Abstract
Inflammation has been implicated in the mechanisms responsible for preterm and term parturition, as well as fetal injury. Out of all of the suspected causes of preterm labor and delivery, infection and/or inflammation is the only pathological process for which both a firm causal link with preterm birth has been established and a molecular pathophysiology defined. Inflammation has also been implicated in the mechanism of spontaneous parturition at term. Most cases of histopathological inflammation and histological chorioamnionitis, both in preterm and term labor, are sub-clinical in nature. The isolation of bacteria in the amniotic fluid, known as microbial invasion of the amniotic cavity, is a pathological finding; the frequency of which is dependent upon the clinical presentation and gestational age. This article reviews the role of inflammation in preterm and term parturition.
Keywords: preterm labor, cytokines, fetal inflammatory response syndrome, chemokines, microbial invasion of the amniotic cavity
Introduction
Inflammation has been implicated in the mechanisms responsible for term and preterm parturition as well as fetal injury.1–10 Of all suspected causes of preterm labor and delivery, infection and/or inflammation is the only pathologic process for which both a firm causal link with preterm birth has been established and a molecular pathophysiology defined.11 Inflammation has also been implicated in the mechanism of spontaneous parturition at term. This article will review the role of inflammation in preterm and term parturition.
The spectrum of inflammation: clinical, histopathological and molecular
Inflammation is the basic process by which tissues of the body respond to insults.12 The first comprehensive description of the clinical signs of inflammation has been attributed to Celsus,13 who introduced four of the five classical signs (calor, dolor, rubor, and tumor), which translate to heat, pain, redness, and swelling. Galen added the fifth sign (function laesa), which means impaired function.13 Since that time, clinical inflammation has been classically defined by the presence of these five cardinal signs, all of which reflect the effects of chemokines, cytokines and other inflammatory mediators on local blood vessels and tissues.14,15 Vasodilatation and increased permeability account for the changes in temperature, redness and swelling (to some extent), while the migration of cells into tissue and the action of their mediators on the nerve endings account for pain and swelling.15 In contrast, histologic inflammation is defined by the infiltration of tissue by neutrophils, macrophages, and lymphocytes.13 The type of cell infiltrate is used to classify inflammation into acute or chronic. However, this classification may have some limitations in reproductive tissues in which there is physiologic infiltration of inflammatory cells. For example, neutrophils are normally present in menstrual endometrium,16 and the differential diagnosis between acute endometritis and perimenstrual endometrium requires examination of the magnitude of the infiltration.17 Pathologic examination has been the gold standard for the diagnosis of inflammation. However, chemotactic signals must be present for the white blood cells to migrate to the site of injury or infection. Thus, there is a window of time in which a “molecular signature of inflammation”, is present before histologic evidence is observed. For example, analysis of the transcriptome (see below)18 or the detection of inflammatory markers in body fluids (e.g. plasma, cerebrospinal fluid or amniotic fluid) may allow detection of early signs of inflammation that may not be detectable by conventional pathology.
A clear understanding of the spectrum of inflammation is important because there is a common misconception that the absence of systemic clinical signs, such as fever, chills, leucocytosis, etc., makes inflammation (and sometimes infection) unlikely. In reality, the evidence, as well as the understanding of pathophysiology, suggests that the opposite is the case. Most cases of histopathological inflammation are subclinical in nature. This is also the case in histologic chorioamnionitis, both at term (B.H. Yoon, R. Romero, et al., unpublished observations) and preterm delivery.19,20
Inflammation: from pathology to physiology and back
Inflammation is widely regarded as the fundamental mechanism of multicellular organisms to deal with insults, both of infectious and non-infectious nature. Inasmuch as the primary force shaping the evolution of the immune system is defense against microorganisms, it is not surprising that the mechanisms of inflammation were discovered when studying infectious diseases.21 However, inflammation also plays a central role in physiologic processes, particularly in the reproductive tract. The rupture of an ovarian follicle,22 the implantation of the blastocyst,23,24 menstruation,25 and parturition26,27 are characterized by cellular and molecular events which are found in pathologic inflammation (i.e. those associated with disease).
The value of inflammation
In the presence of invading microorganisms, inflammation accomplishes three main goals: (1) to deliver cells and molecules to suppress the infection; (2) to generate a physical barrier to the spread of the infection; and (3) to promote repair of the injured tissue.15
Cells called to the site of injury include macrophages, neutrophils, and lymphocytes. Molecules released during the course of inflammation include antimicrobial peptides, cytokines, chemokines, and other inflammatory mediators such as prostaglandins, leukotrienes, complement, etc.15 Some of these molecules change the state of activation of macrophages and neutrophils so that microbial killing is enhanced (i.e. through the release of reactive oxygen species). For example, activation of NADPH oxydase converts molecular oxygen into superoxide, which in turn is transformed by superoxide dismutase into hydrogen peroxide. This molecule has antimicrobial properties and can be transformed by peroxidase to hypochlorite and hydroxyl radicals. Superoxide, hydrogen peroxide, and hydroxyl radicals are called reactive oxygen species or reactive oxygen metabolites, and are important in microbial killing.15 When these molecules are released outside of phagocytes, they can injure other host cells. The state in which there is an excess generation and activity of reactive oxygen metabolites is called oxidative stress and is a mechanism of disease in systemic inflammation (e.g. sepsis,28 and preeclampsia29–32).
A second major goal of inflammation is to prevent the spread of microorganisms, and this is often accomplished by activation of the coagulation system and formation of thrombi in blood vessels draining the infected/inflamed sites. Thrombin, the rate limiting step of coagulation, has also pro-inflammatory properties. Indeed, in vitro experiments had demonstrated that thrombin enhances the LPS-induced interleukin (IL)-1 and tumor necrosis factor-α (TNF-α) in monocytes.33
Inflammation as a response to “danger signals” (microbial or nonmicrobial)
Injury can be the result of exposure to microorganisms or non-microbial-related insults. The consequences of microbial invasion and proliferation are well known and, therefore, will not be discussed in this section. However, the means by which non-microbial insults can injure and be recognized by the host are less well known. For example, exposure to an allergen or a transplanted organ can cause disease because the immune system recognizes the allergen or the organ as non-self. How is this accomplished? The immune system has evolved to identify the non-self using pattern recognition receptors (PRR) which are receptors that can identify repeating patterns of molecular structure, common to most microorganisms.15 However, it is now realized that these pattern recognition receptors can be used not only to sense the presence of microorganisms but also to identify “danger signals”.34 The basic premise of the “danger model” is that the immune system is more concerned with damage than with foreignness (“non-self”), and that an immune reaction is set into action because of “alarm signals” from injured tissues rather than by the recognition of non-self.34
Examples of “alarm signals” are those released by necrotic cells of the host that have been injured by microbial or non-microbial insults. The first pattern recognition receptors identified were Toll-like receptors35,36 and the ligands for these receptors were originally thought to be of microbial origin (e.g. endotoxin, peptidoglicans, viral RNA, etc.).15,35,36 It is now known that Toll-like receptors can recognize not only microbial products but also host signals produced in the context of injury, such as heat-shock proteins.37 Thus, the “danger model” of immunity provides a framework to understand the nature of the immune response and it releases it from the previously held paradigm, which was dependent largely on the self versus non-self concept.34
The immune response has two components: the innate and the adaptive. The innate mechanisms acts immediately, are non-specific, and lack immunological memory. The adaptive immune response, on the other hand, is specific, takes time to develop, and has memory.15 Inflammation is part of the innate immune response. However, it is now known that innate immunity orchestrates an appropriate adaptive immune response, and inflammation would also be part of the adaptive response.15 In conclusion, inflammation can be thought as central to maintaining tissue homeostasis. Exaggerated or prolonged inflammation or lack of an adequate inflammatory response can lead to disease.
Infection as a cause of premature labor
Evidence of causality:
Infection is a frequent and important mechanism of disease in premature labor and delivery.5,8,38,39 The evidence in support of this includes: 1) intrauterine infection or systemic administration of microbial products to pregnant animals can result in preterm labor and delivery;8,40–52 2) extra-uterine maternal infections such as malaria,53–58 pyelonephritis,59–63 pneumonia,64 and periodontal disease have been associated with premature parturition;65–68 3) subclinical intrauterine infections are associated with preterm labor and delivery;69 4) patients with intra-amniotic infection70–72 or intra-uterine inflammation (defined as an elevation of amniotic fluid concentrations of cytokines73,74 and matrix degrading enzymes75) in the midtrimester are at risk for subsequent preterm delivery; 5) antibiotic treatment of ascending intrauterine infections can prevent prematurity in experimental models of chorioamnionitis;49,76 and 5) treatment of asymptomatic bacteriuria prevents prematurity.77,78
The frequency and clinical significance of intrauterine infection:
Intrauterine infections caused by bacteria are considered to be the leading cause of infection-associated preterm birth. The amniotic cavity is considered to be sterile as less than 1% of women not in labor at term will have bacteria in the amniotic fluid. Therefore, the isolation of bacteria in the amniotic fluid is a pathologic finding, which we have defined as microbial invasion of the amniotic cavity, or MIAC. Most of these infections are subclinical in nature and cannot be detected without amniotic fluid analysis. The frequency of MIAC depends upon the clinical presentation and gestational age. In patients with preterm labor with intact membranes, the rate of positive amniotic fluid cultures is 12.8%.39 However, among those patients who have preterm labor with intact membranes and deliver a preterm neonate, the frequency is 22 %. Among women with preterm PROM, the rate of positive amniotic fluid cultures at admission is 32.4%,39 however, at the time of the onset of labor, as many as 75% of patients will have MIAC,79 suggesting that microbial invasion occurs during the latency period.
The frequency of MIAC among women with cervical insufficiency is up to 51%.80,81 If the cervix is short (as determined by sonographic cervical length of less than 25 mm) MIAC occurs in 9% of cases.82 Finally, the frequency of MIAC in twin gestations is 11.9%.83,84 Of interest, in twin gestations in whom MIAC is detected, the presenting sac is nearly always involved, while the other amniotic cavity may not have MIAC.84
Patients with MIAC are more likely to deliver preterm, have spontaneous rupture of the membranes, develop clinical chorioamnionitis, and experience adverse perinatal outcome than patients with preterm labor or preterm PROM with sterile amniotic fluid.
An interesting and consistent observation is that the lower the gestational age at presentation (preterm labor with intact membranes or preterm PROM), the higher the frequency of positive amniotic fluid cultures.85,86 Thus, infection is more prevalent in the earlier spontaneous preterm birth.
Microbiology of intrauterine infection:
The most common microorganisms found in the amniotic cavity are genital Mycoplasmas and, in particular, Ureaplasma urealyticum.38,87 However, other microorganisms found in the amniotic cavity include Mycoplasma hominis, Streptococcus agalactiae, E. coli, Fusobacterium species, and Gardnerella vaginalis.38,87 Of interest, with the use of molecular microbiologic techniques, organisms normally found in the oral cavity have been detected in amniotic fluid of women with preterm labor.88 This observation raises questions as to the pathway used by these organisms to reach the amniotic cavity (see below).
