Abstract
BACKGROUND
The ultrasound-guided retroclavicular block (RCB) is a recently described alternative approach to brachial plexus blockade at the level of the cords. Although more distal blockade of the brachial plexus is thought to be associated with a lower incidence of phrenic nerve block, the impact of RCB on ipsilateral diaphragmatic function has not been formally investigated.
OBJECTIVE
To compare the effects of supraclavicular and retroclavicular brachial plexus block on diaphragmatic function.
SETTING
A single tertiary hospital, study period from December 2017 to May 2019.
DESIGN
Double-blinded, randomised study.
PATIENTS
A total of 40 patients undergoing upper extremity surgery below the axilla. Exclusion criteria included significant pulmonary disease, BMI more than 40 and contraindication to peripheral nerve block.
INTERVENTIONS
Patients were randomised to supraclavicular or retroclavicular brachial plexus block with ropivacaine 0.5%.
OUTCOME MEASURES
Phrenic block was assessed by measuring changes in diaphragmatic excursion using M-mode ultrasound, and maximum inspiratory volume on incentive spirometry from baseline, at 15 and 30 min postblock, and postoperatively. Comparative assessment of block characteristics included timing and distribution of sensory and motor block onset in the upper extremity, and scanning and block performance times.
RESULTS
The incidence of phrenic block in the supraclavicular group was higher by ultrasound imaging (70 vs. 15%) and also by pulmonary function testing (55 vs. 5%), with both diaphragmatic excursion and maximum inspiratory volume decreasing to a greater extent after supraclavicular block (SCB) compared with RCB at 15, 30 min and postoperative time points (repeated measures analysis of variance, P < 0.001). There was no difference in timing and extent of distal arm block, but suprascapular and axillary nerves were more consistently blocked after SCB than after RCB.
CONCLUSION
The current study confirms the hypothesis that a RCB is significantly less likely to affect ipsilateral diaphragmatic function than a SCB.
TRIAL REGISTRATION
Clinicaltrials.gov identifier: NCT02631122
Introduction
A variety of approaches for regional blockade for upper extremity surgery have been described, all centred around parts of the brachial plexus. A more posterior approach to the cords of the brachial plexus may have several notable advantages when compared with more traditional infraclavicular techniques, including improved needle visualisation, a favourable ultrasound-beam-to-needle angle promoting reflection and an easier path to the posterior cord. Hebbard and Royse1 proposed a posterior approach in 2007, depicting the needle trajectory crossing parts of the trapezius, supraspinatus and subscapularis muscles. A further modification, the retroclavicular block (RCB), with a needle insertion point anterior to the edge of the trapezius muscle and immediately posterior to (walked off behind) the clavicle to minimise passage through muscles was proposed2 and validated as a feasible block technique, with a surgical anaesthesia success rate of 96%.3 Comparisons of the RCB with infraclavicular blockade have demonstrated superior needle tip and shaft visibility, reduced performance time and fewer needle passes.4-6 The success rate, onset time and pain relief have also all been found to be similar between RCB and supraclavicular block (SCB).7
One commonly assumed and cited advantage of more distal brachial plexus blocks, such as axillary and infraclavicular, over more proximal blocks, such as supraclavicular, is the lower incidence of phrenic nerve block.8,9 Reported rates of phrenic nerve paralysis vary widely depending on approach and technique (multiple injection vs. single injection, ‘corner pocket’ vs. neural cluster, ultrasound guided vs. nerve stimulation guided),10,11 and volume and concentration of local anaesthetic.12 The SCB has been reported to have anywhere from 0 to a 67% incidence of diaphragmatic paralysis,10-16 while that cited for the infraclavicular approach is 0 to 26%.17-19 A recent study reported decreased diaphragmatic paralysis for the more distal costoclavicular approach, compared with supraclavicular.20 However, the incidence of phrenic nerve block with RCB has not been reported. The objective of this randomised, controlled, double-blind trial was to test the hypothesis that the RCB would have a lower incidence of hemidiaphragmatic dysfunction compared with SCB, while providing similarly effective surgical anaesthesia.
Methods
Recruitment and randomisation
The current double-blind, randomised study was conducted at Brigham and Women’s Hospital between December 2017 and May 2019. Prior to patient enrolment this study was approved by the Institutional Review Board (IRB#2015P001537, 399 Revolution Drive, Somerville, Massachusetts, USA, 11 May 2016), and was registered on clinicaltrials.gov (NCT02631122, Vlassakov, 16 December 2015). Applicable CONSORT guidelines were adhered to.
Patients aged more than 18 years old undergoing upper extremity surgery below the axilla under regional anaesthesia were recruited. Exclusion criteria included BMI more than 40, American Society of Anesthesiologists (ASA) Physical Status class more than 3, significant pulmonary disease, known diaphragmatic dysfunction, pre-existing neuropathy of the surgical arm and contraindications to peripheral nerve block (allergy to local anaesthetics, coagulopathy, local infection). Written informed consent was obtained during the pre-operative evaluation visit, which occurs days to weeks before surgery. Patients were randomly assigned to either RCB or SCB in a 1 : 1 ratio using a predetermined computer-generated randomisation table by one of the investigators (KLS). This allocation was communicated confidentially to the block operator before the block procedure, and was not disclosed to the patient or the co-investigator and study staff performing the assessments of diaphragmatic excursion, respiratory volumes, sensory and motor block, and pain.
