Abstract
Background:
Human adenovirus (HAdV) species B, C, and E are commonly associated with acute respiratory illnesses (ARI). We sought to determine the association between HAdV species and ARI severity in children over one respiratory season at Monroe Carell Jr. Children’s Hospital at Vanderbilt.
Methods:
We conducted a retrospective cohort study of children with HAdV from a provider-ordered BioFire® FilmArray Respiratory Pathogen Panel 2.0 (RPP) from 05/2018–06/2019. Type-specific PCR assays for HAdV-B3, B7, B11, B14, B16, B21, HAdV-C1, C2, C5, C6, and HAdV-E4 were performed. Demographics, clinical characteristics, and outcome data were compared between HAdV species.
Results:
Of 4514 respiratory specimens collected, 2644 (59 %) had at least one pathogen detected by RPP, and 384 (15 %) were HAdV-positive; 342 (89 %) were available for research testing with 306 (89 %) specimens from unique symptomatic individuals; 237 (77 %) were positive for the following species: 104 (44 %) HAdV-B, 114 (48 %) HAdV-C, 9 (4%) HAdV-E, and 10 (4%) with co-detection between species. The majority with identified HAdV species were seen in the ED (62 %), and approximately one-third were hospitalized. Patients with HAdV-C were more likely to be younger, hospitalized, and have a higher frequency of seizures compared to HAdV-B.
Conclusion:
HAdV-C and HAdV-B were the most common species detected, with differences in clinical characteristics and outcomes noted. Additional studies with larger sample sizes focusing on a high-risk pediatric population are necessary to determine if differences in illness severity across individual HAdV types exist to guide further type-specific HAdV vaccine development.
Keywords: Adenovirus, Species, Respiratory, Epidemiology
1. Introduction
Human adenoviruses (HAdVs) are non-enveloped, double-stranded DNA viruses, with at least 90 HAdV types that are categorized into seven species (A–G) [1,2]; with new types continuing to emerge [3]. HAdVs account for approximately 5–10 % of all acute respiratory illnesses (ARI) in children [4–9], and the most common symptoms include fever, coryza, and cough [4,10–12]. Even though the majority of HAdVs cause mild and self-limited upper ARI in immunocompetent children, HAdVs can be associated with lower respiratory tract symptoms, multi-organ failure, and even death [13–25]. Globally, HAdVs attribute to 15 % of ARI in hospitalized children [5,9,26]. The majority of these hospitalizations occur in children with chronic illnesses [10]. Species that are associated with ARI as the most common clinical presentation are HAdV-B (types B3, B7, B11, B14, B16, and B21), HAdV-C (types C1, C2, C5, and C6), and HAdV-E (type E4).
Circulating HAdVs can vary temporally and geographically [27], with different HAdV species predominating. Many reports about HAdV types and/or species have been from outbreak investigations. Only a few studies of hospitalized children with severe lower ARI demonstrated differences between HAdV species. Some studies showed the predominance of HAdV-B and HAdV-C species [28,29]; however, the vast majority of these studies were from outside the United States (US). Therefore, our study aims to define the association between specific HAdV species and hospitalization as a proxy for illness severity in children with the provider-ordered multiplex molecular test.
2. Methods
2.1. Study design and population
We conducted a retrospective cohort study of all children whose nasopharyngeal swab (in viral transport media) were positive for HAdV by a provider-ordered BioFire® FilmArray Respiratory Pathogen Panel 2.0 (RPP) at Monroe Carell Jr. Children’s Hospital at Vanderbilt. Children under 18 years who presented to outpatient (OP), emergency department (ED), or inpatient settings from 5/1/18–6/30/19, and had documented ARI symptoms (fever and/or any of the following: cough, congestion, shortness of breath, or wheezing) were included in the study. Subjects were excluded from our analysis if they were asymptomatic, had two or more HAdV-positive respiratory specimens during the study period, had a healthcare-acquired infection (HAI) defined as HAdV-positive specimen collected 72 h or more after hospital admission, or had a non-ARI indication for hospitalization (Fig. 1).
Fig. 1.
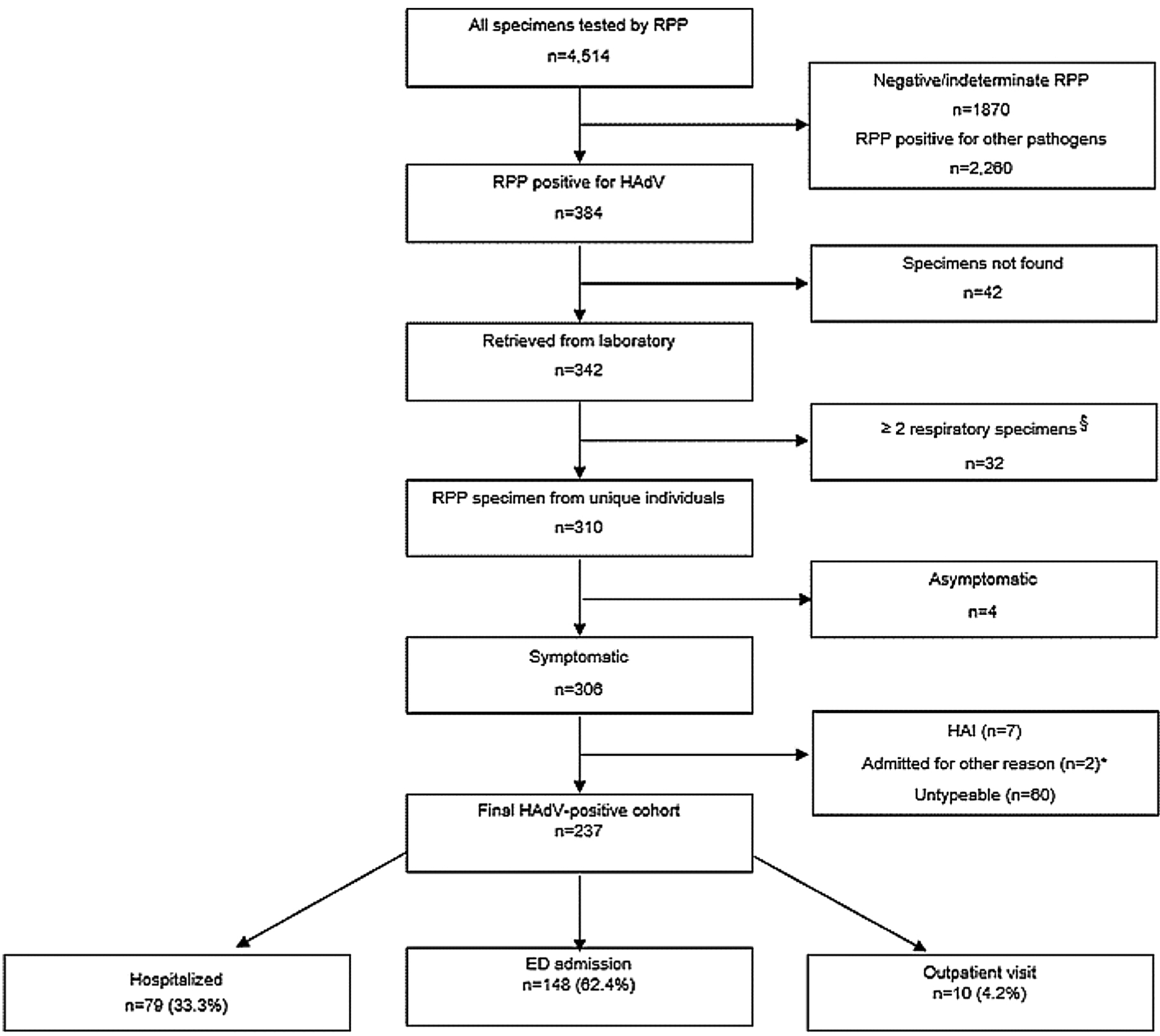
Study Inclusion Algorithm and Results of Respiratory Specimen Testing.
Abbreviation: RPP, BioFire respiratory pathogen panel; HAdV, human adenovirus; HAI, hospital-acquired infection.
§ Two or more respiratory swabs were collected and tested by RPP from the same patient during study period (either during the same or different healthcare encounter).
