ABSTRACT
Background
Identification of nutrients of public health concern has been a hallmark of the Dietary Guidelines for Americans (DGA); however, a formal systematic process for identifying them has not been published.
Objectives
We aimed to propose a framework for identifying “nutrients or food components” (NFCs) of public health relevance to inform the DGA.
Methods
The proposed framework consists of 1) defining terminology; 2) establishing quantitative thresholds to identify NFCs; and 3) examining national data. The proposed framework utilizes available data from 3 key data sources or “prongs”: 1) dietary intakes; 2) biological endpoints; and 3) clinical health consequences such as prevalence of health conditions, directly or indirectly through validated surrogate markers.
Results
In identifying potential NFCs of public health concern, the 2020 DGA Committee developed a decision-tree framework with suggestions for combining the 3 prongs. The identified NFCs of public health concern for Americans ≥1 y old included fiber, calcium (≥2 y old), vitamin D, and potassium for low intakes and sodium, added sugars, and saturated fats (≥2 y old) for high intakes that were associated with adverse health consequences. Iron was identified among infants ages 6–12 mo fed human milk. For reproductive-aged and pregnant females, iron (all trimesters) and folate (first trimester) were identified for low intake, based on dietary and biomarker data (iron) or the severity of the consequence (folic acid and neural tube defects). Among pregnant women, low iodine was of potential public health concern based on biomarker data. Other NFCs that were underconsumed, overconsumed, and pose special challenges were identified across the life course.
Conclusions
The proposed decision-tree framework was intended to streamline and add transparency to the work of this and future Dietary Guidelines Advisory Committees to identify NFCs that need to be encouraged or discouraged in order to help reduce risk of chronic disease and promote health and energy balance in the population.
Keywords: dietary guidelines, nutrient, public health, nutrition policy, nutrition risk
Introduction
The US Dietary Guidelines for Americans (DGA) represent a concerted effort on the part of the Federal government to provide a set of evidence-based dietary recommendations to “help promote health and prevent chronic disease” (1, 2). These Guidelines serve as a cornerstone of Federal nutrition policy, and help to shape nutrition programs in order to best promote human health. The reach of the Guidelines also extends beyond the public sector to the food industry, the health care system, and educational sectors.
The Guidelines have been published every 5 y since their inception in 1980, but the topics addressed and the work of the Dietary Guidelines Advisory Committees (DGACs) have varied across iterations. A hallmark of the work of the DGAC is identification of “nutrients” of public health concern, based on whether they are over- or underconsumed relative to recommendations and that over- or underconsumption is linked to an adverse health outcome, either directly or indirectly. Historically, these have been referred to as “nutrients,” but have included nonnutrients such as fiber. The National Academies of Science, Engineering, and Medicine (NASEM; formerly the Institute of Medicine) defines food substances as being comprised of energy and a range of nutrient and nonnutrient dietary constituents, including, but not limited to, macronutrients, micronutrients, fiber, sugars, or other naturally occurring bioactive food components (3, 4). To be more consistent with the NASEM terminology, the proposed framework utilized the term “nutrients and food components” (NFCs) in lieu of the term “nutrients” alone. Furthermore, we utilize the term “public health relevance” throughout as an umbrella term inclusive of underconsumed, overconsumed, of “public health concern,” of “public health significance,” and those that “posed special challenges.”
Before the 2020 DGAC work, the process for identifying nutrients of public health concern was critiqued by a NASEM Committee on the DGA, because the process had not been consistent or transparent (4). This NASEM Committee recommended the harmonization of terms used to identify nutrients that are under- or overconsumed and that a transparent process be developed moving forward, to facilitate comparison across cycles of the DGA. The NASEM report also recommended that some data analysis and summary of existing data should be completed by Federal staff before convening the DGAC. The purpose of this overview is to propose an in-depth framework for transparently defining and harmonizing terminology as well as for establishing thresholds for identifying NFCs of public health relevance for use in the DGA, although the framework could be applied to other countries and different contexts. The NFCs identified as being of public health relevance by the 2020 DGAC are described to exemplify the outcome of this process.
Methods
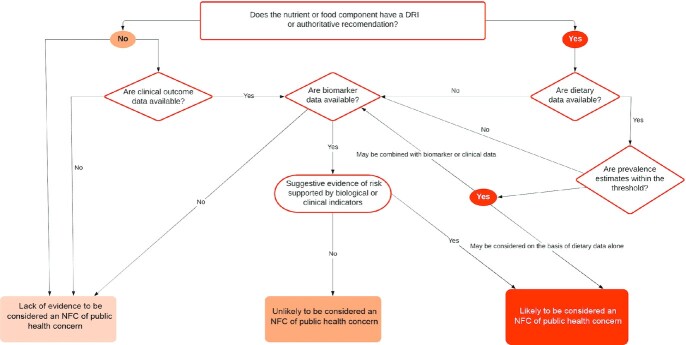
This work represents the work of the 2020 DGAC, a panel of non-Federal scientists under the auspices of the Federal Advisory Committee Act with support from Federal staff, and some portions of this work are published as part of the formal report to the US government (5). We systematically approached the identification of potential NFCs of public health concern before data were examined. We first developed a set of terminology (Table 1). We also developed a decision tree (Figure 1) in order to define NFCs to be considered for potential inadequacy or excess utilizing the “3-pronged approach” developed by previous DGACs (2) that was subsequently endorsed by the NASEM DGA review (6). The 3 prongs broadly represent 1) dietary intakes; 2) biological endpoints; and 3) clinical health consequences such as prevalence of health conditions, assessed directly or indirectly through validated surrogate markers related to NFC exposures (Table 2 provides examples). The DGAC used the totality of evidence from these data sources to the fullest extent possible in addition to information garnered from the extensive scientific efforts of the FDA with regard to labeling standards (7); NFCs for which specific questions were addressed by the 2020 DGAC; and NFCs that were previously identified by the 2015 DGAC report for Americans aged 2 y and older (2). Given that this was the first DGAC to address birth to <24 mo of age (B-24) and owing to a lack of data available that were national in scope, the 3-pronged approach for this age group was also augmented with expert opinion from DGAC subcommittee members and guided by the NASEM report on the Special Supplemental Nutrition Program for Women, Infants, and Children (i.e., WIC Report). The WIC Report identified several NFCs warranting increased or decreased consumption by infants, children, and pregnant and lactating women (Supplemental Table 1) (8).