Significance of MIAC detected only by molecular microbiology techniques:
The prevalence of MIAC is based on the results of standard microbiologic methods (i.e. cultivation techniques). A positive culture can only be obtained if the culture conditions in the laboratory are able to support the growth of a particular microorganism. Inasmuch as the growth requirements of all microorganisms are unknown, a negative culture cannot be taken to exclude definitively the presence of a microorganism. In other words, while a positive culture is indicative of MIAC, a negative culture indicates that the laboratory was not able to grow bacteria from the specimen, either because bacteria was absent (a true negative result) or because the laboratory conditions did not support the growth of a specific microorganism (a false negative result). It is noteworthy that only 1% of the whole microbial world can be detected by cultivation techniques (“the great plate count anomaly”).89–91 Consequently, the frequency of MIAC reported herein represents minimum estimates. These figures are likely to change with the introduction of more sensitive methods for microbial recovery and identification. Indeed, several investigators have demonstrated that the prevalence of MIAC is higher when molecular microbiologic techniques are used to detect conserved sequences in prokaryotes (e.g. bacterial 16S rDNA with PCR).92–95
The clinical significance of MIAC detected purely by molecular microbiology techniques, but which cannot be detected by cultivation techniques, has been recently addressed. Patients with a positive PCR for Ureaplasma urealyticum but negative culture have similar adverse outcomes than patients with a positive amniotic fluid culture for this microorganism and worse outcomes than patients with sterile amniotic fluid and negative PCR.96,97 Moreover, patients with a positive PCR but a negative culture have the same degree of inflammation (amniotic fluid IL-6, histologic chorioamnionitis or funisitis) as those with a positive amniotic fluid culture.97 Collectively, this evidence suggests that the presence of microbial footprints detected by PCR is associated with adverse outcome.
Intrauterine infection can also be present in the absence of a positive amniotic fluid culture for microorganisms, or a negative PCR. Specifically, if the infection is localized to the decidua or the space between amnion and chorion, microorganisms may not be detected in the amniotic cavity.86 There is evidence that the rate of microbial colonization in the chorioamniotic space is higher than that observed in the amniotic cavity.86 Patients with positive amniotic fluid cultures in the membranes, but negative cultures in the amniotic fluid, often have elevation of amniotic fluid concentrations of indicators of inflammation, such as IL-6.86 Therefore, some patients with intra-amniotic inflammation but negative cultures in the amniotic fluid may have intra-uterine infection in the extra-amniotic space.
Microorganisms in the chorioamniotic membranes – is it always indicative of pathology?
The amniotic cavity is normally considered sterile for bacteria, even with the use of molecular microbiologic techniques. In contrast, fluorescent in situ hybridization with a DNA probe specific for conserved regions of bacterial DNA (the 16S ribosomal RNA) has detected bacteria in the fetal membranes of up to 70% of women undergoing elective cesarean section at term.98 Bacteria are often present in the membranes of patients with preterm labor and intact membranes, and patients with preterm PROM.98 These findings suggest that the presence of bacteria alone is not sufficient to cause preterm labor and delivery, and that microbial colonization of the chorioamniotic membranes may not always elicit a fetal or maternal inflammatory response. However, preterm labor is more frequent when a fetal inflammatory response in elicited as diagnosed by an increase in IL-1 and IL-6 and a decrease in IL-10.98
MIAC as a chronic process:
Although chorioamnionitis is traditionally considered an acute process, evidence that MIAC exists for an extended period of time is mounting. Cassell et al.70 were the first to report the recovery of genital Mycoplasmas from 6.6% (4/61) of amniotic fluid samples collected by amniocentesis between 16 and 21 weeks of gestation. Two women had positive cultures for Mycoplasma hominis and two for Ureaplasma urealyticum. Women with M. hominis delivered at 34 and 40 weeks without neonatal complications, while those with U. urealyticum had premature delivery, neonatal sepsis and neonatal death at 24 and 29 weeks. Subsequently, Gray et al.71 reported a 0.37% prevalence (9/2461) of positive cultures for U. urealyticum in amniotic fluid samples obtained during second trimester genetic amniocentesis. After exclusion of a therapeutic abortion case, all women (8/8) with positive amniotic fluid cultures had either a fetal loss within four weeks of amniocentesis (n=6) or preterm delivery (n=2). All had histological evidence of chorioamnionitis. These observations suggest that microbial invasion could be clinically silent in the midtrimester of pregnancy and that pregnancy loss/preterm delivery could take weeks to occur. A similar finding was reported by Horowitz et al.,72 who detected U. urealyticum in 2.8% (6/214) of amniotic fluid samples obtained between 16 and 20 weeks of gestation. The rate of adverse pregnancy outcome (fetal loss, preterm delivery and low birthweight) was significantly higher in women with a positive amniotic fluid culture than in those with a negative culture (3/6 [50%] versus 15/123 [12%]; P=0.035).
Pathways of intra-amniotic infection:
Microorganisms may gain access to the amniotic cavity and fetus using any of the following pathways: (1) ascending from the vagina and the cervix; (2) hematogenous dissemination through the placenta (transplacental infection); (3) retrograde seeding from the peritoneal cavity through the fallopian tubes; and (4) accidental introduction at the time of invasive procedures such as amniocentesis, percutaneous fetal blood sampling, chrorionic villous sampling, or shunting.7 The most common pathway of intrauterine infection is the ascending route (Figure 1).
Figure 1.
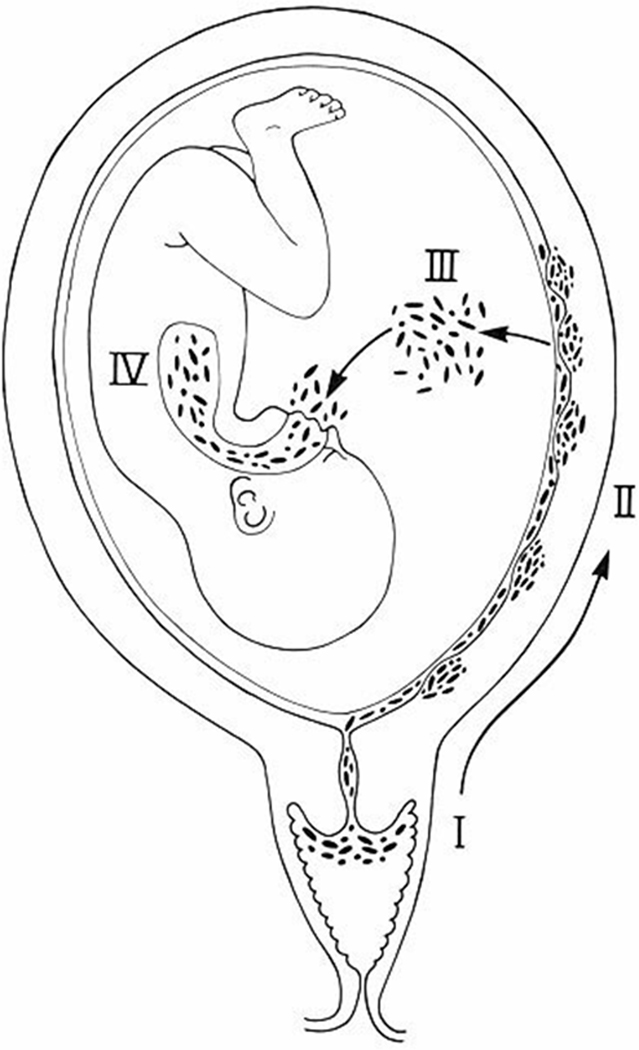
The most common pathway of intrauterine infection is the ascending route. (Reproduced, with permission, from Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynaecol 1982; 9:593–607).
Accumulating evidence supports a relationship between periodontal disease and premature labor and delivery.66,68,99–103 The mechanism underlying this association has not been definitively established, however, there is experimental evidence that microorganisms found in the gingival crevice can be isolated from the amniotic fluid, suggesting that maternal bacteremia and transplacental passage could account for some of these infections. Indeed, a humoral fetal response has been demonstrated by Boggess et al.104
Microbial products in the amniotic cavity:
The adverse events associated with microbial invasion can be due to the proliferation of intact microorganisms or bacterial products. For example, the cell wall of Gram-negative bacteria contains lipopolysaccharide (LPS) or endotoxin. This potent agent is capable of inducing endotoxic shock and death.105 Gram-positive bacteria lack LPS but contain peptidoglycans (PGN) and lipoteichoic acid, essential components of the bacterial wall.106 Mycoplasmas have products such as lipoglycans.107 Many of the effects of microorganisms are mediated by these products, which can be released during bacterial death. Consequently, even nonviable bacteria may exert deleterious effects. LPS, peptidoglycans and lipoglycans are recognized by Toll-like receptors and other pattern recognition molecules and can elicit an inflammatory response.
Bacterial endotoxin in amniotic fluid was first identified in 1987.108 Subsequently, it was found that the concentrations of these microbial products were significantly higher in women with preterm labor than in those with microbial invasion without preterm labor.109 There is a paucity of data about the amniotic fluid concentration of other microbial products. A number of experimental studies have determined that endotoxin administration into the amniotic cavity110,111 or intraperitoneally48,112,113 can result in an inflammatory response and potent biological effects in the fetal lung.114–116 Moreover, intrauterine bacterial inoculation, in an ascending model of intra-amniotic infection, was associated with histologic evidence of brain white matter damage.117
Detection of viral genome in the amniotic fluid and adverse pregnancy outcome:
A viral genome is detected in the amniotic fluid of up to 15% of asymptomatic low-risk pregnancies118–121 and 41% of pregnancies at risk for viral infection.122–124 The most common viral DNA isolates, either in low- or high-risk pregnancies, are adenovirus, cytomegalovirus (CMV) and enterovirus.119–124
Burguete et al125 performed a multicenter study to determine the prevalence of adeno-associated parvovirus (AAV) in midtrimester amniotic fluid samples, using PCR specific for DNA sequences of AAV. The prevalence of amniotic fluid positive for AAV by PCR was 27% (64/238). Moreover, patients with amniotic fluid positive for AAV by PCR had a higher frequency of preterm labor and preterm PROM than those with AF negative for AAV by PCR.125 Reddy et al.124 found an association between detection of a viral genome in the amniotic fluid and adverse pregnancy outcome. PCR was conducted for CMV, parvovirus B19, adenovirus, enterovirus, HSV, EBV, and RSV in 147 pregnancies. One hundred and thirty-eight fetuses were chromosomally normal, and among these, 25 (18%) had a positive amniotic fluid by PCR. These fetuses were more likely to deliver prematurely, have preterm PROM, non-immune hydrops, low birth weight or an intrauterine fetal demise. When only structurally normal fetuses were analyzed, those with a positive amniotic fluid for viral genome by PCR were more likely to die in utero and have both a lower gestational age and lower birth weight at delivery.
