Abstract
Background:
There is a sparsity of data describing the periodontal microbiome in elderly individuals. We analyzed the association of subgingival bacterial profiles and clinical periodontal status in a cohort of participants in the Washington Heights-Inwood Columbia Aging Project (WHICAP).
Methods:
Dentate individuals underwent a full-mouth periodontal examination at six sites/tooth. Up to four subgingival plaque samples per person, each obtained from the mesio-lingual site of the most posterior tooth in each quadrant, were harvested and pooled. Periodontal status was classified according to the Centers for Disease Control/American Academy of Periodontology (CDC/AAP) criteria as well as based on the percentage of teeth/person with pockets ≥4 mm deep. Bacterial DNA was isolated and was processed and analyzed using Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS). Differential abundance across the periodontal phenotypes was calculated using the R package DESeq2. α- and β-diversity metrics were calculated using DADA2-based clustering.
Results:
The mean age of the 739 participants was 74.5 years, and 32% were male. Several taxa including Sneathia amnii-like sp., Peptoniphilaceae [G-1] bacterium HMT 113, Porphyromonas gingivalis, Fretibacterium fastidiosum, Filifactor alocis, and Saccharibacteria (TM7) [G-1] bacterium HMT 346 were more abundant with increasing severity of periodontitis. In contrast, species such as Veillonella parvula, Veillonella dispar, Rothia dentocariosa, and Lautropia mirabilis were more abundant in health. Microbial diversity increased in parallel with the severity and extent of periodontitis.
Conclusions:
The observed subgingival bacterial patterns in these elderly individuals corroborated corresponding findings in younger cohorts and were consistent with the concept that periodontitis is associated with perturbations in the resident microbiome.
Keywords: epidemiology, geriatric dentistry, microbiology, periodontitis, risk factor(s)
1. INTRODUCTION
The human oral microbiome is diverse,1 facilitates multiple essential functions including oral tissue homeostasis, development of mucosal immunity, and food digestion2 and is currently thought to harbor >700 bacterial species.3,4 It is well established that the composition of the periodontal microbiome is not static over time but subject to multiple perturbations due to environmental or endogenous exposures which associate with the clinical periodontal conditions.2 Indeed, multiple studies over the years have used a variety of technologies ranging from bacterial culture, to DNA probes and next generation sequencing and have documented profound differences in the subgingival microbiome between states of periodontal health and disease.5–9 A finite number of “established periodontal pathogens,” that is, bacterial species frequently recovered in higher proportions from deep pockets or from sites with progressive periodontal tissue loss10 have been identified and have been extensively studied with respect to function and virulence properties in in vitro and animal models.11 More contemporary studies have increasingly focused on microbial communities rather than on specific bacterial taxa, in recognition that periodontitis is not a classic microbial infection but is rather associated with a state of microbial dysbiosis.12 Generally speaking, this dysbiosis has been considered either the cause of the disruption of periodontal homeostatic mechanisms that leads to inflammatory responses and results in breakdown of the periodontal tissues13 or, according to an alternative view, the result of inflammatory changes that act as environmental stressors and, in turn, induce bacterial dysbiosis.14 In other words, periodontitis is either viewed as a polymicrobial perturbation of the host homeostasis in a susceptible host, or as an inflammation-driven disruption of the periodontal microbial homeostasis, leading to subgingival dysbiosis and subsequent host-mediated destruction of the periodontal tissues. In our view, both scenarios are biologically plausible and complementary, and in fact converge in the development of periodontitis. While specific bacterial species with disproportionate effects of the microbial habitat have been described,15 the role of the aggregate microbial community at the dento-gingival niche is likely more important than that of individual constituents.16 Therefore, it appears that the quest towards an increased understanding of the determinants of periodontitis, and in particular the contribution of the microbiome to the periodontitis susceptibility puzzle, will be better served by research that studies bacterial communities, rather than individual bacterial species.
Additional studies of the subgingival microbiome in elderly individuals are particularly important for multiple reasons. First, the segment of the world population over 60 years continues to expand and has been projected to almost double between 2015 and 2050, from 12% to 22%.17 With edentulism decreasing, tooth retention in older dentate persons increasing, and age-associated comorbidities on the rise,18 the oral healthcare needs of the elderly continue to grow and to become increasingly complex. From a research perspective, while studies investigating individuals of young age can correctly identify those susceptible to periodontitis on the basis of prevalent pathology, accurate detection of non-susceptible individuals remains problematic since a young periodontally healthy or intact person may still develop periodontitis later in life. Therefore, the risk of misclassification is substantial. In contrast, studying the determinants of susceptibility to periodontitis among elderly individuals with fully developed periodontal phenotypes minimizes the risk for misclassification. Importantly, as pointed out in a fairly recent comprehensive review of available studies examining the subgingival microbiota of the aging mouth,19 data from older cohorts are particularly sparse.
Our group has conducted an Ancillary Study of Oral Health among the participants of the Washington Heights-Inwood Columbia Aging Project (WHICAP), which is a multi-ethnic longitudinal study of aging elderly residents in northern Manhattan in New York. In this work, we present data on the subgingival microbiome of the dentate participants of the ancillary study, and of the association of metrics of bacterial relative abundance and diversity with clinical periodontal status.
2. MATERIALS AND METHODS
2.1. WHICAP ancillary study of oral health
Over the past 20 years, WHICAP has serially assessed ≈6,000 participants aged >65 years with respect to medical, social, and health behavior histories, general medical exams, and neuropsychological testing.20 The WHICAP Ancillary Study of Oral Health is a cross-sectional cohort study that recruited 1,130 individuals among the parent study participants and was conducted between December 2013 and June 2016.21 The mean age of the ancillary study participants was 75.4 years (SD 6.7); 66.6% of the attendees were female, 44.7% were Hispanic, 30.4% black, and 23.3% white. The Institutional Review Board of the Columbia Presbyterian Irving Medical Center approved the design and procedures of the study which was conducted according to the Helsinki Declaration of 1975, as revised in 2013. All participants signed written informed consent forms. The clinical oral examination protocol was described earlier,21 and included full-mouth assessments of probing depth (PD) and clinical attachment level (CAL) at six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, disto-lingual, mid-lingual, and mesio-lingual) at all present teeth, excluding third molars, by a single calibrated dental examiner.
