Abstract
Objectives:
Persons experiencing homelessness (PEH) are disproportionately affected by tuberculosis (TB). We estimate area-specific rates of TB among PEH and characterize the extent to which available data support recent transmission as an explanation of high TB incidence.
Methods:
We estimated TB incidence among PEH using National Tuberculosis Surveillance System data and population estimates for the US Department of Housing and Urban Development’s Continuums of Care areas. For areas with TB incidence higher than the national average among PEH, we estimated recent transmission using genotyping and a plausible source-case method. For cases with ≥ 1 plausible source case, we assessed with TB program partners whether available whole-genome sequencing and local epidemiologic data were consistent with recent transmission.
Results:
During 2011–2016, 3164 TB patients reported experiencing homelessness. National incidence was 36 cases/100,000 PEH. Incidence estimates varied among 21 areas with ≥ 10,000 PEH (9–150 cases/100,000 PEH); 9 areas had higher than average incidence. Of the 2349 cases with Mycobacterium tuberculosis genotyping results, 874 (37%) had ≥ 1 plausible source identified. In the 9 areas, 23%–82% of cases had ≥ 1 plausible source. Of cases with ≥ 1 plausible source, 63% were consistent and 7% were inconsistent with recent transmission; 29% were inconclusive.
Conclusions:
Disparities in TB incidence for PEH persist; estimates of TB incidence and recent transmission vary by area. With a better understanding of the TB risk among PEH in their jurisdictions and the role of recent transmission as a driver, programs can make more informed decisions about prioritizing TB prevention strategies.
Keywords: tuberculosis, transmission, incidence, homelessness, evaluation
Tuberculosis (TB) disease incidence in the United States has declined annually to <3 cases per 100,000, but persons experiencing homelessness (PEH) are disproportionately affected.1,2 Approximately 5% of TB cases have occurred among persons who reported experiencing homelessness in the year before their TB diagnosis.1,2 In contrast, <1% of the adult US population experiences homelessness each year.3 PEH who use shelter services are at increased risk because congregate settings with shared airspace can facilitate Mycobacterium tuberculosis exposure and transmission.2,4 PEH with M. tuberculosis infection can also be at increased risk of rapid progression to TB disease due to an increased prevalence of comorbidities that affect cellular immunity (eg, HIV, diabetes, malnutrition, stress, and substance use).5,6 Inadequate health care access, stigma, and mental health disorders can contribute to delays diagnosing TB disease.5,7 In recent years, several US cities have experienced TB outbreaks among PEH, including transmission of drug resistant strains of M. tuberculosis, which can overwhelm local public health resources.7–10
Because TB incidence and epidemiology vary geographically,4,11,12 TB control activities for PEH are most effective when they are designed using local data. Before 2015, however, the US Department of Housing and Urban Development (HUD) used a nationally representative sample of Continuums of Care (CoCs) to develop their national estimate of homeless population size, and area-specific data were not available. New data and methods that have recently become available provide opportunities to develop area-specific estimates and inform public health decisions. Beginning in 2018, HUD began publishing area-specific estimates of annual homeless population size going back to 2015, allowing for local TB incidence rate calculations among PEH.13 Using genotyping data and a field-validated method published in 2015, the proportions of TB cases attributed to recent TB transmission can also be estimated.4,14 Distinguishing cases of recent transmission from cases that arise due to reactivation of M. tuberculosis infection acquired in the remote past could help identify areas where ongoing transmission is occurring, prioritize prevention strategies and resource allocations accordingly, and monitor changes over time. Using these new data and methods, we estimate area-specific rates of TB among PEH and characterize the extent to which available data support recent transmission as an explanation of high TB incidence locally in this vulnerable population.
METHODS
We estimated national TB incidence per 100,000 PEH annually during 2007–2016 and area-specific TB incidence among PEH averaged for 2011–2016. For both estimates, numerators originated from National Tuberculosis Surveillance System (NTSS) data on verified cases of TB, which state and local health departments from 50 US states and Washington, DC reported to the US Centers for Disease Control and Prevention (CDC) using a standard form. The case report includes the date that the case was counted by the health department and the city and county of the patient’s recorded address at the time of diagnosis, which for patients experiencing homelessness can be the location of a shelter or other temporary location. Limited clinical data and self-reported information on TB risk factors and comorbidities, including whether the patient experienced homelessness in the year before TB diagnosis,15 are included. NTSS defines homelessness as having no fixed, regular, and adequate nighttime residence in the year before TB diagnostic evaluation, and includes living in shelters providing temporary living accommodations, nonresidential structures, and unstable housing situations (eg, alternating between multiple residences for short stays of uncertain duration).
Denominators for the incidence estimates originated from HUD’s Homeless Management Information Systems (HMIS) data. HMIS are locally administered applications used by jurisdictions to track homeless service provision throughout the year. They collect data on client-level characteristics and service needs of persons accessing emergency shelters, transitional housing, permanent supportive housing, rapid rehousing, and other services. HMIS might not include persons experiencing unsheltered homelessness (unless they participate in street outreach or day shelter programs), and does not include survivors of domestic violence served by providers funded under the Violence Against Women Act.16 HUD uses aggregated HMIS data reported from a nationally representative sample of areas to estimate the US homeless population size for the Annual Homeless Assessment Report.17 To estimate national TB incidence among PEH, we divided the annual number of TB cases among persons reporting homelessness in NTSS by the national homeless population size estimate reported in the Annual Homeless Assessment Report. We used incidence rates published annually by CDC’s Division of TB Elimination (DTBE)1 to compare TB incidence among PEH with the entire US population.
