Abstract
In 2019, a large outbreak of a novel coronavirus disease (COVID-19) occurred in China. The purpose of this study is to quantitatively analyze the evolution of chest computed tomography (CT) imaging features in COVID-19. Nine patients with positive real-time reverse-transcriptase polymerase chain reaction results were included in this study. Totally 19 CT scans were analyzed. Lesion density, lesion volume, and lesion load were higher in the severe group than in the mild group. A significantly positive correlation was noted between major laboratory prognosticators with lesion volume and load. Lesion load at the first week of disease was significantly higher in severe group (p = 0.03). Our study revealed that several CT features were significantly different between severely and mildly infected forms of COVID-19 pneumonia. The CT lesion load value at the first week of infection may be applied as an outcome predictor of the disease.
Keywords: COVID19, Coronavirus, Computed tomography, Prognosticator
Introduction
In December 2019, a large outbreak of a novel coronavirus infection occurred in Wuhan, Hubei Province, China.1 , 2 On January 30, 2020, the World Health Organization declared a global public health emergency against the outbreak of coronavirus disease 2019 (COVID-19) due to its rapid spread worldwide.3 Although most patients have mild symptoms and good prognosis, patients with severe COVID-19 may present with acute respiratory distress syndrome (ARDS) and life-threatening systemic inflammation. Because the time between symptom onset and ARDS development can be as short as 9 days, early diagnosis of COVID-19 is essential for appropriate patient management. Several studies have proved the value of different biomarkers in representing disease severity. Moreover, computed Tomography (CT) was reported as a very sensitive tool in disease diagnose and clinical severity indication along the disease course. The major CT features of COVID-19 pneumonia include ground-glass opacities (GGO, 88 %) and consolidations (32 %) distributed bilaterally (88 %) in the peripheral lung zones (76 %).4 Evolution of the CT features along disease course was also delineated.5 In the early phase (0–4 days), GGO is the most common finding. During the progressive phase (5–8 days), progression of the number and size of GGO with gradual transformation into multiple consolidations and the development of a “crazy-paving” pattern. More extensive involvement of bilateral lung with dense consolidations are noted in the peak stage (9–13 days). In the absorption stage, consolidations were resolved with appearance of fibrotic bands. However, the relationship between CT features and important serum prognosticators are still unclear. In this study, we aimed to quantify the evolution of chest CT imaging features in patients with COVID-19 treated in Taiwan. Moreover, several blood-based biomarkers were analyzed and compared with imaging findings. Furthermore, the predictive imaging feature is proposed to predict the clinical course of severe and mild diseases.
Materials and methods
Patient information
The laboratory and CT data of nine patients with confirmed COVID-19 were collected retrospectively in compliance with all applicable laws, regulations, and policies for the protection of human patients. The institutional review board approved this study. A confirmed case was defined as positive for real-time reverse-transcriptase polymerase-chain-reaction (rRT-PCR) assay of nasopharyngeal swab specimens.1 All patients were divided into two groups according to disease severity. Patients experiencing any respiratory distress (increased respiratory rate with decreased oxygen saturation under room air) during the whole course of the disease were assigned to the severe group. Patients with no obvious symptoms and signs of respiratory distress during the entire disease course were assigned to the mild group. The values of several blood-based biomarkers were collected along the entire disease course, including white blood cells (WBC), lactate dehydrogenase (LDH), C-reactive protein (CRP), and ferritin. The first day of the disease was defined as typical symptom onset including cough, fever, myalgia or fatigue, sore throat, dyspnea, diarrhea, and nausea and vomiting.6 For patients with asymptomatic infection, day 1 was defined as the day when the disease was confirmed using a PCR test.
Image analysis
All CT scans were performed on a 64 slice CT unit (SOMATOM Perspective, Siemens Healthcare) at 110 kVp tube voltage, 80 mAs reference tube current, 0.5s gantry rotation time, pitch 0.8, detector collimation of 64 × 0.6 mm with z-flying focal spot. All images were reconstructed with a slice thickness of 5 mm and increment of 5 mm in the axial plane using a corresponding lung kernel. Nonenhanced CT images displayed in lung window (level: −200 Hounsfield units [HU]; width: 2000 HU) were used for image analysis. A board-certified radiologist (K.H., with 14 years of experience) blinded to clinical information and disease severity delineated each lesion manually using OsiriX software (Pixmeo, Geneva, Switzerland). All area with signal intensity higher than adjacent normal-appearing lung parenchyma was included in the regions of interest as the involved parenchyma. The regions of interest were confirmed and revised by another senior radiologist (G.Y.L., with 30 years of experience). Image pixels enclosed by the defined lesion contour were used for further calculation and analysis. The lesion density represents the mean value of the HU measured from all lesions in each case. The lesion volume is the summation of the measured volume of all lesions in each case. The lesion load was defined as {the measured density (HU) − air density} × lesion volume, which stands for the increased density within the pulmonary airspace and the extent of lesion involvement.