2.2. Subgingival plaque sample collection and processing
From all dentate participants, four individual subgingival plaque samples, each from the mesio-lingual surface of the most posterior tooth in each quadrant were obtained before the probing examination. In brief, supragingival plaque was removed from the teeth to be sampled and subgingival plaque was harvested using sterile curets* and was transferred to individual Eppendorf tubes that contained 150 μL of sterile T-E buffer (10 mM Tris HCl, 1.0 mM EDTA, pH 7.6). The plaque pellet was resuspended using a sterile pipette and was vigorously vortexed. Subsequently, one half of each individual plaque sample was transferred into a new sterile tube to create a single, pooled subgingival plaque sample per participant. Samples were kept at −80°C until processing which was performed at the Forsyth Institute, Cambridge by means of Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS), an in silico 16S rDNA-based semi-quantitative analysis, using a modified protocol previously described.22 Briefly, V3–V4 forward (341F) AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTCCTACGGGAGGCAGCAG and reverse (806R) CAAGCAGAAGACGGCATACGAGATNNNNNNNNNNNNAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT primers were used for polymerase chain reaction (PCR)-amplification of 10 to 50 ng of DNA extracted from each sample, and then purified using AMPure beads. A library of 100 ng was then pooled, gel-purified, and subsequently quantified using quantitative PCR; 20% PhiX was added to 12 pM of the library and sequenced.†
2.3. Data analyses
Periodontal status was analyzed using the categorical four level Centers for Disease Control/American Academy of Periodontology (CDC/AAP) classification,23 as well as using continuous measures of extent and severity of periodontitis (percentage of teeth per person with PD ≥4 mm and ≥6 mm, and percentage of teeth per person with CAL ≥3 mm and ≥5 mm). Analyses of the clinical phenotypes were performed using statistical software.‡
Bacterial identification from 16S rRNA gene sequence data were determined using ProbeSeq for HOMINGS, a customized BLAST algorithm that contained species-specific, custom-made 16S rDNA in silico probes (17 to 40 bases), according to the Human Oral Microbiome Database (HOMD).24 Bacterial identification was based on 598 oligonucleotide probes targeting individual oral bacterial species or a cluster of a few closely-related species as well as 94 genus-specific probes, which identified groups of closely related species within the same genus.25,26 An earlier published comparison of HOMINGS with the classical tree-based approach implemented in QIIME showed congruent composition profiles of clinical samples and mock communities as well as similar α- and β- diversity estimates obtained through the two approaches.27
In addition, the Divisive Amplicon Denoising Algorithm version 2 (DADA2 1.12.1) was used for quality-filtering, trimming, error correction, exact sequence inference, chimera removal, and generation of amplicon sequence variant tables (ASV).28 Taxonomic classification was performed using a Naïve Bayes classifier trained using the GreenGenes 97% clustered sequences (version 13_8). The ASV tables were imported into R 3.6.1 to calculate α-diversity29–31 and β-diversity metrics using a function of the phyloseq v1.28.0 package.32 Based on α-diversity rarefaction, samples were included in the analyses if the rarefaction curves were plateaued and a minimum cutoff of 10,000 counts was exceeded. Differential abundance analysis for bacterial ASVs was performed using DESeq2. The relative abundance of each species or genus examined was correlated with CDC/AAP class and with the percentage of teeth with PD ≥4 mm, adjusted for age, sex, education levels, and smoking status. The P values were adjusted by the Benjamini-Hochberg method33 to control the false discovery rate at 5%. β-Diversity was analyzed using permutational multivariate analysis of variance (PERMANOVA), a non-parametric multivariate ANOVA that identifies differences in sample centroids. Test statistics were calculated based on a comparison of dissimilarities among inter-class and intra-class objects. Analyses were adjusted for age, sex, education level, and smoking.
3. RESULTS
3.1. Cohort characteristics and clinical periodontal status
The present report includes data from 739 dentate individuals whose 1) bacterial sample-derived sequencing data met the quality metrics described above, and 2) periodontal status could be classified according to the CDC/AAP criteria. The participants had a mean age of 74.5 years (range 60.2 to 98.2); 31.7% were men; 39.5% were Hispanic, 30.5% black, and 28.5% white; 44.4% were former and 3.5% current smokers; and 60.8% were of middle (12 to 16 years) or high (≥ 17 years) educational attainment (Table 1).
TABLE 1.
Demographic and other characteristics of the study sample (N = 739)
| Age (yrs) | Sex | Race/Ethnicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Male | Female | Hispanic | African-American | Caucasian | Other | |||
| 65–69 | 223 | 30.2 | ||||||||
| 70–74 | 259 | 35.1 | N | 234 | 505 | N | 292 | 225 | 211 | 11 |
| 75–79 | 114 | 15.4 | % | 31.7 | 68.3 | % | 39.5 | 30.5 | 28.5 | 1.5 |
| 80+ | 143 | 19.3 | ||||||||
| Smoking | Educational attainment | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Never | 370 | 50.1 | Low | 240 | 32.5 |
| Former | 328 | 44.4 | Middle | 341 | 46.1 |
| Current | 26 | 3.5 | High | 153 | 20.7 |
| Missing | 15 | 2.0 | Missing | 5 | 0.7 |
Mean 74.5; SD 6.4; Range 60.2*−98.2
The reported age was calculated by subtracting the date of birth from the date of the oral examination. Due to inaccuracies in birthdate data, a total of 17 participants were in fact younger than the minimum stipulated age of 65 years (15 participants were between age 64 and 65 years, two were between 63 and 64 years, and one was 60.2 years)
Table 2 describes the distribution of the samples by CDC/AAP class, as well as by quartiles of percentage of teeth per person with PD ≥4 mm (the distribution by quartile of percentage of teeth per person with PD ≥6 mm was extremely skewed, while the CAL-based quartiles showed only weak associations with the microbial profiles and were thus abandoned in all further analyses). Approximately one-fifth of the participants (20.6%) was classified as periodontally healthy, only 2.7% of the cohort fulfilled the criteria for mild periodontitis, 54.5% had moderate and 22.2% severe periodontitis. People in the first quartile had no teeth with PD ≥4 mm; people in the second quartile had between 0% and 15.38% of their teeth affected at that level of pocketing; people in the third quartile had between 15.39% and 33.33% of their teeth with PD ≥4 mm, while the fourth quartile included individuals with up to 100% of their teeth affected. Table S1 in online Journal of Periodontology shows detailed clinical periodontal data in each CDC/AAP class and quartile. The microbiologically sampled sites had a deeper average probing depth than the full-mouth score (3.06 mm; SD 0.94; range 1.00 to 7.25) versus 2.23 mm; SD 0.59; range 1.08 to 6.35) while the Pearson correlation between the two was 0.81 (P <0.001).