Since 2015, HUD has required each area receiving federal funding to submit annual deduplicated counts of the local homeless population size, which HUD now makes publicly available.13 However, shelters’ participation in HMIS reporting to HUD varies by area; to adjust for this variability in data completeness and estimate the actual number of PEH in each area, we divided the 2016 HMIS count by the annual bed-coverage rate using the area’s Housing Inventory Count (ie, the percentage of beds available year round within the area that occur in shelters that contributed data to the HMIS).13 To estimate area-specific TB incidence rates, we linked the state, county, and city of each reported case of TB to HUD’s CoC service areas.18 CoCs vary in size and can include 1 or multiple counties or public health jurisdictions; in limited situations, CoCs cross state borders.19 We estimate area-specific TB incidence per 100,000 PEH by dividing the average annual number of TB cases reporting homelessness in NTSS during 2011–2016 by the estimated number of PEH in 2016. We analyzed all data by fiscal year, which is the reporting period used by HUD. Our analyses include CoCs that represent urban areas with ≥10,000 PEH, excluding those that span more than 1 state, had an annual bed-coverage rate <20%, and Balance of State CoCs (ie, all jurisdictions in the state that are not included in another other CoC).
For areas with TB incidence estimates among PEH that were higher than the national average, we evaluated the role of recent transmission as a driver of incidence. We used a plausible source-case method, published by DTBE in 2015, to estimate TB cases attributed to recent transmission with national molecular surveillance data.4,14 DTBE has supported genotyping for at least 1 M. tuberculosis isolate from each culture-positive TB case in the United States since 2004. GENTypes are defined as unique combinations of spacer oligonucleotide typing (spoligotyping) and 24-locus mycobacterial interspersed repetitive unit-variable number tandem repeat typing results.20 The plausible source-case method searches for a plausible source case with an M. tuberculosis isolate of the same GENType and a respiratory form of TB disease in a patient 10 years of age or older who was diagnosed within 2 years before the given case and whose reported address was within 10 miles.4,14 Using this method, we estimated the area-specific numbers and percentages of TB cases among PEH that had a plausible source. We then repeated these plausible source-case analyses limiting plausible sources to include only those source cases that also reported homelessness (ie, potential recent transmission among patients experiencing homelessness).
To assess potential misclassification of cases with plausible sources, we collaborated with state and local TB program partners to review additional data as available, including results of whole-genome sequencing (WGS) and epidemiologic investigations. WGS can provide greater resolution than spoligotyping and mycobacterial interspersed repetitive unit-variable number tandem repeat for examining genetic relatedness of isolates. DTBE uses WGS data to perform whole-genome single nucleotide polymorphism (wgSNP) analysis, which identifies mutations at single positions in the genome (ie, nucleotides) to compare isolates with matching or similar genotypes. Cases with the same GENType can be distantly related by wgSNP analysis. Occasionally, nominally different GENTypes are shown to be closely related by wgSNP analysis and could be considered part of the same TB cluster or outbreak. Availability of wgSNP analysis results from WGS data varied by area because DTBE prioritized sequencing resources selectively for suspected outbreaks and clusters of public health importance during 2012–2017.
As state and local TB programs often have more detailed information on epidemiologic links among patients in their jurisdictions, we provided line lists of cases with plausible sources and invited further program review using local epidemiologic data to share additional relevant information. After reviewing available WGS and local epidemiologic data, we categorized cases as consistent with, not consistent with, or unknown/inconclusive with respect to recent transmission based on established criteria (Table 1). Categorizations were reviewed by state and local partners to resolve discrepancies.
TABLE 1.
WGS and Epidemiologic Criteria for Categorizing TB Cases Among PEH With Plausible Sources as Consistent, Not Consistent, or Unknown/Inconclusive With Respect to Recent Transmission
| Consistent with recent transmission |
| Mycobacterium tuberculosis isolate ≤5 SNPs from any recent case’s isolate by wgSNP analysis* |
| Epidemiologic links documented |
| WGS not available, but GENType known to be associated with a large outbreak affecting PEH† |
| Diagnostic test for M. tuberculosis infection documenting recent conversion‡ |
| Not consistent with recent transmission |
| M. tuberculosis isolate >5 SNPs from all recent cases’ isolates by wgSNP analysis |
| Previously diagnosed and untreated latent TB infection |
| Strong epidemiologic evidence suggesting transmission occurred in the remote past, outside the United States, or both |
| Unknown or inconclusive |
| WGS and epidemiologic data unavailable |
| M. tuberculosis isolate >5 SNPs from all recent cases’ isolates, but many cases/isolates missing from the wgSNP analysis |
| Conflicting epidemiologic information about timing of exposure or infection |
Recent is defined as ≤ 2 years between TB diagnoses.
GENTypes are defined as unique combinations of spacer oligonucleotide typing (spoligotyping) and 24-locus MIRU-VNTR typing results.
Refers to a patient who had a baseline negative test for M. tuberculosis infection and a recent positive test for M. tuberculosis infection, suggesting that the patient became infected recently. For more information on diagnostic testing for M. tuberculosis infection see Lewinsohn et al.21
MIRU-VNTR indicates mycobacterial interspersed repetitive unit-variable number tandem repeat; PEH, persons experiencing homelessness; SNPs, single nucleotide polymorphisms; TB, tuberculosis; WGS, whole-genome sequencing; wgSNP, whole-genome single nucleotide polymorphism.