Statistical analysis
Since patients not always have CT and blood test at the same day, both the imaging and serum data were separated into 4 time periods along the disease course: day 1–3, day 4–7, day 8–14 and after day 15th. The mean values of the biomarkers in each time period of each case were calculated. And the correlation tests between CT features and serum biomarkers were performed according to the abovementioned data subsets.
The Spearman's rank-order correlation test was used to calculate the correlation between blood-based biomarkers and imaging features. The nonparametric Mann–Whitney U test was used for comparing the values of blood-based biomarkers and different imaging features between patients with severe and mild disease. All statistical calculation was conducted using Prism software (release 8.0, GraphPad Software Inc., La Jolla, CA, USA). A p value of 0.05 was considered statistically significant.
Results
Nine patients with confirmed COVID-19 pneumonia were included in this study. Two of them presented with asymptomatic infection. The first patient was admitted to our hospital on March 16, 2020, and the last patient was admitted on April 25, 2020. The mean admission days was 23.6 (20–42) days. The cohort consisted of two men and seven women with a mean age of 41.2 (±16.2, 22–66) years. No COVID-19-related deaths was detected. Complete laboratory data, including nasopharyngeal RT-PCR test for COVID-19, complete blood count, blood chemistry tests, and necessary serum inflammatory biomarkers, were collected from all patients. Serial CT scans were conducted at every 1–3 week interval, and in total, 19 chest CT examinations were performed in all these nine patients.
Blood-based biomarker findings
High values of LDH, CRP, and ferritin were noted in patients of the severe group, especially of ferritin. These biomarker levels increased from day 3 of symptom onset and remained constant until day 12. The values of all biomarkers returned to normal after day 20, except ferritin, which remained at an abnormally high level until day 40. The WBC count was not considerably different between both groups.
Chest CT imaging findings
All our patients had typical CT presentations of COVID-19: multifocal ground-glass opacities with peripheral distribution.7 , 8 Consolidation and subpleural atelectasis were prevalent in severe cases. No pleural effusion could be identified in any of our patients (Fig. 1 ). In quantitative analysis, lesion density, volume, and load were higher in the severe group than in the mild group. These values increased at disease onset and reached the peak approximately 7–9 days after disease onset.
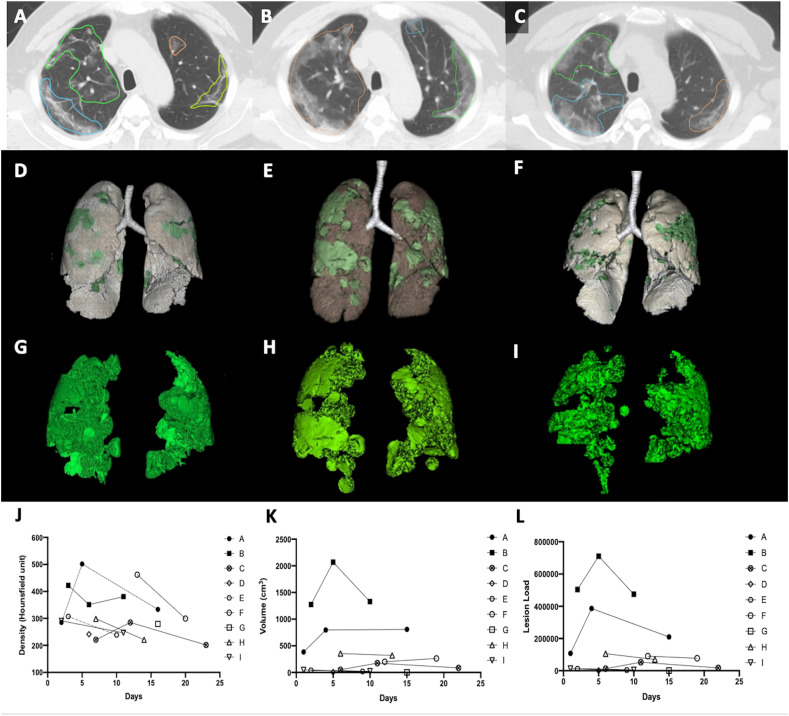
Figure 1.
Serial change in the chest computed tomography image features of a patient with severe coronavirus disease 2019 pneumonia. Increase in lesion volume was noted on day 6 (B, E, H) compared with that on day 3 (A, D,G) and then decreased on day 11 (C, F, I). The individual values of lesion density (J), lesion volume (K), and lesion load (L) were higher in the severe group (black symbols) than in the mild group (white symbols) along the entire course of disease. The phenomenon is most significant in lesion load feature (L).