Block procedure
Ultrasound-guided brachial plexus block was performed prior to surgery in the pre-operative holding area by an attending anaesthesiologist with formal regional anaesthesia training and extensive experience with both techniques. Intravenous access was established. Facial oxygen was given and an ECG, pulse oximeter and blood pressure monitor were attached. The patient was positioned supine with the head of bed elevated to a 30-degree angle and the head turned to the contralateral side. Sedation with midazolam was administered and also fentanyl if required, for patient anxiety according to perceived clinical need, and baseline measurement of diaphragmatic excursion with ultrasound, maximal inspiratory volumes (MIV) with the incentive spirometer, and sensory and motor testing were performed. The block site was then sterilised with a solution of chlorhexidine 2% in isopropyl alcohol 70%. A high-frequency linear array transducer (15 to 6 MHz, HFL50xp, SonoSite X-Porte; SonoSite, Inc., Bothell, Washington, USA) was used to guide the plexus block procedure.
For the SCB, the transducer was placed in the supraclavicular fossa, and the subclavian artery and brachial plexus were visualised in short axis above the first rib. After infiltration of the skin with lidocaine 1% 1 to 2 ml with a 29-gauge needle, a 22-gauge 50 mm block needle (SonoPlex; Pajunk GmbH, Geisingen, Germany) was advanced under direct visualisation using in-plane technique. Ropi-vacaine 0.5% 25 ml was injected incrementally after negative aspiration to obtain spread to the brachial plexus trunks. Although redirection and optimal positioning was at the discretion of the operator, the above volume was typically divided between the ‘corner pocket’ and the often distinctly separate superior trunk.21-23
For the RCB, the transducer was placed on the chest just caudal to the clavicle in a sagittal orientation and medial to the coracoid process. The axillary artery and the brachial plexus cords were identified in short axis. The needle insertion point was immediately behind (posterior to) the clavicle in the anterior supraclavicular fossa. A 21-gauge 100-mm needle (SonoPlex; Pajunk GmbH) was walked off underneath the clavicle and advanced toward the plexus cords and axillary artery. In this technique, the ultrasound probe was initially angled towards the clavicle to identify the needle approaching from behind the clavicle and minimise the inherent ‘blind spot’. The needle was then advanced in-plane under direct visualisation to reach the brachial plexus surrounding the axillary artery. Increments of ropivacaine 0.5% 25 ml were injected after negative aspiration at the lateral cord, and then again after needle redirection between the posterior cord and the axillary artery to a position underneath the axillary artery, to achieve upward (anterior) displacement of the artery by the injectate and also to promote spread just caudal to the vessel towards the medial cord.
Both SCB and RCB techniques were followed by ultrasound-guided infiltration block of the intercostobrachial and medial brachial cutaneous nerves in the proximal axilla with 5 ml ropivacaine 0.5% to reduce tourniquet pain. A provision was made that if the attending anaesthesiologist providing intra-operative patient care considered a plexus catheter beneficial for postoperative pain control, an 18-gauge 100-mm needle (SonoLong; Pajunk GmbH) would be used for the initial block, and after completion of the standardised local anaesthetic injection via the needle (ropivacaine 0.5%, 25 ml, as described above), a 20-gauge (Perifix; B. Braun Melsungen AG, Melsungen, Germany) catheter would be inserted to a position adjacent to the brachial plexus and secured in place. No local anaesthetic was infused through the catheter until after all study measurements were obtained. Strict aseptic technique was used for all injections, with additional sterile drapes in the case of continuous catheter insertion.
Diaphragmatic excursion measurement and respiratory function assessment
All measurements (diaphragmatic excursion measurement and respiratory function assessment, and the assessment of sensory and motor block) were obtained by the same anaesthesiologist (PG) who was blind to the block technique and was not present at the bedside during the block procedure. Baseline measurements of both diaphragmatic excursion and MIV were obtained for each patient prior to the nerve blocks, but after the administration of procedural sedation for nerve block. Subsequent measurements were obtained at 15 min after completion of block, 30 min postblock and postoperatively in the postanaesthesia care unit (PACU). The head of the bed was elevated 30 degrees for all measurements.
Diaphragmatic excursion was assessed as described by Boussuges et al.24 using a phased array probe (5 to 1 MHz, rP19xp, SonoSite X-Porte; SonoSite, Inc.). The probe was initially placed along the anterior axillary line at the level of the costal margin, with slight adjustments made for optimisation of image. The location of the probe at best image was marked with a skin marker for consistency of subsequent measurements. Diaphragm excursion was measured using M-mode while the patient took a deep breath. Three measurements were obtained, recorded and averaged at each time point.
Respiratory function was assessed by measuring MIV using the Voldyne 5000 incentive spirometer (Hudson RCI; Teleflex, Morrisville, North Carolina, USA). Patients were asked to exhale maximally and then take a maximal breath in through the device mouthpiece, and volume was recorded. Three attempts were recorded and averaged.
