Abstract
Background:
Abnormal eye gaze perception is related to symptoms and social functioning in schizophrenia. However, little is known about the brain network mechanisms underlying these abnormalities. Here, we employed dynamic causal modeling (DCM) of fMRI data to discover aberrant effective connectivity within networks associated with eye gaze processing in schizophrenia.
Methods:
Twenty-seven patients (schizophrenia/schizoaffective disorder, SZ) and 22 healthy controls (HC) completed an eye gaze processing task during fMRI. Participants viewed faces with different gaze angles and performed explicit gaze discrimination (Gaze: “Looking at you?” yes/no) or implicit gaze processing (Gender: “male or female?”). Four brain regions, the secondary visual cortex (Vis), posterior superior temporal sulcus (pSTS), inferior parietal lobule (IPL), and posterior medial frontal cortex (pMFC) were identified as nodes for subsequent DCM analysis.
Results:
SZ and HC showed similar generative model structure, but SZ showed altered connectivity for specific self-connections, inter-regional connections during all gaze processing (reduced excitatory bottom-up and enhanced inhibitory top-down connections), and modulation by explicit gaze discrimination (increased frontal inhibition of visual cortex). Altered effective connectivity was significantly associated with poorer social cognition and functioning.
Conclusions:
General gaze processing in SZ is associated with distributed cortical dysfunctions and bidirectional connectivity between regions, while explicit gaze discrimination involves predominantly top-down abnormalities in the visual system. These results suggest plausible neural mechanisms underpinning gaze processing deficits and may serve as bio-markers for intervention.
Keywords: psychosis, neuroimaging, fMRI, effective connectivity, social cognition, face processing
1. INTRODUCTION
Social cognitive impairment is a prominent feature of schizophrenia and a critical determinant of functional outcome (Fett et al., 2011). It shows limited response to currently available medication treatments (e.g., antipsychotics), leaving a major treatment gap. In view of this, considerable effort has been made to better understand the mechanisms of social cognitive deficits in schizophrenia in order to develop better interventions and improve outcome. Neuroimaging studies have consistently revealed altered brain activation in schizophrenia patients when processing of social information, particularly in regions associated with early visual processing, salience detection, face processing, cognitive control, and mentalizing (Dong et al., 2018; Green et al., 2015). Yet, how these regional functional abnormalities interact and collectively contribute to the observed behavior remain unclear. Because schizophrenia is seen as fundamentally a disorder of dysfunctional brain connectivity (or “dysconnection”) rather than resulting from regional brain dysfunction (Friston et al., 2016a), a network perspective is needed to fully appreciate the neural underpinnings of social cognitive deficits. This study aims to delineate the aberrant brain dynamics underlying a critical social cognitive process—eye gaze processing—in schizophrenia using dynamic causal modeling (DCM).
Eye gaze is a ubiquitous social cue that conveys one’s attention and mental state. Our ability to accurately and efficiently discriminate others’ eye gaze direction, especially gaze directed toward self, is critical for understanding intent and navigating the social environment. Gaze perception emerges in early infancy, and its development is foundational to subsequent social adaptation in primates, including humans (Emery, 2000; Farroni et al., 2002). In fact, multiple neuropsychiatric disorders accompanied by significant social dysfunction—including schizophrenia (Hooker and Park, 2005; Rosse et al., 1994) as well as autism-spectrum disorders and social phobia (Schulze et al., 2013; Senju et al., 2003)— are characterized by altered perception of self-directed gaze. In schizophrenia, deficits in eye-contact perception significantly explain variance in socio-emotional impairment, even after accounting for the effects of neurocognitive impairment (Tso et al., 2012). Thus, eye-contact perception is relevant to functional outcome, motivating the need for a better understanding of its neural mechanisms.
Eye-contact perception involves both bottom-up and top-down processes: encoding and integrating visual stimuli (position of the iris in the context of the eyes and the face) and making a judgment whether the gaze is self-referential or not. An important question in schizophrenia is whether abnormal gaze perception reflects deficits in bottom-up, or top-down processing, or some combination of both. Numerous studies have documented disruptions in basic visual perception, supporting a ‘bottom-up’ model of processing deficits (Silverstein and Keane, 2011). Neuroimaging studies of face processing in schizophrenia show abnormal activation in the occipital cortex (Kohler et al., 2008; Taylor et al., 2011) and impaired early stages of visual processing as indexed by the P100 event-related potential component (Earls et al., 2016). When eye-contact perception is assessed with a psychophysical method, individuals with schizophrenia showed reduced visual perceptual sensitivity (Tso et al., 2012; Yao et al., 2018), which is significantly associated with poor visual integration—the ability to link individual local visual elements to form a holistic representation (Tso et al., 2014). These findings together support the notion that abnormal gaze perception in schizophrenia may begin with impaired visual processing. However, deficits in higher-level cognitive processes, including cognitive control and mentalizing, both localized to the medial frontal cortex, are highly prevalent (Sugranyes et al., 2011; Van Veen and Carter, 2002). Thus, it is possible that impaired top-down modulation of the visual system (Dima et al., 2009) contributes to altered gaze perception and more generally face processing. Therefore, both bottom-up (deficits in basic visual perception lead to abnormal gaze perception) and top-down theories (compromised higher-level cognition fails to properly regulate visual processing) are plausible and not mutually exclusive. A brain network model encompassing dynamic interactions between key brain regions involved in gaze processing is needed to address this question of directional influences in connectivity, which can guide future interventions to address poor social cognition.
In this study, we used dynamic causal modeling (DCM) to discover disordered functional architecture of eye gaze processing in schizophrenia. DCM has been used to demonstrate the contribution of functional dysconnectivity to cognitive abnormalities in schizophrenia such as attention, working memory, prediction, and visual illusion (Dima et al., 2010, 2009; Fogelson et al., 2014; Roiser et al., 2013; Zhou et al., 2018). It uses differential equations to model causal interactions between neuronal populations that are generative of the observed BOLD response in an experimental context (Friston et al., 2003). Neural connections are estimated using a Bayesian framework, which integrates prior probabilities and observed data to evaluate the credibility of the connections based on posterior probabilities. In this study, fMRI data were acquired while patients and healthy controls undertook an eye gaze processing task. DCM was applied to the fMRI time series to discover aberrant connectivity during processing of eye gaze in the context of faces, as well as modulation of connectivity by explicit gaze discrimination in schizophrenia.
2. METHODS AND MATERIALS
2.1. Participants
Twenty-nine individuals with schizophrenia or schizoaffective disorder (SZ) and 22 healthy controls (HC) completed the study. Diagnoses were established using the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-I) (First et al., 1995). See Supplemental Information for details of recruitment procedure and inclusion/exclusion criteria. Two SZ participants’ data were discarded due to chance-level performance on the gaze processing task. Characteristics of the remaining 27 SZ and 22 HC participants are summarized in Table 1.
Table 1.
Participant characteristics
| SZ (n = 27) | HC (n = 22) | t | χ2 | p | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Demographics | |||||
| Age | 33.6 ± 10.7 | 32.1 ± 13.3 | 0.44 | -- | .664 |
| Sex (male/female) | 12 / 15 | 11 / 11 | -- | 0.15 | .698 |
| Education, years | 14.6 ± 2.3 | 15.7 ± 1.9 | −1.86 | -- | .069 |
| Parental education, years | 16.0 ± 2.7 | 15.6 ± 3.7 | 0.45 | -- | .655 |
| Age of onset | 15.5 (4.8) | -- | -- | -- | -- |
| Duration of psychosis | 18.6 (10.2) | -- | -- | -- | -- |
| Comorbidity (n, %) | |||||
| Mood disorders | 5 (18.5%) | -- | -- | -- | -- |
| Anxiety disorders | 20 (74.1%) | -- | -- | -- | -- |
| Substance use disorders (past) | 9 (33.3%) | -- | -- | -- | -- |
| Medications | |||||
| CPZeq (mg daily) | 318 ± 318 | -- | -- | -- | -- |
| Mood stabilizer user (n, %) | 8 (29.6%) | -- | -- | -- | -- |
| Antidepressant user (n, %) | 13 (48.1%) | -- | -- | -- | -- |
| Benzodiazepine user (n, %) | 7 (25.9%) | -- | -- | -- | -- |
| Anticholinergic user (n, %) | 4 (14.8%) | -- | -- | -- | -- |
| Clinical Assessments | |||||
| PANSS positivea | 8.7 ± 3.7 | -- | -- | -- | -- |
| PANSS negativea | 10.4 ± 4.0 | -- | -- | -- | -- |
| PANSS disorganizationa | 3.9 ± 1.6 | -- | -- | -- | -- |
| PANSS excitementa | 4.8 ± 2.2 | -- | -- | -- | -- |
| PANSS depressiona | 7.3 ± 2.2 | ||||
| Functional Assessments | |||||
| WRAT3-R | 49.8 ± 5.8 | 50.2 ± 5.4 | −0.23 | -- | .819 |
| MCCB | 24.5 ± 24.2 | 72.1 ± 22.8 | −7.03 | -- | < .001 |
| MSCEIT.exp | 101.1 ± 16.0 | 115.4 ± 18.4 | −2.88 | -- | .005 |
| SAS-SR | 2.76 ± 0.54 | 3.38 ± 0.30 | 5.04 | -- | < .001 |
Note. CPZeq = antipsychotic dose in chlorpromazine equivalent mg daily. PANSS = Positive and Negative Symptoms Scale; WRAT3-R = Wide Range Achievement Test, revised, Reading subtest. MCCB = MATRICS Consensus Cognitive Battery; MSCEIT.exp = Mayer-Salovey-Caruso Emotional Intelligence Test Experiential Emotional Intelligence Quotient; SAS-SR = Social Adjustment Scale—Self-report.