TABLE 2.
Clinical periodontal status in the sample according to the Centers for Disease Control/American Academy of Periodontology (CDC/AAP) classification, as well as according to percentage of teeth per person with PD ≥4 mm
| CDC/AAP classes | n | % |
|---|---|---|
| No periodontitis | 152 | 20.6 |
| Mild | 20 | 2.7 |
| Moderate | 403 | 54.5 |
| Severe | 164 | 22.2 |
| Quartiles of percentage of teeth/person with PD ≥4 mm | Quartile limits |
|---|---|
| Q1 | 0.00%−0.00% |
| Q2 | 0.00%−15.38% |
| Q3 | 15.39%−33.33% |
| Q4 | 33.34%−100.00% |
3.2. Relationship between bacterial relative abundance and clinical periodontal status
Table 3 presents the 10 most abundant taxa by AAP/CDC class (top panel) as well as by quartile of teeth per person with PD ≥4 mm (bottom panel), as identified using the HOMINGS pipeline. Streptococcal species, Leptotrichia wadei, and Rothia dentocariosa were consistently among the most abundant taxa across all phenotypes. At the bottom of each panel, rankings and relative abundance are presented for four established “periodontal pathogens” (Treponema denticola, Tannerella forsythia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans) according to each clinical phenotypes. With the exception of T. denticola, which ranked third in abundance in severe periodontitis and among the fourth quartile of percentage of ≥4 mm pockets per person, these bacterial taxa ranked low in abundance in clinical states suggestive of clinical periodontal pathology. The complete rankings and relative abundance of all taxa identified are presented in Table S2 in online Journal of Periodontology.
TABLE 3.
The 10 most abundant taxa by Centers for Disease Control/American Academy of Periodontology (CDC/AAP) class (top panel) and by quartile of percentage of teeth per person with PD ≥4 mm (bottom panel)
| Rank by CDC/AAP class | Healthy | Relative abundance (%) | Mild | Relative abundance (%) | Moderate | Relative abundance (%) | Severe | Relative abundance (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Rothia dentocariosa | 7.15 | Streptococcus Genus probe 4 | 12.86 | Streptococcus Genus probe 4 | 7.65 | Streptococcus Genus probe 4 | 6.84 |
| 2 | Streptococcus Genus probe 4 | 7.06 | Leptotrichia wadei | 6.15 | Leptotrichia wadei | 6.42 | Leptotrichia wadei | 4.12 |
| 3 | Leptotrichia wadei | 5.33 | Corynebacterium matruchotii | 4.71 | Rothia dentocariosa | 5.09 | Treponema denticola | 3.21 |
| 4 | Corynebacteriun matruchotii | 4.11 | Leptotrichia hongkongensis | 2.81 | Corynebacterium matruchotii | 4.43 | Leptotrichia Genus probe 3 | 3.05 |
| 5 | Veillonella dispar | 2.40 | Fusobacterium Genus probe 4 | 2.73 | Leptotrichia shahii | 3.16 | Corynebacterium matruchotii | 2.94 |
| 6 | Leptotrichia shahii | 2.14 | Prevotella nigrescens | 2.52 | Fusobacterium Genus probe 4 | 1.97 | Saccharibacteria (TM7) [G-5]-like sp. | 2.21 |
| 7 | Fusobacterium Genus probe 4 | 2.00 | Prevotella denticola | 2.09 | Leptotrichia sp HTM 417 | 1.81 | Rothia dentocariosa | 2.15 |
| 8 | Leptotrichia hongkongensis | 1.98 | Leptotrichia shahii | 2.09 | Leptotrichia hongkongensis | 1.76 | Parvimonas micra | 2.15 |
| 9 | Prevotella nigrescens | 1.82 | Parvimonas micra | 1.74 | Leptotrichia Genus probe 3 | 1.70 | Peptidiphaga sp HTM 183 | 1.94 |
| 10 | Veillonella Genus probe 2 | 1.60 | Bacteroidales [G-2] sp HTM 274 | 1.42 | Veillonella dispar | 1.68 | Peptoniphilaceae [G-1] bacterium HMT 113 | 1.84 |
| Treponema denticola | 21st | 1.16 | 54th | 0.47 | 35th | 0.79 | 3rd | 3.21 |
| Tannerella forsythia | 108th | 0.18 | 60th | 0.38 | 87th | 0.24 | 73rd | 0.32 |
| Porphyromonas gingivalis | 133rd | 0.12 | 158th | 0.07 | 149th | 0.09 | 89th | 0.25 |
| Aggregatibacter actinomycetemcomitans | 297th | 0.00 | 299th | 0.00 | 282nd | 0.01 | 261st | 0.03 |
| Rank by quartile | Q1 | Relative abundance (%) | Q2 | Relative abundance (%) | Q3 | Relative abundance (%) | Q4 | Relative abundance (%) |
| 1 | Streptococcus Genus probe 4 | 8.30 | Leptotrichia wadei | 7.42 | Streptococcus Genus probe 4 | 7.60 | Streptococcus Genus probe 4 | 6.76 |
| 2 | Rothia dentocariosa | 7.83 | Streptococcus Genus probe 4 | 7.24 | Leptotrichia wadei | 5.29 | Leptotrichia wadei | 4.94 |
| 3 | Leptotrichia wadei | 4.96 | Rothia dentocariosa | 5.79 | Corynebacterium matruchotii | 4.18 | Treponema denticola | 3.18 |
| 4 | Corynebacterium matruchotii | 4.36 | Corynebacterium matruchotii | 4.89 | Rothia dentocariosa | 2.46 | Corynebacterium matruchotii | 2.66 |
| 5 | Leptotrichia shahii | 2.67 | Leptotrichia shahii | 3.46 | Leptotrichia Genus probe 3 | 2.45 | Saccharibacteria (TM7) [G-5}-like sp. | 2.58 |
| 6 | Leptotrichia hongkongensis | 2.37 | Leptotrichia sp HTM 417 | 2.10 | Leptotrichia sp HTM 498 | 2.40 | Rothia dentocariosa | 2.58 |
| 7 | Veillonella dispar | 2.34 | Fusobacterium Genus probe 4 | 1.87 | Leptotrichia shahii | 2.38 | Leptotrichia Genus probe 3 | 2.14 |
| 8 | Fusobacterium Genus probe 4 | 1.79 | Veillonella dispar | 1.86 | Fusobacterium Genus probe 4 | 2.14 | Parvimonas micra | 2.07 |
| 9 | Streptococcus Genus probe 1 | 1.55 | Leptotrichia hongkongensis | 1.84 | Leptotrichia sp HTM 417 | 1.93 | Leptotrichia shahii | 1.80 |
| 10 | Prevotella denticola | 1.50 | Leptotrichia Genus probe 3 | 1.79 | Peptidiphaga sp HTM 183 | 1.84 | Peptoniphilaceae [G-1] bacterium HMT 113 | 1.79 |
| Treponema denticola | 25th | 0.96 | 50th | 0.48 | 26th | 0.99 | 3rd | 3.18 |
| Tannerella forsythia | 101st | 0.18 | 115th | 0.15 | 69th | 0.32 | 69th | 0.35 |
| Porphyromonas gingivalis | 125th | 0.13 | 212th | 0.03 | 122nd | 0.16 | 97th | 0.22 |
| Aggregatibacter actinomycetemcomitans | 297th | 0.00 | 300th | 0.00 | 237th | 0.03 | 267th | 0.02 |
The Table also lists the rankings and relative abundance of four “established periodontal pathogens” (Treponema denticola, Tannerella forsythia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans) according to clinical periodontal phenotype. Note that genus level probes capture several species within the genus other than those for which specific species-level probes were used.