TB incidence and plausible source-case analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC), and program review data were tabulated in Excel for Office 365 version 1902 (Microsoft Corporation, Redmond, WA). This project was determined not to be human subjects research by the US CDC and did not require approval by an institutional review board because data were collected and analyzed as part of routine public health surveillance.
RESULTS
The national incidence of TB disease among PEH decreased from 46 cases per 100,000 in 2007 to 31 cases per 100,000 in 2016, which was a 32% decrease. However, the incidence of TB disease among PEH was 11 times higher compared with the US population in 2016 (Fig. 1).
FIGURE 1.
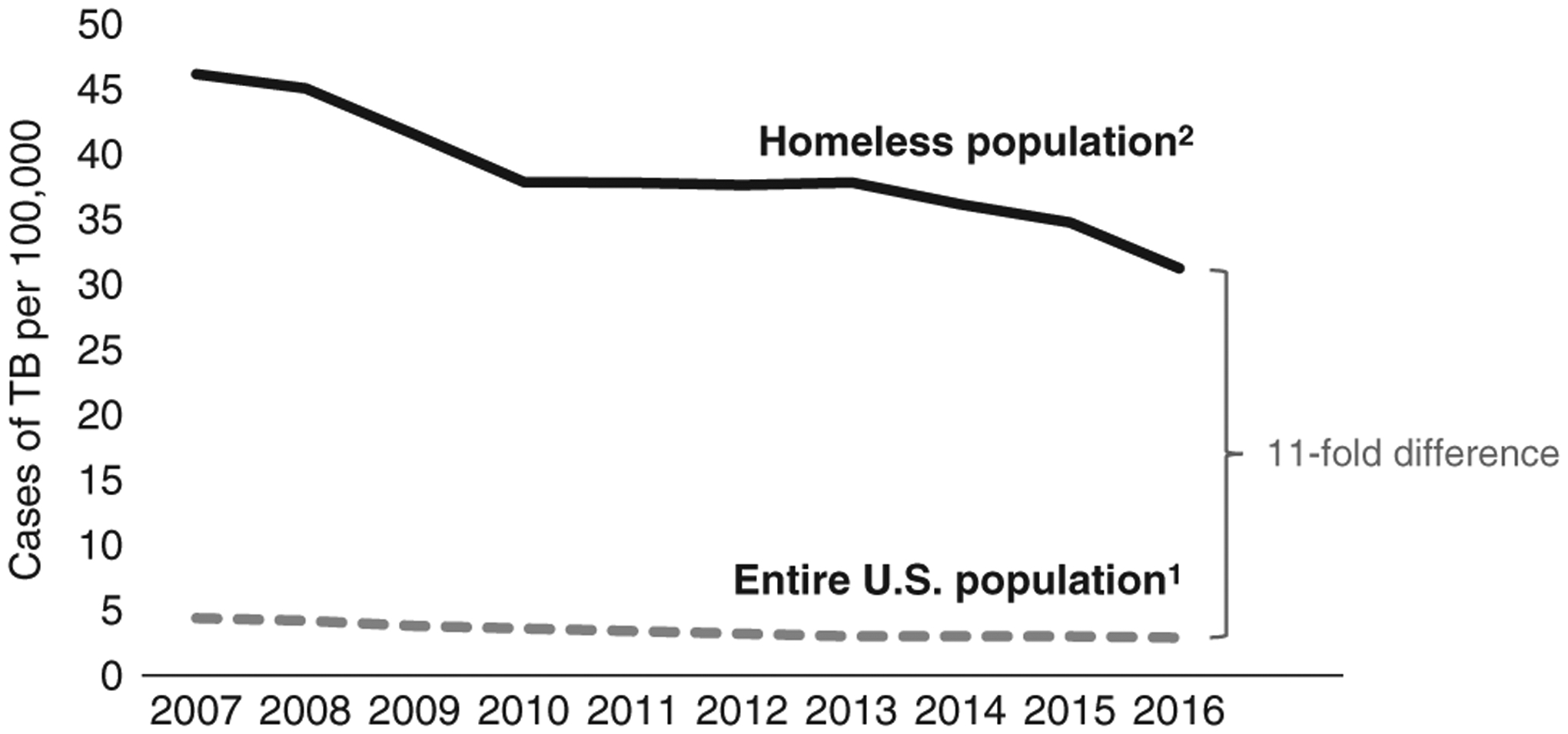
Change in tuberculosis (TB) incidence over time among entire US population1 and persons experiencing homelessness,2 United States, 2007–2016. 1TB incidence among the entire US population is shown for comparison based on published case rates.1 2To estimate national TB incidence among persons experiencing homelessness (PEH), we divided the mean number of TB cases among persons with reported homelessness in the National Tuberculosis Surveillance System during fiscal years 2011–2016 by the national homeless population size estimate during 2016 reported in the Annual Homeless Assessment Report.17
During 2011–2016, 3164 TB cases were reported among PEH in the year before TB diagnosis, which was an average of 527 cases per year. Twenty-one areas were estimated to have ≥10,000 PEH (population range, 10,095–148,312). Among these areas, the mean numbers of TB cases per year (range, 1–49), and TB incidence rates (range, 9–150 cases per 100,000 persons) among PEH varied substantially (Table 2). The national average TB incidence rate during 2011–2016 was 36 cases per 100,000 PEH; 9 areas had TB incidence rates among PEH that were higher than this national estimate.
