ABSTRACT
Background
Liver cancer incidence and mortality are escalating globally. Magnesium intake has been studied extensively in nonmalignant liver pathology, but the association between dietary intake of magnesium and primary liver malignancy has not been previously evaluated.
Objectives
We aimed to determine the association between total magnesium intake and primary liver cancer risk.
Methods
Using the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study prospective cohort, we estimated the association between magnesium intake and the risk of incident primary liver cancer using Cox proportional hazard modeling adjusted for relevant confounders. Comprehensive stratified and sensitivity analyses were performed.
Results
During 6.4 million person-years of follow-up time, 1067 primary liver cancers occurred in 536,359 participants. Higher magnesium intake was independently associated with a lower risk of liver cancer (P-trend = 0.005), with intakes in the highest compared with lowest quartile associated with 35% lower risk (HR: 0.65; 95% CI: 0.48, 0.87). The dose-related inverse association was more pronounced in moderate and heavy alcohol users (HR: 0.54; 95% CI: 0.35, 0.82; P-trend = 0.006), and this interaction was statistically significant (P-interaction = 0.04).
Conclusions
Based on a prospective cohort analysis, we demonstrated that magnesium intake is associated with a lower risk of primary liver cancer, which was more pronounced among moderate and heavy alcohol users. Robust experimental and mechanistic data provide a biological basis to support these findings.
Keywords: hepatocellular carcinoma, epidemiology, public health, digestive system neoplasm, nutrition therapy
Introduction
Liver cancer remains one of the most common and deadliest cancers worldwide. Rates of primary liver cancer increased by an estimated 75% between 1990 and 2015 and accounted for >840,000 new cases and 781,000 related deaths globally in 2018 alone (1). Hepatocellular carcinoma (HCC) comprises the vast majority of primary liver cancers. Although cirrhosis is the strongest risk determinant, HCC also occurs in the absence of cirrhosis. Globally, the most common etiologies for cirrhosis and HCC are chronic viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), and alcohol-related liver disease (2). The obesity epidemic has heralded a parallel rise in NAFLD prevalence, which is now estimated to affect 20% of the global population and >34% of the US population (3, 4). In many industrialized countries, NAFLD is now the leading cause of HCC and can even occur in individuals without cirrhosis. Despite the observation that 70% of all liver cancers diagnosed in the US are attributable to preventable factors (5) and despite established HCC screening guidelines, the incidence and mortality rates of liver cancer in the US since 1980 have nevertheless tripled and doubled, respectively (6, 7). As such, there is clearly an unmet need to identify other adjunctive modifiable measures to reduce the growing burden. Dietary factors are estimated to account for ∼30% of cancer mortality in Western countries, including the US, second only to tobacco smoking and by a small margin (8, 9).
Magnesium, a divalent cation, is an essential micronutrient in the human diet because of its fundamental role in maintaining physiologic homeostasis through the regulation of several biological processes and pathways, including DNA replication and repair, maintenance of genomic stability and fidelity, signal transduction, cell proliferation and differentiation, angiogenesis, apoptosis, and inflammatory responses (8, 10). Previous studies have consistently demonstrated that increased magnesium intake is inversely associated with the risk of metabolic syndrome and type 2 diabetes (11, 12). Based on randomized controlled clinical trials, magnesium supplementation is associated with improved insulin resistance among diabetics (13) and with reduced systemic inflammatory markers (14). Relatedly, higher magnesium intake is associated with not only a lower likelihood of developing NAFLD (15) and, among alcohol users, alcohol-related liver disease, but also lower risk of liver-related mortality, irrespective of the etiology of liver disease. It is well recognized, however, that several liver diseases, particularly alcohol-related liver disease, are associated with magnesium deficiency and that magnesium deficiency, in turn, exacerbates liver pathology and disease progression (8, 10, 16, 17). The underlying mechanisms are not completely defined, but they relate to altered magnesium homeostasis in the liver and subsequent dysregulation of immune, inflammatory, and, relatedly, potentially carcinogenic pathways. In vitro data also demonstrate an inhibitory effect of magnesium on experimental hepatoma and HCC development (18, 19).
We therefore hypothesized that magnesium intake may be inversely associated with incident primary liver cancer and that this protective risk might be more pronounced among those who use alcohol. We secondarily hypothesized that calcium intake might confound or interact with these associations given the close physiologic relation between these 2 divalent cations (10). This interaction has been previously demonstrated in studies of other gastrointestinal (GI) diseases, including GI neoplasia (20–23). To evaluate these hypotheses, we conducted an analysis of the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study prospective cohort with the primary objective to define the association between magnesium and the risk of incident primary liver cancer. Better defining the health outcomes associated with altered magnesium intake could have important public health implications because at least half of the US population does not meet the daily RDA for magnesium (24–26).
Methods
Data source and study population
The NIH-AARP Diet and Health Study cohort is one of the largest prospective cohorts with dietary data and cancer outcomes in the United States and is supported by the National Cancer Institute (NCI). The NCI developed the baseline questionnaire, which included a self-administered FFQ. Between 1995 and 1996, the questionnaire was mailed to ∼3.5 million AARP members aged 50–71 y who primarily resided in 1 of 6 states (California, Florida, Pennsylvania, New Jersey, North Carolina, and Louisiana) or 2 metropolitan areas (Detroit, MI, and Atlanta, GA). A total of 566,398 participants returned the questionnaires. Additional details of this cohort as well as comparability of respondents compared with nonrespondents have been previously described and published, along with external validation of the NIH-AARP Diet and Health Study cohort (23, 27). The cohort was followed prospectively through December 2011. Figure 1 illustrates construction of the analytic cohort for this analysis (n = 536,359 respondents).
