Since 2000, collaborative and multidisciplinary field inventories have helped quadruple park coverage in Peru’s richest region.
Abstract
Meeting international commitments to protect 17% of terrestrial ecosystems worldwide will require >3 million square kilometers of new protected areas and strategies to create those areas in a way that respects local communities and land use. In 2000–2016, biological and social scientists worked to increase the protected proportion of Peru’s largest department via 14 interdisciplinary inventories covering >9 million hectares of this megadiverse corner of the Amazon basin. In each landscape, the strategy was the same: convene diverse partners, identify biological and sociocultural assets, document residents’ use of natural resources, and tailor the findings to the needs of decision-makers. Nine of the 14 landscapes have since been protected (5.7 million hectares of new protected areas), contributing to a quadrupling of conservation coverage in Loreto (from 6 to 23%). We outline the methods and enabling conditions most crucial for successfully applying similar campaigns elsewhere on Earth.
INTRODUCTION
Achieving Aichi Biodiversity Target 11, the protection of at least 17% of Earth’s terrestrial ecosystems, will require the creation of new parks and reserves covering an area larger than India (1). While research to determine which new areas should be protected is common, research examining how those new areas might achieve protection, and might do so in a socially equitable fashion, is not. For example, protected area coverage in Amazonia has grown markedly over the past two decades, but the scientific, political, and historical processes behind that growth have been poorly documented (2).
Here, we report on one process behind the historic shift in Amazonian land use: a campaign of collaborative conservation science designed to provide the information needed to protect megadiverse landscapes. We worked in Peru’s Loreto department, a 37 million ha expanse of Amazonian lowlands and Andean foothills bordering Ecuador, Colombia, and Brazil, which is one of the most biologically diverse regions on Earth. Accounting for only 5% of the Amazon basin by area, Loreto harbors 20 to 40% of the Amazon’s terrestrial vertebrate species (Fig. 1) (3); is traversed by eight major Amazonian rivers; and sits at the convergence of global maxima for mammal, bird, amphibian, tree, and aquatic fauna diversity (4–10). Loreto boasts 96% forest cover within the world’s largest block of intact forest (11–13), the largest peat deposits in the Amazon basin (14), >300 endemic plant and vertebrate species (3, 15), and >50% of Peru’s aboveground carbon stocks (11) and freshwater fish species (16). It is also a hot spot of cultural diversity, home to >170,000 indigenous inhabitants belonging to 24 groups (fig. S1).
Fig. 1. Maps illustrating the distribution of species richness of four taxonomic groups across South America from highest (red) to lowest (blue).
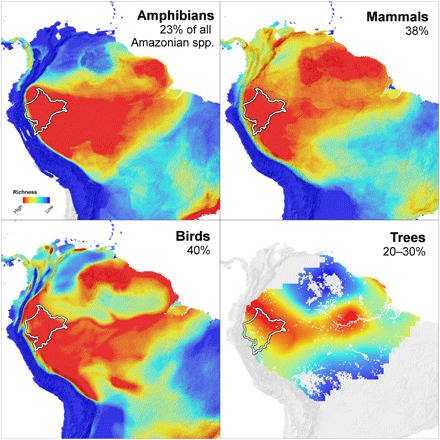
The white border outlines Peru’s Loreto region, which accounts for 5% of the Amazon basin’s area. Percentages in each panel indicate the proportion of all Amazonian species expected to occur in Loreto. For amphibians, mammals, and birds, maps and percentages were based on (4); the tree map was based on (5), and the tree percentage is an estimate based on (3, 6).
In 2000, Loreto had a single protected area covering only 5.6% of its territory and three provisional areas awaiting official designation (an additional 2.3%). Today, Loreto has 14 protected areas covering 23.0% of its territory (and two provisional areas covering 0.8%). This recent expansion of Loreto’s conservation coverage can serve as a roadmap for conservationists in incompletely protected landscapes elsewhere, especially since much of it occurred in the wake of replicable and well-documented conservation interventions. More generally, the rapid inventory methods and results described here contribute to the larger project of rigorously documenting conservation interventions and their outcomes in a way that helps conservationists identify successful strategies and avoid mistakes (17).
RESULTS
Conservation results
Of the 14 unprotected landscapes in Loreto visited by rapid inventories in the period 2000–2016, 9 are now partly or entirely inside new protected areas (a 64% success rate). Of the 8.9 million ha studied in rapid inventories, 5.7 million ha were subsequently designated as 11 new protected areas (a 64% success rate). Between 2000 and 2019, the proportion of Loreto in protected areas increased from 6 to 23%, as its protected area coverage grew from 2 to 8.5 million ha (Fig. 2 and table S1). These protected areas have lower rates of deforestation and forest degradation compared to unprotected forests (18).
Fig. 2. Map of Peru’s Loreto region contrasting protected area coverage in 2000 and 2019.
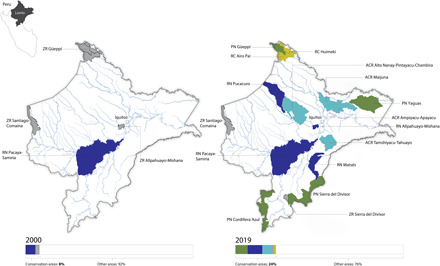
Colors indicate the different types of conservation areas: green, national parks (PN); gray, national-level reserved zones (ZR); dark blue, national reserves (RN); yellow, national-level communal reserves (RC); and light blue, regional conservation areas (ACR).
The 11 new protected areas declared following rapid inventories in Loreto represent 84.6% of the 13 new protected areas declared in Loreto during that period and account for 89% of the newly protected land by area. They include four different categories of national- and regional-level areas, classified in the International Union for Conservation of Nature Protected Area Management categories II (4) and VI (7) (Fig. 2 and table S1) (19). The 11 protected areas declared following rapid inventories contain 89% of Loreto’s terrestrial vertebrate species (mol.org/places), ~650 Tg of aboveground carbon (11), and representative areas of the region’s key terrestrial and aquatic habitats (white sand forest, blackwater rivers, peatland swamps and other large wetlands, montane and lowland forests, and headwater streams). Two of the areas protect an additional 1.1 million ha outside of Loreto, in neighboring regions of Peru.
On average, 5 years passed between the conclusion of rapid inventory field work in a landscape and the declaration of a new protected area there (Fig. 3). The most recent five rapid inventories (field work in 2012–2016) have not yet resulted in new protected areas, but the regional and national governments are currently making progress toward protecting three of those landscapes.