* 2 symptomatic patients were admitted for surgical procedure (liver biopsy and appendectomy).
2.2. Data collection
Demographic, social, clinical, and laboratory data were collected through medical chart abstractions and were entered into a standardized, secured electronic Research Electronic Data Capture database [30]. The study protocol was approved by the Institutional Review Board at Vanderbilt University.
Patients were considered to have underlying medical conditions if they had asthma, airway diseases, cystic fibrosis, bronchopulmonary dysplasia, chronic pulmonary disorders, congenital or acquired heart diseases, liver and gastrointestinal diseases, kidney diseases, diabetes and other endocrine diseases, blood disorders including sickle cell disease, cancer, history of organ transplant, immunodeficiency including asplenia, cerebral palsy, neurological disorders, genetic and metabolic disorders, Trisomy-21 syndrome, and/or complex care conditions.
2.3. Laboratory testing
The original HAdV-positive clinical specimens were frozen at −80 °C after clinical-use FilmArray testing. For this study, the thawed specimens underwent DNA extraction by QIAGEN QIAcube. Repeat testing was performed to confirm non-type-specific HAdV by single-plex qPCR followed by type-specific qPCR assay targeting the hexon gene using a standardized CDC protocol [31,32] for the most common circulating HAdV types: HAdV-B3, B7, B11, B14, B16, B21; HAdV-C1, C2, C5, C6; and HAdV-E4 [33]. Laboratory supplies were funded by VICTR (Vanderbilt Institute for Clinical and Translational Research) and NIH-sponsored T32 grants.
2.4. Statistical analyses
Analyses were performed using Stata/IC 15.0 (StatCorp LLC, College Station, TX). For descriptive analysis, the Pearson χ2 test for categorical variables, and median test or t-test with unequal variances for continuous variables were used for pairwise comparison of demographic, social, and clinical characteristics between HAdV-B and HAdV-C, as single species detected. Subjects with HAdV-E and co-detection between species were excluded from our analysis due to the small sample size of these groups. We used logistic regression to address our primary goal of evaluating the association between hospitalization and HAdV species (HAdV-B and HAdV-C), adjusting for age, gender, race, ethnicity, childcare attendance, having siblings at home, underlying medical condition, seizure as presenting symptom, and co-detection with other respiratory viruses on provider-ordered RPP. We performed a secondary analysis to examine the association between co-detection with other respiratory viruses and HAdV species (HAdV-B and HAdV-C) using logistic regression, adjusting for age, gender, race, ethnicity, childcare attendance, having siblings at home, and underlying medical condition. A chained-equations multiple imputation procedure was performed with 500 imputation iterations to account for missing values for childcare attendance (33 %) and siblings at home (22 %). Cycle threshold (Ct) values were calculated from non-type-specific HAdV qPCR, as a surrogate marker for a viral load, with higher Ct values indicating lower viral load. Specimens with HAdV co-detections between species were excluded from the Ct value analysis. We used a significance level of α = 0.05 for all analyses and robust standard errors to formulate confidence intervals and perform robust Wald-based hypothesis tests.
3. Results
3.1. Study population
Over the study period, a total of 4514 specimens were tested by RPP; of which, 2644 (59 %) were positive for at least one pathogen, and 384 (15 %) were HAdV-positive (Fig. 1). Three hundred and forty-two (89 %) specimens were retrieved from the clinical laboratory, which represented 310 subjects. After applying exclusion criteria, the final cohort included 237 unique subjects with an ARI and typeable HAdV results (Fig. 1). A total of 62.4 % were seen in the ED and discharged home, 33.3 % were admitted to the hospital, and 4.2 % were seen in an OP clinic (Fig. 1).
3.2. HAdV species and type distribution
Among detected species, 115 (46 %) were HAdV-B and 128 (50 %) were HAdV-C (Fig. 2a), with HAdV-B3, HAdV-C2, and HAdV-C1 being the most predominant types (42 %, 25 %, and 17 %, respectively) (Fig. 2b). Co-detections between HAdV species were identified in 10 (4%) subjects; of which, 6 (60 %) had another respiratory pathogen detected (Supplemental Table 1). Among 227 subjects with single HAdV species detection, 111 (49 %) had co-detections with another respiratory pathogen.