FIGURE 1.
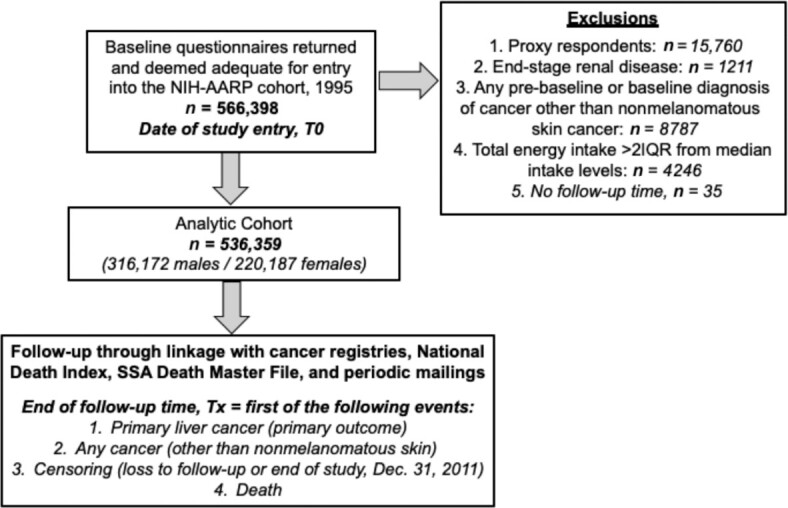
Flow diagram detailing the construction of the analytic cohort from the NIH-AARP Diet and Health Study prospective cohort. NIH-AARP, NIH-American Association of Retired Persons.
Exposure and outcome assessment
Usual intake over the 12 mo prior to enrollment was assessed using a self-administered 124-item semiquantitative baseline FFQ (Supplemental Methods). The food items, standardized frequency and portion categories, and the nutrient database were adapted from the USDA's 1994–1996 Continuing Survey of Food Intake by Individuals, which reflected the most common food intakes reported by the general US population at the time (28). Additional details of the FFQ and conversions are previously described (27, 29).
The primary exposure for this analysis was total magnesium intake. Total intake in milligrams for both magnesium and calcium was calculated as the sum of dietary intake from food or beverages, plus intake from magnesium- or calcium-containing supplements. Supplemental intake represented an aggregate of single and multivitamin/mineral magnesium- or calcium-containing supplements.
The primary outcome was incident diagnosis of liver cancer as the first primary cancer. Incident cancer cases were identified through cancer registry linkage, which included registries from the initial 8 geographies listed previously and, starting in 2003, also Arizona, Nevada, and Texas. Cancer registry linkage for the NIH-AARP Diet and Health Study cohort is excellent, with prior studies confirming ∼90% accuracy for diagnoses (30). Throughout the study period, all registries maintained North American Association of Central Cancer Registry certification as being ≥90% complete within 2 y of cancer diagnosis. Liver cancer cases were classified according to the Surveillance, Epidemiology, and End Results program's International Classification of Disease for Oncology (third edition) site codes C22.0–C22.1 (7). Death was ascertained by linkage to the Social Security Administration Death Master file, linkage with cancer registries, and by NIH-AARP study investigators’ follow-up searches of the National Death Index as well as mailings.
The study follow-up time was defined as cohort entry, T0—the time when the baseline questionnaire was received—until the earliest occurrence of the following: liver cancer diagnosis, any other cancer diagnosis except nonmelanomatous skin cancer, censoring, or death. Censoring occurred with either loss to follow-up due to relocation outside of any of the aforementioned geographic cancer registry regions or with last date of follow-up for the Diet and Health Study cohort (December 31, 2011).
Statistical analysis
Baseline cohort characteristics including demographics, lifestyle factors, and nutritional intakes were compared according to quartiles of magnesium intake. All nutritional intake values were adjusted for age, sex, BMI (in kg/m2), and caloric intake using generalized linear regression modeling.
Total magnesium (primary exposure) and calcium intakes were categorized into quartiles for all analyses. There were no substantive differences when we evaluated sex-specific compared with common quartiles, so we elected to use the latter. Cox proportional hazard models were used to estimate HRs and 95% CIs for risk of incident primary liver cancer. Schoenfeld residuals were used to test the proportional hazards assumption. The reference group was the lowest quartile of magnesium or calcium intake for all analyses. Including the quantile as a continuous variable in the model allowed evaluation for any linear dose responses, which were presented as P-trend values.
We evaluated covariates hypothesized or previously established to be associated with the primary exposures or outcome as potential confounders. Covariates associated with the exposure and outcome (potential confounders) were adjusted for in the models. Minimally adjusted models were adjusted for age (years, continuous), sex (male/female), race/ethnicity (non-Hispanic white compared with non-white), and energy (caloric) intake (kilocalories per day, continuous). Fully adjusted models were additionally adjusted for the following potential confounder covariates: obesity (BMI ≥30 compared with BMI <30), smoking status (current/former or “ever smoker” compared with never smoker), alcohol use by sex-specific standards (moderate/heavy use, yes or no), highest level of education achieved (less than high school degree, completed high school degree, some post–high school training, completed college or higher degree), self-reported health status (fair or poor, good, excellent or very good), and the Healthy Eating Index (31). Models were additionally adjusted for either magnesium or calcium depending on the respective exposure of interest. All covariates had <5% missing data, so multiple imputation for missing data was not needed. Records with missing data were excluded from analyses.