Fig. 3. Timeline showing the dates of 14 rapid inventories in Loreto, Peru and the dates of any subsequent protected area creation.
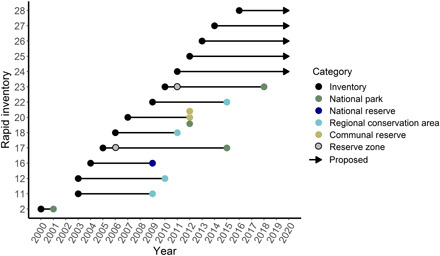
Reserved zones are precursory designations of government intent, not functioning protected areas.
Biological inventory results
The 14 rapid biological inventories in Loreto generated >18,000 museum specimens and >17,000 sighting records, as well as >4300 pages of reports, >10,000 photographs of species in the field, and 23 photographic field guides. In total, we recorded >1800 vertebrate and >2400 vascular plant species. Inventories captured 93% of Loreto’s known terrestrial vertebrate fauna and nearly all of the 75 globally threatened species in that group. All plant and fish occurrence records are available on Global Biodiversity Information Facility (GBIF) (gbif.org), and all terrestrial vertebrate occurrence records are available on Map of Life (mol.org). Plant specimens were deposited at the Herbario Amazonense de la Universidad Nacional de la Amazonía Peruana (AMAZ), Herbario San Marcos de la Universidad Nacional Mayor de San Marcos (USM), Herbario Vargas de la Universidad Nacional San Antonio Abad del Cusco (CUZ), Herbario del Museo de Historia Natural del Ecuador (QCNE), Herbario de la Universidad Estatal Amazónica (ECUAMZ), and Searle Herbarium of the Field Museum (F herbaria); animal specimens were deposited at Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos (MUSM), Centro de Ornitología y Biodiversidad (CORBIDI), Museo de Colecciones Referenciales Biológicas del Instituto de Investigaciones de la Amazonía Peruana (CRBIIAP), Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ), Universidad Nacional de la Amazonía Peruana (UNAP), and the Field Museum.
We recorded a large number of undescribed species, including at least 39 vascular plants (now described; Fig. 4D), 20 fishes (3 described and 17 suspected), 33 amphibians (4 described and 29 suspected), 3 reptiles (suspected), 2 mammals (suspected), and 1 bird (suspected). Rapid inventories in Loreto generated the first Peruvian records for 64 plant species (20), 10 amphibian species, 5 reptile species, and 1 bird species, as well as dozens of range extensions for vertebrates. Biological data collected in the Loreto rapid inventories have been used in >125 peer-reviewed scientific articles to date.
Fig. 4. Photographs of rapid biological inventories and social assessments in Loreto, Peru.

(A) The Maijuna indigenous community of Nueva Vida, visited in 2009. Photo credit: Álvaro del Campo, Field Museum. (B) The biological team and community members on the banks of the Yaguas River, visited in 2010. Photo credit: Álvaro del Campo, Field Museum. (C) A participatory mapping exercise led by the social science team during a rapid inventory in the Ere-Campuya region in 2012. Photo credit: Federico Pardo, Field Museum. (D) Ladenbergia shawistigma Chilq., a shrub found during the rapid inventory of the Cordillera Escalera mountains in 2013 and 1 of 39 new plant species found during the work described here. Photo credit: Luis Torres Montenegro, Peruvian Center for Biodiversity and Conservation.
Social assessment results
Despite the very different cultural and historical backgrounds of the communities we visited, rapid social assessments uncovered a set of consistent social and cultural assets among residents, which we described in ~800 pages of inventory reports. These include a deep knowledge of the landscape and its natural resources (fig. S2), a recognition by residents that quality of life in their communities is high because many needs (such as food, water, medicine, and construction materials) are provided by the natural resources they harvest in healthy forests and rivers, a recognition of communities’ dependence on natural areas adjacent to (i.e., outside of) communal territories (fig. S2), a culture of mutual assistance and reciprocity that underlies a strong capacity for self-organization and collaborative management, and, in indigenous communities, a strong interest in maintaining traditional practices (21). Together, these common assets translated into a strong interest in preventing the degradation of forests and neighboring communal territories, especially by outsiders and commercial interests, and thus, strong support for conservation areas that respected and built on communities’ social assets.
The 11 protected areas declared following rapid inventories directly or indirectly benefited 257 indigenous and campesino communities and towns in Loreto and a total of 32,642 residents of those communities. Seven of the new protected areas are direct-use areas that permit subsistence practices by local residents and prohibit these activities by outsiders. These areas also allow small-scale market-oriented activities—such as fishing, hunting, and harvesting of nontimber forest products—as long as they are carried out with management plans. Four of the new protected areas (all national parks) permit subsistence activities by residents but prohibit direct commercial uses, with some exceptions (19). These national parks provide a suite of additional benefits for local residents, such as the protection of sites that are culturally or historically important and the protection of source populations of game and fish species harvested by residents outside of the park borders, e.g., in neighboring indigenous territories.
DISCUSSION
The methods described in this paper helped both Loreto (23%) and Peru (21%) achieve Aichi Biodiversity Target 11, 17% of terrestrial ecosystems under protection, and are currently being used to help Loreto reach a more ambitious target. Because of their combined social and biological focus, rapid inventories also expedited the creation of protected areas that are equitably established and managed, ecologically representative, and well connected (three other goals of Target 11). In this section, we discuss which aspects of the rapid inventory model made it effective in Loreto, what other factors contributed to the expansion of the protected areas system there, and how the model can be applied to other regions.
Key strategies of the rapid inventory model
We believe that much of the rapid inventory program’s success in conserving landscapes is due to synthesis and consensus building, appreciation of local knowledge, speed, and strong and diverse partners. We relied on five deliberate strategies that go beyond the traditional model of biological inventories (22): (i) building a consensus vision for conservation across a wide cross section of stakeholders and scientists, (ii) ensuring that local communities and organizations were at the forefront of that vision, (iii) basing the vision on both social and biological data with the complementary goals of maintaining healthy ecosystems and guaranteeing local residents’ quality of life, (iv) quickly creating synthetic products that decision-makers need to implement the vision as policy, and (v) maintaining long-term engagement with decision-makers to promote the creation and sustainability of these areas, largely by working with in-country partners who have a long-term commitment to communities, conservation, and territorial planning in the target landscape.