Items based on the 5-factor solution in Wallwork et al. (2012).
The study was conducted in accordance with the protocol approved by the Institutional Review Board of the University of Michigan Medical School. Written informed consent was obtained from each participant after full explanation of the study was provided.
2.2. Experimental Paradigm: Eye Gaze Processing Task
Black-and-white photos of actors (Gur et al., 2002) with 9 different gaze angles were used. Gaze angles represent 9 levels of “eye-contact signal strength” on a scale from 0.2, 0.3, …, to 1.0 (from averted to direct gaze), based on our prior work (Tso et al., 2012). The faces were organized in blocks of 6 images. During Gaze blocks, participants performed explicit gaze discrimination of the faces (“Looking at me?” yes/no). During Gender blocks, participants judged the gender of the faces (“Gender?” male/female). Since gaze processing is an automatic/spontaneous process (Stein et al., 2011), the Gender condition is considered an implicit gaze processing condition in this study. Figure 1 shows task details.
Figure 1. Eye gaze processing task.
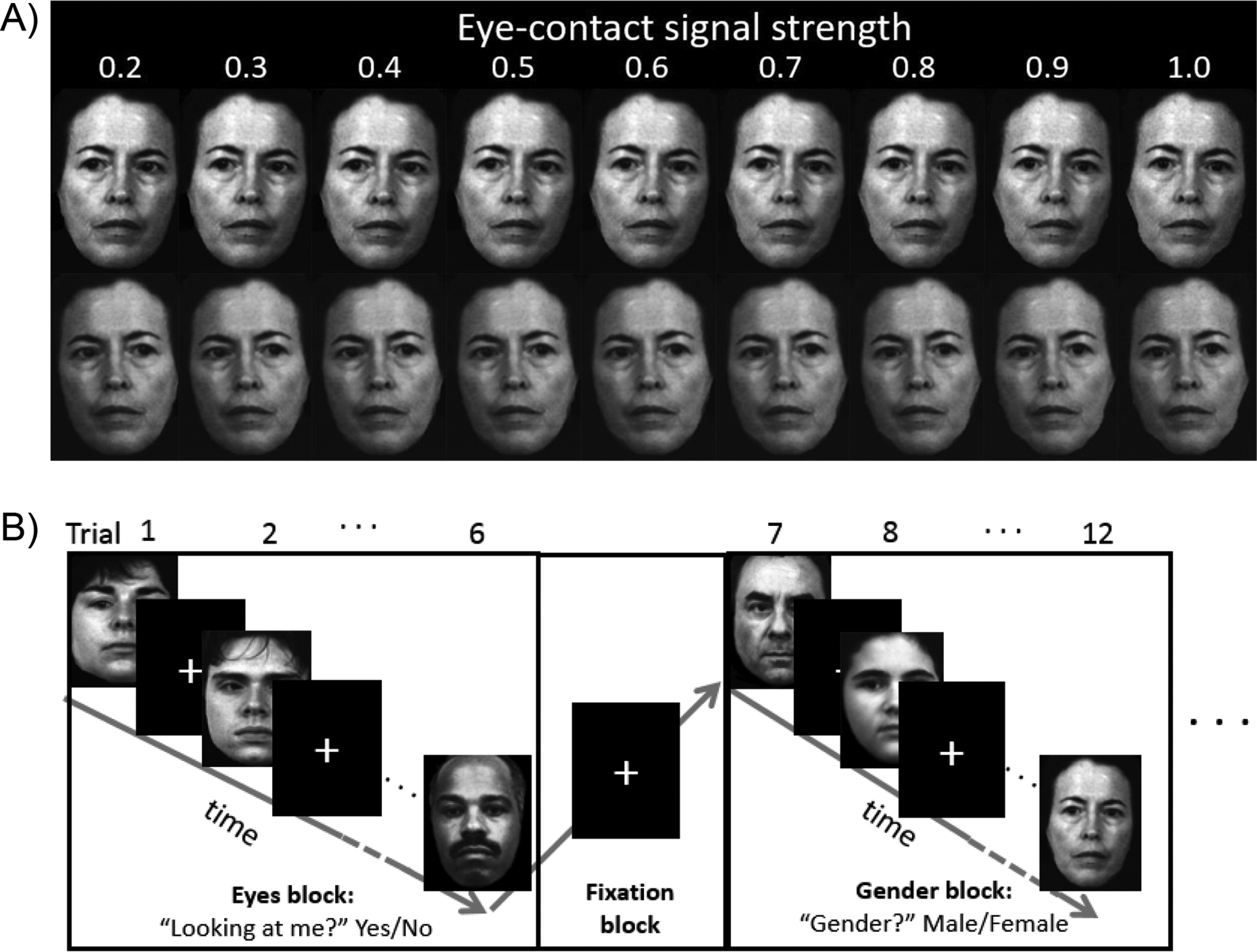
A) Example stimuli used in the task. Each actor had 9 eye-contact signal strengths, with each signal strength presented twice: looking left (upper panel) or looking right (lower panel). The stimulus set contained 6 actors, totaling 9 × 2 × 6 = 108 images. B) Stimuli were presented in a mixed (blocked event-related) design. Gaze and Gender trials were presented in alternating blocks (19.8 – 24.4 s) with a fixation block (11.4 – 15.6 s) between each block. Within each block, gaze angle and gender of the faces were pseudo-randomized. Each face was presented for 1.5 s and separated from the next face by a random jitter (1.6 – 3.9 s). There were totally 108 trials (6 trials × 6 blocks × 3 runs) for each of the conditions (Gaze, Gender).
2.3. Socio-emotional Functioning Assessments
The Mayer–Salovey–Caruso Emotional Intelligence Test (Mayer et al., 1999) Experiential Emotion Intelligence Quotient (MSCEIT.exp) was used to measure participants’ ability to perceiving and using emotions. The Social Adjustment Scale–Self-Report (SAS-SR) (Weissman and MHS Staff, 1999) was used to assess social functioning; the overall score was recoded so that higher scores indicate better social functioning.
2.4. fMRI Data Acquisition and Preprocessing
MRI scanning occurred on a 3.0 T GE MR 750 Discovery scanner (LX [8.3] release, General Electric Healthcare, Waukesha, WI). A T1-weighted image was acquired in the same prescription as the functional images to facilitate co-registration. Functional images were acquired with a T2*-weighted, reverse spiral acquisition sequence. Participants underwent 3 runs (12 task blocks and 11 fixation blocks per run), each consisting of 228 volumes. After acquisition of functional volumes, a high resolution T1 scan was obtained for anatomic normalization. fMRI data were processed using typical methods in Statistical Parametric Mapping (SPM12, Wellcome Institute of Cognitive Neurology, London). Scans with excessive movement within a run were discarded. Functional volumes were then co-registered with T1 image, spatially normalized to the MNI152 brain, and spatially smoothed with an 8 mm isotropic Gaussian kernel. See Supplemental Information 1 for details of the scan acquisition parameters and data preprocessing.
2.5. Behavioral Data Analyses
2.5.1. Gender identification accuracy and reaction time.
Group difference in accuracy in gender identification (an indicator of attention) was examined by t-test. Reaction time (RT) for Gender and Gaze trials was examined with separate linear mixed models (LMMs; methodological details in Supplemental Information 1).
2.5.2. Psychophysical gaze perception metrics.
Two metrics were derived from the behavioral responses on the gaze perception task: slope (indexing perceptual precision) and threshold (indexing self-referential bias). See Figure 2 for explanation of these two gaze perception measures. Hierarchical Bayesian modeling (HBM) was used to derive individual and group estimates of these two gaze perception measures (see Supplemental Information 1 for methodological details).
Figure 2.