Inflammation as a mechanism for preterm parturition
An overview of the inflammatory response:
The first line of defense against infection is provided by the innate immune system. Epithelial surfaces (skin and mucous membranes) represent the first physical barrier between the body and microorganisms. Injuries to the epithelial surface provide a logical point of entry of microorganisms. These injuries can result from accidents or physiologic processes (e.g. menstruation). Thus, a sexually transmitted microorganism may cause infection if it gains access to the endometrial wound during menstruation. However, bacteria can cross intact epithelial barriers. There is experimental126 and clinical evidence38,108 that bacteria can cross intact chorioamniotic membranes. Epithelium, however, represents more than a physical barrier against microorganisms. Most epithelia produce natural anti-microbial peptides (e.g. alpha-defensins and beta-defensins),127 which can kill bacteria by damaging their cell membranes.128–131 For example, the fetal lung produces surfactant proteins (SP-A132,133 and SP-D133), that belong to the collectin family, which can bind microorganisms and facilitate phagocytosis (opsonization). Moreover, SP-A and SP-D have been shown to be involved in clearance of bacteria, fungi, and apoptotic and necrotic cells, down-regulation of allergic reaction, and resolution of inflammation.134
Another mechanism of host defense against infection derives from the metabolic products of bacteria. For example, lactobacilli, which colonize the vagina shortly after birth, produce lactic acid and lower the pH of the vagina. This unique partnership between vaginal tissues and species-specific strains of lactobacilli has been considered responsible for enabling internal fertilization in the evolution of mammals from amphibians.135 In addition to the low pH, some strains of lactobacilli also produce antimicrobial products (bacteriocin-like compounds) which prevent the growth of pathogenic bacteria.136,137
The innate component of the immune system also provides immediate protection from microbial challenge by recognizing the presence of micro-organisms, thus preventing tissue invasion and/or eliciting a host response to limit microbial proliferation (inflammation).15 One of the mechanisms by which the innate immunity recognizes micro-organisms is by using pattern recognition receptors (PRRs) which bind to repeating patterns of molecular structures present in the surfaces of microorganisms.15 PRRs are classified based on their function and subcellular localization into the following groups: (1) soluble PRRs such as “the acute phase proteins” Mannan Binding Lectin (MBL) and C-Reactive Protein (CRP), which act as opsonins to neutralize and clear pathogens through the complement and phagocytic systems; (2) transmembrane PRRs, which include scavenger receptors, C-type lectins and the Toll-like receptors (TLRs); (3) intracellular PRRs, including Nod1 and Nod2, RIG-1 and MDA-5, which mediate recognition of intracellular pathogens (e.g. viruses).138
Ten different TLRs have been recognized in humans.15 TLR-4 recognizes the presence of LPS (Gram-negative bacteria), and TLR-2 recognizes peptidoglycans, lipoproteins, and zymosan (Gram-positive bacteria, mycoplasmas, and fungi). TLR-3 recognizes double-stranded RNA (viruses). The ligand for TLR-5 is flagellin.15,139,140
Ligation of TLRs results in activation of NFκB, which, in turn, leads to the production of cytokines, chemokines, and anti-microbial peptides.15 Moreover, activation of the Toll pathway also induces surface expression of co-stimulatory molecules required for the induction of adaptive immune responses such as CD-80 and CD-86. These molecules, in combination with antigenic microbial peptides, presented by MHC class II proteins in dendritic cells and macrophages can activate naïve CD4 T-cells which, in turn, initiate most adaptive immune responses.15
The genital tract and trophoblasts have innate immune receptors:
Toll-like receptors (TLR)-1, −2, −3, −5, and −6, have been identified in the epithelia from the vagina, ecto- and endocervix, endometrium, and uterine tubes.141 Of note, TLR-4 has only been demonstrated in the endocervix, endometrium, and uterine tubes, but not in the vagina and ectocervix.141 This has been interpreted as evidence that TLR-4 may participate in the modulation of immunological tolerance in the lower parts of the female reproductive tract and in host defense against infection.141 Similarly, trophoblast cells are able to recognize and respond to pathogens through the expression of Toll-like receptors. We have demonstrated that trophoblast cells are able to recognize pathogens through the expression of TLR-2 and TLR-4. However, activation of different TLRs appears to generate distinct trophoblast cell responses. Indeed, in vitro studies have demonstrated that TLR-4 ligation by microbial products (LPS) promotes cytokine production, while ligation of TLR-2 to peptidoglycan and lipoteichoic acid induces apoptosis in first trimester trophoblast cells.142 These findings suggest that a pathogen, through TLR-2, may directly promote trophoblast cell death142 observed in a number of pregnancy complications including spontaneous abortion,143 intrauterine growth restriction144,145 and preeclampsia.144,146
The importance of TLRs in preterm parturition:
Since TLRs are crucial for the recognition of microorganisms, it will be anticipated that defective signaling through this pattern recognition receptor will impair bacteria-induced preterm labor. This is indeed the case. A strain of mice which has a spontaneous mutation for TLR-4 is less likely than wild type mice to deliver preterm after intrauterine inoculation of heat killed bacteria or LPS.110,147 In pregnant women, TLR-2 and TLR-4 are expressed in the amniotic epithelium.148 Moreover, spontaneous labor at term and preterm delivery with histologic chorioamnionitis, regardless of the membrane status (intact or ruptured), are associated with an increased mRNA expression of TLR-2 and TLR-4 in the chorioamniotic membranes.148 These observations suggest that the innate immune system plays a role in parturition, whether or not there is demonstrable intra-amniotic infection/inflammation.
The role of pro-inflammatory cytokines (IL-1 and TNF-α):
A solid body of evidence indicates that cytokines play a central role in the mechanisms of inflammation/infection-induced preterm parturition.2,26,69,149–159 IL-1 was the first cytokine to be implicated in the onset of preterm labor associated with infection.149 Evidence in support of participation of IL-1 included that: (1) IL-1 is produced by human decidua in response to bacterial products;160 (2) IL-1 can stimulate prostaglandin production by human amnion and decidua;161 (3) IL-1 concentration and bioactivity was increased in the amniotic fluid of women with preterm labor and infection;162 (4) IL-1 could stimulate myometrial contractions163 (Bulletti C, personal communication, 2002); and (5) administration of IL-1 to pregnant animals induced preterm labor and delivery,164 a phenomenon that could be blocked by the administration of its natural antagonist, IL-1 receptor antagonist (IL-1ra).165
Similarly, the evidence supporting the role of TNF-α in the mechanisms of preterm parturition includes: (1) TNF-α stimulates prostaglandin production by amnion, decidua, and myometrium;8 (2) human decidua can produce TNF-α in response to bacterial products;166,167 (3) amniotic fluid TNF-α bioactivity and immunoreactive concentrations are elevated in women with preterm labor and intra-amniotic infection;166 (4) in women with preterm PROM and intra-amniotic infection, TNF-α concentrations are higher in the presence of labor;168 (5) TNF-α can induce preterm parturition when administered systemically to pregnant animals;48,169,170 (6) TNF-α can stimulate the production of MMPs,171,172 which may play a role in membrane rupture173–175 and cervical ripening;171,176,177 and (7) TNF-α application in the cervix induces changes that resemble cervical ripening.178
Redundancy in the cytokine network:
Additional cytokines [(IL-6,86,179–183 IL-10,163,184,185 IL-16,186 IL-18,187 colony stimulating factors (CSFs),188–190 macrophage migration inhibitory factor (MIF)191] and chemokines [IL-8,189,192–194 monocyte chemotactic protein-1 (MCP-1),195 epithelial cell-derived neutrophil-activating peptide-78,196 and Regulated on Activation Normal T cell Expressed and Secreted (RANTES)197] had also been implicated in the mechanisms of disease in preterm labor and delivery. The redundancy of the cytokine network implicated in parturition is such that a blockade on a single factor is insufficient to prevent preterm delivery in the context of infection. For example, preterm labor can occur in knockout (KO) mice for the IL-1 type I receptor after exposure to infection, suggesting that IL-1 is sufficient, but not necessary, for the onset of parturition in the context of intraamniotic infection/inflammation.198 However, blockade of both IL-1 and TNF-α production in a double-KO mouse model has been associated with a decreased rate of preterm birth after infection.199
Anti-inflammatory cytokines and preterm labor:
Interleukin-10 is thought to be a key cytokine for the maintenance of pregnancy. Indeed, IL-10 production is significantly reduced in the placenta of patients at term not in labor compared with that from first- and second-trimester tissues, suggesting that down-regulation of IL-10 is a physiologic event that favors an inflammatory state around the time of onset of labor.184 IL-10 has also been implicated in the control of preterm parturition associated with inflammation.185 Indeed, IL-10 expression was reduced in placental tissues of pregnancies complicated by preterm labor and chorioamnionitis when compared to placental tissues from normal controls.185 Importantly, IL-10 inhibited COX-2 mRNA expression in cultured placental explants from preterm labor deliveries, but not in those from labor at term, indicating that the mechanisms involved in the regulation of the inflammatory response during term and preterm parturition may be different.185 Further evidence that IL-10 plays a role in down-regulation of the inflammatory response in preterm labor comes from a study in which pregnant rhesus monkeys (n=13) were allocated to three interventional groups: (1) intra-amniotic IL-1β infusion with maternal dexamethasone intravenously (n = 4), (2) intra-amniotic IL-1β + interleukin-10 (n = 5), and (3) intra-amniotic IL-1β administered alone (n = 5). Dexamethasone and interleukin-10 treatment significantly reduced IL-1β-induced uterine contractility (P<0.05) Tumor necrosis factor-alpha levels and leukocyte counts were also attenuated by interleukin-10 treatment (P <0.05).163 The administration of IL-10 in animal models of infection has been associated with improved pregnancy outcome.200,201
Inflammation and fetal injury: the fetal inflammatory response syndrome
While the traditional definition of inflammation describes “localized inflammation” to a particular tissue, it is now recognized that inflammation may be present in circulating blood. Such a state is referred as the “Systemic Inflammatory Response Syndrome.” This condition was originally described in adults and is often referred to by the acronym “SIRS.” SIRS was introduced in 1992 by the American College of Chest Physicians and the Society of Critical Care Medicine to describe a complex set of findings, which often involved cardiovascular abnormalities thought to be the result of systemic activation of the innate immune system.202 The changes, which included fever, tachycardia, hyperventilation, and an elevated white blood cell count,202 have been attributed to the effects of cytokines and other pro-inflammatory mediators.203 In 2001, the same organization noted that the elevation of certain mediators, such as IL-6, may be associated with SIRS and that this observation may bring about a new definition of the syndrome in adult patients, as the clinical and laboratory findings originally proposed to characterize SIRS were non-specific.204 We defined the fetal counterpart of SIRS for the first time in 1997, using precisely the same parameter that was proposed in adults: an elevated IL-6 concentration (in fetal blood).205 We coined the term, “Fetal Inflammatory Response Syndrome” (FIRS) to refer to the fetal counterpart of SIRS.9
FIRS was originally described in pregnancies complicated by preterm labor and preterm PROM and was operationally defined as a fetal plasma concentration of IL-6 > 11 pg/ml. Fetuses with FIRS had a higher rate of severe neonatal morbidity (respiratory distress syndrome, suspected or proved neonatal sepsis, pneumonia, bronchopulmonary dysplasia. intraventricular hemorrhage, periventricular leukomalacia, or necrotizing enterocolitis)9 and a shorter cordocentesis-to-delivery interval.9,10
The original work describing FIRS was based on fetal blood samples obtained by cordocentesis.9,10 Many of the findings have since been confirmed by studying umbilical cord blood at the time of birth, including the elevation of pro-inflammatory cytokines and the relationship between these cytokines and the likelihood of clinical and suspected sepsis.206–208 Pathological examination of the umbilical cord is an alternative approach to determine whether fetal inflammation was present before birth. Funisitis and chorionic vasculitis are the histopathologic hallmarks of FIRS.209 Funisitis is associated with endothelial activation, a key mechanism in the development of organ damage,210 and neonates with funisitis are at increased risk for neonatal sepsis20 and long-term handicaps, such as bronchopulmonary dysplasia (BPD)206 and cerebral palsy.211 Another approach to detect FIRS is to measure C-reactive protein concentration in umbilical cord blood, which has been shown to be elevated in patients with amniotic fluid infection, funisitis, and congenital neonatal sepsis.212 In addition, since neutrophils in the amniotic fluid are predominantly of fetal origin,213 the amniotic fluid white blood cell count can also be used as an indirect index of fetal inflammation.213 Intra-amniotic inflammation is a risk factor for impending preterm delivery and adverse perinatal outcome in women with preterm PROM, even in the absence of documented intra-amniotic infection.214
Fetal target organs during FIRS
Fetal microbial invasion or other insults can result in a systemic fetal inflammatory response that can progress toward multiple organ dysfunction, septic shock, and perhaps death in the absence of timely delivery. Evidence of multisystemic involvement in cases of FIRS includes increased concentrations of fetal plasma MMP-9,215 an enzyme involved in the digestion of type IV collagen and in the pathophysiology of preterm PROM.175 Moreover, several fetal organs including the hematopoietic system, the adrenals, heart, brain, lungs, and skin have been proposed to be target organs during FIRS (Figure 2).