These strategies adhere closely to the newly described field of translational ecology, “an intentional approach in which ecologists, stakeholders, and decision-makers work collaboratively to develop and deliver ecological research that, ideally, results in improved environment-related decision-making” (23). In general, we believe that translational ecology provides a crucial framework for achieving Aichi Biodiversity Target 11.
A key aspect of our work in Loreto was collaboration with hundreds of experts and dozens of institutions (fig. S3). These partners varied markedly in their knowledge of the landscape, political and economic power, scientific expertise, and experience in conservation. Rather than downplaying these imbalances, we highlighted them in meetings and workshops so that the team understood what was valuable about each partner’s contribution and could build a consensus plan for conservation that was salient (useful for decision-makers) and scientifically credible while also embraced by stakeholders as a legitimate reflection of their knowledge and values (24).
For example, local residents often had years of experience managing a landscape but limited opportunities to share their knowledge with others or to formalize their sustainable management practices. Likewise, staff in government agencies often had deep knowledge of conservation policy in Peru but a limited understanding of the sociocultural, political, and biological contexts in the priority areas they were tasked with protecting. By bringing the two groups together (often for the first time) and treating both as crucial members of the team, we could build a consensus plan that was not only seen as broadly legitimate and, thus, attractive to decision-makers but also that ensured a more effective protected area over the long term by incorporating the knowledge and needs of local people into our recommendations for conservation (25).
Creating an environment in which a large number of very different stakeholders’ contributions were valued required dedicated work in communication, translation, facilitation, and mediation (24). This work was typically performed by the Field Museum and its primary partners whose staff were trained in “boundary-spanning” practices, i.e., a commitment to translate insights among partners and to create products perceived as legitimate by all (26). We found that the Field Museum was especially valued in Loreto as a boundary-spanning organization, because our base at a large natural history museum overseas meant we were seen as (i) scientifically reliable, (ii) respectful of both cultural and biological diversity, (iii) a relatively impartial convener, and (iv) committed to translating rigorous scientific results for a broad audience (27).
Other important enabling conditions
Ours was not an experimental approach, and we cannot draw rigorous conclusions about causality. Confirming a causal link between the methods described here and protected area creation will require more detailed documentation of the processes behind the creation of other protected areas in Peru and in South America during this same time period (2000–2016). In the meantime, at least four indicators offer strong evidence that rapid inventories facilitated the creation of protected areas that might not otherwise have been created or that might have taken many more years to create. First, almost none of the target landscapes had processes in motion to create a new protected area when rapid inventories were carried out there; the inventories initiated those processes or restarted earlier processes that had fallen dormant (see Fig. 3). Second, the official government reports used to formally propose and establish the 11 new areas in Loreto contain substantial amounts of data collected during rapid inventories. Third, personal communications from numerous stakeholders and decision-makers have confirmed the importance of rapid inventories for their work to create new areas in Loreto. Last, all but one of the target landscapes featured competing development proposals or land-use designations at the time of our rapid inventories, including oil concessions, timber concessions, proposed roads, proposed transmission lines, and mining concessions. By providing a compelling counterargument for conservation and well-being of the local people, rapid inventories helped prevent further degradation to these landscapes and their formalization as extractive landscapes, both of which would have hindered protected area creation at a later date.
Independent of the rapid inventories campaign, a number of political and socioeconomic trends clearly contributed to an expansion of the protected areas system in Loreto. For example, in 2000, a large proportion of land in Loreto lacked title or a clear land-use category and was viewed by decision-makers as needing formalization (28). Because this led the Peruvian and Loreto park services to issue maps of conservation priority areas in 2000 and 2009, respectively (28–30), based on the recommendations of scientists, indigenous leaders, nongovernmental organizations (NGOs), and public agencies, our subsequent inventories in those areas were regarded as the continuation of established government policy and also had some degree of popular support.
Over our 20-year campaign, as political leadership in Loreto (and Peru) fluctuated from pro- to anti-conservation, one administration made a key contribution to increasing the region’s protected areas coverage. In 2007–2014, the governor of Loreto promoted a vision of green development that represented an abrupt departure from the region’s traditional extractive model dependent on logging. The new vision reflected a perception at the time among indigenous leaders and left-leaning politicians in Loreto and across western Amazonia that extractive economies had eroded natural resources and quality of life and that environmentally friendly development alternatives and stronger land rights for indigenous peoples [sometimes organized around the concept of buen vivir (31)] were needed to restore them. In 2007, the Loreto government created an agency tasked with creating and managing a network of regional protected areas, open to sustainable use and comanaged by communities, that would complement the parks and reserves managed in Loreto by the national park service. This agency, now known as the Dirección Ejecutiva de Conservación y Diversidad Biológica del Gobierno Regional de Loreto [DICREL, formerly PROCREL (Programa de Conservación, Gestión y Uso Sostenible de la Diversidad Biológica de Loreto)], identified conservation priority landscapes for Loreto (30), strengthened public support for the green development vision, worked closely with decision-makers in the Loreto regional government, and was largely responsible for 4 of the 11 new conservation areas declared in Loreto (see table S1). This regional trend was reinforced by institutional advancements at the national level, e.g., the 2008 establishment of Peru’s Ministry of the Environment and the strengthening of indigenous organizations in Peru.
Rapid inventories of these priority areas revealed another intrinsic incentive for establishing protected areas. While these landscapes were considered “empty” and “uncategorized” by government agencies, most of them were already under the de facto conservation management of nearby indigenous and campesino communities whose titled territories were too small to meet the basic needs for subsistence hunting, fishing, and harvesting (32). These communities were often eager to formalize their unlicensed use and management of adjacent lands to protect the lands they relied on from outside loggers, hunters, and fishermen and to restore resources that had been degraded (33) and were, thus, willing to support the creation of conservation areas whose zoning and management plans allowed sustainable uses of natural resources by local residents.
One additional factor that made possible the expansion of Loreto’s protected areas system was the availability of sustained and coordinated funding for conservation work in the region. In addition to the direct costs of carrying out rapid inventories (activities 1 to 7 in Fig. 5), the Field Museum and primary partners often required substantial additional funds to continue engaging with decision-makers and residents of the target landscapes in the years before an area was declared. Much of this funding came from the Gordon and Betty Moore Foundation (2, 34), which supported at least six organizations advancing conservation work in Loreto, including DICREL, and encouraged coordination among them. For the rapid inventory work itself, the cost was substantially lower than $1/ha protected.
Fig. 5. Ten sequential activities carried out as part of a rapid biological inventory and social assessment designed to facilitate protected area creation in Loreto, Peru.