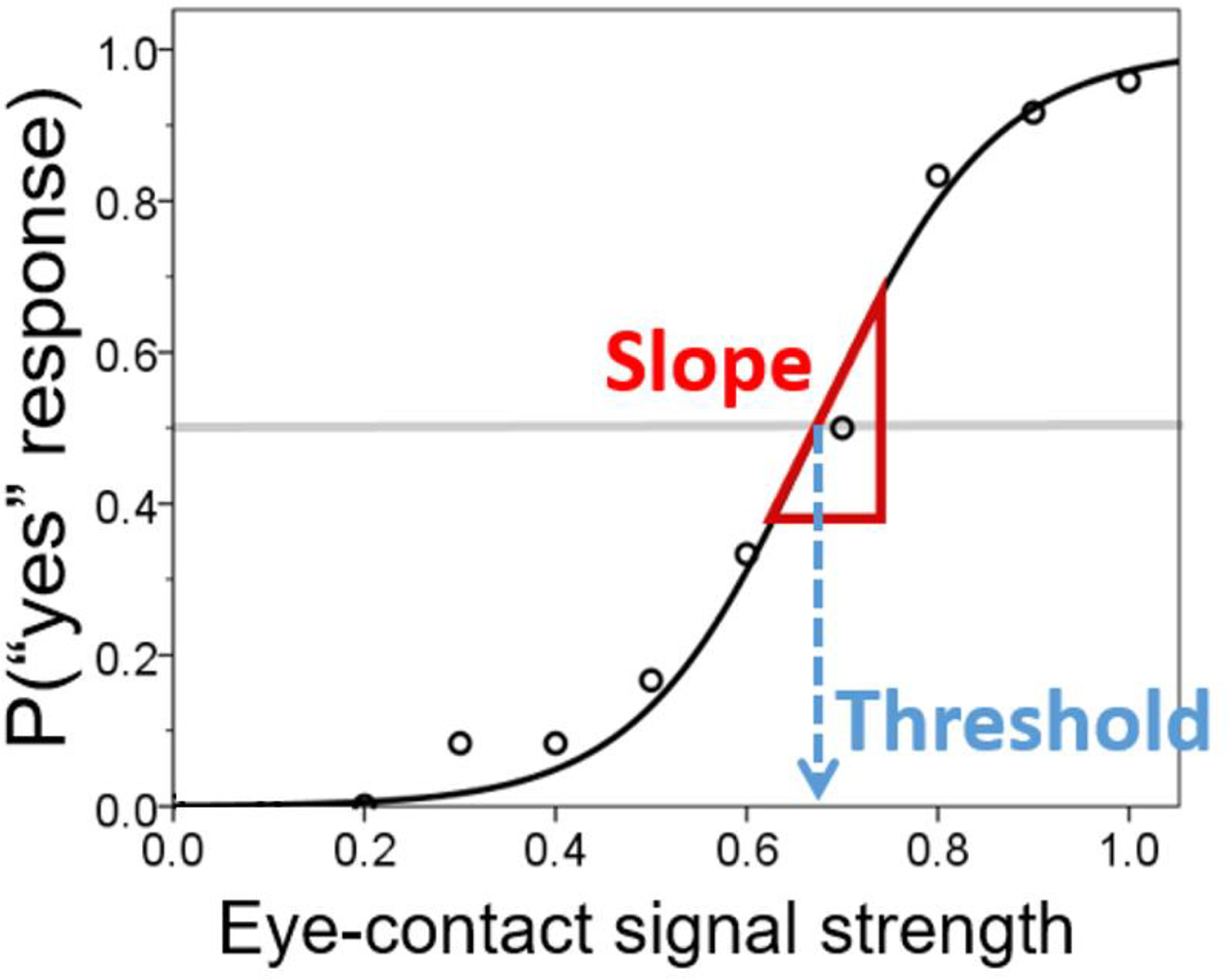
The psychophysical approach to derive gaze perception metrics. Probability of perception of self-directed gaze (“yes” response) is conceptualized as a function of eye-contact signal strength. The curve represents a theoretical, logistic function generative of the data (proportion of number of trials in which self-directed gaze was endorsed out of the total number of trials completed; represented by round markers). The slope of the curve at y = 0.5 indicates one’s perceptual precision (i.e., how sensitive the perception is with respect to change in eye-contact signal strength). The threshold (x value at y = 0.5) represents the intensity of eye-contact signal that elicits eye-contact perception 50% of the time; lower thresholds indicate that weaker signals are needed to perceive gaze as self-directed, thus representing a stronger self-referential bias.
2.6. Effective Connectivity Analysis
2.6.1. Identification of regions of interest (ROIs).
We applied a general linear model (GLM) to the fMRI time-series data to identify ROIs for subsequent DCM analysis. At the individual level, BOLD signals were regressed to two boxcar regressors of interest (Gaze and Gender events, respectively), along with nuisance regressors (3 runs and 6 motion parameters and derivatives), convolved with a hemodynamic response function. Then, individual beta estimates of the Gaze – Gender contrast were forwarded to second-level analysis. We chose this contrast because we were interested in brain regions that play a significant role in explicit eye gaze discrimination. The GLM result is illustrated in Figure 3A (see Supplemental Information 1, Table S1 and Figure S1 and for details). We selected 4 ROIs based on the relevance of their associated cognitive processes to gaze processing: secondary visual cortex (Vis) for early visual processing, posterior superior temporal sulcus (pSTS) for encoding of gaze direction (Boyarskaya et al., 2015; Burra et al., 2017; Carlin et al., 2011; Caruana et al., 2014; Sato et al., 2016; Schobert et al., 2018; Steuwe et al., 2014), inferior parietal lobule (IPL) for visuospatial processing (Itier and Batty, 2009), and posterior medial frontal cortex (pMFC) for cognitive control (Calder et al., 2002; Schilbach et al., 2006; Urakawa et al., 2015). For each ROI, the first principal component (eigenvariate) of a 5 mm radius sphere centered at the individual’s peak coordinate within a 10 mm radius of the group peak was extracted as the time-series data for DCM analysis.
Figure 3. Regions of interest (ROIs) and the full model for dynamic causal modeling analysis (DCM).
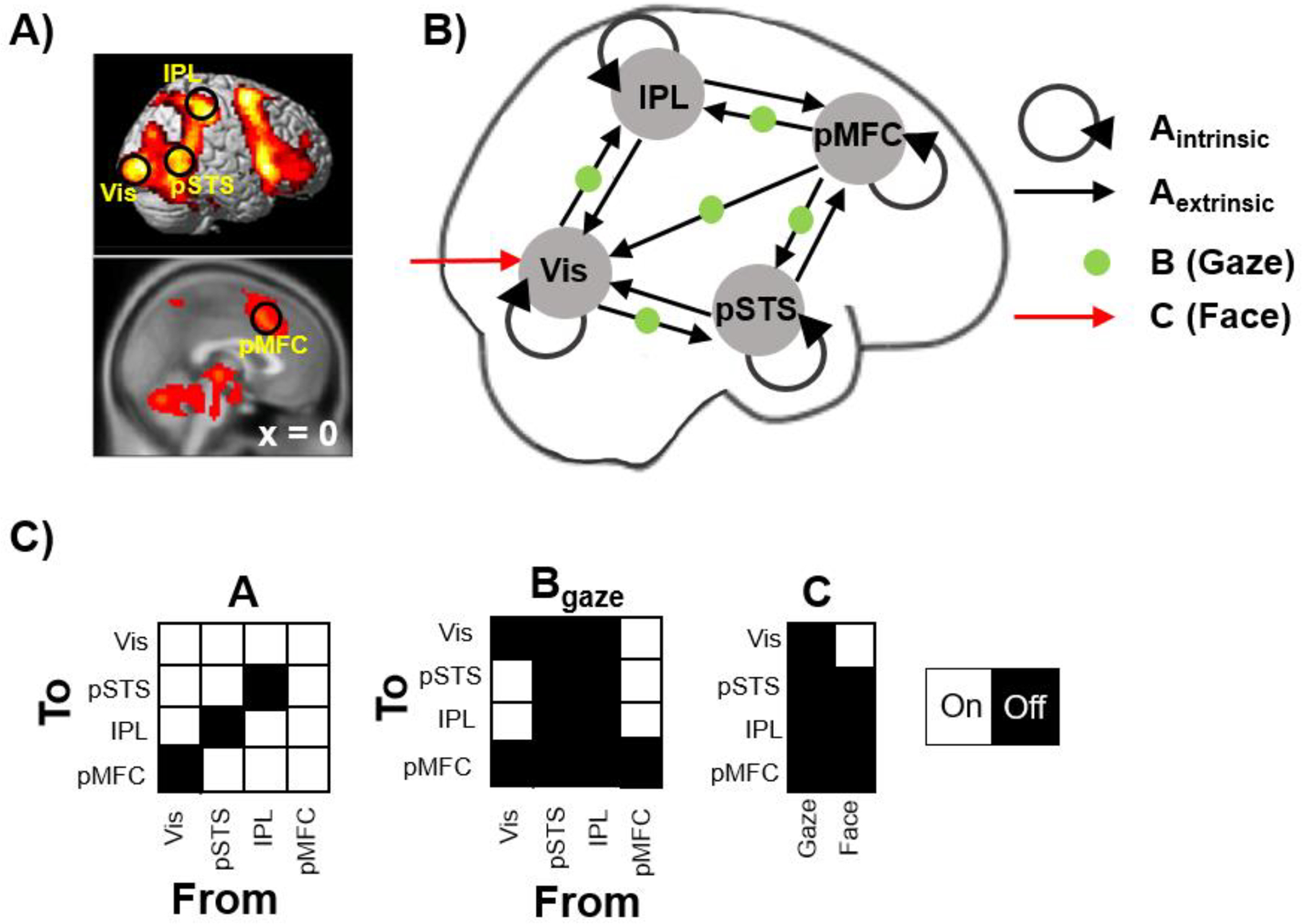
A) Based on the GLM result of the Gaze – Gender contrast across participants, 4 ROIs were selected as the nodes for subsequent DCM analysis: V2 visual cortex (Vis), posterior superior temporal sulcus (pSTS), inferior parietal lobule (IPL), and posterior medial frontal cortex (pMFC). B) The full model used in the DCM analysis. Black arrows indicate connections in during all face events (matrix A), while green dots indicate the connections that were allowed to be modulated by explicit gaze discrimination (matrix B). The red arrow indicates driving input (matrix C). C) The full DCM model in terms of on/off parameters specified in matrices A, B, and C.