Figure 2.
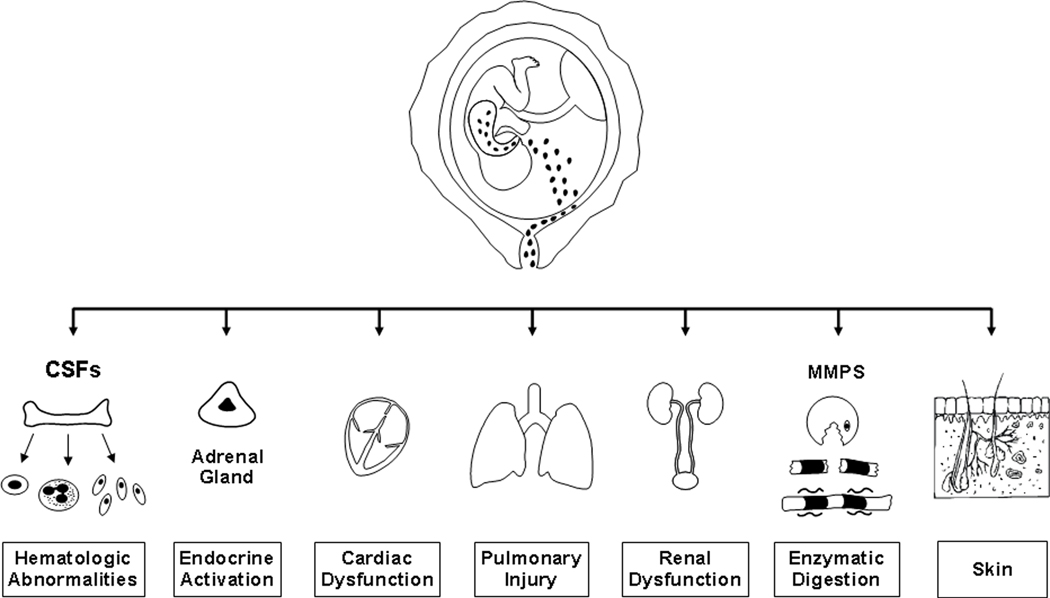
Fetal target organs during the fetal inflammatory response syndrome. CSFs, colony-stimulating factors; MMPs, matrix metalloproteinases.
The hematopoietic system:
The hematological response of the human fetus with FIRS is characterized by significant changes in the granulocyte and red blood cell lineages.216 Indeed, two-thirds of fetuses with FIRS have neutrophilia, defined as a neutrophil blood count above the 95th percentile for the gestational age.216 In contrast, only 7.1% of these fetuses (3/42) had neutropenia.216 The mechanisms responsible for fetal neutrophilia are not completely understood. However, it has been proposed that Granulocyte Colony Stimulating Factor (G-CSF), the primary physiologic regulator of neutrophil production, may participate in these mechanisms.217 Indeed, fetuses with FIRS had a higher median plasma concentration of G-CSF than those without FIRS (median: 714.4 pg/ml, range: 23.3–4229.2 vs. median: 55.7 pg/ml, range: 7.7–411; p<0.01).217
Fetuses with FIRS had a higher median nucleated red blood cell count than those without FIRS (median count: 2.42, range: 0–35 vs. median count: 1.38, range: 0–63.6; p<0.05).216 These changes are not associated with changes in the umbilical vein pH or PO2 levels.218 Thus, metabolic acidemia is unlikely to be the cause of these hematological changes, as described in fetuses with intrauterine growth restriction and abnormal Doppler velocimetry in the middle cerebral artery, inferior vena cava, and ductus venosus.219 Thus, the possibility that elevated nucleated red blood cell counts are a consequence of FIRS should be considered. Evidence in favor of this has been recently reported, indicating that the IL-6 concentration in umbilical cord blood may be an independent explanatory variable for the prediction of high nucleated red blood cell count.220
FIRS has also been associated with changes in markers of monocyte and neutrophil activation.221 Indeed, fetuses that were delivered within 72 hours of cordocentesis had a higher expression of CD11c, CD13, CD15, and CD67 than those delivered at term. In contrast, there were no significant differences in the percentages of CD14 and CD63 between the two groups. Collectively, these results indicate that fetuses destined to deliver prematurely have phenotypic evidence of activation of the monocyte-neutrophil system.221
The fetal thymus:
Thymus involution has been associated with infection in neonates. Recent evidence indicates that this also is the case in the fetus. A sonographically small thymus is found in cases with intra-amniotic infection/inflammation among patients with preterm labor and intact membranes.222 Indeed, the fetal thymus perimeter measured <5th percentile for gestational age in all cases with microbial invasion of the amniotic cavity (10/10), but in only 23.8% (5/21) of cases with negative cultures (P < 0.01). Furthermore, the fetal thymus was <5th percentile for gestational age in 100% (8/8), 71.4% (5/7), and 12.5% (2/16) of fetuses with funisitis, isolated chorioamnionitis, and without histologic signs of infection, respectively. Depletion of thymocytes probably results from glucocorticoid-induced apoptosis of the lymphoid tissue during the acute phase response.223 Thus, thymic involution has been proposed to be the result of lymphocyte depletion from both the thymic cortex and medulla, possibly mediated by activation of the hypotalamo-pituitary-adrenal axis.223
The adrenal glands:
Fetuses with FIRS have endocrine evidence of “stress” expressed as an abnormal cortisol/dehydroepiandrosterone ratio.224 Indeed, Yoon et al.224 reported a significant correlation between fetal plasma cortisol and fetal plasma interleukin-6 (r = 0.3, p<0.05) and a significant association between fetal plasma cortisol/dehydroepiandrosterone sulfate ratio and a shorter interval from cordocentesis to delivery (hazards ratio: 2.9, 95% CI: 1–8.4; p<0.05). Fetal plasma cortisol, but not maternal cortisol, was an independent predictor of the duration of pregnancy, after adjusting for gestational age and the results of amniotic fluid cultures (hazard ratio: 2.9, 95% CI: 1.3–6.7; p<0.05). Patients with preterm PROM who went into spontaneous labor and delivered within 7 days of cordocentesis had a significantly higher median fetal plasma concentration of cortisol but not of dehydroepiandrosterone sulfate than those delivered after 7 days (for fetal plasma cortisol: median 8.35 μg/dl, range: 4.7 to 12.4 μg/dl vs. median 4.75 μg/dl, range: 3.0 to 10.4 μg/dl; p<0.0001; for fetal plasma dehydroepiandrosterone sulfate: median 154.4 μg/dl, range: 8.6 to 333.8 μg/dl vs. median 194.6 μg/dl, range: 96.7 to 402.5 μg/dl; p=0.09).224 Collectively, these results indicate that an elevation in fetal plasma cortisol, but not dehydroepiandrosterone sulfate (DHEA-S), was followed by the onset of spontaneous preterm labor in patients with preterm PROM. Adult patients admitted to an intensive care unit with burns225 or pancreatitis have elevation of the cortisol/DHEA-S ratio just as human fetuses with FIRS. This may have short and long term implications given recent observations about the effect of glucocorticoids in fetal programming of several metabolic functions.226–229
The fetal skin:
The fetal skin is also a target organ during FIRS. Indeed, Kim et al230 studied the expression of TLR-2 and TLR-4 skin samples from fetuses between 21 to 24 weeks of gestation and reported that: 1) the skin from fetuses born to mothers without chorioamnionitis expressed TLR-2 and TLR-4 in the epidermis (TLR-2: median 3%, range 0.4%−7.2% and TLR-4: median 99.5%, range 91%−100%); 2) there was a dramatic increase in the expression of TLR-2, but not in TLR-4, in the epidermis of fetuses born after chorioamnionitis (TLR-2: median 19.6%, range 10.3%−89.6%; p=0.007 and TLR-4: median 100%, range 89.4%−100%; p=0.5); and 3) TLR-2 and TLR-4 were also expressed in the mononuclear inflammatory infiltrate of the dermal-epidermal junction. The authors proposed that the fetal skin is capable of recognizing the presence of microorganisms through the expression of ‘‘pattern-recognition receptors’’ and, thus, participates in a fetal inflammatory response to microbial products.230 The clinical manifestation of the involvement of the fetal skin during FIRS would be a fetal dermatitis.
The fetal kidneys:
Yoon et al. 231 reported that oligohydramnios is associated with FIRS among patients with preterm PROM. Indeed, patients with an amniotic fluid index ≤ 5 cm had: 1) significantly higher IL-6 concentrations in umbilical cord plasma at birth (fetal response); 2) higher concentrations of amniotic fluid pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α (intra-amniotic inflammatory response); and 3) higher rates of histologic and clinical chorioamnionitis (maternal response) than did those with an amniotic fluid index > 5 cm.224,231 These observations are consistent with the report that fetuses with fetal bacteremia diagnosed by cordocentesis had oligohydramnios (amniotic fluid index < 5 cm) more frequently than did those with a sterile blood culture.
The reasons why oligohydramnios in preterm PROM is associated with a higher rate of fetal infection/inflammation remain unclear. Yoon et al.224,231 proposed that, since the amniotic fluid has antimicrobial properties,232,233 oligohydramnios may reduce the protective effect of this component of innate immunity. Alternatively, redistribution of blood flow away from the kidneys may take place as part of the host response to microbial products, leading to oligohydramnios.