Block measurements
The following block performance times were recorded by a research assistant: first, scanning time – time from first contact of ultrasound probe to skin to optimal brachial plexus image obtained; second, needling time – time from the first needle puncture to the end of the block procedure (needle out). At the end of the block procedure, the block operator was asked to rate the block as technically easy or difficult on a scale from 1 to 10 (1 = very easy to 10 = extremely difficult).
Assessment of sensory and motor block was performed by an anaesthesiologist blinded to the block type every 5 min for up to 30 min after injection. Sensory block was tested by response to pinprick in the distribution of the median (sM), radial (sR), ulnar (sU), musculocutaneous (sMC), median antebrachial cutaneous (sMABC), median brachial cutaneous (sMBC), intercostobrachial (sICB), axillary (sA) and suprascapular (sSS) nerves and rated as 1 = normal sensation, 2 = decreased sensation, 3 = no sensation. Motor block was tested using thumb opposition (mM), finger abduction (mU), wrist extension (mR), elbow flexion (mMC), arm abduction at shoulder (mA), and external rotation of the shoulder (mSS) and rated as 1 = full strength, 2 = decreased strength, 3 = unable to move. A clinically successful block was pragmatically defined as sensory and motor deficit in the upper extremity at and below the axilla resulting in ability to perform surgery without a need to convert to general anaesthesia. To compare the block onset and progression dynamics relative to innervation territories, a composite distal upper extremity block score was calculated (sM + mM + sR + mR + sU + mU + sMC + mMC + s-+ sMAC) and compared between the two approaches. Similar comparisons were conducted for the axillary (sA + mA) and suprascapular (sSS + mSS) nerve block scores.
Intra-operative procedure
Intra-operative anaesthesia and analgesia were determined by the primary anaesthesiologist.
Blinding
The primary data collector was blind to group assignment, and was not present at the time of block placement. Although the insertion site is similar between approaches, block assignment was further concealed by covering the ipsilateral neck and clavicle after the block. The patient and surgeons were also blind to the block technique performed. The primary anaesthesia team, which was independent of the study, was aware of the block type.
Outcomes
The primary outcome was phrenic block, measured as the change in diaphragmatic excursion from preblock to postblock, calculated as a percentage change from baseline, and measured at 15 min postblock, 30 min postblock and in PACU, and also the change in MIV from preblock to postblock, calculated as a percentage change from baseline. For clinical interpretability, the presence of phrenic block was defined as a decrease in diaphragmatic excursion of at least 50% from baseline, and decrease in MIV at least 30% (lower threshold for impact on MIV was because it is less specifically affected by ipsilateral block). Secondary outcomes included block success, onset of sensory and motor block, ease of placement (imaging time, needling time, difficulty rating) and pain scores/morphine equivalents used in PACU.
Sample size calculation
Based on previous literature, we assumed an incidence of hemidiaphragmatic paresis of 50% for supraclavicular nerve blocks and 20% for retroclavicular nerve blocks.13,16 Using the proportion of participants with diaphragmatic block as the primary outcome, given the 30% anticipated difference between groups, and setting an alpha significance level of P = 0.05 and power at 80%, it was calculated that a minimum of 36 patients would be required per group. Based on this, initial planned enrolment was for 80 participants.
Statistical analysis
All variables were tested for normality of distribution, and categorical and continuous variables were compared between groups using χ2 or Fisher’s exact test and Student’s t test or Mann–Whitney, as appropriate. Repeated measures analysis of variance (rmANOVA) was used to assess group differences in diaphragmatic excursion and MIV over multiple timepoints (15, 30 min, postop); rmANOVA was also used to assess the differences in sensory and motor block dynamics across multiple timepoints (5, 10, 15, 20, 25, 30 min). The level of significance was set at P less than 0.05. All statistical analyses were performed with SPSS Version 25 (IBM, Armonk, New York, USA).
Results
Patient characteristics
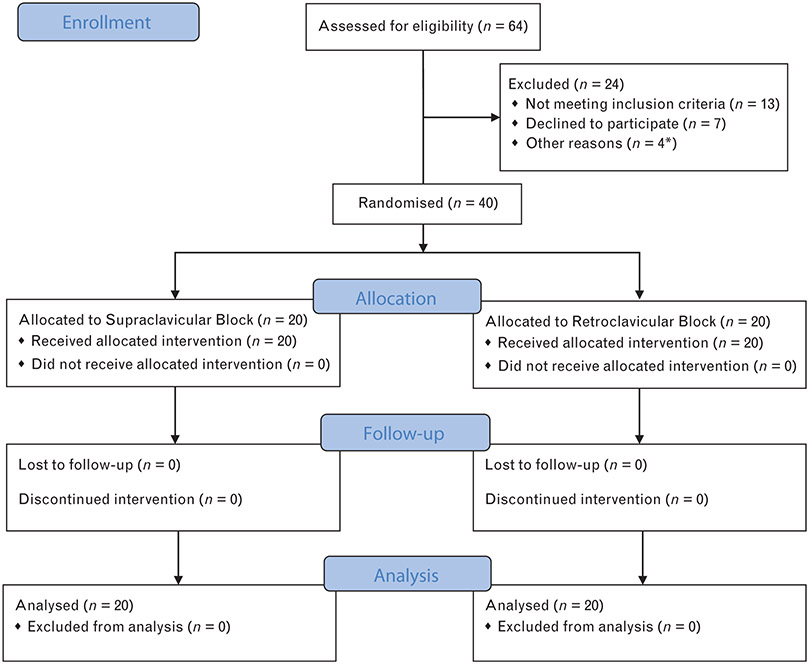
Interim analysis after enrolment of 40 participants revealed a clear difference in primary and several secondary outcomes, and no further patients were recruited. In all, 44 patients were recruited and 40 completed the study (Fig. 1). Three patients were excluded prior to block placement due to surgery being cancelled or postponed (one for elevated potassium, one for neurological deficits and one for cardiac work-up) and one patient was excluded from the study due to pre-operative identification of a large pleural effusion on the operative side (Fig. 1). There were no significant group differences in age, weight, BMI, sex, ASA class (Table 1). Twenty patients were randomised to each group, and all were included in analysis.