TABLE 2.
Area-specific Average Annual TB Case Counts and Incidence Rate Estimates Among PEH, United States, 2011–2016
| Area* | Average Annual Cases of TB Among Persons With Reported Homelessness† | Estimated Population of PEH‡ | Estimated Annual Incidence of TB per 100,000 PEH§ |
|---|---|---|---|
| National | 527 | 1,467,861 | 36 |
| Atlanta, GA | 18 | 14,811 | 120 |
| Boston, MA | 3 | 20,360 | 14 |
| Chicago, IL | 11 | 20,534 | 54 |
| Columbus, OH | 3 | 11,647 | 29 |
| Dallas, TX | 29 | 19,790 | 145 |
| Denver, CO | 2 | 17,233 | 12 |
| District of Columbia | 3 | 19,915 | 13 |
| Fort Worth, TX | 3 | 10,744 | 32 |
| Houston, TX | 17 | 15,876 | 107 |
| Las Vegas, NV | 4 | 11,078 | 35 |
| Los Angeles, CA | 49 | 52,985 | 93 |
| Miami, FL | 7 | 10,120 | 72 |
| Minneapolis, MN | 3 | 14,011 | 23 |
| New York City, NY | 17 | 148,312 | 11 |
| Philadelphia, PA | 4 | 13,259 | 30 |
| Phoenix, AZ | 7 | 19,827 | 34 |
| Salt Lake City, UT | 1 | 13,203 | 9 |
| San Diego, CA | 19 | 12,363 | 150 |
| San Francisco, CA | 9 | 10,095 | 87 |
| Seattle, WA | 3 | 23,187 | 11 |
| St Petersburg, FL | 4 | 10,131 | 39 |
Area defined based on US Department of HUD Continuums of Care.18 Areas with a population of ≥ 10,000 PEH in 2016 are included.
Average annual cases of TB defined as the mean number of TB cases during fiscal years 2011–2016 that were reported to CDC’s NTSS as occurring among persons having experienced homelessness in the year before their TB diagnostic evaluation.
Homeless population size is estimated based on HUD Homeless Management Information System and Housing Inventory Count data for 2016 (see the Methods section).13
TB incidence is estimated using average annual cases of TB during 2011–2016 divided by the homeless population estimate for 2016 per 100,000.
HUD indicates Housing and Urban Development; NTSS, National Tuberculosis Surveillance System; PEH, persons experiencing homelessness; TB, tuberculosis.
Nationally, almost three quarters (74%) of TB cases among PEH in NTSS had genotyped isolates allowing for assessment of plausible sources (n=2349 of 3164 cases), and more than one third (37%) of cases among PEH had 1 or more plausible source cases identified (Table 3). In the 9 higher incidence areas, the number of cases with genotyping (range, 22–240 cases) and proportions of cases with at least 1 plausible source varied substantially (range, 23%–82%); furthermore, the proportions of cases with a plausible source that also reported homelessness varied among these 9 areas (range, 13%–73%). In the 9 higher incidence areas, cases among PEH with plausible sources had 4–28 unique GENTypes (Table 3).
TABLE 3.
Cases of TB Among Persons With Reported Homelessness and a Plausible Source, United States, 2011–2016
| Area* | Genotyped Cases† | Cases Among Persons With Reported Homelessness and a Plausible Source,‡ n (%§) | Cases Among Persons With Reported Homelessness and a Plausible Source‡ Who Reported Homelessness,∥ n (%§) | No. Unique GENTypes¶ |
|---|---|---|---|---|
| National | 2349 | 874 (37) | 569 (24) | NA |
| Atlanta, GA | 77 | 59 (77) | 50 (65) | 10 |
| Chicago, IL | 35 | 16 (46) | 9 (26) | 9 |
| Dallas, TX | 142 | 116 (82) | 104 (73) | 14 |
| Houston, TX | 77 | 51 (66) | 39 (51) | 8 |
| Los Angeles, CA | 240 | 128 (53) | 102 (43) | 28 |
| Miami, FL | 40 | 17 (43) | 6 (15) | 13 |
| San Diego, CA | 80 | 28 (35) | 16 (20) | 13 |
| San Francisco, CA | 39 | 9 (23) | 5 (13) | 6 |
| St Petersburg, FL | 22 | 5 (23) | 5 (23) | 4 |
Area defined based on US Department of HUD Continuums of Care.18 Areas included in the table have an estimated population of PEH ≥ 10,000 in 2016 and TB incidence greater than the national average of 36 cases per 100,000 PEH during 2011–2016.
Total number of TB cases with Mycobacterium tuberculosis isolate genotyping results (ie, complete data for the plausible source-case method to estimate recent transmission).
Cases are assessed using the plausible source-case method to estimate recent transmission. A given case is considered to have a plausible source if any case can be identified in the national TB molecular surveillance database that has the same M. tuberculosis genotype, has an infectious form of TB disease, resides within 10 miles of the case, is 10 years of age or older, and was diagnosed within 2 years before the case’s diagnosis date.
Proportions are calculated using all genotyped cases as the denominators.
For each given case, all plausible source cases identified were further reviewed to determine homelessness status. If any of the plausible source cases also reported homelessness, they were included in these counts.