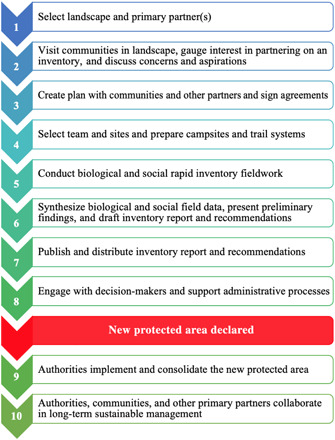
For more details, see table S2.
Rapid inventories that did not result in protection
Not all rapid inventories in Loreto have resulted in protected area creation, and these cases offer insight into the conditions that are most crucial for success. The most common reason protected areas were not created after rapid inventories was the lack of a sufficiently strong consensus for conservation across local communities and government. In these cases, the methods described above were not able to overcome disagreements among local communities or their higher-level organizations (e.g., local indigenous communities favored a protected area, while the regional organization that represents them did not, or some communities favored a protected area, but others wanted a new road), discord among government agencies (e.g., the park service favored a protected area, but another branch of government was promoting forestry or oil concessions there), or mutual distrust between government and local communities.
The most prominent example of the latter is the Santiago-Comaina Reserved Zone in western Loreto, a wilderness area that indigenous communities and the national government are both strongly committed to conserving. The reserved zone was established in 1999, but since then, contrasting visions of how to conserve it by indigenous groups and the park service (a large new indigenous territory and a national-level protected area, respectively) have proven impossible to reconcile. Our 2011 rapid inventory made progress brokering a compromise but not enough to countervail the violent conflict that broke out between the national government and indigenous groups in the region 2 years earlier. This suggests that our strategies demand longer engagement, or are simply less likely to succeed, where violent social discord prevails (a conclusion corroborated so far by our 2016 and 2018 rapid inventories in conflict zones in the Colombian Amazon). On the other hand, all of the landscapes where rapid inventories facilitated new area creation featured some serious disagreement among and between communities and the government, and our consensus-building strategy helped overcome the discord. In our experience, the fundamental prerequisite for success is a willingness by both the government and residents to recognize each other’s authority in land management.
Another lesson learned from rapid inventories that did not result in area creation is that some landscapes require a longer-term investment in postinventory engagement with government decision-makers than our team, and primary partners have been able to maintain. This is especially the case for landscapes in which the work of reconciling existing land-use categories with a new protected area requires long and complicated bureaucratic processes or multiple levels of government. Last, our experience with rapid inventories in Amazonian countries with strongly centralized national governments (e.g., Colombia and Bolivia in the early 2000s) make it clear that Peru’s decentralized government and, particularly, Loreto’s vigorous regional conservation program were important factors in the extension of its protected area coverage.
Contributions to protected area consolidation
While the focus of our work was protected area establishment, we recognize that new protected areas in Loreto require substantial additional work to conserve these landscapes effectively over the long term (34). Rapid inventories contributed to that consolidation by (i) providing sound scientific information needed to establish management priorities; (ii) strengthening working relationships between government personnel and community leaders before an area was declared; (iii) convening stakeholders to draft consensus recommendations on zoning, community participation in management, and other aspects of consolidation before an area was declared; (iv) creating a large and publicly available collection of documentary material that the government and others could use to attract long-term funding for operations; (v) helping local communities map their natural resource use, formalize their vision for the future of the landscape, and document social assets relevant to protected area management; and (vi) supporting the long-term work and commitment of primary partners in the target landscapes.
Another way that the rapid inventories campaign contributed to deepening long-term commitments to conservation was by encouraging communities involved in inventories to carry out quality of life planning. Drafted by communities, often with the support of primary partners, quality of life plans describe residents’ vision of well-being on the landscape and outline the steps necessary to achieve that vision. These plans also provide a foundation for dialog and collaboration between park managers and local communities and facilitate the incorporation of local communities’ needs, priorities, and participation into the protected area management plans and other government planning processes (21).
In addition to protected area creation and consolidation, rapid inventories in Loreto also advanced a number of other important goals in tropical science and conservation (35). These include reducing shortfalls in biodiversity knowledge (36), training Peruvian biologists, contributing specimens to Peruvian museums, providing open-access biodiversity information in Spanish and indigenous languages, and contributing to parallel efforts to protect Loreto’s forests by titling new indigenous and campesino communities (18, 37).
Work to expand Loreto’s protected area coverage and involve local people in the management of protected areas is ongoing. At least 1.7 million ha designated as conservation priorities by the Loreto regional government await categorization. The amount of Loreto’s land in other conservation-friendly land-use categories (indigenous territories, conservation concessions, ecotourism concessions, inactive forestry concessions, and private conservation areas) exceeds that in its protected areas. Together, these lands account for roughly 50% of Loreto’s territory. With continuing investment and coordination, these lands can provide an effective defense against threats, such as road building, plantation farming, and dam building. Meeting both of these challenges, connecting individual areas in integrated conservation corridors that are anchored by engaged and supportive communities, has the potential to provide massive benefits for the Amazon.
MATERIALS AND METHODS
The applied science campaign was organized around rapid biological and social inventories of the target landscapes. Each rapid inventory consisted of a series of sequential activities requiring ~2 years of work. These methods are described in detail below and in table S2 and are summarized in Fig. 5.
Landscape selection, coordination among partners, and team building
We selected landscapes in Loreto that had previously been identified as conservation priorities by working groups of indigenous leaders, NGOs, scientists, and government officials (Fig. 6) (28–30). Large landscapes were preferred over small ones to maximize the potential conservation impact of each inventory. Selected landscapes averaged >1.1 million ha in size and were typically remote and sparsely populated expanses of intact forest adjoining indigenous communities, campesino or ribereño (a mix of indigenous and nonindigenous) communities, or existing protected areas (Fig. 4A).
Fig. 6. Map of Loreto, Peru, showing 14 rapid inventory landscapes (gray polygons), including 45 biological inventory campsites (green dots) and 104 social assessment sites (blue dots).
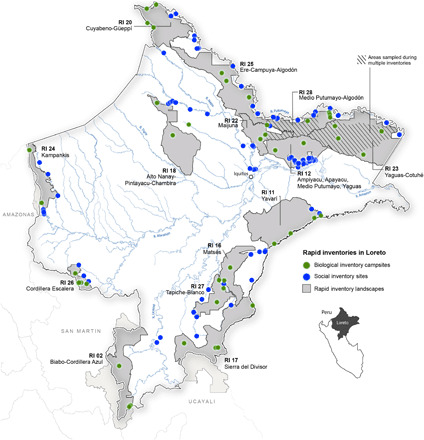
Detailed information on each inventory is given in table S1. Inset shows Loreto’s location in Peru.
