Abstract
Background:
Mindfulness is the practice of awareness and living in the present moment without judgment. Mindfulness-based interventions may improve dementia-related outcomes. Before initiating interventions, it would be beneficial to measure baseline mindfulness to understand targets for therapy and its influence on dementia outcomes.
Objective:
This cross-sectional study examined patient and caregiver mindfulness with patient and caregiver rating scales and patient cognitive performance and determined whether dyadic pairing of mindfulness influences patient outcomes.
Methods:
Individuals (N = 291) underwent comprehensive evaluations, with baseline mindfulness assessed using the 15-item Applied Mindfulness Process Scale (AMPS). Correlation, regression, and mediation models tested relationships between patient and caregiver mindfulness and outcomes.
Results:
Patients had a mean AMPS score of 38.0 ± 11.9 and caregivers had a mean AMPS score of 38.9 ± 11.5. Patient mindfulness correlated with activities of daily living, behavior and mood, health-related quality of life, subjective cognitive complaints, and performance on episodic memory and attention tasks. Caregiver mindfulness correlated with preparedness, care confidence, depression, and better patient cognitive performance. Patients in dyads with higher mindfulness had better cognitive performance, less subjective complaints, and higher health-related quality of life (all p-values<0.001). Mindfulness effects on cognition were mediated by physical activity, social engagement, frailty, and vascular risk factors.
Conclusion:
Higher baseline mindfulness was associated with better patient and caregiver outcomes, particularly when both patients and caregivers had high baseline mindfulness. Understanding the baseline influence of mindfulness on the completion of rating scales and neuropsychological test performance can help develop targeted interventions to improve well-being in patients and their caregivers.
Keywords: Alzheimer’s disease, dementia, inflammation, mild cognitive impairment, mindfulness
INTRODUCTION
Alzheimer’s disease and related dementias (AD RD) are significant public health challenges, affecting more than 5 million people in the US [1] and more than 50 million worldwide [2]. ADRD not only result in progressive cognitive impairment but lead to interference with activities of daily living, behavioral changes, increased caregiver burden and stress, and rising health care costs [3]. By year 2050, the number of ADRD cases is expected to increase as the number of people over 65 years of age grows by 62% and the number over 85 years of age is expected to grow by 84% [1]. More than one in eight adults over age 65 has dementia, and current projections indicate a three-fold increase by 2050 [1]. Mild cognitive impairment (MCI) is a prodromal state that often progresses to ADRD [4]. At present, treatments for ADRD are largely symptomatic with modest effects, and there are no approved treatments for MCI.
A large number of modifiable (e.g., exposures, lifestyle, and social habits) and nonmodifiable (e.g., age, sex, genetics) risk factors has been identified [5-8]. The available literature suggests that up to 30% of ADRD cases could potentially be preventable through modification of risk factors and behavioral changes to mitigate the effect of unmodifiable risk factors [5-8]. Multiple lines of evidence from epidemiological and longitudinal observational studies exist that suggest that the risk of ADRD appears to be reduced in individuals who are physically [9, 10] and cognitively active [11, 12], socially engaged [13, 14], have higher educational attainment and cognitive reserve [15, 16], and eat a heart- and brain-healthy diet [17, 18]. There is increasing interest in lifestyle modification [19-21], integrative medicine [22], and personalized medicine [23] approaches to treat and/or prevent dementia.
Another potential modifiable risk factor is mindfulness [24]. Mindfulness is the practice of awareness and living in the present moment without judgment [25-27]. Mindfulness practice has been associated with cognitive benefits such as improved attention, working memory, and executive function in cognitively healthy older adults [28-30] and includes activities such as yoga, meditation, breathing exercises, and relaxation [27, 31, 32]. However, to date there is limited understanding of the degree to which mindfulness practices and skills are applied in daily life in older adults when life stressors are encountered [33]. In that regard, mindfulness practice refers to the use of formal and informal techniques and approaches to achieving mindfulness as the moment unfolds [28], rather than as the result of any intervention. It is therefore useful to measure an individual’s use of mindfulness practice [34, 35], and how mindfulness influences potential outcomes of interest [36, 37]. Although mindfulness-based research in older adults is still in its infancy, the available evidence support that mindfulness-based interventions may reduce anxiety in older adults [38, 39], and offer cognitive benefits in the areas of memory and attention [40, 41]. Research also suggests that mindfulness-based approaches can reduce stress, depression, insomnia, feelings of loneliness, and social exclusion [39, 43], as well as cardiovascular risk factors [44]—all of which are associated with increased risk for ADRD [45]. Mindfulness practice in individuals with MCI improves memory and brain network efficiency on functional magnetic resonance imaging (MRI) [46]. However, a potential challenge in designing and implementing a mindfulness-based intervention is identifying and quantifying a baseline level of mindfulness practices in older adults before starting the intervention and how mindfulness impacts other ADRD risk factors.
Given the potential relationships between mindfulness and outcomes relevant to dementia and the potential effectiveness of mindfulness-based interventions, we hypothesized that a higher baseline level of mindfulness in the patient would be associated with better cognitive performance, activities of daily living, mood and behavior, and lower caregiver distress. As caregivers play an increasingly important role in day-to-day care management and care coordination of patients with ADRD, we also hypothesized that higher baseline levels of mindfulness in the caregiver would be associated with better patient and caregiver outcomes. We also wished to examine whether baseline mindfulness were positively correlated with other protective or resilience factors associated with ADRD such as physical activity, diet, and social engagement, and negatively correlated with risk or vulnerability factors associated with ADRD such as age, vascular risk factors, physical frailty, and multiple medical co-morbidities. Identification of the relationship between mindfulness and outcomes might provide future targets for intervention. We conducted a cross-sectional study examining baseline patient and caregiver mindfulness in relation to patient and caregiver rating scales and patient outcomes, and whether dyadic pairing of mindfulness influences patient and caregiver outcomes.
METHODS
Participants
This retrospective cross-sectional study was performed on 291 consecutive patients-caregiver dyads attending our center for clinical care or participation in cognitive aging research. During the 3-hour visit, patients and caregivers underwent a comprehensive clinical, cognitive, functional, and behavioral evaluation modeled after the Uniform Data Set (UDS) from the National Institute of Aging Alzheimer Disease Research Center Program with additional components including the primary outcome measure of mindfulness used in this study, the Applied Mindfulness Process Scale (AMPS) [33]. In addition, patients and caregivers independently completed rating scales and were independently interviewed to generate the Clinical Dementia Rating (CDR) [47]. Caregivers completed a psychosocial assessment while the patient underwent neuropsychological testing and physical and neurologic examinations. Finally, a feedback session was conducted with the patient and caregiver to review the results. All components of the assessment are part of standard of care at our center [48] and protocols in the clinic and research projects are identical. The study included older adults ranging from no dementia to individuals with moderate-to-severe dementia of any etiology. Individuals living in skilled nursing facilities were excluded. A waiver of consent was obtained for clinic patients, while prospective research participants provided written informed consent. This study was approved by the University of Miami Institutional Review Board.
Participant characteristics
Demographic information including age, sex, education, socioeconomic status, race, ethnicity, past medical history, medications, alcohol, tobacco, and substance use history, co-morbidities, and family history were collected. Socioeconomic status (SES) was calculated with combined education and occupation scores using the Hollingshead Two-Factor Index of Social Status [49].
Clinical evaluation
Standardized scales from the UDS were administered to the informants to provide ratings of cognition, function, and behavior [50, 51]. The CDR [47] was used to determine the presence or absence of dementia and to stage its severity; a global CDR 0 indicates no dementia; CDR 0.5 represents MCI or very mild dementia; CDR 1, 2, or 3 correspond to mild, moderate, or severe dementia. The CDR-SB was calculated by adding up the individual CDR categories (range: 0–18; higher scores supporting more severe impairment). Because CDR 0 includes individuals with and without subjective cognitive complaints, and CDR 0.5 includes individuals with MCI and very mild dementia, we also staged each individual using the Global Deterioration Scale (GDS) [52]. A GDS 1 indicates no cognitive impairment (NCI); GDS 2 indicates subjective cognitive impairment (SCI); GDS 3 corresponds to MCI; GDS 4–7 corresponds to mild, moderate, moderate-severe, or severe dementia.
Caregiver ratings of patient cognition, function, and behavior
The following scales were completed by the caregiver to rate the patient. Activities of daily living were captured with the Functional Activities Questionnaire (FAQ) [53]. Dementia-related behaviors and psychological features were measured with the Neuropsychiatric Inventory (NPI) [54]. The patient’s health-related quality of life was measured with the Health Utilities Index-Mark 3 (HUI-3) [55].
Cognitive evaluation
The Patient and Caregiver independently completed the Quick Dementia Rating System (QDRS) [56, 57] for a global dementia rating of the patient. The Patient completed the Cognitive Change Index (CCI) [58], the Cognitive Function Instrument (CFI) [59], and the AD8 [60] to assess subjective memory complaints. Verbal IQ was determined with the Test of Premorbid Function (Pearson Assessments, Bloomington, MN). Cognitive testing included the Montreal Cognitive Assessment [61] for a global screen, and the UDS psychometric battery supplemented with additional measures: 15-item Multilingual Naming Test (naming) [51]; Animal naming fluency (verbal fluency) [51]; Hopkins Verbal Learning Task (HVLT, episodic memory for word lists – immediate and delayed recall) [62]; Number forward/backward tests (working memory) [51]; Trailmaking A and B (processing and visuospatial abilities) [63]; the Number-Symbol Coding Test (executive function) [64] and the Noise-Pareidolia test (visual perception) [65]. The cognitive test battery was combined to create a composite sum of z-scores. The Hospital Anxiety and Depression Scale (HADS) [66] was performed for distinct ratings of depression and anxiety.
Diagnoses
Global rating scales (CDR, GDS) were combined with cognitive performance, the neurologic examination, and laboratory tests to assign individuals to the following diagnostic categories after a consensus conference: Cognitively normal controls, MCI [4], or Dementia. Dementia diagnoses were determined using standard criteria for Alzheimer’s disease (AD) [67], dementia with Lewy bodies (DLB) [68], vascular contributions to cognitive impairment and dementia (VCID) [69], and frontotemporal degeneration (FTD) [70].
Ratings of caregiver characteristics
Caregivers completed ratings of care confidence, care preparedness, burden, mood, and appraisals of caregiving. Care Confidence was adapted from the Dementia Care Confidence scale [71] and used in prior studies [72] consisting of 4 questions scored on a 0–4 Likert Scale (range 0–16, higher scores are better). Caregiver preparedness was assessed with the Preparedness for Caregiving Scale [73]. Caregiver burden was captured with the 12-item Zarit Burden Inventory [74]. Caregiver mood was assessed using the Personal Health Questionnaire-4 (PHQ4) [75]. An assessment of the overall caregiver experience was captured with the Positive and Negative Appraisals of Caregiving (PANAC) scale [72], providing separate scores for Positive Appraisals and Negative Appraisals.
Mindfulness evaluation
Mindfulness was measured in this study with the Applied Mindfulness Process Scale (AMPS) [33]. Patients and caregivers were asked independently to indicate how often they used mindfulness principles to confront negative and stressful events in daily life over the past week; however, the AMPS does not require the respondent to specify the type of mindfulness they practice. Responses were reported on a 5-point Likert scale from never to almost always and summed up for a total score that ranges from 0–60 with higher scores indicative of greater use of mindfulness practice. Three factors underly the AMPS including decentering or deidentifying from negative thoughts and feelings; positive emotion regulation, which involves coping by refocusing on positive emotions and positively reappraising adverse life events; and negative emotion regulation, which reflects the use of mindfulness to cope by reducing emotional distress. Besides the total score, scores for each of the three factors were also calculated by summing five items per factor for a score ranging from 0–20. The AMPS was previously reported to have strong internal consistency reliability (Cronbach’s α = 0.91) and item-total reliability (range = 0.51–0.72) in cognitively healthy individuals [33]. Using the lowest quartile of global mindfulness and individual mindfulness factors as a cutoff, we created dichotomous variables to capture low mindfulness status as compared to normal/high values, separately for patients and informants. We then combined these dichotomous mindfulness variables to create a 4-level variable that captures the combination of low patient and/or informant mindfulness: Group 1: high patient and informant mindfulness; Group 2: low patient and high informant mindfulness; Group 3: high patient and low informant mindfulness; Group 4: low patient and informant mindfulness.
Assessment of resilience (protective) factors
Educational attainment was captured as the years of formal schooling ranging from 0–20, with any post-graduate training being capped at 20 years. The Quick Physical Activity Rating (QPAR) [76] was used to determine the dosage of physical activity the patient participates over a 4-week period. Scores range from 0–153 with higher scores representing greater participation in physical activity. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet scoresheet [77] was used to determine the extent to which the patient follows the Mediterranean-DASH diet. The scoresheet captures 15 food categories and frequencies with scores ranging from 0–15. Higher scores represent greater adherence to the MIND diet. Social engagement was captured by an investigator-generated question that ask “How would you rate the participant’s overall socialization?” scored on a Likert scale using anchors (poor, fair, good, excellent) with scores ranging from 1–4 with higher scores representing greater social engagement.
Assessment of vulnerability (risk) factors
Age was reported as years at time of assessment. Medical co-morbidities were captured with the Charlson Comorbidities Index [78]. Vascular risk factors were captured with a modified version of the Cardiovascular Risk Factors, Aging, and Dementia (mCAIDE) scale [79, 80] with scores ranging from 0–14 with higher scores representing higher risk of vascular disease. Physical frailty was assessed with the Fried Frailty Phenotype [81], a five-factor frailty index which includes muscle weakness, slow gait, fatigue, physical inactivity, and weight loss. Scores of 1–2 are rated as pre-frailty and scores of 3 or greater support the presence of frailty.
