Abstract
Prostate cancer (PCa) is the second most common cancer among men in the United States. While the use of prostate-specific antigen has improved the ability to screen and ultimately diagnose PCa, there still remain false positives due to noncancerous conditions in the prostate gland itself and other prognostic biomarkers for PCa are needed. Contents within extracellular vesicles (EVs) have emerged as promising biomarkers that can give valuable information about disease state, and have the additional benefit of being acquired through noninvasive liquid biopsies. Meaningful communication between cancer cells and the microenvironment are carried by EVs, which impact important cellular processes in prostate cancer such as metastasis, immune regulation, and drug resistance.
Keywords: Extracellular vesicles, exosomes, biomarkers, prostate cancer
Extracellular Vesicles
Extracellular vesicles (EVs) are now recognized as important components of intercellular communication in their transfer of proteins, lipids, and nucleic acids (1). These EVs represent a heterogeneous mix of cell-derived membranous vesicles, subclassified as exosomes, microvesicles, and apoptotic bodies that range in size, protein composition, and mechanism of release from cells. Exosomes are the smallest type of EVs, generally ranging from 30 to 120 nm in diameter and are released from the inward budding of endosomal membranes. Microvesicles, also known as ectosomes, can overlap in size with exosomes being 100 to 1000 nm in diameter, but are released from the cell by budding and pinching outward from the plasma membrane (2). Apoptotic bodies range from 500 to 2000 nm in diameter and are released in the final steps of apoptosis during plasma membrane blebbing (3). While there is much yet to be discovered about the role of apoptotic bodies, this subtype seems to have less of a role in cell–cell communication (4). As such, our review will focus on the exosome and microvesicle subpopulations, and we will refer to both simply as EVs. EVs can be found in common bodily fluids such as saliva (5), blood (6), and urine (7). As the content of EVs reflect their cell of origin (8), this gives EVs potential for use as biomarkers derived from noninvasive liquid biopsy techniques. As intercellular communicators, they have been shown to induce phenotypic changes in recipient cells (9) and have multiple roles in cancer progression, including metastasis, immune cell regulation, and drug resistance.
Brief Summary on Prostate Cancer
Prostate cancer (PCa) is the second leading cause of cancer death among men in the United States with approximately 1 in 8 men at risk of developing this disease (10). PCa is a heterogeneous disease as those who are diagnosed vary in age and face different clinical severities. This further complicates the etiology of PCa; however, several risk factors may contribute, including but not limited to age (mortality of PCa correlates with increasing age), race (African Americans have the highest rates of PCa), and a family history of PCa (11-13).
Starting in the early 1990s, PCa incidence spiked to all-time highs and has since plateaued. The spike in incidence has been attributed to the use of prostate-specific antigen (PSA) testing (14). PSA testing was approved by the U.S. Food and Drug Administration in 1994 in conjunction with a digital rectal exam to test asymptomatic men for PCa. While this test is the gold standard for screening men with PCa, PSA levels can increase through noncancerous clinical conditions, such as inflammation, infection, trauma, prostate manipulation, and benign prostatic hypertrophy (15). The positive predictive value for PSA is approximately 25% to 40% (16) and it is known that PSA testing does contribute to an increase in false-positive PCa cases. Additional markers alongside PSA to improve specificity of diagnosing PCa would be beneficial in preventing overdiagnosis and overtreatment. Multiple studies have recognized significant differences in the content of EVs between noncancerous patients and those with PCa (17-19), indicating that EVs have the potential to be clinically relevant in detecting PCa. However, to date there has been little overlap of the nominated biomarkers, and determining the robustness of such biomarkers will be the primary challenge in using EVs in a clinical setting.
Nominated EV biomarkers for prostate cancer
RNA
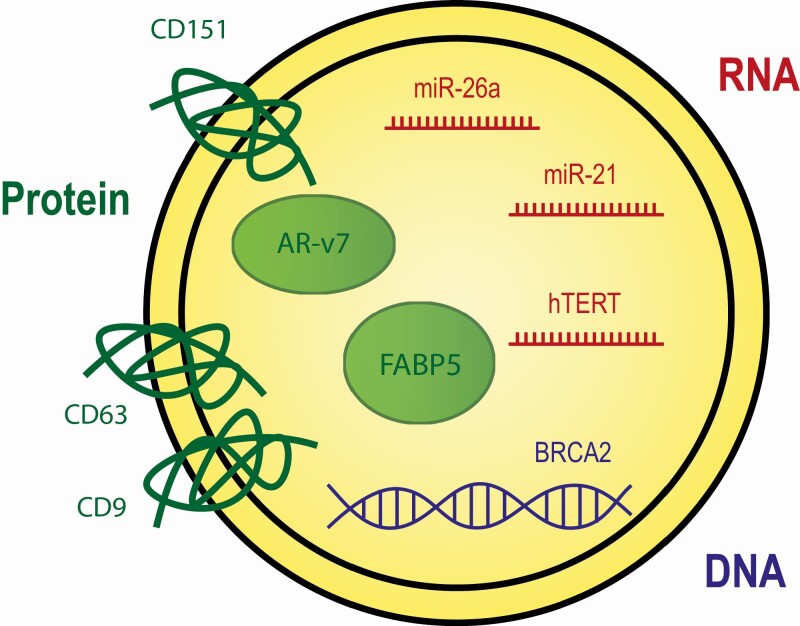
Much work has been done to characterize the RNA cargo in EVs and has identified many mRNA and miRNAs, although other RNA types such as tRNAs and rRNAs have also been found (20). The role of miRNAs in the initiation and progression of PCa (21) has made this type of cargo of particular interest for biomarker development. Here we discuss several miRNAs and mRNAs of interest in the context of their potential use for biomarkers as well as their functional relevance to PCa (Fig. 1). See Table 1 for additional notable RNAs that are upregulated in EVs of PCa.