The essential prerequisite for selecting a landscape was the existence of one or more Loreto-based partners with a long-term commitment to conserving that landscape and often a history of working with local communities there (hereafter referred to as “primary partners”). These partnerships made it possible for the Field Museum team to generate information for a large number of different landscapes, while the primary partners led the follow-through activities for each one. The 14 rapid inventories involved 30 primary partners (see full list in table S1), including 13 local indigenous federations, regional and national government agencies, five Peruvian NGOs, and the Peruvian programs of three international NGOs. For a given rapid inventory, primary partners typically included one or more indigenous federations, an NGO, and a government agency. The most frequent primary partner was the Peruvian NGO Instituto del Bien Común (six inventories), which has worked on territorial planning with Loreto communities since 1998.
Once the Field Museum and the primary partners had agreed to work in a given landscape, we engaged local, regional, and national stakeholders to learn more about the sociopolitical context of the landscape. The most crucial step in this phase was a series of meetings with representatives of communities in the landscape to (i) explain the goals and methods of a rapid inventory; (ii) ask for their permission and support; (iii) hear their concerns, advice, and goals regarding the work; (iv) invite their participation in the inventory, and, if they were interested in participating; (v) draft a consensus plan for the inventory and its follow-up. Decisions were formalized in written agreements (Fig. 5).
Selecting team members for the rapid inventory was another key aspect of this phase. The core team typically consisted of seven to eight Field Museum and seven to eight primary partner staff, many with prior experience in rapid inventories. For every one of these core experts, we typically invited one to two experts associated with Peruvian universities, conservation organizations, indigenous federations, or campesino organizations, for a total team size of 30 to 50. Team building was also used to engage regional and national stakeholders; we included experts from those organizations on the team to increase the number of people who would regard the inventory output as reliable, useful, and an institutional priority. Some individuals and institutions who joined our teams would likely have opposed protected area creation had they not been involved because they would have lacked ownership and a clear view of the process.
Over the 14 rapid inventories, we did not work with the same small circle of partners. Instead, the cumulative number of partners increased at a constant rate throughout the campaign (fig. S3).
Biological inventory methods
The primary goals of the rapid biological inventory were to identify species, natural resources, and landscape features with high conservation value (at global, national, or local scales), to assess the conservation status of those assets (i.e., on a gradient from well preserved to seriously degraded), and to document threats. Before carrying out a rapid biological inventory, we studied satellite images and geological maps, talked with local residents, and flew over the landscape on a small plane or helicopter. One goal of these first steps was to identify three to five campsites that would allow us to visit a diversity of vegetation types and aquatic habitats, including both dominant and rare features on the landscape (Fig. 6). Many of these sites were in remote headwater areas far from local villages and were accessed by helicopter; almost none had been visited by biologists before. To facilitate field work, advance teams with local residents visited each site 1 month before the surveys to open ~20 km of trails.
On a typical inventory, biological teams consisted of 16 professional biologists and one geologist and six to seven residents of nearby villages who were familiar with the landscape (Fig. 4B). A total of 97 professional biologists participated in the 14 rapid inventories in Loreto. Of these, 48% were Peruvian, 24% were from elsewhere in South America, and 28% were from outside South America. In landscapes close to international borders, we made an effort to include experts from Ecuador, Colombia, or Brazil.
We surveyed each site for 1 to 5 days. At each site, one to three geologists sampled surface soils and waters; one to nine botanists collected and surveyed vascular plants; two to three ichthyologists collected fishes; two to three herpetologists collected and surveyed amphibians and reptiles; two to six ornithologists surveyed birds; and two to eight mammalogists surveyed large and medium-sized mammals and sometimes surveyed and collected bats.
Our primary aims were to assemble checklists of recorded and expected species, to describe the most valuable conservation assets observed in the field (e.g., unique habitats, endemic or threatened species, and populations of locally prized species), to assess the conservation status and primary threats to those assets, and to photograph species and habitats. We also highlighted conservation assets that were not well represented in existing protected areas in Loreto or Peru. These descriptive products took priority over hypothesis-driven science not only because of the short amount of time at each site and the astronomical diversity of these communities but also because descriptive data are a key ingredient of the technical proposals (expedientes técnicos) used by government agencies in Peru to establish new protected areas.
Social assessment methods
The primary goals of the rapid social assessment were to document the history, values, practices, and aspirations of local communities; to understand local ecological knowledge; and to establish which of these assets could be incorporated into long-term conservation plans and recognized as vital by decision-makers. These goals were grounded in the recognition that the residents of rural Amazonia have deep knowledge of the landscapes they inhabit, depend on natural systems for their well-being, and have long-standing systems for managing their natural resources (38, 39). We wanted to understand this local knowledge and experience so that our recommendations for a new conservation area would not interfere with community practices and so that decision-makers grasped how those practices and the knowledge and values underlying them would support a new conservation area rather than threaten it (e.g., because residents had a keen interest in preventing overharvest and other destructive activities).
The social science team typically consisted of two to three Field Museum social scientists, three to four primary partner staff (often including additional social scientists), and three to four other community leaders. During the inventory, the social science team typically spent 2 to 3 days at each of three to four communities in or adjacent to the target landscape (Figs. 4, A to C, and 6). We chose communities that offered a representative cross section of local cultures, settlement histories, and resource use. At each community, we divided our time between leading workshops and focus groups, conducting interviews and household economic surveys with residents, and observing and participating in daily activities. The workshops typically lasted 2 to 3 hours and involved 20 to 80 adult women and men, including residents invited from nearby communities. Workshop activities included discussions of the methods and goals of rapid inventories and how these might be related to the concerns of the communities about protecting the sources of their livelihoods. This transparent exchange generated trust in the communities for the inventory team and facilitated support for conservation efforts at the local level. We also carried out a group exercise that quantified how community members rated different aspects of communal living (i.e., the relative quality of natural resources, social relations, and cultural, political, and economic conditions) and invited them to reflect on how these elements influence quality of life (21) and a participatory exercise in which residents mapped forest types and land uses on the landscape (e.g., farming, logging, nontimber forest product harvest, and cattle ranching; Fig. 4C and fig. S2), as well as important places (e.g., salt licks and sacred sites), threats, projects, and stakeholders in the region. To facilitate these activities, we prepared maps and field guides with photos of local animals and plants (http://fieldguides.fieldmuseum.org).