Figure 1 illustrates findings derived from differential abundance analysis using the DESeq2 package with respect to CDC/AAP class (left panel) and probing depth-based quartile (right panel). Depicted taxa had a minimum differential abundance fold change of two with a P value of <0.01. Periodontally healthy conditions and people with no pockets with PD ≥4 mm (Q1) were used at the reference group for all comparisons in the left and right panel, respectively. A pronounced elevation of a number of taxa, including Leptotrichiaceae, Peptoniphilaceae, several species of the genus Treponema, and TM7 was noted both in severe periodontitis and among participants in the fourth quartile of people with pocketing. Interestingly, P. gingivalis was among the species elevated in severe periodontitis but not in the fourth quartile. After adjustments for age, sex, smoking, and education, 54 out of 303 taxa and 35 out of 93 genera analyzed were differentially abundant (DA) between the four CDD/AAP with a false discovery rate (FDR) of <0.05 (Tables S3 and S4 in online Journal of Periodontology). Similar analyses based on quartiles of percentage teeth/person with PD ≥4 mm, identified 54 DA taxa and 56 DA genera (Tables S5 and S6 in online Journal of Periodontology).
FIGURE 1.
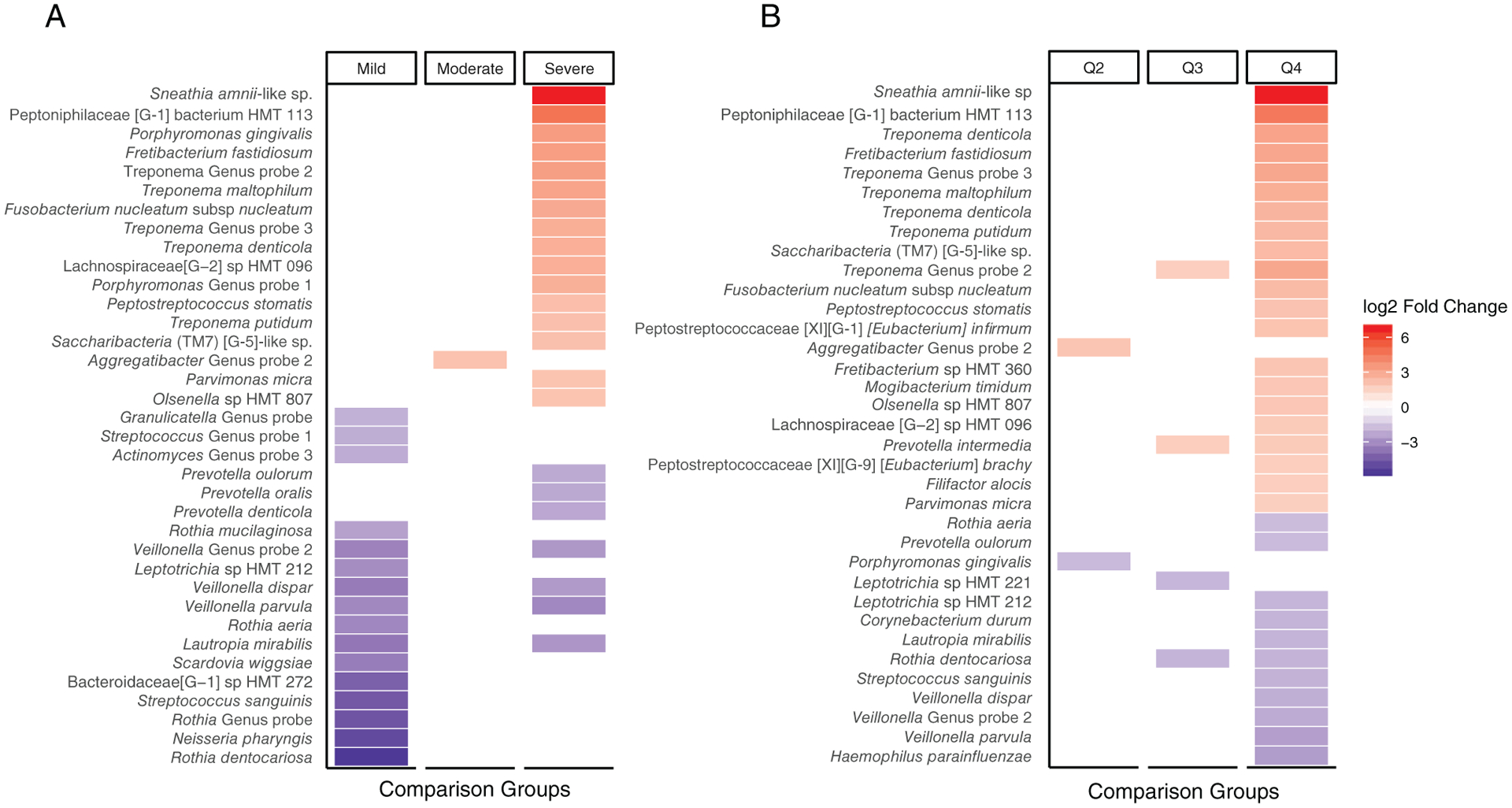
Heatmap of differential abundance between Centers for Disease Control/American Academy of Periodontology (CDC/AAP) classes (A) and quartiles of percentage teeth/person with PD ≥4 mm (B). Differential abundance testing (DESeq2, R package) was performed to determine differences between groups using ProbeSeq for Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS). Periodontal health (for CDC/AAP classes) and Q1 (quartile-based analyses) were used as the comparison groups. Bacterial taxa marked in red were statistically significantly (P <0.01) more abundant, and those marked in blue less abundant than the reference group with absolute log2foldchange >2
3.3. Relationship between bacterial diversity and clinical periodontal status
Figure 2 presents α-diversity metrics (Chao and Shannon indices) in the four CDC/AAP classes (Fig. 2A) and in the quartiles according to percentage of teeth/person with PD ≥4 mm (Fig. 2B). In general, both α-diversity metrics were higher in the presence of periodontal pathology when compared with health, irrespective of whether periodontal status was characterized by means of CDC/AAP classes or by means of quartiles of percentage teeth with ≥4 mm pockets, however, no statistically significant differences were detected between moderate versus severe periodontitis (Fig. 2A) or between Q3 and Q4 (Fig. 2B). Similar patterns, albeit somewhat attenuated, were observed in analyses adjusted for age, sex, smoking, and education level (Figs. 2C and 2D). Note that the “mild periodontitis” category was an outlier in the observed trend.