Figure 1.
Key markers in prostate cancer EVs. The schematic depicts an extracellular vesicle with cargo that references important markers for EVs in prostate cancer. RNA cargo is depicted in red, DNA in blue, and protein in green.
Table 1.
Upregulated RNAs in prostate cancer extracellular vesicles
| RNAs | Functional relevance |
|---|---|
| miR-409(102) | Represses tumor suppressor genes Ras suppressor 1 and stromal antigen 2, contributes to epithelial to mesenchymal transition |
| miR-142-3p(103) | Regulates FOXO1 for cell proliferation (104) |
| miR-106a (105) | Downregulates PTEN (106) |
| miR-1246(107) | Associated with cell proliferation and EMT |
| miR-19b-3p(108) | Targets CFL2, involved with cell proliferation |
| miR-940(109) | Targets ARHGAP1 and FAM134A, promotes osteogenic differentiation |
| miR-141(110) | Associated with metastatic PCa, unknown function |
| miR-1290(111) | Possibly targets NAT1 and FOXA1 |
| miR-2909(112) | Possibly targets MALT1, KLF4, and UPC2, involved in metabolism and immune regulation |
| TMPRSS2:ERG (113) | Promotes invasion |
| BRN2, BRN4(114) | Neural transcription factors that drive neuroendocrine prostate cancer |
List of upregulated RNAs in extracellular vesicles that have been nominated as potential biomarkers for prostate cancer, and includes known functions of the RNAs
Oncogenic miRNAs, such as miR-21, miR-141, and miR-375, were found to be upregulated in EVs from urine in PCa patients compared with healthy controls (22). These 3 miRNAs have been previously suggested to be useful diagnostic markers for PCa, having also been found to be upregulated in serum of PCa patients (23). Both miR-21 and miR-141 are involved in the regulation of osteogenesis (24), and miR-21 can activate the immune system through toll like receptors, which contributes to tumor growth and metastasis (25).
Another miRNA that has been recognized as a potential biomarker is miR-26a, which has been shown to be downregulated in PCa tissue (26). Recently, a study done by Urabe et al. demonstrated that miR-26a is involved in the secretion of EVs in PCa cells, and that miR-26 suppresses the expression of SHC4, PFDN4,and CHODC1 which were shown to be increased in PCa tissues and are involved in the biogenesis of EVs (27). Forced expression of miR-26a in PCa cells inhibited cell proliferation (26) and halted tumor progression in in vivo mouse studies (27), likely through the restoration of regulating EV secretion.
The activation of telomerase (hTERT) is one of the hallmarks of cancer (28), and has been of interest as a potential biomarker for detection of cancer initiation and progression. The prevalence of hTERT in serum has been noted for PCa as well as many other types of cancer including breast, colon, and hepatocellular carcinoma (29-33). A pan-cancer study that included cases with prostate cancer measured the concentration of EV-derived hTERT mRNA in the sera of patients and found that 62% of patients with solid tumors expressed hTERT mRNA in their EVs, 60% with hematological malignancies exhibited hTERT mRNA in their EVs, while no signs of hTERT mRNA were detected in the healthy control group (34).
Proteins
The implementation of mass spectrometry has identified thousands of proteins from EVs (35). These include proteins involved in EV structure, such as the tetraspanins CD9, CD63, and CD81, which are frequently used to identify EVs (36), and also the protein cargo that EVs carry. Here we discuss both structural and cargo proteins that may have utility as biomarkers in PCa (Fig. 1).
For PCa, the activity of androgen receptor (AR) is of particular importance for tumor progression. The truncated splice variant AR-v7 generates a transcript that lacks the C-terminal ligand binding domain, leading to ligand independent activity (37). Expression of AR-v7 is a known biomarker for resistance to androgen-targeted treatments enzalutamide and abiraterone (38-40). Notably, both full length AR and AR-v7 have been identified in PCa EVs (41, 42), and both of these receptors have been shown to be transferred to AR-null cells and stimulate AR signaling (43). This suggests that AR-v7 expression in EVs may be useful in predicting or monitoring resistance to androgen-targeted therapies.