We conducted stratified analyses to evaluate for effect modification by sex, race/ethnicity, alcohol use, obese status, and smoking status. Our prior studies have suggested that the calcium-to-magnesium ratio might modify the association between magnesium (or calcium) and the outcome of interest, and so we also evaluated effect modification by calcium-to-magnesium ratio (22, 23, 32). Calcium-to-magnesium ratio was calculated as the ratio of total calcium intake to total magnesium intake. Thresholds for “high,” “normal,” and “low” were based on our prior studies and were set at calcium-to-magnesium ratios of >2.6, 1.7–2.6, and <1.7, respectively (20, 21).
We lastly conducted 2 sensitivity analyses—1 excluding cases of liver cancer within 12 mo of follow-up, because these might more likely represent prevalent instead of incident cases, and another evaluating the association between supplemental magnesium as the exposure and incident liver cancer. Statistically significant interactions were tested for using the –2 log(likelihood) test comparing models with and without the interaction term. All analyses were performed using SAS software (version 7.1; SAS Institute).
Ethics approval
The study was approved by the Special Studies Institutional Review Board of the NCI. Original consent from participants was obtained from investigators for the NIH-AARP Diet and Health Study at enrollment. No new data collection or participant contact occurred for the purposes of this study. Participants were not involved in the study design, conduct, reporting, or dissemination plans of our research.
Results
Among 536,359 participants meeting inclusion criteria, a total of 1067 cases of primary liver cancer occurred over 6421,022 person-years of follow-up time. Table 1 details the cohort characteristics according to quartile of magnesium intake. The age at cohort entry was similar across quartiles. In the cohort overall, there were more men (316,172; 58.9%) than women (220,187; 41.1%). Except for the lowest quartile of magnesium intake, there were more men than women in all quartiles, with the greatest differential in the highest quartile of magnesium intake. The majority of respondents across all quartiles were non-Hispanic whites, with the largest percentage in the middle quartiles (93.4–94.0%). Compared to individuals in the highest quartile of magnesium intake, individuals in the lowest quartile of magnesium intake were more frequently never smokers and more often reported no alcohol intake; they also more frequently reported less than a high school degree and less frequently reported excellent or very good health. Given the size of the cohort, some differences in baseline characteristics were identified that likely have no biological significance (e.g., BMI).
TABLE 1.
Cohort characteristics stratified by quartiles of magnesium intake, NIH-AARP Diet and Health Study 1995–20111
| Magnesium quartiles | ||||
|---|---|---|---|---|
| Characteristic | Q1: 288 ± 58 mg (n = 134,101) | Q2: 341 ± 65 mg (n = 134,086) | Q3: 387 ± 74 mg (n = 134,087) | Q4: 499 ± 131 mg (n = 134,085) |
| Age at cohort entry, y | 61.7 ± 5.4 | 61.7 ± 5.4 | 61.7 ± 5.3 | 61.5 ± 5.4 |
| Men, % | 48.5 | 55.5 | 61.6 | 70.2 |
| Race/ethnicity, % | ||||
| Non-Hispanic white | 90.6 | 93.4 | 94.0 | 92.4 |
| Non-Hispanic black | 5.3 | 3.4 | 3.0 | 3.9 |
| Hispanic | 2.0 | 1.7 | 1.7 | 2.1 |
| Asian | 1.5 | 1.2 | 1.0 | 1.1 |
| Other | 0.5 | 0.3 | 0.3 | 0.5 |
| BMI, m/kg2 | 27.2 ± 5.3 | 27.0 ± 5.0 | 27.0 ± 5.0 | 27.2 ± 5.1 |
| Obese status (BMI > 30), % | 25.3 | 23.3 | 22.7 | 24.5 |
| Smoking status, % | ||||
| Never smoker | 39.0 | 37.0 | 35.4 | 33.7 |
| Former smoker | 47.8 | 51.0 | 53.0 | 53.6 |
| Current smoker | 13.2 | 12.0 | 11.7 | 12.7 |
| Alcohol use, % | ||||
| None (0 g/d) | 28.2 | 23.8 | 22.6 | 23.6 |
| ≤1 drink/d | 23.4 | 20.5 | 18.2 | 15.1 |
| ≤2 drinks/d | 9.3 | 9.1 | 8.6 | 7.6 |
| 2–3 drinks/d | 6.5 | 6.7 | 6.6 | 5.7 |
| >3 drinks/d | 32.6 | 39.8 | 44.1 | 48.0 |
| Moderate/heavy alcohol use—sex-specific standards, % | 43.3 | 50.3 | 53.9 | 56.0 |
| Highest education achieved, % | ||||
| Less than high school degree | 30.3 | 25.9 | 24.3 | 24.7 |
| Completed high school degree | 10.1 | 10.3 | 10.0 | 10.1 |
| Some post–high school training | 24.6 | 24.0 | 23.6 | 23.9 |
| Completed college or higher degree | 35.0 | 39.8 | 42.1 | 39.7 |
| Self-reported health, % | ||||
| Excellent or very good | 48.3 | 51.3 | 52.8 | 52.5 |
| Good | 35.8 | 34.9 | 33.9 | 33.2 |
| Fair or poor | 16.0 | 13.7 | 13.3 | 14.3 |
| Daily energy intake,2 kcal/d | 1801.5 ± 239.1 | 1830.0 ± 238.2 | 1856.9 ± 233.9 | 1900.5 ± 222.5 |
| Healthy Eating Index3 | 68.9 ± 1.5 | 68.2 ± 1.6 | 67.6 ± 1.7 | 66.0 ± 2.2 |
| Total calcium intake,3 mg | 819.1 ± 186.8 | 933.8 ± 192.9 | 1032.9 ± 208.8 | 1291.4 ± 333.1 |
| Dietary—total, mg | 553.8 ± 144.6 | 683.5 ± 162.7 | 796.5 ± 186.3 | 1077.0 ± 330.4 |
| Supplemental intake, mg | 265.3 ± 123.9 | 250.3 ± 123.1 | 236.4 ± 120.5 | 214.4 ± 113.9 |
| Total magnesium intake,3 mg | 288.4 ± 58.0 | 341.0 ± 65.1 | 386.6 ± 74.3 | 498.7 ± 131.3 |
| Dietary—total, mg | 239.1 ± 57.9 | 291.6 ± 65.4 | 337.3 ± 74.6 | 449.5 ± 131.4 |
| Supplemental intake, mg | 49.4 ± 4.5 | 49.3 ± 4.4 | 49.2 ± 4.3 | 49.1 ± 4.3 |
Values are means ± SDs or percentages. HEI, Healthy Eating Index; NIH-AARP, NIH-American Association of Retired Persons; Q, quartile.