GENTypes are defined as unique combinations of spacer oligonucleotide typing (spoligotyping) and 24-locus MIRU-VNTR typing results. Occasionally, nominally different GENTypes are shown to be closely related by wgSNP analyses and could be considered part of the same TB cluster or chain of transmission.
HUD indicates Housing and Urban Development; MIRU-VNTR, mycobacterial interspersed repetitive unit-variable number tandem repeat; PEH, persons experiencing homelessness; TB, tuberculosis; wgSNP, whole-genome single nucleotide polymorphism.
In the 9 areas, wgSNP analyses were available for review for over half (55%) of cases with a plausible source (n=234 of 429 cases). However, the completeness of these data varied by area (range, 0%–87%). In Los Angeles, for example, 87% of cases had wgSNP analyses available. Epidemiologic data completeness also varied by area (range, 16%–100%) but were available for almost two thirds (62%) of cases overall. Among cases experiencing homelessness with at least 1 plausible source identified, 63% of cases had WGS and/or local epidemiologic data that were consistent with recent transmission, 7% of cases had WGS or local epidemiologic data that were inconsistent with recent transmission, and 29% of cases lacked sufficient WGS and epidemiologic data to assess likelihood of recent transmission, or the review was inconclusive (Table 4).
TABLE 4.
Collaborative Review of WGS and Epidemiologic Data for TB Cases With Plausible Sources Among Persons Who Reported Homelessness, United States, 2011–2016
| Area* | Total Cases Among Persons With Reported Homelessness and a Plausible Source† | WGS or Local Epidemiologic Data Are Consistent With Recent Transmission,‡ n (%) | WGS or Local Epidemiologic Data Not Consistent With Recent Transmission,§ n (%) | WGS or Local Epidemiologic Data Unavailable or Inconclusive,∥ n (%) |
|---|---|---|---|---|
| Atlanta, GA | 59 | 46 (78) | 0 (0) | 13 (22) |
| Chicago, IL | 16 | 7 (44) | 0 (0) | 9 (56) |
| Dallas, TX | 116 | 83 (72) | 0 (0) | 33 (28) |
| Houston, TX | 51 | 16 (31) | 2 (4) | 33 (65) |
| Los Angeles, CA | 128 | 103 (80) | 9 (7) | 16 (13) |
| Miami, FL | 17 | 4 (24) | 0 (0) | 13 (76) |
| San Diego, CA | 28 | 2 (7) | 21 (75) | 5 (18) |
| San Francisco, CA | 9 | 6 (67) | 0 (0) | 3 (33) |
| St Petersburg, FL | 5 | 5 (100) | 0 (0) | 0 (0) |
| Total | 429 | 272 (63) | 32 (7) | 125 (29) |
Area defined based on US Department of Housing and Urban Development (HUD) Continuums of Care.18 Areas included in the table have an estimated population of PEH ≥ 10,000 in 2016 and TB incidence greater than the national average of 36 cases per 100,000 PEH during 2011–2016.
Cases reviewed are the total number of TB cases among persons with reported homelessness and a recent plausible source.
Cases consistent with recent transmission are defined by having an Mycobacterium tuberculosis isolate ≤ 5 SNPs from another recent case’s isolate by wgSNP analysis, epidemiologic links documented among PEH, a genotype known to be associated with a large outbreak affecting PEH, or a diagnostic test for M. tuberculosis infection documenting recent conversion.
Cases not consistent with recent transmission are defined by having an M. tuberculosis isolate > 5 SNPs from all other recent cases’ isolates by wgSNP analysis, previously diagnosed and untreated TB infection, or strong epidemiologic evidence suggesting transmission occurred in the remote past outside the United States, or both.
Inconclusive cases are defined by having WGS and epidemiologic data unavailable, an M. tuberculosis isolate > 5 SNPs from all recent cases’ isolates but many cases/isolates missing from the wgSNP analysis, or conflicting epidemiologic information about timing of exposure or infection.
PEH indicates persons experiencing homelessness; SNPs, single nucleotide polymorphisms; TB, tuberculosis; WGS, whole-genome sequencing.
DISCUSSION
Despite substantial progress reducing TB incidence rates in the United States, we found that disparities among PEH have been persistent. Our national estimate for 2016 was 11 times higher than the estimate for the general US population (2.9 TB cases per 100,000 population), which is similar to a disparity found during 2006–2010 when rates among PEH were 10 times higher.1,2 Furthermore, the percentage of genotyped cases with 1 or more plausible sources (37%) was 2.6 times higher among PEH compared with all genotyped cases nationally (14%)1,4; this disparity indicates that opportunities exist to improve control of M. tuberculosis transmission in this vulnerable population. Our updated estimates can serve as a baseline for collectively monitoring the overall effectiveness of enhanced efforts to address gaps in TB prevention and control and the underlying medical and social risk factors that make this population vulnerable. However, TB epidemiology is geographically heterogeneous, so area-specific analyses of TB incidence among homeless populations are also needed to monitor local progress.