A study by Fujita et al. demonstrated that EVs isolated from urine containing Fatty Acid Binding Protein 5 (FABP5) were significantly associated with Gleason score 7 and higher. The receiving operator characteristic curve analysis in the study showed that the area under the curve for the prediction of Gleason score ≥7 by FABP5 was 0.856 (95% CI 0.708-1.00, P = .002), whereas the area under the curve value for prediction by serum PSA was 0.511 (95% CI 0.280-0.757, P = .87) (44). FABP5 is an intracellular lipid-binding protein that transports fatty acids, and it is known that overexpression of FABP5 in PCa tissues correlates with poor patient survival (45, 46). That FABP5 is also secreted into EVs and therefore capable of being monitored via liquid biopsy makes it a potentially promising prognostic biomarker for advanced or aggressive PCa.
Proteomic analyses of EVs from the PC3 prostate adenocarcinoma cell line identified CDCP1 and CD151 as promising PCa biomarkers due to their specificity in expression compared with epithelial cells (47). The tetraspanin CD151 regulates cell migration through its interaction with matrix metalloproteinases, and is critical in the early steps of forming metastatic lesions (48, 49). Expression of CD151 in PCa tissue increases during disease progression, is associated with poor prognosis, and has a better predictive value for the clinical outcome of low-grade PCa patients than traditional histologic grading methods (47). It is worth exploring if CD151 in PCa EVs also has a similar predictive value as a biomarker in this clinical setting. CDCP1’s main function is as an anti-apoptotic molecule that facilitates tumor cell survival during metastasis (50). In PCa, the inhibition of CDCP1 through the monoclonal antibody 25A11 inhibited metastasis of the PC3 cell line in a subcutaneous mouse xenograft model (51). The expression of CDCP1 in EVs was significantly higher in PC3 cells than in benign prostate or nonmetastatic cells (52), suggesting that levels of CDCP1 could be useful as a marker of metastatic potential.
While individual proteins found in EVs may be useful biomarkers as described above, it is more likely that a panel of EV proteins will provide greater specificity and sensitivity than a single EV protein. Using targeted proteomics on EVs isolated from urine, the combination of adseverin and transglutaminase was able to differentiate benign and PCa, including both low and high risk (53). Additionally, the 5-panel combination of CD63/GLPK5/SPHM/PSA/PAPP was able to discriminate between low-risk and high-risk PCa, and interestingly the expression of all 5 proteins was lower in the high-risk PCa cohort (53). This study speaks to the great potential of EV proteins as versatile biomarkers for many stages of PCa progression.
DNA
A pioneering study in 2011 demonstrated that EVs contain DNA; it was previously thought that only proteins and RNAs were present (54). While it is now well accepted that genomic DNA fragments, including mutations from the cell of origin, are contained in EVs derived from PCa patients (55-57), this is considered to be a little studied area for this cancer type. It must be noted that studies have shifted to analyzing circulating tumor DNA (ctDNA) or cell-free DNA (cfDNA) vs EV-containing DNA. However, ctDNA/cfDNA data would likely include EV-containing DNA, and results of these studies may implicate the important role of EVs when assessing ctDNA/cfDNA. Targeted sequencing of ctDNA from over 800 metastatic PCa samples observed that the most frequent genetic aberrations were from the genes BRCA2, ATM, and CDK12 and that loss of BRCA2 and CDK12 but not ATM was associated with poor prognosis (58). These results may be extrapolated to EVs where ctDNA data and other DNA alteration data could be used as possible biomarkers for PCa. Also, it has been observed that oncosomes, which are large vesicles (1-10 µm), (59) contain more genomic DNA content than exosomes and microvesicles (60) and so may be of more use in developing DNA biomarkers than the types of EVs that we have discussed in this review.
Functional Roles of EVs
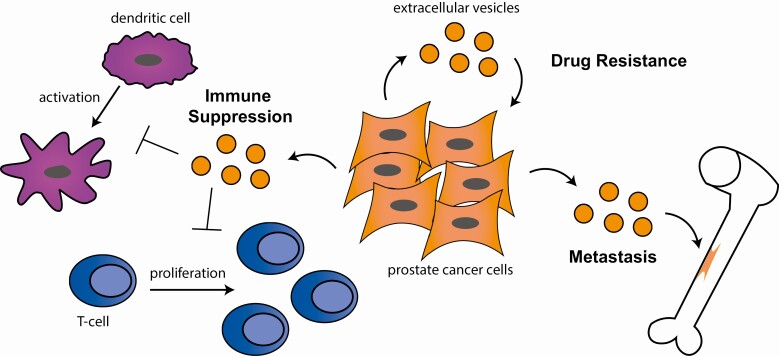
While EVs have potential to be clinically useful as biomarkers for several stages of PCa progression, there is much yet to be discovered about the functional roles EVs may have. We now understand EVs as important mediators of cell to cell communication and not simply exporting “junk” cargo. Here we discuss the impact EVs can have on important aspects of cancer progression such as metastasis, immune activation, and drug resistance (Fig. 2).
Figure 2.
Functional roles of EVs in prostate cancer. Schematic that depicts extracellular vesicles (orange) coming from prostate cancer cells and their role in promoting metastasis to the bone, promoting drug resistance through cell to cell signaling, and suppression of the immune system through inhibiting dendritic cell activation and inhibiting T-cell proliferation.