The fetal heart:
A recent report indicates that fetuses with preterm PROM have changes in the parameters used to evaluate diastolic function of the heart when compared to fetuses of women with uncomplicated pregnancies.234 The changes in the Doppler waveform characteristics in fetuses with preterm PROM are consistent with a high left ventricular compliance, particularly among those with proven intra-amniotic infection. These changes include a higher ratio between the early filling delta E/A ratio in both ventricles and a higher delta E/A VTI in the left ventricle, as compared to normal fetuses. The E wave reflects early diastolic filling, while the A wave represents changes in flow velocity due to atrial contraction. It is possible that these changes represent a compensatory mechanism similar to that observed in adults with sepsis. It is also possible that fetuses unable to change cardiac compliance in the context of a fetal systemic inflammatory response syndrome may not be able to maintain ventricular stroke volume and cardiac output and, hence, may not perfuse the brain adequately, predisposing to hypotension and brain ischemia in utero, which could create conditions for the development of periventricular leukomalacia. These changes in diastolic function may, therefore, have protective and even survival value. In cases of overwhelming fetal sepsis (the pathophysiologic counterpart to septic shock in adults), myocardial depression may lead to fetal death, which we have observed in cases with preterm PROM. The mechanism by which sepsis induces myocardial depression is not completely understood. The most likely explanation is that the myocardium is depressed by the action of soluble factors such as bacterial products and cytokines, which are elevated in the circulation of patients with septic shock.235–237
The observation that fetuses with preterm PROM and intra-amniotic infection undergo changes in cardiac function are consistent with the findings of Yanowitz et al.,238 who reported that neonates born with histologic chorioamnionitis had several hemodynamic abnormalities, including a decreased mean and diastolic blood pressure, and that there was a correlation between mean blood pressure and umbilical cord IL-6 concentrations.238 It is possible that some of these hemodynamic changes are present in utero and may contribute to the pathophysiology of periventricular leukomalacia and cerebral palsy.239 Those conditions were originally considered to be due to ischemia/hypoxia and have recently been linked to chorioamnionitis, infection, and fetal inflammation. In the context of FIRS, the combination of inflammatory changes in the brain and fetal systemic hypotension may increase the likelihood of brain injury. Recent evidence in support of this view is the observation that histologic chorioamnionitis with histologic evidence of placental hypoperfusion (poorly vascularized villi, multiple capillary lumina is some villi, increased intervillous volume and reduced total capillary bed) is associated with and an OR of 15.2 95% CI: 1.3–181) to have abnormal neurological outcome at the corrected age to 24 months.240
Why does the fetus mount an inflammatory response?
When the fetal inflammatory response syndrome was first described, we proposed that in the context of intrauterine infection, the onset of preterm labor would have survival value and that it would be part of the repertoire of host defense mechanisms against infection.9,10 The fetus would use the effector limb of the immune response via the secretion of pro-inflammatory cytokines to signal the onset of labor and exit a hostile intrauterine environment. Evidence in support of this hypothesis has been recently reported by Lahra et al.,241 who compared the frequency of a histological fetal response to chorioamnionitis (umbilical vasculitis with or without funisitis) between infants who survived the neonatal period and cases of perinatal death. Neonatal survivors had a higher prevalence of histological chorioamnionitis (95% CI, 1.02–1.21; p=0.02) and a higher rate of umbilical vasculitis/funisitis at 25 to 29 weeks of gestation (95% CI, 0.33–0.86; p=0.01) and 30 to 34 weeks of gestation (95% CI, 0.18–0.85; p=0.02), when compared to those who died in the perinatal period.
Important long-term consequences of FIRS include chronic lung disease193,194,242–245 and cerebral palsy.117,211,246–249 The readers are referred to the original articles and reviews regarding these transcendental consequences of FIRS.
A role of the fetus in the onset of labor
Among women with preterm PROM, FIRS is associated with the impending onset of preterm labor, regardless of the inflammatory state of the amniotic fluid (Figure 3).9 This suggests that the human fetus plays a role in initiating the onset of labor. However, maternal cooperation must occur for parturition. Thus, it is possible that systemic fetal inflammation may occur in the absence of labor when the inflammatory process does not involve the chorioamniotic membranes and decidua. Such instances may occur in the context of hematogenous viral infections (i.e. CMV infection) or other disease processes (i.e., alloimmunization).
Figure 3.
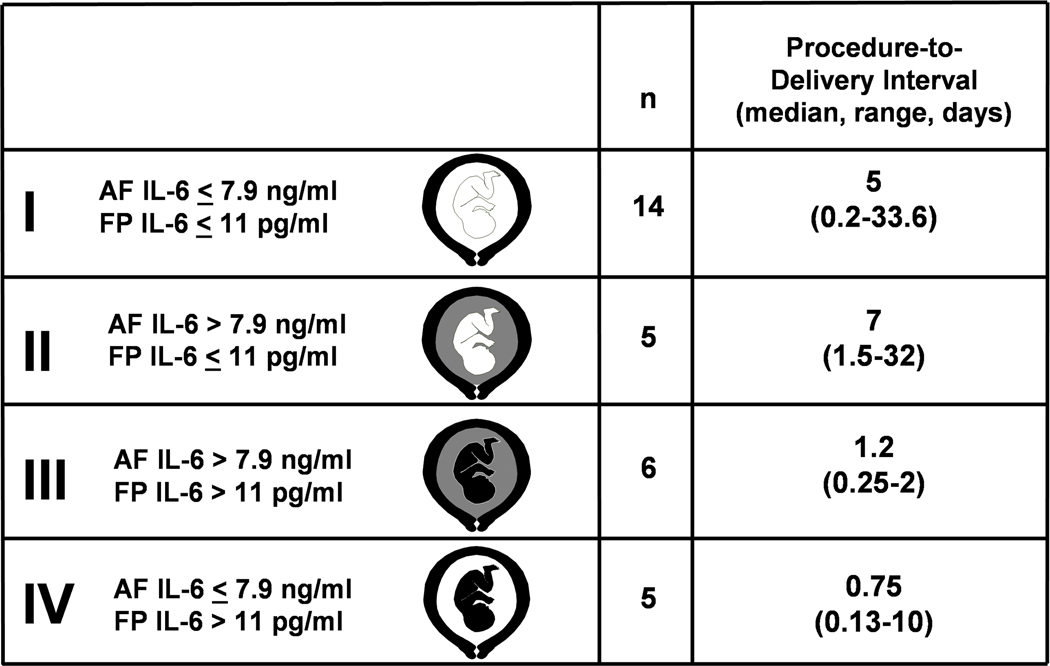
Classification and procedure-to-delivery intervals of patients according to amniotic fluid and fetal plasma interleukin-6 (IL-6) concentrations. A white color in the fetal or amniotic fluid (AF) compartment represents a low fetal plasma (FP) or amniotic fluid IL-6 concentration, respectively. Black in the fetal or amniotic fluid compartment denotes elevated fetal plasma or amniotic fluid IL-6 concentration.
Fetal death and maternal/fetal systemic inflammation
The rate of maternal inflammation is nine times more frequent than that of fetal inflammation in stillbirth. This could have two potential explanations. First, it is possible that fetal infection occurs, but fails to trigger a fetal inflammatory response and the onset of preterm labor. In this case, in utero fetal death would represent failure of the host response mechanisms dealing with intrauterine infection. This concept is supported by evidence that, in some cases of intrauterine death due to group B streptococci (with intact membranes), there may be absence of both a maternal and a fetal inflammatory response despite widespread fetal infection.250,251 Similar observations can be derived from the study of Tafari and colleagues with intrauterine infection by Ureaplasma urealyticum.252 Despite the fact that intrauterine infection was documented by culture of micro-organisms from fetal lung tissue at postmortem examination, fetal death could occur before the onset of labor. The second possible explanation for the discrepant rate of maternal and fetal inflammatory responses is that inflammation of the placental membranes may occur after fetal death and be etiologically unrelated to the fetal demise.253 The possibility that fetal death represent a failure of host defense requires further consideration given the association between homozygosity for the IL-1 receptor antagonist (IL-1ra) allele 2 and the risk of fetal death.254 Carriage of this allele is associated with increased production of IL-1ra.255,256 An excess of IL-1ra in the fetal compartment may limit the ability of the fetus to deploy a pro-inflammatory response and thus limit the repertoire of mechanisms available for host defense, including the ability to exit a hostile intrauterine environment by initiating the onset of labor.9,10,257 However, further studies are required to test this hypothesis.
The maternal systemic inflammatory response in normal pregnancy and preterm labor and delivery
Normal pregnancy has been proposed to be a state of physiologic activation of the innate limb of the immune response. Evidence in support of this view is the observation that normal pregnancy is associated with phenotypic changes in monocytes and granulocytes, as demonstrated by flow cytometry studies showing that granulocytes from normal pregnant women have up-regulation of CD14 (receptor for lipopolysaccharide and its binding protein) and CD64 (high affinity receptor for IgG, mediating the release of IL-1, IL-6 and TNF-α), and down-regulation of CD16 ( low affinity receptor for aggregated IgG) and HLA-DR (class II MCH antigen) than granulocytes of nonpregnant women.258 Similarly, monocytes from pregnant women had up-regulation of CD11b (integrin αM subunit that binds to ICAM-1), CD14, CD18 (Integrin β2 subunit that mediates fir adhesion of leukocytes to endothelium), CD62L (L-selectin, that mediates tethering and rolling of leukocytes), and CD64), and down-regulation of HLA-DR.258 Moreover, baseline iROS, oxidative burst and stimulation index values are increased in both granulocytes and monocytes.258
Preterm parturition with intact or rupture membranes is associated with phenotypic and metabolic changes in maternal monocytes and granulocytes that are consistent with the presence of intravascular maternal inflammation.259,260 Indeed, preterm labor and intact membranes is associated with up-regulation of CD11b, CD15 (fucosylated carbohydrate structure, recognizes endothelial selectins) and CD66 in maternal granulocytes, as well as up-regulation of CD11b and CD15 in maternal monocytes.259 Similarly, preterm PROM is associated with up-regulation of CD11b, CD14, CD64 and CD66b on granulocytes and CD11b on monocytes.260 Moreover, both preterm labor with intact membranes and preterm PROM were associated with increased ratio of oxidative burst over basal intracellular oxygen radical species in both granulocytes and monocytes.259,260
Conclusions
The evidence reviewed in this article indicates that intraamniotic infection/inflammation is causally linked to preterm parturition, fetal injury, and the development of a fetal inflammatory response syndrome, in a subset of patients. Moreover, preterm parturition (with intact or ruptured membranes) is associated with a maternal systemic inflammatory response characterized by phenotypic and metabolic changes of maternal monocytes and granulocytes. It is possible that modulation of inflammation with anti-inflammatory cytokines (i.e. IL-10), corticoids, antioxidants and/or other factors, such as anti-MIF antibodies, may complement antibiotic therapy and limit fetal injury.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Reference List
- 1.Brocklehurst P. Infection and preterm delivery. BMJ 1999;318:548–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–1528 [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507 [DOI] [PubMed] [Google Scholar]
- 4.Ledger WJ. Infection and premature labor. Am J Perinatol 1989;6:234–236 [DOI] [PubMed] [Google Scholar]
- 5.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol 1983;62:137–144 [PubMed] [Google Scholar]
- 6.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol 1982;9:593–607 [PubMed] [Google Scholar]
- 7.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–584 [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Mazor M, Wu YK et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–279 [PubMed] [Google Scholar]
- 9.Gomez R, Romero R, Ghezzi F et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202 [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Gomez R, Ghezzi F et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–193 [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Mazor M, Munoz H et al. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–429 [DOI] [PubMed] [Google Scholar]
- 12.Henson PM. Dampening inflammation. Nat Immunol 2005;6:1179–1181 [DOI] [PubMed] [Google Scholar]
- 13.Ley K. History of inflammation research. 2001;1–10 [Google Scholar]
- 14.Gallin JI, Snyderman R. Inflammation: Historical Perspective. 1999;3rd:5–12 [Google Scholar]