This report offers a framework for sequential use of different types of available data to analyze and understand TB incidence and recent transmission among PEH. First, we used newly available data from HUD to estimate area-specific homeless population sizes and provide the first surveillance-based TB incidence rate estimates for 21 urban areas with 10,000 or more PEH. These urban areas represented 41% of the 527 average annual TB cases reported among PEH during 2011–2016. TB incidence rates in the 21 areas ranged from 4 times higher to 4 times lower than our national estimate (36 cases per 100,000 population). Because the demographic, epidemiologic, and structural factors that affect TB incidence differ locally, these estimates are not intended for direct comparison. Instead, we further characterized the extent to which available data support recent transmission as a source of TB incidence in 9 areas where rate estimates were higher than the national average rate. Using universally available molecular surveillance, we found area-specific variability in the proportions of cases with a plausible source case and the proportions with a plausible source case who also reported homelessness (ie, suggestive of possible recent transmission). Lastly, we described the extent to which WGS or local epidemiologic data were available to determine if there was additional evidence consistent with recent transmission as a driver of TB incidence in each area.
Mismatching definitions of homelessness between the numerator and denominator, inconsistently applied case counting definitions, and methodologic limitations that affect the numerators and denominators of our incidence rate estimates likely explain some of the variability. For incidence numerator estimates, homelessness might be underreported in NTSS, and the extent of underreporting might vary by jurisdiction. In San Diego, cases among PEH might have been overreported if TB cases occurred among patients who lived binationally in Mexico and San Diego during their TB diagnoses (M. Moore, oral and written communication, DTBE medical officer assigned to County of San Diego Health & Human Services Agency, San Diego, CA). For denominator estimates, HMIS data collection and reporting completeness also likely differ by area; areas with lower HMIS coverage might have less precise population estimates because HMIS is designed to capture housing service utilization. Population sizes and duration of homelessness (ie, chronic or short-term) can be differentially underestimated in areas with varying numbers of unsheltered persons or persons using other types of unstable housing, such as “couch surfing” with friends or family, living in vehicles, or occupying other locations in the community (eg, abandoned properties). Our estimates of population size also do not distinguish between a person who spends a single night in a shelter and a person who experiences chronic homelessness. Furthermore, migration or other trends (eg, housing rental costs)22 can lead to imprecise denominator estimates. Investigative journalists documented thousands of instances where PEH were “bussed out” of major cities such as San Francisco, CA and New York City during 2011–2016.23–25 In this 6-year period, we assumed a stable, average population size, which is likely an underestimate of the true population size because national HMIS estimates indicate that homelessness declined by 5.4% during 2011–2016.17 In the future, this simplifying assumption might not be necessary if robust, area-specific estimates are available annually.
Despite limitations, our analyses are an important step to facilitate evidence-based decisions and appropriately prioritize strategies for TB control at the local level. With a better understanding of the TB risk among PEH in their jurisdictions, we demonstrated how state and local health departments can begin to assess the role of recent transmission as a driver of that risk. TB cases resulting from recent transmission are particularly important for public health because they represent opportunities to interrupt further transmission and detect and treat latent TB infection (LTBI) to prevent TB disease. Although our estimates of recent transmission are based on a plausible source-case method and conventional genotyping, which both might be subject to misclassification for several reasons (eg, presumed 10-mile proximity using a shelter or health department address in lieu of a residence),1,14 our detailed, collaborative review demonstrated relatively few examples of misclassification after factoring in WGS and local epidemiologic investigation data. Future introduction of recent transmission estimates using universal WGS data might improve these estimates. In areas where WGS, local epidemiologic data, and analyses of plausible source cases are consistent with recent transmission, preventing new TB infections could have a substantial impact on the TB rate in the local homeless population. Ultimately, the goal of these analyses is to identify the most appropriate TB control strategies for PEH by evaluating the role of recent transmission.7,26 These estimates are not intended for rankings or comparison across jurisdictions. Findings from these analyses will be most useful when TB programs and organizations providing services to PEH work together to interpret them within the context of their jurisdictions, implement strategies for preventing M. tuberculosis transmission among PEH, and monitor changes over time.
Early diagnosis of cases, timely and effective TB treatment, and thorough testing and treatment of contacts are core components of TB prevention.9,27–32 However, common barriers that limit the feasibility and effectiveness of implementing these core components among PEH should be addressed by TB programs and partner organizations.7,9,33,34 PEH often have inadequate access to medical care that leads to delays in diagnosis and longer infectious periods that add to the risk of transmission.35 Adhering to lengthy treatment protocols for TB can be challenging for TB patients experiencing homelessness, especially for those who lack stable housing and are experiencing other comorbidities or conditions.33,36 Barriers that health departments face in providing TB evaluations for contacts of infectious TB patients experiencing homelessness include difficulties eliciting names of potential contacts and locating contacts.10,28,37 Stigma associated with TB and the psychological stress associated with the homeless experience can also have a substantial impact on a patient’s willingness to seek care and adhere to treatment.9,33,38
To help overcome barriers, proven strategies for preventing TB among PEH should be applied. For example, electronic data systems like HMIS can be used to support thorough contact investigations.9,10 TB education campaigns and infection control measures can be implemented systematically in facilities providing services to PEH through collaborations with various stakeholders.9,27 LTBI affects ~1 in 5 PEH39; recent advances in testing for M. tuberculosis infection and treating LTBI address some of the barriers for TB prevention among PEH. Specifically, interferon-γ release assays for diagnosing LTBI, which can be completed in a single visit,21 and LTBI treatment with safe and effective short-course rifamycin-based regimens can improve treatment completion rates among PEH.40–43 Complementary strategies that can impact TB prevention among PEH include acknowledging and addressing stigma and stress and integrating TB prevention with other public health and housing efforts to provide a more comprehensive and synergistic approach to health. In fact, establishing stable housing not only meets the basic human need for shelter, it also empowers and facilitates vulnerable populations to better address their health needs. Several studies have shown that ensuring housing is associated with improved treatment adherence, better health outcomes, and cost savings for TB and other infectious diseases.44–48
Characterizing TB epidemiology among PEH at the local level, combined with adequate resources and political will, provides an opportunity for local jurisdictions to identify the most appropriate combinations of proven TB prevention and complementary strategies to address the disproportionate impact of TB on PEH in their communities.