Semi-structured interviews with key informants and focus groups (often organized by gender, age, or livelihood) helped us document how natural resources are used, how residents perceive their quality of life, how decisions are made in the community, and how natural resources contribute to the household and communal economy. These were supplemented by household economic surveys to capture income, expenses, and economic benefits derived from natural resources and analyses of family relationships and community support networks. The social assessment team also participated in daily activities such as farming, fishing, use of medicinal plants, and communal work projects.
During the campaign, we gathered data in 104 communities, belonging to 20 different indigenous groups, and in 18 other communities with mostly campesino residents (Fig. 6). The communities we visited had population sizes of 10 to1000 and territory sizes of 5000 to 10,000 ha. To quantify the number of people who have benefited from the 14 protected areas established following rapid inventories, we tallied human populations of all titled indigenous communities, campesino communities, and towns within the buffer zone of those 14 areas.
Analysis, reporting, and engagement with decision-makers
On the last day of field work, the social and biological teams met in a local community to present preliminary findings to residents and to continue the dialog about conservation and quality of life on the landscape. Over the next 2 days, the teams convened in the closest large city with other stakeholders to jointly draft documents summarizing the biological and social assets observed in the field, major threats to those assets, primary conservation opportunities, and recommendations for converting those opportunities into action. Because this group included people with different interests, expertise, authority, familiarity with the landscape, and familiarity with workshops, discussions were moderated to ensure that everyone’s input was considered by the group, whether they were a park service employee, a local fisherman, or a senior scientist.
Over the following week, the team analyzed the field data and wrote a rough draft of the inventory report. The rough draft included the documents mentioned above and a 10-page executive summary for decision-makers, detailed maps and satellite images, and technical chapters and appendices focused on individual taxonomic groups or aspects of the social assessment. During this week, the team also presented the preliminary synthesis of results and recommendations to decision-makers, partners, reporters, and others in Iquitos and Lima.
The typical inventory report was an ~370-page printed volume published 6 to 12 months after the conclusion of field work (16, 39). All texts were published in both Spanish and English, with executive summaries in indigenous languages where appropriate (these included Awajún, Bora, Capanahua, Kichwa, Maijuna, Matsés, Murui, Ocaina, Secoya, Shawi, and Wampis). Hard copies of the report were delivered to the communities, other partners, and key decision-makers in the regional and national governments and presented 12 to 24 months after the inventory at formal events in Iquitos and Lima. Digital copies were indexed and made freely available at the Biodiversity Heritage Library (40).
Immediately after the inventory field work, the Field Museum and primary partners began using the preliminary products of the inventory to engage decision-makers and to gauge their capacity for implementing the team’s recommendations. The primary goal was to maintain the momentum created by the rapid inventory so that opportunities to conserve the target landscape were present on the agendas of the decision-makers best positioned to act. Sometimes, this required regular contact with individual decision-makers over several years, in the form of meetings, correspondence, phone calls, or additional overflights of the landscape. We also provided all technical materials requested by the decision-makers to advance the administrative processes of area declaration, such as maps, texts, species lists, infographics, or other data for expedientes técnicos. Another important component of this phase of work was facilitating meetings and travel to ensure that local community leaders were heard by regional and national authorities and could make their own case for conservation and its importance to their quality of life. While our recommendations for conservation were detailed, we left the specific category of protected area to the discretion of the decision-makers.
Acknowledgments
We thank the thousands of people, hundreds of communities, and dozens of institutions that made these rapid inventories possible. R. Foster and T. Wachter were founding members of the rapid inventory team at the Field Museum and led the botanical team and logistics, respectively, in many of the inventories. Other key contributors include W. S. Alverson, B. Rodríguez Grández, L. Chicaje Curay, R. Panduro, E. Tuesta Cerrón, F. Alvarado Sangama, A. Sandoval Estrella, J. Noningo, A. Noningo, R. Tafur, A. Lancha Pizango, D. Huayunga Inuma, E. Miranda, N. Dávila, G. Knell, and C. López Wong. We thank the Peruvian Forest Service (SERFOR) for providing all research, collection, and export permits for the biological portion of the rapid inventory work. We thank the Field Museum Institutional Review Board for approving the social research of rapid inventories in Loreto. We thank five anonymous reviewers whose comments substantially improved this paper. Funding: Rapid inventories in Loreto were funded by the Field Museum, Gordon and Betty Moore Foundation, John D. and Catherine T. MacArthur Foundation, Hamill Family Foundation, Thomas W. Haas Foundation, Margaret A. Cargill Foundation, Blue Moon Fund, Boeing Company, Exelon Corporation, and Nalco Corporation. The writing of this paper was supported by a grant from the Field Museum Grainger Bioinformatics Center. Author contributions: Conceptualization: D.A.R., A.d.C., D.K.M., N.C.A.P., A.D.R., C.F.V., and A.W. Data curation: D.A.R., A.d.C., L.S.d.S., C.C.J., N.K., A.A.L., D.K.M., M.P.F., N.C.A.P., A.R., D.F.S., T.S., M.E.T., C.F.V., A.W., A.R., R.C.S., M.B.M., A.R.S.R., F.R.F.V., T.M.V., D.E.R.G., L.R.C., R.S., M.A.R.P., J.D.A., G.G.-U., R.v.M., L.O.R., J.J.M.-C., M.H.H., R.G.-V., T.P., A.B., A.C., P.J.V., J.A.M.O., L.A.T.M., and G.N.-I. Formal analysis: D.A.R., L.S.d.S., N.K., N.C.A.P., T.S., and M.E.T. Funding acquisition: D.A.R., D.K.M., N.C.A.P., A.D.R., C.F.V., A.W., R.C.S., M.B.M., D.E.R.G., L.R.C., and J.A.A. Investigation: D.A.R., A.d.C., L.S.d.S., C.C.J., N.K., A.A.L., D.K.M., M.P.F., N.C.A.P., D.F.S., M.E.T., C.F.V., A.W., A.R., R.C.S., A.R.S.R., F.R.F.V., T.M.V., D.E.R.G., L.R.C., J.A.A., R.F.S., M.A.R.P., J.D.A., G.G.