Fig. 2.
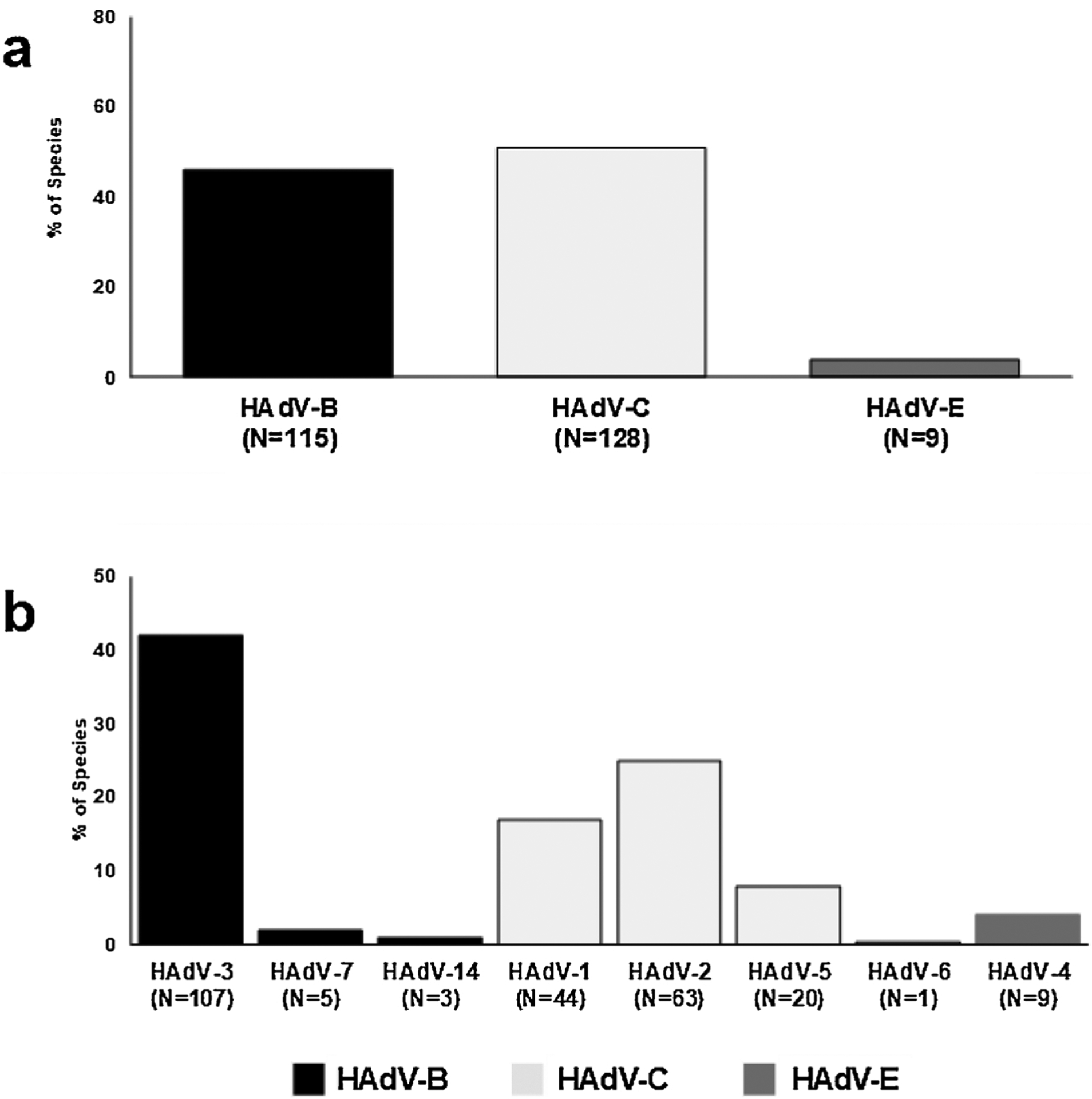
a: Distribution of HAdV Species Among Symptomatic Cases. b. Distribution of HAdV Types Among Symptomatic Cases.
3.3. Demographic and clinical differences between HAdV-B and HAdV-C
Table 1 shows a comparison between HAdV-B and HAdV-C as single species detections. Fever and cough were the most common symptoms in both species groups (Table 1). Children with HAdV-C were more likely to be younger, had a higher frequency of seizures and hospitalizations, but a lower frequency of conjunctivitis, compared to those with HAdV-B (Table 1). Subjects under two years had a higher proportion of HAdV-C detections than HAdV-B (72 % vs. 24 %, p < 0.05). In contrast, older patients had a higher frequency of HAdV-B detection than HAdV-C (56 % vs. 32 % in patients 2–4 years; 80 % vs. 9% in patients 5–9 years; and 64 % vs. 18 % in patients 10–18 year, respectively; p-value<0.05) (Fig. 3a). Dyspnea and chest retractions on physical exam were seen more frequently with HAdV-C than HAdV-B, but this association was not statistically significant (Table 1).
Table 1.
