Abstract
The prevalence of inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn's disease (CD), increases gradually worldwide in the past decades. IBD is generally associated with the change of the immune system and gut microbiota, and the conventional treatments usually result in some side effects. Bifidobacterium longum, as colonizing bacteria in the intestine, has been demonstrated to be capable of relieving colitis in mice and can be employed as an alternative or auxiliary way for treating IBD. Here, the mechanisms of the Bifidobacterium longum in the treatment of IBD were summarized based on previous cell and animal studies and clinical trials testing bacterial therapies. This review will be served as a basis for future research on IBD treatment.
1. Introduction
Inflammatory bowel disease (IBD) is mainly manifested as chronic and recurrent inflammation in the gastrointestinal tract. It includes ulcerative colitis (UC) and Crohn's disease (CD) [1]. Although traditionally regarded as a disease prevalent in Western countries, the incidence of IBD is gradually increasing globally, especially in newly industrialized countries [2]. In the past decade, IBD has become a global public health challenge [3]. Its main symptoms include diarrhea, abdominal cramps, weight loss, fatigue, anemia, and extraintestinal symptoms (especially joint pain or arthritis). These will cause serious obstacles and troubles to human's normal life [4]. Most patients with IBD suffer from fecal incontinence and also face the risk of a weakened immune system and bowel cancer [5]. The occurrence of IBD is closely related to genetic susceptibility, environment, immune regulation dysfunction, gut microbiota, nutrition, and lifestyle [6]. However, the exact cause of IBD has yet to be determined, which makes it difficult to develop targeted treatments [4, 7].
At present, the commonly used drugs for the treatment of IBD include immunosuppressive drugs, biological agents, and antibiotics [8]. Among them, 5-aminosalicylic acid (5-ASA) is widely used in the treatment of IBD due to its good clinical efficacy [9]. However, taking this medicine will cause adverse reactions such as diarrhea, abdominal pain, headache, and nasopharyngitis, making the patient uncomfortable [8]. Monoclonal cytokines such as anti-TNF-α and IL-6 can also treat IBD, but the high production cost of this method makes it unacceptable for some patients [6]. Recently, studies have found that Bifidobacterium longum can be used as an adjuvant treatment for IBD [10]. Bifidobacterium longum belongs to the genera Actinomyces and Bifidobacterium. It is a gram-positive bacterium that performs anaerobic respiration [11]. The genus Bifidobacterium inhabits intestinal tracts of humans and animals. It is one of the first microorganisms to colonize the host gut [12]. It has more than 50 different species, of which, Bifidobacterium longum is one of the most abundant microorganisms in the intestines of infants and adults [8, 9]. It can be separated from a variety of animals, including intestines of babies and long-lived elderly [13]. Diseases inside and outside the intestine are closely related to the changes in the abundance of Bifidobacterium longum. Compared with healthy people, the abundance of Bifidobacterium longum flora in the stool of patients with intestinal diseases is much lower [14]. Bifidobacterium can be stably colonized in the human intestine. It has immune tolerance to the human body and will not cause rejection [15]. A large number of animal experiments and clinical studies have shown that Bifidobacterium longum can reduce the symptoms of colitis and relieve chronic inflammation [16]. However, the mechanisms of Bifidobacterium longum to treat IBD and regulate the intestinal immune system are still unclear. In this review, we will focus on the cell and animal experiments and clinical trials to summarize the mechanisms of Bifidobacterium longum on the prevention and treatment of IBD, which would provide a basis for subsequent therapeutic applications.
2. Interaction between Bifidobacterium longum and the Host
The human gastrointestinal environment can be regarded as a complex ecosystem. It contains trillions of microbes, which are usually called gut microbiota [17, 18]. Scientists have discovered that the composition of the gut microbiota and its metabolites plays an important role in protecting the intestinal barrier and regulating the immune balance [19]. Disturbances of the gut microbiota often occur in patients with intestinal diseases, such as irritable bowel syndrome, idiopathic chronic diarrhea, colorectal cancer, and IBD [20]. Some studies have shown that IBD usually causes general changes in the structure of the gut microbiota of patients, resulting in a decrease in the diversity and species abundance [21, 22]. The anaerobic species and short-chain fatty acid producers depleted, and the facultative anaerobic bacteria increased in the gut of patients [23]. Changes in gut microbiota will affect the normal operation of the mucosal immune system, leading to functional degradation [24]. Probiotics that promote the balance of gut microbiota play an important role in the treatment of IBD [25].
It is reported that the intervention of probiotics improved the gut microbiota and has an effective protective effect on the immune health of the host [26, 27]. The results of animal and clinical studies showed that products containing probiotics or prebiotics improved IBD by regulating proinflammatory signaling pathways and downregulating proinflammatory cytokines [7]. Bifidobacterium longum, as one of the most abundant members in the gut, can protect the intestinal epithelial barrier and tissue structure and balance the gut microbiota to alleviate the symptoms of colitis [28]. Moreover, Bifidobacterium can secrete a variety of active metabolites [29]. They influence the interaction between digestion, endocrine, cardiovascular, immune, and nervous systems to maintain the host in a healthy state [30, 31]. Bifidobacterium longum inhibits inflammation by regulating the balance of the immune system, improving the intestinal barrier function, and increasing acetate production [32]. This species has been widely used as a probiotic because of its beneficial effects on host health and has been recognized as safe by the United States Food and Drug Administration and the European Food Safety Authority [15].
3. Mechanisms of Bifidobacterium longum in Improvement of IBD
3.1. Bifidobacterium Longum and Antioxidant Activity
Oxidative stress has been regarded as one of the major mechanisms involved in the pathophysiology of IBD [33]. It is characterized by the inability of the organism to detoxify reactive oxygen species (ROS) caused by a disequilibrium in the balance between their production and accumulation in cells and tissues [34]. The infiltration of immune cells occurred in active IBD as the prominent feature. More extensive recruitment of neutrophils and less of monocytes are the typical characteristics in lesion location. Myeloperoxidase (MPO), an abundant granule heme enzyme, is unique to both neutrophils and monocytes [35]. Through the halogenation or peroxidase cycle, MPO could generate reactive oxygen species (ROS) effectively [36]. ROS mainly includes the oxygen-containing ions, molecules, or groups with high activity. The abnormal accumulation of ROS will cause serious damage to normal physiological metabolic activity [37]. They induce fatty acid side-chain reactions to create lipid malondialdehyde and hydroperoxides, which results in the damage of biological macromolecules and causes the impairment of cell structure and function [37]. Substantial evidence shows that the imbalance between the accumulation of ROS and antioxidant activity is closely related to the incidence and severity of IBD. For IBD patients, oxidative stress occurs with the raise of ROS levels and decline of antioxidant levels, which leads to chronic tissue damage continuously [38, 39] (Figure 1).
Figure 1.