FIGURE 2.
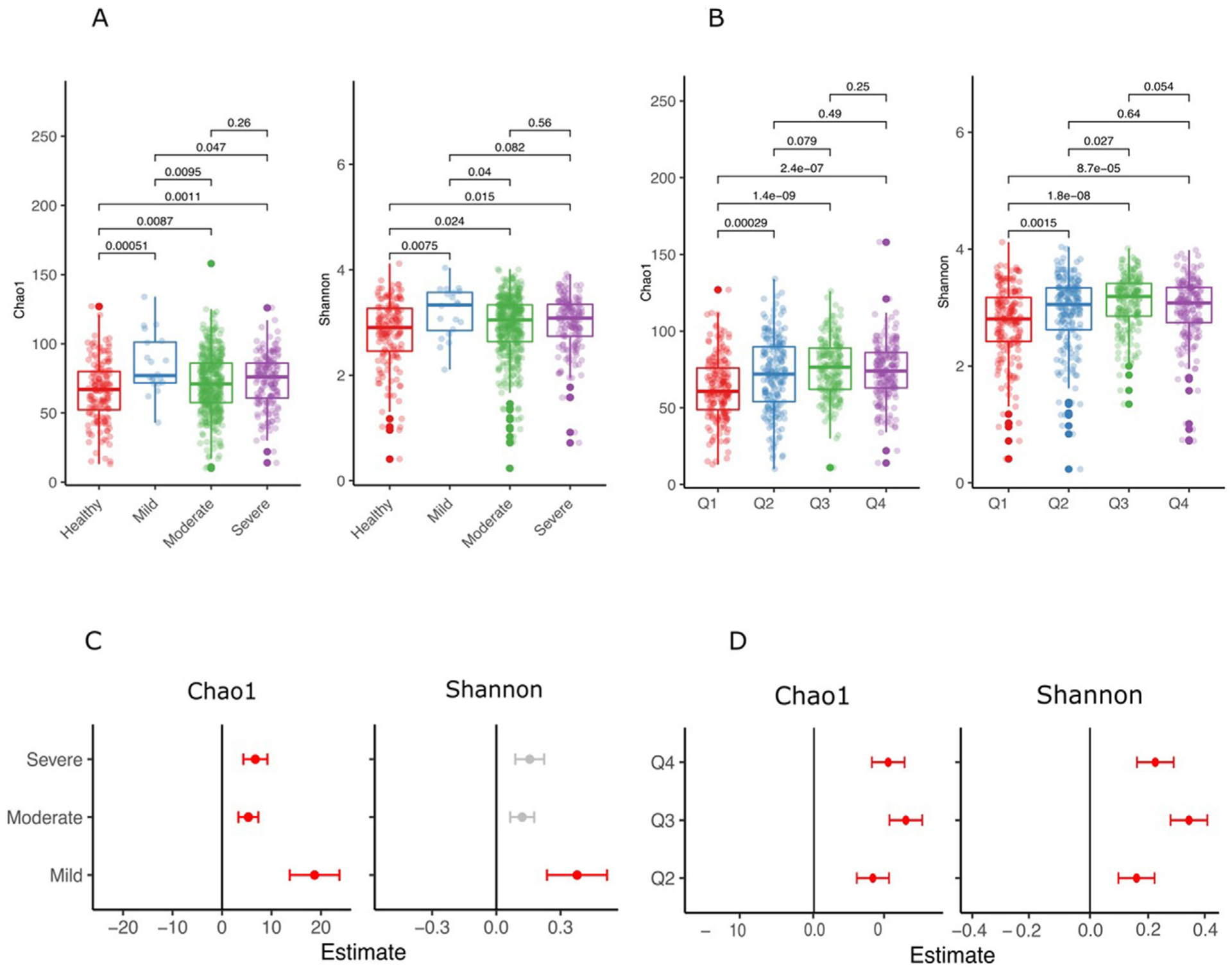
Alpha diversity using the Divisive Amplicon Denoising Algorithm Version 2 (DADA2) pipeline (Chao 1 estimator, left; Shannon index, right) according to Centers for Disease Control/American Academy of Periodontology (CDC/AAP) classes (A and C) and quartiles of percentage teeth/person with PD ≥4 mm (B and D). Statistically significant differences were tested using the non-parametric Wilcoxon Rank Sum test. The lower panels (C and D) represent a linear regression analyses adjusting for age, sex, smoking, and education level. Periodontal health (for CDC/AAP classes) and Q1 (quartile-based analyses) were used as the comparison groups. The plots illustrate the coefficients of the model and are marked in red if significantly different at P <0.05
Figure 3 describes β-diversity metrics (Bray-Curtis dissimilarity) according to periodontal status expressed through CDC/AAP classes (Fig. 3A) or through quartiles of percentage of teeth/person with PD ≥4 mm (Fig. 3B). Statistically significant differences were observed between periodontal health and mild periodontitis (P = 0.019), periodontal health and moderate periodontitis (P = 0.05), periodontal health and severe periodontitis (P = 0.001), and between moderate and severe periodontitis (P = 0.001) but not between mild and moderate periodontitis or between mild and severe periodontitis. In contrast, statistically significant differences in β-diversity emerged in all pair-wise comparisons based on quartiles of percentage of teeth/person with PD ≥4 mm.