Fig. 1. Consolidated Standards of Reporting Trials diagram showing patient recruitment and flow.
*Four patients excluded prior to randomisation. One excluded due to pre-operative identification of large pleural effusion. Three patients were excluded due to surgery being postponed (one for elevated potassium, one for neurologic deficits and one for cardiac work-up). CONSORT, Consolidated Standards of Reporting Trials
Table 1.
Patient and block characteristics
| Patient and surgical characteristics | Supraclavicular | Retroclavicular |
|---|---|---|
| n | 20 | 20 |
| Age | 64.80 ± 17.33 | 60.20 ± 15.46 |
| Weight (kg) | 77.61 ± 14.22 | 76.88 ± 16.56 |
| BMI | 27.27 ± 4.62 | 28.23 ± 3.51 |
| Sex | ||
| Male | 11 (55%) | 11 (55%) |
| Female | 9 (45%) | 9 (45%) |
| ASA | ||
| I | 0 (0%) | 2 (10%) |
| II | 5 (25%) | 3 (15%) |
| III | 15 (75%) | 15 (75%) |
| Surgical classification | ||
| Vascular procedure | 13 | 14 |
| Orthopaedic procedure | 7 | 6 |
| Surgical side | ||
| Left | 8 (40%) | 12 (60%) |
| Right | 12 (60%) | 8 (40%) |
Data are given as mean ± SD or number (%). ASA, American Society of Anesthesiologists physical score.
Phrenic nerve block
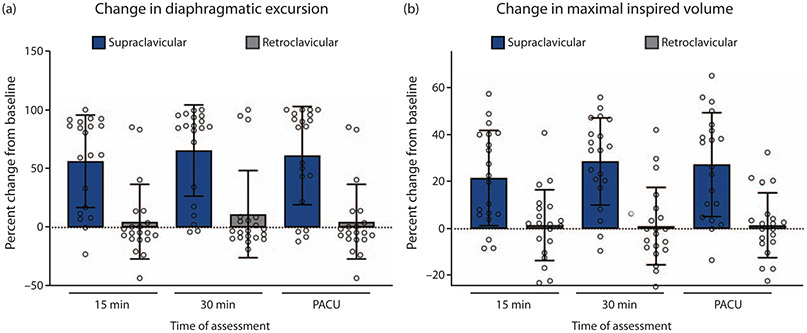
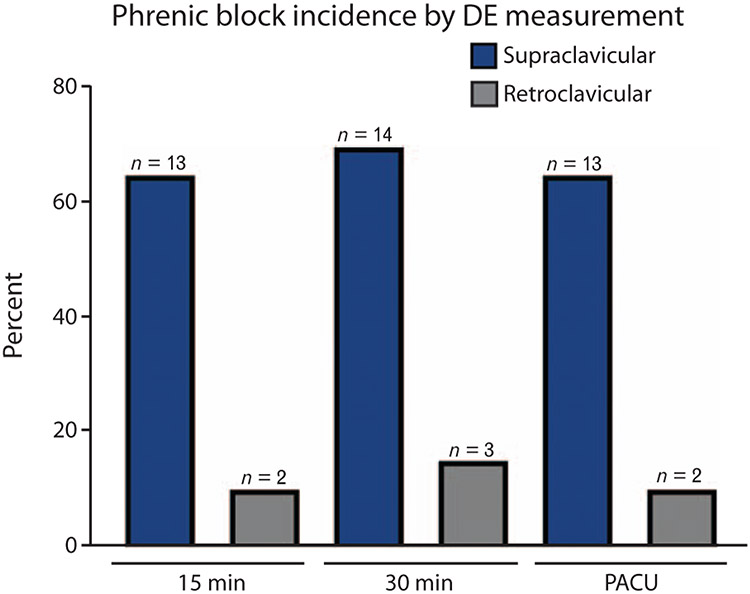
To examine the impact of blocks on diaphragmatic excursion, a percentage decrease in diaphragmatic excursion was calculated from the patient’s baseline at each of three timepoints (15 and 30 min postblock, and PACU). A rmANOVA analysis was performed, which revealed a significant main effect for block group (F = 26.991, P < 0.001), such that those receiving SCB had a greater % decrease in diaphragmatic excursion than those receiving RCB (Fig. 2a; Table 2). Similarly, when the % decrease in maximal inspiratory volume on incentive spirometry from baseline was examined as an outcome, rmANOVA revealed a significant main effect for block group (F = 23.627, P < 0.001), such that those receiving SCB had a greater % decrease in MIV than those receiving RCB (Fig. 2b; Table 2). There was no main effect for time, or time by group interaction for either outcome (% change diaphragmatic excursion or MIV). Diaphragmatic excursion measurements and MIV measurements correlated highly for any given patient at each timepoint (Pearson Rho 0.6 to 0.9, all P < 0.001). Phrenic block was defined as more than 50% decrease in diaphragmatic excursion. χ2 analysis revealed a statistically significant difference in the incidence of phrenic nerve block at timepoints 15, 30 min, and PACU between groups (65, 70 and 65% for the SCB group, and 10, 15 and 10% for the RCB group) (P = 0.001, Fig. 3). Reduction in MIV was defined as more than 30% decrease from baseline. The incidence of reduced MIV was also significantly different (40, 55 and 50% for the SCB group, and 5, 5 and 5% for the RCB group) (χ2, P = 0.001).