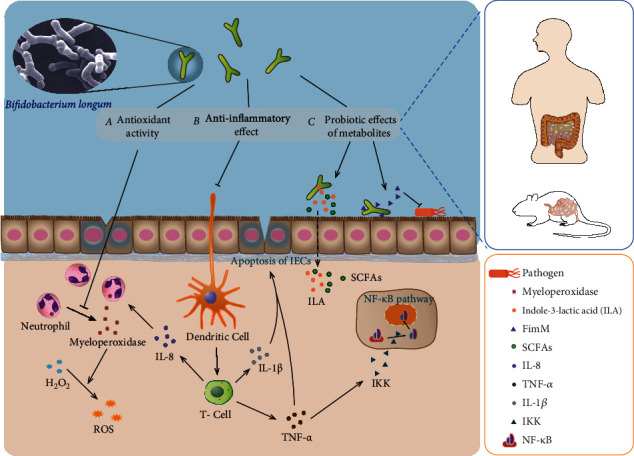
Protective mechanism of Bifidobacterium longum against intestinal inflammation.
Studies in cell and animal experiments have shown that Bifidobacterium longum strains regulate oxidative stress by enhancing the body's antioxidant activity and regulating the production and accumulation of ROS, thereby reducing the symptoms of IBD. B. longum 5(1A) administration in the dextran sulfate sodium- (DSS-) induced colitis in mice abated severe lesions in the colon with the decreased level of eosinophil peroxidase [40] (Figure 1). In addition, oral Bifidobacterium longum is also an effective treatment of ethanol-induced gastritis injury. Application of microbial inoculum downregulates the tumor necrosis factor (TNF) expression, myeloperoxidase activity, and hemorrhagic ulcerative lesions area [41]. Moreover, similar antioxidant effects have been found for the fermented products or metabolites of B. longum YS108R [24]. Without altering cell viability, B. longum CCFM752 supernatants increased intracellular antioxidative capacity with enhanced intracellular catalase activity and reduced NADPH oxidase activation [42].
Many anaerobic microorganisms remove ROS mainly by secreting and producing enzymes, such as NADH oxidase, NADH peroxidase, catalase and superoxide dismutase [43]. Currently, there are few studies concerning oxygen resistance and free radical scavenging genes or enzymes of B. longum, and there have been reports only about the strains NCC2705 [44], BBMN68 [43], and LTBL16 [45]. It has been found that B. longum LTBL16 had three peroxide oxidoreductase coding genes (LTBL16-000027, LTBL16-000028, LTBL16-000976) and one NADH oxidase coding gene (LTBL-001911), which can effectively remove ROS in bifidobacteria and improve oxygen resistance [45]. Recent studies have found that Bifidobacterium longum BBMN68 had an incomplete glutredoxin system. Thioredoxin and glutaredoxin make up the thioredoxin- and glutaredoxin-dependent reduction systems in Escherichia coli and many other bacteria and are responsible for maintaining a reduced environment in the cell cytosol [46]. Under oxidative stress, the genes grxC1- (BBMN68_125-) and grxC2- (BBMN68_1397-) encoding glutaredoxin, trxB1- (BBMN68_1345-) encoding thioredoxin reductase, and BBMN68_991-encoding thioredoxin are all upregulated [43]. Studies have found that when Bifidobacterium is under oxidative stress, thioredoxin reductase can respond positively to its transcription and translation [47]. In addition, the thioredoxin-dependent reduction system can reduce perredoxin and H2O2, scavenging free radicals, quenching singlet oxygen, and then maintaining the intracellular thioldisulfide balance [48]. Thus, the thioredoxin-dependent antioxidant system might be the major redox homeostasis system in strain BBMN68.
In mammals, several longevity proteins of the sirtuin family have been shown to play an antioxidant role by deacetylation activity. The cytosolic isoform SIRT2 is capable of deacetylating forkhead box protein FOXO1a and FOXO3a, thereby increasing FOXO-dependent transcription of antioxidant enzymes and reducing the cellular ROS level [49]. The probiotic (B. longum NCC2705) has the Sir2 gene family and has antioxidant activity in the human body. BL-Sir2 regulated FOXO3a mediated antioxidant genes, deacetylated σH, and increased the activity of manganese superoxide dismutase and catalase and reduced ROS [44]. In addition, a Sir2-encoding gene (LTBL16-002010) was also found in B. longum LTBL16, which could improve FOXO-dependent transcription of antioxidant enzymes encoding genes and reduce ROS levels in cells [45]. Therefore, Bifidobacterium longum can suppress oxidative stress and stimulate the production of antioxidants, thereby reducing the oxidative damage of intestinal tract of IBD (Figure 1).
Bifidobacterium longum can protect intestinal epithelial cells by different mechanisms. These include (a) Bifidobacterium longum can decline myeloperothe xidase activity and the production of ROS, suppress oxidative stress, and reduce the damage of the tintestinal tract. (b) Bifidobacterium longum can downregulate inflammatory cytokines and inhibit NF-κB pathway to regulate the intestinal immune system and protect intestinal epithelial cells. (c) Bifidobacterium longum can produce various metabolites to enhance adhesion to the intestinal tract and inhibit harmful bacteria. It can also participate in immune regulation. Bifidobacterium longum was photographed by Mark Schell, University of Georgia, Athens, GA [50].
3.2. Bifidobacterium longum Reduces the Inflammatory Cytokine Expression in the Intestine
In vitro experiments and animal models indicate that Bifidobacterium longum has anti-inflammatory effects on intestinal diseases (Table 1 and Table 2). Bifidobacterium longum can reduce spontaneous and chemically induced colitis by regulating cytokines or inducing immune regulation mechanisms in a specific way [51]. The intestine is an important immune organ. Goblet cells in the intestine produce mucus to fight off invading pathogens. Under the mucus, intestinal epithelial cells and various immune cells form another defense barrier to prevent the invasion of pathogenic microorganisms [52]. These cells can specifically secrete various cytokines to regulate the immune system. For example, Th1 cells can secrete tumor necrosis factor α (TNF-α) to initiate a variety of proinflammatory responses [53]. Th17 cells are involved in the activation and recruitment of neutrophils [54]. Treg cells can express the transcription factor forkhead box P3 (FOXP3) and secrete the anti-inflammatory cytokine IL-10, thereby inhibiting a strong inflammatory response [55].
Table 1.
Effects of B. longum strains in modulating inflammation based on in vitro and ex vivo studies.
| Strains | Dose | Cell | Effect | Ref. |
|---|---|---|---|---|
| B. longum CECT-7347 | 2 × 109 cells/mL | HT-29 cell | IL-8 ↓ | [62] |
| B. longum Bif10 and Bif16 | 1 × 1010 CFU/mL | RAW264.7 cell | TNF-α, IL-1β, IL-6 ↓ SCFA ↑ |
[59] |
| B. longum BB536 | 5 × 108 cells/mL | PIE cell | TNF-α↓ | [60] |
| B. longum KACC 91563 | 1 × 106, 107, 108 CFU/well | Splenocytes macrophages | TNF-α ↓, IgE ↓ IL-2, 4, 6, 10, IFN-γ ↓ |
[51] |
| B. longum R0033 | 100 : 1 for bacteria to cell ratio | HT-29 cell | TNF-α, IL-8 ↓ | [61] |
| B. longum 51A | 1 × 103, 105 CFU/well | Keratinocyte fibroblast cell | IL-6, IL-8 ↓ | [73] |
| B. longum BL05 | 5 × 104, 105, 106 CFU/well | HT-29 cell THP-1 cell |
IL-10 ↑ IL-1β, IL-6 ↓ |
[67] |
| B. longum LC67 | 1 × 103, 105 CFU/mL | KATO III cells | NF-κB ↓ IL-8 ↓ |
[41] |
| B. longum LC67 | 1 × 104, 106 CFU/mL | Caco-2 cells | NF-κB ↓ | [28, 74] |
Table 2.