Apolipoprotein E genotyping
Apolipoprotein E (APOE) genotyping was performed by True Health Diagnostics LLC (Richmond, VA). Six possible allelic combinations were obtained with individuals dichotomized as being APOE ε4 carriers or non-carriers.
Volumetric MRI
A subset of individuals (n = 76) underwent volumetric MRI with NeuroQuant software (CorTechs Labs, San Diego, CA), an FDA-approved automated quantitative analysis of brain MRI images with normative reference data adjusted for age, sex, and intracranial volume with high correlation to FreeSurfer [82] and visual assessment [83]. Hippocampal volumes were used as a measure of brain health and a biomarker of neurodegeneration [84].
Statistical analysis
Analyses were conducted using SAS University Edition (Cary, NC). Descriptive statistics were used to examine demographic characteristics of patients and caregivers, informant and patient rating scales, dementia staging paradigms, and neuropsychological testing. One-way analysis of variance (ANOVA) with Tukey post-hoc tests were used for continuous data and Chi-square analyses were used for categorical data. Pearson correlation coefficients were used to test strength of association between patient and caregiver AMPS scores and outcome measures. Internal consistency was examined as the proportion of the variability in the responses that is the result of differences in respondents, reported as the Cronbach alpha reliability coefficient. Coefficients greater than 0.7 are good measures of internal consistency [56, 57].
Strength of association was assessed comparing AMPS scores with performance on each Gold Standard measure of cognition (e.g., CDR, neuropsychological testing), function (i.e., FAQ), behavior (e.g., NPI, HADS), caregiver ratings (e.g., ZBI, PHQ-4), resilience (e.g., physical activity, diet), vulnerability (e.g., age, frailty), and hippocampal atrophy using Pearson correlation coefficients following the unified framework of construct validity [85]. Separate analyses were run for total mindfulness score and individual mindfulness factors. AMPS scores were plotted with fitted regression lines against subjective complaints (patient QDRS), global cognition (Mo CA), depression (HADS), and hippocampal volumes by cognitive status (cognitively healthy controls, MCI, dementia) to test whether higher AMPS scores were associated with better outcomes. Known-group validity was assessed by examining the AMPS scores by patient characteristics, APOE status, frailty ratings, CDR and GDS staging, and dementia etiology [57, 76]. Correction for multiple comparisons was performed using Bonferroni corrections.
Patient and caregiver factors and cognitive performance tests, which were significantly associated with both patient and caregiver mindfulness, were further investigated in relation to combined patient/caregiver mindfulness status with linear regression analysis. Three models were investigated, an unadjusted model comparing patient/caregiver mindfulness status on individual reported or performance-based measures of cognition, mood, functionality, quality of life, and caregiving experience. A second model further adjusted for disease severity with CDR global, and a third model added age as a covariate in addition to disease severity.
Mediation analyses for cross-sectional data were used to assess whether protective- and risk factors help explain at least in part the effect of patient mindfulness on cognitive performance as measured by z-scores on MoCA, memory, and attention-executive functionality (i.e., memory recall and delay, Trail Making B, and symbol-number coding task), as well as by the composite z-score for the neuropsychological battery. To reduce the likelihood of type 1 error, only patient-related factors found to be significantly related to mindfulness were assessed as potential mediators. We then used bootstrapping to obtain an estimation of indirect effects (ab path effects), separately by mediator. Bootstrapping involves re-sampling the data multiple times (1,000 resamples were drawn in this study) and estimating an indirect effect across these resamples along with confidence intervals around it to assess its significance. The following effects were also assessed: a path = effect of predictor on mediator; b path = effect of mediator on outcome; c path = total effect of predictor on outcome; c’ path = direct effect of predictor on outcome; and ab/c=proportion of effect that is mediated.
RESULTS
Sample characteristics
Sample characteristics are shown in Table 1. The patients had a mean age of 74.7 ± 9.4 years (range 38–98 y) with a mean education of 15.8 ± 2.9 years (range 6–20 y). The sample was 48.6% female, 39.8% APOE ε4 carriers, and 97.3% White with 5.5% of the sample reporting Hispanic ethnicity. The patients had a mean CDR-SB of 3.9 ± 4.1 (range 0–18) and a mean MoCA score of 19.7 ± 6.8 (range 2–30). The sample covered a range of CDR stages including 50 CDR 0, 125 CDR 0.5, 61 CDR 1, 44 CDR 2, and 10 CDR 3. The sample covered a range of GDS stages including 27 GDS 1, 22 GDS 2, 94 GDS 3, 71 GDS 4, 46 GDS 5, and 30 GDS 6 individuals. Consensus clinical diagnoses included 49 cognitively normal controls, 95 MCI, 56 AD, 70 DLB, 11 VCID, and 9 FTD individuals. One CDR 0 individual had MCI with a non-memory domain impairment. The caregivers had a mean age of 55.9 ± 15.2 years (range 20–76 y) with a mean education of 15.9 ± 2.6 years (range 4–20 y). Caregivers were 64.1% female and 69.5% were spouses. Patients had a mean AMPS score of 38.0 ± 11.9 (range 0–60) and caregivers had a mean AMPS score of 38.9 ± 11.5 (range 0–60). Internal consistency for the patient AMPS was excellent with a Cronbach’s alpha = 0.926 (95% CI:0.906–0.944, p < 0.001). Internal consistency for the caregiver AMPS was also excellent with a Cronbach’s alpha = 0.922 (95% CI:0.899–0.941, p < 0.001).
Table 1.
Sample Characteristics
| Patient Characteristics |
Caregiver Characteristics |
||||
|---|---|---|---|---|---|
| Variable | Mean (SD) | Range | Variable | Mean (SD) | Range |
| Age, y | 74.7 (9.4) | 38–98 | Age, y | 55.9 (15.2) | 20–76 |
| Sex, %F | 48.6 | Sex, %F | 64.1 | ||
| Education, y | 15.8 (2.9) | 6–20 | Education, y | 15.9 (2.6) | 4–20 |
| Race, %White | 97.3 | Race, % White | 91.3 | ||
| Hispanic, % | 5.5 | Hispanic, % | 7.5 | ||
| Hollingshead Index | 24.1 (12.2) | 11–65 | Relationship, %Spouse | 69.5 | |
| QDRS, patient version | 3.9 (4.2) | 0–24.5 | QDRS, caregiver version | 5.4 (5.1) | 0–24 |
| FAQ | 8.1 (9.2) | 0–30 | PANAC – positive | 13.2 (6.8) | 0–32 |
| NPI | 6.2 (5.7) | 0–27 | PANAC – negative | 19.6 (5.8) | 0–31 |
| HUI3 | 0.57 (0.32) | −0.21–1.40 | Preparedness | 21.4 (6.5) | 0–32 |
| Charlson | 2.3 (1.7) | 0–8 | Care Confidence | 10.5 (2.9) | 0–16 |
| CCI | 4.3 (3.7) | 0–80 | Caregiver Burden | 12.2 (11.9) | 0–48 |
| CFI | 24.8 (19.7) | 0–15 | Caregiver Depression | 2.1 (1.6) | 0–12 |
| AD8 | 2.6 (2.2) | 0–8 | Caregiver AMPS total score | 38.9 (11.5) | 0–60 |
| HADS-Anxiety | 5.6 (3.7) | 0–21 | |||
| HADS-Depression | 6.1 (3.9) | 0–19 | |||
| MoCA | 19.7 (6.8) | 2–30 | |||
| CDR-SB | 3.9 (4.1) | 0–18 | |||
| Fried Frailty | 2.1 (1.4) | 0–5 | |||
| Patient AMPS total score | 38.0 (11.9) | 0–60 | |||
Mean (SD) or %. AMPS, Applied Mindfulness Process Scale; QDRS, Quick Dementia Rating System; FAQ, Functional Activities Questionnaire; NPI, Neuropsychiatric Inventory; HUI3, Health Utilities Index Mark 3; CCI, Cognitive Change Index; CFI, Cognitive Function Instrument; HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment; CDR-SB, Clinical Dementia Rating Sum of Boxes; PANAC, Positive and Negative Appraisals of Caregiving.
Patient mindfulness by known groups
Patient mindfulness was compared between patient age, sex, education, race and ethnicity, SES class, APOE status, verbal IQ, frailty status, CDR and GDS stages, and dementia etiologies in Table 2. There were no differences in patient mindfulness scores by age, sex, education, SES, race/ethnicity, verbal IQ, or APOE carrier status. AMPS were significantly different between individuals with no frailty, pre-frailty, and frailty (p < 0.001). AMPS scores were highest in CDR 0 individuals (45.1 ± 10.8) and were different from other CDR stages (p < 0.001), as were CDR 0.5 (38.9 ± 10.9). AMPS were however not different between CDR 1–3. Patient mindfulness was highest in GDS 1 NCI individuals (47.4 ± 8.6) and different from other GDS stages (p < 0.001). While other GDS stages were not significantly different from each other, a trend for a decline in patient AMPS from GDS 2 (SCI) to GDS 6 (moderate dementia) was noted. Cognitively healthy controls had significantly higher AMPS scores (44.8 ± 10.7, p < 0.001) than individuals with dementia etiologies other than FTD but were not different from MCI. Participants with VCID reported the lowest levels of mindfulness, however due to the low absolute numbers of VCID and FTD individuals, results comparing dementia etiologies should be interpreted with caution.
Table 2.
Mindfulness by Patient Characteristics, CDR and GDS Stage, and Dementia Etiology
| Age |
Sex |
|||||||
|---|---|---|---|---|---|---|---|---|
| <60 y | 60–69 y | 70–79 y | 80 + y | p | Male | Female | p | |
| AMPS | 41.3 (8.5) | 38.2 (12.3) | 38.6 (11.9) | 36.4 (12.1) | 0.303 | 36.8 (11.9) | 39.3 (11.8) | 0.081 |
| Race/Ethnicity |
Socioeconomic Class |
|||||||
| White | Black | Hispanic | p | Upper | Middle | Lower | p | |
| AMPS | 38.1 (12.0) | 44.3 (9.5) | 33.1 (9.6) | 0.116a | 38.4 (12.2) | 38.9 (12.1) | 37.6 (11.7) | 0.875 |
| Education |
Verbal IQ |
|||||||
| <12 y | 13–16 y | >16 y | p | <100 | 100–120 | >120 | p | |
| AMPS | 36.6 (11.6) | 37.5 (12.6) | 39.5 (10.9) | 0.271 | 35.9 (14.8) | 39.9 (9.2) | 44.0 (9.0) | 0.092 |
| Fried Frailty Scores |
APOE ε4 Status |
|||||||
| No Frailty | Pre-Frailty | Frailty | p | Non-carrier | Carrier | p | ||
| AMPS | 44.1 (10.3) | 39.0 (11.2) | 34.6 (12.0) | <0.001b | 38.6 (12.0) | 40.0 (11.8) | 0.465 | |
| Staging – Clinical Dementia Rating |
||||||||
| CDR 0 | CDR 0.5 | CDR 1 | CDR 2 | CDR 3 | p | |||
| AMPS | 45.1 (10.8) | 38.9 (10.9) | 33.5 (11.9) | 34.2 (11.5) | 35.8 (13.5) | <0.001c | ||
| Staging – Global Deterioration Scale |
||||||||
| GDS 1 | GDS 2 | GDS 3 | GDS 4 | GDS 5 | GDS 6 | p | ||
| AMPS | 47.4 (8.6) | 41.8 (12.3) | 39.3 (10.9) | 35.7 (11.5) | 34.7 (12.3) | 34.2 (12.1) | <0.001d | |
| Consensus Diagnosis |
||||||||
| Control | MCI | AD | DLB | VCID | FTD | p | ||
| AMPS | 44.8 (10.7) | 39.4 (10.9) | 37.2 (12.4) | 34.1 (10.8) | 28.2 (11.8) | 36.1 (13.2) | <0.001e | |
Mean (SD).
Results should be interpreted with caution as the number of African Americans and Hispanics is low.
Frailty stages are different from each other.
CDR 0 different from other CDR stages, CDR 0.5 different from other CDR stages, CDR 1-3 not different from each other.
GDS 1 different from other GDS stages, other stages not different from each other.
Controls different from AD, DLB and VCID, MCI different from DLB and VCID, FTD not different from other Dx. AMPS, Applied Mindfulness Process Scale; GDS, Global Deterioration Scale; MCI, mild cognitive impairment; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; VCID, vascular contributions to cognitive impairment and dementia; FTD, frontotemporal degeneration.
Association of patient and caregiver mindfulness with caregiver rating scales
The strength of association between patient and caregiver AMPS and caregiver-completed ratings is shown in Table 3. Patient mindfulness showed moderate inverse correlations (all p-values<0.001) with caregiver ratings of global cognitive status (QDRS, R = −0.304), function (FAQ, R = −0.283), behavior (NPI, R = −0.294), dementia severity (CDR-SB, R = −0.255) and a positive correlation with patient health-related quality of life (HUI3, R = 0.326, p < 0.001). Patient mindfulness was inversely correlated with negative appraisals of caregiving (PA NAC-Negative, R = −0.218, p < 0.001) but was not associated with caregiver preparedness, confidence, burden, depression, or positive appraisals. Amongst the AMPS factors, Factor 2 (positive emotional regulation) was most strongly correlated with the same constructs as the total AMPS score.
Table 3.
