D. 2006. Using genomics to study evolutionary origins of seeds. In: Williams C, ed. Landscapes, genomics and transgenic conifers. Dordrecht: Springer, 85–106. [Google Scholar]
- Brown RH, Nickrent DL, Gasser CS. 2010. Expression of ovule and integument-associated genes in reduced ovules of Santalales. Evolution & Development 12: 231–240. [DOI] [PubMed] [Google Scholar]
- Bui LT, Pandzic D, Youngstrom CE, et al. 2017. A fern AINTEGUMENTA gene mirrors BABY BOOM in promoting apogamy in Ceratopteris richardii. Plant Journal 90: 122–132. [DOI] [PubMed] [Google Scholar]
- Bürglin TR. 1997. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Research 25: 4173–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela H, Johnston R, Gerhold A, Foster T, Hake S. 2008. The milkweed pod1 gene encodes a KANADI protein that is required for abaxial/adaxial patterning in maize leaves. Plant Cell 20: 2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CJ. 1935. Gymnosperms. Structure and evolution. Chicago: University of Chicago Press. [Google Scholar]
- Colombo L, Battaglia R, Kater MM. 2008. Arabidopsis ovule development and its evolutionary conservation. Trends in Plant Science 13: 444–450. [DOI] [PubMed] [Google Scholar]
- Crane PR. 1985. Phylogenetic analysis of seed plants and the origin of angiosperms. Annals of the Missouri Botanical Garden 72: 716–793. [Google Scholar]
- Crane PR, Friis EM, Pedersen KR. 1995. The origin and early diversification of angiosperms. Nature 374: 27–33. [Google Scholar]
- Crepet WL, Friis EM, Nixon KC. 1991. Fossil evidence for the evolution of biotic pollination. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 333: 187–95. [Google Scholar]
- Dash M, Malladi A. 2012. The AINTEGUMENTA genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus × domestica Borkh.). BMC Plant Biology 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan HRM. 1920. Contribution to the knowledge of the morphological value and the phylogeny of the ovule and its integuments. Recueil des Travaux des botaniques Neerlandais 17: 219–324. [Google Scholar]
- Dong YH, Yao JL, Atkinson RG, Putterill JJ, Morris BA, Gardner RC. 2000. MDH1: an apple homeobox gene belonging to the BEL1 family. Plant Molecular Biology 42: 623–633. [DOI] [PubMed] [Google Scholar]
- Doyle JA, Donoghue MJ. 1986. Seed plant phylogeny and the origin of angiosperms: an experimental cladistic approach. Botanical Review 52: 321–431. [Google Scholar]
- Doyle JA, Donoghue MJ. 1992. Fossils and seed plant phylogeny reanalyzed. Brittonia 44: 89–106. [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, et al. 1996. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. 1996. Structure and function of female and bisexual organ complexes in Gnetales. International Journal of Plant Sciences 157: S113–S125. [Google Scholar]
- Endress PK. 2010. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden 97: 541–583. [Google Scholar]
- Endress PK. 2011. Angiosperm ovules: diversity, development, evolution. Annals of Botany 107: 1465–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enugutti B, Kirchhelle C, Oelschner M, et al. 2012. Regulation of planar growth by the Arabidopsis AGC protein kinase UNICORN. Proceedings of the National Academy of Sciences of the USA 109: 15060–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enugutti B, Kirchhelle C, Schneitz K. 2013. On the genetic control of planar growth during tissue morphogenesis in plants. Protoplasma 250: 651–661. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Perea JV, Bowman JL. 2001. Establishment of polarity in lateral organs of plants. Current Biology 21: 1251–1260. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. 2004. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131: 2997–3006. [DOI] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, et al. 2003. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell 15: 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz C, Pelaz S, Yanofsky MF. 1999. Control of carpel and fruit development in Arabidopsis. Annual Review of Biochemistry 99: 321–354. [DOI] [PubMed] [Google Scholar]
- Figueiredo DD, Köhler C. 2014. Signalling events regulating seed coat development. Biochemical Society Transactions 42: 358–363. [DOI] [PubMed] [Google Scholar]
- Figueiredo DD, Köhler C. 2016. Bridging the generation gap: communication between maternal sporophyte, female gametophyte and fertilization products. Current Opinion in Plant Biology 29: 16–20. [DOI] [PubMed] [Google Scholar]
- Flynn P, Wongdagger M, Zavar M, Dean NM, Stokoe D. 2000. Inhibition of PDK-1 activity causes a reduction in cell proliferation and survival. Current Biology 10: 1439–1442. [DOI] [PubMed] [Google Scholar]
- Forest F, Moat J, Baloch E, et al. 2018. Gymnosperms on the EDGE. Scientific Reports 8: 6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser CS, Skinner DJ. 2019. Development and evolution of the unique ovules of flowering plants. Current Topics in Developmental Biology 131: 373–399. [DOI] [PubMed] [Google Scholar]