Animal studies of B. longum strain effects in modulating inflammation.
| Strains | Dose | Model | Effect | Ref. |
|---|---|---|---|---|
| B. longum Bif10 and Bif16 | 5 × 109 CFU/mouse/day | DSS-induced colitis in mice | SCFA ↑ TNF-α, IL-1β, IL-6 ↓ |
[59] |
| B. longum 5 (1A) | 1 × 108 CFU/mouse/day | DSS-induced colitis in mice | IL-1 ↓ MPO ↓ |
[40] |
| B. longum YS108R | 1 × 109 CFU/mouse/day | DSS-induced colitis in mice | IL-10 ↑ TNF-α, MPO, IL-1β, IL-6, IL-17A ↓ |
[24, 72] |
| B. longum ATCC 15707 | 1 × 107 CFU/kg/day | DSS-induced colitis in mice | SCFA ↑ TNF-α, IL-6, TGF-β ↓ |
[71] |
| B. longum HB5502 | 4 × 109 CFU/day | TNBS-induced colitis in mice | HMGB1 ↓ | [84] |
| B. Longum LC67 | 1 × 109 CFU/mouse/day | Ethanol-induced gastritis in mice | NF-κB, CXCL4, TNF ↓ | [41] |
| B. longum LC67 | 1 × 109 CFU/mouse/day | High-fat diet-induced colitis in mice | AMPK ↑ NF-κB ↓ |
[74] |
| B. longum LC67 | 1 × 109 CFU/mouse/day | TNBS-induced colitis in mice | NF-κB, MPO ↓ | [79] |
Under normal circumstances, the mucosal cells of the intestine can keep the proinflammatory and anti-inflammatory cytokines in a relatively balanced state [51]. In the intestines of patients with IBD, this balance is disrupted. The increase in the number and activity of proinflammatory cytokines in the mucosa leads to damage and inflammation of the intestinal tissues [56]. In the process of IBD, immune cells are activated after receiving a stimulating signal. A large number of inflammatory cytokines are secreted, including tumor necrosis factor (TNF-α), interleukin (IL-1β), IL-6, and ROS [57] (Figure 1). An increase in intestinal epithelial cell (IEC) apoptosis is a major characteristic of IBD. Studies have shown that excessive TNF-α can destroy the integrity of the intestinal epithelium and induce apoptosis of IECs [58] (Figure 1). The study of T cell metastasis showed that the content of TNF-α in the intestinal tract of colitis increased significantly [59]. In the study of various strains of Bifidobacterium longum, it was found that after incubating cells with probiotics, the level of TNF-α was significantly reduced. The disease can be alleviated by the neutralizing effect of TNF-α [51, 60, 61].
Furthermore, TNF-α induces inflammatory responses with the expression of proinflammatory cytokines, including IL-1β, IL-6, and IL-8 [62]. The IL-1β is produced by IECs in a paracrine manner. It could disrupt the maturation and function of IECs resulting in exerting major epithelial barrier alterations [63]. As a pleiotropic cytokine, IL-6 plays a central role in immunoregulation, inflammation response, and oncogenesis. Anti-IL-6 monoclonal antibody effectively suppresses chronic intestinal inflammation in mouse models [64]. A previous research demonstrates that proinflammatory molecules like IL-8 could be induced by enteropathogenic bacteria colonizing in the gut. As a consequence, neutrophils and other inflammatory cells will be recruited [65]. Infiltration of neutrophils may perpetuate inflammation and result in cell damage, epithelial barrier dysfunction, and diarrhea [66]. Marzia et al. used B. longum and macrophages to conduct a simulation study of the intestinal epithelial barrier function. It was found that IL-10 was induced by probiotics significantly. On the contrary, the production of IL-1β and IL-6 was downregulated by 70% and 80%, respectively [67]. Similarly, after coincubation with B. longum HT-CECT-7347, HT29 cells stimulated by TNF-α displayed a drastic dose-dependent decline in IL-8 production [62]. In addition, it was found that after treatment with Bifidobacterium longum, colitis mice alleviated inflammation, and the content of short-chain fatty acids in the intestinal tract also increased. The regulation of immunity by short-chain fatty acids (SCFAs) is mainly mediated by activation of free fatty acid receptor 2 (FFA2) or inhibition of histone deacetylase (HDAC) [68]. As the main receptor of SCFA, FFA2 is expressed on immune cells and inhibits the NF-κB signaling pathway to produce anti-inflammatory effects [69]. HDACs are generally expressed in immune, endothelial, and vascular smooth muscle cells [70]. Inhibition of HDAC activity causes an open structure of DNA/chromatin, which facilitates the regulation of the expression of transcription factors, such as NF-κB and FOXP3 [68]. Therefore, B. longum can regulate intracellular signaling pathways and decrease the level of IL-1β, IL-6, and IL-8, reduce the alterations of the in vitro epithelial barrier induced by DSS, and regulate the inflammatory response [59, 71, 72] (Figure 1).
NF-κB plays a crucial role in a variety of immune and inflammatory reactions in the intestine. It can participate in the induction and regulation of the related gene expression [75]. Studies have found that TNF-α acts through the activation of TNF receptors. This activation triggers a series of intracellular events that result in the activation of the transcription factor NF-κB [76]. Its activation level is closely related to the severity of intestinal inflammation. Upon receipt of a proinflammatory stimulus, IKK phosphorylates inhibitory kB (IkB) molecules, releases NF-κBp50-p65 heterodimeric protein, migrates to the cell nucleus, and binds to specific kB sites (Figure 1). Genes encoding cytokines and chemokines, cell adhesion molecules, and immune receptors will be activated and transcribed to produce important mediators of inflammation [77, 78]. In an ethanol-induced gastroenteritis study, the oral administration of B. longum LC67 in mice was found to suppress the TNF-α expression and NF-κB activation in mucosal cells, restore the gut microbiota disturbance, and alleviate ethanol-induced GI inflammation [28]. For the mice with high-fat diet- (HFD-) induced obesity, B. longum alleviated colitis by regulating NF-κB activation through the inhibition of the production of harmful substances in the gut microbiota [74]. Further research showed that Bifidobacterium longum could prevent the nuclear localization of NF-κB-p65 in the damaged intestine to a certain extent and increase the expression of NF-κB-p65 in the cytoplasm [62, 79].