Demographic and Clinical Characteristics of HAdV-B and HAdV-C Species in Symptomatic Cases Seen at Vanderbilt Children’s Hospital Over a 14-Month Period (May 2018–June 2019)€.
| Characteristics | All Speciesλ (n = 237) % (n) | HAdV-B (n = 104) % (n) | HAdV-C (n = 114) % (n) | p-value |
|---|---|---|---|---|
| Mean age, months (SD) | 39.2 (39.7) | 55.9 (41.4) | 20.9 (25.6) | <0.005 |
| Male sex | 60 % (142) | 58 % (60) | 60 % (68) | 0.769 |
| Race | ||||
| White | 75 % (165) | 80 % (74/92) | 71 % (77/108) | 0.053 |
| Black/African American | 16 % (35) | 10 % (9/92) | 22 % (24/108) | |
| Other | 9% (19) | 10 % (9/92) | 6% (7/108) | |
| Hispanic ethnicity | 19 % (44/230) | 24 % (24/102) | 17 % (18/109) | 0.202 |
| Childcare attendance | 57 % (90/159) | 60 % (40/67) | 54 % (43/79) | 0.522 |
| Underlying medical conditions§ | 32 % (76) | 31 % (32) | 33 % (38) | 0.685 |
| Fever | 94 % (223) | 95 % (99) | 94 % (107) | 0.667 |
| Rash | 11 % (26) | 14 % (15) | 8% (9) | 0.124 |
| Conjunctivitis | 18 % (42) | 28 % (29) | 11 % (12) | 0.001 |
| Cough | 67 % (158) | 67 % (70) | 69 % (79) | 0.752 |
| Dyspnea on physical exam | 11 % (27) | 8% (8) | 15 % (17) | 0.095 |
| Chest retraction on physical exam | 12 % (29) | 9% (9) | 16 % (18) | 0.110 |
| Wheezing on physical exam | 5% (12) | 4% (4) | 5% (6) | 0.617 |
| Seizure | 6% (14) | 3% (3) | 10 % (11) | 0.042 |
| Admitted in hospital | 33 % (79) | 25 % (26) | 43 % (49) | 0.005 |
| Oxygen requirement | 29 % (23/79) | 23 % (6/26) | 33 % (16/49) | 0.386 |
| ICU admission | 11 % (9/79) | 4% (1/26) | 14 % (7/49) | 0.163 |
| Mean duration of hospital stay, hours | 64 | 67 | 58 | 0.567 |
Pearson χ2 test for categorical variables, and t-test for continuous variables.
Included 104 cases of HAdV-B, 114 cases of HAdV-C, 9 cases of HAdV-E and 10 cases with co-detection between HAdV species.
Underlying medical condition includes chronic pulmonary disease, asthma, airway disease, cardiac disease, renal disease, gastrointestinal disease, endocrine disease, blood disease including sickle cell anemia, immunodeficiency including asplenia, neurological conditions, Trisomy-21, genetic and metabolic disease, complex care patients.
Fig. 3.
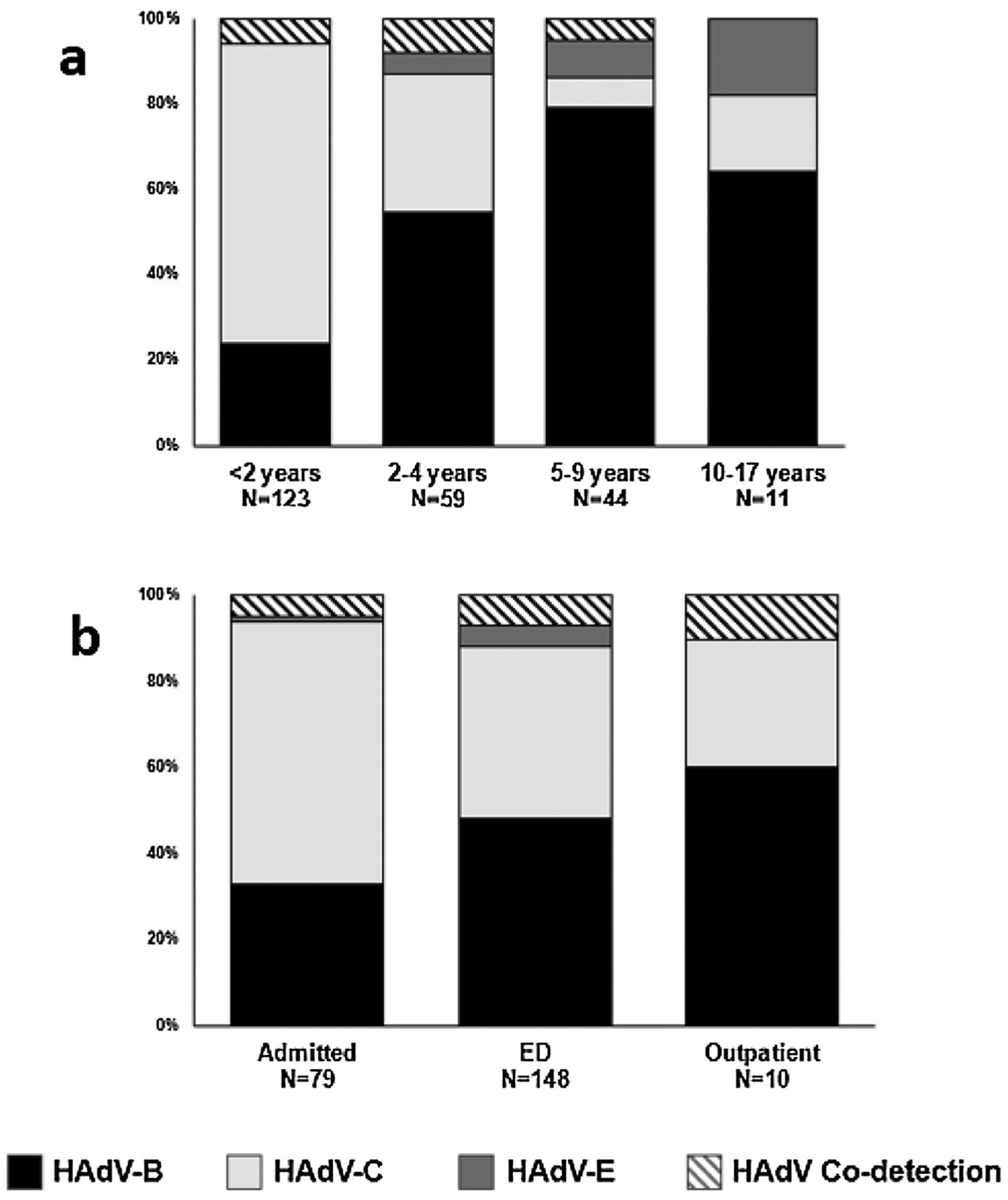
a: Distribution of HAdV Species by Age. b: Distribution of HAdV Species by Healthcare Settings.