- 15.Janeway C, Travers P, Walport M, Schlomchik M. Innate immunity. 2005;6th:37–102 [Google Scholar]
- 16.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update 2000;6:16–27 [DOI] [PubMed] [Google Scholar]
- 17.Kiviat NB, Wolner-Hanssen P, Eschenbach DA et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. AM J SURG PATHOL 1990;14:167–175 [DOI] [PubMed] [Google Scholar]
- 18.Haddad R, Tromp G, Kuivaniemi H et al. Spontaneous labor at term is characterized by a genomic signature of acute inflammation in the chorioamniotic membranes but not in the systemic circulation. Am J Obstet Gynecol 2004;191:S138 [Google Scholar]
- 19.Yoon BH, Romero R, Moon JB et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology 2001;185:1130–1136 [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Romero R, Park JS et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–1129 [DOI] [PubMed] [Google Scholar]
- 21.Metchnikoff E. Lectures on the comparative pathology of inflammation (reprint of the 1983 English translation). 1968;XVII [Google Scholar]
- 22.Brannstrom M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol 2002;57:47–60 [DOI] [PubMed] [Google Scholar]
- 23.Chard T. Cytokines in implantation. Hum Reprod Update 1995;1:385–396 [DOI] [PubMed] [Google Scholar]
- 24.Finn CA. Implantation, menstruation and inflammation. Biol Rev Camb Philos Soc 1986;61:313–328 [DOI] [PubMed] [Google Scholar]
- 25.Kelly RW, King AE, Critchley HO. Inflammatory mediators and endometrial function--focus on the perivascular cell. J Reprod Immunol 2002;57:81–93 [DOI] [PubMed] [Google Scholar]
- 26.Keelan JA, Blumenstein M, Helliwell RJ et al. Cytokines, prostaglandins and parturition--a review. Placenta 2003;24 Suppl A:S33–S46 [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Parvizi ST, Oyarzun E et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990;35:235–238 [PubMed] [Google Scholar]
- 28.Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med 2002;33:1173–1185 [DOI] [PubMed] [Google Scholar]
- 29.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000;21:597–602 [DOI] [PubMed] [Google Scholar]
- 30.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet 1999;354:788–789 [DOI] [PubMed] [Google Scholar]
- 31.Dekker GA, Sibai BM. The immunology of preeclampsia. Semin Perinatol 1999;23:24–33 [DOI] [PubMed] [Google Scholar]
- 32.Morris JM, Gopaul NK, Endresen MJ et al. Circulating markers of oxidative stress are raised in normal pregnancy and pre-eclampsia. Br J Obstet Gynaecol 1998;105:1195–1199 [DOI] [PubMed] [Google Scholar]
- 33.Hoffman M, Cooper ST. Thrombin enhances monocyte secretion of tumor necrosis factor and interleukin-1 beta by two distinct mechanisms. Blood Cells Mol Dis 1995;21:156–167 [DOI] [PubMed] [Google Scholar]
- 34.Matzinger P. The danger model: a renewed sense of self. Science 2002;296:301–305 [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988;52:269–279 [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R, Janeway J. The Toll receptor family and microbial recognition. Trends in Microbiology 2000;8:452–456 [DOI] [PubMed] [Google Scholar]
- 37.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends in Immunology 2002;23:509–512 [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Sirtori M, Oyarzun E et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824 [DOI] [PubMed] [Google Scholar]
- 39.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13 [DOI] [PubMed] [Google Scholar]
- 40.Zahl PA, Bjerknes C. Induction of decidua-placental hemorrhage in mice by the endotoxins of certain gram-negative bacteria. Proc Soc Exper Biol Med 1943;54:329–332 [Google Scholar]
- 41.Takeda Y, Tsuchiya I. Studies on the pathological changes caused by the injection of the Shwartzman filtrate and the endotoxin into pregnant rabbits. Jap J Exper Med 1953;21:9–16 [Google Scholar]
- 42.Rieder RF, Thomas L. Studies on the mechanisms involved in the production of abortion by endotoxin. J Immunol 1960;84:189–193 [PubMed] [Google Scholar]
- 43.McKay DG, Wong TC. The effect of bacterial endotoxin on the placenta of the rat. Am J Pathol 1963;42:357–377 [PMC free article] [PubMed] [Google Scholar]
- 44.Skarnes RC, Harper MJ. Relationship between endotoxin-induced abortion and the synthesis of prostaglandin F. Prostaglandins 1972;1:191–203 [DOI] [PubMed] [Google Scholar]
- 45.Kullander S. Fever and parturition. An experimental study in rabbits. Acta Obstet Gynecol Scand Suppl 1977;77–85 [DOI] [PubMed] [Google Scholar]
- 46.Bang B. The etiology of epizootic abortion. J Comp Anthol Ther 1987;10:125 [Google Scholar]
- 47.McDuffie RS Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol 1992;167:1583–1588 [DOI] [PubMed] [Google Scholar]
- 48.Fidel PL Jr., Romero R, Wolf N et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–1475 [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Munoz H, Gomez R et al. Antibiotic therapy reduces the rate of infection-induced preterm delivery and perinatal mortality. Am J Obstet Gynecol 1994;170:390 [Google Scholar]
- 50.Gravett MG, Witkin SS, Haluska GJ et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–1667 [DOI] [PubMed] [Google Scholar]
- 51.Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol 1996;174:1725–1731 [DOI] [PubMed] [Google Scholar]
- 52.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004;15:479–487 [DOI] [PubMed] [Google Scholar]
- 53.Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol 1969;63:245–263 [DOI] [PubMed] [Google Scholar]
- 54.Herd N, Jordan T. An investigtion of malaria during pregnancy in Zimbabwe. Afr J Med 1981;27:62. [PubMed] [Google Scholar]
- 55.McGuire W, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature 1994;371:508–510 [DOI] [PubMed] [Google Scholar]
- 56.Osman NB, Folgosa E, Gonzales C, Bergstrom S. Genital infections in the aetiology of late fetal death: an incident case-referent study. J Trop Pediatr 1995;41:258–266 [DOI] [PubMed] [Google Scholar]
- 57.Folgosa E, Gonzalez C, Osman NB et al. A case control study of chorioamniotic infection and histological chorioamnionitis in stillbirth. APMIS 1997;105:329–336 [DOI] [PubMed] [Google Scholar]
- 58.Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol Infect 2005;1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hibbard L, Thrupp L, Summeril S, Smale M, Adams R. Treatment of pyelonephritis in pregnancy. Am J Obstet Gynecol 1967;98:609–615 [DOI] [PubMed] [Google Scholar]
- 60.Patrick MJ. Influence of maternal renal infection on the foetus and infant. Arch Dis Child 1967;42:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wren BG. Subclinical renal infection and prematurity. Med J Aust 1969;2:596–600 [DOI] [PubMed] [Google Scholar]
- 62.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol 1973;42:112–117 [PubMed] [Google Scholar]
- 63.Fan YD, Pastorek JG, Miller JM Jr., Mulvey J. Acute pyelonephritis in pregnancy. Am J Perinatol 1987;4:324–326 [DOI] [PubMed] [Google Scholar]
- 64.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol 1982;144:413–417 [DOI] [PubMed] [Google Scholar]
- 65.Goepfert AR, Jeffcoat MK, Andrews WW et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol 2004;104:777–783 [DOI] [PubMed] [Google Scholar]
- 66.Jarjoura K, Devine PC, Perez-Delboy A et al. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol 2005;192:513–519 [DOI] [PubMed] [Google Scholar]
- 67.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 2006;113:135–143 [DOI] [PubMed] [Google Scholar]
- 68.Offenbacher S, Boggess KA, Murtha AP et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol 2006;107:29–36 [DOI] [PubMed] [Google Scholar]
- 69.Gomez R, Ghezzi F, Romero R et al. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995;22:281–342 [PubMed] [Google Scholar]
- 70.Cassell GH, Davis RO, Waites KB et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302 [PubMed] [Google Scholar]
- 71.Gray DJ, Robinson HB, Malone J, Thomson RB Jr., Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn 1992;12:111–117 [DOI] [PubMed] [Google Scholar]
- 72.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995;40:375–379 [PubMed] [Google Scholar]
- 73.Romero R, Munoz H, Gomez R et al. Two thirds of spontaneous abortion/fetal deaths after genetic amniocentesis are the result of a pre-existing sub-clinical inflammatory process of the amniotic cavity. Am J Obstet Gynecol 1995;172:S261 [Google Scholar]
- 74.Wenstrom KD, Andrews WW, Hauth JC et al. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–550 [DOI] [PubMed] [Google Scholar]
- 75.Yoon BH, Oh SY, Romero R et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–1167 [DOI] [PubMed] [Google Scholar]
- 76.Fidel P, Ghezzi F, Romero R et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003;14:57–64 [DOI] [PubMed] [Google Scholar]
- 77.Romero R, Oyarzun E, Mazor M et al. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73:576–582 [PubMed] [Google Scholar]
- 78.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2001;CD000490 [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Quintero R, Oyarzun E et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–666 [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Gonzalez R, Sepulveda W et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–1091 [DOI] [PubMed] [Google Scholar]
- 81.Mays JK, Figueroa R, Shah J et al. Amniocentesis for selection before rescue cerclage. Obstet Gynecol 2000;95:652–655 [DOI] [PubMed] [Google Scholar]
- 82.Hassan S, Romero R, Hendler I et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mazor M, Hershkovitz R, Ghezzi F et al. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand 1996;75:624–627 [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Shamma F, Avila C et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol 1990;163:757–761 [DOI] [PubMed] [Google Scholar]
- 85.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–357 [DOI] [PubMed] [Google Scholar]
- 86.Andrews WW, Hauth JC, Goldenberg RL et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–612 [DOI] [PubMed] [Google Scholar]
- 87.Romero R, Mazor M, Morrotti R et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–133 [DOI] [PubMed] [Google Scholar]
- 88.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG: An International Journal of Obstetrics and Gynaecology 2002;109:527–533 [DOI] [PubMed] [Google Scholar]
- 89.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Reviews 1995;59:143–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Relman DA. The search for unrecognized pathogens. Science 1999;284:1308–1310 [DOI] [PubMed] [Google Scholar]
- 91.Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Research in Microbiology 2000;151:167–177 [DOI] [PubMed] [Google Scholar]
- 92.Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med 1990;323:1573–1580 [DOI] [PubMed] [Google Scholar]
- 93.Jalava J, Mantymaa ML, Ekblad U et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol 1996;103:664–669 [DOI] [PubMed] [Google Scholar]
- 94.Hitti J, Riley DE, Krohn MA et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis 1997;24:1228–1232 [DOI] [PubMed] [Google Scholar]
- 95.Gardella C, Riley DE, Hitti J et al. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol 2004;21:319–323 [DOI] [PubMed] [Google Scholar]
- 96.Yoon BH, Romero R, Kim M et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–1137 [DOI] [PubMed] [Google Scholar]
- 97.Yoon BH, Romero R, Lim JH et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–924 [DOI] [PubMed] [Google Scholar]
- 98.Steel JH, O’donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005;26:672–677 [DOI] [PubMed] [Google Scholar]
- 99.Offenbacher S, Beck JD, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. Journal Of Dental Education 1998;62:852–858 [PubMed] [Google Scholar]