Besides, probiotics can secrete tryptophan metabolites to maintain the healthy homeostasis of the host [80]. It has previously been reported that a number of colonizing intestinal bacteria, particularly Gram-negative organisms, can metabolize the amino acid tryptophan to improve health and provide immune protection [81]. Bifidobacterium longum subsp. infantis can produce indole-3-lactic acid (ILA) in its culture medium as an anti-inflammatory molecule (Figure 1). This molecule reduces the IL-8 response after IL-1β stimulus. It interacts with the transcription factor aryl hydrocarbon receptor (AHR) and prevents transcription of the inflammatory cytokine IL-8 [82]. In addition, it could significantly attenuate lipopolysaccharide- (LPS-) induced activation of NF-κB in macrophages and significantly attenuate TNF-alpha and IL-8 in intestinal epithelial cells to protects gut epithelial cells [82, 83]. ILA increased the mRNA expression of the aryl hydrogen receptor- (AhR-) target gene CYP1A1 and nuclear factor erythroid 2-related factor 2- (Nrf2-) targeted genes glutathione reductase 2 (GPX2), superoxide dismutase 2 (SOD2), and NADPH dehydrogenase (NQO1) and protects gut epithelial cells in culture via activation of the AhR and Nrf2 pathway [83]. Therefore, Bifidobacterium longum can reduce the production of proinflammatory cytokines, inhibit the activation of NF-κB induced by TNF-α, and improve the symptoms of IBD (Figure 1).
3.3. Bifidobacterium longum Enhances the Intestinal Barrier Function
Intact intestinal epithelial cells can ensure the normal intestinal function. It can resist pathogenic microorganisms and harmful substances in the intestinal environment to avoid damage [85]. Good intestinal barrier function requires tight junctions between intestinal epithelial cells [86]. In IBD, the intestinal permeability of the patient's intestinal mucosa increases, and the expression of the tight junction protein (TJP) decreases, which affects the protective function of the intestine and causes inflammation [87]. Inflammation of the intestinal epithelial mucosa will exacerbate this phenomenon, leading to a further decrease in TJP and forming a vicious circle [88]. Studies have shown that feeding mice with B. longum YS108R can improve the mucosal barrier damage induced by DSS and increase the expression of TJP and mucin2 to alleviate colitis [24]. In a similar experiment on 2,4,6-trinitrobenzenesulfonic acid- (TNBS-) induced colitis mice, it was found that the expression of tight junction proteins ZO-1, occluding, and claudin-1 in the colon was significantly reduced, but this phenomenon was alleviated after feeding with B. longum HB5502 [84].
Moreover, some studies have shown that IBD patients were accompanied with weight loss, inflammatory cell infiltration, anemia, decrease of colon length, and damage of the mucosal layer [89]. Mice with colitis induced by chemical reagents are often used as models to obtain symptoms similar to IBD for research on the treatment of related diseases [90]. Feeding mice with Bifidobacterium longum strains Bif10 and Bif16 could reduce their crypt deformation, diarrhea, etc. The decrease in colon length was alleviated, and the survival rate was improved [59]. Compared to the control group, the infiltration of inflammatory cells in the colon tissue of the B. longum ATCC 1570 treatment group was improved. Crypt alterations and ulceration areas were not observed in the epithelium [71]. Similarly, Bifidobacterium longum LC67 can alleviate TNBS-induced colon shortening in mice. Myeloperoxidase activity is also reduced. At the same time, the edema and destruction of colonic epithelial cells have been relieved, and the expression of the colonic tight junction protein has been restored [79]. In addition, the study found that after treatment with Bifidobacterium longum, the content of SCFAs in the intestinal tract of colitis mice also increased. SCFAs, as metabolites of the gut bacteria, are used by epithelial cells as their primary energy source to promote the health of the GI system [91]. SCFAs improves the expression of connexin in intestinal epithelial cells by enhancing the expression of the MUC2 gene and activating the AMP-activated protein kinase (AMPK) pathway [92]. Moreover, SCFA has an impact on the population and function of innate immune cells through G-protein coupled receptor signaling and HDAC inhibition and plays an important role in maintaining the intestinal barrier function [91, 93].
3.4. Bifidobacterium longum Regulates Gut Microbiota
In normal individuals, symbiosis exists between the gut microbiota and the host. This harmonious and stable symbiotic relationship can regulate mucosal immunity and prevent the colonization of pathogens in the intestine [94] (Figure 1). Recently, studies have revealed that gut microbiota imbalance played a vital role in the causation of various diseases including IBD [95]. The improvement of gut microbiota composition has been proposed as an effective auxiliary method for the treatment of certain intestinal inflammatory diseases [96]. A previous research has revealed that the gut flora of DSS-treated mice changed significantly in comparison with the control group. The abundance of gut microbiota was reduced, and the bifidobacteria supplementation alleviated the changes of gut microbiota induced by DSS [72]. B. longum YS108R can produce abundant extracellular polymeric substances (EPS). After feeding the fermented milk to DDS-induced colitis mice, it was found that the gut microbiota was adjusted, and pathogenic bacteria such as Enterobacteriaceae were also suppressed [24].
Probiotics in the intestine can release many biologically active peptides, bringing countless benefits to the health of the host [97]. Adhesion to the gastrointestinal tract is considered to be important for bifidobacteria to colonize the human gut and exert their probiotic effects. FimM is a novel surface adhesin that is mainly present in B. longum strains. Under normal circumstances, FimM may block pathogen access to the mucus layer by binding to mucins. Under pathogen invasion, FimM could competitively inhibit pathogen adhesion by binding to fibronectin and fibrinogen [98]. In addition, Bifidobacterium supplementation increased the level of intestinal SCFAs and inhibited the abundances of pathobionts at the genus level. Bacterial components of B. longum fed mice were slightly different from those of healthy mice [59]. As the final products of anaerobic intestinal microbiota fermentation, SCFAs have beneficial effects in accelerating intestinal movement and modulating the body immune system. They can also increase the risk of metabolic syndrome and reduce plasma cholesterol levels [99, 100] (Figure 1). Another study stated that B. longum KACC 91563 exists favorable impacts on increase of the SCFA content in feces of normal dogs and improves the gut microbiota structure [101]. The study found that B. longum BB536 had a synergistic effect with gut microbiota, which is helpful to maintain body homeostasis, and reduce the probability of gastrointestinal and allergic diseases [18]. These results indicated that Bifidobacterium longum had active influences on host healthy through restoring the gut microbiota balance.
4. Application of Bifidobacterium longum in Clinical Trials
Many clinical trials have shown that using Bifidobacterium longum can effectively improve the symptoms of IBD (Table 3). In comparison with placebo-treated subjects, B. longum 536 can improve the clinical symptoms of patients with mild to moderately active UC. 8 weeks after treatment, disease activity and clinical scores are greatly reduced [102]. 12 weeks after treatment with Bifidobacterium longum in patients with IBS-D, proinflammatory cytokines (IL-6, IL-8, and tumor necrosis factor TNF-α) were decreased, and intestinal permeability and gastrointestinal symptoms were improved [103]. Besides, Bifidobacterium longum is also used together with other ingredients to achieve better results. For example, when it was used together with the prebiotic synergy 1, the CD activity and histological score were reduced [104]. Bifidobacterium longum and inulin-oligofructose were provided to UC patients. 4 weeks after treatment, it was found that the expression of β-defensin, IL-1α, and TNF-α genes was decreased. At the same time, rectal biopsy was improved, inflammation was reduced, and epithelial tissue was regenerated [105].