- Gasser CS, Broadhvest J, Hauser BA. 1998. Genetic analysis of ovule development. Annual Review of Plant Physiology and Plant Molecular Biology 49: 1–24. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Müller M, Affolter M, et al. 1990. The structure of the homeodomain and its functional implications. Trends in Genetics 1: 323–329. [DOI] [PubMed] [Google Scholar]
- Gross-Hardt R, Lenhard M, Laux T. 2002. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes & Development 16: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr JM Jr. 1995. The origin of the ovule. American Journal of Botany 82: 547–564. [Google Scholar]
- Hollander JL, Vander Wall SB. 2009. Dispersal syndromes in North American Ephedra. International Journal of Plant Sciences 170: 323–330. [Google Scholar]
- Hollander JL, Vander Wall SB, Baguley JG. 2010. Evolution of seed dispersal in North American Ephedra. Evolutionary Ecology 24: 333–345. [Google Scholar]
- Horst NA, Katz A, Pereman I, Decker EL, Ohad N, Reski R. 2016. A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nature Plants 2: 15209. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Imamura A, Katoh E, et al. 2002. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14: 2015–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Harrar Y, Lin C, et al. 2014. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. Plant Cell 26: 246–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickert-Bond SM. 2003. Systematics of New World Ephedra L.(Ephedraceae): integrating morphological and molecular data. PhD Thesis, Arizona State University, USA. [Google Scholar]
- Ickert-Bond SM, Rydin C. 2011. Micromorphology of the seed envelope of Ephedra L.(Gnetales) and its relevance for the timing of evolutionary events. International Journal of Plant Sciences 172: 36–48. [Google Scholar]
- Izhaki A, Bowman JL. 2007. KANADI and class III HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell 19: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR, Gasser CS. 2009. Ovule development: genetic trends and evolutionary considerations. Sexual Plant Reproduction 22: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR, Skinner DJ, Gasser CS. 2009. Roles of polarity determinants in ovule development. Plant Journal 57: 1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR, Arreola A, Gallagher TL, Gasser CS. 2012. ETTIN (ARF3) physically interacts with KANADI proteins to form a functional complex essential for integument development and polarity determination in Arabidopsis. Development 139: 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. 1997. The origin and early evolution of plants on land. Nature 389: 33–39. [Google Scholar]
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. 2001. KANADI regulates organ polarity in Arabidopsis. Nature 411: 706–709. [DOI] [PubMed] [Google Scholar]
- Kim S, Soltis PS, Wall K, Soltis DE. 2006. Phylogeny and domain evolution in the APETALA2-like gene family. Molecular Biology and Evolution 23: 107–120. [DOI] [PubMed] [Google Scholar]
- Klucher KM, Chow H, Reiser L, Fischer RL. 1996. The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8: 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Jaramillo MA, Di Stilio VS. 2004. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics 166: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jürgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Keijzer CJ, Koornneef M. 1994. A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loconte H, Stevenson DW. 1990. Cladistics of the Spermatophyta. Brittonia 42: 197–211. [Google Scholar]
- Lora J, Hormaza JI, Herrero M. 2015. Transition from two to one integument in Prunus species: expression pattern of INNER NO OUTER (INO), ABERRANT TESTA SHAPE (ATS) and ETTIN (ETT). New Phytologist 208: 584–595. [DOI] [PubMed] [Google Scholar]
- Losa A, Colombo M, Brambilla V, Colombo L. 2010. Genetic interaction between AINTEGUMENTA (ANT) and the ovule identity genes SEEDSTICK (STK), SHATTERPROOF1 (SHP1) and SHATTERPROOF2 (SHP2). Sexual Plant Reproduction 23: 115–121. [DOI] [PubMed] [Google Scholar]
- Manchado-Rojo M, Weiss J, Egea-Cortines M. 2014. Validation of Aintegumenta as a gene to modify floral size in ornamental plants. Plant Biotechnology Journal 12: 1053–1065. [DOI] [PubMed] [Google Scholar]
- Martens P. 1971. Les Gnétophytes. Handbuch der Pflanzenanatomie, XII (2). 2nd edn. Berlin: Borntraeger. [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- McAbee JM, Hill TA, Skinner DJ, et al. 2006. ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. Plant Journal 46: 522–531. [DOI] [PubMed] [Google Scholar]
- Meeuse ADJ. 1966. Fundamentals of phytomorphology. New York: The Ronald Press Company. [Google Scholar]
- Miller MA.Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA, 1–8.