TABLE 1.
Proposed definitions utilized in the decision-tree framework to identify NFCs1
| NFC of public health relevance: proposed term | Proposed definition |
|---|---|
| Underconsumed nutrient or food component | An NFC that is underconsumed by ≥5% of the population or in specific groups relative to the EAR, AI, or other quantitative authoritative recommendations from the diet alone.2 Underconsumed is used to replace the term “shortfall nutrient.” |
| Overconsumed nutrient or food component | An NFC that is consumed in potential excess of the UL, CDRR, or other quantitative authoritative recommendations by ≥5% of the population or in specific groups from the diet alone.2, 3 |
| Nutrient or food component of public health concern | Underconsumed and overconsumed NFCs with supporting evidence through biochemical indexes or functional status indicators, if available, plus evidence that the inadequacy or excess is directly related to a specific health condition, indicating public health relevance |
| Nutrient or food component that poses special challenges | An NFC for which it was difficult to identify at-risk groups or for which dietary guidance to meet recommended intake levels was challenging to develop |
AI, Adequate Intake, CDRR, Chronic Disease Risk Reduction; EAR, Estimated Average Requirement; NFC, nutrient or food component; UL, Tolerable Upper Intake Level.
Existing authoritative quantitative thresholds include existing Federal guidance, inclusive of the 10% energy recommendations for added sugars and saturated fats from the 2015–2020 Dietary Guidelines for Americans and the DRIs. The 5% threshold was set to identify potential food components that the Committee should examine further if biomarker or clinical data supported the potential for public health concern justification. Note this does not mean that all food components identified by this threshold were considered by the Committee to be inadequate or excessive.
For some nutrients the UL is established only from supplemental sources (e.g., magnesium) or for specific forms (e.g., folic acid).
FIGURE 1.
Decision-tree framework for identifying NFCs of public health concern. The decision path does not always start with dietary data. For example, iodine does not have dietary data available; but, it could be considered in this pathway based on biomarker or clinical data. NFC, nutrient or food component.
TABLE 2.
Life stage approach identifying NFCs of public health concern and related information on food sources by life stage1
| Three prongs used together in decision making | Top main food categories (and subcategories) contributing to current intake3 | |||
|---|---|---|---|---|
| NFC (life stages) | Dietary metric2 | Biochemical or clinical indicator | Associated health condition | |
| Fiber (ages ≥2 y) | >AI | No reliable biochemical marker exists | Coronary heart disease | Mixed dishes (burgers/sandwiches), vegetables (nonstarchy, including peas and beans), fruit (not including juice) |
| Vitamin D4 (ages ≥1 y) | <EAR | Serum 25-hydroxyvitamin D concentrations | Impaired peak bone mass accrual; low bone mass and osteoporosis | Dairy (milk, yogurt), mixed dishes (burgers/sandwiches), and protein foods (eggs) |
| Calcium4 (ages ≥2 y) | <EAR | No reliable biochemical “status” marker exists | Impaired peak bone mass accrual; low bone mass and osteoporosis | Mixed dishes (burgers/sandwiches), dairy (milk, yogurt), beverages other than milk or 100% juice (waters) |
| Potassium4 (ages ≥1 y) | >AI | 24-h urinary excretion | Hypertension and cardiovascular disease | Mixed dishes (burgers/sandwiches), vegetables (nonstarchy, including peas and beans), beverages (coffee/tea) |
| Sodium5 (ages ≥1 y) | >CDRR | 24-h urinary excretion | Hypertension and cardiovascular disease | Mixed dishes (burgers/sandwiches, rice/pasta/other grain-based mixed dishes, pizza) |
| Saturated fat5 (ages ≥2 y) | >10% TE | Total cholesterol; LDL cholesterol | Cardiovascular disease | Solid fats (animals fats/shortening/coconut and palm oils), mixed dishes (burgers/sandwiches), desserts and sweet snacks, dairy (high-fat milk and yogurt) |
| Added sugars5 (ages ≥1 y) | >10% TE | No reliable biochemical marker exists | Overweight and obesity and related comorbidities | Sweetened beverages, desserts and sweet snacks, coffee and tea (with their additions), candy and sugars, breakfast cereals and bars |
| Iron4, 6 (reproductive-aged and pregnant women) | <EAR | Serum ferritin, soluble transferrin receptor, hemoglobin | Iron deficiency and iron deficiency anemia | Various heme and nonheme dietary sources of iron are consumed |
| Iodine7 (pregnancy) | N/A | Urinary iodine concentrations | Impaired neurocognitive development | Data not available to the Committee |
| Folic acid (pregnancy, first trimester) | <EAR | Serum and RBC folate | Neural tube defects | Folic acid is the form of synthetic folate found in fortified foods and dietary supplements |
Life stages are ages 1 y or 2 y and older, including pregnant or lactating women, unless otherwise noted. AI, Adequate Intake; CDRR, Chronic Disease Risk Reduction; EAR, Estimated Average Requirement; N/A, not available; NFC, nutrient or food component; TE, total energy intake.