Table 3.
Clinical evidence for B. longum with IBD.
| Strains | Number of patients (age) | Length of treatment | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| B. longum 536 | 56 (31-58 years old) | 8 weeks | 2 − 3 × 1011 CFU/day | Clinical remission UC disease activity index ↓ |
[102] |
| B. longum ES1 | 16 (16-65 years old) | 12 weeks | 1 × 109 CFU/day | Proinflammatory cytokines ↓ Improved intestinal permeability |
[103] |
| B. longum and synergy 1 | 35 (18-79 years old) | 6 months | 4 × 1011 CFU/day | CD activity ↓ TNF-α ↓ |
[104] |
| B. longum and inuli | 18 (24-67 years old) | 4 weeks | 4 × 1011 CFU/day | Inflammatory parameters ↓ | [105] |
| VSL #3 | 147 (26-52 years old) | 12 weeks | 7.2 × 1012 CFU/day | Induction of remission in mild-to-moderate UC | [106] |
| VSL #3 | 119 (25-49 years old) | 9 months | 1.8 × 1010 CFU/day | Inflammatory cytokine levels ↓ | [107] |
| VSL #3 | 131 (33-62 years old) | 8 weeks | 3.6 × 109 CFU/day | Clinical scores in UC ↓ | [109] |
| B. longum 536 | 12 (28-45 years old) | 1 months | 4 × 109 CFU/day | Improvement of gut microbiota | [112] |
When the probiotic product VSL#3 embodying Bifidobacterium longum was administered to patients with active UC, the symptoms of enteritis were effectively relieved 6 weeks after treatment, and there was no adverse reactions [79]. In 2009, similar results were obtained in experiments on patients with mild to moderate UC, and the disease activity index was also reduced [106]. Fedorak et al. assessed the preventive effect of VSL#3 against postoperative CD recurrence and found that the reduction of the proinflammatory cytokines in the patient's intestinal mucosa was owed to VSL#3, and the postoperative recurrence rate was also remained at a low level [107]. Similarly, the use of VSL#3 was found to be able to alleviate the pain of IBD patients to varying degrees and has great potential for disease treatment [108–111]. Therefore, the results of clinical trials prove that the use of Bifidobacterium longum alone or in combination with other probiotics can effectively improve the symptoms of IBD patients. Bifidobacterium longum can be used as an effective preventive or auxiliary treatment for IBD.
5. Future Perspectives of Bifidobacterium longum-Associated Therapy in IBD
In the past decades, the use of genetic engineering and biological engineering to express proteins or polypeptides with specific functions using bifidobacteria as vectors has become a new therapeutic method [113, 114]. Bifidobacterium is an excellent candidate for the development of living vectors for the production and delivery of heterologous proteins on mucosal surfaces. Bifidobacterium longum, which is a probiotic, can be colonized in the intestine for a long time, and it is immune tolerant to the human body. In the case of long-term use, it will not cause rejection by the human body [115]. However, compared to the use of a single bacterial agent, the effect of using a composite bacterial agent is more significant. The optimal dose and treatment time of the bacterial agent in the course of use and its molecular mechanism of action have not yet been determined. In addition, probiotic preparations take a long time to be effective [116]. In the treatment of severe acute inflammatory bowel disease, chemical drugs and surgical treatment are still the first choice [117]. Therefore, the above issues will be the focus of future research.
6. Conclusions
Bifidobacterium longum is a symbiotic bacterium existed in the human gastrointestinal tract. Both animal and clinical trials have found and demonstrated that Bifidobacterium longum had preventive and protective impacts on IBD. Bifidobacterium longum can change the structure of the gut microbiota, induce and regulate immune responses, and reduce the expression of inflammatory cytokines and ROS in the intestine. Besides, it can also maintain the normal intestinal barrier function by increasing the expression of the TJP protein. Therefore, Bifidobacterium longum has great potential and can be used as a prevention, replacement, or adjuvant treatment for IBD.
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities (Project No. FRF-BR-19-003B and FRF-BR-20-03B).
Conflicts of Interest
The authors declare that there is no conflict of interest to report.
References
- 1.Ananthakrishnan A. N. Epidemiology and risk factors for IBD. Nature Reviews. Gastroenterology & Hepatology. 2015;12(4):205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 2.Ng S. C., Shi H. Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan G. G. The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 4.Parada Venegas D., de la Fuente M. K., Landskron G., et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourie S., Jackson D., Aveyard H. Living with Inflammatory Bowel Disease: A review of qualitative research studies. International Journal of Nursing Studies. 2018;87:149–156. doi: 10.1016/j.ijnurstu.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn W. J., Feagan B. G., Marano C., et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Mijan M. A., Lim B. O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: present status and future trends. World Journal of Gastroenterology. 2018;24(25):2673–2685. doi: 10.3748/wjg.v24.i25.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Parker C. E., Feagan B. G., Macdonald J. K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2016;5 doi: 10.1002/14651858.CD000544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousseaux C., Lefebvre B., Dubuquoy L., et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-γ. Journal of Experimental Medicine. 2005;201(8):1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaza-Díaz J., Ruiz-Ojeda F. J., Vilchez-Padial L. M., Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics ini chronic diseases. Nutrients. 2017;9(6):p. 555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottacini F., van Sinderen D., Ventura M. Omics of bifidobacteria: research and insights into their health-promoting activities. Biochemical Journal. 2017;474(24):4137–4152. doi: 10.1042/BCJ20160756. [DOI] [PubMed] [Google Scholar]
- 12.O'Callaghan A., van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turroni F., Duranti S., Milani C., Lugli G. A., van Sinderen D., Ventura M. Bifidobacterium bifidum: a key member of the early human hut microbiota. Microorganisms. 2019;7(11):p. 544. doi: 10.3390/microorganisms7110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arboleya S., Watkins C., Stanton C., Ross R. P. Gut bifidobacteria populations in human health and aging. Frontiers in Microbiology. 2016;7(1204) doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C. C., Yu Z. M., Zhao J. X., Zhang H., Zhai Q. X., Chen W. Colonization and probiotic function of Bifidobacterium longum. Journal of Functional Foods. 2019;53:157–165. doi: 10.1016/j.jff.2018.12.022. [DOI] [Google Scholar]
- 16.Zhang M., Zhou L., Zhang S., et al. Bifidobacterium longum affects the methylation level of forkhead box P3 promoter in 2, 4, 6-trinitrobenzenesulphonic acid induced colitis in rats. Microbial Pathogenesis. 2017;110:426–430. doi: 10.1016/j.micpath.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Thursby E., Juge N. Introduction to the human gut microbiota. Biochemical Journal. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C. B., Odamaki T., Xiao J.-z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of Functional Foods. 2019;54:506–519. doi: 10.1016/j.jff.2019.02.002. [DOI] [Google Scholar]