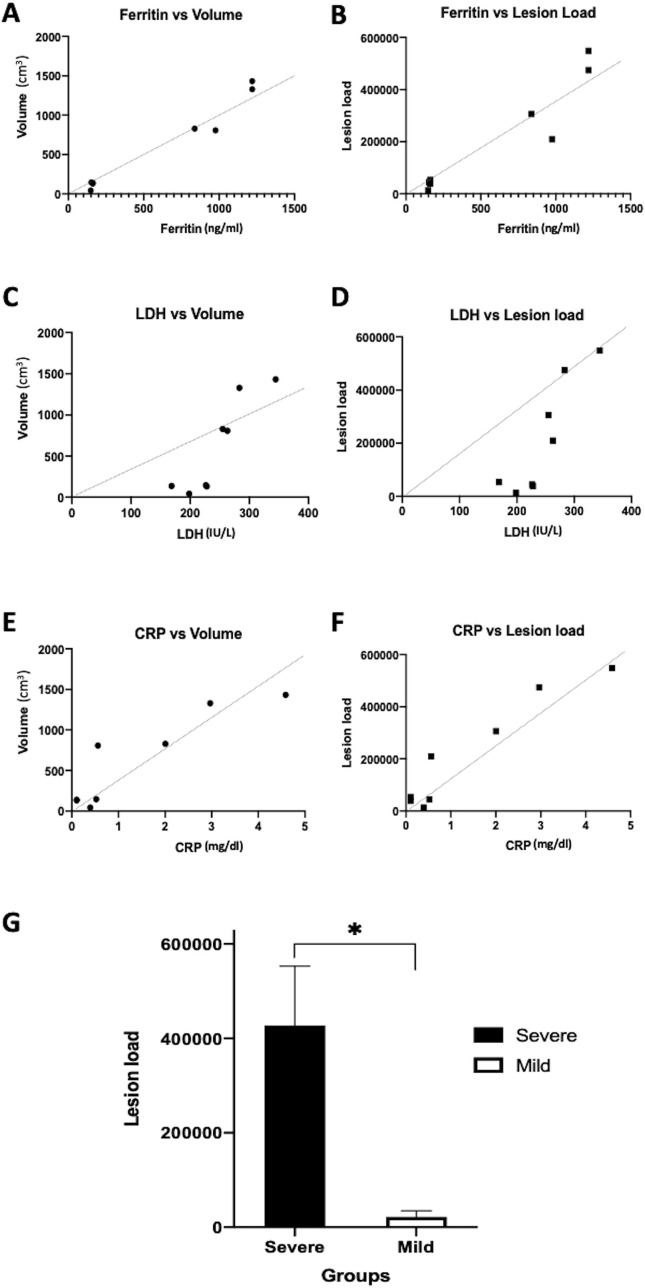
Regarding the association between serum biomarkers and chest CT images, Spearman's correlation results showed a positive correlation between LDH with lesion volume (r = 0.86, p = 0.01), LDH with lesion load (r = 0.81, p = 0.02), ferritin with lesion volume (r = 0.88, p = 0.01), ferritin with lesion load (r = 0.92, p < 0.01), CRP with lesion volume (r = 0.92, p < 0.01), and CRP with lesion load (r = 0.01, p < 0.01) (Fig. 2 ).
Figure 2.
Correlation results between computed tomography findings and major serum prognosticators. Significant correlation between ferritin with lesion volume (A), ferritin with lesion load (B), lactate dehydrogenase with lesion volume (C), lactate dehydrogenase with lesion load (D), C-reactive protein with lesion volume (E), and C-reactive protein with lesion load (F) are noted. (G) Significant difference of lesion load values at the first week between the severely and mildly infected groups is noted, which may be applied a predictor of disease prognosis.
To determine the predictive value of CT imaging features, we compared the mean values of lesion density, volume, and load at the first week of the disease between both groups. Only lesion load at the first week of disease was significantly different between mild and severe groups (445395 vs 13060, p = 0.03, Fig. 2G). The lesion density and volume were not significantly different between both the groups (p = 0.08 and 0.06, respectively).
Discussion
In this retrospective observational study, several imaging biomarkers including lesion density, volume, and load could distinguish between the severe and mild forms of COVID-19 pneumonia. The lesion volume and load were well correlated with ferritin, LDH, and CRP levels along the entire disease course. Moreover, we found that the first week CT lesion load value is a predictor of disease prognosis.