- 100.Jeffcoat MK, Geurs NC, Reddy MS et al. Periodontal infection and preterm birth: results of a prospective study. The Journal Of The American Dental Association 2001;132:875–880 [DOI] [PubMed] [Google Scholar]
- 101.Madianos PN, Lieff S, Murtha AP et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol 2001;6:175–182 [DOI] [PubMed] [Google Scholar]
- 102.Offenbacher S, Lieff S, Boggess KA et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol 2001;6:164–174 [DOI] [PubMed] [Google Scholar]
- 103.Khader YS, Ta’ani Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. Journal of Periodontology 2005;76:161–165 [DOI] [PubMed] [Google Scholar]
- 104.Boggess KA, Madianos PN, Preisser JS, Moise J, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. American Journal of Obstetrics and Gynecology 2005;192:554–557 [DOI] [PubMed] [Google Scholar]
- 105.Downey JS, Han J. Cellular activation mechanisms in septic shock. Front Biosci 1998;3:d468–d476 [DOI] [PubMed] [Google Scholar]
- 106.Wang JE, Dahle MK, McDonald M et al. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 2003;20:402–414 [DOI] [PubMed] [Google Scholar]
- 107.Smith PF. Lipoglycans from mycoplasmas. Crit Rev Microbiol 1984;11:157–186 [DOI] [PubMed] [Google Scholar]
- 108.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol 1987;157:815–819 [DOI] [PubMed] [Google Scholar]
- 109.Romero R, Roslansky P, Oyarzun E et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol 1988;158:1044–1049 [DOI] [PubMed] [Google Scholar]
- 110.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 2003;163:2103–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grigsby PL, Hirst JJ, Scheerlinck JP, Phillips DJ, Jenkin G. Fetal responses to maternal and intra-amniotic lipopolysaccharide administration in sheep. Biol Reprod 2003;68:1695–1702 [DOI] [PubMed] [Google Scholar]
- 112.Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest 1983;15:230–241 [DOI] [PubMed] [Google Scholar]
- 113.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods 1998;39:147–154 [DOI] [PubMed] [Google Scholar]
- 114.Jobe AH, Newnham JP, Willet KE et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 2000;162:1656–1661 [DOI] [PubMed] [Google Scholar]
- 115.Jobe AH, Newnham JP, Willet KE et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182:401–408 [DOI] [PubMed] [Google Scholar]
- 116.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol 2005;25 Suppl 2:S31–S35 [DOI] [PubMed] [Google Scholar]
- 117.Yoon BH, Kim CJ, Romero R et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177:797–802 [DOI] [PubMed] [Google Scholar]
- 118.McLean LK, Chehab FF, Goldberg JD. Detection of viral deoxyribonucleic acid in the amniotic fluid of low-risk pregnancies by polymerase chain reaction. Am J Obstet Gynecol 1995;173:1282–1286 [DOI] [PubMed] [Google Scholar]
- 119.Yankowitz J, Weiner CP, Henderson J, Grant S, Towbin J. Outcome of low-risk pregnancies with evidence of intraamniotic viral infection detected by PCR on amniotic fluid obtained at second trimester genetic amniocenteses. J Soc Gynecol Investig 1996;3:132A [Google Scholar]
- 120.Wenstrom KD, Andrews WW, Bowles NE et al. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol 1998;92:420–424 [DOI] [PubMed] [Google Scholar]
- 121.Baschat AA, Towbin J, Bowles NE, Harman CR, Weiner CP. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med 2003;13:381–384 [DOI] [PubMed] [Google Scholar]
- 122.Van den Veyver IB, Ni J, Bowles N et al. Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab 1998;63:85–95 [DOI] [PubMed] [Google Scholar]
- 123.Baschat AA, Harman CR, Towbin JA, Weiner CP. Fetal sonographic abnormalities and intrauterine viral infection. Am J Obstet Gynecol 2000;182:S95 [Google Scholar]
- 124.Reddy U, Zlatnik M, Baschat AA et al. Detection of viral deoxyribonucleic acid in amniotic fluid: predictor of abnormal pregnancy (abstract # 408). Am J Obstet Gynecol 2001;185:S192. Am J Obstet Gynecol 2001;185: [Google Scholar]
- 125.Burguete T, Rabreau M, Fontanges-Darriet M et al. Evidence for infection of the human embryo with adeno-associated virus in pregnancy. Hum Reprod 1999;14:2396–2401 [DOI] [PubMed] [Google Scholar]
- 126.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 1984;148:915–928 [DOI] [PubMed] [Google Scholar]
- 127.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol 2003;1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1999;1462:1–10 [DOI] [PubMed] [Google Scholar]
- 129.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1999;1462:55–70 [DOI] [PubMed] [Google Scholar]
- 130.Yang L, Weiss TM, Lehrer RI, Huang HW. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 2000;79:2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–395 [DOI] [PubMed] [Google Scholar]
- 132.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004;101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mori K, Kurihara N, Hayashida S, Tanaka M, Ikeda K. The intrauterine expression of surfactant protein D in the terminal airways of human fetuses compared with surfactant protein A. Eur J Pediatr 2002;161:431–434 [DOI] [PubMed] [Google Scholar]
- 134.Kishore U, Bernal AL, Kamran MF et al. Surfactant proteins SP-A and SP-D in human health and disease. Arch Immunol Ther Exp (Warsz ) 2005;53:399–417 [PubMed] [Google Scholar]
- 135.Costerton W, Veeh R, Shirtliff M et al. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003;112:1466–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McGroarty JA, Reid G. Detection of a Lactobacillus substance that inhibits Escherichia coli. Canadian Journal Of Microbiology 1988;34:974–978 [DOI] [PubMed] [Google Scholar]
- 137.Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes and Infection 2002;4:319–324 [DOI] [PubMed] [Google Scholar]
- 138.Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol 2005;25:503–510 [DOI] [PubMed] [Google Scholar]
- 139.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004;6:1382–1387 [DOI] [PubMed] [Google Scholar]
- 140.Way SS, Thompson LJ, Lopes JE et al. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol 2004;6:235–242 [DOI] [PubMed] [Google Scholar]
- 141.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod 2005;20:1372–1378 [DOI] [PubMed] [Google Scholar]
- 142.Abrahams VM, Bole-Aldo P, Kim YM et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol 2004;173:4286–4296 [DOI] [PubMed] [Google Scholar]
- 143.Vadillo OF, Avila Vergara MA, Hernandez GC, Arechavaleta VF, Beltran MJ. [Apoptosis in trophoblast of patients with recurrent spontaneous abortion of unidentified cause]. Ginecol Obstet Mex 2000;68:122–131 [PubMed] [Google Scholar]
- 144.Huppertz B, Hemmings D, Renaud SJ et al. Extravillous trophoblast apoptosis--a workshop report. Placenta 2005;26 Suppl A:S46–S48 [DOI] [PubMed] [Google Scholar]
- 145.Murthi P, Kee MW, Gude NM, Brennecke SP, Kalionis B. Fetal growth restriction is associated with increased apoptosis in the chorionic trophoblast cells of human fetal membranes. Placenta 2005;26:329–338 [DOI] [PubMed] [Google Scholar]
- 146.Neale DM, Mor G. The role of Fas mediated apoptosis in preeclampsia. J Perinat Med 2005;33:471–477 [DOI] [PubMed] [Google Scholar]
- 147.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 2003;69:1957–1963 [DOI] [PubMed] [Google Scholar]
- 148.Kim YM, Romero R, Chaiworapongsa T et al. Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–1355 [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Durum SK, Dinarello CA, et al. Interleukin-1: A signal for the initiation of labor in chorioamnionitis. 1986; [Google Scholar]
- 150.Challis JR, Lye SJ, Gibb W et al. Understanding preterm labor. Ann N Y Acad Sci 2001;943:225–234 [DOI] [PubMed] [Google Scholar]
- 151.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev 2002;60:S19–S25 [DOI] [PubMed] [Google Scholar]
- 152.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med 2004;32:391–399 [DOI] [PubMed] [Google Scholar]
- 153.Mohan AR, Loudon JA, Bennett PR. Molecular and biochemical mechanisms of preterm labour. Semin Fetal Neonatal Med 2004;9:437–444 [DOI] [PubMed] [Google Scholar]
- 154.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol 2004;190:1509–1519 [DOI] [PubMed] [Google Scholar]
- 155.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 2004;82:799–804 [DOI] [PubMed] [Google Scholar]
- 156.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112 Suppl 1:16–18 [DOI] [PubMed] [Google Scholar]
- 157.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig 2005;12:145–155 [DOI] [PubMed] [Google Scholar]
- 158.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction 2005;130:569–581 [DOI] [PubMed] [Google Scholar]
- 159.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand 2005;84:516–525 [DOI] [PubMed] [Google Scholar]
- 160.Romero R, Wu YK, Brody DT et al. Human decidua: a source of interleukin-1. Obstet Gynecol 1989;73:31–34 [PubMed] [Google Scholar]
- 161.Romero R, Durum S, Dinarello CA et al. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 1989;37:13–22 [DOI] [PubMed] [Google Scholar]
- 162.Romero R, Brody DT, Oyarzun E et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–1123 [DOI] [PubMed] [Google Scholar]
- 163.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol 2003;188:252–263 [DOI] [PubMed] [Google Scholar]
- 164.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–971 [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–1045 [DOI] [PubMed] [Google Scholar]
- 166.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest 1989;83:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol 1991;41:123–127 [DOI] [PubMed] [Google Scholar]
- 168.Romero R, Manogue KR, Mitchell MD et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. American Journal of Obstetrics and Gynecology 1989;161:336–341 [DOI] [PubMed] [Google Scholar]
- 169.Silver RM, Lohner S, Chen CL. Tumor necrosis factor-alpha mediates LPS-induced abortion: evidence from the LPS-resistant murine strain C3H/HeJ. J Soc Gynecol Investig 1993;P218 [Google Scholar]
- 170.Fidel PL Jr., Romero R, Cutright J et al. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig 1997;4:22–26 [DOI] [PubMed] [Google Scholar]
- 171.Watari M, Watari H, DiSanto ME et al. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. American Journal Of Pathology 1999;154:1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-[alpha] in the premature rupture of membranes and preterm labor pathways. American Journal of Obstetrics and Gynecology 2002;187:1159–1162 [DOI] [PubMed] [Google Scholar]
- 173.Athayde N, Edwin SS, Romero R et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–1253 [DOI] [PubMed] [Google Scholar]
- 174.Maymon E, Romero R, Pacora P et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000;183:887–894 [DOI] [PubMed] [Google Scholar]
- 175.Romero R, Chaiworapongsa T, Espinoza J et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–1130 [DOI] [PubMed] [Google Scholar]
- 176.Osmers RGW, Adelmann-Grill BC, Rath W et al. Biochemical events in cervical ripening dilatation during pregnancy and parturition. Journal of Obstetrics and Gynaecology 1995;21:185–194 [DOI] [PubMed] [Google Scholar]
- 177.Rath W, Winkler M, Kemp B. The importance of extracellular matrix in the induction of preterm delivery. Journal Of Perinatal Medicine 1998;26:437–441 [DOI] [PubMed] [Google Scholar]
- 178.Chwalisz K, Benson M, Scholz P et al. Cervical ripening with the cytokines interleukin 8, interleukin 1[beta] and tumour necrosis factor [alpha] in guinea-pigs. Hum Reprod 1994;9:2173–2181 [DOI] [PubMed] [Google Scholar]
- 179.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, −1 alpha, and −6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab 1993;77:805–815 [DOI] [PubMed] [Google Scholar]
- 181.Gomez R, Romero R, Galasso M et al. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–210 [DOI] [PubMed] [Google Scholar]