- 19.Liang D., Leung R. K.-K., Guan W., Au W. W. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathogens. 2018;10(1):p. 3. doi: 10.1186/s13099-018-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Y., Zhou G., Qin X., Huang S., Wang B., Cao H. The potential role of gut mycobiome in irritable bowel syndrome. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo T., Ng S. C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Frontiers in Microbiology. 2018;9 doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K., Wan Z., Ou A., et al. Monofloral honey from a medical plant,Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitisviamodulating gut microbial populations in rats. Food & Function. 2019;10(7):3828–3838. doi: 10.1039/C9FO00460B. [DOI] [PubMed] [Google Scholar]
- 23.Vich Vila A., Imhann F., Collij V., et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Science Translational Medicine. 2018;10(472, article eaap8914) doi: 10.1126/scitranslmed.aap8914. [DOI] [PubMed] [Google Scholar]
- 24.Yan S., Yang B., Ross R. P., et al. Bifidobacterium longum subsp. longum YS108R fermented milk alleviates DSS induced colitis via anti-inflammation, mucosal barrier maintenance and gut microbiota modulation. Journal of Functional Foods. 2020;73:p. 104153. doi: 10.1016/j.jff.2020.104153. [DOI] [Google Scholar]
- 25.Sanchez B., Delgado S., Blanco-Miguez A., Lourenco A., Gueimonde M., Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Molecular Nutrition & Food Research. 2017;61(1) doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar A., Lehto S. M., Harty S., Dinan T. G., Cryan J. F., Burnet P. W. J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends in Neurosciences. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma T., Jin H., Kwok L.-Y., Sun Z., Liong M.-T., Zhang H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiology of Stress. 2021;14, article 100294 doi: 10.1016/j.ynstr.2021.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim W. G., Kim H. I., Kwon E. K., Han M. J., Kim D. H. Lactobacillus plantarumLC27 andBifidobacterium longumLC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food & Function. 2018;9(8):4255–4265. doi: 10.1039/C8FO00252E. [DOI] [PubMed] [Google Scholar]
- 29.Chugh B., Kamal-Eldin A. Bioactive compounds produced by probiotics in food products. Current Opinion in Food Science. 2020;32:76–82. doi: 10.1016/j.cofs.2020.02.003. [DOI] [Google Scholar]
- 30.Chen S., Huang G., Liao W., et al. Discovery of the bioactive peptides secreted by Bifidobacterium using integrated MCX coupled with LC -MS and feature-based molecular networking. Food Chemistry. 2021;347, article 129008 doi: 10.1016/j.foodchem.2021.129008. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez A., Vázquez A. Bioactive peptides: a review. Food Quality and Safety. 2017;1(1):29–46. doi: 10.1093/fqs/fyx006. [DOI] [Google Scholar]
- 32.Chichlowski M., Shah N., Wampler J. L., Wu S. S., Vanderhoof J. A. Bifidobacterium longum subspecies infantis (B. infantis) in pediatric nutrition: current state of knowledge. Nutrients. 2020;12(6):p. 1581. doi: 10.3390/nu12061581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J. L., Cai X. J., Gao W., et al. Prussian blue nanozyme with multienzyme activity reduces colitis in mice. ACS Applied Materials & Interfaces. 2018;10(31):26108–26117. doi: 10.1021/acsami.8b10345. [DOI] [PubMed] [Google Scholar]
- 34.Pizzino G., Irrera N., Cucinotta M., et al. Oxidative stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017;2017:13. doi: 10.1155/2017/8416763.8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichele D. D., Kharbanda K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World Journal of Gastroenterology. 2017;23(33):6016–6029. doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chami B., Martin N. J. J., Dennis J. M., Witting P. K. Myeloperoxidase in the inflamed colon: a novel target for treating inflammatory bowel disease. Archives of Biochemistry and Biophysics. 2018;645:61–71. doi: 10.1016/j.abb.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Gu Z., Liu Y., Hu S., et al. Probiotics for alleviating alcoholic liver injury. Gastroenterology Research and Practice. 2019;2019:8. doi: 10.1155/2019/9097276.9097276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rana S. V., Sharma S., Prasad K. K., Sinha S. K., Singh K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from North India. Indian Journal of Medical Research. 2014;139:568–571. [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z. W., Ren Z. P., Zhang J., et al. Role of ROS and nutritional antioxidants in human diseases. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abrantes F. A., Nascimento B. B., Andrade M. E. R., et al. Treatment withBifidobacterium longum51A attenuates intestinal damage and inflammatory response in experimental colitis. Beneficial Microbes. 2020;11(1):47–57. doi: 10.3920/BM2019.0098. [DOI] [PubMed] [Google Scholar]
- 41.Kwon E. K., Kang G. D., Kim W. K., Han M. J., Kim D. H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate ethanol-induced gastritis and hepatic injury in mice. Journal of Functional Foods. 2017;38:389–398. doi: 10.1016/j.jff.2017.09.036. [DOI] [Google Scholar]
- 42.Wang Y. S., Fang Z. F., Zhai Q. X., et al. Supernatants of Bifidobacterium longum and Lactobacillus plantarum strains exhibited antioxidative effects on A7R5 cells. Microorganisms. 2021;9(2):p. 452. doi: 10.3390/microorganisms9020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo F., Yu R., Xiao M., et al. Transcriptomic analysis of Bifidobacterium longum subsp. longum BBMN68 in response to oxidative shock. Scientific Reports. 2018;8(1, article 17085) doi: 10.1038/s41598-018-35286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Q., Li S., Xie Y., et al. The NAD+-dependent deacetylase, Bifidobacterium longum Sir2 in response to oxidative stress by deacetylating SigH (σH) and FOXO3a in Bifidobacterium longum and HEK293T cell respectively. Free Radical Biology and Medicine. 2017;108:929–939. doi: 10.1016/j.freeradbiomed.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Huang G., Pan H., Zhu Z., Li Q. The complete genome sequence of Bifidobacterium longum LTBL16, a potential probiotic strain from healthy centenarians with strong antioxidant activity. Genomics. 2020;112(1):769–773. doi: 10.1016/j.ygeno.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Carmel-Harel O., Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annual Review of Microbiology. 2000;54(1):439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- 47.Xiao M., Xu P., Zhao J. Y., et al. Oxidative stress-related responses of Bifidobacterium longum subsp. longum BBMN68 at the proteomic level after exposure to oxygen. Microbiology. 2011;157(6):1573–1588. doi: 10.1099/mic.0.044297-0. [DOI] [PubMed] [Google Scholar]
- 48.Zeller T., Klug G. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften. 2006;93(6):259–266. doi: 10.1007/s00114-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 49.Kitade M., Ogura Y., Monno I., Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Frontiers in Endocrinology. 2019;10 doi: 10.3389/fendo.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinert B. Friendly tenants in the human gut: The genome of B. longum. Genome News Network; 2002. 2021, http://www.genomenewsnetwork.org/articles/10_02/bifido.shtml. [Google Scholar]
- 51.Choi M., Lee Y., Lee N. K., et al. Immunomodulatory effects by Bifidobacterium longum KACC 91563 in mouse splenocytes and macrophages. Journal of Microbiology and Biotechnology. 2019;29(11):1739–1744. doi: 10.4014/jmb.1812.12002. [DOI] [PubMed] [Google Scholar]
- 52.Tang C., Ding R. X., Sun J., Liu J., Kan J., Jin C. H. The impacts of natural polysaccharides on intestinal microbiota and immune responses - a review. Food & Function. 2019;10(5):2290–2312. doi: 10.1039/C8FO01946K. [DOI] [PubMed] [Google Scholar]