2.6.2. Dynamic causal modeling.
DCM uses bilinear state (differential) equations to estimate effective connectivity between neuronal populations in an experimental context (Friston et al., 2003):
In this state equation, z is a vector representing the neuronal activity of the ROIs (“nodes”) and u is a matrix capturing all experimental events of interest. A contains parameters denoting the connectivity strength within the nodes (“intrinsic” or self-connections) or between the nodes (“extrinsic”). When the input u is mean-centered (which was the case in our analysis), matrix A parameters represent the mean connectivity across all modeled events (Zeidman et al., 2019). In this study, we modeled only the face events (and not fixations). Therefore, in this report, we label the process represented by matrix A as “general gaze processing,” which encompasses both implicit (Gender trials) and explicit (Gaze trials) gaze processing. Matrix B contains parameters denoting changes of connectivity due to specific experimental condition(s); in this case, explicit gaze discrimination during Gaze trials. Finally, matrix C contains parameters denoting the perturbation of neural activity in the nodes by external stimuli (i.e., driving inputs).
In SPM12, this neuronal model is coupled to a hemodynamic model embedding the Balloon-Windkessel model (which translates neuronal activity into hemodynamic responses) to predict the observed BOLD response (Friston et al., 2000). Parameter values in DCM reflect the rates of change (in Hz) in neural activity, with positive values indicating excitatory influences (i.e., connections that cause increased neural activity in a region) and negative values indicating inhibitory influences (causing reduced neural activity). The only exception is the self-connection parameters in matrix A, which undergo a transformation to provide the values used in the DCM model:
where A is the parameter estimated value appearing in matrix A and a is the value used in the model. This transformation restrains the values of self-connections to negative (i.e., always decaying over time). The default (prior) value is −0.5 Hz (i.e., when A parameter is zero). Therefore, positive self-connection parameters in matrix A indicate a faster self-decay process (i.e., stronger self-inhibition), while negative parameter values indicate weaker self-inhibition.
In this study, modeling began with constructing a full model encompassing all connections and psychological modulations of interest (Figure 3B). Specifically, the full model consisted of the 4 nodes each with a self-connection that allows natural decay of activity over time. We allowed bi-directional connections between all nodes, with two exceptions: a direct feedforward connection from Vis to pMFC and connections between IPL and pSTS were omitted (i.e., parameters set to zero), based on the literature of visual processing (Gilbert and Li, 2013). For driving input, we assumed that the sensory stimuli (faces) entered the neural system via the occipital cortex, thus Vis was modeled as the receiver of the driving input, which is a boxcar regressor representing each face presentation. Finally, we allowed explicit gaze discrimination (Gaze trials) to modulate feedforward connections from Vis (to encompass the bottom-up theory) and feedback connections from pMFC (to encompass the top-down theory). Figure 3C illustrates this full model in terms of A, B and C parameters allowed to switch on. See Supplemental Information for prior values of switched on or off parameters.
2.6.3. Connectivity parameter estimation.
Our primary interest was in estimating quantitative differences in connectivity strength between HC and SZ during general gaze processing (matrix A) and how connectivity changes by explicit gaze discrimination (matrix B). Therefore, we focused on quantitative comparisons of the DCM parameters (in particular, those in matrices A and B) between the two groups, rather than determining if a “winning” model for SZ qualitatively differed from that for HC. To derive DCM parameters estimates for hypothesis testing at the group level, the full model described above was first estimated at the individual level (variance explained: SZ = 9.6% ± 6.7%; HC = 6.0% ± 6.4%). Then, Parametric Empirical Bayes (PEB) (Friston et al., 2016b), taking into account the uncertainty of individual estimates, was used to estimate the values and probability distributions of the group-level parameters, separately for HC and SZ. This was done separately to optimize A parameters and B parameters. Finally, a PEB-of-PEB was performed on the HC and SZ PEBs, using one regressor for commonalities across groups (1 for each each) and a second regressor for group differences (1 for SZ, −1 for HC). Again, this was done twice to optimize A and B parameters separately.
2.7. Social Functional Relevance of Effective Connectivity
To investigate the functional relevance of the effective connectivity, canonical correlation analysis (CCA) was conducted to delineate the relationships between two sets of variables of interest. Specifically, DCM parameters that showed credible group differences (i.e., with posterior probability ≥ 95%) were included as covariate variables. Dependent variables included four measures of behavior covering functioning across the domains of gaze perception (gaze slope, gaze threshold), experiential emotional intelligence (MSCEIT.exp), and social functional outcome (SAS-SR).
3. RESULTS
3.1. Behavioral Data
3.1.1. Gender identification accuracy and reaction time (RT).
SZ (0.80 ± 0.10) and HC (0.84 ± 0.10) showed similar gender identification accuracy, t(47) = 1.38, p = .174. For Gaze trials, RT increased as gaze became more direct but reached a peak in the “ambiguous” zone (middle area of the gaze continuum); no group effect or interactions were detected. As expected, RT for Gender trials showed no relationship with Signal Strength, and group difference did not reach statistical significance (Supplemental Information 1, Figure S2).
3.1.2. Psychophysical gaze perception metrics.
Compared with HC, SZ showed lower threshold (i.e., stronger self-referential bias; posterior probability = 85.2%) and reduced slope (i.e., reduced perceptual precision; posterior probability = 99.3%) (Figure 4).
Figure 4. Posterior probability density plots of the psychophysical gaze perception measures.