Technical challenges
A significant barrier in the study of EVs is the heterogeneity of the subpopulations and the lack of ability to easily distinguish between them. Exosomes and microvesicles overlap in particle size, which is the most common type of characterization method, and there are not yet reliable protein content markers that differentiate between these two groups. Currently, techniques such as flow cytometry, dynamic light scattering, and nanoparticle tracking analysis are used for EV analysis (61). However, flow cytometers are unreliable due to their limited resolution, and thus EV-specific assays have been developed (62). Dynamic light scattering is a more reliable technique except when variable sizes of EVs are present in the suspension, which is a frequent occurrence. Smaller EVs remain undetected in the presence of larger sized EVs (63) and are lost from analysis. Nanoparticle tracking analysis can identify a range of EV sizes, but only when the sample has been diluted sufficiently (64). Additionally, most of these techniques are only capable of analyzing the size of the EVs after isolation. The primary difference between exosomes and microvesicles is how they are secreted from the cell (65), but currently we don’t have any markers to distinguish between exosomes and microvesicles after they are secreted and isolated from fluid.
Metastasis
PCa metastasis is a critically lethal stage of cancer progression and most metastatic tumors arise in the bone, as well as the liver and lungs. While several studies have shown EVs as a possible biomarker for metastasis in general, there is also evidence to suggest EVs may be playing a functional role in this development.
It has been shown that EVs are capable of inducing epithelial to mesenchymal transition (EMT) in normal prostate epithelial cell lines, in which the addition of EVs isolated from PCa patients led to increased expression of EMT markers vimentin and N-cadherin and decreased expression of the epithelial marker E-cadherin (66). This suggests that EVs can alter the transcriptional profile of receiving cells and, specifically, genes involved in EMT, a precursor to metastatic progression. This was corroborated in a study that applied EVs derived from a mesenchymal variant of the 22RV1 cell line to the androgen sensitive VCAP PCa cell line (67), and similarly found an induction of vimentin, N-cadherin, and other EMT markers. As inhibition of androgen signaling and an increase in transforming growth factor-β signaling is known to induce the EMT transition in PCa (68-70), the researchers investigated the effect of EVs on these signaling networks and found inhibition of AR, PSA, and ERG and an increase in transforming growth factor-β1 and phosphorylated SMAD2. A cluster of miRNAs carried inside the EVs known to interact with AR may have facilitated this mechanism of EMT transition (67). Additionally, changes in expression of tetraspanin proteins CD9 and CD151 in EVs have been found to influence invasion and migration of the normal prostate epithelial cell line RWPE-1 (71). Reduction in CD9 expression and increased CD151 expression are frequently seen in metastatic PCa (72, 73). EVs that have reduced levels of CD9 or overexpression of CD151 promoted invasion in RWPE1 cells, while only CD151 overexpression increased migration. Interestingly, the altered expression of CD9 and CD151 modified the proteome of the EVs (71), though the mechanism related to how these tetraspanins can affect such changes is unclear.
An important reversal of the EMT process is the mesenchymal to epithelial transition which gives rise to secondary tumor sites. EVs appear capable of preparing the premetastatic niche for cancer cells, likely through promoting inflammation in recipient cells (74, 75). Repeated injections of EVs from a breast cancer cell line that primarily metastasized to the lungs led to enrichment of EVs localized to the lungs and seemed to “train” the tumor microenvironment. When breast cancer cells were later injected, there were significantly more cancer cells that localized to the lungs, from both the breast cancer line that the EVs were derived from as well as a secondary cell line that primarily metastasized to bone (76). Protein analysis by mass spectrometry identified integrin expression profiles that appear capable of distinguishing the tissue-specific homing. Integrins ITGα6, ITGβ4, and ITGβ1 were abundant in EVs that homed to the lungs, whereas EVs that homed to the liver had more abundance of ITGβ5 and ITGαv (76). While it is unclear if there are integrin patterns that predict homing to bone, EVs derived from PCa cell lines seem to preferentially target bone marrow stromal cells (77). The protein transfer of pyruvate kinase M2 from EVs to these recipient cells led to increased levels of the chemokine CXCL12 in a HIF-1α–dependent mechanism (77), which likely contributed to an inflammatory response (78) that prepared the premetastatic niche. Additionally, the introduction of EVs derived from the enzalutamide resistant PCa cell line CW-R1 resulted in NF-κB signaling and enhanced osteoclast differentiation in bone marrow stromal cells (79), which is an initial step in reprogramming the bone microenvironment to allow for tumor cell proliferation and metastasis (80).
Immune response
Immune checkpoint inhibitor therapy targeting the PD-L1/PD-1 axis has shown effectiveness in several cancer types including melanoma, non–small-cell lung cancer, and renal cancer, but the responses in PCa with these therapies remains elusive (81, 82). The mechanisms for why PCa remains largely unresponsive to immunotherapy remain unknown. It is possible that the low mutational burden impedes antitumor immunity, or the presence of suppressive cell populations such as regulatory T-cells (83) or myeloid-derived suppressor cells (84).