Correlation of Patient and Caregiver Mindfulness with Caregiver Ratings
| Caregiver Rating | Patient Mindfulness |
Caregiver Mindfulness |
||||||
|---|---|---|---|---|---|---|---|---|
| AMPS Total | AMPS Factor 1 | AMPS Factor 2 | AMPS Factor 3 | AMPS Total | AMPS Factor 1 | AMPS Factor 2 | AMPS Factor 3 | |
| QDRS, Caregiver | −0.304 (<0.001) | −0.203 (0.007) | −0.292 (<0.001) | −0.197 (0.009) | −0.154 (0.01) | −0.085 (0.26) | −0.204 (0.007) | −0.108 (0.15) |
| FAQ | −0.284 (<0.001) | −0.216 (0.004) | −0.275 (<0.001) | −0.183 (0.01) | −0.162 (0.007) | −0.113 (0.134) | −0.237 (0.002) | −0.140 (0.06) |
| NPI | −0.294 (<0.001) | −0.236 (0.002) | −0.331 (<0.001) | −0.296 (<0.001) | −0.116 (0.06) | −0.079 (0.29) | −0.176 (0.02) | −0.163 (0.03) |
| HUI3 | 0.326 (<0.001) | 0.210 (0.006) | 0.340 (<0.001) | 0.279 (<0.001) | 0.222 (<0.001) | 0.181 (0.02) | 0.289 (<0.001) | 0.169 (0.03) |
| CDR-SB | −0.255 (<0.001) | −0.188 (0.01) | −0.242 (0.001) | −0.164 (0.03) | −0.123 (0.04) | −0.059 (0.43) | −0.157 (0.04) | −0.083 (0.27) |
| PANAC-Positive | 0.105 (0.08) | 0.139 (0.07) | 0.234 (0.002) | 0.156 (0.04) | 0.196 (0.001) | 0.245 (0.001) | 0.278 (<0.001) | 0.051 (0.50) |
| PANAC-Negative | −0.218 (<0.001) | −0.101 (0.190) | −0.267 (<0.001) | −0.204 (0.008) | −0.280 (<0.001) | −0.133 (0.08) | −0.307 (<0.001) | −0.239 (0.002) |
| Caregiver Preparedness | 0.156 (0.01) | 0.105 (0.21) | 0.202 (0.01) | 0.176 (0.04) | 0.249 (<0.001) | 0.188 (0.02) | 0.241 (0.004) | 0.141 (0.09) |
| Care Confidence | 0.038 (0.53) | 0.054 (0.49) | 0.030 (0.70) | 0.032 (0.68) | 0.202 (0.001) | 0.141 (0.07) | 0.256 (0.001) | 0.207 (0.007) |
| Caregiver Burden | −0.055 (0.38) | −0.027 (0.73) | −0.156 (0.05) | −0.085 (0.29) | −0.122 (0.05) | 0.075 (0.35) | −0.222 (0.005) | −0.153 (0.05) |
| Caregiver Depression | −0.110 (0.08) | −0.158 (0.04) | −0.207 (0.007) | −0.177 (0.02) | −0.267 (<0.001) | −0.200 (0.01) | −0.303 (<0.001) | −0.273 (<0.001) |
Pearson coefficient (p-value). Bold signifies significance after Bonferroni Correction for multiple comparisons (corrected p < 0.005). AMPS, Applied Mindfulness Process Scale; QDRS, Quick Dementia Rating System; FAQ, Functional Activities Questionnaire; NPI, Neuropsychiatric Inventory; HUI3, Health Utilities Index Mark 3; CDR-SB, Clinical Dementia Rating Sum of Boxes; PANAC, Positive and Negative Appraisals of Caregiving.
Caregiver mindfulness was correlated with patient health-related quality of life (HUI3, R = 0.222, p < 0.001) but not with caregiver rated QDRS, FAQ, NPI, or CDR-SB scores (Table 3). Higher caregiver mindfulness was correlated with caregiver positive appraisals of caregiving (PANAC-Positive, R = 0.196), caregiver preparedness (R = 0.249), and care confidence (R = 0.202) and inversely correlated with caregiver depression (R = −0.267). Similar to patient mindfulness, in caregivers, AMPS Factor 2 was most strongly correlated with the same constructs as the total AMPS score.
Association of patient and caregiver mindfulness with patient rating scales
The strength of association between patient and caregiver AMPS and patient-completed ratings is shown in Table 4. Patient mindfulness was correlated with lower self-reported global ratings of impairment (QDRS), less subjective cognitive complaints (AD8, CCI, CFI), and lower anxiety and depression (HADS, all p-values<0.001). All three AMPS factors correlated with these constructs.
Table 4.
Correlation of Patient and Caregiver Mindfulness with Patient Ratings
| Patient Rating | Patient Mindfulness |
Caregiver Mindfulness |
||||||
|---|---|---|---|---|---|---|---|---|
| AMPS Total | AMPS Factor 1 | AMPS Factor 2 | AMPS Factor 3 | AMPS Total | AMPS Factor 1 | AMPS Factor 2 | AMPS Factor 3 | |
| QDRS, Patient | −0.462 (<0.001) | −0.282 (<0.001) | −0.364 (<0.001) | −0.316 (<0.001) | −0.152 (0.01) | −0.140 (0.06) | −0.228 (0.002) | −0.210 (0.005) |
| CCI | −0.428 (<0.001) | −0.281 (<0.001) | −0.332 (<0.001) | −0.328 (<0.001) | −0.163 (0.007) | −0.131 (0.08) | −0.236 (0.002) | −0.236 (0.002) |
| CFI | −0.548 (<0.001) | −0.549 (0.005) | −0.473 (0.012) | −0.488 (0.02) | −0.018 (0.842) | −0.101 (0.63) | −0.049 (0.82) | −0.333 (0.10) |
| AD8 | −0.362 (<0.001) | −0.299 (<0.001) | −0.367 (<0.001) | −0.325 (<0.001) | −0.208 (>0.001) | −0.177 (0.02) | −0.220 (0.004) | −0.314 (<0.001) |
| HADS-Anxiety | −0.306 (<0.001) | −0.243 (>0.001) | −0.303 (<0.001) | −0.342 (<0.001) | −0.162 (0.07) | −0.127 (0.09) | −0.236 (0.002) | −0.277 (<0.001) |
| HADS-Depression | −0.377 (<0.001) | −0.279 (<0.001) | −0.457 (<0.001) | −0.362 (<0.001) | −0.188 (0.002) | −0.138 (0.07) | −0.343 (<0.001) | −0.269 (<0.001) |
Pearson coefficient (p-value). Bold signifies significance after Bonferroni Correction for multiple comparisons (corrected p < 0.008). AMPS, Applied Mindfulness Process Scale; QDRS, Quick Dementia Rating System; CCI, Cognitive Change Index; CFI, Cognitive Function Instrument; mPPT, Mini Physical Performance Test; HADS, Hospital Anxiety and Depression Scale.
Total caregiver mindfulness was inversely correlated with AD8 and HADS-Depression but not the other patient-reported outcomes. For caregivers, Factor 2 (positive emotional regulation) and Factor 3 (negative emotional regulation) were most strongly correlated with patient self-reports.
Association of patient and caregiver mindfulness with cognitive performance
Associations between patient and caregiver AMPS and patient neuropsychological testing are presented in Table 5. Higher patient mindfulness is associated with better performance on HVLT immediate and delayed recall, faster times on Trailmaking A and B, higher scores on the MoCA and Number-Symbol Coding Task, better performance on Animal Naming, and higher z-scores. Higher caregiver mindfulness was associated with better MoCA, HVLT, verbal fluency, naming, and z-scores (Table 5). There were very weak or no associations seen with the individual factors for either patient or caregiver and cognitive testing (data not shown).
Table 5.
Correlation of Patient Mindfulness with Patient Cognitive Performance
| Neuropsychological Test |
Patient AMPS | Caregiver AMPS |
|---|---|---|
| MoCA | 0.245 (<0.001) | 0.197 (0.001) |
| Noise Pareidolia | −0.118 (0.05) | −0.116 (0.06) |
| Numbers Forward | 0.145 (0.01) | 0.076 (0.22) |
| Numbers Backward | 0.124 (0.04) | 0.017 (0.78) |
| HVLT-Immediate | 0.265 (<0.001) | 0.201 (0.001) |
| HVLT-Delay | 0.257 (<0.001) | 0.227 (<0.001) |
| HVLT-Recognition | 0.100 (0.09) | 0.147 (0.02) |
| Trailmaking A | −0.200 (0.001) | −0.151 (0.01) |
| Trailmaking B | −0.215 (0.001) | −0.097 (0.16) |
| Number-Symbol Coding | 0.213 (0.001) | 0.158 (0.01) |
| Verbal Fluency | 0.266 (<0.001) | 0.198 (0.001) |
| MINT | 0.094 (0.11) | 0.192 (0.001) |
| Composite z-score | 0.313 (<0.001) | 0.215 (0.002) |
Pearson coefficient (p-value). Bold signifies significance after Bonferroni Correction for multiple comparisons (corrected p < 0.005). AMPS, Applied Mindfulness Process Scale; MoCA, Montreal Cognitive Assessment; HVLT, Hopkins Verbal Learning Task; MINT, Multilingual Naming Test.
Relationship between patient and caregiver mindfulness
We examined the relationship between patient and caregiver mindfulness and whether dyadic mindfulness could influence the results of rating scales and performance. Total patient AMPS scores were moderately correlated with total caregiver AMPS scores (R = 0.301, p < 0.001). Each patient AMPS factor was moderately correlated to the corresponding caregiver AMPS factor: Factor 1 R = 0.277, Factor 2 R = 0.420, Factor 3 R = 0.361 (all p-values<0.001).
Table 6 presents the results of the linear regression analysis assessing the relationship of patient and caregiver mindfulness status and cognitive, functional, and psychosocial outcomes. In unadjusted models, dyads characterized by low patient/high informant mindfulness and low patient/low caregiver mindfulness reported higher levels of cognitive impairment as measured by QDRS and CCI. Similarly, low patient mindfulness or a combination of low patient and low caregiver mindfulness was associated with higher levels of functional impairment as measured by FAQ and poorer performance on the MoCA. When caregiver self-ratings were assessed, we found that low caregiver mindfulness was linked to higher caregiver depression and more negative appraisals of caregiving when accompanied by low patient mindfulness. In addition, low caregiver mindfulness and combined low patient and caregiver mindfulness were associated with lower memory recall, while low patient mindfulness with lower composite z-score. Patient health-related quality of life was the most consistent correlate of patient/caregiver mindfulness status, with all three comparison groups reporting poorer health-related quality of life compared to the high patient and caregiver mindfulness group. Controlling for disease severity diminished effects on caregiver QDRS, FAQ, MoCA, memory recall, and composite z-score but not on patient QDRS, AD8, HADS-D, and caregiver PHQ4 scores. Further adjustment for age did not have an impact on these effects.
Table 6.