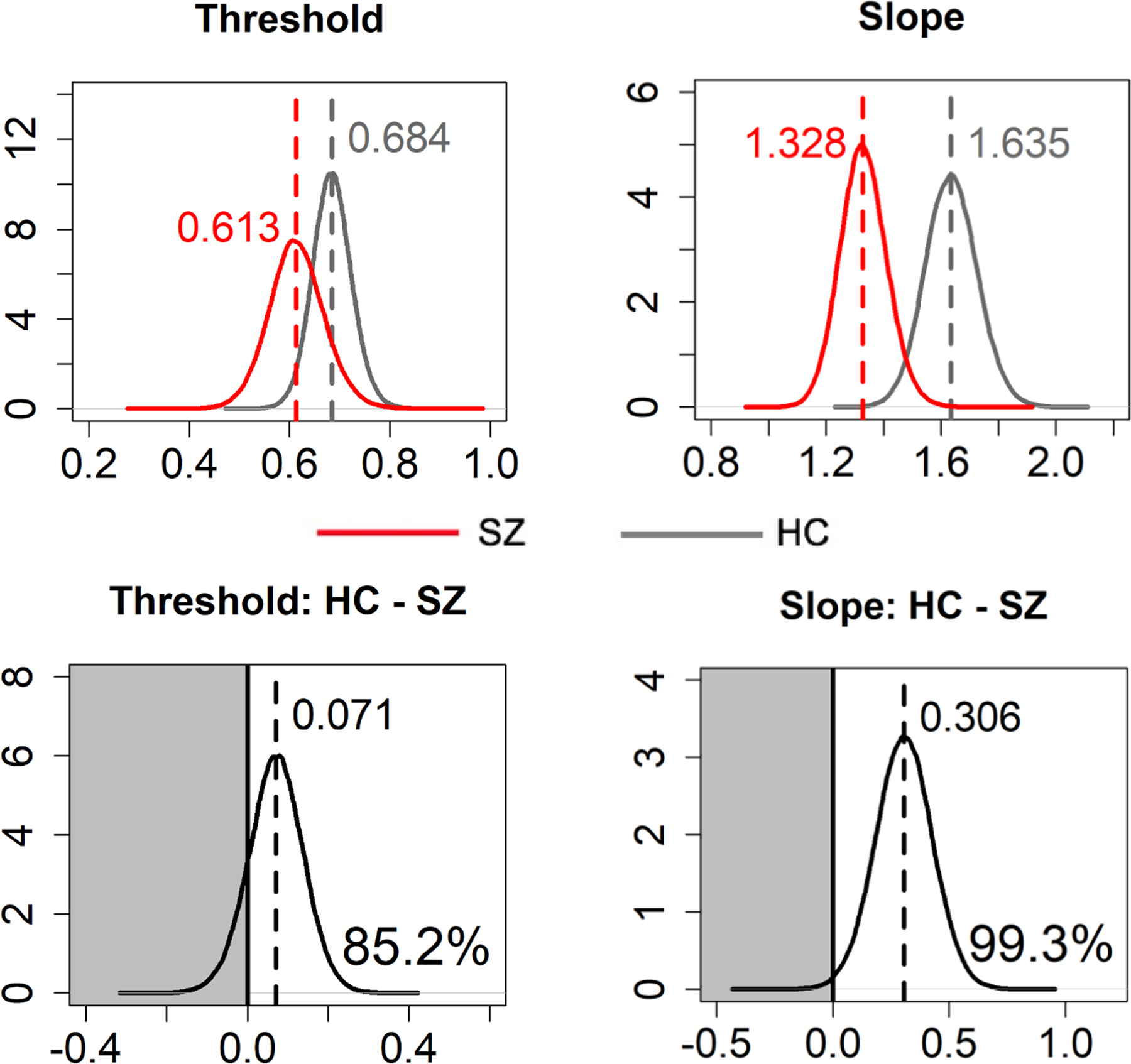
Dashed vertical lines and numbers at the top indicate median values of Markov Chain Monte Carlo (MCMC) samples. Numbers at the bottom right (bottom panel) indicate the posterior probability of the HC > SZ group difference (i.e., area under the curve in the white area).
3.2. Effective Connectivity
The PEB results are illustrated in Figure 5 (parameters estimates in Table 2). Overall, the SZ and HC models show many similarities structurally. During general gaze processing (matrix A), both groups showed excitatory bottom-up connectivity throughout the system, and inhibitory top-down influences of pMFC and IPL on Vis. Explicit gaze discrimination (matrix B) strengthened the excitatory bottom-up connections from Vis, as well as reduced the top-down influences of pMFC on IPL and pSTS in both groups.
Figure 5. Dynamic causal modeling results.
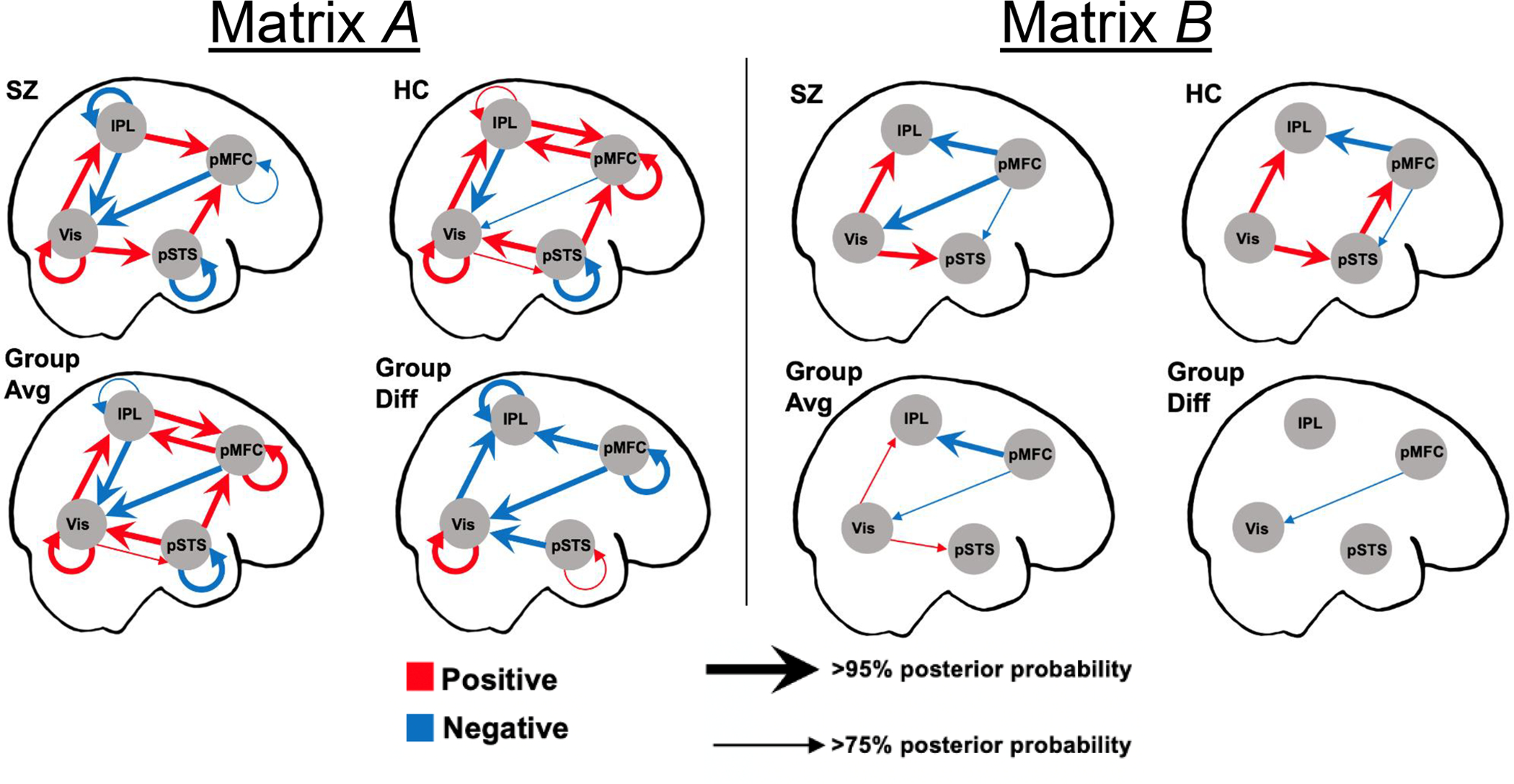
Matrix A represents connectivity during all face events, and matrix B represents connectivity changes due to explicit gaze processing. For clarity, only parameters whose values are non-zero with posterior probability ≥ 75% are shown.
Table 2.
DCM parameter estimates
| To | From | ||||
|---|---|---|---|---|---|
| Vis | pSTS | IPL | pMFC | ||
| Matrix A (connections during all face events) | |||||
| Vis | HC | 0.4292(0.057)* | 0.2405(0.049)* | −0.5653(0.065)* | −0.1046(0.070) # |
| SZ | 0.6344(0.049)* | −0.0220(0.076) | −0.5355(0.077)* | −0.3392(0.058)* | |
| All | 0.5620(0.044)* | 0.1256(0.052)* | −0.6235(0.057)* | −0.2422(0.052)* | |
| SZ-HC | 0.0996(0.044)* | −0.1383(0.052)* | −0.0021(0.057) | −0.1250(0.052)* | |
| pSTS | HC | 0.0223(0.017) # | −0.6165(0.057)* | −0.0083(0.024) | |
| SZ | 0.0464(0.014)* | −0.5096(0.048)* | 0.0030(0.017) | ||
| All | 0.0314(0.024) # | −0.6021(0.044)* | −0.0103(0.026) | ||
| SZ-HC | 0.0112(0.024) | 0.0670(0.044) # | 0.0069(0.026) | ||
| IPL | HC | 0.2739(0.024)* | 0.0551(0.058) # | 0.1872(0.041)* | |
| SZ | 0.1751(0.020)* | −0.1381(0.047)* | 0.0059(0.020) | ||
| All | 0.2179(0.026)* | −0.0418(0.044) # | 0.0998(0.032)* | ||
| SZ-HC | −0.0507(0.026)* | −0.1075(0.044)* | −0.0946(0.032)* | ||
| pMFC | HC | 0.3352(0.057)* | 0.2624(0.036)* | 0.3033(0.062)* | |
| SZ | 0.2972(0.049)* | 0.2229(0.041)* | −0.0691(0.048) # | ||
| All | 0.3395(0.044)* | 0.2463(0.035)* | 0.1436(0.046)* | ||
| SZ-HC | −0.0295(0.044) | −0.0222(0.035) | −0.2093(0.046)* | ||
| Matrix B (changes in connection due to gaze discrimination) | |||||
| Vis | HC | −0.0120(0.144) | |||
| SZ | −0.2999(0.121)* | ||||
| All | −0.1462(0.194) # | ||||
| SZ-HC | −0.1340(0.194) # | ||||
| pSTS | HC | 0.2517(0.077)* | −0.1351(0.111) # | ||
| SZ | 0.2139(0.064)* | −0.1215(0.084) # | |||
| All | 0.2199(0.177) # | −0.1200(0.183) | |||
| SZ-HC | −0.0176(0.177) | 0.0060(0.183) | |||
| IPL | HC | 0.1852(0.090)* | −0.2701(0.137)* | ||
| SZ | 0.2378(0.060)* | −0.4025(0.091)* | |||
| All | 0.1995(0.178) # | −0.3141(0.189)* | |||
| SZ-HC | 0.0253(0.178) | −0.0642(0.189) | |||
| pMFC | HC | ||||
| SZ | |||||
| All | |||||
| SZ-HC | |||||
Note. Values in parentheses are standard deviations. Shaded cells represented switched-off connections (i.e., not allowed) in the DCM model. Parameter value is non-zero with posterior probability > 95% (*) or > 75% (#).