Fig. 2. Impact of supraclavicular and retroclavicular block on diaphragmatic function.
(a) Diaphragmatic excursion measured using M-mode ultrasound was reduced to a greater degree after supraclavicular than after retroclavicular block (repeated measures analysis of variance P < 0.001). (b) Maximal inspired volume as measured by incentive spirometry was reduced to a greater degree after supraclavicular than after retroclavicular block (repeated measures analysis of variance P < 0.001). PACU, postanaesthesia care unit.
Table 2.
Assessment of diaphragmatic function
| Supraclavicular | Retroclavicular | P value | Mean difference (95% CI) |
|||
|---|---|---|---|---|---|---|
| DE (cm)/MIV (ml), n=20 |
Percentage change from baseline, % |
DE (cm)/MIV (ml), n=20 |
Percentage change from baseline (%) |
|||
| Baseline DE (cm) | 5.5 ± 1.5 | 5.1 ± 1.8 | ||||
| DE at 15 min | 2.5 ± 2.4 | 56 ± 39 | 4.8 ± 2.3 | 0.9 ± 27 | <0.001 | 55 (33 to 78) |
| DE at 30 min | 2.0 ± 2.3 | 65 ± 39 | 4.6 ± 2.6 | 7 ± 34 | <0.001 | 58 (34 to 82) |
| DE PACU | 2.0 ± 2.1 | 61 ± 42 | 4.8 ± 2.2 | 5 ± 31 | <0.001 | 56 (32 to 80) |
| Baseline MIV (ml) | 2342 ± 946 | 2175 ± 811 | ||||
| MIV at 15 min | 1823 ± 794 | 22 ± 20 | 2128 ± 847 | 0.2 ± 12 | 0.001 | 21 (10 to 32) |
| MIV at 30 min | 1658 ± 719 | 29 ± 18 | 2160 ± 938 | −0.6 ± 14 | <0.001 | 29 (18 to 39) |
| MIV at PACU | 1699 ± 752 | 27 ± 22 | 2145 ± 904 | 0.9 ± 14 | <0.001 | 26 (14 to 39) |
| Block characteristics | ||||||
| Imaging time (s) | 14 ± 13 | 22 ± 18 | 0.114 | −8 (−18 to 2) | ||
| Needling time (s) | 262 ± 100 | 276 ± 119 | 0.699 | −14 (−84 to 57) | ||
| Block time (s) | 276 ± 106 | 298 ± 128 | 0.566 | −21 (−97 to 54) | ||
| Block rating (0 to 10) | 2.5 ± 1.7 | 3.5 ± 2.1 | 0.141 | −0.9 (−2.1 to 0.3) | ||
| Side effects | ||||||
| Blood aspiration | 2 (10%) | 2 (10%) | 1.0 | |||
| Paraesthesia | 9 (45%) | 4 (20%) | 0.176 | |||
| Horner’s syndrome | 4 (20%) | 0 (0%) | 0.106 | |||
| Dyspnoea | 0 (0%) | 0 (0%) | – | |||
Data presented as mean ± SD and number (%). P values reported from group comparisons using Mann–Whitney U test of % change from baseline. DE, diaphragmatic excursion; MIV, maximal inspiratory volume; PACU, postanaesthesia care unit.
Fig. 3. Incidence of phrenic block after supraclavicular and retroclavicular block.
Phrenic block was defined as more than 50% decrease in diaphragmatic excursion from individual baseline. A greater incidence of phrenic block was observed amongst subjects receiving supraclavicular than retroclavicular block (χ2, P = 0.001). DE, diaphragmatic excursion; PACU, postanaesthesia care unit.
Block characteristics
One patient in the SCB group had a failed block that resulted in unplanned conversion to general anaesthesia. Three patients in the RCB group required a small amount of supplementary local infiltration by surgeon, all in the proximal upper arm.