Relationship Between Patient and Caregiver Mindfulness
| Patient/ informant factors |
Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 2 versus Group 1 |
Group 3 versus Group 1 |
Group 4 versus Group 1 |
Group 2 versus Group 1 |
Group 3 versus Group 1 |
Group 4 versus Group 1 |
Group 2 versus Group 1 |
Group 3 versus Group 1 |
Group 4 versus Group 1 |
|
| β ± SE(p) | β ± SE(p) | β ± SE(p) | β ± SE(p) | β ± SE (p) | β ± SE(p) | β ± SE(p) | β ± SE(p) | β ± SE(p) | |
| Patient QDRS | 3.61 ± 0.73 (<0.001) | 0.61 ± 0.73 (0.404) | 4.43 ± 0.84 (<0.001) | 2.45 ± 0.63 (<0.001) | 0.32 ± 0.62 (0.604) | 3.43 ± 0.72 (<0.001) | 2.47 ± 0.63 (<0.001) | 0.36 ± 0.63 (0.563) | 3.52 ± 0.73 (<0.001) |
| CCI | 10.65 ± 3.41 (0.002) | −0.59 ± 3.49 (0.866) | 16.44 ± 3.94 (<0.001) | 6.59 ± 3.15 (0.038) | −1.56 ± 3.18 (0.624) | 12.81 ± 3.62 (<0.001) | 6.64 ± 3.17 (0.037) | −1.49 ± 3.19 (0.642) | 13.05 ± 3.66 (<0.001) |
| AD8 | 0.77 ± 0.41 (0.034) | 0.34 ± 0.41 (0.406) | 1.60 ± 0.47 (<0.001) | 3.65 ± 0.41 (0.113) | 0.26 ± 0.40 (0.514) | 1.39 ± 0.46 (0.003) | 0.6 ± 0.41 (0.107) | 0.28 ± 0.40 (0.488) | 1.44 ± 0.47 (0.002) |
| HADS-D | 1.59 ± 0.72 (0.029) | 0.81 ± 0.72 (0.265) | 2.83 ± 0.83 (<0.001) | 1.35 ± 0.73 (0.066) | 0.73 ± 0.72 (0.314) | 2.61 ± 0.83 (0.002) | 1.43 ± 0.72 (0.047) | 0.87 ± 0.71 (0.224) | 2.91 ± 0.83 (<0.001) |
| Informant QDRS | 3.45 ± 0.89 (<0.001) | 1.83 ± 0.91 (0.045) | 3.42 ± 1.03 (0.001) | 1.60 ± 0.64 (0.013) | 1.35 ± 0.64 (0.037) | 1.76 ± 0.73 (0.017) | 1.59 ± 0.64 (0.014) | 1.34 ± 0.65 (0.039) | 1.76 ± 0.74 (0.019) |
| FAQ | 5.56 ± 1.62 (<0.001) | 2.76 ± 1.64 (0.094) | 6.45 ± 1.85 (<0.001) | 2.23 ± 0.98 (0.024) | 1.76 ± 0.98 (0.074) | 3.00 ± 1.12 (0.008) | 2.26 ± 0.99 (0.023) | 1.80 ± 0.99 (0.069) | 3.09 ± 1.13 (0.007) |
| HUI3 | −0.19 ± 0.06 (<0.001) | −0.17 ± 0.06 (0.003) | −0.28 ± 0.06 (<0.001) | −0.10 ± 0.05 (0.036) | −0.14 ± 0.05 (0.002) | −0.20 ± 0.05 (<0.001) | −0.09 ± 0.05 (0.042) | −0.14 ± 0.05 (0.003) | −0.19 ± 0.05 (<0.001) |
| PANAC− | 3.05 ± 1.18 (0.010) | 4.27 ± 1.21 (<0.001) | 4.06 ± 1.37 (0.003) | 1.90 ± 1.13 (0.095) | 3.98 ± 1.14 (<0.001) | 3.03 ± 1.30 (0.021) | 1.91 ± 1.14 (0.094) | 4.00 ± 1.15 (<0.001) | 3.06 ± 1.31 (0.021) |
| PHQ4 | 0.97 ± 0.46 (0.037) | 1.94 ± 0.48 (<0.001) | 1.10 ± 0.564 (0.051) | 0.75 ± 0.46 (0.108) | 1.89 ± 0.47 (<0.001) | 0.90 ± 0.56 (0.107) | 0.75 ± 0.47 (0.107) | 1.91 ± 0.48 (<0.001) | 0.94 ± 0.56 (0.097) |
| MoCA | −4.76 ± 1.17 (<0.001) | −1.98 ± 1.20 (0.100) | −4.05 ± 1.35 (0.003) | −2.11 ± 0.77 (0.006) | −1.28 ± 0.78 (0.100) | −1.68 ± 0.89 (0.060) | −1.98 ± 0.76 (0.009) | −1.08 ± 0.76 (0.157) | −1.26 ± 0.87 (0.148) |
| HVLT immediate | −3.70 ± 1.25 (0.004) | −2.59 ± 1.25 (0.040) | −4.79 ± 1.49 (0.002) | −1.28 ± 0.90 (0.156) | −1.65 ± 0.89 (0.065) | −2.92 ± 1.06 (0.006) | −1.1 ± 0.87 (0.205) | −1.42 ± 0.86 (0.100) | −2.44 ± 1.03 (0.019) |
| HVLT delay | −1.96 ± 0.67 (0.004) | −1.96 ± 0.66 (0.003) | −2.21 ± 0.79 (0.005) | −0.89 ± 0.52 (0.088) | −1.51 ± 0.51 (0.003) | −1.31 ± 0.61 (0.032) | −0.71 ± 0.49 (0.143) | −1.32 ± 0.48 (0.006) | −0.92 ± 0.57 (0.109) |
| Trailmaking A | 23.57 ± 8.25 (0.005) | 9.39 ± 8.15 (0.250) | 20.82 ± 9.41 (0.028) | 11.43 ± 6.13 (0.064) | 6.18 ± 6.01 (0.305) | 7.42 ± 6.99 (0.290) | 11.22 ± 6.10 (0.067) | 5.29 ± 5.99 (0.378) | 5.71 ± 6.99 (0.415) |
| Trailmaking B | 14.82 ± 11.26 (0.190) | 10.81 ± 10.69 (0.313) | 22.87 ± 12.54 (0.070) | −1.33 ± 0.69 (0.891) | 5.31 ± 9.06 (0.558) | 7.00 ± 10.74 (0.515) | 4.76 ± 9.05 (0.600) | 0.74 ± 8.49 (0.930) | 1.27 ± 10.07 (0.900) |
| NSCT | −7.36 ± 2.70 (0.007) | −6.87 ± 2.56 (0.008) | −7.24 ± 3.06 (0.019) | −2.60 ± 2.09 (0.215) | −4.92 ± 1.95 (0.012) | −3.86 ± 2.34 (0.101) | −1.92 ± 1.88 (0.310) | −3.87 ± 1.76 (0.029) | −2.14 ± 2.12 (0.315) |
| Composite z-score | −0.57 ± 0.16 (<0.001) | −0.45 ± 0.17 (0.007) | −0.40 ± 0.19 (0.033) | −0.27 ± 0.13 (0.037) | −0.38 ± 0.13 (0.004) | −0.13 ± 0.15 (0.371) | −0.25 ± 0.13 (0.049) | −0.34 ± 0.13 (0.008) | −0.06 ± 0.15 (0.679) |
NOTES: Group 1 = High patient AMPS/high informant AMPS; Group 2 = low patient AMPS/high informant AMPS; Group 3 = high patient AMPS/low informant AMPS; Group 4 = low patient AMPS/low informant AMPS. Model 1: unadjusted; Model 2: adjusted for dementia severity; Model 3 = adjusted for age and dementia severity. Bold signifies significance after Bonferroni Correction for multiple comparisons (p < 0.004). QDRS, Quick Dementia Rating Scale; CCI, Patient Cognitive Change Index; HADS-D, Hospital Anxiety and Dementia Scale – Dementia; FAQ, Functional Assessment Questionnaire; HUI3, Health Utility Index Mark 3; PANAC−, PANAC negative appraisals; PHQ4, Informant depression; MoCA, Montreal Cognitive Assessment; HVLT, Hopkins Verbal Learning Test; NSCT, Number Symbol Coding Task.
Association of patient mindfulness with patient self-ratings, cognitive performance and hippocampal volume
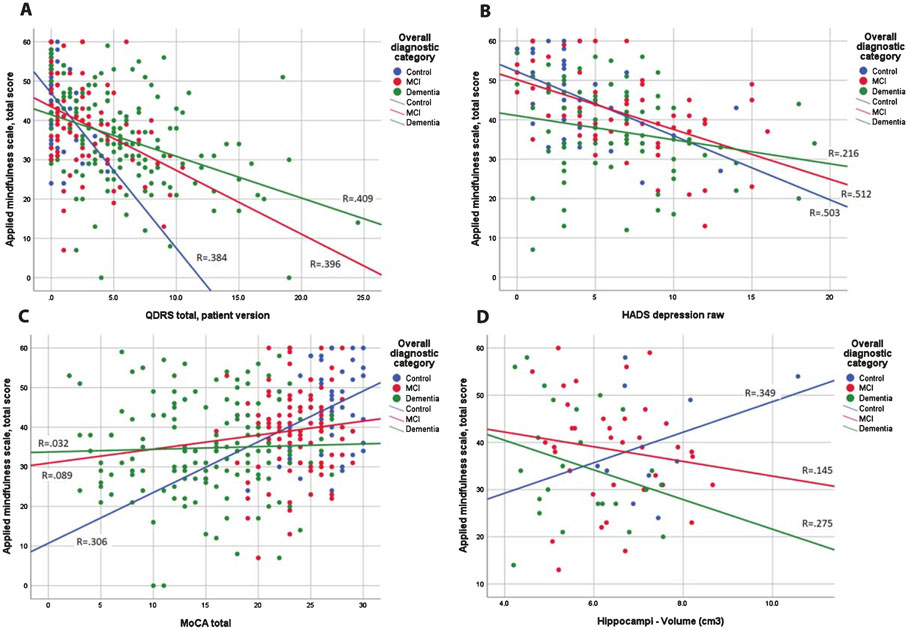
We next examined the relationship (Fig. 1) between patient AMPS scores with self-rated global performance (measured with patient QDRS, Fig. 1A), depression (measured with HADS-Depression, Fig. 1B), overall cognitive performance (measured with MoCA, Fig. 1C), and MRI hippocampal volumes (Fig. 1D). Cases were divided three groups: cognitively normal controls (blue circles), MCI (red circles), and dementia (green circles) with subgroup regression lines. Higher AMPS scores are moderately associated with better self-reported cognitive function in cognitively normal controls (R = 0.384, p < 0.001), MCI (R = 0.396, p < 0.001), and dementia cases (R = 0.409, p < 0.001). Higher AMPS scores are strongly associated with fewer depression symptoms in cognitively normal controls (R = 0.503, p < 0.001) and MCI (R = 0.512, p< 0.001) but not in dementia cases (R = 0.216). Higher AMPS scores are moderately associated with better cognitive performance in cognitively normal controls (R = 0.306, p < 0.001) but not in MCI (R = 0.089) or dementia cases (R = 0.032). Higher AMPS scores are moderately correlated with larger hippocampal volumes in cognitively normal controls (R = 0.349, p < 0.001) but not in MCI (R = 0.145) or dementia cases (R = 0.275).
Fig. 1.
Association of Patient Mindfulness with Cognitive Performance and Hippocampal Volumes. Scatterplots are shown for cognitively normal controls (blue circles), MCI (red circles), and dementia (green circles) with fitted regression lines for the three subgroups. Panel 1A demonstrates the association between AMPS scores (y-axis) and self-rated global performance measured with patient QDRS (x-axis). Higher AMPS scores are moderately associated with better self-rated cognitive status in cognitively normal controls (R = 0.384, p < 0.001), MCI (R = 0.396, p < 0.001), and dementia cases (R = 0.409, p < 0.001). Panel 1B depicts the association between AMPS scores (y-axis) and depression measured by the HADS. Higher AMPS scores are strongly associated with fewer depression symptoms in cognitively normal controls (R = 0.503, p < 0.001) and MCI (R = 0.512, p < 0.001) but not in in dementia cases (R = 0.216). Panel 1C demonstrates the association between AMPS scores (y-axis) and overall cognitive performance measured by the MoCA (x-axis). Higher AMPS scores are moderately associated with better cognitive performance in cognitively normal controls (R = 0.306, p < 0.001) but not in MCI (R = 0.089) or dementia cases (R = 0.032). Panel 1D shows the association between AMPS scores (y-axis) and hippocampal volumes (x-axis). Higher AMPS scores are moderately correlated with larger hippocampal volumes in cognitively normal controls (R = 0.349, p < 0.001) but not in MCI (R = 0.145) or dementia cases (R = 0.275).
Relationship of patient mindfulness with other modifiable resilience and vulnerability factors
We hypothesized that individuals who had higher levels of mindfulness at baseline would likely also have higher ratings in other activities that may offer ADRD protective benefits. We examined six resilience factors in addition to AMPS: education, verbal IQ, physical activity (QPAR), diet (MIND), and social engagement by diagnostic group (Table 7). Controls and MCI were similar but different from dementia on diet and socialization. Controls were different from MCI, and MCI different from dementia on physical activity and mindfulness. We also examined five vulnerability factors: age, depression (HADS), frailty (Fried), vascular risks (mCAIDE), and medical co-morbidities (Charlson) by diagnostic group (Table 7). Controls and MCI were similar but different from dementia on age. Controls were different from MCI and dementia on depression and co-morbidities. Controls were different from MCI, and MCI different from dementia on frailty and vascular risks. Among resilience factors, verbal IQ, physical activity, and social engagement were moderately correlated with AMPS, while among vulnerability factors, depression, frailty, and vascular risks were inversely correlated with AMPS (Table 7). We then evaluated the relationships between AMPS tertiles with distribution of diagnosis, performance on neuropsychological test, and with mean scores for resilience and vulnerability factors, first as unadjusted analyses and then adjusted for dementia severity using the CDR-SB (Table 8). Although there was a higher proportion of cognitively healthy controls in the top AMPS tertile and a higher proportion of dementia cases in the bottom AMPS tertile (p < 0.001), 50% of controls and 50% of dementia cases were distributed in the remaining tertiles. Cognitive performance was highest in the top AMPS tertile for MoCA (p < 0.001) and the composite cognitive z-score (p < 0.001). When examining resilience factors by AMPS tertiles, unadjusted analyses demonstrated differences in physical activity (p < 0.001) and social engagement (p < 0.001). After adjusting for dementia severity, only social engagement remained different (p < 0.001). When examining vulnerability factors by AMPS tertiles, unadjusted analyses demonstrated differences in depression (p < 0.001) and frailty (p < 0.001). These remained significant after adjustment for dementia severity.
Table 7.
Relationship Between Patient Mindfulness and Resilience and Vulnerability Factors
| Scale Characteristics |
Association with AMPS |
Scores by Diagnosis |
|||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | R (p) | Controls | MCI | Dementia | p | |
| Resilience factors | |||||||
| Mindfulness (AMPS) | 38.0 (11.8) | 0–60 | — | 44.8 (10.7) | 39.4 (10.8) | 34.9 (11.8) | <0.001a |
| Education (y) | 15.8 (2.7) | 6–20 | 0.094 (0.110) | 15.9 (2.2) | 16.3 (2.7) | 15.4 (2.8) | 0.100 |
| Verbal IQ | 111.4 (14.6) | 69–133 | 0.332 (0.007) | 118.8 (7.0) | 112.8 (13.8) | 106.1 (20.1) | 0.115 |
| Physical activity (QPAR) | 21.6 (19.4) | 0–132 | 0.272 (<0.001) | 42.2 (26.7) | 24.7 (19.8) | 14.9 (13.8) | <0.001a |
| MIND diet | 8.8 (2.2) | 2.5–14 | 0.115 (0.068) | 9.5 (2.2) | 9.4 (2.2) | 8.3 (2.3) | 0.007b |
| Social Engagement | 2.8 (0.9) | 1–4 | 0.272 (<0.001) | 3.3 (0.6) | 3.0 (0.8) | 2.5 (0.8) | <0.001b |
| Vulnerability factors | |||||||
| Age (y) | 74.7 (9.4) | 38–98 | −0.087 (0.141) | 68.3 (10.2) | 72.3 (8.9) | 78.4 (7.7) | <0.001b |
| HADS-Depression | 6.1 (3.9) | 0–19 | −0.377 (<0.001) | 3.8 (3.2) | 6.4 (4.1) | 6.4 (4.0) | 0.002c |
| Fried Frailty Score | 2.1 (1.4) | 0–5 | −0.330 (<0.001) | 0.8 (1.1) | 1.7 (1.2) | 2.8 (1.2) | <0.001a |
| mCAIDE | 7.4 (2.9) | 0–14 | −0.186 (0.001) | 5.3 (3.3) | 6.9 (3.0) | 8.5 (2.5) | <0.001a |
| Charlson Comorbidity Index | 2.3 (91.7) | 0–8 | −0.026 (0.656) | 1.2 (1.6) | 2.3 (1.6) | 2.8 (1.6) | <0.001c |
Mean (SD) or R (p-value). Bold signifies significance after Bonferroni Correction for multiple comparisons (p < 0.01).