The group difference models revealed multiple credible group differences (> 95% posterior probability) during general gaze processing (matrix A). Specifically, the self-connection parameter of Vis was increased while those of IPL and pMFC were decreased in SZ relative to HC. Because these parameters control the self-inhibition rate in each region, these findings indicate that Vis was more inhibited while IPL and pMFC were less inhibited in SZ. In terms of inter-regional connections, SZ showed less excitatory bottom-up connection from Vis to IPL, a absence of excitatory top-down connections from pMFC to IPL and from pSTS to Vis, and stronger inhibitory top-down influence of pMFC on Vis, relative to HC.
When criterion of at least 75% posterior probability was used, SZ displayed additional connectivity abnormalities. These include: faster self-decay of pSTS and stronger bottom-up excitatory influences of Vis on pSTS during general gaze processing, and stronger inhibitory top-down influence by the pMFC on Vis during explicit gaze discrimination.
3.3. Functional Relevance of Effective Connectivity
The CCA model examining the relationships between the seven effective connectivity parameters with credible group differences (all from matrix A) and four behaviors of interest was statistically significant, Wilk’s Λ = 0.202, p < .001, yielding one significant canonical function (Figure 6; Supplemental Information 1, Table S2). The canonical function suggests that stronger self-inhibition of Vis but reduced self-inhibition of higher-level cortical regions (IPL and pMFC), reduced bottom-up excitatory influence of Vis (on IPL), and stronger top-down inhibition by pMFC (on Vis and IPL) during gaze processing were collectively associated with poorer social cognition and functioning.
Figure 6. Canonical correlation between effective connectivity and social cognition & functioning.
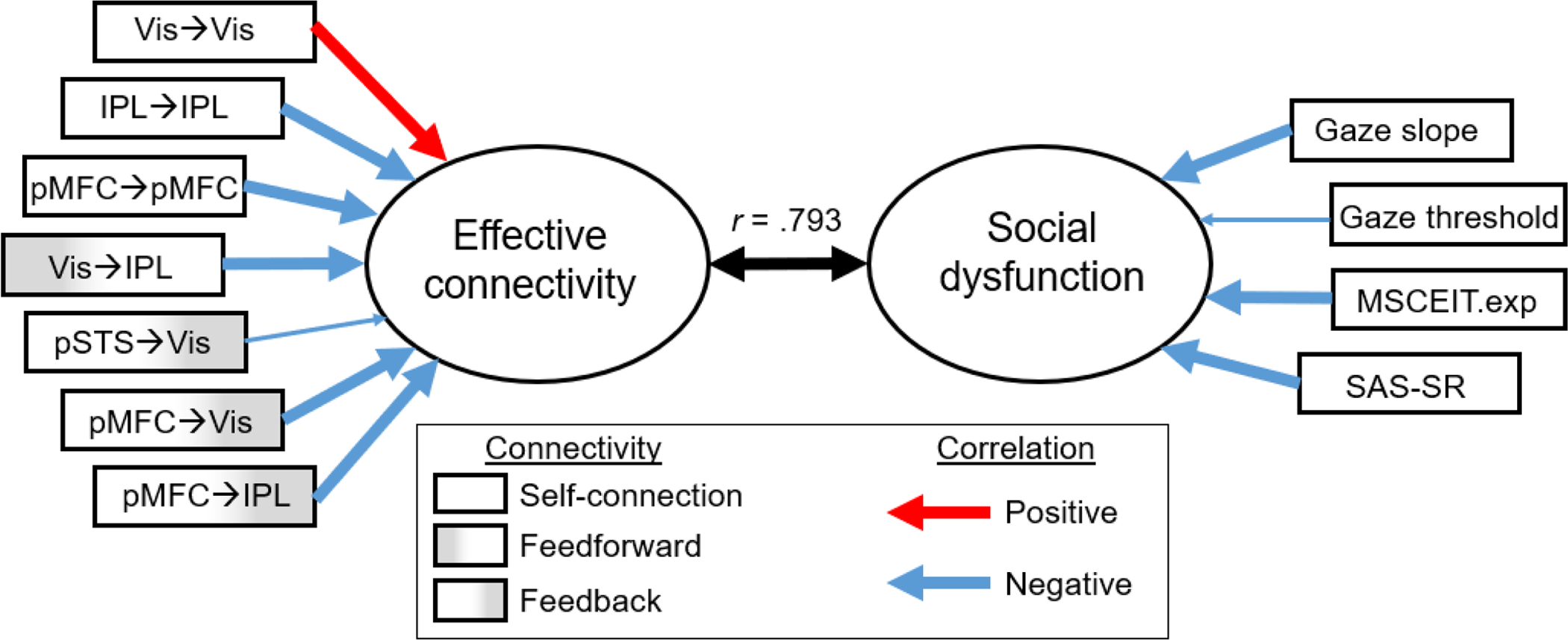
Stronger self-inhibition of Vis, weaker self-inhibition of IPL and pMFC, weaker bottom-up connections from Vis, and stronger top-down inhibition of the dorsal visual system by the medial frontal cortex were associated with abnormal gaze perception, poorer social cognition and social adjustment. Ovals represent synthetic predictors (left) and criterion variables (right). Thick arrows pointing from the predictor/dependent variables to the canonical variates represent structure coefficients > |.30|. Vis = V2 visual cortex; IPL = inferior parietal lobule; pSTS = posterior superior temporal sulcus; pMFC = posterior medial frontal cortex; MSCEIT.exp = Mayer–Salovey–Caruso Emotional Intelligence Test Experiential Emotion Intelligence Quotient; SAS-SR = Social Adjustment Scale–Self-Report.
4. DISCUSSION
This study aimed to identify alterations in brain dynamics during gaze processing in schizophrenia. The group DCM models revealed overall similar functional architectures in SZ and HC. During gaze processing, both groups showed excitatory bottom-up influences of the visual cortex on temporo-parietal regions and of the temporo-parietal regions on the medial frontal cortex. At the same time, parietal and medial frontal cortex exerted inhibitory influences on the visual cortex. When performing explicit gaze discrimination, SZ and HC similarly strengthened excitatory bottom-up connections from the visual cortex to temporo-parietal regions and inhibitory top-down connections from medial frontal cortex to the dorsal visual system (IPL and Vis).
The major differences in brain dynamics between SZ and HC during face and gaze processing lie in connectivity strengths rather than functional organization. The group difference model of general gaze processing (matrix A) reveals that all four brain regions examined showed differential self-connection strength between SZ and HC (3 out of 4 with > 95% posterior probability). Specifically, these finding indicate that in schizophrenia, the visual cortex was more inhibited (i.e., less sensitive to inputs from the network) while higher-cognitive regions were more sensitive to inputs from other regions. Biologically, self-connection parameters can be interpreted as representing excitatory-inhibitory balance (E/I) mediated by the interaction of pyramidal cells and inhibitory interneurons (Bastos et al., 2012; Zeidman et al., 2019). Our finding suggests that disrupted E/I balances are pervasive throughout the visual processing stream in schizophrenia, characterized by increased inhibition in low-level visual cortex and disinhibition in regions higher up in the hierarchy. Such deviations may originate from glutamatergic (Krystal et al., 2017) and GABAergic dysfunctions (Taylor and Tso, 2015; Tso et al., 2015), which both have been hypothesized to contribute to social cognitive impairment and social disability in schizophrenia (Lee and Green, 2016).