Using linear regression modeling, daily energy intake was adjusted for age, sex, and BMI.
Using linear regression modeling, HEI and calcium and magnesium intakes were adjusted for energy intake, age, sex, and BMI.
The mean daily energy intake (adjusted for age, sex, and BMI) was greatest among those in the highest quartile of magnesium intake. Total calcium intake was also greatest among those in the highest quartile of magnesium intake and was driven by dietary intakes; indeed, supplemental calcium intake was lowest among individuals in the highest quartile of magnesium intake, but it was highest among those in the lowest quartile of magnesium intake (214.4 mg compared with 265.3 mg, respectively). The differences in total magnesium intake across magnesium quartiles were also primarily driven by dietary intake, given that median supplemental magnesium intake for all quartiles was similar.
Higher magnesium intake was associated with a lower risk of incident liver cancer (P-trend = 0.005), with magnesium intakes in the highest compared with lowest quartile associated with an overall 35% lower risk of incident liver cancer (HR: 0.65; 95% CI: 0.48, 0.87) (Table 2). The association was also present for men. Among women, although directionality of the association was maintained, the association between magnesium intake and incident liver cancer was not statistically significant. After adjusting for confounders, the associations between calcium and risk of liver cancer were overall null (Supplemental Table 1). There was no significant interaction between magnesium or calcium intake and sex on liver cancer risk (P-interaction = 0.87 and 0.30, respectively). There was a suggestive inverse association between calcium intake and liver cancer risk among women (P = 0.06).
TABLE 2.
Associations between total magnesium intake and risk of liver cancer stratified by sex, NIH-AARP Diet and Health Study 1995–20111
| Quartiles of magnesium intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (low): mean ± SD = 288 ± 58 mg | Q2: mean ± SD = 341 ± 65 mg | Q3: mean ± SD = 387 ± 74 mg | Q4 (high): mean ± SD = 499 ± 131 mg | |||||||
| Liver cancer (1067 cases) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | P-trend | P-interaction |
| Overall | 253 | 254 | 262 | 298 | ||||||
| Minimally adjusted2 | 1.00 (Ref) | 0.87 (0.73, 1.04) | 0.79 (0.66, 0.95) | 0.67 (0.54, 0.84) | <0.001 | |||||
| Fully adjusted3 | 1.00 (Ref) | 0.93 (0.75, 1.15) | 0.83 (0.66, 1.04) | 0.65 (0.48, 0.87) | 0.005 | 0.87 | ||||
| Men | 167 | 193 | 208 | 252 | ||||||
| Minimally adjusted2 | 1.00 (Ref) | 0.92 (0.75, 1.14) | 0.82 (0.66, 1.02) | 0.68 (0.53, 0.88) | 0.002 | |||||
| Fully adjusted3 | 1.00 (Ref) | 0.95 (0.74, 1.21) | 0.86 (0.66, 1.12) | 0.64 (0.45, 0.89) | 0.01 | |||||
| Women | 86 | 61 | 54 | 46 | ||||||
| Minimally adjusted2 | 1.00 (Ref) | 0.77 (0.55, 1.08) | 0.74 (0.51, 1.08) | 0.72 (0.44, 1.18) | 0.13 | |||||
| Fully adjusted3 | 1.00 (Ref) | 0.92 (0.62, 1.38) | 0.76 (0.47, 1.22) | 0.77 (0.40, 1.48) | 0.29 | |||||
NIH-AARP, NIH-American Association of Retired Persons; Q, quartile.
Cox proportional hazard modeling was used for this analysis and adjusted for age, sex, race/ethnicity, and energy intake.
Cox proportional hazard modeling was used for this analysis and adjusted for age, sex, race/ethnicity, energy intake, obesity, smoking, alcohol intake, self-reported health, Healthy Eating Index, educational status, and total calcium intake.