FIGURE 3.
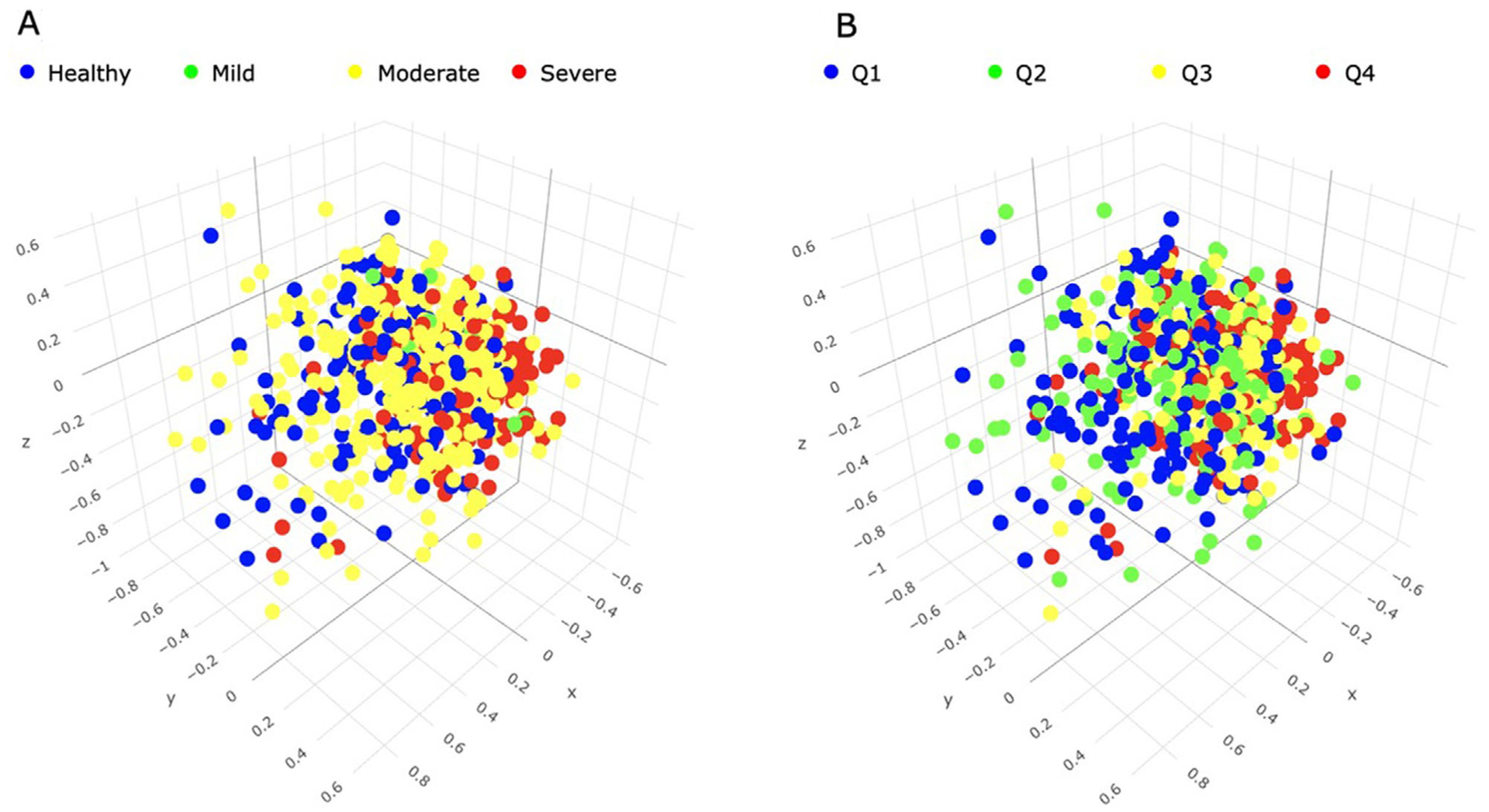
Three-dimensional Principal Coordinates Analysis (PCoA) plots of β-diversity using output from the Divisive Amplicon Denoising Algorithm Version 2 (DADA2) pipeline. Beta diversity is visualized by means of Bray-Curtis distance metrics. A) Centers for Disease Control/American Academy of Periodontology (CDC/AAP) classes; B) quartiles of percentage of teeth/person with PD ≥4 mm
4. DISCUSSION
In this study, we concomitantly examined the clinical periodontal status and the subgingival microbiome of a cohort of elderly individuals. We obtained and analyzed plaque samples by means of next generation sequencing to perform a comprehensive identification of the prevalent bacterial taxa as well as to calculate relative abundance and α- and β-diversity metrics in different states of periodontal health and disease. We classified the clinical periodontal status using both a four-level ordinal scale that is widely used in epidemiological studies (the CDC/AAP classification) and a continuous measure of periodontitis extent and severity based on the percentage of teeth per participant with PD ≥4 mm. Our findings indicate that 1) the most abundant and/or differentially enriched taxa that emerged among the distinct periodontal phenotypes in this cohort of elderly individuals were generally similar to those described in the literature for younger age groups; and 2) subgingival microbial diversity increased in parallel with the severity and extent of periodontitis.
Several methodological aspects of the present study must be emphasized to correctly interpret the findings. First, the individuals involved were a subset of elderly, community-dwelling participants in a longitudinal study of aging in a tri-ethnic population. They were not selected on the basis of any particular periodontal condition, and are thus representative of the source population with respect to both clinical periodontal status and periodontal microbiology. Importantly, the periodontal condition of the participants was assessed through a full-mouth examination (six sites per tooth at all teeth present by a single examiner according to a standardized protocol as earlier described21), therefore, the risk for a biased assessment of periodontal status due to partial recording (a common shortcoming of epidemiologic studies of periodontitis34) is not an issue in the present study.