- Miller MA, Pfeiffer W, Schwartz T. 2012. The CIPRES Science Gateway: enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, 1–8.
- Mizukami Y, Fischer RL. 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proceedings of the National Academy of Sciences of the USA 97: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrusan Z, Reiser L, Feldmann KA, Fischer RL, Haughn GW. 1994. Homeotic transformation of ovules into carpel-like structures in Arabidopsis. Plant Cell 6: 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Wang Y, Franzen R, Santi L, Salamini F, Rohde W. 2001. In vitro interactions between barley TALE homeodomain proteins suggest a role for protein-protein associations in the regulation of Knox gene function. Plant Journal 27: 13–23. [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W. 2012. The invention of WUS-like stem cell-promoting functions in plants predates leptosporangiate ferns. Plant Molecular Biology 78: 123–134. [DOI] [PubMed] [Google Scholar]
- Nardmann J, Reisewitz P, Werr W. 2009. Discrete shoot and root stem cell-promoting WUS/WOX5 functions are an evolutionary innovation of angiosperms. Molecular Biology and Evolution 26: 1745–1755. [DOI] [PubMed] [Google Scholar]
- Neumann U, Hay A. 2020. Seed coat development in explosively dispersed seeds of Cardamine hirsuta. Annals of Botany 126: 39–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson S, Krizek BA. 2006. AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiology 141: 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabón-Mora N, Wong GK, Ambrose BA. 2014. Evolution of fruit development genes in flowering plants. Frontiers in Plant Science 5: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, et al. 2003. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424: 85–88. [DOI] [PubMed] [Google Scholar]
- Ray A, Robinson-Beers K, Ray S, et al. 1994. Arabidopsis floral homeotic gene BELL (BEL1) controls ovule development through negative regulation of AGAMOUS gene (AG). Proceedings of the National Academy of Sciences of the USA 91: 5761–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Liu T, Newell NR, et al. 2013. Establishing a framework for the Ad/abaxial regulatory network of Arabidopsis: ascertaining targets of class III homeodomain leucine zipper and KANADI regulation. Plant Cell 25: 3228–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser L, Modrusan Z, Margossian L, et al. 1995. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83: 735–742. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, et al. 2000. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290: 2105–2110. [DOI] [PubMed] [Google Scholar]
- Robinson-Beers K, Pruitt RE, Gasser CS. 1992. Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell 4: 1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell, GW, Scheckler SE. 1988. Biology of ancestral gymnosperms. In: Beck CD, ed. Origin and evolution of gymnosperms. New York: Columbia University Press. [Google Scholar]
- Rudall PJ. 2021. Evolution and patterning of the ovule in seed plants. Biological Reviews 96: 943–960. [DOI] [PubMed] [Google Scholar]
- Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG. 2014. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evolutionary Biology 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydin C, Pedersen KR, Crane PR, Friis EM. 2006. Former diversity of Ephedra (Gnetales): evidence from Early Cretaceous seeds from Portugal and North America. Annals of Botany 98: 123–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydin C, Khodabandeh A, Endress PK. 2010. The female reproductive unit of Ephedra (Gnetales): comparative morphology and evolutionary perspectives. Botanical Journal of the Linnean Society 163: 387–430. [DOI] [PubMed] [Google Scholar]
- Sánchez-Tinoco MY, Engleman EM. 2004. Seed coat anatomy of Ceratozamia mexicana (Cycadales). Botanical Review 70: 24–38. [Google Scholar]
- Sánchez-Tinoco MY, Engleman EM. 2005. The vascularization of the seed of Ceratozamia meхicana (Zamiaceae). In: Proceedings of Cycad 2005. New York: New York Botanical Garden Press, 223–235. [Google Scholar]
- Schneitz K, Hülskamp M, Pruitt RE. 1995. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. The Plant Journal 7: 731–749. [Google Scholar]