The amount of alcohol use modified the association between magnesium and liver cancer risk (P-interaction = 0.04) (Table 3). The inverse association between higher magnesium intake and lower risk of liver cancer was more pronounced among participants reporting moderate/heavy alcohol use (P-trend = 0.006) but was null among participants with less or no alcohol intake; among moderate/heavy alcohol users, magnesium intake in the highest compared with lowest quartile was associated with a 46% risk reduction (HR: 0.54; 95% CI: 0.35, 0.82) (Table 3). There was no interaction between magnesium intake and smoking on liver cancer risk (P-interaction = 0.14) (Table 3). There was a statistically significant interaction between magnesium and calcium-to-magnesium ratio on liver cancer risk when the interaction was evaluated using the ratio as a continuous measure (P-interaction = 0.005); the magnitude of the associations among individuals with intakes in the highest quartile was similar, however (Table 3). We did not observe effect modification by race/ethnicity or obesity status (Table 3). Supplemental magnesium intake alone was not associated with liver cancer (P-trend = 0.64), irrespective of sex (data not shown).
TABLE 3.
Stratified analyses of the associations between total magnesium intake and risk of liver cancer, NIH-AARP Diet and Health Study 1995–20111
| Quartiles of magnesium intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (low): mean ± SD = 288 ± 58 mg | Q2: mean ± SD = 341 ± 65 mg | Q3: mean ± SD = 387 ± 74 mg | Q4 (high): mean ± SD = 499 ± 131 mg | |||||||
| Liver cancer (1067 cases) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | Cases, n | HR (95% CI) | P-trend | P-interaction |
| Alcohol use | 0.04 | |||||||||
| Not moderate/heavy (n = 571) | 151 | 1.00 (Ref) | 133 | 0.93 (0.69, 1.25) | 126 | 0.86 (0.63, 1.19) | 161 | 0.84 (0.56, 1.26) | 0.34 | |
| Moderate/heavy (n = 496) | 102 | 1.00 (Ref) | 121 | 0.94 (0.69, 1.27) | 136 | 0.82 (0.59, 1.13) | 137 | 0.54 (0.35, 0.82) | 0.006 | |
| Smoking status | 0.14 | |||||||||
| Never smoker (n = 280) | 77 | 1.00 (Ref) | 71 | 0.83 (0.58, 1.20) | 63 | 0.68 (0.45, 1.03) | 69 | 0.53 (0.30, 0.92) | 0.02 | |
| Ever smoker (n = 745) | 162 | 1.00 (Ref) | 177 | 0.99 (0.76, 1.28) | 190 | 0.91 (0.69, 1.20) | 216 | 0.72 (0.50, 1.02) | 0.07 | |
| Calcium-to-magensium intake ratio | 0.005 | |||||||||
| <1.7 (low) | 52 | 1.00 (Ref) | 38 | 0.81 (0.49, 1.34) | 50 | 1.00 (0.56, 1.76) | 54 | 0.72 (0.30, 1.68) | 0.71 | |
| 1.7–2.6 (normal) | 115 | 1.00 (Ref) | 134 | 1.06 (0.77, 1.46) | 115 | 0.80 (0.54, 1.19) | 123 | 0.72 (0.41, 1.28) | 0.17 | |
| ≥2.6 (high) | 86 | 1.00 (Ref) | 82 | 0.95 (0.65, 1.39) | 97 | 0.97 (0.66, 1.43) | 121 | 0.75 (0.46, 1.24) | 0.35 | |
| Race | 0.28 | |||||||||
| Non-white (n = 129) | 34 | 1.00 (Ref) | 36 | 1.53 (0.85, 2.77) | 28 | 1.07(0.52, 2.19) | 31 | 0.77 (0.29, 2.10) | 0.66 | |
| White (n = 922) | 215 | 1.00 (Ref) | 216 | 0.87 (0.69, 1.09) | 231 | 0.80 (0.63, 1.02) | 260 | 0.63 (0.46, 0.86) | 0.005 | |
| Obese status | 0.52 | |||||||||
| Nonobese (n = 699) | 168 | 1.00 (Ref) | 159 | 0.82 (0.64, 1.06) | 181 | 0.78 (0.60, 1.02) | 191 | 0.59 (0.42, 0.84) | 0.006 | |
| Obese (n = 338) | 76 | 1.00 (Ref) | 89 | 1.22 (0.83, 1.79) | 77 | 0.96 (0.63, 1.47) | 96 | 0.81 (0.47, 1.39) | 0.34 | |
Cox proportional hazard modeling was used for this analysis and adjusted for age, sex, race, energy intake, obesity, alcohol intake (for smoking model), smoking status (for alcohol model), self-reported health, educational status, and total calcium intake. Note that missing cases for smoking status (n = 42, 3.9%), race/ethnicity (n = 16, 1.5%), and obesity status (n = 30, 2.8%) were excluded from the respective analyses. NIH-AARP, NIH-American Association of Retired Persons; Q, quartile.
The mean time to liver cancer occurrence ranged from 7.9 to 8.8 y, depending on the magnesium quartile (Supplemental Table 2). Removing participants diagnosed with primary liver cancer within 12 mo of follow-up (n = 40) did not change our conclusions (Supplemental Table 3).
Discussion
We demonstrated a significant association between magnesium intake and risk of primary liver cancer. Based on a cohort analysis of 536,359 NIH-AARP Diet and Health Study participants with >6.4 million person-years of follow-up time, we confirmed our hypothesis that higher magnesium intake is associated with a lower risk of liver cancer. We demonstrated that after adjusting for relevant confounders including calcium intake, there was a dose-related overall 35% reduced risk of incident liver cancer among participants with magnesium intake in the highest compared with lowest quartile, with greater benefit among those reporting moderate/heavy alcohol use. We confirmed interactions between magnesium and alcohol intake on liver cancer risk. Notably, the association between magnesium and reduced risk of luminal GI cancers, including colorectal cancer and noncardia gastric cancer, has also been reported (20, 21, 23).