- 182.Hillier SL, Witkin SS, Krohn MA et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–948 [PubMed] [Google Scholar]
- 183.Messer J, Eyer D, Donato L et al. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr 1996;129:574–580 [DOI] [PubMed] [Google Scholar]
- 184.Hanna N, Hanna I, Hleb M et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 2000;164:5721–5728 [DOI] [PubMed] [Google Scholar]
- 185.Hanna N, Bonifacio L, Weinberger B et al. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol 2006;55:19–27 [DOI] [PubMed] [Google Scholar]
- 186.Athayde N, Romero R, Maymon E et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000;182:135–141 [DOI] [PubMed] [Google Scholar]
- 187.Pacora P, Romero R, Maymon E et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol 2000;183:1138–1143 [DOI] [PubMed] [Google Scholar]
- 188.Saito S, Kato Y, Ishihara Y, Ichijo M. Amniotic fluid granulocyte colony-stimulating factor in preterm and term labor. Clin Chim Acta 1992;208:105–109 [DOI] [PubMed] [Google Scholar]
- 189.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–88 [DOI] [PubMed] [Google Scholar]
- 190.Goldenberg RL, Andrews WW, Mercer BM et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:625–630 [DOI] [PubMed] [Google Scholar]
- 191.Chaiworapongsa T, Romero R, Espinoza J et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005;18:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Romero R, Ceska M, Avila C et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–820 [DOI] [PubMed] [Google Scholar]
- 193.Yoon BH, Romero R, Jun JK et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–830 [DOI] [PubMed] [Google Scholar]
- 194.Ghezzi F, Gomez R, Romero R et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10 [DOI] [PubMed] [Google Scholar]
- 195.Esplin MS, Romero R, Chaiworapongsa T et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–373 [DOI] [PubMed] [Google Scholar]
- 196.Keelan JA, Yang J, Romero RJ et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod 2004;70:253–259 [DOI] [PubMed] [Google Scholar]
- 197.Athayde N, Romero R, Maymon E et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999;181:989–994 [DOI] [PubMed] [Google Scholar]
- 198.Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol 2002;186:523–530 [DOI] [PubMed] [Google Scholar]
- 199.Hirsch E, Filipovich Y. The IL-1 type I and the TNF type I receptors are essential mediators of bacterially induced preterm labor in a murine model. J Soc Gynecol Inv 2004;11: [Google Scholar]
- 200.Terrone DA, Rinehart BK, Granger JP et al. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–480 [DOI] [PubMed] [Google Scholar]
- 201.Rodts-Palenik S, Wyatt-Ashmead J, Pang Y et al. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol 2004;191:1387–1392 [DOI] [PubMed] [Google Scholar]
- 202.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874 [PubMed] [Google Scholar]
- 203.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood 1999;93:425–439 [PubMed] [Google Scholar]
- 204.Levy MM, Fink MP, Marshall JC et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530–538 [DOI] [PubMed] [Google Scholar]
- 205.Gomez R, Ghezzi F, Romero R, et al. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J Obstet Gynecol 1997;176:514 [Google Scholar]
- 206.Yoon BH, Romero R, Kim KS et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–779 [DOI] [PubMed] [Google Scholar]
- 207.Chaiworapongsa T, Romero R, Kim JC et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 2002;186:1178–1182 [DOI] [PubMed] [Google Scholar]
- 208.Witt A, Berger A, Gruber CJ et al. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med 2005;33:22–26 [DOI] [PubMed] [Google Scholar]
- 209.Pacora P, Chaiworapongsa T, Maymon E et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25 [DOI] [PubMed] [Google Scholar]
- 210.D’Alquen D, Kramer BW, Seidenspinner S et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res 2005;57:263–269 [DOI] [PubMed] [Google Scholar]
- 211.Yoon BH, Romero R, Park JS et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–681 [DOI] [PubMed] [Google Scholar]
- 212.Yoon BH, Romero R, Shim JY et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90 [DOI] [PubMed] [Google Scholar]
- 213.Sampson JE, Theve RP, Blatman RN et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997;176:77–81 [DOI] [PubMed] [Google Scholar]
- 214.Shim SS, Yoon BH, Romero R et al. The frequency and clinical significance on intra-amniotic inflammation in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 2003;189:S83. [DOI] [PubMed] [Google Scholar]
- 215.Romero R, Athayde N, Gomez R et al. The fetal inflammatory response syndrome is characterized by the outpouring of a potent extracellular matrix degrading enzyme into the fetal circulation. Am J Obstet Gynecol 1998;178:S3 [Google Scholar]
- 216.Gomez R, Berry S, Yoon BH et al. The hematologic profile of the fetus with systemic inflammatory response syndrome. Am J Obstet Gynecol 1998;178:S202 [Google Scholar]
- 217.Berry SM, Gomez R, Athayde N et al. The role of granulocyte colony stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;178:S202 [Google Scholar]
- 218.Gomez R, Romero R, Ghezzi F et al. Are fetal hypoxia and acidemia causes of preterm labor and delivery? Am J Obstet Gynecol 1997;176:S115 [Google Scholar]
- 219.Baschat AA, Gembruch U, Reiss I et al. Neonatal nucleated red blood cell counts in growth-restricted fetuses: relationship to arterial and venous Doppler studies. Am J Obstet Gynecol 1999;181:190–195 [DOI] [PubMed] [Google Scholar]
- 220.Ferber A, Minior VK, Bornstein E, Divon MY. Fetal “nonreassuring status” is associated with elevation of nucleated red blood cell counts and interleukin-6. Am J Obstet Gynecol 2005;192:1427–1429 [DOI] [PubMed] [Google Scholar]
- 221.Berry SM, Romero R, Gomez R et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995;173:1315–1320 [DOI] [PubMed] [Google Scholar]
- 222.Di Naro E, Cromi A, Ghezzi F et al. Fetal thymic involution: A sonographic marker of the fetal inflammatory response syndrome. American Journal of Obstetrics and Gynecology 2006;194:153–159 [DOI] [PubMed] [Google Scholar]
- 223.Haeryfar SM, Berczi I. The thymus and the acute phase response. Cellular and Molecular Biology 2001;47:145–156 [PubMed] [Google Scholar]
- 224.Yoon BH, Romero R, Jun JK et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998;179:1107–1114 [DOI] [PubMed] [Google Scholar]
- 225.Dolecek R. Endocrine changes after burn trauma--a review. Keio J Med 1989;38:262–276 [DOI] [PubMed] [Google Scholar]
- 226.Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int 2000;57:1412–1417 [DOI] [PubMed] [Google Scholar]
- 227.Dodic M, Hantzis V, Duncan J et al. Programming effects of short prenatal exposure to cortisol. FASEB J 2002;16:1017–1026 [DOI] [PubMed] [Google Scholar]
- 228.Nathanielsz PW, Berghorn KA, Derks JB et al. Life before birth: effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr 2003;92:766–772 [PubMed] [Google Scholar]
- 229.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci 2004;1032:63–84 [DOI] [PubMed] [Google Scholar]
- 230.Kim YM, Kim GJ, Kim MR et al. Skin: An Active Component of The Fetal Innate Immune System. American Journal of Obstetrics and Gynecology 2003;189:S74 [Google Scholar]
- 231.Yoon BH, Kim YA, Romero R et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1999;181:784–788 [DOI] [PubMed] [Google Scholar]
- 232.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis 1998;27:513–518 [DOI] [PubMed] [Google Scholar]
- 233.Espinoza J, Chaiworapongsa T, Romero R et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21 [DOI] [PubMed] [Google Scholar]
- 234.Romero R, Espinoza J, Goncalves LF et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–157 [DOI] [PubMed] [Google Scholar]
- 235.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care 2002;6:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kumar A, Krieger A, Symeoneides S, Kumar A, Parrillo JE. Myocardial dysfunction in septic shock: Part II. Role of cytokines and nitric oxide. J Cardiothorac Vasc Anesth 2001;15:485–511 [DOI] [PubMed] [Google Scholar]
- 237.Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock: Part I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth 2001;15:364–376 [DOI] [PubMed] [Google Scholar]
- 238.Yanowitz TD, Jordan JA, Gilmour CH et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51:310–316 [DOI] [PubMed] [Google Scholar]
- 239.Garnier Y, Coumans AB, Jensen A, Hasaart TH, Berger R. Infection-related perinatal brain injury: the pathogenic role of impaired fetal cardiovascular control. J Soc Gynecol Investig 2003;10:450–459 [DOI] [PubMed] [Google Scholar]
- 240.Kaukola T, Herva R, Perhomaa M et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res 2006;59:478–483 [DOI] [PubMed] [Google Scholar]
- 241.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 2004;190:147–151 [DOI] [PubMed] [Google Scholar]
- 242.Yoon BH, Romero R, Shim JY et al. “Atypical” chronic lung disease of the newborn is linked to fetal systemic inflammation. Am J Obstet Gynecol 2002;187:S129 [Google Scholar]
- 243.Jobe AH. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol 2003;8:9–17 [DOI] [PubMed] [Google Scholar]
- 244.Dammann O, Allred EN, Van Marter LJ, Dammann CE, Leviton A. Bronchopulmonary dysplasia is not associated with ultrasound-defined cerebral white matter damage in preterm newborns. Pediatr Res 2004;55:319–325 [DOI] [PubMed] [Google Scholar]
- 245.Dammann O, Leviton A, Bartels DB, Dammann CE. Lung and brain damage in preterm newborns. Are they related? How? Why? Biol Neonate 2004;85:305–313 [DOI] [PubMed] [Google Scholar]
- 246.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8 [DOI] [PubMed] [Google Scholar]
- 247.Yoon BH, Romero R, Kim CJ et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 1997;177:406–411 [DOI] [PubMed] [Google Scholar]
- 248.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol 2005;58:821–828 [DOI] [PubMed] [Google Scholar]
- 249.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med 2006;34:5–12 [DOI] [PubMed] [Google Scholar]
- 250.Christensen KK. Infection as a predominant cause of perinatal mortality. Obstet Gynecol 1982;59:499–508 [PubMed] [Google Scholar]
- 251.Christensen KK, Christensen P, Hagerstrand I et al. The clinical significance of group B streptococci. J Perinat Med 1982;10:133–146 [PubMed] [Google Scholar]
- 252.Tafari N, Ross S, Naeye RL, Judge DM, Marboe C. Mycoplasma T strains and perinatal death. Lancet 1976;1:108–109 [DOI] [PubMed] [Google Scholar]
- 253.Blackwell S, Romero R, Chaiworapongsa T et al. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med 2003;14:151–157 [DOI] [PubMed] [Google Scholar]
- 254.Gerber S, Yih M, Vardhana S et al. Relation between fetal interleukin-1 receptor antagonist (IL-1RA) gene polymorphism and unexplained fetal death. Am J Obstet Gynecol 2002;187:S58. [DOI] [PubMed] [Google Scholar]
- 255.Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis 2002;34:204–209 [DOI] [PubMed] [Google Scholar]
- 256.Vamvakopoulos J, Green C, Metcalfe S. Genetic control of IL-1beta bioactivity through differential regulation of the IL-1 receptor antagonist. Eur J Immunol 2002;32:2988–2996 [DOI] [PubMed] [Google Scholar]
- 257.Romero R, Mazor M, Brandt F et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–123 [DOI] [PubMed] [Google Scholar]
- 258.Naccasha N, Gervasi MT, Chaiworapongsa T et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001;185:1118–1123 [DOI] [PubMed] [Google Scholar]
- 259.Gervasi MT, Chaiworapongsa T, Naccasha N et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1124–1129 [DOI] [PubMed] [Google Scholar]
- 260.Gervasi MT, Chaiworapongsa T, Naccasha N et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:171–175 [DOI] [PubMed] [Google Scholar]