Second, our sampling strategy called for collection of four subgingival plaque samples (each harvested from the mesio-lingual surface of the most posterior tooth in each quadrant) which were subsequently pooled into a single sample/participant. Given the established association between PD and microbial profiles,8,35–37 the mesio-lingual, rather than the commonly used mesio-buccal site was purposefully selected to avoid microbial sampling from shallower pockets due to gingival recession. Importantly, our data showed that the probing depths of the sampled sites were, on average, deeper than, but strongly positively correlated with, the whole mouth scores. Thus, a potential concern that the microbial sampling strategy used that involved fixed sites would bias the harvested microbiome towards periodontal health cannot be substantiated. However, pooling of microbial samples clearly distorts the correlation between the microbial community and the clinical characteristics of the sampled sites, and allows the most numerous and abundant taxa to “overpower” those present in lower proportions. Hence, the reported relative abundance values (Table 3 and Table S2 in online Journal of Periodontology) represent proportions of the aggregate of four individual samples and do not reflect the actual relative abundance of the particular taxa in their original habitat.
Lastly, all diversity metrics were calculated by mapping of ASV sequences to the GreenGenes database which, although inferior to HOMD with respect to precision in the taxonomy of oral bacteria, resulted in an average of only 4% unmatched reads on the species level among the 739 pooled samples analyzed.
As shown in Table 3, species of the genus Leptotrichia, Streptococcus, and Corynebacterium were among the most abundant taxa in moderate and severe periodontitis according to the CDC/AAP classification as well as in the third and fourth quartiles of percentage of teeth/person with PD ≥4 mm. In contrast, with the notable exception of Treponema denticola which ranked third in abundance in severe periodontitis and in the Q4 of pocketing, all other established periodontal pathogens were conspicuously absent from the high ranked abundant species. Thus, Tannerella forsythia ranked 73rd and 69th in severe periodontitis and Q4, respectively, with relative abundance between 0.32% and 0.35%. Corresponding values for P. gingivalis which ranked 89th in severe periodontitis and 97th in Q4 were 0.25% and 0.22%. Lastly, A. actinomycetemcomitans ranked 261st and 267th in severe periodontitis and Q4, respectively, with relative abundance of 0.03% to 0.02%. The fact that these “periodontal pathogens” comprised only a very small proportion of the subgingival microbiome is in accordance with earlier studies that reached similar conclusions using a variety of techniques including checkerboard hybridizations,38,39 16s rRNA sequencing9,40–44 and metagenomics/metatranscriptomics.45,46
Perhaps not surprisingly, given the fact that we analyzed pooled samples, there was considerable overlap between the 10 top most abundant taxa encountered in periodontal health/mild periodontitis or in Q1/Q2 and those in more severe states of disease, an observation suggesting that most of the taxa likely represent constituents of the resident microbial periodontal habitat. However, as illustrated in Figure 1, several conspicuous differences in relative abundance were noted between periodontal health and severe periodontitis and between Q1 and Q4 for several species. Although depicted differences between periodontal health and mild periodontitis should be interpreted with caution because the latter group included only 20 individuals, species such as Sneathia amnii-like sp. and Peptoniphilaceae [G-1] bacterium HMT113 were noticeably more abundant in severe periodontitis than in health, and in Q4 than in Q1. This was also observed for P. gingivalis in severe periodontitis versus health, for Filifactor alocis in Q4 versus Q1 and for Fretibacterium fastidiosum and Saccharibacteria (TM7) [G-5]-like sp. in both the severe periodontitis versus health, and the Q4 versus Q1 comparisons. In contrast, species such as Veillonella parvula, Veillonella dispar, Rothia dentocariosa, and Lautropia mirabilis were significantly less abundant in severe periodontitis and Q4 when compared with periodontal health and Q1, respectively. However, the differences in bacterial profiles between the various periodontitis-related phenotypes observed in the present study were generally less pronounced than those earlier documented in studies of rather limited size (such as a comparison between 29 periodontally healthy individuals with 29 chronic periodontitis patients,41 and a comparative analysis of plaque samples from 30 post-menopausal women with or without periodontitis16), or those detected in a recent large study of 1,206 women aged >50 years.47
What is in agreement with the above studies and additional publications in the literature48,49 is the significant association between α- (Fig. 2) and β-diversity (Fig. 3) and the extent and severity of periodontitis, suggesting that periodontitis is not a “classical infection” where a single, exogenous pathogen dominates the niche in the state of disease but is rather characterized by progressive microbial dysbiosis characterized by relative enrichment of the habitat by resident bacterial species. However, a decrease in α-diversity from health to periodontitis has also been documented in the literature50 and has been proposed to support the keystone species hypothesis.15 As expected, the observed differences in the diversity metrics in our study became more pronounced when comparing extreme rather that consecutive phenotypic classes, and remained statistically significant for both the ordinal and the continuous phenotypes after adjustment for age, sex, smoking, and education level.
Lastly, the findings of the present study suggest that segregation of the participants according to periodontal status by means of a continuous measure of extent and severity of periodontitis (i.e., percentage of teeth/person with ≥4 mm deep pockets) than by an ordinal, categorical scale (i.e., the CDC/AAP classes) seemed to translate in more distinct bacterial profiles between the clinical phenotypes. Indeed, differences in bacterial abundance (Fig. 1) and α-diversity (Fig. 2) were more pronounced when performing quartile-based than CDC/AAP class-based comparisons. This appears reasonable, as increased pocketing creates an environment conducive of ecological shifts towards dysbiosis, while attachment loss per se (a component in the CDC/AAP classification) does not. The latter finding was also substantiated by exploratory analyses with attachment loss-based quartiles that did not correlate with distinct microbial profiles (data not shown).
5. CONCLUSIONS
The present findings, derived from a sizeable cohort of elderly, community-dwelling individuals who were not pre-selected on the basis of their clinical periodontal status, add to the sparse literature on the bacterial ecology of the aging mouth, and are consistent with the concept of periodontitis being associated with perturbations in the resident subgingival microbiome.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by grants from the National Institutes of Health (DE022568 and AG037212) and the National Center for Advancing Translational Sciences (TR000040), Bethesda, MD. Dr. Annavajhala was supported by a training grant TL1 TR001875 through the Irving Institute Clinical and Translational Science Award, Columbia University, New York, NY. The authors report no conflicts of interest related to this study. Portions of this paper were presented by Dr. Papapanou at the Day of Celebration for Steven Offenbacher. The symposium was hosted in October 2019 by the University of North Carolina Adams School of Dentistry and supported by Colgate-Palmolive Company and the American Academy of Periodontology. Colgate-Palmolive Company provided generous support for publication of this Journal of Periodontology supplement but had no involvement with the content.