In addition to abnormal self-connections, SZ also exhibited altered strengths in inter-regional connections during gaze processing. Overall, SZ displayed weaker feedforward connection from the visual cortex but stronger inhibitory top-down influences of the frontal cortex compared with HC. When engaging in explicit gaze discrimination, SZ further increased top-down inhibition of Vis; this modulation was not observed in HC. Stronger down-regulating influences of pMFC on Vis during explicit gaze discrimination could reflect increased reliance on higher-level cognition to determine the self-referential nature of gaze. For example, manipulation of prior self-referential belief can change the tendency to perceive self-directed gaze (Stoyanova et al., 2010), showing that higher cognition can override sensory data. Our finding that SZ exhibited excessive top-down control to determine eye contact is consistent with the behavioral observation of a self-referential bias (lower eye contact perception threshold) in SZ (Tso et al., 2012; Yao et al., 2018). However, this finding should be interpreted with caution due to its lower level of evidence (posterior probability merely exceeding 75%), which may be because the estimation of matrix B was based on fewer trials compared to matrix A.
The DCM parameters that were altered in SZ seem to play a significant role in social dysfunction. We found a significant canonical correlation function linking these effective connectivity parameters to social cognition/functioning. Inspection of the variables loading on the effective connectivity variate suggests that altered cortical self-inhibition, weaker bottom-up connections from Vis and stronger top-down inhibition of the dorsal visual system by the medial frontal cortex were the primary contributors to poorer social cognition and functioning. This provides preliminary support for the validity of the DCM model. However, it should be noted that although CCA is a powerful multivariate tool, it tends to overfit data and overestimate correlations between the predictor and dependent variates, especially when sample-size-to-number-of-variables ratio is low. Therefore, when interpreting CCA results, it is advisable to focus on the meaning of the dimensions (i.e., how the variables load onto the variates) rather than the magnitudes of the canonical correlations. Independent studies are needed to replicate these findings.
The findings of this study should be interpreted in light of its limitations. First, the samples were small. Larger studies are needed to replicate the findings, as well as to conduct subgroup or covariate analyses to evaluate the effects of other important factors such as sex, age, and cultural differences. Additionally, it would be important to examine intra-individual stability of these DCM parameters, if they are to be used as biomarkers of social deficits or treatment response. Thus far, very few studies have examined such a reliability measure. Encouragingly, at least one study has reported sufficient rest-retest reliability for DCM parameter estimates obtained during a face perception paradigm in healthy individuals (Frässle et al., 2016). Second, most of the SZ participants were medicated, leaving it impossible to determine whether the findings reflect genuine endophenotypes of the disorder or a result of medications (or both). Although no correlations between effective connectivity and antipsychotic dose were found in this and other studies (Mukherjee et al., 2014, 2012; Potvin et al., 2017), extending this investigation to high-risk (e.g., schizotypy, psychosis risk syndromes) and early-psychosis populations with limited medication exposure would help clarify this question. Third, our 4-node model was by no means a comprehensive depiction of the brain, although the ROIs selected should be reasonably representative of the core brain regions involved in gaze processing. For example, our IPL and pMFC nodes are very similar to, respectively, the IPL B cluster defined by Mars et al. (Mars et al., 2011) and the SMA/pre-SMA clusters identified by Sallet et al. (2013). Our pMFC and pSTS nodes are also very similar to the clusters showing preferential activation in Reading the Mind in the Eyes test (Schurz et al., 2014). Recent developments, such as regression DCM constrained by sparsity (sparse rDCM), enable efficient effective connectivity analyses in complex brain networks and have shown preliminary face validity at a single-subject level (Frässle et al., 2018). Further validation of similar methods would allow applications in clinical research that requires valid group-level comparisons. Finally, the mean variance in BOLD signal explained by the DCM model was low in this study (< 10%), partially because fixations (> half of the task duration) were not modeled. Future investigation should consider using an A-B-N design (A and B = experimental blocks; N = null block), instead of an A-N-B-N design as used in this study, to reduce the proportion of not-modeled (null) events without sacrificing the sensitivity of the A-B contrast in the GLM analysis (Maus et al., 2010).
5. CONCLUSIONS
Using DCM this study found that gaze processing in schizophrenia involves abnormal intra- and inter-cortical dynamics. Both bottom-up and top-down abnormalities were indicated during general gaze processing, and explicit gaze discrimination was characterized by excessive top-down suppression of the visual cortex in schizophrenia. Overall, the findings provided preliminary evidence for altered gaze processing network dynamics in schizophrenia and its functional relevance.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (5KL2TR000434 to I.F.T.) and the National Institute of Mental Health (5K23MH108823 to I.F.T.). The authors thank the research volunteers for their participation, and Beier Yao, Savanna Mueller, Tyler Grove, Erica Shulte, and Zarina Kraal for their assistance in clinical assessment and data collection. Preliminary analyses of this data were previously published in an abstract in Neuropsychopharmacology, 40, S233.
Footnotes
DISCLOSURES
The authors reported no biomedical financial interests or potential conflicts of interest relevant to this research.
References
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ, 2012. Canonical Microcircuits for Predictive Coding. Neuron 76, 695–711. 10.1016/j.neuron.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarskaya E, Sebastian A, Bauermann T, Hecht H, Tüscher O, 2015. The Mona Lisa effect: Neural correlates of centered and off-centered gaze. Hum. Brain Mapp 36, 619–632. 10.1002/hbm.22651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra N, Baker S, George N, 2017. Processing of gaze direction within the N170/M170 time window: A combined EEG/MEG study. Neuropsychologia 100, 207–219. 10.1016/j.neuropsychologia.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Keane J, Scott SK, Owen AM, Christoffels I, Young AW, 2002. Reading the mind from eye gaze. Neuropsychologia 40, 1129–1138. 10.1016/S0028-3932(02)00008-8 [DOI] [PubMed] [Google Scholar]
- Carlin JD, Calder AJ, Kriegeskorte N, Nili H, Rowe JB, 2011. A Head View-Invariant Representation of Gaze Direction in Anterior Superior Temporal Sulcus. Curr. Biol 21, 1817–1821. 10.1016/j.cub.2011.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana F, Cantalupo G, Russo G. Lo, Mai R, Sartori I, Avanzini P, 2014. Human cortical activity evoked by gaze shift observation: An intracranial EEG study. Hum. Brain Mapp 35, 1515–1528. 10.1002/hbm.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Dietrich DE, Dillo W, Emrich HM, 2010. Impaired top-down processes in schizophrenia: A DCM study of ERPs. Neuroimage 52, 824–832. 10.1016/j.neuroimage.2009.12.086 [DOI] [PubMed] [Google Scholar]
- Dima D, Roiser JP, Dietrich DE, Bonnemann C, Lanfermann H, Emrich HM, Dillo W, 2009. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage 46, 1180–1186. 10.1016/j.neuroimage.2009.03.033 [DOI] [PubMed] [Google Scholar]
- Dong D, Wang Y, Jia X, Li Y, Chang X, Vandekerckhove M, Luo C, Yao D, 2018. Abnormal brain activation during threatening face processing in schizophrenia : A meta-analysis of functional neuroimaging studies. Schizophr. Res 197, 200–208. 10.1016/j.schres.2017.11.013 [DOI] [PubMed] [Google Scholar]
- Earls HA, Curran T, Mittal VA, 2016. Deficits in Early Stages of Face Processing in Schizophrenia : A Systematic Review of the P100 Component. Schizophr. Bull 42, 519–527. 10.1093/schbul/sbv096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev 24, 581–604. 10.1016/S0149-7634(00)00025-7 [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH, 2002. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci 99, 9602–9605. 10.1073/pnas.152159999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez M. de G., Penn DL, van Os J, Krabbendam L, 2011. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev 35, 573–588. 10.1016/j.neubiorev.2010.07.001 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB., 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. (SCID-P), version 2.0. Biometrics Research, New York, NY. [Google Scholar]
- Fogelson N, Litvak V, Peled A, Fernandez-del-Olmo M, Friston K, 2014. The functional anatomy of schizophrenia: A dynamic causal modeling study of predictive coding. Schizophr. Res 158, 204–212. 10.1016/j.schres.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frässle S, Lomakina EI, Kasper L, Manjaly ZM, Leff A, Pruessmann KP, Buhmann JM, Stephan KE, 2018. A generative model of whole-brain effective connectivity. Neuroimage 179, 505–529. 10.1016/j.neuroimage.2018.05.058 [DOI] [PubMed] [Google Scholar]