EVs may drive aspects of immune escape through immune suppression. EVs derived from the LNCaP cell line model were shown to be capable of not only inhibiting proliferation of T-cells but also cause apoptosis (85). EVs have also been shown to suppress the function of natural killer cells (86) and inhibit differentiation of dendritic cells (87). Undifferentiated dendritic cells are incapable of presenting antigens to T-cells, leading to a suppressed immune response. Manipulating the activation of dendritic cells is an area of interest in increasing the effectiveness of immunotherapy (88), and EVs isolated from mature dendritic cells have been used in a phase II clinical trial with non-small cell lung cancer patients to stimulate the immune response. Unfortunately, this trial did not see a significant effect on T-cell responses though natural killer activity was enhanced (89).
Importantly, EVs have been shown to activate the PD-L1/PD-1 axis. PD-L1 (CD274) on the surface of tumor cells binds its receptor PD-1 on effector T-cells which serves to suppress T-cell activity. PD-L1 has also been found on the surface of EVs in several cancer types (90-92) and shown to be capable of binding PD-1 on T-cells (93). Notably, work done by Poggio et al. showed that PD-L1 from EVs derived from PCa cells were capable of suppressing T-cell activation. Knocking out PD-L1 and thereby removing PD-L1-containing EVs inhibited tumor growth. This inhibition was reversed when EVs containing PD-L1 were reintroduced into the mouse model (94). Additionally, these researchers showed that knocking out the genes Rab27a and nSMase2, which are involved in the biogenesis of EVs, in combination with anti-PD-L1 treatment was capable of extending mouse survival. This offers a potential avenue of increasing effectiveness of immunotherapies in PCa where both cell surface and EV PD-L1 can be targeted.
Drug resistance
There are multiple avenues in which EVs can contribute to drug resistance in cells, such as transferring drug efflux pumps, apoptotic modulators, and the drugs themselves (95). In PCa, it has been shown that EVs from docetaxel resistant PCa cell lines can confer that resistance to docetaxel sensitive lines (96). Comparison of EV proteins between docetaxel sensitive or resistant DU145 PCa cells nominated a potential predictive signature (97) including increased expression of multidrug resistance genes MDR1 and MDR3, but there has been no functional follow-up to this finding.
PCa cell lines that are resistant to enzalutamide (Enz) were found to exhibit higher secretion of EVs than their parental Enz-sensitive lines. Additionally, inhibiting EV secretion by small molecule inhibitors or siRNA knockdown of syntaxin 6 significantly reduced the viability of Enz-resistance lines (98). These data suggest that EVs can contribute to cell proliferation in drug resistant lines, though it is unclear in this study if inhibiting EV secretion is the main contributor to resistance or the lack of EV signals from surrounding resistant cells.
Conclusions
The intercellular interactions that EVs mediate have been repeatedly shown to play important roles in PCa initiation and progression. Remodeling the microenvironment to prepare a premetastatic niche, suppressing the immune system, and facilitating resistance to therapies are key functions that contribute to cancer cell survival. Understanding not only the mechanism behind these functions but also how to combat these effects are important considerations for future studies. Blocking these abilities of EVs is a potential avenue to significantly improve current therapies.
EVs may also be a therapy in and of themselves. EVs derived from a fibrosarcoma cell line were engineered to encapsulate the chemotherapeutic doxorubicin and homed to the cancer cell of origin, successfully delivering the drug and inhibiting tumor growth in mouse models (99). That EVs can function as shuttles back to their parent cancer cell of origin opens up new ways to deliver therapies with specificity, including treatments with RNAi. EVs have been modified to include siRNAs or shRNAs targeting the mutated form of KRAS, a driver in many cancer types. KRAS has been historically difficult to target, however, the first KRAS-targeted therapy, sotorasib, was recently approved by the FDA for non–small-cell lung cancer (100). These modified EVs, known as iExosomes, were able to successfully inhibit mutant KRAS signaling in a pancreatic cancer mouse model and increase survival (101). This success has led to a clinical trial using iExosomes in metastatic pancreatic cancer patients (NCT03608631). For PCa, perhaps EVs can offer a new mechanism to deliver agents that specifically target androgen receptor signaling that drive the majority of PCa cases.
The cargo that EVs carry have been shown to have phenotypic impacts whereby cancer cells can utilize this cargo to aid in either proliferation and metastasis or by researchers for targeting and inhibition. But, no matter the function, the contents of EVs can also be used as a noninvasive means to monitor the disease state. Several RNA and protein biomarkers have been nominated as being able to help diagnose PCa or distinguish a subtype. To move forward and positively impact patient care, we need to identify robust markers that are repeatable across several research groups. Ideally, the markers would be able to overcome the variety of ways to isolate and evaluate EVs. That EVs can offer information about tumor progression through a noninvasive liquid biopsy is of huge benefit for PCa.
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Acknowledgments
Financial Support: M.L. is supported by the Targets of Cancer Training Program grant T32 CA009138. J.M.D. is supported by the Department of Defense Prostate Cancer Research Program W81XWH-18-1-0542.