- 53.Jang D. I., Lee A. H., Shin H. Y., et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. International Journal of Molecular Sciences. 2021;22(5):p. 2719. doi: 10.3390/ijms22052719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Passelli K., Billion O., Tacchini-Cottier F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Frontiers in Immunology. 2021;12(446) doi: 10.3389/fimmu.2021.649348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tangye S. G., Ma C. S., Brink R., Deenick E. K. The good, the bad and the ugly -- TFH cells in human health and disease. Nature Reviews. Immunology. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 56.Zhao L., Wu H., Zhao A., et al. The in vivo and in vitro study of polysaccharides from a two-herb formula on ulcerative colitis and potential mechanism of action. Journal of Ethnopharmacology. 2014;153(1):151–159. doi: 10.1016/j.jep.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Zhang T., Jiang J., Liu J., et al. MK2 is required for neutrophil-derived ROS production and inflammatory bowel disease. Frontiers in Medicine. 2020;7 doi: 10.3389/fmed.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pott J., Kabat A. M., Maloy K. J. Intestinal epithelial cell autophagy is required to protect against TNF- induced apoptosis during chronic colitis in mice. Cell Host & Microbe. 2018;23(2):191–202.e4. doi: 10.1016/j.chom.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Singh S., Bhatia R., Khare P., et al. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-75702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato N., Yuzawa M., Aminul M. I., et al. Evaluation of porcine intestinal epitheliocytes as an in vitro immunoassay system for the selection of probiotic bifidobacteria to alleviate inflammatory bowel disease. Probiotics and Antimicrobial Proteins. 2021;13(3):824–836. doi: 10.1007/s12602-020-09694-z. [DOI] [PubMed] [Google Scholar]
- 61.MacPherson C. W., Shastri P., Mathieu O., Tompkins T. A., Burguiere P. Genome-wide immune modulation of TLR3-mediated inflammation in intestinal epithelial clls differs between single and multi-strain probiotic combination. Plos One. 2017;12(1, article e0169847) doi: 10.1371/journal.pone.0169847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martorell P., Alvarez B., Llopis S., et al. Heat-treated Bifidobacterium longum CECT-7347: a whole-cell postbiotic with antioxidant, anti-inflammatory, and gut-barrier protection properties. Antioxidants. 2021;10(4):p. 536. doi: 10.3390/antiox10040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowarski R., Jackson R., Gagliani N., et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163(6):1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bevivino G., Monteleone G. Advances in understanding the role of cytokines in inflammatory bowel disease. Expert Review of Gastroenterology & Hepatology. 2018;12(9):907–915. doi: 10.1080/17474124.2018.1503053. [DOI] [PubMed] [Google Scholar]
- 65.Saxena A., Lopes F., McKay D. M. Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli. Inflammation Research. 2018;67(10):829–837. doi: 10.1007/s00011-018-1172-5. [DOI] [PubMed] [Google Scholar]
- 66.Lohrasbi V., Abdi M., Asadi A., et al. The effect of improved formulation of chitosan-alginate microcapsules of Bifidobacteria on serum lipid profiles in mice. Microbial Pathogenesis. 2020;149, article 104585 doi: 10.1016/j.micpath.2020.104585. [DOI] [PubMed] [Google Scholar]
- 67.Sichetti M., De Marco S., Pagiotti R., Traina G., Pietrella D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis and B. longum) Nutrition. 2018;53:95–102. doi: 10.1016/j.nut.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Li M., van Esch B., Wagenaar G. T. M., Garssen J., Folkerts G., Henricks P. A. J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. European Journal of Pharmacology. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol. 2012;3 doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amin S. A., Adhikari N., Jha T. Structure-activity relationships of HDAC8 inhibitors: non-hydroxamates as anticancer agents. Pharmacological Research. 2018;131:128–142. doi: 10.1016/j.phrs.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Celiberto L. S., Bedani R., Dejani N. N., et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. Plos One. 2017;12(4):p. e0175935. doi: 10.1371/journal.pone.0175935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan S., Yang B., Zhao J. C., et al. A ropy exopolysaccharide producing strainBifidobacterium longumsubsp.longumYS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food & Function. 2019;10(3):1595–1608. doi: 10.1039/C9FO00014C. [DOI] [PubMed] [Google Scholar]
- 73.Silva A. K. S., Silva T. R. N., Nicoli J. R., Vasquez-Pinto L. M. C., Martins F. S. In vitroevaluation of antagonism, modulation of cytokines and extracellular matrix proteins byBifidobacteriumstrains. Letters in Applied Microbiology. 2018;67(5):497–505. doi: 10.1111/lam.13062. [DOI] [PubMed] [Google Scholar]
- 74.in Kim H., Kim J. K., Kim J. Y., Jang S. E., Han M. J., Kim D. H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutrition Research. 2019;67:78–89. doi: 10.1016/j.nutres.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2(1, article 17023) doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Oliveira D. C., Hastreiter A. A., Mello A. S., et al. The effects of protein malnutrition on the TNF-RI and NF-κB expression via the TNF-α signaling pathway. Cytokine. 2014;69(2):218–225. doi: 10.1016/j.cyto.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Neurath M. F., Becker C., Barbulescu K. Role of NF-κB in immune and inflammatory responses in the gut. Gut. 1998;43(6):856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Viatour P., Merville M. P., Bours V., Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends in Biochemical Sciences. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Jang S. E., Jeong J. J., Kim J. K., Han M. J., Kim D. H. Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Scientific Reports. 2018;8(1):p. 7500. doi: 10.1038/s41598-018-25775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakurai T., Odamaki T., Xiao J. Z. Production of indole-3-lactic acid by bifidobacterium strains isolated fromhuman infants. Microorganisms. 2019;7(9):p. 340. doi: 10.3390/microorganisms7090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roager H. M., Licht T. R. Microbial tryptophan catabolites in health and disease. Nature Communications. 2018;9(1):p. 3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng D., Sommella E., Salviati E., et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatric Research. 2020;88(2):209–217. doi: 10.1038/s41390-019-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehrlich A. M., Pacheco A. R., Henrick B. M., et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiology. 2020;20(1):p. 357. doi: 10.1186/s12866-020-02023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X. H., Fu Y., Wang L. L., Qian W., Zheng F., Hou X. H. Bifidobacterium longum and VSL3 ® amelioration of TNBS-induced colitis associated with reduced HMGB1 and epithelial barrier impairment. Developmental and Comparative Immunology. 2019;92:77–86. doi: 10.1016/j.dci.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Strober W., Fuss I., Mannon P. The fundamental basis of inflammatory bowel disease. The Journal of Clinical Investigation. 2007;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanderpool C., Yan F., Polk B. D. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflammatory Bowel Diseases. 2008;14(11):1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 87.Buckley A., Turner J. R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harbor Perspectives in Biology. 2018;10(1) doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber L.-M., Fischer A., Scholz S., Metzke D., Baumgart D. C. Impact of proinflammatory cytokines and mesalamine on the expression of the tight junction protein claudin-1 in intestinal epithelial cells. Gastroenterology. 2017;152(5):p. S740. doi: 10.1016/S0016-5085(17)32573-8. [DOI] [Google Scholar]