3.4. Hospitalization association by HAdV species
Fig. 3b displays the distribution of species by clinical settings. HAdV-C was the most common species identified among patients admitted to the hospital, representing 62 % hospitalized cases. HAdV-B and HAdV-C were detected with nearly equal frequency in ED (49 % and 42 %, respectively). HAdV-B was the most common species identified in the OP setting (60 %) (Fig. 3b).
Children with HAdV-C had 2.52 (95 % CI: 1.22, 5.19) times higher odds of being hospitalized compared to children with HAdV-B, adjusted for covariates in Table 2. Having an underlying medical condition was independently associated with hospital admission (Table 2).
Table 2.
Association Between HAdV Species (HAdV-B and HAdV-C) and Hospitalization in Symptomatic Patients at Vanderbilt Children’s Hospital€.
| Adjusted OR | 95 % CI | p-value | |
|---|---|---|---|
| HAdV-B | Ref. | Ref. | Ref. |
| HAdV-C | 2.52 | 1.22–5.19 | 0.013 |
| Age, months | 1.00 | 0.99–1.01 | 0.476 |
| Male sex | 1.08 | 0.57–2.07 | 0.808 |
| Race | |||
| White | Ref. | Ref. | Ref. |
| Black/African American | 0.81 | 0.34–1.91 | 0.625 |
| Other | 1.47 | 0.44–4.87 | 0.529 |
| Hispanic ethnicity | 0.62 | 0.25–1.57 | 0.316 |
| Childcare attendance | 0.89 | 0.40–2.01 | 0.795 |
| Siblings | 1.99 | 0.82–4.84 | 0.126 |
| Underlying medical condition | 2.72 | 1.37–5.42 | 0.004 |
| Seizure | 3.41 | 0.89–13.05 | 0.073 |
| Co-detection with other respiratory viruses | 1.45 | 0.75–2.82 | 0.269 |
Multivariable logistic regression.
3.5. HAdV species and viral Co-detection
Children with HAdV-C had a higher frequency of viral co-detections compared to those with HAdV-B (60 % vs. 39 %, p < 0.05). The most common co-detected respiratory pathogen with either HAdV-B or HAdV-C was human rhinovirus (HRV) (61 % vs. 46 %, respectively), followed by co-detections with ≥2 other respiratory pathogens (17 % vs. 22 %, respectively). However, after adjusting for covariates, no sufficient evidence of an association between HAdV species and co-detection with other viruses was found (Table 3). Of note, patients of younger age and those who attended childcare had higher odds of having co-detection with other respiratory pathogens (Table 3).
Table 3.
Association Between HAdV Species (HAdV-B and HAdV-C) and Co-detection with Other Respiratory Pathogens at Vanderbilt Children’s Hospital€.
| Adjusted OR | 95 % CI | p-value | |
|---|---|---|---|
| HAdV-B | Ref. | Ref. | Ref. |
| HAdV-C | 1.78 | 0.89–3.58 | 0.105 |
| Age, months | 0.99 | 0.98–0.99 | 0.020 |
| Male sex | 1.04 | 0.56–1.91 | 0.911 |
| Race | |||
| White | Ref. | Ref. | Ref. |
| Black/African American | 0.85 | 0.35–2.07 | 0.721 |
| Other | 3.45 | 0.91–13.06 | 0.068 |
| Hispanic ethnicity | 0.66 | 0.27–1.59 | 0.349 |
| Childcare attendance | 2.61 | 1.17–5.81 | 0.019 |
| Siblings | 0.71 | 0.31–1.61 | 0.413 |
| Underlying medical condition | 0.83 | 0.44–1.59 | 0.584 |
Multivariable logistic regression.
3.6. HAdV species ct values
We did not find a significant difference in median Ct value between HAdV single detection vs. HAdV co-detected with other respiratory viruses for all HAdV in our final cohort (Fig. 4a). This remained true for separately analyzed cohorts of HAdV-B and HAdV-C (23 [IQR = 20–30] vs. 22 [IQR = 18–30], and 32 [IQR = 26–36] vs. 31 [IQR = 27–36]; respectively; p-value >0.05). However, amongst HAdV specimens detected as a single pathogen on RPP, the Ct value in specimens with HAdV-B was significantly lower than with HAdV-C or HAdV-E (Fig. 4b). Ct value in specimens with either HAdV-B or HAdV-C as single detection was significantly lower in patients seen in OP and ED settings compared to those admitted to the hospital (Fig. 4c).
Fig. 4.