ACKNOWLEDGMENTS
The authors thank William Snow and Marlisa Grogan from US Department of Housing and Urban Development for helping us appropriately apply HUD estimates of homelessness for these analyses. We thank the public health staff, laboratory personnel, and clinicians who contributed data to the US National TB Surveillance System, and especially those who reviewed WGS and epidemiologic data for the collaborative review. The authors also thank T. Navin, J.S. Kammerer, A. Hill, S. Marks, K. Raz, and S. Talarico and other colleagues from Division of TB Elimination and the Outbreak Detection Working Group for providing helpful discussions about these analyses and Christine Olagun-Samuel for conducting a literature review on stigma associated with TB and homelessness.
Footnotes
Publisher's Disclaimer: Disclaimer:
Publisher's Disclaimer: The views and opinions expressed in this article are those of the authors and do not necessarily represent an official position of the US Centers for Disease Control and Prevention.
The authors declare no conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2016. Available at: https://www.cdc.gov/tb/statistics/reports/2016/default.htm. Accessed July 23, 2018.
- 2.Bamrah S, Yelk Woodruff RS, Powell K, et al. Tuberculosis among the homeless, United States, 1994–2010. Int J Tuberc Lung Dis. 2013;17: 1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Housing and Urban Development. The 2015 Annual Homeless Assessment Report (AHAR) to congress, part 2: estimates of homelessness in the United States. Available at: https://www.hudexchange.info/resource/5162/2015-ahar-part-2-estimates-of-homelessness/. Accessed December 4, 2017.
- 4.Yuen CM, Kammerer JS, Marks K, et al. Recent transmission of tuberculosis—United States, 2011–2014. PLoS One. 2016;11:e0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duarte R, Lönnroth K, Carvalho C, et al. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology. 2018;24: 115–119. [DOI] [PubMed] [Google Scholar]
- 6.Committee on Infectious Diseases, American Academy of Pediatrics. Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 report of the committee on infectious diseases, 31st ed. Itasca, IL: American Academy of Pediatrics; 2018. Available at: https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640207&bookid=2205. Accessed October 8, 2019. [Google Scholar]
- 7.Powell KM, VanderEnde DS, Holland DP, et al. Outbreak of drug-resistant Mycobacterium tuberculosis among homeless people in Atlanta, Georgia, 2008–2015. Public Health Rep. 2017;132:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mindra G, Wortham JM, Haddad MB, et al. Tuberculosis outbreaks in the United States, 2009–2015. Public Health Rep. 2017;132:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Division of Tuberculosis Elimination. Workshop on Tuberculosis and Homelessness: Infection Control Measures in Homeless Shelters and Other Overnight Facilities That Provide Shelter: Summary of the Workshop Held September 28–29, 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Infectious Diseases, NCHHSTP; 2018. Available at: https://www.cdc.gov/tb/topic/populations/homelessness/TB_and_Homelessness_2015_Summit.pdf. Accessed October 8, 2019. [Google Scholar]
- 10.Tibbetts KK, Ottoson RA, Tsukayama DT. Public health response to tuberculosis outbreak among persons experiencing homelessness, Minneapolis, Minnesota, USA, 2017–2018. Emerg Infect Dis. 2020;26:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherng ST, Shrestha S, Reynolds S, et al. Tuberculosis incidence among populations at high risk in California, Florida, New York, and Texas, 2011–2015. Am J Public Health. 2018;108(S4):S311–S314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha S, Hill AN, Marks SM, et al. Comparing drivers and dynamics of tuberculosis in California, Florida, New York, and Texas. Am J Respir Crit Care Med. 2017;196:1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Housing and Urban Development. National summary—system performance measures 2015–2017. Available at: https://www.hudexchange.info/resource/5793/national-summary-system-performance-measures-2015-2017/. Accessed October 17, 2019.
- 14.France AM, Grant J, Kammerer JS, et al. A field-validated approach using surveillance and genotyping data to estimate tuberculosis attributable to recent transmission in the United States. Am J Epidemiol. 2015;182:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Report of Verified Case of Tuberculosis (RVCT) Instruction Manual. 2009. Available at: https://www.cdc.gov/tb/programs/rvct/default.htm. Accessed August 20, 2018.
- 16.US Department of Housing and Urban Development. 2020 HMIS Data Standards—HUD Exchange. Available at: https://www.hudexchange.info/resource/3824/hmis-data-dictionary/. Accessed February 18, 2020.
- 17.US Department of Housing and Urban Development. The 2016 Annual Homeless Assessment Report (AHAR) to congress, part 2: estimates of homelessness in the United States. 2017. Available at: https://www.hudexchange.info/resources/documents/2016-AHAR-Part-2.pdf. Accessed August 17, 2018.
- 18.US Department of Housing and Urban Development. Continuum of Care (CoC) Program. Available at: https://www.hudexchange.info/programs/coc/. Accessed October 15, 2019.