Low proportion exceeding the AI; high proportion exceeding the CDRR; high proportion below the EAR; high proportion exceeding 10% of TEs.
Dietary data come from NHANES 2013–2016 (n = 15,807) from day one 24-h recall; dietary data were prepared by the Federal Data Analysis Team and exclude pregnant and lactating women and children consuming breast milk. Baby foods and infant formulas were excluded from this analysis. See the Food Category Sources of Food Groups and Nutrients Data Supplement in the 2020 Dietary Guidelines Advisory Committee Report.
The FDA has also designated this NFC as of “public health significance.”
NFCs encouraged to limit. Food sources are provided as food category subgroups.
Iron requirements are higher for vegan and vegetarian diets, owing to lower iron bioavailability from plant sources.
Iodine dietary data were not available in the USDA Food and Nutrient Database for Dietary Studies at the time of the Committee's work. Goitrogens in the diet are also relevant for bioavailability.
All data examined were previously in the public domain or commissioned by the Federal government under existing Institutional Review Board approvals. No other ethical or human subject approval was required to carry out the work of the DGAC.
Terminology and quantitative thresholds
The terms “underconsumed” and “overconsumed” have been consistently utilized in the last 3 DGAC reviews (2005, 2010, and 2015); however, no quantitative thresholds were applied to define these terms. For underconsumed NFCs, the NASEM report identified a range of risk of dietary inadequacy from 9% to 69% [Table 7-2 from NASEM (6)]. Similarly, previous DGACs did not establish thresholds for the term overconsumed, but the NASEM report suggested that previous committees identified NFCs when ≥50% of 1 or multiple population groups exceeded the Tolerable Upper Intake Level (UL) or another standard for excessive intake.
The 2020 DGAC decided to use a 5% cutoff for the assessment of risk of dietary inadequacy or excess in the population in order to provide a threshold that was sufficiently low and therefore adequately sensitive as the screening criteria for the dietary data; however, this is an arbitrary value. Across all previous DGAC reports, a NFC was not elevated to be of “public health concern” unless low or high intakes (relative to the standards) were observed across the population of Americans aged 2 y and older, and unless under- or overconsumption had been linked to adverse health outcomes in the scientific literature (9).
“Nutrient that poses special challenges” was a phrase developed by the 2005 DGAC to define the food components for which dietary guidance to meet recommended intake levels was challenging to develop. The 2020 DGAC extended the term to be inclusive of NFCs for which it is difficult to identify potentially at-risk groups based on unavailability of dietary data (e.g., iodine was not in the USDA Food and Nutrient Database for Dietary Studies) or a biomarker or other endpoint to link low intakes directly to adverse outcomes (e.g., choline), but for which monitoring of low intakes should continue.
Three-pronged approach to available data
The dietary data considered by the DGAC came from the most recently available What We Eat in America portion of the NHANES, a federally coordinated program of studies designed to assess the health and nutritional status of children and adults in the United States. NHANES is a nationally representative, cross-sectional survey that samples noninstitutionalized civilian US residents using a complex, stratified, multistage probability cluster sampling design and includes interviews, questionnaires, and physical examinations conducted by the National Center for Health Statistics of the CDC. Complete details of the NHANES methodology are publicly available (10). For ages 1 y and older, the most recently available data are from NHANES 2013–2016 (n = 16,379); pregnant (n = 125) and lactating women (n = 78) were examined separately. Given the smaller sample size of the B-24 life stage, NHANES 2007–2016 were combined to achieve a sample size sufficient to examine the data together (n = 988) or stratified by infants receiving human milk (n = 141) and infants receiving any infant formula (n = 847).
Means and usual intake distributions of energy, macronutrients, and selected NFCs were computed from two 24-h recalls (24HRs). In NHANES, the first 24HR is collected in person and the second is collected by telephone, both by trained interviewers using the validated Automated Multiple-Pass Method to systematically help participants report their food and beverage intake in great detail while minimizing respondent burden (11, 12). Sample weights were used to account for differential nonresponse and noncoverage, to adjust for planned oversampling of some groups, and to adjust for uneven representation of days of the week relative to collection of the 24HR. The USDA Food and Nutrient Database for Dietary Studies was used to convert foods and beverages as consumed into their respective energy and food component values (13). Usual intake distributions were estimated using the National Cancer Institute method (14) with inclusion of covariates for the mode of administration (in person or via telephone) and the day of the week on which the 24HR was collected, dichotomized as the weekend (Friday–Sunday) or a weekday (Monday–Thursday). Population prevalence estimates of potential risk of inadequacy or excess were evaluated in comparison to the DRIs (15, 16) or other authoritative recommendations when such standards existed (2) (Box 1) for all age groups except birth to 6 mo.