Post-hoc analyses: Controls, MCI, and Dementia all different from each other.
Post-hoc analyses: Controls and MCI not different from each other; Dementia different from Controls and MCI.
Post-hoc analyses: Controls different from MCI and Dementia; MCI and Dementia not different from each other. AMPS, Applied Mindfulness Process Scale; MCI, mild cognitive impairment; QPAR, Quick Physical Activity Rating; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; HADS, Hospital Anxiety and Depression Scale; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Dementia.
Table 8.
Relationship Between Patient Mindfulness Tertiles and Resilience and Vulnerability Factors
| Bottom Tertile | Middle Tertile | Top Tertile | p | ||
|---|---|---|---|---|---|
| Diagnosis | <0.001 | ||||
| Controls, % | 23.7 | 26.3 | 50.0 | ||
| MCI, % | 22.6 | 45.2 | 32.3 | ||
| Dementia, % | 50.0 | 25.6 | 24.5 | ||
| Neuropsychologic Tests | Mean (SD) | Mean (SD) | Mean (SD) | p | |
| MoCA | 18.0 (6.6) | 20.6 (6.5) | 21.5 (6.6) | 0.001 | |
| Cognitive z-score | −0.17 (0.99) | 0.19 (0.94) | 0.42 (0.89) | 0.001 | |
| Resilience factors | Mean (SD) | Mean (SD) | Mean (SD) | p | Adj p |
| Education (y) | 15.5 (2.8) | 15.9 (2.7) | 15.9 (2.4) | 0.311 | 0.803 |
| Verbal IQ | 105.7 (17.2) | 113.0 (13.8) | 115.4 (13.2) | 0.130 | 0.700 |
| Physical activity (QPAR) | 17.7 (14.4) | 20.7 (19.7) | 29.9 (24.9) | <0.001 | 0.104 |
| MIND diet | 8.6 (1.9) | 8.6 (2.3) | 9.3 (2.3) | 0.081 | 0.415 |
| Social Engagement | 2.6 (0.8) | 2.8 (0.9) | 3.1 (0.9) | <0.001 | 0.004 |
| Vulnerability factors | Mean (SD) | Mean (SD) | Mean (SD) | p | Adj p |
| Age (y) | 75.3 (8.7) | 74.1 (11.3) | 74.2 (8.2) | 0.595 | 0.794 |
| HADS-Depression | 7.3 (4.0) | 6.5 (3.7) | 3.8 (2.9) | <0.001 | <0.001 |
| Fried Frailty Score | 2.5 (1.4) | 2.1 (1.3) | 1.5 (1.3) | <0.001 | 0.006 |
| mCAIDE | 7.9 (2.6) | 7.3 (3.1) | 6.9 (3.2) | 0.028 | 0.017 |
| Charlson Comorbidity Index | 2.4 (1.4) | 2.5 (2.0) | 2.1 (1.6) | 0.274 | 0.574 |
Means (SD) or %. Adj p: ANCOVA controlling for dementia severity with Clinical Dementia Rating Sum of Boxes. Bold signifies significant p-value after controlling for multiple comparisons. MCI, mild cognitive impairment; MoCA, Montreal Cognitive Impairment; QPAR, Quick Physical Activity Rating; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; HADS, Hospital Anxiety and Depression Scale; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Dementia.
Association between patient mindfulness, resilience and vulnerability factors, and cognition
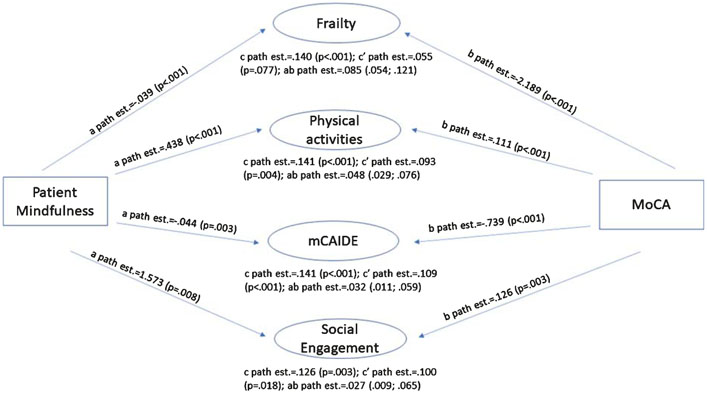
Finally, we used mediation analysis to test whether resilience and vulnerability factors explain the effect of mindfulness on cognitive tests of global function (MoCA, z-score), episodic memory (HVLT immediate and delayed recall), and executive function (Trailmaking B, Number Symbol Coding Test) (Table 9). As an example, mediation results are presented in Fig. 2 for the MoCA. Two resilience and two vulnerability factors were found to help explain the relationship between patient mindfulness and performance on the MoCA: physical activity as measured by the QPAR and socialization (resilience factors) and frailty as measured by the Fried Frailty Score and mCAIDE (vulnerability factors). As indicated by the proportion of mindfulness effect on MoCA that is mediated by individual patient-related factors, higher levels of physical activity and socialization observed in participants high on mindfulness explained 34% and 21%, respectively, of its positive impact on Mo CA score. Similarly, lower frailty and mCAIDE scores explained 61% and 23%, respectively, of the effect of mindfulness on MoCA. Significant indirect (ab path) effects were 0.085 for frailty; 0.048 for physical activity; 0.032 for mCAIDE score; and 0.027 for socialization as indicated by their 95% CI. These results were consistent across the other cognitive measures assessed, with indirect effects ranging from 0.007–0.20 for physical activity; 0.003–0.009 for socialization; 0.008–0.026 for frailty; and 0.004–0.011 for mCAIDE. The proportion of the total effect that is mediated (ab/c) ranged from 0.405–0.438 for physical activity; 0.205–0.339 for socialization; 0.513–0.703 for frailty; and 0.229–0.360 for mCAIDE (data not shown).
Table 9.
Mediation Analyses for Association Between Patient Mindfulness, Resilience and Vulnerability Factors, and Cognition
| MoCA | Z score | HVLT Recall | HVLT delay | Trailmaking B | NSCT | ||
|---|---|---|---|---|---|---|---|
| Education | a path (p) | 0.023 (0.105) | 0.023 (0.106) | 0.024 (0.104) | 0.025 (0.096) | 0.014 (0.432) | 0.014 (0.393) |
| b path (p) | 0.471 (<0.001) | 0.048 (0.014) | 0.084 (0.017) | 0.098 (0.027) | −0.100 (0.028) | 0.060 (0.045) | |
| c path (p) | 0.139 (<0.001) | 0.013 (0.007) | 0.038 (<0.001) | 0.046 (<0.001) | −0.036 (0.003) | 0.023 (0.002) | |
| c’ path (p) | 0.128 (<0.001) | 0.012 (0.013) | 0.036 (<0.001) | 0.043 (<0.001) | −0.034 (0.004) | 0.023 (0.003) | |
| ab path | 0.011 | 0.001 | 0.002 | 0.002 | −0.001 | 0.001 | |
| (95% CI) | (−0.0001; 0.030) | (−0.000; 0.004) | (0.0001; 0.006) | (−0.0001; 0.007) | (−0.006; 0.002) | (−0.0001; 0.004) | |
| ab/c | 0.079 | 0.077 | 0.053 | 0.043 | 0.028 | 0.043 | |
| QPAR | a path (p) | 0.438 (<0.001) | 0.437 (<0.001) | 0.429 (<0.001) | 0.426 (<0.001) | 0.493 (<0.001) | 0.457 (<0.001) |
| b path (p) | 0.111 (<0.001) | 0.017 (<0.001) | 0.037 (<0.001) | 0.047 (<0.001) | −0.030 (<0.001) | 0.026 (<0.001) | |
| c path (p) | 0.141 (<0.001) | 0.013 (0.005) | 0.039 (<0.001) | 0.048 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.093 (0.004) | 0.006 (0.187) | 0.023 (0.005) | 0.028 (0.008) | −0.022 (0.062) | 0.014 (0.068) | |
| ab path | 0.048 | 0.007 | 0.016 | 0.020 | −0.015 | 0.012 | |
| (95% CI) | (0.029; 0.076) | (0.004; 0.011) | (0.009; 0.022) | (0.011; 0.032) | (−0.024; −0.007) | (0.006; 0.020) | |
| ab/c | 0.340 | 0.538 | 0.410 | 0.417 | 0.405 | 0.408 | |
| MIND diet | a path (p) | 0.021 (0.074) | 0.021 (0.075) | 0.021 (0.073) | 0.024 (0.041) | 0.039 (0.006) | 0.033 (0.012) |
| b path (p) | 0.463 (0.016) | 0.061 (0.024) | 0.148 (0.004) | 0.235 (<0.001) | −0.210 (0.002) | 0.149 (<0.001) | |
| c path (p) | 0.144 (<0.001) | 0.014 (0.005) | 0.040 (<0.001) | 0.048 (<0.001) | −0.033 (0.012) | 0.025 (0.003) | |
| c’ path (p) | 0.135 (<0.001) | 0.013 (0.011) | 0.037 (<0.001) | 0.042 (<0.001) | −0.025 (0.057) | 0.020 (0.014) | |
| ab path | 0.010 | 0.001 | 0.003 | 0.006 | −0.008 | 0.005 | |
| (95% CI) | (0.0004; 0.026) | (−0.000; 0.004) | (0.0002; 0.009) | (0.001; 0.013) | (−0.018; −0.003) | (0.001; 0.011) | |
| ab/c | 0.069 | 0.071 | 0.075 | 0.125 | 0.242 | 0.200 | |
| Social Engagement | a path (p) | 0.017 (0.001) | 0.017 (0.001) | 0.017 (0.002) | 0.017 (0.002) | 0.019 (0.002) | 0.019 (<0.001) |
| b path (p) | 1.573 (0.008) | 0.182 (0.032) | 0.554 (<0.001) | 0.562 (0.006) | −0.448 (0.021) | 0.253 (0.058) | |
| c path (p) | 0.126 (0.003) | 0.013 (0.032) | 0.034 (0.003) | 0.044 (0.002) | −0.026 (0.064) | 0.023 (0.014) | |
| c’ path (p) | 0.100 (0.018) | 0.010 (0.109) | 0.025 (0.029) | 0.035 (0.017) | −0.018 (0.216) | 0.019 (0.056) | |
| ab path | 0.027 | 0.003 | 0.009 | 0.009 | −0.009 | 0.005 | |
| (95% CI) | (0.009; 0.065) | (0.001; 0.007) | (0.003; 0.019) | (0.003; 0.021) | (−0.019; −0.002) | (0.001; 0.011) | |
| ab/c | 0.214 | 0.231 | 0.265 | 0.205 | 0.339 | 0.217 | |
| Age | a path (p) | −0.062 (0.187) | −0.062 (0.185) | 0.055 (0.253) | −0.060 (0.216) | −0.067 (0.242) | −0.060 (0.249) |
| b path (p) | −0.305 (<0.001) | −0.043 (<0.001) | −0.080 (<0.001) | −0.118 (<0.001) | 0.097 (<0.001) | −0.080 (<0.001) | |
| c path (p) | 0.139 (<0.001) | 0.013 (0.006) | 0.039 (<0.001) | 0.047 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.121 (<0.001) | 0.010 (0.015) | 0.035 (<0.001) | 0.040 (<0.001) | −0.030 (0.004) | 0.020 (0.002) | |
| ab path | 0.019 | 0.003 | 0.004 | 0.007 | −0.006 | 0.005 | |
| (95% CI) | (−0.005; 0.051) | (−0.001; 0.006) | (−0.003; 0.012) | (−0.002; 0.018) | (−0.016; 0.003) | (−0.002; 0.012) | |
| ab/c | 0.137 | 0.231 | 0.103 | 0.149 | 0.162 | 0.200 | |
| HADS-Depression | a path (p) | −0.126 (<0.001) | −0.126 (<0.001) | −0.131 (<0.001) | −0.124 (<0.001) | 0.123 (<0.001) | −0.115 (<0.001) |
| b path (p) | −0.087 (0.401) | 0.004 (0.775) | −0.061 (0.029) | −0.055 (0.122) | 0.037 (0.307) | −0.026 (0.255) | |
| c path (p) | 0.138 (<0.001) | 0.012 (0.008) | 0.040 (<0.001) | 0.048 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.127 (<0.001) | 0.013 (0.010) | 0.032 (<0.001) | 0.041 (<0.001) | −0.033 (0.010) | .0022 (0.007) | |
| ab path | 0.011 | −0.001 | 0.008 | 0.007 | −0.005 | 0.003 | |
| (95% CI) | (−0.014; 0.038) | (−0.004; 0.004) | (0.001; 0.016) | (−0.003; 0.016) | (−0.014; 0.004) | (−0.003; 0.009) | |
| ab/c | 0.080 | 0.083 | 0.200 | 0.146 | 0.135 | 0.120 | |
| Fried Frailty | a path (p) | −0.039 (<0.001) | −0.039 (<0.001) | −0.037 (<0.001) | −0.036 (<0.001) | −0.038 (<0.001) | −0.036 (<0.001) |
| b path (p) | −2.189 (<0.001) | −0.214 (<0.001) | −0.543 (0.005) | −0.737 (<0.001) | 0.682(<0.001) | −0.475 (<0.001) | |
| c path (p) | 0.140 (<0.001) | 0.013 (0.006) | 0.039 (<0.001) | 0.047 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.055 (0.077) | 0.005 (0.311) | 0.019 (0.024) | 0.021 (0.042) | −0.011 (0.315) | 0.008 (0.271) | |
| ab path | 0.085 | 0.008 | 0.020 | 0.026 | −0.026 | 0.017 | |
| (95% CI) | (0.054; 0.0121) | (0.005; 0.013) | (0.012; 0.030) | (0.016; 0.038) | (−0.040; −0.015) | (0.010; 0.024) | |
| ab/c | 0.607 | 0.615 | 0.513 | 0.553 | 0.703 | 0.680 | |
| mCAIDE | a path (p) | −0.044 (0.003) | −0.044 (0.003) | −0.042 (0.005) | −0.044 (0.004) | −0.041 (0.022) | −0.042 (0.011) |
| b path (p) | −0.739 (<0.001) | −0.091 (<0.001) | −0.198 (<0.001) | −0.243 (<0.001) | 0.261 (<0.001) | −0.209 (<0.001) | |
| c path (p) | 0.141 (<0.001) | 0.013 (0.005) | 0.039 (<0.001) | 0.048 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.109 (<0.001) | 0.009 (0.043) | 0.031 (<0.001) | 0.037 (<0.001) | −0.027 (0.015) | 0.016 (0.019) | |
| ab path | 0.032 | 0.004 | 0.008 | 0.011 | −0.011 | 0.009 | |
| (95% CI) | (0.011; 0.059) | (0.002; 0.008) | (0.003; 0.017) | (0.004; 0.020) | (−0.021; −0.002) | (0.003; 0.017) | |
| ab/c | 0.227 | 0.308 | 0.250 | 0.229 | 0.297 | 0.36 | |
| Charlson Comorbidities | a path (p) | −0.003 (0.739) | −0.003 (0.734) | −0.004 (0.633) | −0.004 (0.684) | 0.003 (0.811) | −0.000 (0.988) |
| b path (p) | −0.892 (<0.001) | −0.102 (0.002) | −0.315 (<0.001) | −0.386 (<0.001) | 0.321 (<0.001) | −0.203 (<0.001) | |
| c path (p) | 0.141 (<0.001) | 0.013 (0.005) | 0.039 (<0.001) | 0.048 (<0.001) | −0.037 (0.002) | 0.025 (0.001) | |
| c’ path (p) | 0.139 (<0.001) | 0.013 (0.005) | 0.038 (<0.001) | 0.047 (<0.001) | −0.038 (<0.001) | 0.025 (<0.001) | |
| ab path | 0.003 | 0.0003 | 0.001 | 0.001 | 0.001 | 0.000 | |
| (95% CI) | (−0.011; 0.019) | (−0.001; 0.002) | (−0.004; 0.007) | (−0.004; 0.008) | (−0.005; 0.006) | (−0.003; 0.004) | |
| ab/c | 0.021 | 0.023 | 0.026 | 0.021 | 0.027 | − |
Bold signifies significant p-value after controlling for multiple comparisons. MoCA, Montreal Cognitive Impairment; HVLT, Hopkins Verbal Learning Task; NSCT, Number Symbol Coding Task; QPAR, Quick Physical Activity Rating; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; HADS, Hospital Anxiety and Depression Scale; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Dementia.