-U., R.v.M., L.O.R., J.J.M.-C., M.H.H., R.G.-V., T.P., A.B., A.C., P.J.V., J.A.M.O., G.N.-I., M.E.D.N., C.N.G.T., T.T.T., L.A.T.M., and I.M.A. Methodology: D.A.R., A.d.C., L.S.d.S., C.C.J., N.K., A.A.L., D.K.M., M.P.F., N.C.A.P., A.D.R., D.F.S., T.S., M.E.T., C.F.V., A.W., A.R., R.C.S., M.B.M., A.R.S.R., F.R.F.V., D.E.R.G., L.R.C., N.B.R., and J.A.A. Project administration: D.A.R., A.d.C., D.K.M., N.C.A.P., A.D.R., D.F.S., M.E.T., C.F.V., A.W., R.C.S., M.B.M., D.E.R.G., L.R.C., N.B.R., M.E.D.N., C.N.G.T., T.T.T., and J.A.A. Supervision: D.A.R., D.K.M., N.C.A.P., A.D.R., M.E.D.N., C.N.G.T., T.T.T., and C.F.V. Validation: D.A.R., N.K., D.K.M., N.C.A.P., and C.F.V. Visualization: D.A.R., N.K., N.C.A.P., T.S., M.E.T., R.C.S., M.B.M., A.R.S.R., and F.R.F.V. Writing: D.A.R., C.C.J., A.A.L., N.C.A.P., and C.F.V. Writing (review and editing): D.A.R., A.d.C., L.S.d.S., C.C.J., N.K., A.A.L., D.K.M., M.P.F., N.C.A.P., A.D.R., D.F.S., T.S., M.E.T., C.F.V., A.W., A.R., R.C.S., M.B.M., A.R.S.R., F.R.F.V., T.M.V., D.E.R.G., L.R.C., J.A.A., R.S., M.A.R.P., J.D.A., G.G.-U., R.v.M., L.O.R., J.J.M.-C., M.H.H., R.G.-V., T.P., A.B., A.C., P.J.V., M.E.D.N., C.N.G.T., T.T.T., L.A.T.M., and G.N.-I. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data have been published in 14 rapid inventory reports, which are available as digital files at the Biodiversity Heritage Library (https://biodiversitylibrary.org/bibliography/99291#/summary and https://biodiversitylibrary.org/bibliography/99292#/summary) and as physical volumes at University of Chicago Press (https://press.uchicago.edu/ucp/books/series/FM-RBSI.html). All plant and fish occurrence records are additionally available on GBIF (gbif.org), and all terrestrial vertebrate occurrence records are available on Map of Life (mol.org). Plant specimens were deposited at the AMAZ, USM, CUZ, QCNE, ECUAMZ, and F herbaria, and animal specimens were deposited at MUSM, CORBIDI, CRBIIAP, QCAZ, UNAP, and the Field Museum. All other data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/31/eabe2998/DC1
REFERENCES AND NOTES
- 1.Lewis E., MacSharry B., Juffe-Bignoli D., Harris N., Burrows G., Kingston N., Burgess N. D., Dynamics in the global protected-area estate since 2004. Conserv. Biol. 33, 570–579 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hardner J., Gullison R. E., O’Neill E., Staying the course: How a long-term strategic donor initiative to conserve the Amazon has yielded outcomes of global significance. Found. Rev. 9, 10.9707/1944-5660.1371, (2017). [Google Scholar]
- 3.N. Pitman, G. Gagliardi Urrutia, C. Jenkins, "La biodiversidad de Loreto, Perú: El conocimiento actual de la diversidad de plantas y vertebrados terrestres" (Center for International Environmental Law, 2013).
- 4.International Union for the Conservation of Nature, "The IUCN Red List of Threatened Species" (Version 2019–1); http://iucnredlist.org.
- 5.ter Steege H., Pitman N., Sabatier D., Castellanos H., van der Hout P., Daly D. C., Silveira M., Phillips O., Vasquez R., van Andel T., Duivenvoorden J., de Oliveira A. A., Ek R., Lilwah R., Thomas R., van Essen J., Baider C., Maas P., Mori S., Terborgh J., NúÑez Vargas P., Mogollón H., Morawetz W., A spatial model of tree alpha-diversity and density for the Amazon region. Biodivers. Conserv. 12, 2255–2277 (2003). [Google Scholar]
- 6.ter Steege H., de Oliveira S. M., Pitman N. C. A., Sabatier D., Antonelli A., Guevara Andino J. E., Aymard G. A., Salomão R. P., Towards a dynamic list of Amazonian tree species. Sci. Rep. 9, 3501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreft H., Jetz W., Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. U.S.A. 104, 5925–5930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass M. S., Finer M., Jenkins C. N., Kreft H., Cisneros-Heredia D. F., McCracken S. F., Pitman N. C. A., English P. H., Swing K., Villa G., Di Fiore A., Voigt C. C., Kunz T. H., Global conservation significance of Ecuador’s Yasuní National Park. PLOS ONE 5, e8767 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins C. N., Pimm S. L., Joppa L. N., Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. U.S.A. 110, E2602–E2610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collen B., Whitton F., Dyer E. E., Baillie J. E. M., Cumberlidge N., Darwall W. R. T., Pollock C., Richman N. I., Soulsby A.-M., Böhm M., Global patterns of freshwater species diversity, threat, and endemism. Global Ecol. Biogeogr. 23, 40–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.G. P. Asner, D. E. Knapp, R. E. Martin, R. Tupayachi, C. B. Anderson, J. Mascaro, F. Sinca, K. D. Chadwick, S. Sousan, M. Higgins, W. Farfan, M. R. Silman, W. A. Llactayo León, A. F. Neyra Palomino, "The high-resolution carbon geography of Peru" (A collaborative report of the Carnegie Airborne Observatory and the Ministry of Environment of Peru, 2014).
- 12.Watson J. E. M., Evans T., Venter O., Williams B., Tulloch A., Stewart C., Thompson I., Ray J. C., Murray K., Salazar A., McAlpine C., Potapov P., Walston J., Robinson J. G., Painter M., Wilkie D., Filardi C., Laurance W. F., Houghton R. A., Maxwell S., Grantham H., Samper C., Wang S., Laestadius L., Runting R. K., Silva-Chávez G. A., Ervin J., Lindenmayer D., The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Draper F. C., Roucoux K. H., Lawson I. T., Mitchard E. T. A., Honorio Coronado E. N., Lähteenoja O., Montenegro L. T., Valderrama Sandoval E., Zaráte R., Baker T. R., The distribution and amount of carbon in the largest peatland complex in Amazonia. Environ. Res. Lett. 9, 124017 (2014). [Google Scholar]
- 15.León B., Roque J., Ulloa Ulloa C., Pitman N., Jørgensen P. M., Cano A., Libro rojo de las plantas endémicas del Perú. Rev. Peru. Biol. 13, 1–976 (2006). [Google Scholar]
- 16.N. Pitman, C. Vriesendorp, D. K. Moskovits, R. von May, D. Alvira, T. Wachter, D. F. Stotz, Á. del Campo, Eds., Perú: Yaguas-Cotuhé (Rapid Biological and Social Inventories report 23, Field Museum, Chicago, IL, 2011). [Google Scholar]
- 17.Sutherland W. J., Pullin A. S., Dolman P. M., Knight T. M., The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Schleicher J., Peres C. A., Amano T., Llactayo W., Leader-Williams N., Conservation performance of different conservation governance regimes in the Peruvian Amazon. Sci. Rep. 7, 11318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.N. Dudley, Ed., "Guidelines for applying protected area management categories" (Best Practice Protected Area Guidelines series no. 21. International Union for the Conservation of Nature, 2008).