BOX 1.
For NFCs with an Estimated Average Requirement (EAR), the estimated prevalence of inadequate intakes (<EAR) was determined using the cutoff method.
For NFCs with an Adequate Intake (AI), mean nutrient intakes were compared to the AI to determine the estimated prevalence >AI.
For NFCs with a UL or Chronic Disease Risk Reduction (CDRR) intake, the estimated prevalence of potentially excessive intakes was determined by examining the percentage of the population with intakes >UL or >CDRR.
For macronutrients, the estimated prevalence of the population with intakes outside of the Acceptable Macronutrient Distribution Range was evaluated.
Percentage energy contributed from added sugars and saturated fat was compared to the 2015–2020 DGA recommendations of <10% of total energy from each food component.
The DGAC first evaluated intakes from foods and beverages alone, and, when available, total usual intake distributions inclusive of dietary supplements (17). Dietary data from those who were aged birth to 12 mo, pregnant, and lactating were provided to the DGAC by the Federal Data Analysis Team.
When available, the committee considered biological endpoints or validated surrogate endpoints such as biochemical indexes to dietary intakes of nutrients (Figure 1). The DGAC further considered scientific evidence on the relation between NFC inadequacy or excess and biomarker data (e.g., LDL cholesterol, RBC folate) and/or clinical health consequences (e.g., cardiovascular disease, cancer) from multiple sources in the Federal domain [see (5) for complete details]. However, for most concentration biomarkers of nutrient status, the 2020 DGAC relied on the Second National Report on Biochemical Indicators of Diet and Nutrition, which represents data from NHANES 2003–2006 (18).
Results
Overview
To the extent possible, a life stage approach was utilized when examining the data, recognizing the special needs for certain periods of development like growth, pregnancy, and lactation. The life stages were defined either by the NASEM age groupings or by the age and life stage groupings utilized in existing Federal reports. The 2020 DGAC did not examine data on younger infants (birth to 5.9 mo). Our life stage work began with older infants (6–11.9 mo) and toddlers (12–23.9 mo), adding to the longstanding work of previous DGACs for all Americans 2 y and older. In addition, within the 2 y and older age group, several life stage subgroups were separately examined including, but not limited to, pregnant and lactating women; adolescents; and older adults. Although data were also examined by race and Hispanic origin and income based on the income-to-poverty ratio, it should be noted that decisions on which NFCs were of public health concern were largely based on the life stage approach.
NFCs identified by life stage
The Committee identified a number of NFCs of public health concern in the general population or within certain life stages based on the proposed framework utilizing the 3-pronged data approach (Table 2). The 2020 DGAC first examined and confirmed previous NFCs of public health concern among all Americans, ages 2 y and older, identified by the 2015–2020 DGA including fiber, calcium, vitamin D, and potassium due to low consumption and sodium, saturated fats, and added sugars due to overconsumption (Supplemental Tables 2, 3, as summarized from Reference 5). Table 2 outlines the health outcomes associated with these NFCs. Calcium and saturated fat were not identified as NFCs of public health concern for children under the age of 2 y.
For other NFCs that are currently underconsumed from foods and beverages, either among all Americans or within certain population subgroups (in addition to those identified as of concern), dietary intakes were not directly or indirectly confirmed through validated surrogate biomarkers and were identified only as under- or overconsumed NFCs (Table 3). Depending on life stage, some NFCs were listed as special challenges, owing to either lack of health outcome data (e.g., choline) or lack of available EAR values, which was the case among infants.
TABLE 3.
NFCs examined and identified as of public health relevance in the 2020 Dietary Guidelines Advisory Committee Report according to category and life stage among the US population1
| Underconsumed | Overconsumed | Special challenges | Public health concern |
|---|---|---|---|
| Compared to EAR• Protein2 | Compared to UL2 (ages 6–12 mo consuming infant formula) | 6–12 mo old• Vitamin D | 6–12 mo old• Iron (breastfed infants) |
| • Vitamin A2 | • Retinol | • Potassium | All Americans, ≥1 y old |
| • Thiamin2 | • Zinc | • Choline | • Vitamin D |
| • Riboflavin2 | Compared to UL2 (ages 1–3 y) | 12–24 mo | • Calcium |
| • Niacin2 | • Copper | • Choline | • Potassium |
| • Vitamin B-62 | • Retinol | • Linoleic acid | • Sodium |
| • Folate2 | • Zinc | All Americans, ≥1 y old | • Added sugars |
| • Vitamin B-122 | • Selenium | • Choline | All Americans, ≥2 y old |
| • Vitamin C2 | Compared to CDRR | • Saturated fats | |
| • Vitamin D | • Sodium | • Fiber | |
| • Vitamin E2 | Compared to 2015–2020 DGA | Reproductive-aged women3 | |
| • Calcium | • Saturated fat2 | • Iron | |
| • Copper2 | • Added sugars | Pregnant women3 | |
| • Iron2 | • Folic acid (first trimester) | ||
| • Magnesium2 | • Iron | ||
| • Phosphorus2 | • Iodine | ||
| • Selenium2 | |||
| • Zinc2 | |||
| Compared to AI | |||
| • Fiber | |||
| • Choline | |||
| • Potassium | |||
| • Vitamin K2 | |||
| Compared with biomarker data | |||
| • Iodine |
Children aged 12–24 mo were most often categorized with the ages ≥1 y for analysis of the dietary data. The term “public health relevance” is used throughout as an umbrella term inclusive of underconsumed, overconsumed, of “public health concern,” of “public health significance,” and “posed special challenge.” AI, Adequate Intake; CDRR, Chronic Disease Risk Reduction; DGA, Dietary Guidelines for Americans; EAR, Estimated Average Requirement; NFC, nutrient or food component; UL, Tolerable Upper Intake Level.