Fig. 2.
Mediation Analyses Patient Mindfulness and Global Cognition. Cross-sectional mediation analyses were employed to assess whether protective- and risk factors help explain at least in part the effect of patient mindfulness on cognitive function. Two resilience and two vulnerability factors were found to help explain the relationship between patient mindfulness and performance on the MoCA: physical activity as measured by the QPAR and socialization (resilience factors) and frailty as measured by the Fried Frailty Score and mCAIDE (vulnerability factors). Most path effects were significant at p < 0.001 indicating highly significant relationships between AMPS score, individual mediators, and MoCA. Higher levels of physical activity and socialization observed in participants high on mindfulness explained 34% and 21%, respectively, of AMPS positive impact on MoCA score. Similarly, lower frailty and mCAIDE scores explained 61% and 23%, respectively, of the effect of mindfulness on MoCA. See text for further details.
DISCUSSION
We found that measurement of baseline mindfulness in older adults was feasible using the AMPS and that patient and caregiver mindfulness correlate with various patient and caregiver health outcomes including cognitive performance, neurodegeneration, function, behavior, mood, quality of life, and caregiving experiences. In addition, mindfulness varied by cognitive status but not dementia etiology and that when coupled with caregiver mindfulness, patient mindfulness level can help predict performance on most patient and caregiver ratings. Finally, we found evidence that patient mindfulness is linked to resilience and vulnerability factors providing support for potential pathways to explain its link to cognitive performance.
Collectively, our findings indicate the importance of considering baseline mindfulness on the observed reporting of cognition, function, and behavior by patients and caregivers and for patient cognitive performance. Our clinical and research goals are to establish what activities people do, how often they do them, and if they are open to the concept of lifestyle modification prior to offering any specific intervention. Greater self-reported cognitive complaints (patient QDRS and AD8) and patient and caregiver depression found in patients with low mindfulness, especially when accompanied by low caregiver mindfulness were unexplained by disease severity and should be the focus of further investigation to identify the specific mechanisms through which low mindfulness leads to subjective cognitive and mood dysfunction. These findings suggest that patient mindfulness may be an useful baseline predictor of response on outcomes and could serve as target for intervention in conjunction with other resilience factors (e.g., exercise, diet, cognitive activities) to prevent dementia in cognitively normal controls, reduce symptoms, and slow progression of cognitive impairment and depression in individuals with MCI by promoting mindfulness practice and adoption of positive lifestyle changes (physical activity, diet, social engagement) in both ADRD patients and their caregivers. In addition, our results support that multimodal approaches for lifestyle that are focused on resilience factors and risk reduction efforts focused on vulnerability factors could serve as targets for intervention in primary and secondary prevention studies.
Mechanisms underlying the potential benefits of mindfulness are not well studied. Using mediation analysis, we found that participants with greater levels of mindfulness are more likely to report higher levels of engagement in physical activities, which in turn was related to better global cognitive performance and performance on measures of memory and attention-executive function. The positive relationship between mindfulness and physical activity is supported by a recent systematic review (N = 20) of mostly cross-sectional studies, which found evidence that mindfulness is positively associated with physical activity (both self-reported and performance-based) and that mindfulness-based interventions are likely to be more effective when they are physical activity-specific [86]. This was interpreted as implying that individuals with higher mindfulness may be able to better translate physical activity intentions into behavior and may be more likely to be motivated to engage in and enjoy participating in physical activity [86]. Furthermore, physical activity has been reported to improve cognition particularly memory and executive function via cardiovascular mechanisms, increased inflammation in the brain and reduced cerebral blood flow but also by increasing neurotrophic factors which promote brain health [87]. Future studies are needed to determine the specific types of physical activity likely to be impacted by higher levels of mindfulness in an effort to maximize its benefits on brain health.
We also found that the higher cognitive performance observed in our participants with higher mindfulness scores was explained in part by lower frailty. The observed relationships between mindfulness, frailty, and cognitive performance in our study are supported by reports of improved physical functionality and cognitive performance in older adults with cognitive impairment who participate in mindfulness meditation interventions [88] and particularly improvement in memory and executive functioning [89]. These findings suggest benefits of both trait and state mindfulness in terms of improved cognitive function via reduced physical impairment/frailty in older adults with cognitive impairment.
In addition, the lower mCAIDE score found in our study among those with higher levels of mindfulness is in line with prior reports. Results from a cross-sectional analysis of data from the New England Family Study (NEFS) suggest that dispositional (trait) mindfulness is positively associated with cardiovascular health, particularly characteristics such as lower BMI and higher physical activity [90], which are factors that are included in the modified CAIDE measure used in our study. Higher CAIDE scores in turn have been linked to poorer cognitive performance including executive function [91], a faster rate of cognitive decline, and an increased risk of dementia [92, 93] supporting the negative relationship found in the current study. Given the significance of cardiovascular risk factors in the development of dementia, research effort needs to be put into the identification of the specific types of mindfulness practices that can help prevent cognitive decline and impairment and preserve cognitive function in those with cognitive impairment by reducing their cardiovascular risk.
We also found higher socialization among our participants who scored higher on mindfulness, which in turn was linked to better cognitive performance. While less studied, the importance of mindfulness on loneliness and social isolation has been highlighted in a recent randomized clinical trial of a mindfulness intervention [94]. In this study of 153 community adults, the group assigned to a monitor and accept present-moment experiences intervention group was more likely to experience decreased loneliness and increased social contact in daily life compared to a monitor only group or a control group. The reported positive relationship between patient socialization and cognitive performance is in line with previously published reports. For example, in an analysis of 3,617 personal interviews of adults aged 24–96 years participating in the Survey of American’s Changing Lives, the more socially engaged participants were, the higher their cognitive performance on the Mini-Mental State Exam was, regardless of age [95]. In addition, a second study following up on these results found that receiving a short social interaction intervention can facilitate cognitive performance and was similar in its effect to an intellectual activity intervention. Findings suggest the importance of mindfulness on cognitive function by highlighting social interaction as a significant mechanism malleable to change.
Finally, mindfulness-based interventions may produce clinical benefits through reduction of negative perseverative thought processes (i.e., dwelling on negative thoughts, worry) [96-98]. This is consistent with our findings that positive emotional regulation had the greatest effect on patient and caregiver outcomes. When mindfulness-based stress reduction was applied to patients with MCI, results showed decreased hippocampal atrophy and increased functional connectivity between the posterior cingulate cortex and bilateral medial prefrontal cortex [99]. Meditators in another study were shown to have less age-associated neurological decline, in terms of grey matter volume and attention performance than non-meditators [100].
One of the goals of this study was to understand the role baseline mindfulness may play in the presentation and capture of dementia-related symptoms. Given the multifactorial nature of ADRD and how it is experienced by patients and caregivers, we were interested in assessing the relationship of patient and caregiver mindfulness with multiple variables to better understand how baseline mindfulness may impact how patients and caregiver respond and how mindfulness-based interventions could potentially benefit patients and caregivers. In addition, as there are many different types of mindfulness-based interventions that could be offered to patients and caregivers, we assessed mindfulness in terms of positive emotional regulation, negative emotional regulation, and decentering to understand which factor(s) might have the most significant effect in order to tailor a future mindfulness-based intervention around these results. Our study suggests that interventions that address positive emotional regulation (AMPS factor 2) may offer potential benefits to patients reducing subjective cognitive complaints, improving mood and health-related quality of life, and influencing performance on episodic memory and attention-executive tasks. Our study also suggests that interventions that address positive emotional regulation may offer potential benefits to caregivers including reducing negative appraisals and depression and improving preparedness and confidence. Because we also examined patient-caregiver dyadic pairings of mindfulness, interventions could also be designed that offer dyad programs rather than just on an individual basis.
Operationalization of the construct of mindfulness is important in order to determine not only where patients and caregivers start, but also to explore underlying mechanisms of therapeutic benefits that could be elicited by mindfulness practice [33]. Another value of a measuring baseline mindfulness is to determine the practical differences in mindfulness between persons, how it may influence data collection on important ADRD outcomes [36, 37], and how to best place mindfulness with other resilience and vulnerability factors. We chose the AMPS as the measure of mindfulness since it was designed to assess how individuals apply mindfulness concepts to cope with stress in their daily lives and could be used as an outcome for a mindfulness-based intervention.
Besides the cognitive symptoms, MCI and ADRD patients often manifest neuropsychiatric symptoms such as depression [101]. In MCI subjects, mindfulness meditation was reported to positively influence emotional regulation [102], general mental health and well-being [103], and depression [104]. Different meditation techniques have also been studied in patients with ADRD and their family caregivers, with benefits reported in terms of decreased cognitive decline, stress reduction, improved quality of life, as well as biomarker changes on structural and functional MRI including increased functional connectivity, increased cerebral blood flow, and decreased brain volume changes [105-110].
Although benefits in terms of cognitive functioning may be smaller than those for psychosocial outcomes [27, 111], mindfulness-based interventions were reported to improve performance on memory, language, and executive functional tasks [107] and stabilize global scores such as the Mini-Mental State Exam [112]. Effectiveness in patients with MCI and ADRD may be difficult to gage given the diversity of outcome measures used across studies [113] but it can likely be improved if tailored to their level of cognitive impairment. In addition, we found higher mindfulness corresponded to a lower score on the FAQ and NPI, which aligns well with prior research that mindfulness may improve functionality and psychopathology [112]. In our study, higher levels of patient and caregiver mindfulness corresponded with lower levels of mood disturbances. This supports prior research showing that mindfulness can improve depression and anxiety in cognitively healthy people [114] and could provide benefit for patients with progressive cognitive decline [113] or their caregivers [115]. When compared to cognitive stimulation therapy and progressive muscle relaxation, mindfulness showed significant improvements in cognitive function in the mindfulness group compared with controls, with the effect size being the largest of the three interventions after 2 years [112]. Collectively, these different studies suggest that participating in mindfulness-based interventions may be associated with changes in cognitive (attention, verbal and recognition memory, executive function), biological (amplitudes for event related potentials, percent change in brain volume on MRI, diurnal cortisol levels, inflammatory markers, resting state functional connectivity changes, and telomerase activity) and psychosocial functioning (perceived stress, symptoms of depression and anxiety, caregiver burden, quality of life, and self-efficacy) [27].