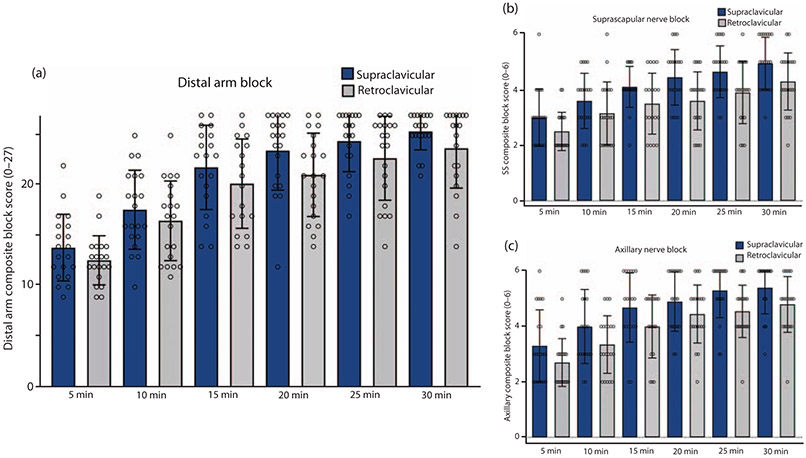
As part of an exploratory secondary analysis of block onset, progression and completeness, we created a distal arm composite block score, which included sensory and motor scores in the radial, ulnar, median, musculocutaneous nerve distributions and sensory for medial antebrachial cutaneous nerve distribution. This distal arm block score could have a maximal score of 27 [three points for each of nine components (four motor, five sensory)]. A rmANOVA of these scores at the three timepoints revealed no significant group difference (F = 1.559, P = 0.22, Fig. 4a). We were also interested whether blocks differentially affected more proximal aspects of the brachial plexus. In particular, a greater impact on composite scores of motor and sensory testing of the suprascapular nerve was seen in the supraclavicular group (rmANOVA, F = 5.065, P = 0.031, Fig. 4b), also for the axillary nerve (rmANOVA, F = 4.342, P = 0.045, Fig. 4c). Individual sensory and motor scores for each tested nerve are shown in the Appendix, http://links.lww.com/EJA/A357. The incidence of positive blood aspiration, paraesthesia, Horner’s syndrome and dyspnoea, associated with the nerve block, was not significantly different between groups (Table 1). Oxygen saturation breathing room air was not statistically different between groups at any time point.
Fig. 4. Extent, timing and distribution of supraclavicular and retroclavicular blocks.
(a) No difference was observed in the onset and extent of sensory and motor block of nerves in the distal arm between groups (repeated measures analysis of variance F = 1.559, P = 0.22). Distal arm block score maximal score 27 (three points for each of nine components). (b) Onset and extent of suprascapular nerve block was greater in the supraclavicular group (repeated measures analysis of variance F = 5.065, P = 0.031). (c) Onset and extent of axillary nerve block was greater in the supraclavicular group (repeated measures analysis of variance, F = 4.342, P = 0.045).
Discussion
The incidence of ipsilateral phrenic block following a variety of brachial plexus block techniques continues to be of great interest to the practicing anaesthesiologist. This is of particular importance when caring for patients with significant pulmonary disease, some of whom may not be able to tolerate inadvertent unilateral diaphragmatic weakness. This randomised, controlled, double-blind trial examines for the first time the incidence of ipsilateral phrenic nerve block after RCB and compares it with that observed after SCB. It reveals that RCB is associated with a lower incidence and degree of ipsilateral hemidiaphragmatic weakness when compared with SCB, as measured by ultrasound-based assessment of diaphragmatic excursion and by incentive spirometry (MIV). Phrenic nerve block after SCB is not surprising and has been reported to cause dyspnoea and even necessitate mechanical ventilation.25,26 In this study, phrenic nerve block after RCB was less common than after SCB, but still present in 3/20 vs. 14/20, by diaphragmatic excursion measurement and in 1/20 vs. 11/20, by MIV spirometry. Further investigations would be required to determine if a lower volume of local anaesthetic could reduce or even eliminate the risk of diaphragmatic weakness with RCB.
These findings are in agreement with a recent study that also employed ultrasound assessment of diaphragmatic function and found a lower rate of phrenic nerve block after another distal approach to the brachial plexus, the costoclavicular approach,20 when compared with that after SCB. This is not surprising, since with the costoclavicular block the recommended block needle tip location at the time of injection is very similar to that with the RCB, just caudal from the clavicle and in immediate proximity to the axillary artery.