Only low dietary estimates in certain age-sex groups that were not confirmed with other prongs of the framework to enable elevation of under- or overconsumption to “public health concern.”
In addition to NFCs identified for all Americans.
The Committee discussed at length whether energy (i.e., caloric intake) should be labeled as being of public health concern given the extent and severity of overweight and obesity, but ultimately decided against labeling it specifically in this way given the complex and multifactorial causes of these conditions, and recognizing the substantial measurement error of assessing total energy intakes through existing dietary assessment methods. Nevertheless, energy balance is a key component of weight status, and options for highlighting this, specifically in the DGA, should be considered.
Birth to 24 mo
The DGAC identified NFCs of public health concern that were primarily but not perfectly consistent with those listed by the NASEM WIC panel for infants, toddlers, young children, and pregnant and lactating women (Supplemental Table 1). For older infants aged 6–11.9 mo, EAR values only exist for iron, zinc, and protein. For older infants fed human milk, iron was identified by the 2020 DGAC as an NFC of public health concern based on a high prevalence of dietary inadequacy (77%), combined with biomarker data supportive of iron inadequacy in older age groups (19). However, although dietary intakes of protein (27% <EAR) and zinc (54% <EAR) were identified as low among human milk–fed infants, dietary estimates are not supported by biochemical, clinical, or health consequences to date to confirm that low dietary intakes are of public health concern among human milk–fed infants. In terms of NFCs without established EAR values, the AI is assumed to exceed the RDA for a nutrient, if one could be established. Thus, in applying the AI, the proportion of a group that exceeds the AI should reflect those who have adequate intakes, but there is no scientific basis to state that the proportion of intakes lower than the AI is an estimate of the prevalence of inadequacy (20). Nevertheless, the DGAC identified potassium, vitamin D, and choline as NFCs being low relative to the AI among all infants, regardless of primary milk feeding mode; no clinical or biomarker data are available in this age group that are national in scope. Moreover, infants fed infant formula (including mixed-fed infants) were at risk of potentially high intakes of zinc (77% >UL) and retinol (23% >UL) from foods (and formula) alone; but, again, no overt clinical data supported the need to label these high dietary intakes as being of public health concern among older infants.
Starting at 12 mo, when the primary source of energy for young children transitions from human milk or infant formula to “table foods,” higher than recommended intakes of sodium and added sugars, and low intakes of potassium, fiber, and vitamins D and E from foods alone were observed in the NHANES and other data sources (21). Based on nutrient intake distributions from foods alone, choline (49% >AI) and linoleic acid (18:2n–6) (39% >AI) were also shortfall dietary components that require further investigation. Between 12 and 24 mo, calcium intake is generally adequate. The NASEM panel also prioritized the nutrients to limit, including sodium and added sugars for toddlers (Supplemental Table 1) (8). Our review confirmed these high intakes from the Federal data sources. Whereas saturated fat limits have been recommended for ages 2 y and older, the DGAC did not make recommendations on saturated fat intakes among those in the B-24 population subgroup. Young children, ages 1–3 y, overconsume retinol, zinc, selenium, and copper relative to the UL, but these intakes have not been linked directly to adverse health outcomes and should continue to be monitored.
Pregnancy and lactation
The same nutrients of public health concern identified among all Americans 2 y and older were identified among pregnant and lactating women. Biomarker data, together with dietary data for nonpregnant similarly aged women, suggest that iron is of public health concern during pregnancy across all trimesters and among reproductive-aged women. Based on the high risk of neural tube defects, folate/folic acid should remain of concern given the high prevalence of dietary inadequacy among pregnant women (first trimester only). Iodine was identified as a potential NFC of public health concern among pregnant women given that some biomarker data suggest low dietary intakes, and that inadequate iodine intake during pregnancy is related to irreversible impaired neurological and behavioral development for the offspring. Although dietary data are not available for iodine, median urinary iodine concentrations (UICs) of pregnant women remain below the WHO cutoff for “insufficiency” (<150 μg/L) (22); depending on the NHANES survey years used the UIC is 144 μg/L (23) or 148 μg/L (24).
Adolescents and reproductive-aged women
In addition to those noted for all Americans, adolescents (ages 9–18 y) have a constellation of dietary risk including low dietary intakes from foods and beverages of iron (girls), protein (girls), folate (girls), vitamin B-6, vitamin B-12 (girls), phosphorus, magnesium, and choline. Among adolescents, <3% exceed the AI for choline, demonstrating widespread underconsumption. Although none of these individual NFCs alone was elevated to public health concern, the widespread low intakes of many NFCs in this life stage should continue to be monitored.