There may be a use for mindfulness-based interventions in caregivers of dementia patients as well. In caregivers, treatment effects were medium-to-large for stress and burden, and large for quality of life [26, 27]. Mindfulness-based interventions including stress reduction and cognitive therapy programs developed for caregivers were well received and showed improvements in stress, depression, anxiety, and perceived caregiver burden [115, 116]. This is consistent with our findings that higher caregiver mindfulness is associated with better preparedness and care confidence while lower caregiver mindfulness is associated with negative appraisals and depression. These effects crossed over to patients with reported improvement in mental health related quality of life and fewer behaviors [116]. When patients and caregivers participate in these activities together, mindfulness-based interventions were found to improve quality of life and depressive symptoms in both [117].
There are several limitations in this study. We studied baseline mindfulness and its association with patient and caregiver outcomes prior to any intervention. We did not ask patients or caregivers to specify how they practiced mindfulness but rather collected information on their thought processes to different life stressors. We also did not discriminate between different forms of mindfulness practices individuals may participate in (e.g., yoga versus hot yoga). This could be a focus of future research. As this is a cross-sectional study, cause and effect cannot be established. The longitudinal effects of mindfulness on patient and caregiver outcomes still need to be elucidated and whether baseline mindfulness leads to “good habits” or the converse. Participants and their caregivers were seen in the context of an academic memory disorders clinic and research program where the prevalence of MCI and dementia are high and both patients and caregivers tend to be better educated and predominantly White. Validation of our findings in other settings where dementia prevalence is lower (i.e., community samples) or where the sample is more diverse is needed. Our research projects and clinic focus on healthy aging, MCI, and early stage ADRD so fewer moderate to severe patients are seen by us. Strengths of this study include the use of a comprehensive evaluation that is part of standard of care with measurement of multiple patient and caregiver constructs using Gold Standard measures.
As pharmacologic approaches continue to offer only modest benefit to patients with dementia, non-pharmacologic, multimodal approaches [19-21] offer potentially promising and cost-effective additions to the treatment regimen for individuals with ADRD and for the prevention of ADRD [5]. Mindfulness practices and other mind/body practices have the potential to improve functioning and quality of life in these patients with negligible risks or side effects. Further studies should incorporate mindfulness-based approaches with a focus on positive emotional regulation to determine short- and long-term effects that these practices may have on patients with ADRD and their caregivers.
ACKNOWLEDGMENTS
This study was supported by grants to JEG from the National Institute on Aging (R01 AG040211 and R01 NS101483), the Research Center of Excellence Program from the Lewy Body Dementia Association, the Harry T. Mangurian Foundation, and the Leo and Anne Albert Charitable Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
JEG is the creator of the QDRS, AD8, and PANAC.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1292r2).
REFERENCES
- [1].Alzheimer Association (2019) 2019 Alzheimer’s Disease Facts and Figures. https://alz.org/alzheimers-dementia/facts-figures. Accessed on 16 October 2020.
- [2].World Health Organization (2020) https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed on 16 October 2020.
- [3].Galvin JE, Valois L (2014) Caregiving issues in dementia. In Dementia-A Comprehensive Update, Atri A, Dickerson B, eds. Oxford University Press, New York, pp. 609–621. [Google Scholar]
- [4].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Galvin JE (2017) Prevention of Alzheimer’s disease: Lessons learned and applied. J Am Geriatr Soc 65, 2128–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, Pan A, Zhang C, Jia J, Feng L, Kua EH, Wang YJ, Wang HF, Tan MS, Li JQ, Hou XH, Wan Y, Tan L, Mok V, Tan L, Dong Q, Touchon J, Gauthier S, Aisen PS, Vellas B (2020) Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry 91, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hodes JF, Oakley CI, O’Keefe JH, Lu P, Galvin JE, Siaf N, Bellara S, Rahman A, Kaufman Y, Hristov H, Rajji T, Frosnacht Morgan AM, Patel S, Merrill DA, Kaiser S, Melendez-Cabrero J, Melendez JA, Krikorian R, Isaacson RS (2019) Alzheimer’s “prevention” vs. “risk reduction”: Transcending semantic for clinical practice. Front Neurology 9, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitchell S, Ridley SH, Sancho RM, Norton M (2017) The future of dementia risk reduction research: Barriers and solutions. J Public Health 39, e275–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gaitán JM, Boots EA, Dougherty RJ, Ma Y, Edwards DF, Mitchell CC, Christian BT, Cook DB, Okonkwo OC (2020) Protocol of Aerobic Exercise and Cognitive Health (REACH): A pilot study. J Alzheimers Dis Rep 4, 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vidoni ED, Perales J, Alshehri M, Giles AM, Siengsukon CF, Burns JM (2019) Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer disease. J Geriatr Phys Ther 42, E129–E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng ST (2016) Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr Psychiatry Rep 18, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galvin JE, Tolea MI, Chrisphonte S (2020) The Cognitive & Leisure Activity Scale (CLAS): A new measure to quantify cognitive activities in older adults with and without cognitive impairment. Alzheimers Dement (N Y), in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hackett RA, Steptoe A, Cadar D, Fancourt D (2019) Social engagement before and after dementia diagnosis in the English Longitudinal Study of Ageing. PLoS One 14, e0220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Biddle KD, d’Oleire Uquillas F, Jacobs HIL, Zide B, Kirn DR, Rentz DM, Johnson KA, Sperling RA, Donovan NJ (2019) Social engagement and amyloid-β-related cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry 27, 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stern Y, Barulli D (2019) Cognitive reserve. Handb Clin Neurol 167, 181–190. [DOI] [PubMed] [Google Scholar]
- [16].Chapko D, McCormack R, Black C, Staff R, Murray A (2018) Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia - a systematic literature review. Aging Ment Health 22, 915–926. [DOI] [PubMed] [Google Scholar]
- [17].Gustafson DR, Bäckman K, Scarmeas N, Stern Y, Manly JJ, Mayeux R, Gu Y (2020) Dietary fatty acids and risk of Alzheimer’s disease and related dementias: Observations from the Washington Heights-Hamilton Heights-Inwood Columbia Aging Project (WHICAP). Alzheimers Dement, doi 10.1002/alz.12154 [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC (2020) Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 95, e374–e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kivipelto M, Mangialasche F, Snyder HM, llegri R, Andrieu S, Arai H, Baker L, Belleville S, Brodaty H, Brucki SM, Calandri I, Caramelli P, Chen C, Chertkow H, Chew E, Choi SH, Chowdhary N, Crivelli L, Torre R, Du Y, Dua T, Espeland M, Feldman HH, Hartmanis M, Hartmann T, Heffernan M, Henry CJ, Hong CH, Håkansson K, Iwatsubo T, Jeong JH, Jimenez-Maggiora G, Koo EH, Launer LJ, Lehtisalo J, Lopera F, Martínez-Lage P, Martins R, Middleton L, Molinuevo JL, Montero-Odasso M, Moon SY, Morales-Pérez K, Nitrini R, Nygaard HB, Park YK, Peltonen M, Qiu C, Quiroz YT, Raman R, Rao N, Ravindranath V, Rosenberg A, Sakurai T, Salinas RM, Scheltens P, Sevlever G, Soininen H, Sosa AL, Suemoto CK, Tainta-Cuezva M, Velilla L, Wang Y, Whitmer R, Xu X, Bain LJ, Solomon A, Ngandu T, Carrillo MC (2020) World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 16, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S; European Prevention of Alzheimer’s Dementia (EPAD) Consortium (2016) Development of interventions for the secondary prevention of Alzheimer’s dementia: The European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 3, 179–186. [DOI] [PubMed] [Google Scholar]
- [21].Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M (2020) Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis 7, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahn HI, Hyun MK (2019) Effectiveness of integrative medicine program for dementia prevention on cognitive function and depression of elderly in a public health center. Integr Med Res 8, 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Galvin JE (2018) Advancing personalized treatment of Alzheimer’s disease: A call for the N-of-1 trial design. Future Neurol 13, 151–160. [Google Scholar]
- [24].Khalsa DS (2015) Stress, meditation, and Alzheimer’s disease prevention: Where the evidence stands. J Alzheimers Dis 48, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kabat-Zinn J, Hanh TN (2013) Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Little, Brown Book Group, Revised Edition. [Google Scholar]
- [26].Shim M, Tilley JL, Im S, Price K, Gonzalez A (2020) A systematic review of mindfulness-based interventions for patients with mild cognitive impairment or dementia and caregivers. J Geriatr Psychiatry Neurol, doi: 10.1177/0891988720957104 [DOI] [PubMed] [Google Scholar]
- [27].Larouche E, Hudon C, Goulet S (2015) Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: An interdisciplinary perspective. Behav Brain Res 276, 199–212. [DOI] [PubMed] [Google Scholar]
- [28].McHugh L, Simpson A, Reed P (2010) Mindfulness as a potential intervention for stimulus over-selectivity in older adults. Res Dev Disabil 31, 178–184. [DOI] [PubMed] [Google Scholar]
- [29].Lao SA, Kissane D, Meadows G (2016) Cognitive effects of MBSR/MBCT: A systematic review of neuropsychological outcomes. Conscious Cogn 45, 109–123. [DOI] [PubMed] [Google Scholar]
- [30].Moynihan JA, Chapman BP, Klorman R, Krasner MS, Duberstein PR, Warren Brown K, Talbot NL (2013) Mindfulness-based stress reduction for older adults: Effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology 68, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Segal ZV, Teasdale JD, Williams JMG (2004) Mindfulness-based cognitive therapy: Theoretical rationale and empirical status. In Mindfulness and Acceptance: Expanding the Cognitive-Behavioral Tradition, Hayes SC, Follette VM, Linehan MM, eds. Guilford Press New York, NY, pp. 45–65. [Google Scholar]
- [32].Newland P, Bettencourt BA (2020) Effectiveness of mindfulness-based art therapy for symptoms of anxiety, depression, and fatigue: A systematic review and meta-analysis. Complement Ther Clin Pract 41, 101246. [DOI] [PubMed] [Google Scholar]
- [33].Li MJ, Black DS, Garland EL (2016) The Applied Mindfulness Process Scale (AMPS): A process measure for evaluating mindfulness-based interventions. Pers Individ Dif 93, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chiesa A, Anselmi R, Serretti A (2014) Psychological mechanisms of mindfulness-based interventions: What do we know? Holistic Nurs Pract 28, 124–148. [DOI] [PubMed] [Google Scholar]
- [35].Chiesa A, Malinowski P (2011) Mindfulness-based approaches: Are they all the same? J Clin Psychol 67, 404–424. [DOI] [PubMed] [Google Scholar]
- [36].Chiesa A (2013) The difficulty of defining mindfulness: Current thought and critical issues. Mindfulness 4, 255–268. [Google Scholar]
- [37].Grossman P, Van Dam NT (2011) Mindfulness, by any other name…: Trials and tribulations of sati in western psychology and science. Contemporary Buddhism 12, 219–239. [Google Scholar]
- [38].Chen KW, Berger CC, Manheimer E, Forde D, Magidson J, Dachman L (2012) Meditative therapies for reducing anxiety: A systematic review and meta-analysis of randomized controlled trials. Depress Anxiety 29, 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V (2013) Mindfulness-based therapy: A comprehensive meta-analysis. Clin Psychol Rev 33, 763–771. [DOI] [PubMed] [Google Scholar]
- [40].Gard T, Holzel BK, Lazar SW (2014) The potential effects of meditation on age-related cognitive decline: A systematic review. Ann N Y Acad Sci 1307, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marciniak R, Sheardova K, Cermakova P, Hudecek D, Sumec R, Hort J (2104) Effect of meditation on cognitive functions in context of aging and neurodegenerative diseases. Front Behav Neurosci 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Khoury B, Sharma M, Rush SE, Fournier C (2015) Mindfulness-based stress reduction for healthy individuals: A meta-analysis. J Psychosom Res 78, 519–528. [DOI] [PubMed] [Google Scholar]
- [43].Innes KE, Selfe TK (2014) Meditation as a therapeutic intervention for adults at risk for Alzheimer’s disease - potential benefits and underlying mechanisms. Front Psychiatry 5, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schneider RH, Grim CE, Rainforth MV, Kotchen T, Nidich SI, Gaylord-King C (2012) Stress reduction in the secondary prevention of cardiovascular disease: Randomized, controlled trial of transcendental meditation and health education in Blacks. Circ Cardiovasc Qual Outcomes 5, 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. [DOI] [PubMed] [Google Scholar]
- [46].Fam J, Sun Y, Qi P, Lau RC, Feng L, Kua EH, Mahendran R (2020) Mindfulness practice alters brain connectivity in community-living elders with mild cognitive impairment. Psychiatry Clin Neurosci 74, 257–262. [DOI] [PubMed] [Google Scholar]
- [47].Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [48].Galvin JE, Valois L, Zweig Y (2014) Collaborative transdisciplinary team approach for dementia care. Neurodegener Dis Manag 4, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hollingshead AB (1957) Two factor index of social position. Yale University Press, New Haven. [Google Scholar]
- [50].Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA, NIA Alzheimer’s Disease Centers (2007) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21, 249–258. [DOI] [PubMed] [Google Scholar]