Glossary
Abbreviations
- AR
androgen receptor
- EMT
epithelial to mesenchymal transition
- EV
extracellular vesicle
- FABP5
Fatty Acid Binding Protein 5
- Enz
enzalutamide ;
- PCa
prostate cancer
- PSA
prostate-specific antigen
Additional Information
Disclosure Statement: The authors declare no conflict of interest.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiang CY, Chen C. Toward characterizing extracellular vesicles at a single-particle level. J Biomed Sci. 2019;26(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology. 2020;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31(6):1059-1062. [DOI] [PubMed] [Google Scholar]
- 6. Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang BX, Zhang L, Wang YJ, et al. Epidemiology of syphilis infection among drug users at methadone maintenance treatment clinics in China: systematic review and meta-analysis. Int J STD AIDS. 2014;25(8):550-558. [DOI] [PubMed] [Google Scholar]
- 8. Laulagnier K, Motta C, Hamdi S, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380(Pt 1):161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maacha S, Bhat AA, Jimenez L, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. [DOI] [PubMed] [Google Scholar]
- 11. Ronquist G, Nilsson BO. The Janus-faced nature of prostasomes: their pluripotency favours the normal reproductive process and malignant prostate growth. Prostate Cancer Prostatic Dis. 2004;7(1):21-31. [DOI] [PubMed] [Google Scholar]
- 12. Winters SJ, Troen P. Altered pulsatile secretion of luteinizing hormone in hypogonadal men with hyperprolactinaemia. Clin Endocrinol. 1984;21(3):257-263. [DOI] [PubMed] [Google Scholar]
- 13. Kiciński M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One. 2011;6(10):e27130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delongchamps NB, Singh A, Haas GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13(3):158-168. [DOI] [PubMed] [Google Scholar]
- 15. Loeb S, Catalona WJ. What to do with an abnormal PSA test. Oncologist. 2008;13(3):299-305. [DOI] [PubMed] [Google Scholar]
- 16. Schröder FH, Carter HB, Wolters T, et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008;53(3):468-477. [DOI] [PubMed] [Google Scholar]
- 17. Øverbye A, Skotland T, Koehler CJ, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6(30):30357-30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodríguez M, Bajo-Santos C, Hessvik NP, et al. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer. 2017;16(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Driedonks TAP, Nolte-‘t Hoen ENM. Circulating Y-RNAs in extracellular vesicles and ribonucleoprotein complexes; implications for the immune system. Front Immunol. 2018;9:3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ChunJiao S, Huan C, ChaoYang X, GuoMei R. Uncovering the roles of miRNAs and their relationship with androgen receptor in prostate cancer. IUBMB Life. 2014;66(6):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foj L, Ferrer F, Serra M, et al. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate. 2017;77(6):573-583. [DOI] [PubMed] [Google Scholar]
- 23. Porzycki P, Ciszkowicz E, Semik M, Tyrka M. Combination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognition. Int Urol Nephrol. 2018;50(9):1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smieszek A, Marcinkowska K, Pielok A, Sikora M, Valihrach L, Marycz K. The role of miR-21 in osteoblasts-osteoclasts coupling in vitro. Cells. 2020;9(2):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110-E2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kato M, Goto Y, Matsushita R, et al. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int J Oncol. 2015;47(2):710-718. [DOI] [PubMed] [Google Scholar]
- 27. Urabe F, Kosaka N, Sawa Y, et al. miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1. Sci Adv. 2020;6(18):eaay3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phatak P, Burger AM. Telomerase and its potential for therapeutic intervention. Br J Pharmacol. 2007;152(7):1003-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miura N, Osaki Y, Nagashima M, et al. A novel biomarker TERTmRNA is applicable for early detection of hepatoma. BMC Gastroenterol. 2010;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. March-Villalba JA, Martínez-Jabaloyas JM, Herrero MJ, Santamaria J, Aliño SF, Dasí F. Cell-free circulating plasma hTERT mRNA is a useful marker for prostate cancer diagnosis and is associated with poor prognosis tumor characteristics. PLoS One. 2012;7(8):e43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dasí F, Lledó S, García-Granero E, et al. Real-time quantification in plasma of human telomerase reverse transcriptase (hTERT) mRNA: a simple blood test to monitor disease in cancer patients. Lab Invest. 2001;81(5):767-769. [DOI] [PubMed] [Google Scholar]
- 32. Pucciarelli S, Rampazzo E, Briarava M, et al. Telomere-specific reverse transcriptase (hTERT) and cell-free RNA in plasma as predictors of pathologic tumor response in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2012;19(9):3089-3096. [DOI] [PubMed] [Google Scholar]
- 33. Terrin L, Rampazzo E, Pucciarelli S, et al. Relationship between tumor and plasma levels of hTERT mRNA in patients with colorectal cancer: implications for monitoring of neoplastic disease. Clin Cancer Res. 2008;14(22):7444-7451. [DOI] [PubMed] [Google Scholar]
- 34. Goldvaser H, Gutkin A, Beery E, et al. Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: a potential pan-cancer marker. Br J Cancer. 2017;117(3):353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34(4):474-490. [DOI] [PubMed] [Google Scholar]
- 36. Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gillette P, Zmijewski M, Shelton MB. The evolution of computer application to assist during clinical electrophysiologic testing. J Electrocardiol. 1989;22(Suppl):218-222. [DOI] [PubMed] [Google Scholar]