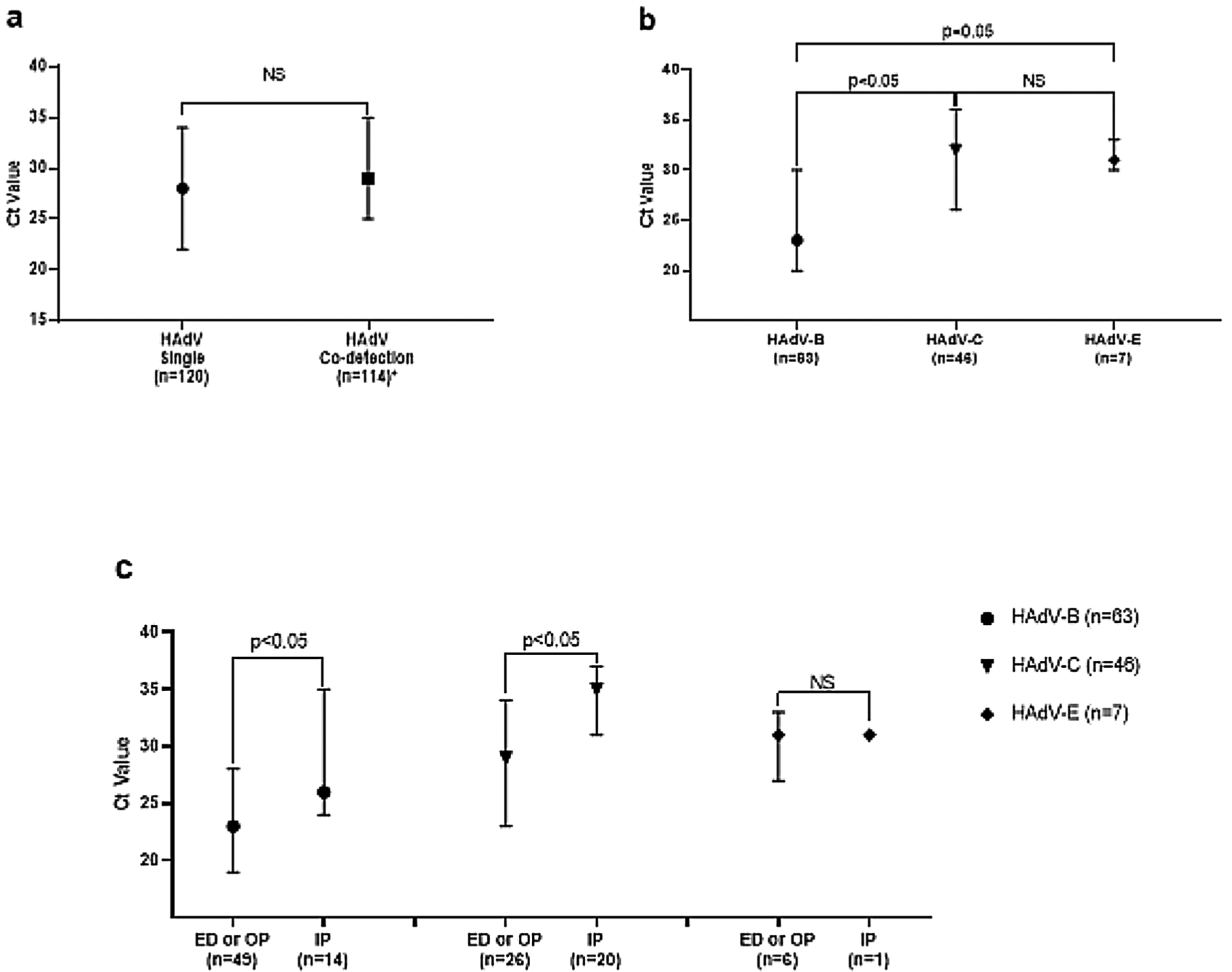
a: Comparison of Cycle Threshold (Ct) Value of Nontype-specific HAdV Between Single HAdV.
Dectecion and HAdV Co-detection with Other Respiratory Infections in Symptomatic Cases. b: Comparison. of Ct Value Between HAdV Species in Symptomatic HAdV-single Cases. c: Comparison of Ct Value Between.
Healthcare Settings by HAdV Species Groups in Symptomatic HAdV-single Cases.
Data are median with IQR; p-values calculated using median test. Analysed Ct Value of Non-type.
Specific HAdV.
Abbreviation: NS, Not Significant.
* 3 cases with type-specific HAdV positive PCR were exclluded from analysis given negative.
HAdV nontype-specific PCR.
4. Discussion
Our study revealed that HAdV-B and HAdV-C were the most common HAdV species detected. One-third of our cohort of HAdV-positive children required hospitalization. The hospitalization frequencies of HAdV-positive children vary in previous studies with reported ranges from 28 % to 56 % [10,34]. Furthermore, we found that children with HAdV-C were more likely to be hospitalized after adjusting for confounding covariates and were younger than those with HAdV-B. These findings are consistent with other studies that reported the same difference in age distribution across HAdV-B and HAdV-C species [11,35,36]. However, unlike an Italian study that reported a higher frequency of hospitalization in children with HAdV-B [37], our study highlights that clinical outcomes and severity are different between HAdV species, and further studies are needed to confirm these findings in pediatric patients.
We noted that fever was the most frequent symptom observed in our subjects for both species, which is similar to the same above mentioned Italian study of 61 HAdV-positive pediatric subjects, with three-quarters of their subjects that reporting high-grade fever [37]. Seizures were also noted in 6% of the cohort of HAdV-positive children, but those with HAdV-C had a higher frequency of seizures compared to HAdV-B. Many other studies have reported HAdV as one of the most common respiratory viruses detected in children with febrile seizures [38–42]. Specifically, one study of 109 children with central nervous system symptoms reported equal distribution of HAdV-B and HAdV-C in children with febrile seizures, with HAdV-B3 as the most commonly detected type [41]. In addition, children with HAdV-C in our study had a lower frequency of conjunctivitis compared to those with HAdV-B, which is in agreement with a study by Tabain et. al of 135 hospitalized HAdV-positive Croatian children under 10 years [11]. The higher frequency of conjunctivitis may be due to tissue tropism by specific HAdV type (s) within the HAdV-B species, as evident by endemic viral conjunctivitis associated with HAdV-D species, specifically types 8, 19, and 37 [43]. Additional studies are needed to understand the differences in clinical characteristics between HAdV species and types.
Many studies have reported HAdV co-detections with other respiratory pathogens ranging from 12 % to 80 % [5,29,44]. Global reports have demonstrated that HAdV-B and HAdV-C were the most common species identified in co-detected specimens, while others show the association of HAdV-C and HAdV-E with co-detections [36]; however, limited data exist about the association of HAdV species with co-detections in US pediatric cohorts. We found that co-detection with other respiratory pathogens occurred in nearly half of our cohort, with the most commonly co-detected pathogen being HRV. These findings are consistent with the Italian study in which they noted that HRV was the most common virus co-detected [37]. In our unadjusted analysis, we found that patients with HAdV-C had significantly higher proportion of co-detections with other respiratory pathogens compared to HAdV-B. Similar findings from the univariate analysis were shown in a Taiwanese study of 531 children which demonstrated a higher proportion of viral co-detections in those with HAdV-C compared to HAdV-B (22 % vs.3%), with the most commonly co-detected virus being influenza (74 %) [36]. However, no association between co-detections and HAdV species was found after adjusting for confounding variables in our study. Given data from other studies demonstrating HAdV positivity in healthy controls [45], it is possible that in some symptomatic patients with HAdV co-detected with other respiratory pathogens, specific HAdV types within HAdV-C species could be due to incidental finding rather than a pathogen causing clinical disease. This may create difficulty in distinguishing true HAdV infection from viral shedding not contributing to current illness given earlier reports of higher rates of detection of HAdV in children with chronic adenotonsillar diseases [46,47]. More comprehensive and longitudinal pediatric studies are needed to understand which specific HAdV types are associated with asymptomatic shedding and which contribute to ARI severity.