The burden of liver cancer and related mortality is steadily rising. This is compounded by a rapidly expanding pool of at-risk individuals due to the obesity epidemic and rising prevalence of NAFLD, adding to the more traditional etiologies of chronic liver disease and cirrhosis in the United States, such as alcohol use and chronic hepatitis C viral infection. When likewise considering that dietary modifications, such as increasing total dietary magnesium intake, are generally safe, inexpensive, and achievable interventions, the potential public health implications of these findings, if confirmed, are appealing. Indeed, only a minority of participants in the NIH-AARP cohort met the age- and gender-specific RDAs for magnesium (34.7% women and 25.2% men) or calcium (11.2% women and 24.7% men). This high prevalence of inadequate intake is consistent with other population-based analyses, including NHANES, which have reported that, on average, 46–52.2% of US adults do not meet the daily RDA for magnesium (24–26).
Our main findings are supported by prior clinical studies demonstrating a protective effect of magnesium on nonmalignant liver-related outcomes, as well as a large body of experimental and translational data defining inflammatory and carcinogenic pathways activated by or aggravated by magnesium deficiency (10). Magnesium is a critical cofactor for enzymes involved in DNA replication, repair, and gene expression, as well as cell proliferation, differentiation, and migration, among other biological processes implicated in carcinogenesis (8, 33). In fact, magnesium administration inhibited experimentally induced colon cancer, and other cancers, in rats (8, 34, 35). Furthermore, in vivo studies demonstrate that magnesium administration might improve liver cirrhosis and reduce fibrosis progression (10). Of relevance, low hepatic magnesium stores, as might occur among chronic alcohol users, lead to local activation of inflammatory cells, recruitment, and significant increases in inflammatory cytokines, which subsequently initiates a detrimental cycle of damage–repair–fibrosis and progression to or worsening of cirrhosis, the strongest risk factor for primary liver cancer (10, 16, 36). These data provide a biological basis for our observation that the dose-related protective association between higher intakes of magnesium and liver cancer was more pronounced among participants reporting moderate/heavy alcohol use.
Our primary findings among moderate/heavy alcohol users are also supported by a platform of clinical data. Two small randomized controlled clinical trials from Finland and Norway demonstrated improved liver chemistries among alcohol users randomly assigned to short-term magnesium supplementation or placebo after 6–8 wk, even after adjusting for age, BMI, and amount of alcohol consumption (37, 38). The only randomized controlled clinical trial of magnesium supplementation compared with placebo conducted among patients with NAFLD, however, found no difference in metabolic parameters or liver chemistries at 12 wk follow-up; however, the authors note that all randomized participants had normal magnesium concentrations at study entry (39). Note, however, that serum magnesium (and calcium) do not accurately reflect dietary intake or total body stores; in fact, only 0.3% of total body magnesium is represented in the serum (38). Instead, dietary intake is the major determinant of total body stores (40, 41). In our cohort, respondents with above median total magnesium intake more frequently reported moderate/heavy alcohol use compared with those with below median magnesium intakes. An analysis of 13,504 participants in the NHANES III cohort who had undergone hepatic steatosis assessment found that for every 100-mg increase in magnesium intake, there was a 49% reduction in liver-related mortality—presented as a composite of benign and malignant related causes—with more pronounced effects among alcohol users and those with hepatic steatosis (42). A more recent analysis of this same cohort demonstrated that magnesium intake was independently associated with a lower likelihood of hepatic steatosis and prediabetes, which conceivably might translate to reduced risk of NAFLD-associated liver neoplasia (15). Furthermore, in the stratified analysis by alcohol use status in that study, the inverse association between the intake of magnesium and risk of NAFLD only appeared in former and current users, but not never users. Similar to our study, both NHANES III studies appropriately evaluated calcium intake as a potential confounder or effect modifier in analyses involving magnesium. In the NHANES III study cited previously, the inverse association between magnesium intake and risk of NAFLD was observed only in those with total daily calcium intake <1200 mg/d (15). Although we did demonstrate an interaction between magnesium and calcium-to-magnesium intake ratio in relation to primary liver cancer risk, this was likely driven by the middle quartiles (quartiles 2 and 3) given that the effect estimates were similar among individuals with magnesium intake in the highest quartile (quartile 4). Notably, calcium-to-magnesium intake ratio modifies the association between magnesium intake and other GI neoplasia, including colorectal adenomas and Barrett's esophagus (20, 22).
There are mixed data regarding dietary calcium intake and risk of liver cancer, and none of the previously discussed studies acknowledge magnesium intake as a potential confounder or effect modifier (32, 43–45). The reasons for these differences are unclear, but differences in the study populations are likely relevant.