Significantly, we found a higher incidence of phrenic nerve block after SCB than the commonly cited rate of 50%.14,16 Reported rates of phrenic nerve block after SCB vary greatly, something that may be related to the choice of local anaesthetic, mix, concentration and volume used,12 in addition to the specifics of nerve block approach and injection technique.10,11 The methods chosen to assess diaphragmatic function also vary and the participant numbers are relatively small to reflect the effects of variability in patient anatomy. In this study, the observed rate of phrenic nerve block, 70% as defined by diaphragmatic excursion, and 55% phrenic block when defined by MIV, may possibly be attributed to the relatively high local anaesthetic volume, 25 ml, but also to the technique of injecting at multiple locations around the plexus, both in the ‘corner pocket’ and the superior portion of the neural cluster, as previously described.10,22,23
To assess diaphragmatic function, we employed both a high-tech, more sensitive and specific outcome (diaphragmatic excursion measurement with M-mode ultrasound), and a low-tech, yet clinically relevant outcome requiring only a simple incentive spirometer (MIV). These high-tech and low-tech outcomes correlated highly for any given individual patient. Although the high-tech outcome (diaphragmatic excursion) could in fact be easily measured in most block patients, given that an ultrasound machine is used for block placement in most cases, the low-tech outcome (MIV) could be measured by any healthcare provider or even by the patient her/himself. Notably, none of the study patients who were found to have diaphragmatic weakness displayed any dyspnoea; however, patients with pulmonary disease were excluded from recruitment.
One previously expressed concern regarding the relatively new RCB is that hypothetically it poses a greater risk for neural or vascular injury than the more traditional SCB.7,27 The current study is underpowered to detect increase in risk, but transient paraesthesia was not observed more frequently (four in RCB group, nine in SCB group) and no patient developed persistent paraesthesia. Similarly, previous investigations of RCB have not reported an increase in complications.3,4 Another concern raised about the RCB in a recent anatomical study is that the suprascapular nerve and vein lie in the needle path at a point which is blinded to ultrasound guidance,27 perhaps referring to the posterior approach described by Hebbard et al. where the proposed needle trajectory crossed parts of the trapezius, supraspinatus and subscapularis muscles.1,28 The RCB trajectory employed in the current study has been consistently described to start anterior to the trapezius muscle and immediately posterior to (just off) the clavicle,2-7 thus avoiding all muscles except for the subclavius muscle, and therefore a safer anatomical path relative to the suprascapular nerve and vein. Significantly, sensory and motor block in the suprascapular nerve distribution were also observed in the RCB patients, but with a consistent and significant delay when compared with the SCB group (Fig. 4b). Such indirect evidence that the risk of suprascapular nerve injury is not increased with RCB, needs further investigation.
The current study presents several limitations and challenges. First, because of most patients were having ambulatory surgery, the duration of nerve blocks, including the phrenic nerve block, was not examined. Second, the relatively low number of participants precluded the power to detect differences in complications. Similarly, while no significant differences in clinical effectiveness were observed, this study was not designed to evaluate differences in success rate, pain scores or opioid consumption between the two techniques.
Conclusion
The incidence of inadvertent ipsilateral phrenic nerve block and its degree after RCB is lower than after SCB.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: none.
Footnotes
Conflicts of interest: none.
Presentation: preliminary data for this study were presented as a poster presentation at the IARS meeting in Montreal, Canada 16 to 20 May 2019, at the ASRA meeting in Las Vegas, USA 11 to 13 April 2019, and at the ISURA meeting in Porto, Portugal 9 to 11 May 2019.
References
- 1.Hebbard P, Royse C. Ultrasound guided posterior approach to the infraclavicular brachial plexus. Anaesthesia 2007; 62:539. [DOI] [PubMed] [Google Scholar]
- 2.Vlassakov K, Janfaza D. Ultrasound-guided retroclavicular approach to the brachial plexus cords [ASRA Abstract A-122]. Presented at the ASRA 33rd Annual Regional Anesthesia Meeting and Workshops, 1 – 4 May 2008, in Playa Del Carmen (Cancun) Mexico. Available at: https://asra.megahosters.com/display_spring_2008.php?id=160. [Google Scholar]
- 3.Charbonneau J, Frechette Y, Sansoucy Y, et al. The ultrasound-guided retroclavicular block: a prospective feasibility study. Reg Anesth Pain Med 2015; 40:605–609. [DOI] [PubMed] [Google Scholar]
- 4.Kavrut Ozturk N, Kavakli AS. Comparison of the coracoid and retroclavicular approaches for ultrasound-guided infraclavicular brachial plexus block. J Anesth 2017; 31:572–578. [DOI] [PubMed] [Google Scholar]