Although the risk of inadequate dietary intake of iron is low in the general population (6% <EAR), iron intake is problematic among adolescent girls (14–18 y) and reproductive-aged women (19–50 y): ∼20% of these population subgroups are at risk of inadequate intake of dietary iron and there is evidence of iron deficiency based on low serum ferritin concentrations (20% among ages 12–19 y and 16% for ages 20–49 y). Therefore, iron was identified to be of public health concern among adolescent and premenopausal females.
Older adults
Older adults may be at risk of low intakes or status for protein, vitamin B-12, and vitamin B-6. The Committee noted a high prevalence of sarcopenia, as assessed by age-adjusted prevalence of reduced muscle strength among older adults (25). Calcium and vitamin D continue to be of public health concern in this age group, especially among women, because of the high prevalence of low bone mass and osteoporosis (26). About 1 in 4 older women (23%) has at-risk dietary intakes of vitamin B-6 (17). Previous NHANES analyses identified 13% of older women with low pyridoxal 5′-phosphate, an indicator of vitamin B-6 status (18); however, no adverse clinical indicators were documented. Similarly, vitamin B-12 status has been related to cognitive function; although this committee did not specifically address cognitive health data and biomarker data from NHANES, 8% of older women have low dietary intakes of B-12, and prevalence estimates for low B-12 status are difficult to interpret owing to limitations in existing biomarkers (18, 27). Thus, future DGACs may wish to further examine data on these 2 vitamins, if available.
Foods and beverage intake patterns
The NFCs identified by this framework result from the fact that half or more of energy intake comes from a limited number of food sources including burgers and sandwiches, desserts and sweet snacks, rice/pasta/other grain-based mixed dishes, sweetened beverages, and chips/crackers/savory snacks (5) (see Table 2). This lack of dietary diversity is driving the imbalances noted in NFCs because these foods contribute high amounts of sodium, saturated fats, and added sugars, often with little contribution to vitamins, minerals, and fiber. For example, burgers and sandwiches are ubiquitously consumed and so are a primary source of many NFCs, but are not necessarily highly ranked in amounts provided per 100 g for some NFCs like calcium, vitamin D, or potassium (2). Fruits and vegetables are concentrated sources of potassium (2) and dairy is a rich source of calcium and vitamin D (2), but the current consumption patterns of Americans are low in fruits, vegetables, and dairy.
Discussion
The DGA recommend dietary patterns that are developed based on nutrient adequacy, energy balance, and reducing risk of diet-related chronic diseases. The focus on dietary patterns recognizes that foods and beverages are not consumed in isolation and the health benefits of dietary patterns are attributable to the cumulative relation among NFCs in relation to health status and risk of chronic disease. Evaluation of the NFCs in dietary patterns can provide a tool to assess certain aspects of the overall nutritional quality of the dietary pattern. In addition, the DGA include recommendations specific to NFCs as part of healthful dietary patterns.
The purpose of developing a framework is to have a transparent and systematic process to identify NFCs and scientific gaps that informs the development of the DGA and the work of future DGACs. This framework is a proposal that will harmonize terminology and provide quantitative thresholds for prevalence estimates obtained from dietary data. The proposal defines the terms and describes the DGAC process to confirm the impact of over- or underconsumed self-reported dietary intakes with a biomarker or clinical or health outcome data. Many NFCs were identified as underconsumed or overconsumed but, when examined relative to other metrics, were not elevated to “public health concern.” For example, vitamins A and E were identified as underconsumed (the previous terminology was shortfall) relative to the EAR; however, when biochemical indexes were examined, <1% of the US population had low biomarker values, and adverse clinical outcomes (upon which the DRIs were established) were not observed broadly in the population. A disconnect between the prevalence of “at risk” based on dietary intakes and the prevalence of “deficiency” based on biochemical estimates was also observed for several other NFCs (e.g., vitamin D, folate, vitamin B-12) among certain population subgroups. The DGAC had to rely on biochemical estimates from NHANES 2003–2006 in making such determinations in some cases, and more recent biochemical data are needed.
In developing this systematic framework, several data gaps or scientific issues emerged. First, some DRI values may not reflect the totality of more current evidence. Second, in many cases an EAR has not been established and the DGAC had to rely on AI values. AI values exist when data are not sufficient to identify an EAR and thus an assessment of low dietary intakes is difficult; this was especially true for infants. Third, valid nutritional biomarkers do not exist for some nutrients, and this hampered our ability to confirm whether low intakes were indeed problematic (e.g., choline, zinc). Fourth, with the exception of vitamin D, the cutoff used to define low status has not been aligned with the EAR values. Most cutoffs for nutritional biomarkers are utilized to identify frank deficiency whereas the EAR is used to identify potential risk of inadequacy, representing different points on the spectrum of true status for an NFC (28). Even in the case of vitamin D, for which there are concerted efforts to harmonize the DRI cutoffs used for the biomarker and the dietary data, large discrepancies in prevalence estimates exist, given that there is wide variation in serum 25-hydroxyvitamin D by geographic location (i.e., UV exposure), adiposity, and race and ethnicity. Fifth, only small sample sizes were available for infants, and pregnant and lactating women, which hampered a thorough assessment among these age groups. Sixth, the DGAC generally examined exposures to NFCs from the diet alone, although for population subgroups with high prevalence of use of dietary supplements, such as during infancy, pregnancy, and lactation, total intake distributions were examined when available or compared with other NHANES publications (29, 30). Not including intakes of NFCs from dietary supplements would overestimate the prevalence of inadequacy and underestimate the population prevalence of intakes exceeding the UL (31–34). Both the 2010 and 2015 DGACs used a >3% cutoff for potentially excessive intakes, whereas the 2020 DGAC used a >5% cutoff as the threshold. Finally, the DGAC relied only on information in the Federal domain and published by Federal authors.