Our study has several strengths but also notable limitations. Our use of the NIH-AARP Diet and Health Study, which is one of the largest and longest prospective cohorts with dietary data and cancer outcomes in the United States, ensured adequate power for our primary analysis, particularly because liver cancer is a rare diagnosis. The prospective design overcomes the potential for recall bias and significantly minimizes the chance of reverse causality, both of which are limitations of case–control studies (27). We further limited the likelihood of reverse causality—that is, altered magnesium or calcium intake due to an already prevalent cancer—influencing our findings by removing all cases diagnosed within 12 mo of the baseline assessment. In addition to adjusting for other relevant factors, we accounted for the relation between magnesium and calcium in maintaining normal physiologic homeostasis, with disrupted balance associated with initiating and propagating aberrant inflammatory and immune stress response pathways (10, 46). Prior analyses of the NIH-AARP Diet and Health Study reported 91–100% power to detect an observed relative risk difference of 20–30% for certain dietary intakes and risk of cancers with similar rarity as liver cancer at 10 y of follow-up; given that our median follow-up time exceeded 15 y, we can reasonably expect that our study was appropriately powered for our primary analysis. For all groups analyzed, there was a dose-related inverse relation between higher magnesium intake and risk of incident liver cancer. However, type II error related to insufficient power for some strata in the subanalyses is still possible. In addition to the very large cohort size, the Diet and Health Study investigators’ rigorous process to develop, test, and refine the 124-food item questionnaire might partly mitigate expected error in dietary measurements (27, 29). Notably, in the validation study comparing the FFQ performance to 24-h diet recalls, magnesium was one of the best-performing nutrients (29). In addition, we cannot rule out residual confounding, which would have an unpredictable impact on the effect estimates. We are unable to comment on generalization to other groups, including non-US populations, where geographic and cultural differences might also be relevant. Finally, because repeated measurements over time are not available, we are unable to comment on how any change from baseline parameters and responses influenced our conclusions.
In conclusion, based on an analysis of the NIH-AARP Diet and Health Study prospective cohort, we demonstrated that higher magnesium intake is associated with a dose-related, lower risk of liver cancer, which was particularly pronounced among moderate/heavy alcohol users. These findings add clinical value to the current expansive body of translational literature defining the mechanisms through which this essential micronutrient mediates inflammatory and antineoplastic pathways, particularly within the liver.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the content expertise provided by hepatologist Youngmin Lee.
The authors’ responsibilities were as follows—SCS: developed the study concept, drafted the manuscript, and is guarantor of the article; SCS, QD, and MJS: designed the study; SCS and XZ: performed statistical analysis; SCS, XZ, QD, and MJS: interpreted the data; QD, RMP, and MJS: reviewed the manuscript for important intellectual content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
SCS is partly funded by the Agency for Healthcare Research and the Quality and Patient-Centered Outcomes Research Institute under award K12 HS026395, a 2019 American Gastroenterological Association Research Scholar Award, and Veterans Affairs Career Development Award ICX002027A. The content is solely the responsibility of the listed authors and does not necessarily represent the official views of the funding agencies listed.
Supplemental Methods and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The data that support the findings of this study are available from the NIH-AARP Diet and Health Study. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with the permission of the NIH-AARP.
Abbreviations used: GI, gastrointestinal; HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute; NIH-AARP, NIH-American Association of Retired Persons.
Contributor Information
Shailja C Shah, Division of Gastroenterology, Hepatology, and Nutrition, Vanderbilt University Medical Center, Nashville, TN, USA; Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA.
Xiangzhu Zhu, Division of Epidemiology, Vanderbilt Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Qi Dai, Division of Epidemiology, Vanderbilt Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Richard M Peek, Jr, Division of Gastroenterology, Hepatology, and Nutrition, Vanderbilt University Medical Center, Nashville, TN, USA.
Martha J Shrubsole, Division of Epidemiology, Vanderbilt Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
References
- 1. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman Aet al. ; Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–7. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM. The epidemiology of nonalcoholic steatohepatitis. Clin Liver Dis. 2018;11:92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Islami F, Goding Sauer A, Miller KD, Siegel R, Fedewa SA, Jackobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram Iet al. . Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 6. Endeshaw M, Hallowell BD, Razzaghi H, Senkomago V, McKenna MT, Saraiya M. Trends in liver cancer mortality in the United States: dual burden among foreign- and US-born persons. Cancer. 2019;125:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Cancer Institute. Cancer stat facts: liver and intrahepatic bile duct cancer. [Internet]. [Cited 30 October, 2019]. Available from: https://seer.cancer.gov/statfacts/html/livibd.html. [Google Scholar]
- 8. Blaszczyk U, Duda-Chodak A. Magnesium: its role in nutrition and carcinogenesis. Rocz Panstw Zakl Hig. 2013;64:165–71. [PubMed] [Google Scholar]
- 9. Doll R, Peto R.. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1192. [PubMed] [Google Scholar]
- 10. Liu M, Yang H, Mao Y. Magnesium and liver disease. Ann Transl Med. 2019;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract. 2008;23:142–51. [DOI] [PubMed] [Google Scholar]