The association between viral load and disease severity remains controversial, with some studies correlating a high viral load with severity [48–50], while others do not find an association [50]. In our study, we found that among single HAdV, the median Ct value of HAdV-B was statistically lower compared to the Ct value of HAdV-C, indicating higher viral load, which theoretically may be positively associated with illness severity. This is contrary to a study that demonstrated a higher viral load in HAdV-C compared to HAdV-B as single detection [29]. Our data showed higher Ct values in both single HAdV-B and HAdV-C in hospitalized children compared to those seen in ED or OP settings, that could be due to incidental findings from prolonged shedding rather than true clinically significant infection, or a lack of positive correlation between viral load and illness severity. Furthermore, Ct values are influenced by specimen handling, PCR techniques, host immune response on viral clearance, and the pathogenicity of individual virus types, leading to challenges in interpreting Ct value as a surrogate for viral load. More studies are needed to investigate the association between viral load and illness severity in children with HAdV infection.
Our study had some limitations. Since this is a single-center study over one year, these findings may not be generalizable to other pediatric populations across the US. Not all specimens were able to be typed either due to suboptimal specimen collection, the specificity of HAdV type-specific qPCR, or specimen degradation. In addition, type sequencing was not performed on these specimens which could possibly change the species distribution. Routine RPP testing of children with ARI is not universal and consistent in OP settings, leading to the under-representation of this group in our study. Furthermore, since this was a retrospective chart review, systematic collection of data was not performed; therefore, not all symptoms and signs were recorded, potentially leading to exposure misclassification. Besides, hospital admission could be not necessarily for respiratory infection management, or HAdV could be incidentally detected in children with prolong shedding reflecting latency, therefore leading to outcome misclassification. Finally, due to small sample sizes, we were unable to perform analyses within single types and to detect differences in respiratory symptoms between HAdV species.
In summary, HAdV-B and HAdV-C were the most common species in children who had a provider-ordered RPP. Children with HAdV-C were more likely to be younger, more frequently to present with seizure, and more likely to be hospitalized compared to children with HAdV-B. Future surveillance pediatric studies with a larger cohort are needed to further elucidate clinical presentations and severity factor differences amongst the specific HAdV types, which can serve as a platform for vaccine development in high-risk groups.
Supplementary Material
Acknowledgment
supported in part by the Vanderbilt CTSA grant UL1TR002243 from NCATS/NIH, and Conducting Child Health Care Research in Vulnerable Population5T32HD060554-09 Training grant 2018-2019.
Declaration of Competing Interest
Dr. Natasha Halasa received grant support from Quidel, Sanofi, and educational grant supported by Genotech. Otherwise, the authors have no conflicts of interest to disclose.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104716.
References
- [1].Tam CC, et al. , Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease, Clin. Infect. Dis 54 (9) (2012) 1275–1286. [DOI] [PubMed] [Google Scholar]
- [2].Ismail AM, et al. , Adenoviromics: mining the human adenovirus species d genome, Front. Microbiol 9 (2018) 2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kajan GL, et al. , A multigene typing system for human adenoviruses reveals a new genotype in a collection of Swedish clinical isolates, PLoS One 13 (12) (2018) e0209038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Edwards KM, et al. , Adenovirus infections in young children, Pediatrics 76 (3) (1985) 420–424. [PubMed] [Google Scholar]
- [5].Jain S, et al. , Community-acquired pneumonia requiring hospitalization among U.S. children, N. Engl. J. Med 372 (9) (2015) 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Azevedo AM, et al. , Detection of influenza, parainfluenza, adenovirus and respiratory syncytial virus during asthma attacks in children older than 2 years old, Allergol. Immunopathol. (Madr) 31 (6) (2003) 311–317. [DOI] [PubMed] [Google Scholar]
- [7].Kokturk N, et al. , Detection of adenovirus and respiratory syncytial virus in patients with chronic obstructive pulmonary disease: exacerbation versus stable condition, Mol. Med. Rep 12 (2) (2015) 3039–3046. [DOI] [PubMed] [Google Scholar]
- [8].Marinheiro JC, et al. , Duplex-PCR assay for the detection of adenovirus and respiratory syncytial virus in nasopharyngeal samples, Mem. Inst. Oswaldo Cruz 104 (1) (2009) 118–120. [DOI] [PubMed] [Google Scholar]
- [9].Finianos M, et al. , Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon, J Med Virol 88 (11) (2016) 1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rocholl C, et al. , Adenoviral infections in children: the impact of rapid diagnosis, Pediatrics 113 (1 Pt 1) (2004) e51–6. [DOI] [PubMed] [Google Scholar]
- [11].Tabain I, et al. , Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes, Pediatr. Infect. Dis. J 31 (7) (2012) 680–684. [DOI] [PubMed] [Google Scholar]
- [12].Lin CH, et al. , A cluster of adenovirus serotype 3 infections in children in northern Taiwan: clinical features and laboratory findings, J. Microbiol. Immunol. Infect 40 (4) (2007) 302–309. [PubMed] [Google Scholar]
- [13].Sun CC, Duara S, Fatal adenovirus pneumonia in two newborn infants, one case caused by adenovirus type 30, Pediatr. Pathol 4 (3–4) (1985) 247–255. [DOI] [PubMed] [Google Scholar]
- [14].Brown M, et al. , Fatal adenovirus type 35 infection in newborns, Pediatr. Infect. Dis. J 10 (12) (1991) 955–956. [DOI] [PubMed] [Google Scholar]
- [15].Henquell C, et al. , Fatal adenovirus infection in a neonate and transmission to health-care workers, J. Clin. Virol 45 (4) (2009) 345–348. [DOI] [PubMed] [Google Scholar]
- [16].Xu J, et al. , Disseminated adenovirus infection in heart and kidney transplant, Turk Kardiyol. Dern. Ars 46 (3) (2018) 231–233. [DOI] [PubMed] [Google Scholar]