- 89.Schoultz I., Keita Å. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells. 2019;8(2):p. 193. doi: 10.3390/cells8020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wirtz S., Popp V., Kindermann M., et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nature Protocols. 2017;12(7):1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 91.Fattahi Y., Heidari H. R., Khosroushahi A. Y. Review of short-chain fatty acids effects on the immune system and cancer. Food Bioscience. 2020;38 doi: 10.1016/j.fbio.2020.100793. [DOI] [Google Scholar]
- 92.Ghosh S., Whitley C. S., Haribabu B., Jala V. R. Regulation of intestinal barrier function by microbial metabolites. Cellular and Molecular Gastroenterology and Hepatology. 2021;11(5):1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chambers E. S., Preston T., Frost G., Morrison D. J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Current Nutrition Reports. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang K., Jin X., Li Q., et al. Propolis from different geographic origins decreases intestinal inflammation and bacteroidesspp. Populations in a model of DSS-induced colitis. Molecular Nutrition & Food Research. 2018;62(17, article 1800080) doi: 10.1002/mnfr.201800080. [DOI] [PubMed] [Google Scholar]
- 95.Khan I., Ullah N., Zha L. J., et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019;8(3):p. 126. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans C. C., LePard K. J., Kwak J. W., et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. Plos One. 2014;9(3):p. e92193. doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanders M. E., Merenstein D. J., Reid G., Gibson G. R., Rastall R. A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nature Reviews Gastroenterology & Hepatology. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 98.Xiong Y., Zhai Z. Y., Lei Y. Q., Xiao B. B., Hao Y. L. A novel major pilin subunit protein FimM is involved in adhesion of Bifidobacterium longum BBMN68 to intestinal epithelial cells. Frontiers in Microbiology. 2020;11 doi: 10.3389/fmicb.2020.590435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemalatha R., Ouwehand A. C., Saarinen M. T., Prasad U. V., Swetha K., Bhaskar V. Effect of probiotic supplementation on total lactobacilli, bifidobacteria and short chain fatty acids in 2–5-year-old children. Microbial Ecology in Health and Disease. 2017;28(1, article 1298340) doi: 10.1080/16512235.2017.1298340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song W. S., Park H. G., Kim S. M., et al. Chemical derivatization-based LC-MS/MS method for quantitation of gut microbial short-chain fatty acids. Journal of Industrial and Engineering Chemistry. 2020;83:297–302. doi: 10.1016/j.jiec.2019.12.001. [DOI] [Google Scholar]
- 101.Park H. E., Kim Y. J., Kim M., et al. Effects of Queso Blanco cheese containing Bifidobacterium longum KACC 91563 on fecal microbiota, metabolite and serum cytokine in healthy beagle dogs. Anaerobe. 2020;64:p. 102234. doi: 10.1016/j.anaerobe.2020.102234. [DOI] [PubMed] [Google Scholar]
- 102.Tamaki H., Nakase H., Inoue S., et al. Efficacy of probiotic treatment withBifidobacterium longum 536for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Digestive Endoscopy. 2016;28(1):67–74. doi: 10.1111/den.12553. [DOI] [PubMed] [Google Scholar]
- 103.Caviglia G. P., Tucci A., Pellicano R., et al. Clinical response and changes of cytokines and zonulin levels in patients with diarrhoea-predominant irritable bowel syndrome treated with Bifidobacterium Longum ES1 for 8 or 12 weeks: a preliminary report. Journal of Clinical Medicine. 2020;9(8):p. 2353. doi: 10.3390/jcm9082353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steed H., Macfarlane G. T., Blackett K. L., et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn's disease. Alimentary Pharmacology & Therapeutics. 2010;32(7):872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- 105.Furrie E., Macfarlane S., Kennedy A., et al. Synbiotic therapy (Bifidobacterium longum/synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54(2):242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sood A., Midha V., Makharia G. K., et al. The probiotic preparation, VSL3 induces remission in patients with mild-to- moderately active ulcerative colitis. Clinical Gastroenterology and Hepatology. 2009;7(11):1202–1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 107.Fedorak R. N., Feagan B. G., Hotte N., et al. The probiotic VSL3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clinical Gastroenterology and Hepatology. 2015;13(5):928–935.e2. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 108.Miele E., Pascarella F., Giannetti E., Quaglietta L., Baldassano R. N., Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. The American Journal of Gastroenterology. 2009;104(2):437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 109.Tursi A., Brandimarte G., Papa A., et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. The American Journal of Gastroenterology. 2010;105(10):2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tursi A., Brandimarte G., Giorgetti G. M., Forti G., Modeo M. E., Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Medical Science Monitor. 2004;10(11):PI126–PI131. [PubMed] [Google Scholar]
- 111.Bibiloni R., Fedorak R. N., Tannock G. W., et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. The American Journal of Gastroenterology. 2005;100(7):1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 112.Toscano M., De Grandi R., Stronati L., De Vecchi E., Drago L. Effect ofLactobacillus rhamnosusHN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: a preliminary study. World Journal of Gastroenterology. 2017;23(15):2696–2704. doi: 10.3748/wjg.v23.i15.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mauras A., Chain F., Faucheux A., et al. A new Bifidobacteria Expression SysTem (BEST) to produce and deliver interleukin-10 in Bifidobacterium bifidum. Frontiers in Microbiology. 2018;9 doi: 10.3389/fmicb.2018.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dosoky N. S., May-Zhang L. S., Davies S. S. Engineering the gut microbiota to treat chronic diseases. Applied Microbiology and Biotechnology. 2020;104(18):7657–7671. doi: 10.1007/s00253-020-10771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ventura M., Turroni F., van Sinderen D. Chapter 4 - Bifidobacteria of the Human Gut: Our Special Friends. In: Tuohy K., Rio D., editors. Diet-Microbe Interactions in the Gut. San Diego: Academic Press; 2015. pp. 41–51. [Google Scholar]
- 116.Gao R. Y., Zhang X. H., Huang L. S., Shen R. R., Qin H. L. Gut microbiota alteration after long-term consumption of probiotics in the elderly. Probiotics and Antimicrobial Proteins. 2019;11(2):655–666. doi: 10.1007/s12602-018-9403-1. [DOI] [PubMed] [Google Scholar]
- 117.Kaur M., Dalal R. L., Shaffer S., Schwartz D. A., Rubin D. T. Inpatient management of inflammatory bowel disease-related complications. Clinical Gastroenterology and Hepatology. 2020;18(6):1346–1355. doi: 10.1016/j.cgh.2019.12.040. [DOI] [PubMed] [Google Scholar]