- 13. Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 14. Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Zambrano-Galvan G, Guerrero-Romero F. Effect of magnesium supplementation on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. 2017;23:4678–86. [DOI] [PubMed] [Google Scholar]
- 15. Li W, Zhu X, Song Y, Song Y, Fan L, Wu L, Kabagambe EK, Hou L, Shrubsole MJ, Liu Jet al. . Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public Health Nutr. 2018;21:2088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romani AMP. Magnesium homeostasis and alcohol consumption. Magnes Res. 2008;21:197–204. [PubMed] [Google Scholar]
- 17. Young A, Cefaratti C, Romani A. Chronic EtOH administration alters liver Mg2+ homeostasis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G57–67. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Li X, Zou Q, Liu L, Zhu X, Jia Q, Wang L, Yan R. Inhibitory effect of magnesium cantharidate on human hepatoma SMMC-7721 cell proliferation by blocking MAPK signaling pathway. Chinese J Cell Mol Immunol. 2017;33:347–51. [PubMed] [Google Scholar]
- 19. Liu Y, Xu Y, Ma H, Zou Q, Liu L, Zhu X, Jia Q, Wang L, Yan R. Hepatitis B virus X protein amplifies TGF-β promotion on HCC motility through down-regulating PPM1a. Oncotarget. 2016;7:33125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, Li M, Shyr Y, Zheng W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr. 2007;86:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dai Q, Sandler R, Barry E, Summers R, Grau M, Baron J. Calcium, magnesium, and colorectal cancer. Epidemiology. 2012;23:504–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai Q, Cantwell MM, Murray LJ, Zheng W, Anderson LA, Coleman HG; FINBAR Study Group. Dietary magnesium, calcium:magnesium ratio and risk of reflux oesophagitis, Barrett's oesophagus and oesophageal adenocarcinoma: a population-based case-control study. Br J Nutr. 2016;115:342–50. [DOI] [PubMed] [Google Scholar]
- 23. Shah SC, Dai Q, Zhu X, Peek RM, Smalley W, Roumie C, Shrubsole MJ. Associations between calcium and magnesium intake and the risk of incident gastric cancer: a prospective cohort analysis of the National Institutes of Health–American Association of Retired Persons (NIH-AARP) Diet and Health Study. Int J Cancer. 2020;146:2999–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bailey RL, Dodd KW, Goldman JA, Gahache JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace TC, McBurney M, Fulgoni VL. Multivitamin/mineral supplement contribution to micronutrient intakes in the United States, 2007–2010. J Am Coll Nutr. 2014;33:94–102. [DOI] [PubMed] [Google Scholar]
- 26. Ford ES, Mokdad AH.. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–82. [DOI] [PubMed] [Google Scholar]
- 27. Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DSet al. . Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Agriculture . Design and operation: the continuing survey of food intake by individuals and the Diet and Health Knowledge Survey, 1994–96. [Internet]. [Cited 12 October, 2019]. Available from: www.ars.usda.gov/ARSUserFiles/80400530/pdf/Dor9496.pdf. [Google Scholar]
- 29. Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, Brown CC, Butcher MS, Mouw T, Leitzmann Met al. . Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health–American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11:183–95. [DOI] [PubMed] [Google Scholar]
- 30. Michaud D, Midthune D, Hermansen S, Leitzmann M, Harlan L, Kipnis V, Michaud DS, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registr Manag. 2005;32(2):70–5. [Google Scholar]
- 31. Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index–2005. J Am Diet Assoc. 2008;108:1854–64. [DOI] [PubMed] [Google Scholar]
- 32. Dai Q, Shu XO, Deng X, Xiang YB, Li H, Yang G, Shrubsole MJ, Ji B, Cai H, Chow WHet al. . Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open. 2013;3:e002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rayssiguier Y, Durlach J, Gueux E, Rock E, Mazur A. Magnesium and ageing: I. Experimental data: importance of oxidative damage. Magnes Res. 1993;6:369–78. [PubMed] [Google Scholar]
- 34. Wang A, Yoshimi N, Tanaka T, Mori H. Inhibitory effects of magnesium hydroxide on c-myc expression and cell proliferation induced by methylazoxymethanol acetate in rat colon. Cancer Lett. 1993;75:73–8. [DOI] [PubMed] [Google Scholar]
- 35. Tanaka T, Shinoda T, Yoshimi N, Niwa K, Iwata H, Mori H. Inhibitory effect of magnesium hydroxide on methylazoxymethanol acetate-induced large bowel carcinogenesis in male F344 rats. Carcinogenesis. 1989;10:613–6. [DOI] [PubMed] [Google Scholar]
- 36. Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, Lebreton J, Mazur A, Rayssiguier Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta. 2000;1501:91–8. [DOI] [PubMed] [Google Scholar]
- 37. Gullestad L, Dolva LO, Søyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16:986–90. [DOI] [PubMed] [Google Scholar]
- 38. Poikolainen K, Alho H.. Magnesium treatment in alcoholics: a randomized clinical trial. Subst Abuse Treat Prev Policy. 2008;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT. The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. J Res Med Sci. 2013;18:573–9. [PMC free article] [PubMed] [Google Scholar]
- 40. Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S23–30. [DOI] [PubMed] [Google Scholar]
- 41. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5:i3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu L, Zhu X, Fan L, Kabagambe EK, Song Y, Tao M, Zhong X, Hou L, Shrubsole MJ, Liu Jet al. . Magnesium intake and mortality due to liver diseases: results from the Third National Health and Nutrition Examination Survey Cohort. Sci Rep. 2017;7:17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang W, Shu X-O, Li H, Yang G, Cai H, Ji BT, Gao J, Gao YT, Zheng W, Xiang YB. Vitamin intake and liver cancer risk: a report from two cohort studies in China. J Natl Cancer Inst. 2012;104:1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duarte-Salles T, Fedirko V, Stepien M, Trichopoulou A, Bamia C, Lagiou P, Lukanova A, Trepo E, Overvad K, Tjonneland Aet al. . Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2014;135:1662–72. [DOI] [PubMed] [Google Scholar]
- 46. Malpuech-Brugère C, Rock E, Astier C, Nowacki W, Mazur A, Rayssiguier Y. Exacerbated immune stress response during experimental magnesium deficiency results from abnormal cell calcium homeostasis. Life Sci. 1998;63:1815–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