The framework has other limitations that must be considered. Although the proposed decision tree outlines quantitative limits on dietary data, quantitative thresholds for the other 2 prongs were not proposed, and are likely needed moving forward. Furthermore, the framework was designed to be used for each individual NFC. Our Committee identified adolescent girls as having low intakes of several nutrients, but the framework, as proposed, only examines 1 NFC at a time, rather than multiple NFCs in a particular population subgroup. Future frameworks may need to consider patterns of low or high dietary intakes of multiple NFCs simultaneously in population subgroups.
In summary, in identifying potential NFCs of public health concern, there is not a single “best” approach recommended. Instead, we provide a framework with suggestions for combining multiple types of available data to make decisions for DGAC reports. We propose that this framework will facilitate a transparent and streamlined approach to examining the totality of evidence to identify NFCs of public health concern and to identify data gaps across the life course. Toward that end, we built a core set of terminology to standardize the language, informed by the work of prior DGACs, and establish quantitative thresholds for terminology. The proposed framework is intended to streamline the work of future Committees to identify the critical NFCs that need to be encouraged or discouraged in order to fulfill the purpose of the DGA, which is to help reduce risk of chronic disease and promote health and energy balance in the US population.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—RLB: wrote the paper and has primary responsibility for the final content; and all authors: worked to develop the proposed framework, reviewed the data prepared by the Federal Data Analysis Team and other existing sources, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: the authors report no conflicts of interest. RLB, TAD, and JSS are current members of the Editorial Board of The Journal of Nutrition.
The findings and conclusions are those of the authors and do not represent the views of their respective universities or any entity of the US Government. This work was completed as part of the Federal Advisory Committee Act.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AI, Adequate Intake; B-24, birth to <24 mo of age; CDRR, Chronic Disease Risk Reduction; DGA, Dietary Guidelines for Americans; DGAC, Dietary Guidelines Advisory Committee; EAR, Estimated Average Requirement; NASEM, National Academies of Science, Engineering, and Medicine; NFC, nutrient or food component; UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children; 24HR, 24-h recall.
Contributor Information
Regan L Bailey, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Jamy D Ard, Department of Epidemiology and Prevention, Wake Forest School of Medicine, Winston Salem, NC, USA.
Teresa A Davis, USDA/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Tim S Naimi, Section of General Internal Medicine, Boston University Medical Center, Boston, MA, USA.
Barbara O Schneeman, (Emeritus) Department of Nutrition, University of California, Davis, Davis, CA, USA.
Jaime S Stang, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota—Twin Cities, Minneapolis, MN, USA.
Kathryn G Dewey, (Emeritus) Department of Nutrition, University of California, Davis, Davis, CA, USA.
Sharon M Donovan, Department of Food Science and Human Nutrition, University of Illinois, Urbana-Champaign, IL, USA.
Rachel Novotny, Department of Human Nutrition, Food and Animal Sciences, University of Hawaiʻi at Mānoa, Honolulu, HI, USA.
Linda G Snetselaar, Department of Preventive Nutrition Education, University of Iowa, Iowa City, IA, USA.
Janet de Jesus, Office of Disease Prevention and Health Promotion, NIH, US Department of Health and Human Services, Rockville, MD, USA.
Kellie O Casavale, Office of Nutrition and Food Labeling, Center for Food Safety and Applied Nutrition, FDA, US Department of Health and Human Services, College Park, MD, USA.
TusaRebecca Pannucci, Center for Nutrition Policy and Promotion, Food and Nutrition Services, USDA, Alexandria, VA, USA.
Eve E Stoody, Center for Nutrition Policy and Promotion, Food and Nutrition Services, USDA, Alexandria, VA, USA.