COVID-19 is associated with variable prognosis. Most patients presented flu-like symptoms only. However, up to 17 % of patients may experience respiratory failure and require artificial ventilation. The overall mortality of patients with COVID-19 pneumonia ranges from 11 % to 15 %.1 , 9 In our cohort, only two patients (22.3 %) experienced respiratory distress. Two patients remained asymptomatic during the entire admission course. All patients were discharged uneventfully within 42 days.
In recent studies, positive chest CT findings have been proposed to be a diagnostic criterion for COVID-19 pneumonia.10 However, wide application of CT scan is limited by poor specificity and high false-positive rate of CT findings related to COVID-19. Different quantification methods of determining disease extent through chest CT have been proposed, including emphysema, pulmonary fibrosis, and ARDS.11 , 12 Li et al. proposed triaging patients based on the visual quantitative score of CT findings.13 Although the score correlated with clinical severity well, the study did not indicate the timing of CT image acquisition. Therefore, whether the score can be used to predict the clinical disease course is unclear. Davide et al. applied the proportion of disease-involved volume as a predictor of patient outcome.14 However, determining “involved” lung parenchyma is sometimes subjective because ground glass opacities can be faint, and the density may be close to normal parenchyma. Wang et al. applied an artificial intelligence (AI) system to auto-segment the COVID-19 related CT lesions and also found difference of the volume, density, and location of the pulmonary opacity between different disease severity.15 However, the accuracy of the AI-algorithm may need more verification in COVID-19 pneumonia. Therefore, we proposed that the lesion load is a reliable method of quantifying disease severity, considering both the involved lung volume and the amount of fluid accumulation within pulmonary air spaces. Our result revealed that the value of lesion load is higher in the severe group than that in the mild group (Fig. 1L). Moreover, it correlated with CRP, LDH, and ferritin very well (Fig. 2B,D,F). Furthermore, it is the only imaging method that can predict disease prognosis at an early stage of the disease (Fig. 2G).
Elevated levels of CRP, LDH, and ferritin have been reported as poor prognosticators in patients with COVID-19.1 , 16, 17, 18 The serum levels of LDH, CRP, and ferritin in our cohort markedly increased in patients in the severe group as compared with patients in the mild group, which is compatible with previous studies. Additionally, our results revealed that both lesion volume and load obtained through CT scans significantly correlated with the aforementioned serum markers. This finding reinforced our assumption that imaging features can be prognosticators of COVID-19 pneumonia. Other serum prognosticators including lymphopenia, neutrophil/lymphocyte ratio, interleukin-6, procalcitonin, D-Dimer, and other coagulation abnormalities were also reported before.17
Our study has several limitations. First, the retrospective design and the small patient cohort may limit the generalizability of our results. Second, the delineation of all pulmonary lesions in CT images was conducted manually with the consensus of two radiologists. Subjective interpretation of lesion territories can lead to bias in this study. Third, comorbidities such as hypertension, diabetes mellitus, and smoking history were not considered in our analysis.
In conclusion, our study revealed that lesion density, volume, and load detected by CT images were significantly different between severe and mild forms of COVID-19 pneumonia. The lesion volume and load were well correlated with several serum prognostic markers along the entire disease course. The CT lesion load value at the first week may be applied as a predictor of disease prognosis.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing as- sistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgments and have given us their written permission to be named. If we have not included an Acknowledgments, then that indicates that we have not received substantial contributions from non-authors.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majumder M., Mandl K.D. Early transmissibility assessment of a novel coronavirus in wuhan, China. SSRN. 2020:3524675. [Google Scholar]
- 3.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Trav Med. 2020;27 doi: 10.1093/jtm/taaa021. taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 5.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (covid-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 7.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., et al. CT imaging features of 2019 novel coronavirus (2019-NCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020;295:18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin G.D., Ryerson C.J., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158:106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romei C., Tavanti L.M., Taliani A., De Liperi A., Karwoski R., Celi A., et al. Automated computed tomography analysis in the assessment of idiopathic pulmonary fibrosis severity and progression. Eur J Radiol. 2020;124:108852. doi: 10.1016/j.ejrad.2020.108852. [DOI] [PubMed] [Google Scholar]
- 13.Li K., Fang Y., Li W., Pan C., Qin P., Zhong Y., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombi D., Bodini F.C., Petrini M., Maffi G., Morelli N., Milanese G., et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:186–196. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y.C., Luo H., Liu S., Huang S., Zhou Z., Yu Q., et al. Dynamic evolution of COVID-19 on chest computed tomography: experience from Jiangsu Province of China. Eur Radiol. 2020;30:6194–6203. doi: 10.1007/s00330-020-06976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bataille S., Pedinielli N., Bergougnioux J.-P. Could ferritin help the screening for COVID-19 in hemodialysis patients? Kidney Int. 2020;98:235–236. doi: 10.1016/j.kint.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]