- 5.Sinha C, Kumar N, Kumar A, et al. Comparative evaluation of two approaches of infraclavicular brachial plexus block for upper-limb surgeries. Saudi J Anaesth 2019; 13:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton EM, Bullock WM, Gadsden J. The retroclavicular brachial plexus block: additional advantages. Reg Anesth Pain Med 2015; 40:733–734. [DOI] [PubMed] [Google Scholar]
- 7.Grape S, Pawa A, Weber E, et al. Retroclavicular vs supraclavicular brachial plexus block for distal upper limb surgery: a randomised, controlled, single-blinded trial. Br J Anaesth 2019; 122:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koscielniak-Nielsen ZJ, Frederiksen BS, Rasmussen H, et al. A comparison of ultrasound-guided supraclavicular and infraclavicular blocks for upper extremity surgery. Acta Anaesthesiol Scand 2009; 53:620–626. [DOI] [PubMed] [Google Scholar]
- 9.Neal JM, Gerancher JC, Hebl JR, et al. Upper extremity regional anesthesia: essentials of our current understanding, 2008. Reg Anesth Pain Med 2009; 34:134–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang RA, Chung YH, Ko JS, et al. Reduced hemidiaphragmatic paresis with a ‘corner pocket’ technique for supraclavicular brachial plexus block: single-center, observer-blinded, randomized controlled trial. Reg Anesth Pain Med 2018; 43:720–724. [DOI] [PubMed] [Google Scholar]
- 11.Renes SH, Spoormans HH, Gielen MJ, et al. Hemidiaphragmatic paresis can be avoided in ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2009; 34:595–599. [DOI] [PubMed] [Google Scholar]
- 12.Bao X, Huang J, Feng H, et al. Effect of local anesthetic volume (20 mL vs 30mL ropivacaine) on electromyography of the diaphragm and pulmonary function after ultrasound-guided supraclavicular brachial plexus block: a randomized controlled trial. Reg Anesth Pain Med 2019; 44:69–75. [DOI] [PubMed] [Google Scholar]
- 13.Ueshima H, Otake H. Incidence of phrenic nerve paralysis after ultrasound-guided supraclavicular brachial plexus block. J Clin Anesth 2019; 56:37–38. [DOI] [PubMed] [Google Scholar]
- 14.Mak PH, Irwin MG, Ooi CG, et al. Incidence of diaphragmatic paralysis following supraclavicular brachial plexus block and its effect on pulmonary function. Anesthesia 2001; 56:352–356. [DOI] [PubMed] [Google Scholar]
- 15.Pham-Dang C, Gunst JP, Gouin F, et al. A novel supraclavicular approach to brachial plexus block. Anesth Analg 1997; 85:111–116. [DOI] [PubMed] [Google Scholar]
- 16.Neal JM, Moore JM, Kopacz DJ, et al. Quantitative analysis of respiratory, motor, and sensory function after supraclavicular block. Anesth Analg 1998; 86:1239–1244. [DOI] [PubMed] [Google Scholar]
- 17.Dullenkopf A, Blumenthal S, Theodorou P, et al. Diaphragmatic excursion and respiratory function after the modified Raj technique of the infraclavicular plexus block. Reg Anesth Pain Med 2004; 29:110–114. [DOI] [PubMed] [Google Scholar]
- 18.Petrar SD, Seltenrich ME, Head SJ, et al. Hemidiaphragmatic paralysis following ultrasound-guided supraclavicular versus infraclavicular brachial plexus blockade: a randomized clinical trial. Reg Anesth Pain Med 2015; 40:133–138. [DOI] [PubMed] [Google Scholar]
- 19.Rettig HC, Gielen MJ, Boersma E, et al. Vertical infraclavicular block of the brachial plexus: effects on hemidiaphragmatic movement and ventilatory function. Reg Anesth Pain Med 2005; 30:529–535. [DOI] [PubMed] [Google Scholar]
- 20.Sivashanmugam T, Maurya I, Kumar N, et al. Ipsilateral hemidiaphragmatic paresis after a supraclavicular and costoclavicular brachial plexus block: a randomised observer blinded study. Eur J Anaesthesiol 2019; 36:787–795. [DOI] [PubMed] [Google Scholar]
- 21.Tran DQ, Dugani S, Correa JA, et al. Minimum effective volume of lidocaine for ultrasound-guided supraclavicular block. Reg Anesth Pain Med 2011; 36:466–469. [DOI] [PubMed] [Google Scholar]
- 22.Techasuk W, González AP, Bernucci F, et al. A randomized comparison between double-injection and targeted intracluster-injection ultrasound-guided supraclavicular brachial plexus block. Anesth Analg 2014; 118:1363–1369. [DOI] [PubMed] [Google Scholar]
- 23.Sivashanmugam T, Ray S, Ravishankar M, et al. Randomized comparison of extrafascial versus subfascial injection of local anesthetic during ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2015; 40:337–343. [DOI] [PubMed] [Google Scholar]
- 24.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by M-mode ultrasonography: methods, reproducibility, and normal values. Chest 2009; 135:391–400. [DOI] [PubMed] [Google Scholar]
- 25.Song JG, Kim SK, Jeon DG, et al. Dyspnea after supraclavicular brachial plexus block in a morbidly obese patient due to phrenic nerve block: a case report. Korean J Anesthesiol 2009; 57:511–514. [DOI] [PubMed] [Google Scholar]
- 26.Jennes E, Vriens PWHE, Heyligers JMM. Acute dyspnoea during brachial plexus blockade. Ned Tijdschr Geneeskd 2017; 161:D1216; [Article in Dutch]. [PubMed] [Google Scholar]
- 27.Sancheti SF, Uppal V, Sandeski R, et al. A cadaver study investigating structures encountered by the needle during a retroclavicular approach to infraclavicular brachial plexus block. Reg Anesth Pain Med 2018; 43:752–755. [DOI] [PubMed] [Google Scholar]
- 28.Beh ZY, Hasan MS, Lai HY, et al. Posterior parasagittal in-plane ultrasound-guided infraclavicular brachial plexus block-a case series. BMC Anesthesiol 2015; 15:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.