- 38. Zhang T, Karsh LI, Nissenblatt MJ, Canfield SE. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2020;18(1):1-10. [DOI] [PubMed] [Google Scholar]
- 39. Pacheco-Orozco RA, Montealegre-Páez L, Cayol F, et al. AR-V7 as a biomarker for resistance to treatment with abiraterone/enzalutamide in three Latin American countries: a hypothetical cost-saving analysis. Oncologist. 2020;25(12):e1990-e1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu Y, Dalrymple SL, Coleman I, et al. Role of androgen receptor splice variant-7 (AR-V7) in prostate cancer resistance to 2nd-generation androgen receptor signaling inhibitors. Oncogene. 2020;39(45):6935-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mizutani K, Terazawa R, Kameyama K, et al. Isolation of prostate cancer-related exosomes. Anticancer Res. 2014;34(7):3419-3423. [PubMed] [Google Scholar]
- 42. Del Re M, Conteduca V, Crucitta S, et al. Androgen receptor gain in circulating free DNA and splicing variant 7 in exosomes predict clinical outcome in CRPC patients treated with abiraterone and enzalutamide. Prostate Cancer Prostatic Dis. 2021;24(2):524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Read J, Ingram A, Al Saleh HA, et al. Nuclear transportation of exogenous epidermal growth factor receptor and androgen receptor via extracellular vesicles. Eur J Cancer. 2017;70:62-74. [DOI] [PubMed] [Google Scholar]
- 44. Fujita K, Kume H, Matsuzaki K, et al. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci Rep. 2017;7:42961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O’Sullivan SE, Kaczocha M. FABP5 as a novel molecular target in prostate cancer. Drug Discov Today. 2020:S1359-6446(20)30375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Myers JS, von Lersner AK, Sang QX. Proteomic upregulation of fatty acid synthase and fatty acid binding protein 5 and identification of cancer- and race-specific pathway associations in human prostate cancer tissues. J Cancer. 2016;7(11):1452-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ang J, Lijovic M, Ashman LK, Kan K, Frauman AG. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1717-1721. [PubMed] [Google Scholar]
- 48. Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9(1):40-55. [DOI] [PubMed] [Google Scholar]
- 49. Testa JE, Brooks PC, Lin JM, Quigley JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59(15):3812-3820. [PubMed] [Google Scholar]
- 50. Deryugina EI, Conn EM, Wortmann A, et al. Functional role of cell surface CUB domain-containing protein 1 in tumor cell dissemination. Mol Cancer Res. 2009;7(8):1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siva AC, Wild MA, Kirkland RE, et al. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68(10):3759-3766. [DOI] [PubMed] [Google Scholar]
- 52. Sandvig K, Llorente A. Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol Cell Proteomics. 2012;11(7):M111.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sequeiros T, Rigau M, Chiva C, et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget. 2017;8(3):4960-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ronquist GK, Larsson A, Stavreus-Evers A, Ronquist G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate. 2012;72(16):1736-1745. [DOI] [PubMed] [Google Scholar]
- 56. Olsson I, Ronquist G. Nucleic acid association to human prostasomes. Arch Androl. 1990;24(1):1-10. [DOI] [PubMed] [Google Scholar]
- 57. Lázaro-Ibáñez E, Sanz-Garcia A, Visakorpi T, et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74(14):1379-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Warner E, Herberts C, Fu S, et al. BRCA2, ATM, and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clin Cancer Res. 2021;27(6):1650-1662. [DOI] [PubMed] [Google Scholar]
- 59. Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vagner T, Spinelli C, Minciacchi VR, et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles. 2018;7(1):1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nolan JP, Duggan E. Analysis of Individual Extracellular Vesicles by Flow Cytometry. Methods Mol Biol. 2018;1678:79-92. [DOI] [PubMed] [Google Scholar]
- 63. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yuana Y, Oosterkamp TH, Bahatyrova S, et al. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8(2):315-323. [DOI] [PubMed] [Google Scholar]
- 65. Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659-1668. [DOI] [PubMed] [Google Scholar]
- 66. Souza AG, B Silva IB, Campos-Fernández E, et al. Extracellular vesicles as drivers of epithelial-mesenchymal transition and carcinogenic characteristics in normal prostate cells. Mol Carcinog. 2018;57(4):503-511. [DOI] [PubMed] [Google Scholar]
- 67. El-Sayed IY, Daher A, Destouches D, et al. Extracellular vesicles released by mesenchymal-like prostate carcinoma cells modulate EMT state of recipient epithelial-like carcinoma cells through regulation of AR signaling. Cancer Lett. 2017;410:100-111. [DOI] [PubMed] [Google Scholar]
- 68. Byrne NM, Nesbitt H, Ming L, McKeown SR, Worthington J, McKenna DJ. Androgen deprivation in LNCaP prostate tumour xenografts induces vascular changes and hypoxic stress, resulting in promotion of epithelial-to-mesenchymal transition. Br J Cancer. 2016;114(6):659-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun Y, Wang BE, Leong KG, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72(2):527-536. [DOI] [PubMed] [Google Scholar]
- 70. Cao Z, Kyprianou N. Mechanisms navigating the TGF-β pathway in prostate cancer. Asian J Urol. 2015;2(1):11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brzozowski JS, Bond DR, Jankowski H, et al. Extracellular vesicles with altered tetraspanin CD9 and CD151 levels confer increased prostate cell motility and invasion. Sci Rep. 2018;8(1):8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang JC, Bégin LR, Bérubé NG, et al. Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin Cancer Res. 2007;13(8):2354-2361. [DOI] [PubMed] [Google Scholar]