- 19.US Department of Housing and Urban Development. Balance of State Continuum of Care Toolkit. 2018. Available at: https://files.hudexchange.info/resources/documents/Balance-of-State-Continuum-of-Care-Toolkit.pdf. Accessed October 18, 2019.
- 20.Ghosh S, Moonan PK, Cowan L, et al. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol. 2012;12:782–788. [DOI] [PubMed] [Google Scholar]
- 21.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glynn C, Fox EB. Dynamics of homelessness in urban America. Ann Appl Stat. 2019;13:573–605. [Google Scholar]
- 23.Baker M Homeless residents got one-way tickets out of town. Many returned to the streets. The New York Times. 2019. Available at: https://www.nytimes.com/2019/09/14/us/homeless-busing-seattle-san-francisco.html. Accessed February 12, 2020.
- 24.Thadani T A ticket out of town. San Francisco Chronicle. 2019. Available at: https://www.sfchronicle.com/bayarea/article/Hundreds-of-homeless-people-board-a-bus-out-of-SF-14188436.php. Accessed February 12, 2020.
- 25.Outside in America Team. Bussed out. The Guardian. 2017. Available at: https://www.theguardian.com/us-news/ng-interactive/2017/dec/20/bussed-out-america-moves-homeless-people-country-study. Accessed February 12, 2020.
- 26.Azevedo MJ, Conwill DE, Lawrence S, et al. Tuberculosis containment among the homeless in Metropolitan Jackson, Mississippi. J Miss State Med Assoc. 2015;56:243–248. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Prevention and control of tuberculosis among homeless persons: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1992;41(RR-5):13–23. [PubMed] [Google Scholar]
- 28.Reichler MR, Khan A, Sterling TR, et al. Risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. J Infect Dis. 2018;218:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Tuberculosis Controllers Association, Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54(RR-15):1–47. [PubMed] [Google Scholar]
- 30.Hamilton K, Tolfree R, Mytton J. A systematic review of active case-finding strategies for tuberculosis in homeless populations. Int J Tuberc Lung Dis. 2018;22:1135–1144. [DOI] [PubMed] [Google Scholar]
- 31.Kong P-M, Tapy J, Calixto P, et al. Skin-test screening and tuberculosis transmission among the homeless. Emerg Infect Dis. 2002;8:1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard C, Sougakoff W, Fournier A, et al. Impact of a 14-year screening programme on tuberculosis transmission among the homeless in Paris. Int J Tuberc Lung Dis. 2012;16:649–655. [DOI] [PubMed] [Google Scholar]
- 33.Connors WJ, Hussen SA, Holland DP, et al. Homeless shelter context and tuberculosis illness experiences during a large outbreak in Atlanta, Georgia. Public Health Action. 2017;7:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC). Notes from the field: tuberculosis cluster associated with homelessness—Duval County, Florida, 2004–2012. MMWR Morb Mortal Wkly Rep. 2012;61:539–540. [PubMed] [Google Scholar]
- 35.Heuvelings CC, de Vries SG, Greve PF, et al. Effectiveness of interventions for diagnosis and treatment of tuberculosis in hard-to-reach populations in countries of low and medium tuberculosis incidence: a systematic review. Lancet Infect Dis. 2017;17:e144–e158. [DOI] [PubMed] [Google Scholar]
- 36.Gupta V, Sugg N, Butners M, et al. Tuberculosis among the homeless—preventing another outbreak through community action. N Engl J Med. 2015;372:1483–1485. [DOI] [PubMed] [Google Scholar]
- 37.Yun LWH, Reves RR, Reichler MR, et al. Outcomes of contact investigation among homeless persons with infectious tuberculosis. Int J Tuberc Lung Dis. 2003;7(12 suppl 3):S405–S411. [PubMed] [Google Scholar]
- 38.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep. 2010;125(suppl 4):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinfurter P, Blumberg HM, Goldbaum G, et al. Predictors of discordant tuberculin skin test and QuantiFERON®-TB Gold In-Tube results in various high-risk groups. Int J Tuberc Lung Dis. 2011;15:1056–1061. [DOI] [PubMed] [Google Scholar]
- 40.Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandul AL, Nwana N, Holcombe JM, et al. High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine for latent Mycobacterium tuberculosis infection. Clin Infect Dis. 2017;65:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nwana N, Marks SM, Lan E, et al. Treatment of latent Mycobacterium tuberculosis infection with 12 once weekly directly-observed doses of isoniazid and rifapentine among persons experiencing homelessness. PLoS One. 2019;14:e0213524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. Morb Mortal Wkly Rep Recomm Rep. 2020;69:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Alliance to End Homelessness. Housing First. Available at: https://endhomelessness.org/resource/housing-first/. Accessed October 8, 2019.
- 45.LoBue PA, Cass R, Lobo D, et al. Development of housing programs to aid in the treatment of tuberculosis in homeless individuals: a pilot study. Chest. 1999;115:218–223. [DOI] [PubMed] [Google Scholar]
- 46.Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11:531–535. [DOI] [PubMed] [Google Scholar]
- 47.Kim H, Choi H, Yu S, et al. Impact of housing provision package on treatment outcome among homeless tuberculosis patients in South Korea. Asia Pac J Public Health. 2019;31:603–611. [DOI] [PubMed] [Google Scholar]
- 48.Wolitski RJ, Kidder DP, Pals SL, et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14:493–503. [DOI] [PubMed] [Google Scholar]