- [51].Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou XH, Shirk SD, Atri A, Kukull WA, Phelps C, Morris JC (2018) Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 32, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Reisberg B (2007) Global measures: Utility in defining and measuring treatment response in dementia. Int Psychogeriatr 19, 421–456. [DOI] [PubMed] [Google Scholar]
- [53].Tappen RM, Rosselli M, Engstrom G (2010) Evaluation of the Functional Activities Questionnaire (FAQ) in cognitive screening across four American ethnic groups. Clin Neuropsychol 24, 646–661. [DOI] [PubMed] [Google Scholar]
- [54].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST (2000) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12, 233–239. [DOI] [PubMed] [Google Scholar]
- [55].Gamst-Klaussen T, Chen G, Lamu AN, Olsen JA (2016) Health state utility instruments compared: Inquiring into nonlinearity across EQ-5D-5L, SF-6D, HUI-3 and 15D. Qual Life Res 25, 1667–1678. [DOI] [PubMed] [Google Scholar]
- [56].Galvin JE (2015) The Quick Dementia Rating System (QDRS): A rapid dementia staging tool. Alzheimers Dement (Amst) 1, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Galvin JE, Tolea MI, Chrisphonte S (2020) Using a patient-reported outcome to improve detection of cognitive impairment and dementia: The patient version of the quick dementia rating system (QDRS). PLoS One 15, e0240422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, Unverzagt FW, Apostolova LG, Saykin AJ, Farlow MR (2016) The Cognitive Change Index as a measure of self and informant perception of cognitive decline: Relation to neuropsychological tests. J Alzheimers Dis 51, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li C, Neugroschl J, Luo X, Zhu C, Aisen P, Ferris S, Sano M (2017) The utility of the Cognitive Function Instrument (CFI) to detect decline in non-demented older adults. J Alzheimers Dis 60, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC (2005) The AD8: A brief informant interview to detect dementia. Neurology 65, 559–594. [DOI] [PubMed] [Google Scholar]
- [61].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [62].Shapiro AM, Benedict RH, Schretlen D, Brandt J (1999) Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 13, 348–358. [DOI] [PubMed] [Google Scholar]
- [63].Reitan RM (1958) Validity of the trail making test as an indication of organic brain damage. Perceptual Motor Skills 8, 271–276. [Google Scholar]
- [64].Galvin JE, Tolea MI, Moore C, Chrisphonte S (2020) The Number Symbol Coding Task: A brief measure of executive function to detect dementia and cognitive impairment. PLoS One 15, e0242233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yokoi K, Nishio Y, Uchiyama M, Shimomura T, Iizuka O, Mori E (2014) Hallucinators find meaning in noises: Pareidolic illusions in dementia with Lewy bodies. Neuropsychologia 56, 245–254. [DOI] [PubMed] [Google Scholar]
- [66].Snaith RP (2003) The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Many JF, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin JE, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I; VICCCS group, Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG (2017) The vascular impairment of cognition classification consensus study. Alzheimers Dement 13, 624–633. [DOI] [PubMed] [Google Scholar]
- [70].Olney NT, Spina S, Miller BL (2017) Frontotemporal dementia. Neurol Clin 35, 339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Connell CM, Boise L, Stuckey JC, Holmes SB, Hudson ML (2004) Attitudes toward the diagnosis and disclosure of dementia among family caregivers and primary care physicians. Gerontologist 44, 500–507. [DOI] [PubMed] [Google Scholar]
- [72].Galvin JE, Tolea MI, Chrisphonte S (2020) The positive and negative appraisals of caregiving (PANAC) scale: A new measure to examine the caregiving experience in Alzheimer’s disease and related dementias. Alzheimer Dement (N Y) 6,e12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Archbold PG, Stewart BJ, Greenlick MR, Harvath TA (1992) The clinical assessment of mutuality and preparedness in family caregivers to frail older people. In Key Aspects of Elder Care: Managing Falls, Incontinence and Cognitive Impairment, Funk SG, Tornquist EMT, Champagne MT, Wiese RA, eds. Springer, New York, pp. 328–339. [Google Scholar]
- [74].Zarit SH, Todd PA, Zarit JM (1987) Subjective burdens of husbands and wives as caregivers: A longitudinal study. Alzheimer Dis Assoc Disord 1, 109–110. [DOI] [PubMed] [Google Scholar]
- [75].Kroenke K, Spitzer RL, Williams JB, Löwe B (2009) An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics 50, 613–621. [DOI] [PubMed] [Google Scholar]
- [76].Galvin JE, Tolea MI, Rosenfeld A, Chrisphonte S (2020) The Quick Physical Activity Rating (QPAR) scale: A brief assessment of physical activity in older adults with and without cognitive impairment. PLoS One 15, e0241641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG Jr (1986) Assessing illness severity: Does clinical judgment work? J Chronic Dis 39, 439–452. [DOI] [PubMed] [Google Scholar]
- [79].Barbera M, Kulmala J, Lisko I, Pietila E, Rosenberg A, Hallikainen I, Hallikainen M, Laatikainen T, Lehtisalo J, Neuvonen E, Ruasnen M, Soininen H, Tuomilehto J, Ngandi T, Solomon A, Kivipelto M (2020) Third follow-up of the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) cohort investigating determinants of cognitive, physical, and psychosocial wellbeing among the oldest old: The CAIDE85+study protocol. BMC Geriatr 20, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Heo J, Tolea MI, Galvin JE (2017)Anew non-invasive and brief CVD risk score system to predict cognitive decline. Innov Aging 1(Suppl 1), 586. [Google Scholar]
- [81].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56, M146–156. [DOI] [PubMed] [Google Scholar]
- [82].Ross DE, Ochs AL, Tate DF, Tokac U, Seabaugh J, Abildskov TJ, Bigler ED (2018) High correlations between MRI brain volume measurements based on NeuroQuant(®) and FreeSurfer. Psychiatry Res Neuroimaging 278, 69–76. [DOI] [PubMed] [Google Scholar]
- [83].Persson K, Barca ML, Cavallin L, Brækhus A, Knapskog AB, Selbæk G, Engedal K (2018) Comparison of automated volumetry of the hippocampus using NeuroQuant® and visual assessment of the medial temporal lobe in Alzheimer’s disease. Acta Radiol 59, 997–1001. [DOI] [PubMed] [Google Scholar]
- [84].Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK (2011) Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology 77, 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Messick S (1995) Standards of validity and the validity of standards in performance assessment. Educ Measure Issues Pract 14, 5–8. [Google Scholar]
- [86].Schneider J, Malinowski P, Watson PM, Lattimore P (2019) The role of mindfulness in physical activity: A systematic review. Obesity Rev 20, 448–463. [DOI] [PubMed] [Google Scholar]
- [87].Nuzum H, Stickel A, Corona M, Zeller M, Melrose RJ, Wilkins SS (2020) Potential benefits of physical activity in MCI and dementia. Behav Neurol 2020, 7807856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wong WP, Coles J, Chambers R, Wu DBC, Hassed C (2017) The effects of mindfulness on older adults with mild cognitive impairment. J Alzheimers Dis Rep 1, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ng KST, Chan HY, Wee ST, Goh LG, Nur F, Tan CTY, Cheah IK-M, Tan CT, Nur G, Wee ST, Goh LG, Chow WL, Ho RC, Kua EH, Larbi A, Mahendran R (2016) Mindful awareness practice (MAP) to improve the cognition of Singaporean elderly with mild cognitive impairment (MCI): A randomized controlled trial (RCT). Alzheimers Dement 12, 1180–1181. [Google Scholar]
- [90].Loucks EB, Britton WB, Howe CJ, Eaton CB, Buka SL (2015) Positive associations of dispositional mindfulness with cardiovascular health: The New England Family Study. Int J Behav Med 22, 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ecay-Torres M, Estanga A, Tainta M, Izagirre A, Garcia-Sebastian M, Villanova J, Clerigue M, Iriondo A, Urreta I, Arrospide A, Diaz-Mardomingo C, Kivipelto M, Martinez-Lage P (2018) Increased CAIDE dementia risk, cognition, CSF biomarkers, and vascular burden in health adults. Neurology 91, e217–226. [DOI] [PubMed] [Google Scholar]
- [92].Kivipelto M, Ngandu T, Laatikainen T, Winbald B, Soininen H, Tuomilehto J (2006) Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol 5, 735–741. [DOI] [PubMed] [Google Scholar]
- [93].Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimaki M, Singh-Manoux A (2013) Predicting cognitive decline: A dementia risk score vs the Framingham vascular risk scores. Neurology 80, 1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lindsay EK, Young S, Brown KW, Smyth JM, Creswell JD (2019) Mindfulness training reduces loneliness and increases social contact in a randomized controlled trial. Proc Natl Acad Sci U S A 116, 3488–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ybarra O, Burnstein E, Winkielman P, Keller MC, Manis M, Chan E, Rodriguez J (2008) Mental exercising through simple socializing: Social interaction promotes general cognitive functioning. Personality Soc Psychol Bull 34, 248–259. [DOI] [PubMed] [Google Scholar]
- [96].Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D (2008) Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord 22, 716–721. [DOI] [PubMed] [Google Scholar]
- [97].Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GER (2007) A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med 33, 11–21. [DOI] [PubMed] [Google Scholar]
- [98].Shapiro SL, Brown KW, Biegel GM (2007) Teaching self-care to caregivers: Effects of mindfulness-based stress reduction on the mental health of therapists in training. Train Educ Prof Psychol 1, 105. [Google Scholar]
- [99].Wells RE, Kerr CE, Wolkin J, Dossett M, Davis RB, Walsh J, Wall RB, Kong J, Kaptchuk T, Press D, Phillips RS, Yeh G (2013) Meditation for adults with mild cognitive impairment: A pilot randomized trial. J Am Geriatr Soc 61, 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pagnoni G, Cekic M (2007) Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging 28, 1623–1627. [DOI] [PubMed] [Google Scholar]
- [101].Orgeta V, Qazi A, Spector A, Orrell M (2015) Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: Systematic review and meta-analysis. Br J Psychiatry 207, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tang YY, Hölzel BK, Posner MI (2015) The neuroscience of mindfulness meditation. Nat Rev Neurosci 16, 213–225. [DOI] [PubMed] [Google Scholar]
- [103].Russell-Williams J, Jaroudi W, Perich T, Hoscheidt S, El Haj M, Moustafa AA (2018) Mindfulness and meditation: Treating cognitive impairment and reducing stress in dementia. Rev Neurosci 29, 791–804. [DOI] [PubMed] [Google Scholar]
- [104].Hofmann SG, Gómez AF (2017) Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am 40, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gu J, Strauss C, Bond R, Cavanagh K (2015) How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev 37, 1–2. [DOI] [PubMed] [Google Scholar]
- [106].Innes KE, Selfe TK, Brown CJ, Rose KM, Thompson-Heisterman A (2012) The effects of meditation on perceived stress and related indices of psychological status and sympathetic activation in persons with Alzheimer’s disease and their caregivers: A pilot study. Evid Based Complement Alternat Med 2012, 927509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR (2010) Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: A preliminary study. J Alzheimers Dis 20, 517–526. [DOI] [PubMed] [Google Scholar]
- [108].Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P (2010) Cortical thickness and pain sensitivity in zen meditators. Emotion 10, 43. [DOI] [PubMed] [Google Scholar]
- [109].Luders E, Clark K, Narr KL, Toga AW (2011) Enhanced brain connectivity in long-term meditation practitioners. Neuroimage 57, 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, Spaeth R, Wall RB, Walksh J, Katchuk TJ, Press D, Phillips RS, Kong J (2013) Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: A pilot study. Neurosci Lett 556, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Marciniak R, Šumec R, Vyhnáiek M, Bendíčková K, Lázničkovα P, Forte G, Jeleník A, Římalová V, Frič J, Hort J, Sheardová K (2020) The effect of mindfulness-based stress reduction (MBSR) on depression, cognition, and immunity in mild cognitive impairment: A pilot feasibility study. Clin Interv Aging 15, 1365–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Quintana-Hernandez DJ, Miro-Barrachina MT, Ibanez-Fernandez IJ, Pino AS, Quintana-Montesdeoca MP, Rodriguez-de Vera B, Moreales-Caanova D, Perez-Vieitez MDC, Rodriguea-Garcia J, Bravo-Caraduje N (2016) Mindfulness in the maintenance of cognitive capacities in Alzheimer’s disease: A randomized clinical trial. J Alzheimers Dis 50, 217–232. [DOI] [PubMed] [Google Scholar]
- [113].Chan J, Leung DKY, Walton H, Wong GHY, Spector A (2020) Can mindfulness-based interventions benefit people with dementia? Drawing on the evidence from a systematic review in populations with cognitive impairments. Expert Rev Neurother 20, 1143–1156. [DOI] [PubMed] [Google Scholar]
- [114].Chiesa A, Serretti A (2009) Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. J Altern Complement Med 15, 593–600. [DOI] [PubMed] [Google Scholar]
- [115].Cheung DSK, Kor PPK, Jones C, Davies N, Moyle W, Chien WT, Yip ALK, Chambers S, Yu CTK, Lai C (2020) The use of modified mindfulness-based stress reduction and mindfulness-based cognitive therapy program for family caregivers of people living with dementia: A feasibility study. Asian Nurs Res (Korean Soc Nurs Sci) 14, 221–230. [DOI] [PubMed] [Google Scholar]
- [116].Kor PPK, Liu JYW, Chien WT (2020) Effects of a modified mindfulness-based cognitive therapy for family caregivers of people with dementia: A randomized clinical trial. Gerontologist, doi: 10.1093/geront/gnaa125 [DOI] [PubMed] [Google Scholar]
- [117].Paller KA, Creery JD, Florczak SM, Weintraub S, Mesulam MM, Reber PJ, Kiragu J, Rooks J, Safron A, Morhardt D, O’Hara M, Gigler KL, Molony JM, Maslar M (2015) Benefits of mindfulness training for patients with progressive cognitive decline and their caregivers. Am J Alzheimers Dis Other Demen 30, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]