- [17].Vyas JM, Marasco WA, Fatal fulminant hepatic failure from adenovirus in allogeneic bone marrow transplant patients, Case Rep. Infect. Dis 2012 (2012) 463569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stalder H, Hierholzer JC, Oxman MN, New human adenovirus (candidate adenovirus type 35) causing fatal disseminated infection in a renal transplant recipient, J. Clin. Microbiol 6 (3) (1977) 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sedlacek P, et al. , Incidence of adenovirus infection in hematopoietic stem cell transplant recipients: findings from the AdVance study, Biol. Blood Marrow Transplant (2018). [DOI] [PubMed] [Google Scholar]
- [20].Rosario RF, et al. , Fatal adenovirus serotype-5 in a deceased-donor renal transplant recipient, Transpl. Infect. Dis 8 (1) (2006) 54–57. [DOI] [PubMed] [Google Scholar]
- [21].Kang JM, et al. , Prospective monitoring of adenovirus infection and type analysis after allogeneic hematopoietic cell transplantation: a single-center study in Korea, Transpl. Infect. Dis 20 (3) (2018) e12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kajon AE, et al. , Fatal disseminated adenovirus infection in a young adult with systemic lupus erythematosus, J. Clin. Virol 50 (1) (2011) 80–83. [DOI] [PubMed] [Google Scholar]
- [23].Hubmann M, et al. , Occurrence, risk factors and outcome of adenovirus infection in adult recipients of allogeneic hematopoietic stem cell transplantation, J. Clin. Virol 82 (2016) 33–40. [DOI] [PubMed] [Google Scholar]
- [24].Hough R, et al. , Fatal adenovirus hepatitis during standard chemotherapy for childhood acute lymphoblastic leukemia, J. Pediatr. Hematol. Oncol 27 (2) (2005) 67–72. [DOI] [PubMed] [Google Scholar]
- [25].Tortora RP, et al. , Adenovirus species C detection in children under four years of age with acute bronchiolitis or recurrent wheezing, J. Clin. Virol 73 (2015) 77–80. [DOI] [PubMed] [Google Scholar]
- [26].Xie L, et al. , Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China, J Med Virol 91 (3) (2019) 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scott MK, et al. , Human adenovirus associated with severe respiratory infection, Oregon, USA, 2013–2014, Emerg Infect Dis. 22 (6) (2016) 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li Y, et al. , Molecular typing and epidemiology profiles of human adenovirus infection among paediatric patients with severe acute respiratory infection in China, PLoS One 10 (4) (2015) e0123234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barrero PR, et al. , Molecular typing of adenoviruses in pediatric respiratory infections in Buenos Aires, Argentina (1999–2010), J. Clin. Virol 53 (2) (2012) 145–150. [DOI] [PubMed] [Google Scholar]
- [30].Harris PA, et al. , Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu X, Erdman DD, Quantitative real-time PCR assays for detection and type-specific identification of the endemic species C human adenoviruses, J. Virol. Methods 237 (2016) 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu X, et al. , Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses, J. Clin. Microbiol 51 (4) (2013) 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Binder AM, et al. , Human adenovirus surveillance - United States, 2003–2016, MMWR Morb. Mortal. Wkly. Rep 66 (39) (2017) 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin GL, et al. , Molecular epidemiology and clinical features of adenovirus infection in Taiwanese children, 2014, J. Microbiol. Immunol. Infect 52 (2) (2019) 215–224. [DOI] [PubMed] [Google Scholar]
- [35].Nakamura H, et al. , Species differences in circulation and inflammatory responses in children with common respiratory adenovirus infections, J. Med. Virol 90 (5) (2018) 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lin GL, et al. , Molecular epidemiology and clinical features of adenovirus infection in Taiwanese children, 2014, J. Microbiol. Immunol. Infect (2018). [DOI] [PubMed] [Google Scholar]
- [37].Esposito S, et al. , Epidemiology and clinical characteristics of respiratory infections due to adenovirus in children living in Milan, Italy, during 2013 and 2014, PLoS One 11 (4) (2016) e0152375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carman KB, et al. , Viral etiological causes of febrile seizures for respiratory pathogens (EFES Study), Hum. Vaccin. Immunother 15 (2) (2019) 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chiu SS, et al. , Influenza A infection is an important cause of febrile seizures, Pediatrics 108 (4) (2001) E63. [DOI] [PubMed] [Google Scholar]
- [40].Chung B, Wong V, Relationship between five common viruses and febrile seizure in children, Arch. Dis. Child 92 (7) (2007) 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huang YC, et al. , Adenovirus infection associated with central nervous system dysfunction in children, J. Clin. Virol 57 (4) (2013) 300–304. [DOI] [PubMed] [Google Scholar]
- [42].Tang J, et al. , Relationship between common viral upper respiratory tract infections and febrile seizures in children from Suzhou, China, J Child Neurol 29 (10) (2014) 1327–1332. [DOI] [PubMed] [Google Scholar]
- [43].Jhanji V, et al. , Adenoviral keratoconjunctivitis, Surv. Ophthalmol 60 (5) (2015) 435–443. [DOI] [PubMed] [Google Scholar]
- [44].Meyers L, et al. , Automated real-time collection of pathogen-specific diagnostic data: syndromic infectious disease epidemiology, JMIR Public Health Surveill. 4 (3) (2018) e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Song E, et al. , Diagnosis of Pediatric Acute Adenovirus Infections: Is a Positive PCR Sufficient? Pediatr. Infect. Dis. J 35 (8) (2016) 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Proenca-Modena JL, et al. , Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children, J. Med. Virol 91 (7) (2019) 1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Proenca-Modena JL, et al. , High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease, PLoS One 7 (8) (2012) e42136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Buckingham SC, Bush AJ, Devincenzo JP, Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants, Pediatr. Infect. Dis. J 19 (2) (2000) 113–117. [DOI] [PubMed] [Google Scholar]
- [49].Li CC, et al. , Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children, Emerg Infect Dis 16 (8) (2010) 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wright PF, et al. , Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus, J. Infect. Dis 185 (8) (2002) 1011–1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