Footnotes
Gracey 11/12, Hu-Friedy, Chicago, IL.
MiSeq, Illumina, San Diego, CA.
R Statistical Package, version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 2016;221:657–666. [DOI] [PubMed] [Google Scholar]
- 3.Methé BA, Nelson KE, Pop M, et al. A framework for human microbiome research. Nature. 2012;486:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems. 2018;3(6):e00187–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Listgarten MA, Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978;5:115–132. [DOI] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD, Dzink JL. Relationship of subgingival microbial complexes to clinical features at the sampled sites. J Clin Periodontol. 1988;15:440–444. [DOI] [PubMed] [Google Scholar]
- 7.Papapanou PN, Sellén A, Wennström JL, Dahlén G. An analysis of the subgingival microflora in randomly selected subjects. Oral Microbiol Immunol. 1993;8:24–29. [DOI] [PubMed] [Google Scholar]
- 8.Papapanou PN, Baelum V, Luan W-M, et al. Subgingival microbiota in adult Chinese: prevalence and relation to periodontal disease progression. J Periodontol. 1997;68:651–666. [DOI] [PubMed] [Google Scholar]
- 9.Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socransky SS, Haffajee AD, Cugini MA, Smith C. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. [DOI] [PubMed] [Google Scholar]
- 11.Kuramitsu HK. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit Rev Oral Biol Med. 2003;14:331–344. [DOI] [PubMed] [Google Scholar]
- 12.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. [DOI] [PubMed] [Google Scholar]
- 13.Darveau RP. Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. [DOI] [PubMed] [Google Scholar]
- 14.Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013;62:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaMonte MJ, Genco RJ, Zheng W, et al. Substantial differences in the subgingival microbiome measured by 16S metagenomics according to periodontitis status in older women. Dent J (Basel). 2018;6(4):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ageing and Health. Fact Sheet # 404. Available at: http://www.who.int/mediacentre/factsheets/fs404/en/. Accessed April 30, 2020.
- 18.Lamster IB. Geriatric periodontology: how the need to care for the aging population can influence the future of the dental profession. Periodontol 2000. 2016;72:7–12. [DOI] [PubMed] [Google Scholar]
- 19.Feres M, Teles F, Teles R, Figueiredo LC, Faveri M. The subgingival periodontal microbiota of the aging mouth. Periodontol 2000. 2016;72:30–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- 21.Shariff JA, Burkett S, Watson CW, Cheng B, Noble JM, Papapanou PN. Periodontal status among elderly inhabitants of northern Manhattan: the WHICAP ancillary study of oral health. J Clin Periodontol. 2018:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in surveillance of periodontitis: the Centers for Disease Control and Prevention periodontal disease surveillance project. J Periodontol. 2012;83:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford). 2010;2010:baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ. Microbiomes of endodontic-periodontal lesions before and after chemomechanical preparation. J Endod. 2015;41:1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belstrøm D, Paster BJ, Fiehn NE, Bardow A, Holmstrup P. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J Oral Microbiol. 2016;8:30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer RJ, Cotton SL, Kokaras AS, et al. Analysis of oral bacterial communities: comparison of HOMINGS with a tree-based approach implemented in QIIME. J Oral Microbiol. 2019;11:1586413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon CE. A mathematical theory of communication. Bell Syst Techn J. 1948;27:379–423. [Google Scholar]
- 30.Chao A Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11:265–270. [Google Scholar]
- 31.Spellerberg IF, Fedor PJ. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Global Ecol Biogeogr. 2003;12:177–179. [Google Scholar]
- 32.McMurdie PJ, Phyloseq HS. An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 34.Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal disease. J Periodontol. 2005;76:262–267. [DOI] [PubMed] [Google Scholar]
- 35.Papapanou PN, Behle JH, Kebschull M, et al. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol. 2009;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Chaparro PJ, McCulloch JA, Mamizuka EM, et al. Do different probing depths exhibit striking differences in microbial profiles. J Clin Periodontol. 2018;45:26–37. [DOI] [PubMed] [Google Scholar]
- 37.Shi M, Wei Y, Hu W, Nie Y, Wu X, Lu R. The Subgingival microbiome of periodontal pockets with different probing depths in chronic and aggressive periodontitis: a pilot study. Front Cell Infect Microbiol. 2018;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. [DOI] [PubMed] [Google Scholar]
- 39.Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 2013;62:95–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and site-specificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009;28:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruyama N, Maruyama F, Takeuchi Y, Aikawa C, Izumi Y, Nakagawa I. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci Rep. 2014;4: 6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong KW, Shin MS, Ahn YB, Lee HJ, Kim HD. Genomewide association study on chronic periodontitis in Korean population: results from the Yangpyeong health cohort. J Clin Periodontol. 2015;42(8):703–710. [DOI] [PubMed] [Google Scholar]
- 44.Boutin S, Hagenfeld D, Zimmermann H, et al. Clustering of subgingival microbiota reveals microbial disease ecotypes associated with clinical stages of periodontitis in a cross-sectional study. Front Microbiol. 2017;8:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duran-Pinedo AE, Chen T, Teles R, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duran-Pinedo AE, Yost S, Frias-Lopez J. Small RNA Transcriptome of the oral microbiome during periodontitis progression. Appl Environ Microbiol. 2015;81(19):6688–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genco RJ, LaMonte MJ, McSkimming DI, et al. The subgingival microbiome relationship to periodontal disease in older women. J Dent Res. 2019;98(9):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu G, Dye BA, Gail MH, et al. The association between the upper digestive tract microbiota by HOMIM and oral health in a population-based study in Linxian, China. BMC Public Health. 2014;14:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meuric V, Le Gall-David S, Boyer E, et al. Signature of microbial dysbiosis in periodontitis. Appl Environ Microbiol. 2017;83(14):e00462–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai D, Huang R, Wen J, Li C, Zhu J, Xia LC. Integrated metagenomic data analysis demonstrates that a loss of diversity in oral microbiota is associated with periodontitis. BMC Genomics. 2017;18(Suppl 1):1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