- Schneitz K, Hülskamp M, Kopczak SD, Pruitt RE. 1997. Dissection of sexual organ ontogenesis: a genetic analysis of ovule development in Arabidopsis thaliana. Development 124: 1367–1376. [DOI] [PubMed] [Google Scholar]
- Scholz S, Pleßmann J, Enugutti B, Hüttl R, Wassmer K, Schneitz K. 2019. The AGC protein kinase UNICORN controls planar growth by attenuating PDK1 in Arabidopsis thaliana. PLoS Genetics 15: e1007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Sieber P, Gheyselinck J, Gross-Hardt R, Laux T, Grossniklaus U, Schneitz K. 2004. Pattern formation during early ovule development in Arabidopsis thaliana. Developmental Biology 273: 321–334. [DOI] [PubMed] [Google Scholar]
- Skinner DJ, Hill TA, Gasser CS. 2004. Regulation of ovule development. Plant Cell 16 (Suppl): S32–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner DJ, Brown RH, Kuzoff RK, Gasser CS. 2016. Conservation of the role of INNER NO OUTER in development of unitegmic ovules of the Solanaceae despite a divergence in protein function. BMC Plant Biology 16: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Strasburger E. 1879. Die Angiospermen und die Gymnospermen. Jena: Fisher. [Google Scholar]
- Takaso T. 1984. Structural changes in the apex of the female strobilus and the initiation of the female reproductive organ (ovule) in Ephedra distachya L. and E. equisetina Bge. Acta Botanica Neerlandica 33: 257–266. [Google Scholar]
- Takaso T. 1985. A development study of the integument in gymnosperms. 3. Ephedra distachya L. and E. equisetina Bge. Acta Botanica Neerlandica 34: 33–48. [Google Scholar]
- Takaso T, Bouman F. 1986. Ovule and seed ontogeny in Gnetum gnemon L. Botanical Magazine. Tokyo 99: 241–266. [Google Scholar]
- Takhtajan AL. 1981. Flowering plants: origin and dispersal. Edinburgh, Scotland: Oliver and Boyd, 310–313.
- Tomlinson PB, Braggins JE, Rattenbury JA. 1991. Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. American Journal of Botany 78: 1289–1303. [Google Scholar]
- Vasco A, Smalls TL, Graham SW, et al. 2016. Challenging the paradigms of leaf evolution: Class III HD-Zips in ferns and lycophytes. New Phytologist 212: 745–758. [DOI] [PubMed] [Google Scholar]
- Walton J. 1953. Evolution of the ovule in pteridosperms. Nature 172: 435–436. [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences of the USA 111: E4859–E4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Li FW, Kramer EM. 2019. Large-scale phylogenomic analysis suggests three ancient superclades of the WUSCHEL-RELATED HOMEOBOX transcription factor family in plants. PloS one 14: e0223521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ito M, Kato M. 2003. Expression pattern of INNER NO OUTER homologue in Nymphaea (water lily family, Nymphaeaceae). Development Genes and Evolution 213: 510–513. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hirayama Y, Imaichi R, Kato M. 2008. AINTEGUMENTA homolog expression in Gnetum (gymnosperms) and implications for the evolution of ovulate axes in seed plants. Evolution & Development 10: 280–287. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sasaki Y, Hashimoto K, Nakajima K, Gasser CS. 2016. CORONA, PHABULOSA and PHAVOLUTA collaborate with BELL1 to confine WUSCHEL expression to the nucellus in Arabidopsis ovules. Development 143: 422–426. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sasaki Y, Sakata K, Gasser CS. 2019. Possible roles of BELL 1 and class III homeodomain-leucine zipper genes during integument evolution. International Journal of Plant Sciences 180: 623–631. [Google Scholar]
- Youngstrom CE, Geadelmann LF, Irish EE, Cheng CL. 2019. A fern WUSCHEL-RELATED HOMEOBOX gene functions in both gametophyte and sporophyte generations. BMC Plant Biology 19: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW. 2009. SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21: 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumajo-Cardona C, Ambrose BA. 2020. Phylogenetic analyses of key developmental genes provide insight into the complex evolution of seeds. Molecular Phylogenetics and Evolution 147: 106778. [DOI] [PubMed] [Google Scholar]
- Zumajo-Cardona C, Vasco A, Ambrose BA. 2019. The evolution of the KANADI gene family and leaf development in lycophytes and ferns. Plants 8: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.