- 20.Torres-Montenegro L. A., Ríos Paredes M. A., Pitman N. C. A., Vriesendorp C. F., Hensold N., Acuy Í. M., Cardozo N. D., Huamantupa I., Beltrán H. W., García-Villacorta R., Mori Vargas T. J., Neill D. A., Fine P. V. A., López-López J. T., Iturri G. N., Palacios W., Revilla N. S., Calderón W. T., Sesenta y cuatro nuevos registros para la flora del Perú a través de inventarios biológicos rápidos en la Amazonía peruana. Rev. Peru. Biol. 26, 379–392 (2019). [Google Scholar]
- 21.Wali A., Alvira D., Tallman P. S., Ravikumar A., Macedo M. O., A new approach to conservation: Using community empowerment for sustainable well-being. Ecol. Soc. 22, 6 (2018). [Google Scholar]
- 22.L. E. Alonso, J. L. Deichmann, S. A. McKenna, P. Naskrecki, S. J. Richards, Eds., Still Counting...: Biodiversity Exploration for Conservation: The First 20 Years of the Rapid Assessment Program (Conservation International, Arlington, VA, 2011). [Google Scholar]
- 23.Enquist C. A. F., Jackson S. T., Garfin G. M., Davis F. W., Gerber L. R., Littell J. A., Tank J. L., Terando A. J., Wall T. U., Halpern B., Hiers J. K., Morelli T. L., McNie E., Stephenson N. L., Williamson M. A., Woodhouse C. A., Yung L., Brunson M. W., Hall K. R., Hallett L. M., Lawson D. M., Moritz M. A., Nydick K., Pairis A., Ray A. J., Regan C., Safford H. D., Schwartz M. W., Shaw M. R., Foundations of translational ecology. Front. Ecol. Environ. 15, 541–550 (2017). [Google Scholar]
- 24.Cash D. W., Clark W. C., Alcock F., Dickson N. M., Eckley N., Guston D. H., Jäger J., Mitchell R. B., Knowledge systems for sustainable development. Proc. Natl. Acad. Sci. U.S.A. 100, 8086–8091 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda J. J., Corral L., Blackman A., Asner G., Lima E., Effects of protected areas on forest cover change and local communities: Evidence from the Peruvian Amazon. World Dev. 78, 288–307 (2016). [Google Scholar]
- 26.Safford H. D., Sawyer S. C., Kocher S. D., Hiers J. K., Cross M., Linking knowledge to action: The role of boundary spanners in translating ecology. Front. Ecol. Environ. 15, 560–568 (2017). [Google Scholar]
- 27.McCarter J., Boge G., Darlow G., Safeguarding the world’s natural treasures. Science 294, 2099–2101 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez L. O., Young K. R., Biological diversity of Peru: Determining priority areas for conservation. Ambio 29, 329–337 (2000). [Google Scholar]
- 29."Plan director de las áreas naturales protegidas (Estrategia nacional)," Servicio Nacional de Áreas Naturales Protegidas por el Estado (SERNANP) (Ministerio del Ambiente, Peru, 2009).
- 30."Estrategia para la gestión de las Áreas de Conservación Regional de Loreto," Programa de Conservación, Gestión y Uso Sostenible de la Diversidad Biológica de Loreto (PROCREL) (Gobierno Regional de Loreto, Peru, 2009).
- 31.Gudynas E., Buen vivir: Today’s tomorrow. Development 54, 441–447 (2011). [Google Scholar]
- 32.Naidoo R., Gerkey D., Hole D., Pfaff A., Ellis A. M., Golden C. D., Herrera D., Johnson K., Mulligan M., Ricketts T. H., Fisher B., Evaluating the impacts of protected areas on human well-being across the developing world. Sci. Adv. 5, eaav3006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Álvarez Alonso J., Sobre el futuro de las comunidades amazónicas: En busca del paraíso perdido. Folia Amazónica 28, 85–111 (2019). [Google Scholar]
- 34.Gullison R. E., Hardner J., Progress and challenges in consolidating the management of Amazonian protected areas and indigenous territories. Conserv. Biol. 32, 1020–1030 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Colvin J. G., A code of ethics for research in the third world. Conserv. Biol. 6, 309–311 (1992). [Google Scholar]
- 36.Hortal J., de Bello F., Diniz-Filho J. A. F., Lewinsohn T. M., Lobo J. M., Ladle R. J., Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. S. 46, 523–549 (2015). [Google Scholar]
- 37.Oliveira P. J. C., Asner G. P., Knapp D. E., Almeyda A., Galván-Gildemeister R., Keene S., Raybin R. F., Smith R. C., Land-use allocation protects the Peruvian Amazon. Science 317, 1233–1236 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Mistry J., Berardi A., Bridging indigenous and scientific knowledge. Science 352, 1274–1275 (2016). [DOI] [PubMed] [Google Scholar]
- 39.N. Pitman, C. Vriesendorp, D. Alvira, J. A. Markel, M. Johnston, E. Ruelas Inzunza, A. Lancha Pizango, G. Sarmiento Valenzuela, P. Álvarez-Loayza, J. Homan, T. Wachter, Á. del Campo, D. F. Stotz, S. Heilpern, Eds., Perú: Cordillera Escalera-Loreto (Rapid Biological and Social Inventories report 26, Field Museum, Chicago, IL, 2014). [Google Scholar]
- 40.Gwinn N. E., Rinaldo C., The Biodiversity Heritage Library: Sharing biodiversity literature with the world. IFLA J. Int. Fed. Libr. 35, 25–34 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/31/eabe2998/DC1