- 73. Detchokul S, Newell B, Williams ED, Frauman AG. CD151 is associated with prostate cancer cell invasion and lymphangiogenesis in vivo. Oncol Rep. 2014;31(1):241-247. [DOI] [PubMed] [Google Scholar]
- 74. Deng ZB, Zhuang X, Ju S, et al. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J Immunol. 2013;190(7):3579-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bondar T, Medzhitov R. The origins of tumor-promoting inflammation. Cancer Cell. 2013;24(2):143-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dai J, Escara-Wilke J, Keller JM, et al. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med. 2019;216(12):2883-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. 2018;15(4):299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Henrich SE, McMahon KM, Plebanek MP, et al. Prostate cancer extracellular vesicles mediate intercellular communication with bone marrow cells and promote metastasis in a cholesterol-dependent manner. J Extracell Vesicles. 2020;10(2):e12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maurizi A, Rucci N. The osteoclast in bone metastasis: player and target. Cancers. 2018;10(7):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goswami S, Aparicio A, Subudhi SK. Immune checkpoint therapies in prostate cancer. Cancer J. 2016;22(2):117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Venturini NJ, Drake CG. Immunotherapy for prostate cancer. Cold Spring Harb Perspect Med. 2019;9(5):a030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lopez-Bujanda Z, Drake CG. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. J Leukoc Biol. 2017;102(2):393-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35(2):169-173. [DOI] [PubMed] [Google Scholar]
- 86. Liu C, Yu S, Zinn K, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375-1385. [DOI] [PubMed] [Google Scholar]
- 87. Yu S, Liu C, Su K, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867-6875. [DOI] [PubMed] [Google Scholar]
- 88. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7-24. [DOI] [PubMed] [Google Scholar]
- 89. Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ricklefs FL, Alayo Q, Krenzlin H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4(3):eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yang Y, Li CW, Chan LC, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Poggio M, Hu T, Pai CC, et al. Suppression of exosomal pd-l1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414-427.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimarães JE, Vasconcelos MH. The role of extracellular vesicles in the hallmarks of cancer and drug resistance. Cells. 2020;9(5):1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Corcoran C, Rani S, O’Brien K, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7(12):e50999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kharaziha P, Chioureas D, Rutishauser D, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6(25):21740-21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Peak TC, Panigrahi GK, Praharaj PP, et al. Syntaxin 6-mediated exosome secretion regulates enzalutamide resistance in prostate cancer. Mol Carcinog. 2020;59(1):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qiao L, Hu S, Huang K, et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10(8):3474-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. FDA Approves First KRAS Inhibitor: Sotorasib. Cancer Discov. 2021. doi: 10.1158/2159-8290.CD-NB2021-0362. [DOI] [PubMed] [Google Scholar]
- 101. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Josson S, Gururajan M, Sung SY, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34(21):2690-2699. [DOI] [PubMed] [Google Scholar]
- 103. Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9(1):13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tan YF, Chen ZY, Wang L, Wang M, Liu XH. MiR-142-3p functions as an oncogene in prostate cancer by targeting FOXO1. J Cancer. 2020;11(6):1614-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Moltzahn F, Olshen AB, Baehner L, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011;71(2):550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu J, Mu X, Yin Q, Hu K. miR-106a contributes to prostate carcinoma progression through PTEN. Oncol Lett. 2019;17(1):1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bhagirath D, Yang TL, Bucay N, et al. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018;78(7):1833-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Osip’yants AI, Knyazev EN, Galatenko AV, et al. Changes in the level of circulating hsa-miR-297 and hsa-miR-19b-3p miRNA are associated with generalization of prostate cancer. Bull Exp Biol Med. 2017;162(3):379-382. [DOI] [PubMed] [Google Scholar]
- 109. Hashimoto K, Ochi H, Sunamura S, et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci U S A. 2018;115(9):2204-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang X, Liang M, Dittmar R, Wang L. Extracellular microRNAs in urologic malignancies: chances and challenges. Int J Mol Sci. 2013;14(7):14785-14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang X, Yuan T, Liang M, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67(1):33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017;259:135-139. [DOI] [PubMed] [Google Scholar]
- 113. Motamedinia P, Scott AN, Bate KL, et al. Urine exosomes for non-invasive assessment of gene expression and mutations of prostate cancer. PLoS One. 2016;11(5):e0154507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bhagirath D, Yang TL, Tabatabai ZL, et al. BRN4 is a novel driver of neuroendocrine differentiation in castration-resistant prostate cancer and is selectively released in extracellular vesicles with BRN2. Clin Cancer Res. 2019;25(21):6532-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.