References
- 1. US Department of Health and Human Services and US Department of Agriculture . About the Dietary Guidelines. [Internet]. Rockville (MD): Office of Disease Prevention and Health Promotion; October 2019. [Cited 2020 Jul 16]. Available from: https://health.gov/our-work/food-nutrition/about-dietary-guidelines. [Google Scholar]
- 2. US Department of Health and Human Services and US Department of Agriculture . Dietary Guidelines for Americans 2015–2020. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 3. Yetley EA, MacFarlane AJ, Greene-Finestone LS, Garza C, Ard JD, Atkinson SA, Bier DM, Carriquiry AL, Harlan WR, Hattis Det al. Options for basing Dietary Reference Intakes on chronic disease endpoints: report from a joint US-/Canadian-sponsored Working Group. Am J Clin Nutr. 2017;105:249S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Academy of Sciences, Engineering, and Medicine . Food and Nutrition Board: guiding principles for developing Dietary Reference Intakes based on chronic disease. Washington (DC): Institute of Medicine; 2017. [PubMed] [Google Scholar]
- 5. The 2020 Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee. Washington (DC): USDA, Agricultural Research Service; 2020. [Google Scholar]
- 6. National Academy of Sciences, Engineering, and Medicine (NASEM) . Recent approaches to assessing nutritional adequacy and exploring chronic disease. In: Redesigning the process for establishing the Dietary Guidelines for Americans. Washington (DC): The National Academies Press; 2017. p. 189–210. [Google Scholar]
- 7. Food and Drug Administration, HHS . Food labeling: revision of the Nutrition and Supplement Facts Labels. Final rule. Fed Regist. 2016;81:33741–999. [PubMed] [Google Scholar]
- 8. National Academies of Sciences, Engineering, and Medicine (NASEM) . Review of WIC food packages: improving balance and choice: final report. Washington (DC): The National Academies Press; 2017. [PubMed] [Google Scholar]
- 9. The Secretary of Health and Human Services and the Secretary of Agriculture . Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Washington (DC): Office of Disease Prevention and Health Promotion, US Department of Health and Human Services; 2015. [Google Scholar]
- 10. National Center for Health Statistics (NCHS) . About the National Health and Nutrition Examination Survey. [Internet]. Hyattsville (MD): NCHS; 2017. [Cited 2020 Aug 17]. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 11. Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136:2594–9. [DOI] [PubMed] [Google Scholar]
- 12. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LAet al. The USDA Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 13. USDA Agricultural Research Service . USDA Food and Nutrient Database for Dietary Studies 2011–2012. [Internet]. Food Surveys Research Group Home Page. Beltsville (MD): Food Surveys Research Group; 2017. [Cited 2020 Jun 11]. Available from: http://www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
- 14. Tooze J, Midthune D, Dodd K, Freedman L, Krebs-Smith S, Subar A, Guenther P, Carroll R, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc. 2006;106:1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Academies of Sciences, Engineering, and Medicine, Food and Nutrition Board . Dietary Reference Intakes: applications in dietary assessment. Washington (DC): Institute of Medicine; 2000. [Google Scholar]
- 16. National Academies of Sciences, Engineering, and Medicine . Stallings VA, Harrison M, OriaM,editors. Dietary Reference Intakes for sodium and potassium. Washington (DC): The National Academies Press; 2019. [PubMed] [Google Scholar]
- 17. USDA Agricultural Research Service . Usual nutrient intake from food and beverages, by gender and age, What We Eat in America, NHANES 2013–2016. Beltsville (MD): Food Surveys Research Group; 2019. [Google Scholar]
- 18. Pfeiffer CM, Sternberg MR, Schleicher RL, Haynes BM, Rybak ME, Pirkle JL. The CDC's second national report on biochemical indicators of diet and nutrition in the U.S. population is a valuable tool for researchers and policy makers. J Nutr. 2013;143:938S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta PM, Hamner HC, Suchdev PS, Flores-Ayala R, Mei Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am J Clin Nutr. 2017;106:1640S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey RL, Murphy SP, Weaver CM. Using the Dietary Reference Intakes to assess intakes. In: Van Horn L, BetoJA,editors. Research: successful approaches in nutrition and dietetics. 4th ed. Chicago (IL): Academy of Nutrition and Dietetics; 2019.p. 294–310. [Google Scholar]
- 21. Ahluwalia N, Herrick KA, Rossen LM, Rhodes D, Kit B, Moshfegh A, Dodd KW. Usual nutrient intakes of U.S. infants and toddlers generally meet or exceed Dietary Reference Intakes: findings from NHANES 2009–2012. Am J Clin Nutr. 2016;104:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization . Urinary iodine concentrations for determining iodine status in populations. [Internet]. Geneva: WHO; 2013. [Cited 2020 Apr 1]. Available from: http://www.who.int/nutrition/vmnis/indicators/urinaryiodine. [Google Scholar]
- 23. Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid. 2013;23:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gahche JJ, Bailey RL, Mirel LB, Dwyer JT. The prevalence of using iodine-containing supplements is low among reproductive-age women, NHANES 1999–2006. J Nutr. 2013;143:872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Looker AC, Wang C-Y. Prevalence of reduced muscle strength in older U.S. adults: United States, 2011–2012. NCHS Data Brief. 2015;(179):1–8. [PubMed] [Google Scholar]
- 26. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94:552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raghavan R, Ashour FS, Bailey RL. A review of cutoffs for nutritional biomarkers. Adv Nutr. 2016;7:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey RL, Pac SG, Fulgoni VL 3rd, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open. 2019;2:e195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, Cowan AE, Jun S, Eicher-Miller HA, Guenther PM, Bhadra A, Thomas PRet al. Best practices for dietary supplement assessment and estimation of total usual nutrient intakes in population-level research and monitoring. J Nutr. 2019;149:181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bailey RL, Fulgoni VL 3rd, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents?. J Pediatr. 2012;161:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bailey RL, Fulgoni VL 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr. 2011;94:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bailey RL, Fulgoni VL 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet. 2012;112:657–63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.