- Frässle S, Paulus FM, Krach S, Jansen A, 2016. Test-retest reliability of effective connectivity in the face perception network. Hum. Brain Mapp 37, 730–744. 10.1002/hbm.23061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Brown HR, Siemerkus J, Stephan KE, 2016a. The dysconnection hypothesis (2016). Schizophr. Res 176, 83–94. 10.1016/j.schres.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W, 2003. Dynamic causal modelling. Neuroimage 19, 1273–1302. 10.1016/S1053-8119(03)00202-7 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Litvak V, Oswal A, Razi A, Stephan KE, Van Wijk BCM, Ziegler G, Zeidman P, 2016b. Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage 128, 413–431. 10.1016/j.neuroimage.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ, 2000. Nonlinear Responses in fMRI: The Balloon Model, Volterra Kernels, and Other Hemodynamics. Neuroimage 12, 466–477. 10.1006/nimg.2000.0630 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Li W, 2013. Top-down influences on visual processing. Nat Rev Neurosci 14, 350–363. 10.1038/nrn3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J, 2015. Social cognition in schizophrenia. Nat Rev Neurosci 16, 620–631. 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE, 2002. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods 115, 137–143. 10.1016/S0165-0270(02)00006-7 [DOI] [PubMed] [Google Scholar]
- Hooker C, Park S, 2005. You must be looking at me: The nature of gaze perception in schizophrenia patients. Cogn. Neuropsychiatry [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M, 2009. Neural bases of eye and gaze processing: The core of social cognition. Neurosci. Biobehav. Rev 33, 843–863. 10.1016/j.neubiorev.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Loughead J, Ruparel K, Indersmitten T, Barrett FS, Gur RE, Gur RC, 2008. Brain activation during eye gaze discrimination in stable schizophrenia. Schizophr. Res 99, 286–293. 10.1016/j.schres.2007.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Anticevic A, Yang GJ, Dragoi G, Driesen NR, Wang XJ, Murray JD, 2017. Impaired Tuning of Neural Ensembles and the Pathophysiology of Schizophrenia: A Translational and Computational Neuroscience Perspective. Biol. Psychiatry 81, 874–885. 10.1016/j.biopsych.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Green MF, 2016. Social Preference and Glutamatergic Dysfunction: Underappreciated Prerequisites for Social Dysfunction in Schizophrenia. Trends Neurosci 39, 587–596. 10.1016/j.tins.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Noonan MAP, Bergmann C, Mitchell AS, Baxter MG, Behrens TEJ, Johansen-Berg H, Tomassini V, Miller KL, Rushworth MFS, 2011. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J. Neurosci 31, 4087–4100. 10.1523/JNEUROSCI.5102-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus B, van Breukelen GJP, Goebel R, Berger MPF, 2010. Optimization of Blocked Designs in fMRI Studies. Psychometrika 75, 373–390. 10.1007/s11336-010-9159-3 [DOI] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, 1999. Mayer-Salovey-Caruso Emotional Intelligence Test. Multi-Health Systems Inc., North Tonawanda, NY. [Google Scholar]
- Mukherjee P, Whalley HC, McKirdy JW, McIntosh AM, Johnstone EC, Lawrie SM, Hall J, 2012. Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophr. Res 134, 118–124. 10.1016/j.schres.2011.09.033 [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Whalley HC, McKirdy JW, Sprengelmeyer R, Young AW, McIntosh AM, Lawrie SM, Hall J, 2014. Altered amygdala connectivity within the social brain in schizophrenia. Schizophr. Bull 40, 152–160. 10.1093/schbul/sbt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Lungu O, Tikàsz A, Mendrek A, 2017. Abnormal effective fronto-limbic connectivity during emotion processing in schizophrenia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 72, 1–8. 10.1016/j.pnpbp.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Roiser JP, Wigton R, Kilner JM, Mendez MA, Hon N, Friston KJ, Joyce EM, 2013. Dysconnectivity in the frontoparietal attention network in schizophrenia. Front. Psychiatry 4, 1–13. 10.3389/fpsyt.2013.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse RB, Kendrick K, Wyatt RJ, Isaac A, 1994. Gaze discrimination in patients with schizophrenia: preliminary report. Am. J. Psychiatry 151, 919–921. 10.1176/ajp.151.6.919 [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Neubert FX, Jbabdi S, O’Reilly JX, Filippini N, Thomas AG, Rushworth MF, 2013. The organization of dorsal frontal cortex in humans and macaques. J. Neurosci 33, 12255–12274. 10.1523/JNEUROSCI.5108-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Toichi M, 2016. Neural mechanisms underlying conscious and unconscious attentional shifts triggered by eye gaze. Neuroimage 124, 118–126. 10.1016/j.neuroimage.2015.08.061 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, Vogeley K, 2006. Being with virtual others: Neural correlates of social interaction. Neuropsychologia 44, 718–730. 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Schobert AK, Corradi-Dell’Acqua C, Frühholz S, van der Zwaag W, Vuilleumier P, 2018. Functional organization of face processing in the human superior temporal sulcus: A 7T high-resolution fMRI study. Soc. Cogn. Affect. Neurosci 13, 102–113. 10.1093/scan/nsx119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Lobmaier JS, Arnold M, Renneberg B, 2013. All eyes on me?! Social anxiety and self-directed perception of eye gaze. Cogn. Emot 27, 1305–1313. 10.1080/02699931.2013.773881 [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J, 2014. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Senju A, Yaguchi K, Tojo Y, Hasegawa T, 2003. Eye contact does not facilitate detection in children with autism. Cognition 89, B43–B51. 10.1016/S0010-0277(03)00081-7 [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, 2011. Perceptual Organization Impairment in Schizophrenia and Associated Brain Mechanisms: Review of Research from 2005 to 2010. Schizophr. Bull 37, 690–699. 10.1093/schbul/sbr052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Senju A, Peelen MV, Sterzer P, 2011. Eye contact facilitates awareness of faces during interocular suppression. Cognition 119, 307–311. 10.1016/j.cognition.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Pannasch S, Beblo T, Reiss J, Lanius RA, 2014. Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Soc. Cogn. Affect. Neurosci 9, 88–97. 10.1093/scan/nss105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova RS, Ewbank MP, Calder AJ, 2010. “You Talkin’ to Me?”: Self-Relevant Auditory Signals Influence Perception of Gaze Direction. Psychol. Sci 21, 1765–1769. 10.1177/0956797610388812 [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S, 2011. Autism spectrum disorders and schizophrenia: Meta-analysis of the neural correlates of social cognition. PLoS One 6. 10.1371/journal.pone.0025322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC, 2011. Social appraisal in chronic psychosis: Role of medial frontal and occipital networks. J. Psychiatr. Res 45, 526–538. 10.1016/j.jpsychires.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Tso IF, 2015. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr. Res 10.1016/j.schres.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Carp J, Taylor SF, Deldin PJ, 2014. Role of visual integration in gaze perception and emotional intelligence in schizophrenia. Schizophr. Bull 40, 617–625. 10.1093/schbul/sbt058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Fang Y, Phan KL, Welsh RC, Taylor SF, 2015. Abnormal GABAergic function and face processing in schizophrenia: A pharmacologic-fMRI study. Schizophr. Res 168, 338–344. 10.1016/j.schres.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Mui ML, Taylor SF, Deldin PJ, 2012. Eye-contact perception in schizophrenia: Relationship with symptoms and socioemotional functioning. J. Abnorm. Psychol 121, 616–627. 10.1037/a0026596 [DOI] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Ishikawa A, Ono T, Nishijo H, 2015. Selective Medial Prefrontal Cortex Responses During Live Mutual Gaze Interactions in Human Infants: An fNIRS Study. Brain Topogr 28, 691–701. 10.1007/s10548-014-0414-2 [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS, 2002. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiol. Behav 77, 477–482. 10.1016/S0031-9384(02)00930-7 [DOI] [PubMed] [Google Scholar]
- Weissman MM, MHS Staff, 1999. Social Adjustment Scale–Self-Report (SAS) Multi-Health Systems Inc., Tonawanda, NY. [Google Scholar]
- Yao B, Mueller SA, Grove TB, McLaughlin M, Thakkar K, Ellingrod V, McInnis MG, Taylor SF, Deldin PJ, Tso IF, 2018. Eye gaze perception in bipolar disorder: Self-referential bias but intact perceptual sensitivity. Bipolar Disord 20, 60–69. 10.1111/bdi.12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Jafarian A, Corbin N, Seghier ML, Razi A, Price CJ, Friston KJ, 2019. A guide to group effective connectivity analysis, part 1: First level analysis with DCM for fMRI. Neuroimage 200, 174–190. 10.1016/j.neuroimage.2019.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zeidman P, Wu S, Razi A, Chen C, Yang L, Zou J, Wang G, Wang H, Friston KJ, 2018. Altered intrinsic and extrinsic connectivity in schizophrenia. NeuroImage Clin 17, 704–716. 10.1016/j.nicl.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


